Abstract
The immune system of female H-2b (C57BL/6) mice is a strong responder against the male minor-H antigen. However rejection or acceptance of such weakly mismatched grafts depends on the type of tissue transplanted. The mechanism responsible for such spontaneous graft acceptance, and its relationship to the natural mechanisms of tolerance of self antigens is unknown. Co-inhibitory molecules negatively regulate immune responses, and are important for self tolerance. We examined whether co-inhibitory molecules play a critical role in “spontaneous” allograft tolerance. Naïve or donor sensitized diabetic female C57BL/6 (B6) wild type (WT), PD-1−/−, and BTLA−/− mice were transplanted with freshly isolated syngeneic male islet grafts. The role of co-inhibitors during priming of anti-donor responses and graft challenge was also assessed using monoclonal antibodies targeting co-inhibitory receptors. Among the co-inhibitor (CTLA-4, PD-1) specific antibodies tested, only anti-PD-1 showed some potential to prevent spontaneous acceptance of male islet grafts. All BTLA−/− and almost all PD-1−/− recipients maintained the ability to spontaneously accept male islet grafts. While spontaneous graft acceptance in naïve recipients was only weakly PD-1 dependent, tolerance induced by the accepted islets was found to be highly PD-1 dependent. Furthermore, spontaneous graft acceptance in pre-sensitized recipients showed an absolute requirement for recipient PD-1 but not BTLA. Thus, the PD-1 pathway, involved in self tolerance, plays a critical role in spontaneous tolerance induced by weakly mismatched grafts in naïve recipients and spontaneous graft acceptance in pre-sensitized recipients.
Keywords: Co-inhibitory molecules, HY antigen, islet transplantation, PD-1, Tolerance
Introduction
The success of MHC matched transplants is impeded by immune responses to minor-H antigens (Dierselhuis and Goulmy, 2009). For instance, bone marrow transplants between HLA matched siblings induced GVH disease due to immune responses against minor-H antigens (Simpson, et al., 2002). Autosomal and Y-chromosome genes encode H-antigens (Simpson and Roopenian, 1997). The male specific antigen (HY) triggers rejection of male syngeneic skin grafts in certain inbred strains of female mice (Eichwald and Silmser, 1955). Naïve H-2b females can reject male skin grafts and thus are referred to as strong responders. Although H-2b females reject male skin or bone marrow (Simpson and Roopenian, 1997, Sireci, et al., 2009), they could not reject male islet (Luo, et al., 2007, Yoon, et al., 2008), kidney (Moxham, et al., 2008) or cardiac grafts (He, et al., 2004). Spontaneous acceptance of male islet grafts may also induce dominant tolerance to male antigens (Yoon, et al., 2008). Adoptive transfer of T cells from tolerant mice to female neonates allowed acceptance of male skin grafts in the adoptive recipients, although third party grafts were not tested. Islet or heart grafts given time to heal into the recipient before the recipient's immune system develops can also lead to tolerance of donor antigens (Chan, et al., 2007). This `natural tolerance' of a transplant given pre-immunocompetence is designed to mimic the conditions that allow establishment of tolerance to peripheral self antigens. However, spontaneous tolerance of male islet grafts by adult mice, i.e. tolerance of grafts given post-immunocompetence of the recipient, may not involve precisely the same mechanisms, due to the inflammatory conditions associated with grafts given to immunocompetent adult recipients and the presence of an established adaptive immune system. It is therefore of interest to determine whether the primary mechanisms of peripheral self tolerance, such as those mediated by co-inhibitory signals, are also involved in spontaneous tolerance of weakly mismatched grafts in immunocompetent recipients.
The balance between co-stimulation and co-inhibition influences the outcome of immune responses by allowing strong immune response against appropriate foreign antigens and maintaining tolerance to self-antigens (Sinclair and Anderson, 1996, Thangavelu, et al., 2010). Cytotoxic lymphocytic antigen-4 (CTLA-4; CD152), programmed death-1 (PD-1; CD279) and B and T lymphocyte attenuator (BTLA; CD272) are some of the major co-inhibitory molecules that have been shown to be involved in immunological tolerance. CTLA-4 is expressed by activated T cells and Tregs and can compete with the co-stimulatory molecule CD28 to bind with its ligands B7.1 (CD80) and B7.2 (CD86). Lack of CTLA-4 induced a fatal lymphoprolifertive disorder in mice (Waterhouse, et al., 1995) that was recently shown to be due to autoreactive T cells (Ise, et al., 2010). PD-1 shares 23% amino acid sequence homology with CTLA-4 and is expressed by activated T cells, B cells and myeloid cells. Lack of PD-1 leads to a narrow spectrum of autoimmune disease that varies based on the strain background (Nishimura, et al., 1999, Nishimura, et al., 2001). PD-1 binds PD-L1(B7H1; CD274) and PD-L2 (B7DC; CD273), with PD-L1 being widely expressed in hematopoietic and non-hematopoietic cells, whereas PD-L2 expression is restricted to dendritic cells and macrophages (Chen, et al., 2007, Keir, et al., 2008). Deficiency of BTLA induced autoimmune hepatitis and anti-nuclear antibodies in aged mice (Oya, et al., 2008) and increased susceptibility to experimental autoimmune encephalomyelitis (Watanabe, et al., 2003) and allergic airway inflammation (Deppong, et al., 2006).
We tested whether the “spontaneous tolerance” of weakly mismatched transplants is mediated by co-inhibitory pathways. Herein, we show that PD-1 plays a critical role in the tolerance of single minor mismatched islet transplants.
Materials and methods
Mice
Adult wild type C57BL/6, (B6; H-2b) mice were obtained from NCI (Frederick, MD). B6.129S7-Rag1tm1mom/J (abbreviated as Rag−/−) mice from Jackson were bred in house. We generated MHC class-I and Rag deficient mice (C57BL/6 H-2Kbtm1-H-2Dbtm1N12 Rag−/−; abbreviated as class-I−/− Rag−/−) by crossing the two knockout lines obtained originally from Jackson. MHC class-II deficient B6.129-H2-Ab1tm1Gru (abbreviated as class-II−/−) mice were from Taconic Farms. C57BL/6-Pdcd1−/− (PD-1−/−) mice, originally generated by Prof. T. Honjo and colleagues (Nishimura, et al., 1998, Nishimura, et al., 1999) in embryonic stem cells of the 129 background and backcrossed 11 generations to C57BL/6, and C57BL/6-BTLA−/− (BTLA−/− mice (Watanabe, et al., 2003)) were bred at the University of Alberta. All protocols on care and handling of animals were carried out in CCAC accredited facilities at the University of Alberta.
Diabetes induction and islet transplantation
Diabetes was chemically induced by a single intraperitoneal injection of STZ; 200 mg/kg; Sigma-Aldrich) in female recipients. Diabetes was confirmed as blood glucose of >20 mmol/L twice on consecutive days. Diabetic recipients were transplanted with 400 male donor islets into the renal subcapsular space. The function of the graft was monitored by blood glucose; rejection was defined as blood glucose exceeding 15 mmol/L on two consecutive days. Nephrectomy was performed to assess whether transplanted islets were responsible for the normoglycemic state.
In vivo antibody treatment and immunization
We used 250 μg anti-mouse PD-1 mAb (J43), anti-mouse PD-L1 (10F.9g2), anti-mouse PD-L2 (TY25), anti-mouse BTLA (6F7) and isotype control (Rat IgG2b) given every other day, beginning on the day of transplantation, for a total of six injections (last injection on day 10). Anti-CTLA-4 (4F10), at a dose of 100 μg of blocking antibody was injected every 2 days from the day of transplantation for a total of six injections. Anti-mouse PD-1, was used at 250 μg mAb (J43) per injection, and given twice, with six days between injections, to block PD-1 signaling. For sensitization, 4x106 male B6 or PD-1−/− splenocytes were injected into female B6 or PD-1−/− mice respectively. Fourteen days post immunization, all recipients were made diabetic, as described above, and transplanted with male islet grafts. WT B6 recipients received male islets from WT B6 donors, and PD-1−/− recipients received islets from PD-1−/− male donors.
Pentamer studies
We used PE labelled H-2Db (WMHHNMDLI; UTY 246–254; Proimmune, Bradenton, FL) pentamers together with antibodies to mouse CD8, pan TCRβ and CD19 (eBioscience, San Diego, CA) to detect the frequency of anti-HY CD8 T cells. Non-specific binding was blocked with anti-CD16/32 antibody (2.4G2; Bio Express, West Lebanon, NH), and mouse, rat and hamster sera. Data acquisition and analysis was on a FACSCalibur™ flow cytometer (BD Biosciences).
Histology and Immunofluorescence
Kidney with islet grafts were fixed in formalin, embedded in paraffin, sectioned, and stained with anti-insulin, hematoxylin and eosin. For immunofluorescence 5μm crosssections were fixed with acetone and blocked with 20% normal goat serum (Jackson ImmunoResearch Westgrove, PN). Staining was with rat anti-mouse CD4 or CD8α, (1:200; Biolegend, San Deigo, CA) followed by goat anti-rat Alexaflour 488 (1:200; Invitrogen Laboratories, Burlington, ON). Slides were coverslipped using Prolong Gold Anti-fade with DAPI (Invitrogen), and visualized on a compound flourscent microscope (Axioplan, Axiovision 4.1 software, Carl Zeiss, Toronto, ON).
Statistical analysis
Statistical analyses were done using GraphPad Prism Software (San Diego, CA). The Kaplan-Meier method and the log rank test were used for graft survival and a Student's t-test for pentamer studies.
Results
Tolerance induced by spontaneously accepted islet grafts is PD-1 dependent
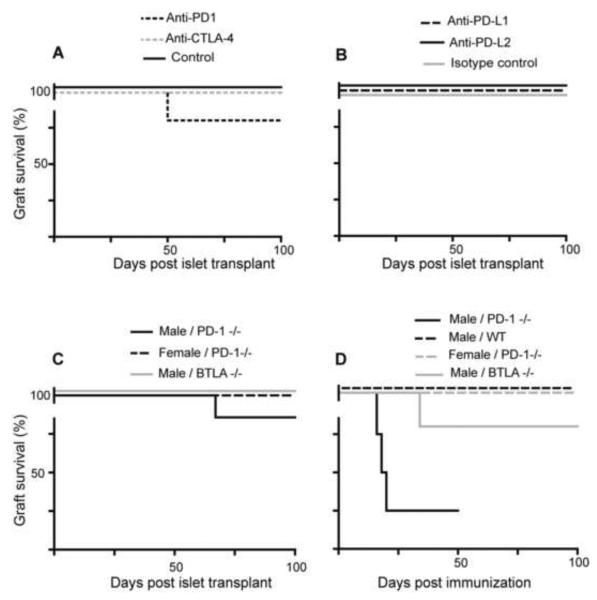
Female WT mice accepted male islet grafts indefinitely (Fig. 1A), in agreement with previous studies (Luo, et al., 2007, Yoon, et al., 2008). We tested whether targeting co-inhibitory pathways prevents spontaneous acceptance of male islets, by treating mice with mAb that block either CTLA-4 or PD-1 (Agata, et al., 1996, van Wijk, et al., 2007). While we have never observed rejection of `syngeneic' male islets by WT mice, one of the recipients treated with anti-PD-1 rejected its male islet graft (Fig. 1A), suggesting that PD-1 may have some role in spontaneous acceptance. A preliminary analysis using anti-PD-L1 or anti-PD-L2 treatment did not provide further support for this hypothesis, as these antibodies did not prevent spontaneous graft acceptance (Fig. 1B). Therefore, we tested the role of PD-1 by two additional approaches. In the first approach, we gave female PD-1−/− mice male islets. In order to ensure the only antigenic mismatches were derived from the Y chromosome, we used PD-1−/− mice as the donors of male islets. Again, lack of PD-1 function appeared to have at most a small effect, with only one out of seven recipients rejecting their graft (Fig. 1C). We also tested the role of BTLA, using BTLA−/− recipients. There was no increase in rejection of male islet grafts in BTLA−/− females (Fig. 1C).
Fig. 1.
PD-1 is required for tolerance but not acceptance of a weakly mismatched islet allograft. (A) Male B6 islets were transplanted to syngeneic female mice that were either untreated (black solid line; n=4; Control) or treated with blocking antibodies to CTLA-4 (grey dashed line; n=5) or PD-1 (black dashed line; n=5). (B) B6 male islets were transplanted to syngeneic female mice that were treated with anti-PD-L1 (black dashed line; n=5), anti-PD-L2 (black solid line; n=5) or isotype control (grey solid line; n=5). (C) Female PD-1−/− mice were transplanted with male PD-1−/− or WT islet grafts (black solid line; n=7) or female PD-1−/− islet grafts (black dashed line; n=3). Also, female BTLA−/− mice were transplanted with male BTLA−/− islet grafts (grey solid line; n=5). (D) 100–105 days post transplantation, female recipients were immunized with 4 × 106 male splenocytes. The groups included female PD-1−/− mice transplanted with male (black solid line; n=4) or female (grey dashed line; n=3) islets or female B6 (WT) mice transplanted with male islets (black dashed line; n=4) and female BTLA−/− mice transplanted with male islets (grey solid line; n=5).
Our previous studies (Luo, et al., 2007) indicated that diabetes (as induced by STZ) could suppress the anti-HY immune response, and this diabetes-induced immunosuppression might have reduced the ability to detect a role for these receptors in spontaneous graft acceptance. We therefore tested whether the ability to maintain the graft after immunization with donor antigen may depend on PD-1 function. We immunized female WT, PD-1−/− or BTLA−/− recipients with long-term accepted grafts (100–105 days) to test if the grafts had induced tolerance to HY or if instead the immunization would trigger islet rejection. Interestingly, almost all of the PD-1−/− recipients, but not WT or BTLA−/− recipients, rejected their long-term established graft (Fig. 1D). Together these results indicate that despite the spontaneous islet allograft acceptance not being strongly PD-1/PD-L1/PD-L2 dependent, the tolerance induced by the long-term presence of the islet graft is highly dependent on PD-1 but not BTLA function.
Spontaneous graft acceptance in sensitized recipients is PD-1 dependent
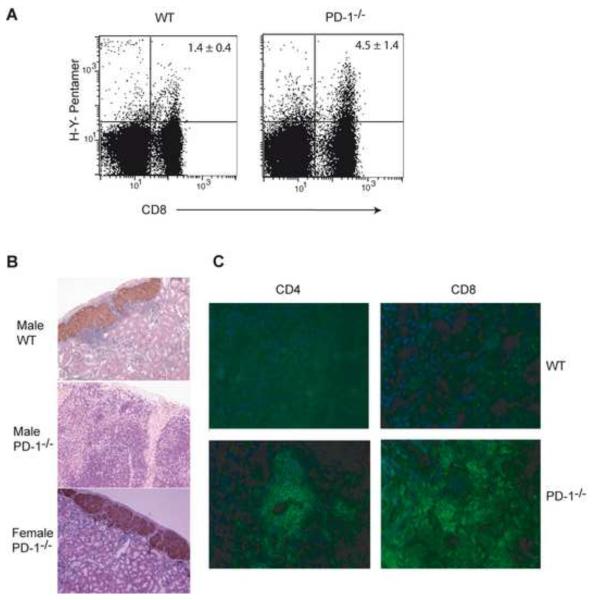
Having found that immunization with male antigen breaks the long-term acceptance of male islet grafts in female PD-1−/− mice, it suggested the possibility that PD-1 may play a more critical role in controlling the response of sensitized recipients. We therefore tested the effect of sensitization with donor antigen prior to transplantation on spontaneous graft acceptance in WT vs. PD-1−/− or BTLA−/− recipients. Female WT, PD-1−/− or BTLA−/− mice were immunized with male splenocytes before islet transplantation. Similar to the immunization post transplantation data, there was consistent rejection (100%) of male, but not control female, islet grafts only in the sensitized female PD-1−/− mice (Table 1). Sensitized female PD-1−/− recipients rejected donor male islet from either WT B6 or PD-1−/− B6 donors, indicating that rejection was not due to potential additional minor antigen mismatches between WT B6 and PD-1−/− B6 mice. Lack of female islet rejection showed the response was donor specific and not due to potential autoreactivity in PD-1−/− mice. Similarly, we found that the PD-1−/− recipients that rejected male islets would accept a female islet graft and reject a second male islet graft when re-transplanted in contralateral kidney (data not shown). Histological examination confirmed the rejection of male islets in sensitized PD-1−/− but not WT recipients, the latter showing strong insulin staining and only a peri-islet infiltrate (Fig. 2B). Male islet grafts were infiltrated with CD4 and CD8 cells in sensitized PD-1−/− mice (Fig. 2C).
Table 1.
PD-1 is required to prevent rejection of male islet grafts in sensitized recipients.
| Group | Islet Donor | Recipients | Anti-PD-1 treatment | Graft Survival (d) | % Graft survival |
|---|---|---|---|---|---|
| 1 | Male | PD-1−/− | N/Aa | 10 × 2, 14, 18, 20 × 2, 28, 76b | 0 |
| 2 | Female | PD-1−/− | N/A | > 100 × 5 | 100 |
| 3 | Male | BTLA−/− | None | 44, > 100 × 3 | 75 |
| 4 | Male | WT | None | > 100 × 7 | 100 |
| 5 | Male | WT | At Immunization | 15, 16, > 100 × 3 | 60 |
| 6 | Male | WT | At Transplantation | 20, > 100 × 4 | 80 |
| 7 | Male | WT | At Immunization & Transplantation | 17, 19, 28, > 100 | 25 |
Female WT or PD-1−/− or BTLA−/− mice were immunized with male splenocytes two weeks before transplantation of islet grafts. The sensitized PD-1−/− mice given male islets (group 1) were significantly different (P< 0.01) from groups 2, 3, and 4. For groups 5–7, female WT mice were treated with anti-PD-1 at the time of immunization with male splenocytes or at the time of transplantation of male islets, 2 weeks after immunization or at both stages. Group 7 vs. 4 (P> 0.05).
N/A, not applicable
Group 1 recipients (n=8) of male islets received islets from WT B6 (n=4) or PD-1−/− B6 (n=4) donors.
Fig 2.
Lack of PD-1 increased the frequency of anti-HY CD8 T cells. (A) Female B6 or PD-1−/− mice were immunized with male splenocytes and pentamer staining was performed in splenocytes 2 weeks after immunization. Plots show the frequency of TCR gated pentamer positive anti-HY CD8 T cells. (B) Histological examination of representative islet grafts from sensitized female WT (not rejected; top) showed numerous intact islets that contain insulin granules (brown) surrounded with a mononuclear cell infiltrate. Male islet grafts in sensitized female PD-1−/− recipients (rejected; middle) had substantial infiltrates that penetrated the islets and little if any insulin staining. Female islet grafts with insulin granules (brown) in sensitized female PD-1−/− recipients (not rejected; bottom). Histology was assessed 5 days post-islet graft rejection and at 105 days post islet transplantation for mice that accepted the islet graft. (C) Immunofluorescence pictures of male islet grafts in sensitized B6 (not rejected) or PD-1−/− (rejected) mice. Blue: staining of nucleus with 4',6'-diamidino-2- phenylindole (DAPI); green: CD4 or CD8 staining. Immunoflluorescence was assessed 2 weeks post islet graft rejection and 130 days post islet transplantation for mice that accepted the islet graft.
Blockade of PD-1 signaling can result in the expansion of anti-donor CD8+ T cells (Koehn, et al., 2008). We examined the frequency of anti-HY CD8 T cells using pentamers, in sensitized female WT or PD-1−/− mice. There was an increase in the percentage of anti-HY CD8 T cells in sensitized female PD-1−/− mice compared to WT mice (P< 0.05; Fig. 2A), suggesting a role for PD-1 in dampening the priming of anti-donor T cells.
Our data indicated that PD-1 is important during the priming stage, with increased accumulation of donor specific CD8 T cells when PD-1 is absent during immunization. However, ligands for PD-1 are also expressed within the islets themselves (Wang, et al., 2005) and therefore lack of PD-1 signaling during the response to the transplant could potentially also contribute to the rejection. To examine this question, we targeted PD-1 with mAb specifically at the immunization stage before transplantation or at the transplantation stage or at both stages. Targeting the PD-1 pathway at both the stages tended to be more effective than blocking at either one of the stages alone (Table 1). Taking these results together, PD-1 may be required at both the priming and transplantation stages for spontaneous allograft acceptance in sensitized recipients.
Loss of PD-1 leads to donor MHC class-I but not class-II dependent rejection
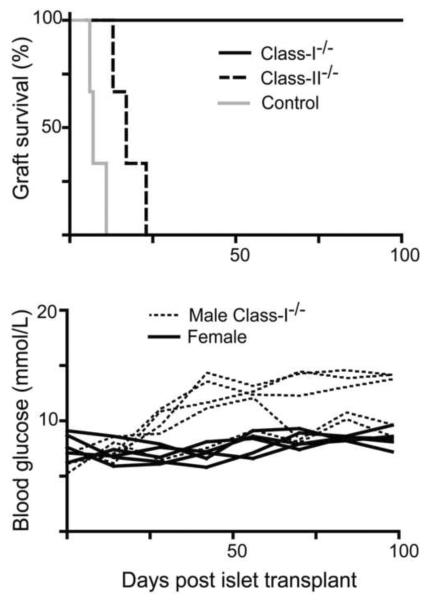
Whether direct presentation of donor antigens by islets is critical for their rejection is controversial. One study suggested donor MHC class-I is a major pathway in islet rejection, as class-I deficient islets were accepted in the majority of recipients (Markmann, et al., 1992). In contrast, Gill and colleagues (Kupfer, et al., 2008) showed that absence of donor class-I and II, but not either alone, prevented rejection. Given the increased frequency of anti-donor CD8 T cells in sensitized PD-1−/− recipients (Fig. 2), we hypothesized that PD-1 deficiency would lead to rejection targeting donor class-I molecules. We compared class-I−/− vs. class-II−/− donor islet grafts in pre-sensitized PD-1−/− recipients. Acute graft rejection was indeed dependent on donor class-I but not class-II (Fig. 3). However, the recipients of class-I−/− donors did exhibit blood glucose levels close to the rejection level (Fig. 3), suggesting some attack on the islets could occur even in the absence of donor class-I.
Fig. 3.
Absence of donor class-I prevented the rejection of male islet grafts in sensitized female PD-1−/− recipients. Top: Chemically induced diabetic female PD-1−/− mice, previously sensitized with male spleen cells were transplanted with male class-I−/− Rag−/− (black solid line; n=5), class-II−/− (black dashed line; n=3) or control Rag−/− (Class-I & II+/+) islet grafts (grey solid line; n=3). Bottom: Islets from male class-I−/− Rag−/− mice were transplanted to sensitized diabetic female B6 PD-1−/− recipients (black dashed lines; n=5). Data shown are blood glucose levels of individual mice, and values for sensitized diabetic female B6 PD-1−/− recipients of control female islets (black solid lines; n=5) are shown for comparison.
Discussion
PD-1 has been shown to play an important role in the maintenance of immunological tolerance (Nishimura, et al., 1999, Nishimura, et al., 2001). Previous studies have reported that deficiency or blockade of the PD-1/PD-L1 pathway prevented the prolongation or acceptance of MHC mismatched skin (Dai, et al., 2009) and cardiac (Wang, et al., 2007, Wang, et al., 2008) allografts, which were achieved with various tolerogenic regimens. Whether such induced transplant acceptance and spontaneous acceptance would involve the same tolerance mechanisms was unknown. We have shown here the significance of the PD-1 pathway in the spontaneous acceptance of weakly mismatched transplants. Female H-2b mice spontaneously accepted syngeneic male islet grafts and an earlier study (Yoon, et al., 2008) reported that the spontaneous acceptance of male islet grafts could induce tolerance to male antigen. We tested whether co-inhibitory molecules are involved in the induction of this spontaneous acceptance of male islet grafts. Our studies represent only an initial test of the role of co-inhibitory molecules such as CTLA-4 and PD-1 by using specific blocking antibodies. While only anti-PD-1 had any discernable effect in allowing rejection of male islets by naïve recipients, and CTLA-4 seemed not to be involved, our studies using anti-CTLA-4 are too limited to completely exclude a role for this pathway in spontaneous allograft acceptance. A larger study and examination of presensitized recipients is required to fully evaluate this possibility. In the case of BTLA deficiency, only a weak effect was discernable, and even then only in the sensitized recipients.
The frequency of T cells against HY antigen in naïve female mice is low (Simpson, 1983) and CD4 T cell help is critical in the CD8 T cell response to HY (Guerder and Matzinger, 1992, Keene and Forman, 1982). Blocking or loss of PD-1 signaling in naïve female mice did not induce rejection of male islet grafts in the majority of naïve female mice. This may indicate that the HY antigens alone are insufficient to trigger islet rejection. However, an earlier study (Luo, et al., 2007) from our laboratory had shown that non-diabetic female recipients induced stronger anti-HY immune responses and more peri-islet infiltration of grafts than those of diabetic female recipients. Thus, lack of rejection may also be due to the immunosuppressive effects of STZ induced diabetes on anti-HY immune responses (Luo, et al., 2007). Hence, we tested whether immunization with donor antigen in the absence of PD-1 signaling would break the spontaneous acceptance of male islet grafts. Immunization did indeed trigger rejection of accepted grafts in PD-1−/− recipients. This rejection was not a result of potential additional minor antigens on the immunizing male spleen cells, as the immunizing cells were also from PD-1−/− mice. A second objective of our experiment was to mimic the situation of islet transplant recipients, in which the recipient's immune system may already be sensitized to islet and/or donor antigens. Interestingly, we found that PD-1 has a crucial role in both the long-term acceptance of the graft after immunization with donor antigen and in initial graft acceptance in pre-sensitized recipients.
There are at least two possibilities that may explain the rejection of male islet grafts in the absence or blockade of PD-1. The first possibility is by increasing the frequency of anti-HY CD8 T cells, as we observed using HY/Db pentamers. Increased CD8 T cells could be due to reduced PD-1 signals in the CD8s themselves, reduced PD-1 signals to HY specific helper T cells that promote CD8 expansion, or due to a reduced ability to generate adaptive Treg cells (Wang, et al., 2008). In accord with our results, previous studies have shown that loss of PD-1 or blockade of the PD-1 pathway increased the clonal expansion or percentage of the anti-donor T cell population, respectively (Koehn, et al., 2008, Kroner, et al., 2009). A second possibility is the absence of PD-1/PD-L1 ligation in the target of rejection, the islets. PD-L1 is expressed in many cells, including beta cells of the islets (Wang, et al., 2005). Lack of PD-L1/PD-1 in NOD mice (Keir, et al., 2006, Wang, et al., 2005), or in an induced diabetes model (Rajasalu, et al., 2010), potentiated the onset of autoimmune diabetes, which suggested that PD-L1 and PD-1 interaction has a protective role in islets. Therefore, we speculated that the absence of PD-1/PD-L1 interactions within parenchymal tissues might play a role in the rejection of male islet grafts. An earlier study (Keir, et al., 2007) had shown the importance of PD-1 at both priming and effector stages of CD8 T cell responses, supporting this possibility. Our data suggest that both an increased frequency of anti-donor T cells and a lack of PD-1 signals at the graft site contribute to the loss of spontaneous tolerance. Consistent with a role for CD8 T cells, class-I expression in donor islets was required for acute male islet allograft rejection in sensitized female PD-1−/− recipients. However, a previous study demonstrated that class-I deficient MHC mismatched islet allografts could be rejected in wild-type mice (Kupfer, et al., 2008). A potential explanation for the different outcomes could be the degree of mismatches and the high frequency of donor islet reactive T cells in the previous study. In addition, we did see increased blood glucose levels in several recipients of class-I deficient islets, suggesting some attack on the islets had occurred. Blockade of PD-1 can increase DTH like immune responses (Salama, et al., 2003) that may have contributed to killing of islets by a donor class-I independent mechanism.
Although sensitized WT B6 recipients had a much lower frequency of HY specific CD8 T cells compared to the sensitized PD-1−/− recipients, they nevertheless had a relatively high frequency of these cells. Thus, it is not yet fully clear why sensitized WT B6 mice are unable to reject a male islet graft. However, increased frequencies of HY specific T cells, compared to naïve mice, has been observed in mice made tolerant to HY through peptide administration (Chai, et al., 2004, James, et al., 2002). These data suggest that HY specific T cell function (e.g. cytokine production or regulatory function) is likely to be just as important as the frequency of specific T cells in determining whether HY expressing target cells are eliminated.
Our studies also have implications for understanding the regulation of immune responses to chronically persistent antigen. Failure of immune responses to clear microbes may lead to persistent or chronic infections, which is mainly associated with T cell dysfunction. The PD-1/PD-L1 pathway is involved in the impairment of T cell function during chronic viral infections; blocking the PD-1/PD-L1 pathway reversed the T cell dysfunction (Barber, et al., 2006, Trautmann, et al., 2006). These studies of chronic antigen exposure, involve responses to systemic viral antigens. Under certain conditions, systemic alloantigens can stimulate immunity and yet not be cleared by the immune system. Examples include systemically injected donor hematopoietic cells and host alloantigens targeted by donor T cells during GVH reactions (Chan, et al., 2008, Rivas, et al., 2009). Such persistent systemic histocompatibility antigens can switch alloimmunity (anti-donor or anti-host) into tolerance; a tolerance that may involve a number of mechanisms, including loss of CD4 co-receptor expression (Chan, et al., 2008), and tolerogenic signals from PD-1 (Rivas, et al., 2009). Liver allografts can lead to systemic donor antigens via migration of passenger leukocytes (Starzl, et al., 1993), and the spontaneous tolerance of liver allografts also appears to be dependent on co-inhibitory signals, including both PD-1 and CTLA-4 (Li, et al., 2005, Morita, et al., 2010). From these virus and alloantigen studies, it might be assumed that it is the systemic nature of persistent antigens that triggers the tolerogenic co-inhibitory pathway. Our results suggest that spontaneous PD-1 dependent tolerance may not be limited to situations with high levels of systemic antigens. We found that donor alloantigen, in the form of an islet graft under the kidney capsule, resists rejection and induces a form of tolerance that is highly dependent on PD-1 function. Success of allograft transplantation is influenced by various factors. The current studies have shown that PD-1 plays a critical role in the spontaneous acceptance of weakly mismatched allografts and thus supports the idea that potentiation of naturally induced co-inhibitory signals (Thangavelu, et al., 2010), such as via PD-1 (Gao, et al., 2003), could be exploited as a mechanism to achieve transplantation tolerance.
Acknowledgements
We thank Deb Dixon for technical assistance and William Chan for discussion. This work was supported by grants from the Canadian Institutes of Health Research MOP 79521 (C.C.A) and the Juvenile Diabetes Research Foundation, 1-2008-98 (C.C.A), and a scholar award from the Alberta Heritage Foundation for Medical Research (C.C.A.), and doctoral studentships from the Muttart Diabetes Research and Training Centre (G.T.) and Alberta Diabetes Institute (G.T.).
Abbreviations
- WT
Wild Type
- STZ
streptozotocin mAb: monoclonal antibody
- GVH
graft versus host
- PD-1
programmed death-1
- BTLA
B and T lymphocyte attenuator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Chai JG, James E, Dewchand H, Simpson E, Scott D. Transplantation tolerance induced by intranasal administration of HY peptides. Blood. 2004;103:3951–3959. doi: 10.1182/blood-2003-11-3763. [DOI] [PubMed] [Google Scholar]
- Chan WF, Perez-Diez A, Razavy H, Anderson CC. The ability of natural tolerance to be applied to allogeneic tissue: determinants and limits. Biol. Direct. 2007;2:10. doi: 10.1186/1745-6150-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WF, Razavy H, Anderson CC. Differential susceptibility of allogeneic targets to indirect CD4 immunity generates split tolerance. J. Immunol. 2008;181:4603–4612. doi: 10.4049/jimmunol.181.7.4603. [DOI] [PubMed] [Google Scholar]
- Chen C, Qu QX, Huang JA, Zhu YB, Ge Y, Wang Q, Zhang XG. Expression of programmed-death receptor ligands 1 and 2 may contribute to the poor stimulatory potential of murine immature dendritic cells. Immunobiology. 2007;212:159–165. doi: 10.1016/j.imbio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Dai H, Zhu H, Lei P, Yagita H, Liu J, Wen X, Zhou W, Gong F, Shen G, Fang M. Programmed death-1 signaling is essential for the skin allograft protection by alternatively activated dendritic cell infusion in mice. Transplantation. 2009;88:864–873. doi: 10.1097/TP.0b013e3181b6ea74. [DOI] [PubMed] [Google Scholar]
- Deppong C, Juehne TI, Hurchla M, Friend LD, Shah DD, Rose CM, Bricker TL, Shornick LP, Crouch EC, Murphy TL, Holtzman MJ, Murphy KM, Green JM. Cutting edge: B and T lymphocyte attenuator and programmed death receptor-1 inhibitory receptors are required for termination of acute allergic airway inflammation. J. Immunol. 2006;176:3909–3913. doi: 10.4049/jimmunol.176.7.3909. [DOI] [PubMed] [Google Scholar]
- Dierselhuis M, Goulmy E. The relevance of minor histocompatibility antigens in solid organ transplantation. Curr. Opin. Organ. Transplant. 2009;14:419–425. doi: 10.1097/MOT.0b013e32832d399c. [DOI] [PubMed] [Google Scholar]
- Eichwald EJ, Silmser CR. Skin. Transplant. Bull. 1955;2:148–149. [PubMed] [Google Scholar]
- Gao W, Demirci G, Strom TB, Li XC. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation. 2003;76:994–999. doi: 10.1097/01.TP.0000085010.39567.FB. [DOI] [PubMed] [Google Scholar]
- Guerder S, Matzinger P. A fail-safe mechanism for maintaining self-tolerance. J Exp Med. 1992;176:553–564. doi: 10.1084/jem.176.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Schenk S, Zhang Q, Valujskikh A, Bayer J, Fairchild RL, Heeger PS. Effects of T cell frequency and graft size on transplant outcome in mice. J. Immunol. 2004;172:240–247. doi: 10.4049/jimmunol.172.1.240. [DOI] [PubMed] [Google Scholar]
- Ise W, Kohyama M, Nutsch KM, Lee HM, Suri A, Unanue ER, Murphy TL, Murphy KM. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat. Immunol. 2010;11:129–135. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James E, Scott D, Chai JG, Millrain M, Chandler P, Simpson E. HY peptides modulate transplantation responses to skin allografts. Int Immunol. 2002;14:1333–1342. doi: 10.1093/intimm/dxf093. [DOI] [PubMed] [Google Scholar]
- Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–782. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J. Immunol. 2007;179:5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehn BH, Ford ML, Ferrer IR, Borom K, Gangappa S, Kirk AD, Larsen CP. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J. Immunol. 2008;181:5313–5322. doi: 10.4049/jimmunol.181.8.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner A, Schwab N, Ip CW, Ortler S, Gobel K, Nave KA, Maurer M, Martini R, Wiendl H. Accelerated course of experimental autoimmune encephalomyelitis in PD-1-deficient central nervous system myelin mutants. Am. J. Pathol. 2009;174:2290–2299. doi: 10.2353/ajpath.2009.081012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer T, Beilke JN, Pham K, Buhrman J, Gill RG. “Indirect” acute islet allograft destruction in nonobese diabetic mice is independent of donor major histocompatibility complex and requires host B lymphocytes. Transplant. Proc. 2008;40:462–463. doi: 10.1016/j.transproceed.2008.01.054. [DOI] [PubMed] [Google Scholar]
- Li W, Zheng XX, Kuhr CS, Perkins JD. CTLA4 engagement is required for induction of murine liver transplant spontaneous tolerance. Am. J. Transplant. 2005;5:978–986. doi: 10.1111/j.1600-6143.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- Luo B, Chan WF, Lord SJ, Nanji SA, Rajotte RV, Shapiro AM, Anderson CC. Diabetes induces rapid suppression of adaptive immunity followed by homeostatic T-cell proliferation. Scand. J. Immunol. 2007;65:22–31. doi: 10.1111/j.1365-3083.2006.01863.x. [DOI] [PubMed] [Google Scholar]
- Markmann JF, Bassiri H, Desai NM, Odorico JS, Kim JI, Koller BH, Smithies O, Barker CF. Indefinite survival of MHC class I-deficient murine pancreatic islet allografts. Transplantation. 1992;54:1085–1089. doi: 10.1097/00007890-199212000-00025. [DOI] [PubMed] [Google Scholar]
- Morita M, Fujino M, Jiang G, Kitazawa Y, Xie L, Azuma M, Yagita H, Nagao S, Sugioka A, Kurosawa Y, Takahara S, Fung J, Qian S, Lu L, Li XK. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am. J. Transplant. 2010;10:40–46. doi: 10.1111/j.1600-6143.2009.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxham VF, Karegli J, Phillips RE, Brown KL, Tapmeier TT, Hangartner R, Sacks SH, Wong W. Homeostatic proliferation of lymphocytes results in augmented memory-like function and accelerated allograft rejection. J. Immunol. 2008;180:3910–3918. doi: 10.4049/jimmunol.180.6.3910. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- Oya Y, Watanabe N, Owada T, Oki M, Hirose K, Suto A, Kagami S, Nakajima H, Kishimoto T, Iwamoto I, Murphy TL, Murphy KM, Saito Y. Development of autoimmune hepatitis-like disease and production of autoantibodies to nuclear antigens in mice lacking B and T lymphocyte attenuator. Arthritis Rheum. 2008;58:2498–2510. doi: 10.1002/art.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasalu T, Brosi H, Schuster C, Spyrantis A, Boehm BO, Chen L, Reimann J, Schirmbeck R. Deficiency in B7-H1 (PD-L1)/PD-1 coinhibition triggers pancreatic beta cell-destruction by insulin-specific, murine CD8 T cells. Diabetes. 2010;59:1966–1973. doi: 10.2337/db09-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas MN, Weatherly K, Hazzan M, Vokaer B, Dremier S, Gaudray F, Goldman M, Salmon I, Braun MY. Reviving function in CD4+ T cells adapted to persistent systemic antigen. J. Immunol. 2009;183:4284–4291. doi: 10.4049/jimmunol.0901408. [DOI] [PubMed] [Google Scholar]
- Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, Azuma M, Yagita H, Sayegh MH, Khoury SJ. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. Review lecture. Immunology of H-Y antigen and its role in sex determination. Proc. R. Soc. Lond. B Biol. Sci. 1983;220:31–46. doi: 10.1098/rspb.1983.0087. [DOI] [PubMed] [Google Scholar]
- Simpson E, Roopenian D. Minor histocompatibility antigens. Curr. Opin. Immunol. 1997;9:655–661. doi: 10.1016/s0952-7915(97)80045-3. [DOI] [PubMed] [Google Scholar]
- Simpson E, Scott D, James E, Lombardi G, Cwynarski K, Dazzi F, Millrain M, Dyson PJ. Minor H antigens: genes and peptides. Transpl. Immunol. 2002;10:115–123. doi: 10.1016/s0966-3274(02)00057-6. [DOI] [PubMed] [Google Scholar]
- Sinclair NR, Anderson CC. Co-stimulation and co-inhibition: equal partners in regulation. Scand. J. Immunol. 1996;43:597–603. doi: 10.1046/j.1365-3083.1996.d01-267.x. [DOI] [PubMed] [Google Scholar]
- Sireci G, Barera A, Macaluso P, Di Sano C, Bonanno CT, Pio La Manna M, Di Liberto D, Dieli F, Salerno A. A continuous infusion of a minor histocompatibility antigen-immunodominant peptide induces a delay of male skin graft rejection. Immunobiology. 2009;214:703–711. doi: 10.1016/j.imbio.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, Ramos H, Todo S, Tzakis A, Fung JJ, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- Thangavelu G, Smolarchuk C, Anderson CC. Co-inhibitory molecules: Controlling the effectors or controlling the controllers? Self/Nonself. 2010;1:77–78. doi: 10.4161/self.1.2.11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- van Wijk F, Nierkens S, de Jong W, Wehrens EJ, Boon L, van Kooten P, Knippels LM, Pieters R. The CD28/CTLA-4-B7 signaling pathway is involved in both allergic sensitization and tolerance induction to orally administered peanut proteins. J. Immunol. 2007;178:6894–6900. doi: 10.4049/jimmunol.178.11.6894. [DOI] [PubMed] [Google Scholar]
- Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc. Natl. Acad. Sci. U S A. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Han R, Hancock WW. Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur. J. Immunol. 2007;37:2983–2990. doi: 10.1002/eji.200737583. [DOI] [PubMed] [Google Scholar]
- Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc. Natl. Acad. Sci. U S A. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Carper K, Malone F, Latchman Y, Perkins J, Fu Y, Reyes J, Li W. PD-L1/PD-1 signal deficiency promotes allogeneic immune responses and accelerates heart allograft rejection. Transplantation. 2008;86:836–844. doi: 10.1097/TP.0b013e3181861932. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Yoon IH, Choi SE, Kim YH, Yang SH, Park JH, Park CS, Kim Y, Kim JS, Kim SJ, Simpson E, Park CG. Pancreatic islets induce CD4(+) [corrected] CD25(−)Foxp3(+) [corrected] T-cell regulated tolerance to HY-mismatched skin grafts. Transplantation. 2008;86:1352–1360. doi: 10.1097/TP.0b013e31818aa43c. [DOI] [PubMed] [Google Scholar]