Abstract
Background
Myofascial pain syndrome is a regional condition of muscle pain and stiffness and is classically characterized by the presence of trigger points in affected musculature. Botulinum toxin type A (BoNT-A) has been shown to have antinociceptive properties and elicit sustained muscle relaxation, thereby possibly affording even greater relief than traditional strategies. Our goal in this study was to determine whether direct injection of BoNT-A into painful muscle groups is effective for cervical and shoulder girdle myofascial pain.
Methods
An enriched protocol design was used wherein 114 patients with cervical and shoulder girdle myofascial pain underwent injection of BoNT-A to determine their response to the drug. Fifty-four responders were then enrolled in a twelve-week, randomized, double-blind, placebo-controlled trial. Pain scales and quality of life measures were assessed at baseline and at routine follow-up visits until completion of the study after 26 weeks.
Results
Injection of BoNT-A into painful muscle groups improved average visual numerical pain scores in subjects who received a second dose of BoNT-A compared to placebo (p = 0.019 (0.26, 2.78)). Subjects who received a second dose of BoNT-A had a reduced number of headaches per week (p = 0.04 (0.07, 4.55)). Brief Pain Inventory interference scores for general activity and sleep were improved (p = 0.046 (0.038, 3.7) and 0.02 (0.37, 4.33), respectively) in those who received a second dose of BoNT-A.
Conclusion
Botulinum toxin type A injected directly into painful muscle groups improves average pain scores and certain aspects of quality of life in patients suffering from severe cervical and shoulder girdle myofascial pain.
Introduction
Myofascial pain syndrome is a common painful condition encountered in the general population. It is a localized muscle condition that presents with skeletal muscle pain and stiffness. Classically, it is defined by the presence of trigger points in specific musculature. Myofascial trigger points are hypersensitive, palpable, and focal taut bands of muscle. Upon palpation of myofascial trigger points, they can produce radiating, referred pain and muscle twitch.1,2 Despite myofascial pain syndromes being quite common, they are most often under-diagnosed or misdiagnosed conditions.3
The exact pathophysiology and etiology of myofascial trigger points and myofascial pain syndrome is still unknown. However, many proposed mechanisms have been studied and reported in the literature. It has been suggested that the development of myofascial trigger points is related to an excess release of acetylcholine, leading to sustained contraction of the muscle and formation of a trigger point.4 This sustained contraction of muscle can lead to a significant increase in the concentration of inflammatory and nociceptive transmitters within the trigger point, as measured by real-time microdialysis in a landmark study by Shah et al.5 Persistent peripheral muscle nociceptive activation by these inflammatory and nociceptive compounds is converted into a permanent stimulus that facilitates pain neurotransmission and leads to central sensitization and glial activation.6–8
Traditional therapeutic approaches for the treatment of myofascial pain have included pharmacotherapy (nonsteroidal antiinflammatory drugs, steroids, tricyclic antidepressants, vasodilators, oral skeletal muscle relaxants), injection therapy (trigger point injection of local anesthetic with and without corticosteroid, or “dry” needling), physical therapy, and behavioral modification.9 At best, long-term benefit with the aforementioned therapies is transient, and treatment outcomes may also be incomplete or nonexistent, with varying degrees of improvement.10–14 Botulinum toxin type A (BoNT-A) injections may offer advantageous treatment for myofascial pain as its effects are prolonged (3–4 months duration), compared with traditional modalities, including trigger point injections, whose effects tend to be exerted over a much shorter time period (several days duration).15 While physical therapy has been shown in many studies to be beneficial for myofascial pain syndrome,2,16 some patients have difficulty completing physical therapy due to severe, refractory pain and spasm. Thus, the sustained muscle relaxation as a result of BoNT-A leading to prolonged pain relief may allow a patient to be able to better participate in physical rehabilitation, which will aid in long-term recovery and pain relief.9
Over the past few decades, BoNT-A has been used clinically to significantly improve and manage certain movement disorders, spasticity, and syndromes of autonomic hyperactivity.17 BoNT-A has been shown to be an analgesic, with direct antinociceptive effects in an inflammatory pain model.18 It has also been shown that BoNT-A directly inhibits the release of pain mediators such as substance P, bradykinin, calcitonin gene related peptide, and glutamate.19,20 Prospective, placebo-controlled studies in humans evaluating the efficacy of BoNT-A for treatment of myofascial pain are limited, with variable results.21 Results from these studies are conflicting due to the differences seen in multiple methodologic variables including: diagnostic selection and eligibility criteria, muscles injected, injection procedure, number of trigger points injected, dose of BoNT-A used, control group treatments, outcome measures, and length of followup.8
The goal of this study was to determine the analgesic effect of BoNT-A injections directly into painful muscle groups in the treatment of cervical and shoulder girdle myofascial pain using an enriched protocol design.
Methods
Subjects
After receiving approval from the IRB at the David Geffen School of Medicine at the University of California, Los Angeles (UCLA), subjects were recruited at the UCLA Pain Management Center from 2005 – 2010. Upon meeting inclusion and exclusion criteria, subjects were enrolled to participate in the study. All subjects gave written informed consent to participate in research.
Enrollment in the study was restricted to male or female patients, ages 18–65 years, with myofascial pain of the neck and shoulders of at least eight months duration. Painful muscle involvement included scapular stabilizers, anterior neck flexors, and posterior cervical musculature. Other inclusion criteria were: A Visual Numerical Scale (VNS) pain score 4 or higher at baseline, no prior treatments with BoNT-A, willingness to discontinue all pain medications except ibuprofen and tramadol for the duration of the study, and women of child-bearing potential must be using a reliable means of contraception and have a negative urine pregnancy test before participation. Exclusion criteria were the following: history of injections of BoNT-A (any serotype), pregnant or breastfeeding women, history of alcohol or drug abuse, use of investigational drugs within one month of study, significant medical or psychiatric disease, and no new medications or change in medications within two months of screening or throughout the study.
Study Design
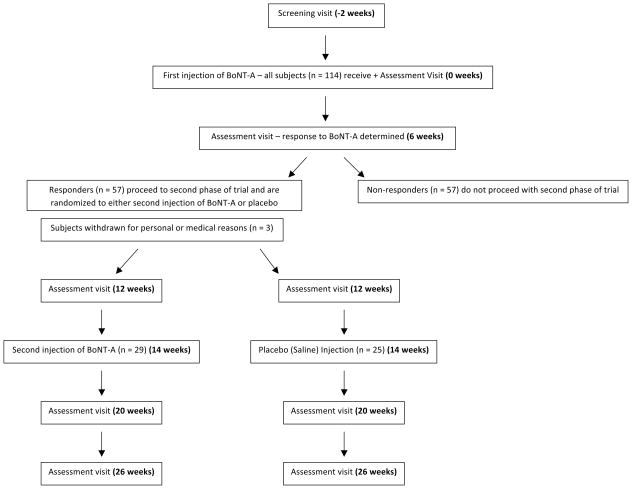
This study can be characterized as using an enriched protocol design22 (Figure 1) wherein all enrolled subjects received one BoNT-A injection during the first phase of the study. Six weeks after this injection, subjects were then categorized as having a clinically significant response to BoNT-A if they had a reduction of two points or more or a 30% reduction in the VNS compared to baseline.23 Subjects deemed to be “responders” to BoNT-A then entered the second phase of the study. The second phase of the study was prospective, randomized, double-blind, and placebo-controlled. Subjects entering the second phase of the study were randomized to receive either BoNT-A or saline injection into painful muscle groups of the neck and shoulders at 14 weeks after the first injection.
Figure 1.
CONSORT diagram showing the flow of subjects through the enriched randomized, double-blind, placebo-controlled trial. BoNT-A = botulinum toxin type A.
Assessment visits during the first phase of the study were done at baseline, 6 weeks and 12 weeks after the first injection. Assessment visits for the second phase of the study were done at 6 weeks and 12 weeks after the second randomized injection. At each assessment visit, the subject had a physical examination and was administered pain scales and questionnaires. The monitoring intervals were chosen to capture peak treatment effects based on clinical practice experience and observations.
Randomization, Blinding, and Rating Scales
Randomization, blinding, and drug preparation was performed by the UCLA Investigational Pharmacy. Each subject identified their areas of pain by marking an anatomical head, neck, and shoulders diagram. The primary outcome measure for pain was characterized by intensity using a VNS from 0 to 10 in the Brief Pain Inventory (BPI).24 This was self reported by the patient as “best,” “worst,” and “average” pain scores “over the last 24 hours.”
Postural analysis, health-related quality of life, disability, and headache were assessed as secondary outcomes. Physical examinations were performed on each patient to evaluate for physical signs of myofascial pain including number of trigger points and their locations, forward head syndrome,25 internal rounding of shoulders, and range of motion of the cervical spine. Health-related quality of life was assessed as a secondary outcome using the 36-item Short-Form Health Survey (SF-36)26 and interference outcomes from the BPI.24 Disability as a secondary outcome was assessed via the Neck Disability Index (NDI).27 Finally, the presence, frequency, and duration of headaches were evaluated as secondary outcomes through patient report.
Dosing Paradigm
At least two weeks before the first injection, subjects were weaned from their existing pain medications with the exception of as needed tramadol or ibuprofen. Throughout the duration of the study, subjects were allowed to use this pharmacologic regimen as needed. During the first phase of the study, all subjects received injections of BoNT-A. The same dosing and injection techniques from the first phase of the study were used in the second phase of the study. For the second phase of the study, subjects received either placebo (saline) or BoNT-A into each painful muscle in a randomized, double-blind fashion.
A fixed pattern, variable dose injection paradigm was used (Table 1). Anterior neck flexor and posterior neck extensor painful muscles were always injected and scapular stabilizer muscles were never injected, due to concern for possible weakness and worsening of the pain. Only painful muscles were injected in the mid-belly, irrespective of the presence or absence or location of trigger points. BoNT-A was prepared for injection by placing 4 mL of sterile saline in a 100 unit vial to make a dilution of 25 units/mL. A maximum of 300 units was injected in any subject, using a 27-gauge needle affixed to an insulin syringe inserted to a depth of 1 cm. Specific doses of BoNT-A were administered depending on the muscle being injected (Table 1) and the operator’s estimation of the individual muscle’s contribution to the total pain (Table 1). For instance, if a subject complained of more intense pain on one side, the operator could increase the dose on that side at his or her discretion.
Table 1.
BoNT-A Dosages based on Muscle Group
| Muscle Group | BoNT-A Dosage (Units) |
|---|---|
| Anterior Musculature | |
| Anterior and Middle Scalenes | 6.25 per scalene |
| Pectoralis Major | 12.5 – 25 |
| Pectoralis Minor | 25 |
| Sternocleidomastoid | 12.5 – 25 |
| Posterior Musculature | |
| Levator Scapulae (Scapular Insertion) | 6.25 – 25 |
| Levator Scapulae (Cervical Origin) | 12.5 – 25 |
| Trapezius (Anterior Border) | 12.5 – 25 |
| Trapezius (Main Body) | 12.5 – 50 |
| Splenius Capitis | 12.5 – 25 |
| Semispinalis Capitis | 6.25 – 25 |
| Scapular Stabilizers | |
| Supraspinatus | 0 |
| Infraspinatus | 0 |
| Rhomboids | 0 |
BoNT-A = botulinum toxin type A
Statistical Analysis
All statistical analyses were performed using JMP 10 (Cary, NC), IBM SPSS V22 (Armonk, NY) and R 3.01 (www.R-project.org; Vienna, Austria). Categorical demographic variables were compared using the chi-square test or Fisher’s exact test as appropriate. Student’s t-test was used to compare quantitative variables between the BoNT-A and placebo groups. Before analysis, histograms were performed for the outcome data to determine the appropriate analysis. All outcome data were found to be approximately normally distributed, which allowed us to use parametric analytic methods for inferential purposes. Student’s t-test was used to measure the significance of mean change for the study drug versus placebo for the primary and secondary outcome measures from Week 26 to baseline and Week 26 to Week 14. To compare mean average BPI VNS scores over time between the BoNT-A and placebo groups, a generalized estimating equation (GEE) model with an autoregressive correlation structure was used. The terms of the GEE model were time, group, and a group/time interaction. Residual analysis on this model was performed and no violations were apparent. The correlations between the BPI VNS response variables were calculated using Pearson’s correlation coefficient.
Unless otherwise indicated, the mean ± SD was reported as the measure of central tendency for parametric data. The median with range was reported as the measure of central tendency for ordinal data. A p value of ≤ 0.05 was chosen to indicate statistical significance. False discovery rates were computed for Tables 5 and 7 to correct for multiple comparisons using the R software (qvalue package).
Table 5.
Analysis of Secondary Outcome Measures – Change in Mean Score from Week 26 compared to Baseline
| Placebo (n = 25) | BoNT-A (n = 29) | P value | 95% CI | |
|---|---|---|---|---|
| SF-36 | ||||
| Physical Functioning | 3.46 ± 7.95 | 2.07 ± 8.77 | 0.55 | (−3.32, 6.11) |
| General Health | 3.93 ± 8.20 | 5.00 ± 9.85 | 0.68 | (−6.18, 4.04) |
| Bodily Pain | 5.75 ± 11.7 | 7.85 ± 12.2 | 0.53 | (−8.83, 4.62) |
| Vitality | 4.88 ± 15.4 | 5.77 ± 10.6 | 0.81 | (−8.31, 6.54) |
| Social Functioning | 9.60 ± 14.7 | 6.65 ± 13.7 | 0.46 | (−5.06, 10.9) |
| Mental Health | 7.43 ± 14.3 | 3.11 ± 13.8 | 0.28 | (−3.57, 12.2) |
| BPI Interference | ||||
| General Activity | −1.80 ± 2.80 | −3.65 ± 3.60 | 0.046 | (0.038, 3.7) |
| Mood | −1.72 ± 3.08 | −3.23 ± 3.19 | 0.09 | (−0.25, 3.28) |
| Walking | −1.16 ± 2.85 | −1.88 ± 2.46 | 0.34 | (−0.77, 2.22) |
| Work | −2.40 ± 2.57 | −4.04 ± 3.41 | 0.06 | (−0.07, 3.34) |
| Relationships | −1.88 ± 3.23 | −2.15 ± 3.54 | 0.77 | (−1.64, 2.18) |
| Sleep | −0.84 ± 3.26 | −3.19 ± 3.73 | 0.02 | (0.37, 4.33) |
| Enjoyment | −2.16 ± 3.35 | −3.92 ± 3.14 | 0.06 | (−0.08, 3.61) |
| Headaches | ||||
| # of headaches per week | 0.58 ± 5.00 | −1.73 ± 2.64 | 0.04 | (0.07, 4.55) |
| Duration of headache (hr) | −8.26 ± 25.7 | −10.6 ± 18.0 | 0.71 | (−10.3, 14.9) |
| Headache VNS (Worst) | −1.20 ± 3.42 | −3.02 ± 3.51 | 0.07 | (−0.15, 3.79) |
| Headache VNS (Best) | −0.52 ± 2.37 | −0.96 ± 2.59 | 0.53 | (−0.97, 1.85) |
| Headache VNS (Average) | −0.92 ± 2.60 | −1.88 ± 3.03 | 0.24 | (−0.65, 2.57) |
All analyses performed using Student’s t-test. FDR = 16.1%
BoNT-A = botulinum toxin type A; CI = confidence interval; VNS = visual numerical scale pain score; BPI= Brief Pain Inventory
Table 7.
Analysis of Secondary Outcome Measures, Change in Mean Score from Week 26 compared to Week 12
| Placebo (n = 25) | BoNT-A (n = 29) | P value | 95% CI | |
|---|---|---|---|---|
| SF-36 | ||||
| Physical Functioning | −1.52 ± 7.67 | −0.30 ± 9.12 | 0.015† | (−1.30, −0.16) |
| General Health | −0.23 ± 9.4 | 3.93 ± 8.04 | 0.10 | (−9.08, 0.76) |
| Bodily Pain | −0.58 ± 10.7 | 1.63 ± 11.5 | 0.48 | (−8.44, 4.04) |
| Vitality | −0.24 ± 13.5 | 0.003 ± 10.0 | 0.94 | (−6.91, 6.42) |
| Social Functioning | 3.49 ± 12.3 | 2.61 ± 13.7 | 0.81 | (−6.44, 8.20) |
| Mental Health | 2.03 ± 11.0 | −2.5 ± 11.5 | 0.16 | (−1.80, 10.9) |
| BPI Interference | ||||
| General Activity | 0.6 ± 3.63 | −0.19 ± 2.79 | 0.38 | (−1.02, 2.61) |
| Mood | 0.36 ± 3.55 | −0.38 ± 2.71 | 0.40 | (−1.03, 2.52) |
| Walking | 0.4 ± 2.47 | 0.08 ± 2.06 | 0.61 | (−0.95, 1.60) |
| Work | 0.28 ± 3.62 | −0.5 ± 3.11 | 0.41 | (−1.12, 2.68) |
| Relationships | 0.4 ± 3.57 | 0.15 ± 2.57 | 0.78 | (−1.50, 1.99) |
| Sleep | 0.48 ± 3.61 | −0.46 ± 2.96 | 0.31 | (−0.91, 2.79) |
| Enjoyment | 0.32 ± 3.9 | −0.42 ± 2.98 | 0.48 | (−1.21, 2.69) |
| Headaches | ||||
| # of headaches per week | 1.77 ± 3.75 | −0.037 ± 2.57 | 0.049 | (0.007, 3.61) |
| Duration of headache (hr) | −1.80 ± 12.82 | 0.58 ± 5.92 | 0.41 | (−8.18, 3.42) |
| Headache VNS (Worst) | 1.08 ± 4.03 | −0.72 ± 3.03 | 0.08 | (−0.23, 3.83) |
| Headache VNS (Best) | 0.4 ± 2.04 | −0.32 ± 1.68 | 0.18 | (−0.34, 1.78) |
| Headache VNS (Average) | 0.24 ± 2.39 | −0.68 ± 2.44 | 0.18 | (−0.45, 2.29) |
All analyses performed using Student’s t-test. FDR = 19.6%
log-transformation required to allow for parametric analysis
BoNT-A = botulinum toxin type A; CI = confidence interval; VNS = visual numerical scale pain score; Brief Pain Inventory = BIP
Results
One hundred-fourteen subjects were enrolled in the study and underwent injection of BoNT-A during the first phase. No a priori power analysis was performed and enrollment was ceased at 114 patients because the supply of BoNT-A had been exhausted. No interim analyses were performed.
At the six week follow up visit, 57 subjects were determined to be responders and 57 subjects were nonresponders. Of the subjects characterized as responders, 29 were randomized to receive a second BoNT-A injection and 25 were randomized to receive placebo injection. One subject who was a responder withdrew from the study before the second phase because she desired to become pregnant. Another responder was withdrawn from the study for cervical muscle weakness and began a workup for myasthenia gravis. A subject who was a responder was withdrawn from the study when the patient was found to be abusing illicit drugs. Data for these subjects were not used in the statistical analysis for demographic and inferential purposes.
Demographics
Demographic data for the study populations from the first and second phase of the study are presented in Tables 2 and 3, respectively. There was no difference between the placebo group and the BoNT-A treatment group at baseline with respect to age, gender, height, ethnicity, weight, duration of pain, history of cervical fusion, history of injury at work, history of pain after motor vehicle accident, history of pain after other injury, baseline average VNS pain score, Beck Depression score, prior positive response to trigger point injections, or involvement in litigation or workers compensation cases. Thus, at week 14 (blinded injection), the subjects to be injected were a homogenous group with respect to demographics.
Table 2.
Baseline Demographic Data for Phase I Subjects
| All Subjects (n=111) | |
|---|---|
| Age, mean ± SD | 47.8 ± 14.9 |
| Gender, n (%) | |
| Male | 27 (24) |
| Female | 84 (76) |
| Height (cm), mean ± SD | 167 ± 9.86 |
| Weight (kg), mean ± SD | 68.9 ± 15.3 |
| Ethnicity, n (%) | |
| Caucasian | 84 (76) |
| Asian | 9 (8) |
| Hispanic | 15 (13) |
| African-American | 3 (3) |
| Duration of Pain (years), mean ± SD | 7.74 ± 9.75 |
| Prior Cervical Fusion, n (%) | 11 (10) |
| Injury at work, n (%) | 50 (45) |
| Injury from MVA, n (%) | 31 (28) |
| Injury from other trauma, n (%) | 10 (9) |
| Baseline Beck Depression score, mean ± SD | 11.8 ± 7.47 |
| Baseline Average VNS score, mean ± SD | 5.84 ± 1.74 |
| Prior positive response to TPI, n (%) | 63 (57) |
| Litigation, n (%) | 5 (5) |
| Workers compensation, n (%) | 3 (3) |
BoNT-A = botulinum toxin type A; MVA = motor vehicle accident; TPI = trigger point injections; VNS = visual numerical scale pain score.
Table 3.
Baseline Demographic Data for Phase II Subjects
| Placebo (n = 25) | BoNT-A (n = 29) | p-value | 95% CI | |
|---|---|---|---|---|
| Age, mean ± SD | 47.4 ± 14.9 | 48.8 ± 16.2 | 0.73 | (−10.02, 7.09) |
| Gender, n (%) | ||||
| Male | 6 (24) | 6 (21) | 0.77 | (−0.19, 0.26) |
| Female | 19 (76) | 23 (79) | 0.77 | (−0.26, 0.19) |
| Height (cm), mean ± SD | 164.3 ± 6.45 | 165.6 ± 8.28 | 0.53 | (−5.41, 2.80) |
| Weight (kg), mean ± SD | 66.7 ± 15.1 | 68.3 ± 13.6 | 0.68 | (−9.43, 6.24) |
| Ethnicity, n (%) | ||||
| Caucasian | 19 (76) | 19 (65) | 0.36 | |
| Asian | 2 (8) | 2 (7) | ||
| Hispanic | 3 (12) | 8 (28) | ||
| African-American | 1 (4) | 0 (0) | ||
| Duration of Pain (years), mean ± SD | 10.4 ± 11.8 | 6.28 ± 10.5 | 0.18 | (−1.92, 10.24) |
| Prior Cervical Fusion, n (%) | 4 (16) | 2 (7) | 0.40 | (−0.10, 0.26) |
| Injury at work, n (%) | 15 (60) | 15 (52) | 0.59 | (−0.18, 0.35) |
| Injury from MVA, n (%) | 4 (16) | 10 (34) | 0.21 | (−0.41, 0.04) |
| Injury from other trauma, n (%) | 2 (8) | 3 (10) | >0.99 | - |
| Baseline Beck Depression score, mean ± SD | 11.5 ± 7.62 | 12.7 ± 7.57 | 0.59 | (−5.47, 3.17) |
| Baseline Average VNS score, mean ± SD | 5.78 ± 1.74 | 5.84 ± 1.82 | 0.80 | (−1.04, 0.81) |
| Prior positive response to TPI, n (%) | 20 (80) | 19 (66) | 0.36 | (−0.09, 0.38) |
| Litigation, n (%) | 1 (4) | 1 (3) | >0.99 | - |
| Workers compensation, n (%) | 0 (0) | 1 (3) | >0.99 | - |
BoNT-A = botulinum toxin type A; CI = confidence interval; MVA = motor vehicle accident; TPI = trigger point injections; VNS = visual numerical scale pain score.
Analysis of Outcomes: Entire Study
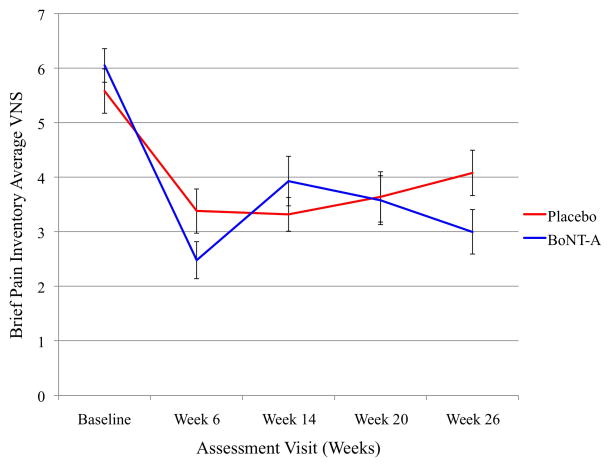
Pain outcomes as assessed by VNS scores from the Brief Pain Inventory (BPI) were analyzed to determine differences between treatment groups from each visit compared to the baseline visit. The Week 12 assessment visit was not compared to baseline between BoNT-A and placebo groups because at that visit all patients had only received BoNT-A. The Week 20 assessment visit did not have significant differences compared to baseline for pain score outcomes. As noted in Table 4 and Figure 2 for Week 26 compared to baseline, subjects who received BoNT-A had improved average pain scores (p=0.019 (0.26, 2.78)) as measured by the BPI. There was a trend towards improvement in worst BPI pain scores (p = 0.052 (-0.019, 3.46)). No significant changes in “best” VNS pain scores or NDI were found.
Table 4.
Analysis of Primary Outcome Measures (Pain Scores) – Change in Mean Score from Week 26 compared to Baseline
| Placebo (n = 25) | BoNT-A (n = 29) | P value | 95% CI | |
|---|---|---|---|---|
| BPI VNS (Best)+ | −1.54 ± 0.53 | −2.11 ± 0.52 | 0.44 | (−0.92, 2.07) |
| BPI VNS (Worst)+ | −1.74 ± 0.62 | −3.46 ± 0.61 | 0.052 | (−0.019, 3.46) |
| BPI VNS (Average)+ | −1.50 ± 0.45 | −3.02 ± 0.44 | 0.019* | (0.26, 2.78) |
| NDI | −11.4 ± 3.27 | −18.1 ± 3.26 | 0.15 | (−2.53, 16.05) |
All analyses performed using Student’s t-test.
- best to worst .42 (p<0.01)
- worst to average .79 (p<0.01)
- best to average .64 (p<0.01)
denotes statistical significance
BoNT-A = botulinum toxin type A; CI = confidence intervalVNS = visual numerical scale pain score; BPI = Brief Pain Inventory
Figure 2.
Brief Pain Inventory (BPI) Average Visual Numerical Score pain scale scores at each assessment visit time point for BoNT-A and placebo groups. VNS = visual numerical scale pain score, BoNT-A = botulinum toxin type A.
There were no significant differences between BoNT-A and placebo groups using the SF-36 scale for quality of life measures (Table 5) comparing Week 26 to baseline. However, as noted in Table 5, improvement in the interference scores for general activity (p=0.046 (0.038, 3.7)) and sleep (p = 0.02 (0.37, 4.33)) were found in subjects who received BoNT-A compared to placebo. Other BPI interference scores that appeared to trend with an improvement of a larger degree in the BoNT-A group compared to placebo over the 26 week time period were mood, work, and enjoyment.
There were no significant associations found between treatment groups and physical examination findings such as internal rounding of shoulders, forward head syndrome,25 coracoid-to-tragus measurements plumb line, and number of painful trigger points.
Subjects who received BoNT-A had a reduction in the number of headaches experienced per week (p = 0.04 (0.07, 4.55)) (Table 5), when comparing Week 26 to baseline. A decrease in the worst headache VNS pain scores from week 0 to week 26 (p = 0.07) was found to trend towards a greater reduction in the BoNT-A group compared to placebo. Best and average headache VNS pain scores and duration of headache were not found to have any significant change between treatment groups from Week 26 compared to baseline.
To compare mean average BPI VNS scores over time between the BoNT-A and placebo groups, a GEE model was used. The terms of the GEE model were time, group, and a group/time interaction. The group effect tests whether there was an overall mean difference between BPI pain scores between the BoNT-A and placebo groups over time. The hypothesis was not that there was an overall difference in the BPI pain scores between groups, but that the decrease in BPI pain scores was “steeper” in the BoNT-A group than the placebo group over time (interaction term). From the GEE model we found a significant time*treatment interaction (−0.27 (0.51, −0.03)) p-value =0.028. This means that for each timepoint, the difference in mean average BPI VNS score between BoNT-A and placebo decreases by about 0.27 units. Thus, in the presence of a significant interaction term, the group main effect is no longer relevant because the interaction term is telling us that the group effect does matter and that it depends on another variable, time. The interpretation of this finding is that both groups decrease over time, but that the BoNT-A group decreases significantly more than the placebo group over time.
Outcomes Analysis: Phase Two
The second phase of the study was analyzed separately from the first phase of the study to determine if there was further significant improvement that could be appreciated after a second dose of BoNT-A. The Week 26 visit was compared to the Week 12 visit (this visit can be considered the “new baseline” because all patients entering the second phase of the trial had received BoNT-A).
Analysis of the outcome measures to distinguish an effect of the BoNT-A injections revealed that there was a significant decrease in the average (p = 0.02 (0.30, 2.91)) and worst (p = 0.03 (0.21, 3.86)) VNS pain scores from Week 26 to Week 12, as measured by the BPI in the BoNT-A-injected group compared to placebo (Table 6). No other significant changes were found in best VNS pain scores, postural analysis, and NDI.
Table 6.
Analysis of Primary Outcome Measures (Pain Scores), Change in Mean Score from Week 26 compared to Week 12
| Placebo (n = 25) | BoNT-A (n = 29) | P value | 95% CI | |
|---|---|---|---|---|
| BPI (Best)+ | 0.04 ± 2.34 | −0.77 ± 2.50 | 0.24 | (−0.55, 2.17) |
| BPI (Worst)+ | 0.92 ± 2.72 | −1.11 ± 3.68 | 0.03* | (0.21, 3.86) |
| BPI (Average)+ | 0.76 ± 1.69 | −0.84 ± 2.78 | 0.02* | (0.30, 2.91) |
| NDI | −0.48 ± 17.5 | −5.52 ± 20.1 | 0.35 | (−5.67, 15.8) |
All analyses performed using Student’s t-test.
- best to worst .39 (p<0.01)
- worst to average .83 (p<0.01)
- best to average .65 (p<0.01)
denotes statistical significance
BoNT-A = botulinum toxin type A; CI = confidence interval; VNS = visual numerical scale pain score; BPI = Brief Pain Inventory; NDI = Neck Disability Index
Physical functioning as measured by the SF-36 scale was found to be significantly different in those who received placebo compared to BoNT-A indicating that physical functioning worsened after they received a placebo injection (p = 0.02(−1.30, −0.16)) (Table 7) for Week 26 compared to Week 12. The General Health SF-36 quality of life outcome appeared to trend towards improvement compared to placebo (p = 0.10). No other significant associations were found with regards to quality of life outcomes using the SF-36 or BPI Interference scores.
Subjects who received placebo were found to have an increased number of headaches per week compared to subjects who received BoNT-A injection (p = 0.049 (0.007, 3.61)) (Table 7). There was a decrease in the worst headache VNS pain scores from Week 26 to Week 12 (p = 0.08) in those who received a second dose of BoNT-A, however, an increase in this pain measure was found in those who received placebo injection. Best and average headache VNS pain scores and duration of headache were not found to have any significant change between treatment groups.
False discovery rates (FDR) were computed on the four exploratory secondary outcome measures as found in Tables 5 and 7, to assess the possibility of false positives from running multiple tests. The calculated FDRs for these Tables were 16.1% and 19.6%, respectively. With the FDRs in this range, this allows for a reasonable level of confidence that there is an overall treatment effect signal within the secondary outcome measure analyses. Thus, among the results found to be significant by the traditional standard alpha level of 0.05, it can be expected that a clear majority (80–85%) of these are true effects rather than false positives.
Discussion
The results of this study suggest that injection of BoNT-A into painful muscle groups of the neck and shoulder area improves pain relief in subjects with cervical and shoulder girdle myofascial pain syndrome. In a study by Ferrante et al,9 no significant improvement in cervicothoracic myofascial pain was found when patients underwent injection of BoNT-A directly into painful trigger points. The conclusions of that study were twofold in terms of the mechanism of inefficacy: either BoNT-A lacked efficacy in treating cervicothoracic myofascial pain or the lack of efficacy could be methodological, meaning the optimal way to treat myofascial pain with BoNT-A is to not inject directly into trigger points. The latter conclusion is most likely, given the findings of our present study. Our results advocate using a combined follow-the-pain and pattern injection technique in lieu of direct trigger point injections.
The enriched protocol design used for this study is unique and may have advantages over the standard randomized clinical trial design for studies evaluating the effect of an intervention for chronic pain.22 One advantage is that it provides information of a response rate after phase one of the study. In our study, 50% of subjects were characterized as responders. This is useful information in its own right, as it serves to be an important predictor of what will happen with the drug in clinical practice.28 Also, in standard randomized clinical trials, there is always the possibility that the true effect size may be underestimated due to the inclusion of patients who are unlikely to respond to therapy. This could possibly lead to the appearance of a negative trial, when in fact a subgroup may have experienced a genuine benefit. With the enriched protocol design, only the responders are included in the randomized controlled trial which may lead to a more accurate representation of the true effect and response of the treatment.
In the present study, subjects who received a second dose of BoNT-A in the second phase of the study had continued dramatic improvement in their pain scores, which was statistically significant compared to those who received placebo. Subjects who received placebo injections in the second phase of the study had worsening of their pain scores in the twelve weeks after the second injection. Given that the subgroup of patients included in the second phase of the study were responders to BoNT-A, this fact serves to support the statement that there is a true treatment effect and that the difference is not purely a placebo effect. This also may indicate that repetitive dosing of BoNT-A may be indicated to provide sustained pain relief in clinical practice.
Quality of life remains a very important secondary outcome to measure in chronic pain populations and the results of this study suggest that BoNT-A reduces the interference of chronic pain in certain facets of everyday living. There was a reduction over the 26-week time period in the interference of chronic pain for general activity and sleep, as measured by BPI interference scores. Many other BPI interference scores (mood, work, and enjoyment) appear to trend with an improvement of a larger degree in the BoNT-A group compared to placebo over the 26 week time period. When the second phase of the study (week 12 to week 26) was analyzed for quality of life measures, there was a worsening in physical functioning in those subjects who received placebos compared to BoNT-A. All other quality of life measures, as measured by BPI interference scores and SF-36 scores, failed to show any significant improvement in the second phase of the study for those subjects who received BoNT-A. However, the trend towards improvement continued after a second injection.
Given that quality of life measures were secondary, hypothesis-generating outcomes, it is possible that the current study was not adequately powered to reveal associations across all quality of life measures. Substantial improvement was found in two BPI interference scores and many interference scores were improved to a larger degree when compared to the placebo group. This trend suggests that BoNT-A may improve multiple facets of quality of life. Thus, further large-scale studies that are adequately powered to reveal associations for quality of life outcome measures are warranted to confirm this. It is also notable that most quality of life scores worsened for those who received placebo injection. This likely represents a decrease in the efficacy of the BoNT-A toxin over time and likely supports the notion that repetitive injections are necessary for continued and sustained improvement. Analysis of headaches as a secondary endpoint demonstrated that subjects who received a second dose of BoNT-A had a significantly decreased number of headaches per week when compared to those who received placebo. However, it deserves mention that subjects who received placebo actually had an increase in the number of headaches per week compared to baseline, which may indicate that repetitive dosing of BoNT-A may be indicated to treat this condition.
Given the correlation of depression with chronic pain, there was concern for confounding of results due to underlying depression.29 However, baseline Beck Depression scores were less than 13 in both placebo and BoNT-A groups, which correlates to minimal depression.30 Because there was no significant depression in the study cohort, the likelihood of depression being a confounder of the results of this study is unlikely.
Adverse effects related to BoNT-A injections have been well reported in the literature and include excessive weakness of injected muscles, weakness of uninjected muscles through regional spread, weakness of remote muscles due to hematogenous spread, dry mouth, reduced sweating, reduced lacrimation, skin rash, flu-like illness, brachial neuritis-like syndrome, bruising, bleeding, and pain at injection site.31–33 Most adverse effects are related to muscle weakness, either those injected or those nearby, which become weak due to local spread of the toxin.34 The specific symptoms that arise tend to correlate to the region the toxin is injected. Injections in the neck can produce weakness of neck movement, difficulty holding up the head against gravity, difficulty swallowing, and weakness of the voice. In the present study, there was a low incidence of these well-described adverse effects including: flu-like illness (n = 9), arthralgias (n = 1), and fatigue (n = 4). Twenty-nine patients reported a mild and vague sensation of weakness in the neck; however, of these, 4 patients reported significant weakness. These four patients described the weakness sensation as being such that when they would bend forward to brush their teeth, they would have a sensation that their head was “flopping forward.” All of the patients who reported significant sensation of weakness in the neck were urgently seen and examined. None of these four patients, nor any of the patients in the present study, developed paralysis of any injected or uninjected muscles, and all had full range of motion and control. In patients who reported a sensation of weakness in the neck, the effects were transient and resolved within 7–10 days.
In patients with chronic pain, there are many outcomes by which treatment success can be measured. In the present study, pain scores and quality of life measures were used to determine proof-of-concept for the injection method used. In future studies, an outcome that would be of utmost importance to measure is evaluation of medication usage (analgesics, topicals, and muscle relaxants) and its change with treatment. It would be expected for medication usage to decrease or cease upon successful treatment of myofascial pain. Additionally, recovery of function (physical and mental) would be essential to measure in this population. Successful treatment of myofascial pain should yield improved physical functioning such as activity level and ability to work as well as improved mental functioning by way of enriched mood, interpersonal relationships, and sleep.
Prospective, placebo-controlled studies evaluating the efficacy of BoNT-A for treatment of myofascial pain are limited, with contradicting results. Freund and Schwartz performed a double-blind, randomized, placebo-controlled trial of direct trigger point injection in patients with chronic whiplash injuries, showing a significant reduction in pain and improved cervical range of motion four weeks after BoNT-A injection.35 Göbel et al36 performed a double-blind, randomized, placebo-controlled trial which showed significant improvement in pain levels 4–6 weeks after treatment using a single trigger point injection technique. A recent study by Benecke et al37 showed significant improvement in pain levels for patients receiving BoNT-A using a fixed-location injection technique, however, the authors note that the length of time to significant pain relief was longer in those who received the fixed-location injections compared to injection directly into trigger points. Miller et al performed a prospective, double-blind, placebo-controlled study using a “follow the pain” and fixed-location injection technique.38 They found a significant reduction in pain intensity that lasted for at least two months.
Wheeler et al39 performed a double-blind, randomized, placebo-controlled trial of direct trigger point injection without significant positive results after a single injection session. However, in that study, a majority of the patients only had BoNT-A injected into their trapezius muscle, with the remainder having injections into seven different neck sites. Injection sites did not include the upper or middle cervical regions. Ojala et al40 performed a randomized, double-blind, placebo-controlled, crossover trial which showed no significant improvement in cervical and shoulder girdle myofascial pain syndrome. This study used small doses of BoNT-A (5 units per trigger point), which may have been too small to provide a clinical benefit.
A minority of the published data evaluating the efficacy of BoNT-A for myofascial pain have used trigger point injections with local anesthetic as the control treatment, because these injections are the more common form of treatment. In a prospective crossover study by Graboski et al, subjects received 25 units of BoNT-A or 0.5 mL of 0.5% bupivacaine in a maximum of 8 trigger points and after pain returned for at least two consecutive weeks with an additional two week “washout” period, received injections of the other substance.41 They found no statistically significant difference between the two treatments and deemed bupivacaine to be more cost effective. Despite no clinical significance, there was a definite trend toward a greater decrease in pain and longer duration of action of BoNT-A. Possible limitations of this study leading to a negative result were a small sample size and the limited number of trigger points injected. Kamanli et al compared BoNT-A trigger point injections with dry needling and lidocaine injections.42 Pain scores were lower in subjects treated with BoNT-A and dry needling and at a 4 week follow up, the lidocaine and BoNT-A groups had significantly decreased visual analogue scale scores. BoNT-A was not found to be inferior to lidocaine or dry needling in this study. Possible issues in the interpretation of the results of this study were that only a single trigger point was treated and the sample size per group was relatively low.
In conclusion, we examined the efficacy of BoNT-A, not only with respect to pain relief, but also examined the constructs of postural analysis, health-related quality of life, disability, and headache. The results of the present study suggest that injection of BoNT-A into painful cervical and shoulder girdle muscle groups, as opposed to direct injection of painful trigger points, provides improvement in average pain scores, a reduction in the number of headaches per week, and improvement in certain facets of quality of life for at least 12 weeks. Further larger-scale studies using the technique of injecting painful muscle groups instead of painful trigger points are warranted given our positive findings. Further studies evaluating whether repetition of BoNT-A injections has a synergistic or additive effect may be of more than passing interest.37
Acknowledgments
Funding: Research support provided by Allergan, Inc. 2525 Dupont Drive, Irvine, CA 92612. The research and statistical analysis described was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124.
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
DISCLOSURES:
Name: Andrea L. Nicol, MD, MS
Contribution: Dr. Nicol helped in data collection, data analysis, and manuscript preparation.
Attestation: Dr. Nicol approved the final manuscript. Dr. Nicol attests to the integrity of the original data and the analysis reported in this manuscript.
Name: Irene I. Wu, MD
Contribution: Dr. Wu helped in data collection and manuscript preparation.
Attestation: Dr. Wu approved the final manuscript. Dr. Wu attests to the integrity of the original data and the analysis reported in this manuscript.
Name: F. Michael Ferrante, MD
Contribution: Dr. Ferrante helped in design the study, the conduct of the study, data collection, data analysis, and manuscript preparation.
Attestation: Dr. Ferrante approved the final manuscript. Dr. Ferrante attests to the integrity of the original data and the analysis reported in this manuscript. Dr. Ferrante is the archival author.
This manuscript was handled by: Spencer S. Liu, MD
Contributor Information
Andrea L. Nicol, Department of Anesthesiology, David Geffen School of Medicine at UCLA, Los Angeles, California (Current Affiliation: Department of Anesthesiology, University of Kansas School of Medicine, Kansas City, Kansas).
Irene I. Wu, Department of Anesthesiology, David Geffen School of Medicine at UCLA, Los Angeles, California.
F. Michael Ferrante, Department of Anesthesiology, David Geffen School of Medicine at UCLA, Los Angeles, California.
References
- 1.Borg-Stein J, Simons DG. Focused review: myofascial pain. Arch Phys Med Rehabil. 2002 Mar;83:S40–47. S48–49. doi: 10.1053/apmr.2002.32155. [DOI] [PubMed] [Google Scholar]
- 2.Simons DG, Travell JG, Simons LS. Myofascial Pain and Dysfunction: The Trigger Point Manual. Upper Half of Body. 2. Vol. 1. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 3.Cummings M, Baldry P. Regional myofascial pain: diagnosis and management. Best Pract Res Clin Rheumatol. 2007 Apr;21(2):367–387. doi: 10.1016/j.berh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004 Feb;14(1):95–107. doi: 10.1016/j.jelekin.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM, Gerber LH. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008 Jan;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Mense S. Muscle pain: mechanisms and clinical significance. Dtsch Arztebl Int. 2008 Mar;105(12):214–219. doi: 10.3238/artzebl.2008.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007 Feb;21(2):131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Climent JM, Kuan TS, Fenollosa P, Martin-Del-Rosario F. Botulinum toxin for the treatment of myofascial pain syndromes involving the neck and back: a review from a clinical perspective. Evid Based Complement Alternat Med. 2013;2013:381459. doi: 10.1155/2013/381459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrante FM, Bearn L, Rothrock R, King L. Evidence against trigger point injection technique for the treatment of cervicothoracic myofascial pain with botulinum toxin type A. Anesthesiology. 2005 Aug;103(2):377–383. doi: 10.1097/00000542-200508000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Aker PD, Gross AR, Goldsmith CH, Peloso P. Conservative management of mechanical neck pain: systematic overview and meta-analysis. BMJ. 1996 Nov 23;313(7068):1291–1296. [PMC free article] [PubMed] [Google Scholar]
- 11.Gross AR, Aker PD, Goldsmith CH, Peloso P. Physical Medicine Modalities for Mechanical Neck Disorders. Cochrane Database Syst Rev. 2000;2(CD000961) doi: 10.1002/14651858.CD000961. [DOI] [PubMed] [Google Scholar]
- 12.Hoving JL, Gross AR, Gasner D, Kay T, Kennedy C, Hondras MA, Haines T, Bouter LM. A critical appraisal of review articles on the effectiveness of conservative treatment for neck pain. Spine (Phila Pa 1976) 2001 Jan 15;26(2):196–205. doi: 10.1097/00007632-200101150-00015. [DOI] [PubMed] [Google Scholar]
- 13.Lang AM. A pilot study of Bolulinum toxin type A (Botox), administered using a novel injection technique, for the treatment of myofascial pain. Am J Pain Manage. 2000;(10):108–112. [Google Scholar]
- 14.Wheeler AH, Goolkasian P, Gretz SS. A randomized, double-blind, prospective pilot study of botulinum toxin injection for refractory, unilateral, cervicothoracic, paraspinal, myofascial pain syndrome. Spine (Phila Pa 1976) 1998 Aug 1;23(15):1662–1666. doi: 10.1097/00007632-199808010-00009. discussion 1667. [DOI] [PubMed] [Google Scholar]
- 15.Borodic GE, Acquadro M, Johnson EA. Botulinum toxin therapy for pain and inflammatory disorders: mechanisms and therapeutic effects. Expert Opin Investig Drugs. 2001 Aug;10(8):1531–1544. doi: 10.1517/13543784.10.8.1531. [DOI] [PubMed] [Google Scholar]
- 16.Hou CR, Tsai LC, Cheng KF, Chung KC, Hong CZ. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger-point sensitivity. Arch Phys Med Rehabil. 2002 Oct;83(10):1406–1414. doi: 10.1053/apmr.2002.34834. [DOI] [PubMed] [Google Scholar]
- 17.Jankovik J, Albanese A, Atassi MZ, Dolly JO, Hallett M, Mayer NH. Botulinum Toxin: Therapeutic Clinical Practice and Science. New York: Saunders; 2009. [Google Scholar]
- 18.Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004 Jan;107(1–2):125–133. doi: 10.1016/j.pain.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005 Oct;26(5):785–793. doi: 10.1016/j.neuro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Jabbari B. Botulinum neurotoxins in the treatment of refractory pain. Nat Clin Pract Neurol. 2008 Dec;4(12):676–685. doi: 10.1038/ncpneuro0948. [DOI] [PubMed] [Google Scholar]
- 21.Jabbari B, Machado D. Treatment of Refractory Pain with Botulinum Toxins-An Evidence-Based Review. Pain Med. 2011 Sep;12(11):1594–1606. doi: 10.1111/j.1526-4637.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- 22.Katz N. Enriched enrollment randomized withdrawal trial designs of analgesics: focus on methodology. Clin J Pain. 2009 Nov-Dec;25(9):797–807. doi: 10.1097/AJP.0b013e3181b12dec. [DOI] [PubMed] [Google Scholar]
- 23.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001 Nov;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 24.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004 Mar;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Griegel-Morris P, Larson K, Mueller-Klaus K, Oatis CA. Incidence of common postural abnormalities in the cervical, shoulder, and thoracic regions and their association with pain in two age groups of healthy subjects. Phys Ther. 1992 Jun;72(6):425–431. doi: 10.1093/ptj/72.6.425. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–483. [PubMed] [Google Scholar]
- 27.En MC, Clair DA, Edmondston SJ. Validity of the Neck Disability Index and Neck Pain and Disability Scale for measuring disability associated with chronic, non-traumatic neck pain. Man Ther. 2009 Aug;14(4):433–438. doi: 10.1016/j.math.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 28.McQuay HJ, Derry S, Moore RA, Poulain P, Legout V. Enriched enrollment with randomised withdrawal (EERW): Time for a new look at clinical trial design in chronic pain. Pain. 2008 Apr;135(3):217–220. doi: 10.1016/j.pain.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003 Nov 10;163(20):2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 30.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996 Dec;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 31.Cote TR, Mohan AK, Polder JA, Walton MK, Braun MM. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005 Sep;53(3):407–15. doi: 10.1016/j.jaad.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia KP, Munchau A, Thompson PD, Houser M, Chauhan VS, Hutchinson M, Shapira AH, Marsden CD. Generalised muscular weakness after botulinum toxin injections for dystonia: a report of three cases. J Neurol Neurosurg Psychiatry. 1999 Jul;67(1):90–3. doi: 10.1136/jnnp.67.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheean GL, Murray NM, Marsden CD. Pain and remote weakness in limbs injected with botulinum toxin A for writer’s cramp. Lancet. 1995 Jul;346(8968):154–6. doi: 10.1016/s0140-6736(95)91212-6. [DOI] [PubMed] [Google Scholar]
- 34.Shaari CM, George E, Wu BL, Biller HF, Sanders I. Quantifying the spread of botulinum toxin through muscle fascia. Laryngoscope. 1991 Sep;101(9):960–4. doi: 10.1288/00005537-199109000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Freund BJ, Schwartz M. Treatment of whiplash associated neck pain [corrected] with botulinum toxin-A: a pilot study. J Rheumatol. 2000 Feb;27(2):481–484. [PubMed] [Google Scholar]
- 36.Göbel H, Heinze A, Reichel G, Hefter H, Benecke R. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: results from a randomized double-blind placebo-controlled multicentre study. Pain. 2006 Nov;125(1–2):82–88. doi: 10.1016/j.pain.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Benecke R, Heinze A, Reichel G, Hefter H, Gobel H. Botulinum Type A Toxin Complex for the Relief of Upper Back Myofascial Pain Syndrome: How Do Fixed-Location Injections Compare with Trigger Point-Focused Injections? Pain Med. Nov. 2011;12(11):1607–1614. doi: 10.1111/j.1526-4637.2011.01163.x. [DOI] [PubMed] [Google Scholar]
- 38.Miller D, Richardson D, Eisa M, Bajwa RJ, Jabbari B. Botulinum neurotoxin-A for treatment of refractory neck pain: a randomized, double-blind study. Pain Med. 2009 Sep;10(6):1012–1017. doi: 10.1111/j.1526-4637.2009.00658.x. [DOI] [PubMed] [Google Scholar]
- 39.Wheeler AH, Goolkasian P, Gretz SS. Botulinum toxin A for the treatment of chronic neck pain. Pain. 2001 Dec;94(3):255–260. doi: 10.1016/S0304-3959(01)00358-X. [DOI] [PubMed] [Google Scholar]
- 40.Ojala T, Arokoski JP, Partanen J. The effect of small doses of botulinum toxin a on neck and shoulder myofascial pain syndrome: a double-blind, randomized, and controlled crossover trial. Clin J Pain. 2006 Jan;22(1):90–96. doi: 10.1097/01.ajp.0000151871.51406.c3. [DOI] [PubMed] [Google Scholar]
- 41.Graboski CL, Gray DS, Burnham RS. Botulinum toxin A versus bupivacaine trigger point injections for the treatment of myofascial pain syndrome: a randomised double blind crossover study. Pain. 2005 Nov;118(1–2):170–175. doi: 10.1016/j.pain.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Kamanli A, Kaya A, Ardicoglu O, Ozgocmen S, Zengin FO, Bayik Y. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005 Oct;25(8):604–611. doi: 10.1007/s00296-004-0485-6. [DOI] [PubMed] [Google Scholar]