Abstract
Objective
This study tested the effect of beginning treatment with a speech-generating device in the context of a blended, adaptive treatment design for improving spontaneous, communicative utterances in school-aged, minimally verbal children with autism.
Method
Sixty-one minimally verbal children with autism, aged 5 to 8 years, were randomized to a blended developmental/behavioral intervention (JASP+EMT) with or without the augmentation of a speech-generating device (SGD) for 6 months with a 3-month follow-up. The intervention consisted of two stages. In Stage 1 all children received two sessions per week for 3 months. Stage 2 intervention was adapted (increased sessions or adding the SGD) based on the child’s early response. The primary outcome was the total number of spontaneous communicative utterances; secondary measures were total number of novel words and total comments from a natural language sample.
Results
Primary aim results found improvements in spontaneous communicative utterances, novel words, and comments that all favored the blended behavioral intervention that began by including an SGD (JASP+EMT+SGD) as opposed to spoken words alone (JASP+EMT). Secondary aim results suggest that the adaptive intervention beginning with JASP+EMT+SGD and intensifying JASP+EMT+SGD for children who were slow responders led to better post-treatment outcomes.
Conclusion
Minimally verbal school-aged children can make significant and rapid gains in spoken spontaneous language with a novel, blended intervention that focuses on joint engagement and play skills and incorporates an SGD. Future studies should further explore the tailoring design used in this study to better understand children’s response to treatment.
Clinical trial registration information—Developmental and Augmented Intervention for Facilitating Expressive Language (CCNIA); http://clinicaltrials.gov/; NCT01013545.
Keywords: autism spectrum disorders, minimally-verbal, school-aged, communication intervention, SMART design
Introduction
Communication impairment is a core deficit of children diagnosed with autism spectrum disorders (ASD). While most children learn to communicate with spoken language, approximately 25–30% of children with ASD remain minimally verbal, even after years of intervention.1,2 Exact numbers are unknown largely because research studies often exclude children due to limited verbal abilities.1 Failure to develop spoken language by age 5 increases the likelihood of a poor long-term prognosis for social and adaptive functioning.2,3
Some children can learn spoken language after the age of 5 years, but the window of opportunity may be small.4 A recent review of studies of language acquisition in individuals with ASD reported on 167 individuals who started speaking after age 5.5 The majority of individuals who acquired spoken language did so between 5 and 7 years of age and had nonverbal IQs over 50. These individuals often received behavioral interventions targeting production of sounds and words and learned to produce single words to request needs and wants. Only one third who began to use spoken language progressed to phrase speech. Because participant and outcome descriptors were often limited, the extent to which word production was communicative (i.e., socially directed to others) is unknown.
One approach to providing minimally verbal children a means to communicate is to use augmentative or alternative communication (AAC) approaches, most often a picture symbol system or speech generating device (SGD). While AAC intervention studies demonstrate improvements in communication, few have demonstrated changes in spoken language. For example, the Picture Exchange Communication System (PECS) is a visually-based augmentative communication system in which children exchange pictures in order to communicate with others. One study randomized 84 children to PECS or control conditions and found that children with PECS training initiated communicative requests at a higher rate.6 Vocalizations also improved, especially for children who had some spoken language at baseline. 7 Language test scores, however, did not improve.6
Another AAC intervention approach involves a speech-generating device (SGD). SGDs display symbols that produce voice output communication when selected. A review of 23 studies that employed an SGD included a total of 51 children with ASD between the ages of 3 and 16 years8. All studies were single subject designs, and most focused on teaching, requesting, or responding to questions using the SGD. Few studies assessed maintenance and generalization. While using a SGD appears to increase communication, particularly requesting in individual children with ASD,8 no rigorous group designs have replicated these findings, and few studies have demonstrated varied communicative functions beyond requesting (e.g., commenting) or an increase in spoken language. Because of the importance of increasing social use of spoken language, in the current study our primary outcome measure was total spontaneous, communicative utterances (SCU) coded from a standardized Natural Language Sample (NLS). SCUs are unprompted, generative (non-scripted) communicative utterances that are directed to a partner for the purpose of sharing information (comments), requests and questions.
Given the lack of spoken language progress for some children with ASD who have had access to early intervention services, we considered novel approaches to intervention in this study. We blended two communication-focused and evidence-based early interventions for preschool children—JASPER (Joint Attention Symbolic Play Engagement and Regulation)9,10 and EMT (Enhanced Milieu Teaching)11,12 hereafter JASP+EMT. JASPER is a naturalistic behavioral intervention focused on the development of prelinguistic gestures (joint attention, requesting) and play skills within the context of play-based interactions as a means to increase joint engagement between an adult and child with ASD.9,10 EMT is a naturalistic behavioral intervention that uses responsive interaction and systematic modeling and prompting to promote spontaneous, functional spoken language.11,12 Both JASPER and EMT have shown efficacy in preschool-aged, minimally verbal children with ASD.10,12–13
Further, given the promising but limited data on the effectiveness of SGDs for children with ASD, we sought to understand the role of SGDs as a treatment component in the context of JASP+EMT. Because not all children were expected to benefit equally from these components, we employed adaptive intervention designs.14 In an adaptive intervention, treatment may be adapted (e.g., by intensifying the dosage or augmenting the spoken intervention with SGD) to address the specific needs of the child (e.g., if the child is making slow progress in spoken communication).
The overarching aim of this study was to construct an adaptive intervention that utilized JASP+EMT and varied the addition of a SGD with minimally verbal school-aged children. Three adaptive interventions were considered in the context of a sequential multiple assignment randomized trial (SMART)15–19:(a) one which began with JASP+EMT and intensified JASP+EMT for children who were slow responders; (b) a second adaptive intervention which began with JASP+EMT and augmented JASP+EMT with SGD for children who were slow responders; and (c) a third which began with JASP+EMT+SGD and intensified JASP+EMT+SGD for children who were slow responders. The SMART design addressed two aims. The primary aim was to examine the effect of the adaptive intervention beginning with JASP+EMT+SGD vs. the adaptive interventions beginning with JASP+EMT alone. A secondary aim was to compare outcomes across the aforementioned three adaptive interventions.
METHOD
Study Design
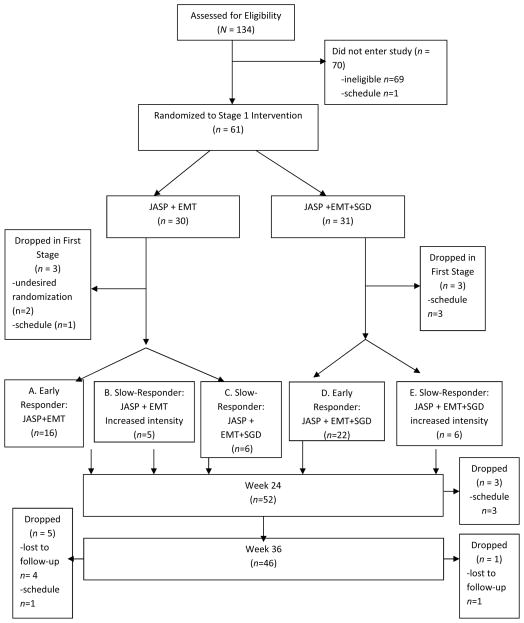
This study was a longitudinal (repeated outcome measures at baseline and weeks 12, 24 and 36), three-site SMART design. This SMART included two stages of treatment (Figure 1). Each stage of treatment was 12 weeks in duration. At the beginning of Stage 1 (baseline), all children meeting inclusion criteria were randomized with equal probability to JASP+EMT vs. JASP+EMT+SGD. At the end of 12 weeks, children were assessed for early response vs. slow-response (defined in section “Stage 2 Treatments: Weeks 13–24”) to Stage 1 treatment. At the beginning of Stage 2 (beginning of week 13), the subsequent treatments were adapted based on response status. All early responders continued with the same treatment for another 12 weeks. For slow-responders to JASP+EMT+SGD, treatment was intensified (3 sessions per week). Slow-responders to JASP+EMT were re-randomized with equal probability to intensified JASP+EMT or augmented JASP+EMT+SGD (see Figure 1). The Institutional Review Board at each site approved the study protocol. Randomization was conducted by an independent data-coordinating center.
Figure 1.
Consort Chart
Note: JASP+EMT =spoken mode of JASPER plus Enhanced Milieu Teaching; JASP+EMT+SGD =spoken mode of JASPER plus Enhanced Milieu Teaching plus Speech Generating Device.
Participants
Inclusion criteria were: (1) previous clinical diagnosis of ASD, confirmed by research-reliable staff using the Autism Diagnostic Observational Schedule (ADOS-Generic)20 Module 1 appropriate for children without phrase speech; (2) chronological age between 5 and 8 years; (3) evidence of being minimally verbal, with fewer than 20 spontaneous different words used during the 20-minute NLS; (4) at least 2 years of prior intervention, per parent report; (5) Receptive language age of at least 24 months (based on performance of 2 out of 3 assessments given potential difficulty complying with standardized test conditions. Exclusion criteria were: (i) major medical conditions other than ASD; (ii) sensory disabilities, e.g., deafness; (iii) motor disabilities, e.g., cerebral palsy; (iv) uncontrolled seizure disorders; and (v) proficient use of an SGD based on parent report and observation during study administration of the Natural Language Sample (NLS).
61 children were randomized in the SMART. At baseline, participants completed diagnostic and standardized cognitive and language assessments. 60 of the children met ADOS20 criteria for autism, and 1 met criterion for ASD. Participants were mean age 6.31 years old (SD=1.16), had an average of 17.23 (SD=16.44) different words on the baseline language sample (NLS) administered by an unfamiliar and blinded assessor. 5 children screened with more than 20 words (range 26–51) and were included due to low intelligibility and predominance of scripted language. Nonverbal cognitive scores averaged 4.00 years (SD=1.12), with an average Brief-IQ standard score of 68.18 (SD= 18.96). Table 1 shows the baseline (pre-treatment) characteristics in the overall sample (n=61) and for Stage 1 treatment groups. Baseline child characteristics did not differ by treatment group assignment.
Table 1.
Participant baseline demographic and developmental variables, by initial treatment assignment
| Total N=61 |
JASP+EMT n=30 |
JASP+EMT+SGD n=31 |
|||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| % | n | % | n | % | n | p-value | |
|
|
|||||||
| Gender | |||||||
| Male | 83% | 51 | 87% | 26 | 79% | 25 | .44 |
| Female | 17% | 10 | 13% | 4 | 21% | 6 | |
| Race | .92 | ||||||
| White | 48% | 29 | 47% | 14 | 48% | 15 | |
| African-American | 23% | 14 | 21% | 6 | 25% | 8 | |
| Asian American | 19% | 12 | 21% | 7 | 16% | 5 | |
| Hispanic | 5% | 3 | 4% | 1 | 7% | 2 | |
| Other | 5% | 3 | 7% | 2 | 4% | 1 | |
| Mother’s Education | .17 | ||||||
| ≤ High School | 5% | 3 | 3% | 1 | 8% | 3 | |
| College | 38% | 23 | 47% | 14 | 27% | 8 | |
| Graduate School | 57% | 35 | 50% | 15 | 65% | 20 | |
| Site | .98 | ||||||
| UCLA | 39% | 24 | 40% | 12 | 39% | 12 | |
| VU | 33% | 20 | 33% | 10 | 32% | 10 | |
| KKI | 28% | 17 | 27% | 8 | 29% | 9 | |
|
| |||||||
| Mean | SD | Mean | SD | Mean | SD | p-value | |
|
|
|||||||
| Age (years) | 6.31 | 1.16 | 6.18 | 1.08 | 6.44 | 1.23 | .37 |
| Language Sample | |||||||
| TSCU | 29.44 | 25.37 | 28.37 | 29.96 | 30.48 | 20.35 | .75 |
| TDWR | 17.20 | 16.44 | 16.78 | 19.52 | 17.61 | 12.91 | .27 |
| TCOM | 6.02 | 8.17 | 7.01 | 11.00 | 5.07 | 3.80 | .86 |
| Standardized Assessments | |||||||
| TELD-3 | |||||||
| Receptive AE | 2.03 | .62 | 1.94 | .52 | 2.12 | .71 | .26 |
| Expressive AE | 1.73 | .39 | 1.70 | .37 | 1.75 | .42 | .68 |
| PPVT-4 | |||||||
| AE | 2.64 | .67 | 2.55 | .66 | 2.72 | .68 | .34 |
| Leiter-R | |||||||
| Brief IQ | 68.18 | 18.96 | 68.73 | 21.26 | 67.65 | 16.77 | .83 |
| AE | 4.00 | 1.12 | 3.93 | 1.12 | 4.07 | 1.14 | .63 |
| ADOS | 20.02 | 4.37 | 20.60 | 4.47 | 19.55 | 4.27 | .35 |
Note. p-values examine whether significant differences between first-stage treatment assignments exist. Chi-squared tests were used, except in cases where n<5, Fisher’s exact test was used. ADOS=Autism Diagnostic Observation Schedule; AE = “age equivalent” in years; JASP+EMT = spoken mode of JASPER plus Enhanced Milieu Teaching; JASP+EMT+SGD = spoken mode of JASPER plus Enhanced Milieu Teaching plus Speech Generating Device; KKI = Kennedy Krieger Institute; PPVT-4=Peabody Picture Vocabulary Test, 4th Edition; TCOM = Total number Comments; TDWR = Total Different Word Roots; TELD-3=Test of Early Language Development, 3rd Edition; TSCU = Total Socially Communicative Utterances; UCLA = University of California-Los Angeles; VU = Vanderbilt University.
Intervention Procedures
The core intervention in all components was the JASP+EMT naturalistic communication intervention that taught joint attention, symbolic play, and social use of language during child-preferred play activities. All training occurred in university clinic playrooms. Each child was assigned a therapist (speech clinician, special educator or child psychologist) who was trained to criterion fidelity (>.90) on all elements of the treatment variations. During the first phase of intervention (12 weeks), all children received 24 1-hour sessions of treatment in either the JASP + EMT spoken mode or JASP+ EMT+ SGD mode. During the JASP+EMT+SGD condition, the SGD was used to model a minimum of 50% of all spoken communication. In the second phase of intervention, parents were included in the second 24 treatment sessions. Following a manualized protocol, the child’s therapist implemented systematic parent training consistent with the treatment variation to which the child was assigned. Throughout both treatment phases and all adaptive treatment variations, fidelity of therapist implementation was assessed for 20% of the sessions.
Measures
Natural Language Sample (NLS)
The NLS was a 20-minute standardized, naturalistic adult-child interaction in which an adult and child played with a specific set of toys. The adult was responsive to child verbal and nonverbal communication but did not prompt the child to talk. The NLS provides a standard context (time, materials, interaction style) that can be used to evaluate a child’s spontaneous expressive language ability.21 Standardized NLS can be used to collect repeated measures of child language production to index growth over time. Such measures provide stable estimates of child expressive abilities, are appropriate for repeated measures, and have been shown to be sensitive to changes associated with language interventions.21 NLS-based measures have been recommended to index productive language abilities of children with autism relative to age-typical normative development.21,22
Research staff blind to treatment condition administered the NLS; staff were trained to 90% fidelity criterion on the NLS interaction procedures prior to assessment. A 24-item checklist based on procedures used in prior research studies 23 was used to assess fidelity. Fidelity was rated on 20% of the sessions by independent and blinded coders. Staff transcribed the samples using Systematic Analysis of Language Transcripts22 (SALT) conventions. Separate, blinded coders verified the transcripts and coded each child utterance for generativity (not scripted), and communicative function (e.g., comment, request, other). Both spoken and SGD-produced utterances were transcribed and coded; mode was noted. Reliability of coding of utterances was determined using an exact agreement formula for each individual code which was then aggregated across codes; overall reliability was 88.1%.
From the NLS, the primary outcome was the total number of spontaneous communicative utterances (TSCU), which included comments, requests, and protests and excluded scripted and nonsocial utterances. Secondary outcomes were total number of different word roots (TDWR) and number of comments (TCOM). Both spoken and SGD-produced utterances were included for all variables. Measures were derived from NLS collected at baseline (pre-treatment) and at weeks 12, 24, and 36 (follow-up). Along with TSCU, TDWR, and TCOM, data on the following additional language variables were collected to derive the early/slow-response measure used to trigger stage 2 treatments: proportion of all utterances that were socially communicative (PSCU), words per minute (WPM), mean length utterance in words (MLUw), and number of unique word combinations (NUWC).
Intervention Session Transcripts
Intervention sessions were transcribed and coded following the same conventions and including the same seven variables (TSCU, TDWR, TCOM, PSCU, WPM, MLUw, and NUWC) as with the NLS. 10-minute sections (minutes 2–12) were transcribed during the first week of intervention and the 12th week of intervention. These seven session transcript measures were used (along with the seven from the NLS) for determining early/slow response to treatment.
Autism Diagnostic Observation Schedule (ADOS), Module 1.20
The ADOS is a 30–45 minute semi-structured play-based assessment from which operationally-defined behaviors associated with ASD are rated on a 0-to-2 or 3-point scale. An algorithm score provides cut-off scores for ASD and autism classifications. All participants completed Module 1 of the ADOS, designed for children at the nonverbal or single word phase. The ADOS was completed at baseline to confirm eligibility.
Leiter International Performance Scale-Revised (Leiter-R) is a nonverbal cognitive assessment for individuals 2–20 years old. Tasks include matching, pattern completion, and sequential order and do not require verbal responses. This test, completed at baseline to confirm eligibility, yields global IQ and mental age equivalence scores.
Peabody Picture Vocabulary Test, Fourth Edition (PPVT-4)
This test of receptive vocabulary development is appropriate for children aged 2 years and older. From an array of four pictures, the child identifies (by pointing) the one that best illustrates the word pronounced by the examiner. The PPVT-4 was completed at baseline to determine eligibility and yields age-equivalent and standard scores.
Test of Early Language Development Third Edition (TELD-3)
The TELD-3 is a standardized assessment with subtests for receptive and expressive vocabulary development. The assessment is normed for children aged 2 years and older. The TELD-3, given at baseline, yields age equivalent and standardized scores; the receptive subtest was used to confirm eligibility.
Demographic Questionnaire
Parents completed a brief demographic questionnaire providing information about the child and family, including child’s previous early intervention and current services.
Intervention Stages
There were two intervention stages (early and adapted) in the study, for a total of 24 weeks of intervention. Interventionists were trained to criterion across sites and closely supervised both on-site and through site visits, weekly conference calls, video feedback and fidelity checks for 20% of sessions that were rated using an exact agreement formula (agreements divided by agreements plus disagreements). Fidelity of treatment implementation averaged 94.26% (SD = 5%) for JASP+EMT and 93.69% (SD = 4%) for JASP+EMT+SGD.
Stage 1 Treatments: Weeks 1 – 12
JASP+EMT
Participants initially randomized to this condition received two, hour-long sessions per week, for 12 weeks. JASP (based on JASPER) focuses on early social-communication skills including coordinated joint attention gestures known to be associated with the development of later spoken language of children with autism.9,10 Intervention ingredients include the creation of contextually relevant and meaningful learning opportunities during interactions with adult partners (therapists, parents) who are responsive to child interests and actions, who model and expand play and gesture use and maintain joint engagement. The second intervention focuses on spoken language acquisition, Enhanced Milieu Teaching (EMT).11,12 EMT is a naturalistic early language intervention that uses seven core strategies to teach language in social interaction: following the child’s lead in conversation and play, responding to communicative initiations from the child with target language, expanding child utterances by adding words to increase complexity while maintaining the child’s meaning, arranging the environment to support and elicit communication from the child, and systematic use of prompts (model, time delays and prompts). Both JASPER and EMT are manualized; the blended intervention was called JASP+EMT.
JASP+EMT+SGD
Participants initially randomized to this condition received two, hour-long sessions per week, for 12 weeks. All aspects of the intervention were the same as JASP+EMT, except for the addition of a speech-generating device (SGD), such as an iPad or DynaVox. The SGD was programmed with vocabulary relevant to the toys/activities used during each treatment session. The interventionist modeled and expanded target language on the SGD in conjunction with spoken language following the modeling and expansion protocols of JASP+EMT; the SGD intervention followed a written protocol. To ensure fidelity of use of the SGD, the interventionist was required to use the SGD at least 50% of the time when modeling language. If the child initiated a communication bid using the SGD, the interventionist expanded this bid at least 80% of the time.
Stage 2 Treatments: Weeks 13–24
Response/Slow-response Measure
Stage 2 treatments depended on early vs. slow response to Stage 1 treatments (JASP+EMT or JASP+EMT+SGD). Early vs. slow response was defined based on fourteen measures from two sources: the seven communication variables (TSCU, PSCU, MLUw, TDWR, WPM, TCOM, and NUWC) from the NLS with blinded assessor, and the same seven communication variables from the intervention transcripts. For each of the fourteen variables, we calculated percent change from baseline to week 12. If the child demonstrated 25% or greater change on at least half of the variables (7 out of 14), then the participant was considered an early responder; otherwise, the child was considered a slow responder. Early responders continued their Stage 1 Intervention assignment for an additional 12-weeks in Stage 2.
Children who were slow responders at the end of Stage 1 received a modified intervention to improve outcomes in spontaneous communication. Slow responders to Stage 1 JASP+EMT intervention were re-randomized with equal probability to either intensified JASP+EMT (increased dose of intervention) or the addition of the SGD (augmenting the intervention). Slow responders to JASP+EMT+SGD intervention for Stage 1 were assigned to intensified JASP+EMT+SGD.
Intensified JASP+EMT+SGD
This intervention was identical in content to JASP+EMT+SGD but occurred for a total of 3 hours per week for an additional 12 weeks.
Intensified JASP+EMT
This intervention was identical in content to JASP+EMT but occurred for a total of 3 hours per week for another 12 weeks.
Augmented JASP+EMT+SGD
This intervention was identical to the Stage 1 JASP+EMT+SGD intervention. It consisted of two, hour-long sessions per week for another 12 weeks.
Parent Participation in the Intervention
Parents were involved in all stages of the study. During Stage 1, parents watched intervention sessions through one-way mirrors. In Stage 2, all parents were provided training concurrent with the intervention sessions in all three adapted conditions. Parents joined the child and therapist in the clinic room, observed the therapist working with the child for part of each session, and then practiced the intervention with the child while the therapist coached their implementation. Fidelity of parent implementation was assessed in 20% of the sessions and averaged 67.38 (SD=11.07) for parents in JASP+EMT and 66.46 (SD=13.08) for parents in JASP+EMT+SGD.
Aims
Primary aim of study was to examine the effectiveness of the adaptive intervention beginning with JASP+EMT+SGD vs. beginning with JASP+EMT on longitudinal outcomes at weeks 12, 24 (primary endpoint) and 36 (follow-up). This corresponds to testing the main effect of Stage 1 treatment (initial JASP+EMT vs. initial JASP+EMT+SGD). Secondary aim of study was to compare mean outcomes at weeks 24 and 36 among the three embedded adaptive interventions.
Sample Size
The planned sample size was based on the primary aim, using the primary outcome (TSCU): a between-groups comparison of Stage 1 treatment (JASP+EMT+SGD vs. JASP+EMT) of the average TSCU at week 24 (the primary endpoint). Using a two-sided, two-sample t test with a Type-I error rate of 5% and assuming an attrition rate of 10% by week 24, the planned total sample size for this study was n=97 to detect a moderate effect size of 0.6 in TSCU with at least 80% power.
Statistical Analysis
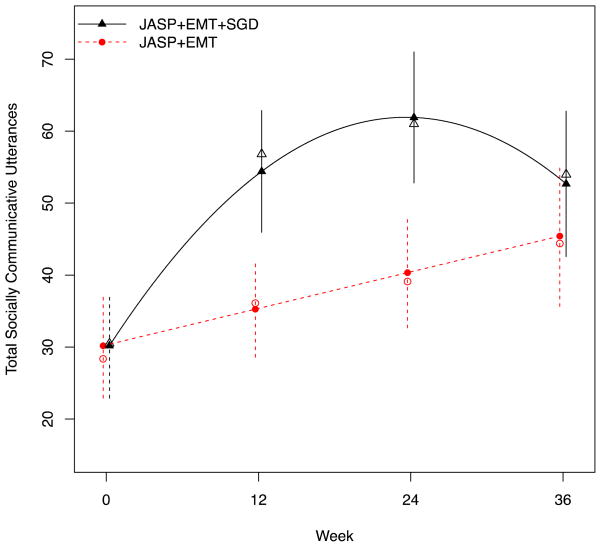
Longitudinal regression models were used to examine mean differences in the primary outcome (TSCU) and secondary outcomes (TDWR, TCOM) between the two Stage 1 treatments (JASP+EMT+SGD vs. JASP+EMT) at weeks 0, 12, 24, and 36. Separate models were fit for each longitudinal outcome. Model diagnostics were used to choose model specifications for time (in weeks): for TSCU, a quadratic model for JASP+EMT+SGD and a linear model for JASP+EMT fit best (see Figure 2); for TDWR and TCOM, piecewise linear models with knot at week 12 fit best. Each model included the following baseline covariates: age (years), gender (female as referent), ethnicity (indicators for African-American, Caucasian, Hispanic, Asian, with other as the referent), site (indicators for sites 1 and 2, site 3 as referent), and total ADOS at baseline. In each model, the residual error terms were assumed to follow a mean-zero normal distribution with a compound symmetric covariance structure used to capture the within-person correlation over time (except for TCOM, for which an unstructured covariance structure led to better fit). Fitted models were used to calculate (and plot) mean scores (marginal over baseline covariates) at each time point and to report between-groups comparisons at weeks 12 and 24 (primary endpoint) and at 36 (follow-up). We also report within-treatment group change 1) from baseline to week 24 (during treatment) and 2) from week 24 to week 36 (post-treatment follow-up). In post hoc analyses, we examined site-by-time-by-treatment interaction effects.
Figure 2.
Primary aim results for the primary outcome (Total Social Communicative Utterances)
Note: Open plotting characters denote observed means; closed denote model-estimated means. Error bars denote 95% confidence intervals for the model-estimated means. JASP+EMT = spoken mode of JASPER plus Enhanced Milieu Teaching; JASP+EMT+SGD = spoken mode of JASPER plus Enhanced Milieu Teaching plus Speech Generating Device.
For the secondary aim analysis, a weighted regression16 was used to compare means in the primary and secondary outcomes between the three embedded adaptive interventions at weeks 24 and 36. A separate model was fit for each outcome. Each model included an indicator for time (week 24 as referent), an indicator for Stage 1 treatment (JASP+EMT vs. JASP+EMT+SGD), an indicator for second-stage treatment (intensify JASP+EMT vs. augment with JASP+EMT+SGD) nested within JASP+EMT, and time-by-treatment interaction terms. Each model included the baseline covariates noted above, plus baseline TSCU, PSCU, TDWR, and TCOM. Non-responders to JASP+EMT were assigned a weight of 4 to account for having 1/4 chance (non-responders to JASP+EMT were randomized twice with probability of 1/2) of following their assigned sequence of treatments. All other children were assigned a weight of 2. Robust standard errors, which account for sampling variation in the distribution of the weights, were used. We also report the rate of response/slow-response at week 12 by Stage 1 treatment assignment.
All randomized participants were included in all analyses in accordance with intention-to-treat principles. In order to enhance interpretation of the results for the primary and secondary aims, we report treatment effect sizes (Cohen’s d)24 at each time point, defined as the estimated mean difference divided by the standard deviation in the outcome. For the response rate at week 12, we report the number of children who need to be treated (NNT)25 initially with JASP+EMT+SGD rather than JASP+EMT for one additional child to benefit. A p value < 0.05 (two-sided) was considered statistically significant; 95% confidence intervals (95%CI) were calculated for all point estimates. Data were analyzed using the nlme (primary aim) and the geepack (secondary aim) packages in R.
Missing Data
Multiple imputation was used to replace missing values in the outcomes and other measures. A sequential regression multivariate imputation algorithm was implemented using the mice package for R. The imputation model used was congenial with all analysis models: it included all longitudinal outcome measures, treatment indicators, response/slow-response measures at week 12, the baseline covariates listed above, and other time-varying measures thought to be correlated with outcomes. Twenty imputed data sets were generated. Point estimates, standard errors, and all tests were calculated using Rubin’s rules for combining the results of identical analyses performed on each of the 20 imputed data sets. Sensitivity analyses26 were conducted to assess the robustness of the results to the missing-at-random assumption. This assumption states that, given the observed data included in the imputation model, the reason for missing data does not depend on data that is unobserved.
RESULTS
Recruitment and Retention
Of the 134 children examined for eligibility, 61 children met criteria and were randomized at Stage 1 (Figure 1). Due to difficulties in recruiting participants who met the inclusion criteria, it was not possible to meet the total planned sample size of 97 (see Discussion). Many of the ineligible participants were below the developmental cut-off of 24 months. All missing values in this study were due to participant attrition from the study; attrition rates were 10% by week 12, 14% by week 24, and 25% by week 36. Attrition did not differ by Stage 1 randomized treatment assignment (p=0.71). Among participants still in the study at week 12, attrition during the follow-up period (weeks 24–36) did not differ by response/slow-response status (p=0.86), nor by Stage 2 randomized treatment assignment among slow responders to JASP+EMT (p=0.89). In sensitivity analyses26 concerning the missing data, results reported below were robust to violations of the missing-at-random assumption.
Primary Aim: Main Effect of Stage 1 Treatment (JASP+EMT+SGD vs. JASP+EMT)
Primary Contrast
Intervening with JASP+EMT+SGD initially (vs. starting with JASP+EMT alone) led to greater TSCU at week 24 (p<0.01; see Table 2). Specifically, the average TSCU at week 24 for JASP+EMT+SGD was 61.9 utterances (95%CI, 52.8 to 71.0) vs. 40.3 utterances (95%CI, 32.7 to 48.0) for JASP+EMT, a clinically significant average difference of 21.6 utterances (95%CI, 10.7 to 32.4). This difference is approximately double the rate of communicative utterance per minute from baseline, which is considerable for this sample of low-rate communicators. This corresponds to a moderate-large treatment effect size of 0.62. On average, 92.1% (95%CI, 87.8% to 96.5%) of the total TSCU (across all time points) in the JASP+EMT+SGD group were spoken utterances. In additional analyses in which only spoken utterances were used for the JASP+EMT+SGD group, the effect of SGD on TSCU at week 24 was attenuated (effect size of 0.51) but remained statistically significant (p<0.05).
Table 2.
Estimated means for the primary Total Socially Communicative Utterances (TSCU) and secondary Total Different Word Roots (TDWR) and Total number Comments (TCOM) outcomes at week 12, 24, and 36, by stage 1 treatment assignment.
| JASP+EMT | JASP+EMT+SGD | Difference | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95%CI | Mean | 95%CI | Mean | 95%CI Effect Size | ||
| Week 12 | |||||||
| TSCU | 35.26 | (28.58,41.94) | 54.40 | (45.94,62.86) | 19.14* | (10.85,27.44) | 0.57 |
| TDWR | 24.32 | (16.87,31.78) | 33.11 | (26.94,39.27) | 8.78* | (.44,17.12) | 0.34 |
| TCOM | 8.10 | (4.83,11.37) | 14.09 | (10.88,17.30) | 5.99* | (1.62,10.36) | 0.51 |
| Week 24 | |||||||
| TSCU | 40.34 | (32.67,48.01) | 61.90 | (52.80,71.00) | 21.56* | (10.70,32.42) | 0.62 |
| TDWR | 25.62 | (19.11,32.14) | 33.11 | (26.94,39.27) | 7.48* | (.20,14.77) | 0.29 |
| TCOM | 8.10 | (4.83,11.37) | 14.09 | (10.88,17.30) | 5.99* | (1.62,10.36) | 0.44 |
| Week 36 | |||||||
| TSCU | 45.42 | (65.64,55.19) | 52.68 | (42.58,62.77) | 7.26 | (−6.16,20.68) | 0.22 |
| TDWR | 26.93 | (18.62,35.23) | 33.11 | (26.94,39.27) | 6.18* | (2.44–14.40) | 0.21 |
| TCOM | 8.10 | (4.93,11.37) | 14.09 | (10.88,17.30) | 5.99* | (1.62–10.36) | 0.54 |
Note: JASP+EMT = spoken mode of JASPER plus Enhanced Milieu Teaching; JASP+EMT+SGD = spoken mode of JASPER plus Enhanced Milieu Teaching plus Speech Generating Device.
denotes p<0.05
Additional Contrasts
Similarly, for the secondary outcomes, Stage 1 JASP+EMT+SGD led to greater TDWR (p=0.04) and TCOM (p<0.01) at week 24. Treatment effect sizes for the secondary outcomes at week 24 were small (0.29 for TDWR) to small-moderate (0.44 for TCOM). For all outcomes, Stage 1 JASP+EMT+SGD was superior to JASP+EMT at week 12. For TDWR and TCOM, treatment effects peaked at week 12, whereas for TSCU, effects peaked at week 24. By week 36, treatment effects were maintained for TCOM, but attenuated for TSCU and TDWR. In post hoc analyses, there was no evidence of significant site-by-treatment interactions effects at any time point: TSCU (p=0.45), TDWR (p=0.78), TCOM (p=0.28).
Secondary Aim: Comparison of Embedded Adaptive Interventions
Consistent with the results for the primary aim, the adaptive interventions leading to the greatest TSCU at week 24 were the adaptive interventions that began with JASP+EMT+SGD and intensified JASP+EMT+SGD among children who were slow responders (see Table 3). Among the two adaptive interventions beginning with JASP+EMT, the adaptive intervention which augmented JASP+EMT with SGD among slow responders led to greater TSCU (42.7; 95%CI, 33.2 to 52.3) than the adaptive intervention, which intensified JASP+EMT for slow responders (39.6; 95%CI, 28.5 to 50.7); however, the mean difference in outcome was not significant clinically or statistically (ES, 0.10; 95%CI, −4.2 to 10.5; p=.40). Results were similar at week 36.
Table 3.
Estimated means for the primary Total Socially Communicative Utterances (TSCU) and secondary Total Different Word Roots (TDWR) and Total Number Comments (TCOM) outcomes at week 24 and 36, for each of the three adaptive interventions (AI).
| JASP+EMT then Intensified JASP+EMT | JASP+EMT then JASP+EMT+SGD | JASP+EMT+SGD then Intensified JASP+EMT+SGD | Differences | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AI1 (A+C)a | AI2 (A+B) | AI3 (D+E) | AI3 vs. AI1 | AI3 vs. AI2 | ||||||||
| Mean | 95%CI | Mean | 95%CI | Mean | 95%CI | Mean | 95%CI | Effect Size | Mean | 95%CI | Effect Size | |
| Week 24 | ||||||||||||
| TSCU | 42.74 | (33.22,52.27) | 39.58 | (28.50,50.66) | 58.45 | (46.71,70.18) | 15.70 | (−0.45,31.85) | 0.45 | 18.87* | (1.45,36.29) | 0.55 |
| TDWR | 27.51 | (21.65,33.38) | 26.46 | (19.48,33.43) | 33.48 | (26.05,40.91) | 5.97 | (−3.53,15.47) | 0.23 | 7.03 | (−3.78–17.83) | 0.27 |
| TCOM | 10.79 | (7.44,14.14) | 9.25 | (5.85,12.66) | 11.90 | (8.10,15.70) | 1.11 | (−4.05–6.27) | 0.08 | 2.65 | (−2.70–8.00) | 0.19 |
| Week 36 | ||||||||||||
| TSCU | 45.58 | (35.93,55.24) | 44.51 | (32.06,56.96) | 52.42 | (44.37,60.46) | 6.83 | (−5.87–19.53) | 0.20 | 7.91 | (−7.42–23.23) | 0.23 |
| TDWR | 29.75 | (22.85,36.64) | 26.36 | (18.13,34.59) | 31.94 | (26.66,38.21) | 2.19 | (−6.53–10.91) | 0.09 | 5.58 | (−4.72–15.88) | 0.22 |
| TCOM | 8.03 | (4.70,11.37) | 8.24 | (5.25,11.23) | 13.77 | (10.21,17.33) | 5.74* | (0.82–10.67) | 0.45 | 5.53* | (0.80–10.27) | 0.44 |
Note:
denotes p<0.05.
See Figure 1, consort chart for definitions of A-E.
All variables are frequency counts.
The overall rate of early response for all participants at week 12 was 70% (95%CI, 57.9% to 82.3%). Children assigned to JASP+EMT had a response rate of 62.2% (95%CI, 45% to 79.3%), whereas children assigned to JASP+EMT+SGD had a response rate of 77.7% (95%CI, 60.6% to 95.0%). This 15.6% difference is clinically, but not statistically, significant (95%CI, −8.7% to 39.9%; p=0.20). Approximately 6.5 children need to be treated26 (95%CI, number needed to harm [NNTH] 11.5 to ∞ to number needed to treat for benefit [NNTB] 2.5) initially with JASP+EMT+SGD rather than JASP+EMT alone for one additional child to respond by week 12.
DISCUSSION
The current study focused on increasing spontaneous communicative, spoken language in minimally verbal, school-aged children with ASD. Using a novel blended, adaptive intervention, children improved over a six-month treatment and 3-month follow-up. These findings are particularly important because the intervention was provided to children who were minimally verbal after early intervention, and in most cases, after at least two years of early intensive behavioral interventions. Children showed significant gains in spontaneous communication in a short period of time in a relatively low intensity developmental and behaviorally-based intervention of 2–3 hours per week.
There were three main findings:
First, there was a robust and consistent finding that beginning intervention with the SGD integrated into the blended intervention was superior in producing more spontaneous communicative utterances than beginning intervention with the blended intervention and spoken language only. These data are especially important given our current knowledge of effective interventions for minimally verbal children with ASD. One randomized trial and several single case studies have found benefit in augmentative communication approaches for increasing requesting but note limitations on improving spoken language. A recent study with preschool-aged, minimally verbal children found gains in spoken language for two oral language-based interventions.27 This small scale RCT with 17 minimally verbal preschoolers with ASD and with mental ages over 12 months did not yield significant effects of two behavioral interventions (one naturalistic and one discrete trial teaching); rather both interventions doubled spoken communication during a 20-minute behavior sample, from 2 words at baseline to 4–5 words at exit. A moderator analysis found that children who had more joint attention skills pre-treatment responded better in both treatments. Joint attention skills have predicted spoken language outcomes in several previous intervention studies,27–29 including one that followed children over a five-year period after receiving JASPER during preschool.30 Thus, the current study that focused on developmental pre-requisites to spoken language, including joint attention, joint engagement, and play along with systematic modeling and prompting for spoken language may have provided the combination of supports needed for minimally verbal children with ASD to successfully increase their spoken communication.
Second, this is one of the first studies to show increases in minimally verbal children’s spontaneous communication including different types of words and functions beyond requesting. Positive outcomes in previous studies generally have been limited to increases in requesting behavior when an augmentative means of communication is introduced.7–9 The focus on requesting is expected in an adult-directed intervention approach in which the child is often prompted to comply with instructions and there are strong external reinforcement strategies in place. The current intervention approach utilized a developmental, child-directed approach with strong naturalistic reinforcement strategies in place. Adults in the intervention were contingently responsive to child attempts at communication and provided expansion of language through models that matched the child’s communicative intent. Children in the current study demonstrated increases in the spontaneous use of language such as commenting and novel words; these outcomes were significantly greater for the group beginning with JASP+EMT+SGD.
Finally, this study employed unique intervention designs that tailored Stage 2 treatment dependent on the child’s response to Stage 1 treatment. Adding in the SGD later for slow responders to JASP+EMT alone did not provide the same benefit as when adding in the SGD from the beginning of treatment. These data are provocative and suggest that including a speech-generating device along with naturalistic behavioral interventions at the start of treatment may be most beneficial to minimally verbal children. The impact of the SGD on spoken language outcomes is consistent with findings from both single case and group design studies.31 Modest increases in spoken language have been associated with SGD and manual sign interventions; however, no previous studies have implemented SGD training using naturalistic approaches such as the blended JASP+EMT intervention, and most studies have been limited to exploring requesting rather than use of spoken language for the full range of pragmatic functions. Based on previous research,32 three pathways have been suggested by which SGD intervention might promote spoken language: by increasing the frequency of communication; by reducing the motor response demands and pressure to communicate; and by altering the acoustic effects of the child’s communication. Of these potential pathways, we posit that changes in the acoustic signal provided to the child through increasing the number and phonological consistency of models when using the SGD and pairing the acoustic signal with the graphic SGD symbol are likely to have influenced the outcomes in the current study. Further research examining the effects of increased models and auditory/visual pairing on spoken language clearly is needed.
A limitation of the current study is that while the sample size is relatively large for a randomized trial in autism research (particularly for this sub-population of children), we enrolled approximately two-thirds of our recruitment target. One of the implications of this may have been a limited ability to distinguish between the two adaptive interventions beginning with JASP+EMT (secondary aim). Thus, the fine-grained analysis comparing the two adaptive interventions that differ by Stage 2 treatment requires replication with a larger sample.
Despite this limitation, this study is one of the first studies to develop and evaluate the components of an adaptive intervention based on children’s early response for improving spoken language outcomes in the understudied and underserved population of minimally verbal school-aged children with ASD. The results of the study suggest that improvements in spontaneous, communicative utterances, novel words, and comments all favored the blended behavioral intervention that began with the addition of a SGD (JASP+EMT+SGD) as opposed to JASP+EMT with spoken words only. Secondary aim results suggest that the adaptive intervention beginning with JASP+EMT+SGD and intensifying the JASP+EMT+SGD treatment for children who were slow responders led to better post-treatment outcomes. There was insufficient evidence that the adaptive intervention that introduces SGD among children who respond slowly to JASP+EMT differed from the adaptive intervention that intensifies JASP+EMT for these children. It is important that in this sample, children who had already had an average of two years of prior treatment made progress in spoken language across all conditions. There is much more to be learned about effective communication intervention for this population. Future studies should test adaptive interventions based on various interventions in an effort to further understand what progress in spoken communication is possible.
Acknowledgments
This study was funded by Autism Speaks #5666, Characterizing Cognition in Nonverbal Individuals with Autism, an initiative begun by Ms. Portia Iverson and Cure Autism Now. Grant support was also provided by the National Institute of Child Health and Human Development (NICHD) R01HD073975-02 (C.K., A.K., S.M., D.A.), and R03MH097954-02 and RC4MH092722-01 from the National Institute of Mental Health (NIMH, D.A.).
Drs. Murphy and Almirall served as the statistical experts for this research.
We would like to thank the families and children who participated in this study and the team of interventionists, coders, transcribers, and data analysts at our three sites: Ya-Chih Chang, Stephanie Patterson, Kathryne Krueger, Charlotte Mucchetti, Dalia Kabab, Caitlin McCracken, Julia Kim, Alison Holbrook, Abbey Hye, and Kelsey Johnson from UCLA; Stephanie Jordan, Courtney Wright, Blair Burnette, and Ann Simonson from Vanderbilt University; Philip Menard, Emily Watkins, Kerry Buechler, Christine Hess, and Sarah Gardner from Kennedy Krieger Institute; and Xi Lu from University of Michigan.
Footnotes
Disclosure: Dr. Kasari has received salary support from grants from the Health Resources and Services Administration (HRSA), the National Institutes of Health (NIH), and Autism Speaks. Dr. Kaiser has received support from NIH and the Institute of Education Sciences (IES). Ms. Nietfeld also has received support from NIH and IES. Dr. Landa has received support from NIMH, the Centers for Disease Control and Prevention (CDC), Autism Speaks, the Simons Foundation Autism Research Initiative (SFARI), IES, HRSA, and Ride On For Autism (ROAR). Drs. Almirall and Murphy have received support from NIH and the National Institute on Drug Abuse (NIDA). Drs. Goods and Mathy report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Connie Kasari, University of California-Los Angeles (UCLA) Semel Institute for Neuroscience and Human Behavior.
Dr. Ann Kaiser, Vanderbilt University.
Dr. Kelly Goods, First Five Los Angeles.
Ms. Jennifer Nietfeld, Vanderbilt University.
Dr. Pamela Mathy, Speech, Language and Hearing Clinic, University of Utah.
Dr. Rebecca Landa, Kennedy Krieger Institute.
Dr. Susan Murphy, University of Michigan.
Dr. Daniel Almirall, University of Michigan
References
- 1.Tager-Flusberg H, Kasari C. Minimally Verbal School-Aged Children with Autism: The Neglected End of the Spectrum. Autism Research. 2013;6 (6):468–478. doi: 10.1002/aur.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson DK, Oti RS, Lord C, Welch K. Patterns of growth in adaptive social abilities among children with autism spectrum disorders. J of Abnorm Child Psychol. 2007;37:1019–1034. doi: 10.1007/s10802-009-9326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutter M, Greenfeld D, Lockyer L. A five to fifteen year follow-up of infantile psychosis: II. Social and behavioral outcome. Brit J Psychiatry. 1967;113:1183–1189. doi: 10.1192/bjp.113.504.1183. [DOI] [PubMed] [Google Scholar]
- 4.Wodka E, Mathey P, Kalb L. Predictors of phrase and fluent speech in children with autism and severe language delay. Pediatrics. 2013;131:1–7. doi: 10.1542/peds.2012-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pickett E, Pullara O, O’Grady J, Gordon B. Speech acquisition in older nonverbal individuals with autism: A review of features, methods and prognosis. Cog Behav Neurol. 2009;22:1–21. doi: 10.1097/WNN.0b013e318190d185. [DOI] [PubMed] [Google Scholar]
- 6.Howlin P, Gordon RK, Pasco G, Wade A, Charman T. The effectiveness of picture exchange communication system (PECS) training for teachers of children with autism: A pragmatic group randomized controlled trial. J Child Psychol Psychiatry. 2007;48:473–481. doi: 10.1111/j.1469-7610.2006.01707.x. [DOI] [PubMed] [Google Scholar]
- 7.Gordon K, Pasco G, McElduff F, Wade A, Howlin P, Charman T. A communication-based intervention for nonverbal children with autism: What changes? Who benefits? J of Consult and Clin Psych. 2011;79:447–457. doi: 10.1037/a0024379. [DOI] [PubMed] [Google Scholar]
- 8.Van Der Meer L, Rispoli M. Communication interventions involving speech-generating devices for children with autism: A review of the literature. Dev Neurorehab. 2010;13:294–306. doi: 10.3109/17518421003671494. [DOI] [PubMed] [Google Scholar]
- 9.Kasari C, Gulsrud AC, Wong C, Kwon S, Locke J. A randomized controlled caregiver mediated joint engagement intervention for toddlers with autism. J Autism Dev Disord. 2010;40:1045–1056. doi: 10.1007/s10803-010-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasari C, Paparella T, Freeman S, Jahromi LB. Language outcome in autism: Randomized comparison of joint attention and play interventions. J Consul Clin Psych. 2008;76:125–137. doi: 10.1037/0022-006X.76.1.125. [DOI] [PubMed] [Google Scholar]
- 11.Kaiser AP, Goetz L. Enhancing communication with persons labeled severely disabled. J Assoc Persons Severe Handicaps. 1993;18:137–142. [Google Scholar]
- 12.Kaiser AP, Hancock TB, Nietfeld JP. The effects of parent-implemented enhanced milieu teaching on the social communication of children who have autism. J Early Ed Dev. 2000;11:423–446. [Google Scholar]
- 13.Goods K, Ishijima E, Chang Y-C, Kasari C. Preschool based JASPER intervention in minimally verbal children with autism: Pilot RCT. J of Autism Dev Disord. 2013;43:1050–1056. doi: 10.1007/s10803-012-1644-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins LM, Murphy SA, Bierman KA. A Conceptual Framework for Adaptive Preventive Interventions. Prevention Science. 2004;5:185–196. doi: 10.1023/b:prev.0000037641.26017.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy SA. An Experimental Design for the Development of Adaptive Treatment Strategies. Statistics in Medicine. 2005;24:1455–1481. doi: 10.1002/sim.2022. [DOI] [PubMed] [Google Scholar]
- 16.Collins LM, Murphy SA, Strecher V. The Multiphase Optimization Strategy (MOST) and the Sequential Multiple Assignment Randomized Trial (SMART): New Methods for More Potent e-Health Interventions. Am J Prev Medicine. 2007;32:S112–118. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahum-Shani I, Qian M, Almirall D, Pelham W, Gnagy B, Fabiano G, Waxmonsky J, Yu J, Murphy SA. Experimental Design Primary Data Analysis Methods for Comparing Adaptive Interventions. Psychological Methods. 2012;17:457–477. doi: 10.1037/a0029372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almirall D, Compton SN, Rynn MA, Walkup JT, Murphy SA. SMARTer Discontinuation Trial Designs for Developing an Adaptive Treatment Strategy. J Child and Adol Psychopharmacology. 2012;22(5):364–374. doi: 10.1089/cap.2011.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almirall D, Compton SN, Gunlicks-Stoessel M, Duan N, Murphy SA. Designing a Pilot Sequential Multiple Assignment Randomized Trial for Developing an Adaptive Treatment Strategy. Statistics Medicine. 2012;31:1887–1902. doi: 10.1002/sim.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- 21.Tager-Flusberg H, Rogers S, Cooper J, Landa R, Lord C, Paul R, Rice M, Stoel-Gammon C, Wetherby A, Yoder P. Defining Spoken Language Benchmarks and Selecting Measures of Expressive Language Development for Young Children With Autism Spectrum Disorders. J Speech, Lang Hear Resch. 2009;52:643–652. doi: 10.1044/1092-4388(2009/08-0136). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J, Iglesias A. SALT: Systematic Analysis of Language Transcripts. Software for the analysis of oral language. Middleton, WI: SALT Software, LLC; 2012. [Google Scholar]
- 23.Kaiser AP, Roberts MY. Parent-implemented enhanced milieu teaching with preschool children with intellectual disabilities. J Speech, Lang, Hear Resch. 2013;56:295–309. doi: 10.1044/1092-4388(2012/11-0231). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, N.J: L. Erlbaum Associates; 1988. p. xxi.p. 567. [Google Scholar]
- 25.Altman D. Confidence intervals for the number needed to treat. British Medical Journal. 1998;317(7168):1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpenter JR, Kenward MG, White IR. Sensitivity analysis after multiple imputation under missing at random: a weighting approach. Statistical Methods in Medical Research. 2007;16:259–275. doi: 10.1177/0962280206075303. [DOI] [PubMed] [Google Scholar]
- 27.Paul R, Campbell D, Gilbert K, Tsiouri I. Comparing spoken language treatments for minimally verbal preschoolers with autism spectrum disorders. J of Autism and develop disord. 2013;43(2):418–431. doi: 10.1007/s10803-012-1583-z. [DOI] [PubMed] [Google Scholar]
- 28.Kaale A, Fagerland W, Martinsen EW, Smith L. Preschool-based social-communication treatment for children with autism: 12-month follow-up of a randomized trial. J Am Acad Child Adolesc Psychiatry. 2014;53(2):188–98. doi: 10.1016/j.jaac.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Yoder P, Stone WL. Randomized comparison of two communication interventions for preschoolers with autism spectrum disorders. J Consult and Clin Psych. 2006;74:426–435. doi: 10.1037/0022-006X.74.3.426. [DOI] [PubMed] [Google Scholar]
- 30.Kasari C, Gulsrud A, Freeman S, Paparella T, Hellemann G. Longitudinal follow up of children with autism receiving targeted interventions on joint attention play. J Am Acad Child Adolesc Psychiatry. 2012;51:487–495. doi: 10.1016/j.jaac.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlosser RW, Wendt O. Effects of augmentative and alternative communication intervention on speech production in children with autism: A systematic review. Am J Speech-Lang Path. 2008;17(3):212–230. doi: 10.1044/1058-0360(2008/021). [DOI] [PubMed] [Google Scholar]
- 32.Blischak DM, Lombardino LJ, Dyson AT. Use of speech-generating devices: In support of natural speech. Augment Altern Com. 2003;19:29–35. doi: 10.1080/0743461032000056478. [DOI] [PubMed] [Google Scholar]