Abstract
Introduction
Parkinson’s Disease (PD) is a progressive neurodegenerative disease. Increasing evidence shows that physical exercise is beneficial for motor and non-motor symptoms of PD, and animal models suggest that it may help slow progression of disease.
Methods
Using a randomized delayed-start design, 31 patients were randomized to an early start group (ESG) or a delayed start group (DSG) exercise program. The ESG underwent a rigorous formal group exercise program for 1 h, three days/week, for 48 weeks (November 2011–October 2012). The DSG participated in this identical exercise program from weeks 24–48. Outcome measures included the Unified Parkinson’s Disease Rating Scale (UPDRS), Walking Test (get-up-and-go), Tinetti Mobility Test, PDQ-39 Questionnaire, and the Beck Depression Inventory.
Results
There was minimal attrition in this study, with only one patient dropping out. Results did not show improvement in total UPDRS scores with early exercise. At week 48, the mean change from baseline total UPDRS score was 6.33 in the ESG versus 5.13 in the DSG (p = 0.58). However, patients randomized to the ESG scored significantly better on the Beck Depression Inventory, with a mean improvement of 1.07 points relative to those in the DSG (p = 0.04).
Conclusions
The findings demonstrate that long-term, group exercise programs are feasible in the Parkinson’s disease population, with excellent adherence and minimal drop out. While the outcome measures used in our study did not provide strong evidence that exercise has a neuroprotective effect on motor function, earlier participation in a group exercise program had a significant effect on symptoms of depression.
Keywords: Parkinson’s disease, Exercise, Tinetti, Neuroprotection, Depression
1. Introduction
Parkinson’s disease (PD) is the second most common progressive neurodegenerative condition in the United States, characterized by the motor symptoms of bradykinesia, rigidity, and resting tremor. It has been estimated that approximately 630,000 people in the United States had the diagnosis of PD in 2010, and prevalence of PD is expected to double by 2040, which will substantially increase the economic burden of this disease [1]. While motor symptoms and the dopaminergic system have long been the primary focus of this disease, it is now recognized that widespread involvement of various non-dopaminergic pathways also contribute to the symptoms of PD. Furthermore, it is increasingly clear that the non-motor symptoms of PD, including depression and anxiety, are often more bothersome to patients than their motor symptoms. Recently, the National Parkinson’s Foundation Quality Improvement Initiative (QII) data demonstrated that the depression affects health status almost twice as much as motor impairment [2].
Countless studies have shown that a variety of exercises improve the symptoms of PD, including home based exercise [3], treadmill [4], resistance exercise [5], tango dancing [6], tai chi [7], and robot-assisted gait training [8]. The LSVT®BIG therapy is derived from the Lee Silverman Voice Treatment, and focuses on intensive exercising of high-amplitude movements. This therapy has been shown to be an effective technique for improving motor performance in patients with PD, with significant improvements seen in Unified Parkinson’s Disease Rating Scale (UPDRS) motor scores [9]. At this time, there are no specific recommendations on what type of exercise is most beneficial in PD, leading most clinicians to suggest any routine leading to improved physical fitness.
While there has been a strong research interest in identifying potential “neuroprotective” therapies that might slow down progression of PD, currently none have proven clinically effective. Large cohort studies have shown that vigorous exercise in midlife significantly reduces risk of developing PD [10–12]. In addition, longevity in PD has been associated with exercise [13]. Thus, if exercise may be involved in reducing the risk of PD, it is possible that it may play a role in slowing down disease progression. In 6-OH-DA rodent models of PD, studies have shown that parkinsonian deficits are attenuated by exercise [14]. Conversely, nonuse via cast immobilization of the parkinsonian side significantly exacerbates motor deficit [15], suggesting that limb disuse may lead to further neurodegeneration. In MPTP rodent models, exercise appears to have a protective effect on dopamine neurons from acute MPTP toxicity [16]. Additional findings have suggested that exercise may attenuate the hyperexcitability of striatal neurons seen after dopamine depletion, possibly via modulation of glutamatergic receptor subunit expression [17]. It is known that vigorous exercise induces brain neurotrophic factor expression [18], and both brain derived neurotrophic factor (BDNF) and glial cell line-derived neurotropic factor (GDNF) have been shown to be decreased in the substantia nigra of patients with PD [19]. It may be that neurotrophic growth factors reduce the vulnerability of DA neurons, thus conferring neuroprotective benefit.
To our knowledge, this is the first study to look at the feasibility of conducting a long-term formal group exercise program in PD, using a randomized delayed start design. This type of study design aims to separate disease modifying/neuroprotective effects from symptomatic effects. Thus, our goal was to gain information on the potential neuroprotective effects of exercise, with the primary outcome measure being total UPDRS score. Furthermore, we explored whether early exercise may confer non-motor benefit in terms of depression and quality of life.
2. Methods
Thirty-one patients with idiopathic PD were selected over a six month period. All consecutive patients referred to our movement disorder center who met inclusion criteria were approached for enrollment. The following inclusion criteria were chosen: 1) Age 40–70 years old diagnosed with PD within three years of symptom onset with a Hoehn and Yahr stage 1 or 2, 2) Participants met the UK Parkinson’s Disease Brain Bank criteria [20], 3) Subjects could be on either no anti-parkinsonian medications, or could be taking amantadine, monoamine oxidase B inhibitors, and/or dopamine agonists, and 4) All subjects must have had adequate vision and English sufficient for compliance with testing and surveys. Exclusion criteria were: 1) Hoehn and Yahr stage 3 or higher, 2) Atypical or secondary parkinsonism, 3) Any other condition (other than the primary indications) which in the opinion of the investigators might contribute to gait or balance impairments or complicate its assessment, and 4) Subjects who have been or are on any formulation of levodopa.
Using a delayed start design, participants were randomized to receive either the exercise intervention for both of the 24-week phases (early start group or ESG), or to receive the exercise intervention in the second phase, weeks 24–48, only (delayed start group or DSG). The two phases were designed to capture any symptomatic benefit of the exercise intervention at the end of the first phase, and also any sustained benefit by the end of the study. Research visits were done at baseline, and at weeks 8, 16, 24, 32, 40 and 48 weeks. Attendance was taken at each exercise session, and all participants were required to participate in at least 70% of the exercise sessions in order to remain in the study. At each visit, participants provided a home exercise diary and an updated list of current PD medications. In addition, blinded clinicians conducted the Unified Parkinson’s Disease Rating Scale (UPDRS) [21], Timed Walk [22] to monitor speed of movement and the Tinetti test [23], which assesses gait and balance status, and has been associated with changes in fall risk [24]. To minimize inter-rater variability, only three clinically experienced raters were used for these tests at all visits. During the baseline visit and at the 48-week visit, participants filled out the PDQ-39 questionnaire [25], which is a disease-specific measure of subjective health status, and the Beck Depression Inventory [26], an instrument to assess the severity of depression. In addition, at the baseline visit participants completed a brief demographic survey, and at week 48, participants filled out a brief post-exercise program survey.
The formal group exercise program was led by a personal trainer, and was based on two, 12-week fitness cycles as follows.
2.1. First 12-week cycle (done in a group setting)
Weeks 1–6 concentrated on each participant achieving a baseline fitness level to allow each person to safely begin the formal strength program. This portion of the fitness agenda consisted of a cardiovascular, core strength, and joint integrity plan.
During weeks 7–12, formal strength training was added with a focus on increasing weight intensity while repetitions decrease (repetition numbers from 25 decreasing to 15). The goal was that each participant came to muscle fatigue/failure with each set.
2.2. Second 12-week cycle (done in a group setting)
Weeks 13–14 consisted of cardio/core/joint integrity work without formal strength training.
During weeks 15–24, formal strength training was added, however weight intensity increased further as repetitions decreased to a smaller number (repetition numbers from 25 decreasing to 10), again with the goal of muscle fatigue/failure with each set.
All sessions lasted 1 h, and occurred three times per week for 48 weeks. These 12-week cycles were identical for both the ESG and DSG. After week 24, the ESG repeated the two, 12-week cycles over again. During cardiovascular training, attempts were made to have each participant achieve 75%–85% of their maximum heart rate for a 1-min interval. A CPR/ACLS certified RN was in attendance during each exercise session to further ensure participant safety.
Ethical permission to conduct this study was obtained from the Institutional Review Board of The Ohio State University. Written informed consent was obtained from each participant prior to enrollment. This study was conducted in full accordance with the Declaration of Helsinki.
3. Statistical analysis
For all the randomized subjects, baseline demographics and clinical characteristics were summarized between groups. For each outcome measure, group mean and standard deviation of the change in scores from baseline was reported at each post-randomization visit.
Our primary outcome was change in total UPDRS score from baseline. To assess neuroprotective effect in this delayed start design [27], we tested three endpoints simultaneously, each at the 0.05 significance level. This was done to determine whether any differences seen between the groups was enduring (as would be expected with a disease-modifying effect) and not diminishing (as would be expected with an intervention that had a prolonged and cumulative symptomatic effect). The objective was to test the following hypotheses: (1) superiority of ESG over DSG at week 24 using data from the first phase, (2) superiority of ESG over DSG at week 48 using data from the second phase, and (3) non-inferiority of the rate of change for ESG to DSG for the second phase. Using the upper limit of the one-sided 95% confidence interval (CI) for change in total UPDRS scores between the ESG and DSG during phase 2, a margin of 3.6 UPDRS points was used (0.15 points per week). Endpoints were analyzed through linear mixed effects models [28], using group, week, week-by-group interaction, and the baseline UPDRS score as the fixed effects. Within-subject correlation among the repeated measures was taken into account by an unstructured variance covariance matrix. Other secondary repeated outcomes were analyzed in a similar way. For BDI and PDQ-39, analysis of covariance (ANCOVA) was used to compare the change in score from baseline between groups. All statistical analyses were conducted in SAS (version 9.2, SAS Institute Inc., Cary, NC).
4. Results
Fifteen participants were randomized to the ESG and sixteen to the DSG. For the 48-week course of the study, only one patient dropped out at week 32 due to extensive travel resulting in missing too many visits. All other patients completed the study and had no missing data. There were no adverse events. Patient baseline characteristics were comparable between the two intervention groups in terms of age, gender, weight and employment status. Other characteristics such as hours of exercise per week and previous PD exercise education were not as comparable. The ESG reported more exercise per week at baseline than the DSG (mean number of hours of exercise per week being 6.8 and 4.6 for the ESG and DSG, respectively). Also, the ESG had fewer participants with previous PD exercise education than the DSG (31% and 53%, respectively) (Table 1). Neither of these observed differences was statistically significant.
Table 1.
Baseline demographics and clinical characteristics of the 31 study participants.
| Variable | Level | Group
|
Total | |
|---|---|---|---|---|
| DSG (N = 15) | ESG (N = 16) | |||
| Age | Mean (SD) (min, max) | 60.1 (6.6) (50, 73) | 59.8 (6.3) (51, 69) | 59.9 (6.3) (50, 73) |
| Weight (in pounds) | Mean (SD) (min, max) | 176.8 (20.4) (150, 235) | 178.1 (36.0) (110, 230) | 177.5 (29.1) (110, 235) |
| Falls in last montha | No Falls | 12 (80%)a | 14 (88%) | 26 (84%) |
| Falls in last 6 monthsa | No Falls | 10 (67%)a | 13 (81%) | 23 (74%) |
| Hours of exercise per week | Mean (SD) (min, max) | 4.6 (3.3) (0, 12) | 6.8 (5.2) (1, 17) | 5.8 (4.5) (0, 17) |
| Gender | Male | 10 (67%) | 10 (63%) | 20 (65%) |
| Currently driving | Yes | 14 (93%) | 15 (94%) | 29 (94%) |
| Length of diagnosis | <1 year | 3 (20%) | 1 (6%) | 4 (13%) |
| 1 to <5 years | 11 (73%) | 11 (69%) | 22 (71%) | |
| 5–10 years | 1 (7%) | 4 (25%) | 5 (16%) | |
| Dyskinesia | Yes | 2 (13%) | 2 (13%) | 4 (13%) |
| Depression | Yes | 4 (27%) | 2 (13%) | 6 (19%) |
| Anxiety | Yes | 3 (20%) | 4 (25%) | 7 (23%) |
| Tobacco use | No | 8 (53%) | 12 (75%) | 20 (65%) |
| Previous | 6 (40%) | 4 (25%) | 10 (32%) | |
| Current | 1 (7%) | 0 (0%) | 1 (3%) | |
| Previous PD exercise education? | Yes | 8 (53%) | 5 (31%) | 13 (42%) |
| Currently attend physical therapy | Yes | 1 (7%) | 3 (19%) | 4 (13%) |
| Ever competed in a sport | Yes | 10 (67%) | 12 (75%) | 22 (71%) |
| Marital status | Married | 14 (93%) | 12 (75%) | 26 (83%) |
| Currently working | Yes | 8 (53%) | 10 (63%) | 18 (58%) |
Note: One patient from the DSG reported having fallen 10 times in the last month and 25 times in the last 6 months.
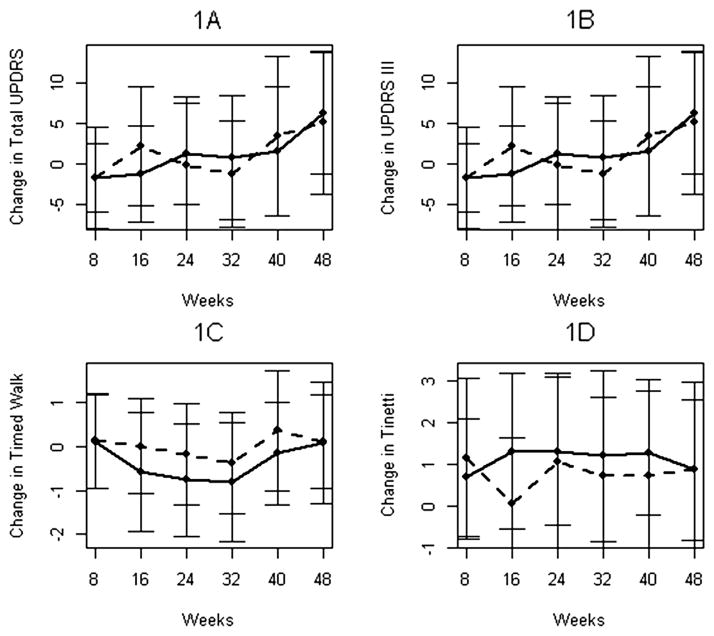
Changes in outcome variables are summarized in Table 2. The group mean plot showed no clear separation between the groups over time (Fig. 1A). For total UPDRS, although the ESG tended to improve more at week 16 (−1.19 ± 5.98) compared to the DSG (2.27 ± 7.35), this was not significant (p = 0.15), and this difference diminished by week 24 (1.31 ± 6.29 for the ESG and −0.13 ± 8.43 for the DSG, p = 0.65). At week 48, both groups had higher UPDRS scores, but the DSG had less increase in total UPDRS (5.13 ± 8.75) than the ESG (6.33 ± 7.49). This was not statistically significant (p = 0.58). During the second phase of the study, the ESG showed a smaller rate of increase in UPDRS scores between weeks 32 and 48 than the DSG (raw mean difference being 5.53 ± 1.84 versus 6.40 ± 1.84 for the ESG and DSG, respectively). The 95% one-sided confidence interval for this difference of −0.87 was (−5.70, 3.57). Given that the upper limit of 3.57 is less than the pre-specified margin of 3.6 UPDRS points over weeks 32–48, this indicates the non-inferiority of the ESG to DSG. A similar pattern was observed looking at change in UPDRS III scores (Fig. 1B). Results of the comparisons, and estimates with upper confidence interval limits for non-inferiority testing are listed in Table 2.
Table 2.
Summary statistics of the efficacy outcomes in the early start group (ESG) and the delayed start group (DSG).
| Outcome variable |
Groupa | Baseline raw score (SD) |
Mean raw score (SD)
|
Mean change from baseline (SD)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1
|
Phase 2
|
Phase 1
|
Phase 2
|
Non- inferiority (NI)d |
|||||||||||||
| Week 8 | Week 16 | Week 24 | Week 32 | Week 40 | Week 48 | Week 8 | Week 16 | Week 24 | p-0valueb | Week 32 | Week 40 |
Week 48 | p-valuec | ||||
| Total UPDRS | ESG | 21.88 (6.73) | 20.19 (7.68) | 20.69 (9.34) | 23.19 (8.54) | 23.07 (11.99) | 23.93 (11.30) | 28.60 (11.20) | −1.69 (4.22) | −1.19 (5.98) | 1.31 (6.29) | 0.65 | 0.80 (7.62) | 1.67 (7.93) | 6.33 (7.49) | 0.58 | −0.87 3.57 |
| DSG | 26.07 (9.95) | 24.40 (11.33) | 28.33(11.56) | 25.93 (12.09) | 24.80 (11.92) | 29.53 (13.21) | 31.20 (13.69) | −1.67 (6.23) | 2.27 (7.35) | −0.13 (8.43) | −1.27 (6.55) | 3.47 (9.76) | 5.13 (8.75) | ||||
| UPDRS III | ESG | 14.00 (5.73) | 13.38 (5.97) | 14.56 (6.87) | 16.13 (6.86) | 16.53 (7.97) | 16.80 (7.74) | 20.40 (7.01) | −0.63 (3.58) | 0.56 (3.37) | 2.13 (5.43) | 0.49 | 1.93 (5.09) | 2.20 (4.33) | 5.80 (4.02) | 0.80 | −0.93 2.56 |
| DSG | 16.60 (7.02) | 16.00 (7.11) | 19.47 (8.43) | 16.60 (8.72) | 17.07 (8.49) | 21.47 (9.68) | 21.87 (10.43) | −0.60 (5.25) | 2.87 (7.37) | 0.00 (7.45) | 0.47 (5.82) | 4.87 (8.31) | 5.27 (7.42) | ||||
| Tinetti | ESG | 26.00 (3.52) | 26.69 (3.50) | 27.31 (1.99) | 27.31 (2.02) | 27.07 (2.09) | 27.13 (2.59) | 26.73 (3.13) | 0.69 (1.40) | 1.31 (1.85) | 1.31 (1.78) | 0.80 | 1.20 (2.04) | 1.27 (1.49) | 0.87 (1.68) | 0.69 | −0.47 0.23 |
| DSG | 26.27 (3.17) | 27.40 (2.06) | 26.33 (2.41) | 27.33 (1.59) | 27.00 (1.56) | 27.00 (1.46) | 27.13 (1.46) | 1.13 (1.92) | 0.07 (1.58) | 1.07 (2.12) | 0.73 (1.87) | 0.73 (2.28) | 0.87 (2.10) | ||||
| Timed Walk | ESG | 6.04 (1.95) | 6.16 (2.03) | 5.46 (1.68) | 5.29 (1.24) | 5.31 (1.15) | 5.97 (2.37) | 6.21 (2.41) | 0.12 (1.07) | −0.58 (1.37) | −0.76 (1.28) | 0.08 | −0.81 (1.35) | −0.16 (1.16) | 0.08 (1.39) | 0.86 | 0.41 1.12 |
| DSG | 6.26 (2.04) | 6.39 (1.83) | 6.27 (1.98) | 6.09 (1.85) | 5.89 (1.66) | 6.63 (2.02) | 6.37 (1.74) | 0.13 (1.08) | 0.01 (1.08) | −0.17 (1.16) | −0.37 (1.15) | 0.37 (1.37) | 0.11 (1.08) | ||||
| BDI | ESG | 6.81 (4.13) | – | – | – | – | – | 3.87 (2.56) | – | – | – | – | – | – | −2.67 (2.55) | 0.04 | – |
| DSG | 9.53 (4.79) | – | – | – | – | – | 7.93 (4.73) | – | – | – | – | – | −1.60 (3.42) | ||||
| PDQ-39 | ESG | 12.55 (8.03) | – | – | – | – | – | 7.27 (6.42) | – | – | – | – | – | – | −5.05 (6.49) | 0.21 | – |
| DSG | 15.51 (12.26) | – | – | – | – | – | 13.29 (12.82) | – | – | – | – | – | −2.73 (9.08) | ||||
DSG: n = 15, ESG: n = 16, 1 patient withdrew at week 32.
p-value is for the between group comparison of the change from baseline to week 24.
p-value is for the between group comparison of the change from baseline to week 48.
The top number is the estimate for the difference in the change between weeks 32 and 48 between the ESG and DGS. The bottom number is the upper limit of the one-sided 95% confidence interval for that estimate.
Fig. 1.
Longitudinal mean changes in four efficacy outcomes (1A. Total UPDRS; 1B. UPDRS III; 1C. Timed Walk; 1D. Tinetti) in the early start group (solid line) and delayed start group (dashed line).
For Timed Walk, the ESG tended to have better scores during the entire study period (Fig. 1C). They demonstrated improved performance at the end of the first phase (−0.76 ± 1.28) compared to the DSG (−0.17 ± 1.16), but this was not statistically significant (p = 0.08). This trend was not sustained for the duration of the study (p = 0.86). For Tinetti, the group mean plot shows that the ESD did better (Fig. 1D), but none of the superiority tests achieved statistical significance (p = 0.69 at week 48).
ANCOVA results showed that at the end of the study, the Beck Depression Index mean change from baseline values decreased more in the ESG (− 2.67) versus the DSG (− 1.60), and this was statistically significant (p = 0.04).
Home exercise diary data was analyzed, and out of 168 days (i.e. the total number of days the DSG had prior to starting the formal exercise program), the DSG had an average of 69 days of exercise, compared to 45 days in the ESG. Using the Wilcoxon Rank Sum Test, this was not statistically significant (p = 0.15).
In the post-exercise program survey, patients were asked to rate how they liked the exercise class overall on a scale from 1 to 5, 5 being the best, and all but one participant answered 5 (the other answered 4).
5. Discussion
Physical activity has been shown to have a positive influence in neurodegenerative diseases, with exercise being correlated with a reduced incidence of cognitive decline and Alzheimer’s disease, and an improvement of motor symptoms in PD. It is possible that these benefits occur via mechanisms that reduce inflammation in the central nervous system, thus promoting neuronal resilience. Furthermore, animal models suggest that exercise may confer a “neuroprotective” benefit in PD, possibly delaying disease progression. This randomized clinical trial uses a delayed start design to see if long-term group exercise is, 1) feasible in Parkinson’s disease patients, and, 2) if this analysis could detect a neuroprotective effect in the early exercise group versus the delayed exercise group.
We were able to demonstrate that patients could adhere to a long-term group exercise program for 48 weeks, with only one patient dropping out. Furthermore, the enthusiasm that these PD patients had for this group exercise program was sustained based on the results of the post-exercise program survey. Many of these patients continue to exercise as a group after the completion of this study.
While this study did not provide strong evidence that the exercise program utilized is neuroprotective, at least as measured by objective change in total and motor UPDRS scores, the lack of effect may have been partially explained by the relatively small sample size for a study of this duration. Another study limitation is that this was a single-blinded study, however this was inevitable since the participants had to be aware of whether they were in the ESG or DSG. The home exercise diary data did not show that the DSG exercised significantly more than the ESG before they started the formal exercise program on week 24, however our home exercise diary data was limited in that we only collected information on whether participants exercised on a particular day or not. We do not know what kind of exercise, the duration or the activity level. Therefore, any exercise done outside of the formal exercise program is a potential confounding issue, and could explain the lack of differences seen between groups in this study.
Finally, it is important to note the inherent difficulties in assessing a neuroprotective effect in PD, as currently we only have indirect measures of progression, and no reliable biomarker. Given that PD is known for its variable progression and heterogenous presentation, despite using a delayed-start design for a long duration, capturing this “neuroprotective” effect may remain elusive and further studies are recommended.
Exercise has been shown to be helpful for mood disorders such as anxiety and depression [29], behavioral symptoms that are common in PD. Exercise has been hypothesized to improve depression through a number of mechanisms of action, including regulation of central monoamines (serotonin, noradrenaline and dopamine), balancing hypothalamic–pituitary–adrenal axis functioning, and increasing levels of β-endorphin. Recently it has been shown that Parkinson’s patients doing higher levels of physical activity had significantly less fatigue, and trends for less apathy and depression [30]. Our study demonstrated that patients who started the group exercise earlier had significantly fewer self-reported symptoms of depression than those in the delayed start group. As the National Parkinson’s Foundation Quality Improvement Initiative (QII) data has noted, depression affects health status almost twice as much as motor impairment [2]. Thus, the differences noted in this study support early interventions of this type. However, the present study cannot differentiate between the benefits of exercise compared to the benefits of the support of the regular group peer meetings on symptoms of depression, as these may also have impacted feelings of isolation or depression.
It is a common recommendation for PD patients to get regular exercise, and while this study was conducted on relatively early onset PD patients, individual exercise regimens can be tailored to one’s physical capabilities and limitations, even in more advanced disease. Not only can PD patients adhere to a long-term group exercise program, exercise can be an inexpensive and fun intervention free of the side effects of current anti-parkinsonian medications. The positive and supportive environment provided by a formal group exercise program cannot be ignored, as it helped to improve attitudes, fostered optimism and was a positive force for not only the patients with PD, but their circle of support as well.
Acknowledgments
Madden Center Development Fund.
OSU Parkinson’s Disease Our Goal is a Cure Fund.
Columbus branch of the National Parkinson’s Foundation.
OSU Center for Clinical and Translational Science CTSA Grant number UL1TR000090.
Paige Pancake, RN, Clinical Research Coordinator.
References
- 1.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord. 2013;28:311–8. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- 2.Nutt JG, Siderowf A, Guttman M, Nelson EC, Schmidt P, Zamudio J, et al. Correlates of health related quality of life (HRQL) in Parkinson’s disease (PD) [abstract] Mov Disord. 2012;27(Suppl 1):546. [Google Scholar]
- 3.Ashburn A, Fazakarley L, Ballinger C, Pickering R, McLellan LD, Fitton C. A randomised controlled trial of a home based exercise programme to reduce the risk of falling among people with Parkinson’s disease. J Neurol Neurosurg Psychiatr. 2007;78:678–84. doi: 10.1136/jnnp.2006.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shulman LM, Katzel LI, Ivey FM, Sorkin JD, Favors K, Anderson KE, et al. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol. 2013;70:183–90. doi: 10.1001/jamaneurol.2013.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcos DM, Robichaud JA, David FJ, Leurgans SE, Vaillancourt DE, Poon C, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord March. 2013;28(9):1230–40. doi: 10.1002/mds.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster ER, Golden L, Duncan RP, Earhart GM. Community-based Argentine tango dance program is associated with increased activity participation among individuals with Parkinson’s disease. Arch Phys Med Rehabil. 2013;94:240–9. doi: 10.1016/j.apmr.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366:511–9. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picelli A, Melotti C, Origano F, Neri R, Waldner A, Smania N. Robot-assisted gait training versus equal intensity treadmill training in patients with mild to moderate Parkinson’s disease: a randomized controlled trial. Parkinsonism Relat Disord March. 2013;19(6):605–10. doi: 10.1016/j.parkreldis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Ebersbach G, Ebersbach A, Edler D, Kaufhold O, Kusch M, Kupsch A, et al. Comparing exercise in Parkinson’s disease–the Berlin LSVT®BIG study. Mov Disord. 2010;25:1902–8. doi: 10.1002/mds.23212. [DOI] [PubMed] [Google Scholar]
- 10.Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, et al. Physical activities and future risk of Parkinson disease. Neurology. 2010;75:341–8. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thacker EL, Chen H, Patel AV, McCullough ML, Calle EE, Thun MJ, et al. Recreational physical activity and risk of Parkinson’s disease. Mov Disord. 2008;23:69–74. doi: 10.1002/mds.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–9. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- 13.Kuroda K, Tatara K, Takatorige T, Shinsho F. Effect of physical exercise on mortality in patients with Parkinson’s disease. Acta Neurol Scand. 1992;86:55–9. doi: 10.1111/j.1600-0404.1992.tb08054.x. [DOI] [PubMed] [Google Scholar]
- 14.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience. 2003;119:899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 15.Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced nonuse in unilateral parkinsonian rats exacerbates injury. J Neurosci. 2002;22:6790–9. doi: 10.1523/JNEUROSCI.22-15-06790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010;1341:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanLeeuwen JE, Petzinger GM, Walsh JP, Akopian GK, Vuckovic M, Jakowec MW. Altered AMPA receptor expression with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci Res. 2010;88:650–68. doi: 10.1002/jnr.22216. [DOI] [PubMed] [Google Scholar]
- 18.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 19.Chauhan NB, Siegel GJ, Lee JM. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson’s disease brain. J Chem Neuroanat. 2001;21:277–88. doi: 10.1016/s0891-0618(01)00115-6. [DOI] [PubMed] [Google Scholar]
- 20.Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, et al. Movement disorders Society Scientific issues Committee report: SIC Task force appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord. 2003;18:467–86. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- 21.Fahn S, Elton RL. UPDRS Program Members. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease. Vol. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987. [Google Scholar]
- 22.Macleod AD, Counsell CE. Timed tests of motor function in Parkinson’s disease. Parkinsonism Relat Disord. 2010;16:442–6. doi: 10.1016/j.parkreldis.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34:119–26. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- 24.Kegelmeyer DA, Kloos AD, Thomas KM, Kostyk SK. Reliability and validity of the Tinetti mobility test for individuals with Parkinson disease. Phys Ther. 2007;87:1369–78. doi: 10.2522/ptj.20070007. [DOI] [PubMed] [Google Scholar]
- 25.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s disease questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing. 1997;26:353–7. doi: 10.1093/ageing/26.5.353. [DOI] [PubMed] [Google Scholar]
- 26.Visser M, Leentjens AF, Marinus J, Stiggelbout AM, van Hilten JJ. Reliability and validity of the Beck depression inventory in patients with Parkinson’s disease. Mov Disord. 2006;21:668–72. doi: 10.1002/mds.20792. [DOI] [PubMed] [Google Scholar]
- 27.D’Agostino RB., Sr The delayed-start study design. N Engl J Med. 2009;361:1304–6. doi: 10.1056/NEJMsm0904209. [DOI] [PubMed] [Google Scholar]
- 28.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. Cary, NC: SAS Institute Inc; 2006. [Google Scholar]
- 29.De Moor MH, Beem AL, Stubbe JH, Boomsma DI, De Geus EJ. Regular exercise, anxiety, depression and personality: a population-based study. Prev Med. 2006;42:273–9. doi: 10.1016/j.ypmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Abrantes AM, Friedman JH, Brown RA, Strong DR, Desaulniers J, Ing E, et al. Physical activity and neuropsychiatric symptoms of Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25:138–45. doi: 10.1177/0891988712455237. [DOI] [PubMed] [Google Scholar]