Abstract
We have previously demonstrated that brain-derived neurotrophic factor (BDNF) interacts with testosterone to regulate dendritic morphology of motoneurons in the highly androgen-sensitive spinal nucleus of the bulbocavernosus (SNB). Additionally, in adult male rats testosterone regulates BDNF in SNB motoneurons and its target muscle, the bulbocavernosus (BC). Because BDNF is retrogradely transported from skeletal muscles to spinal motoneurons, we hypothesized that testosterone could regulate BDNF in SNB motoneurons by acting locally at the BC muscle. To test this hypothesis, we restricted androgen manipulation to the SNB target musculature. After castration, BDNF immunolabeling in SNB motoneurons was maintained at levels similar to those of gonadally intact males by delivering testosterone treatment directly to the BC muscle. When the same implant was placed interscapularly in castrated males it was ineffective in supporting BDNF immunolabeling in SNB motoneurons. Furthermore, BDNF immunolabeling in gonadally intact adult males given the androgen receptor blocker hydroxyflutamide delivered directly to the BC muscle was decreased compared with that of gonadally intact animals that had the same hydroxyflutamide implant placed interscapularly, or when compared with castrated animals that had testosterone implants at the muscle. These results demonstrate that the BC musculature is a critical site of action for the androgenic regulation of BDNF in SNB motoneurons and that it is both necessary and sufficient for this action. Furthermore, the local action of androgens at the BC muscle in regulating BDNF provides a possible mechanism underlying the interactive effects of testosterone and BDNF on motoneuron morphology.
Keywords: neurotrophic factors, bulbocavernosus, testosterone, SNB
INTRODUCTION
The importance of neuron-target interactions and the role of the periphery in maintaining neuron survival, morphology, and function is well-established (Hamburger, 1939; Hamburger and Levi-Montalcini, 1949; Kuno, 1990; Lubischer and Arnold, 1995a; de la Cruz et al., 1996). For example, reductions in neuron number (Oppenheim, 1991) and soma size (Yang and Arnold, 2000a), cytoskeletal elements (McIlwain and Hoke, 2005), axonal transport (Tseng et al., 1996), number of synaptic contacts (Brannstrom and Kellerth, 1998), hormone receptors (Lubischer and Arnold, 1995b), and electrophysiological properties (Zhang et al., 1997) can occur after connections to the peripheral target are severed by axotomy. Peripheral targets of neurons have also been implicated in the regulation of their dendritic morphology (Sumner and Watson, 1971; Yawo, 1987; Andrews et al., 1996; Tseng and Hu, 1996). For example, axotomy decreases the amount of dendritic arbor in spinal motoneurons (Yang et al., 2004), and increasing or decreasing the size of the peripheral target tissue results in a corresponding change in sympathetic ganglion dendritic morphology (Voyvodic, 1989).
The lumbar spinal cord of the rat contains a sexually dimorphic motor nucleus, the spinal nucleus of the bulbocavernosus (SNB; Breedlove and Arnold, 1980). In males, SNB motoneurons innervate the bulbocavernosus and levator ani (BC/LA) muscles of the penis (McKenna and Nadelhaft, 1986), and control penile reflexes important for copulatory behavior (Sachs, 1982). The motoneurons of the SNB are highly androgen-sensitive in that androgens maintain a variety of their features, including soma size and dendritic length (Kurz et al., 1986), the number and size of synapses (Leedy et al., 1987) and gap junction plaques (Matsumoto et al., 1988), androgen receptor nuclear immunoreactivity (Matsumoto et al., 1996), and mRNA expression of the cytoskeletal elements β-actin (Matsumoto et al., 1993) and β-tubulin (Matsumoto et al., 1993) in adult males.
The BC/LA musculature is a critical site of action for androgenic effects in the SNB neuromuscular system in adulthood. BC/LA muscles express high levels of androgen receptors (Breedlove and Arnold, 1980; Matsumoto et al., 1996) and previous studies have suggested that androgenic regulation of BC/LA muscle weight occurs locally at the muscle (Rand and Breedlove, 1992; Monks et al., 2004). For example, BC muscle weight can be regulated by local administration of testosterone or the anti-androgen hydroxyflutamide directly to the muscle (Rand and Breedlove, 1992). Androgenic effects on SNB soma size are also influenced by contact with the BC/LA muscles (Araki et al., 1991), and removal of the target musculature in early postnatal life down-regulates androgen receptor expression and blocks androgen sensitivity in SNB motoneurons (Lubischer and Arnold, 1995a). In addition, androgens can regulate SNB dendritic morphology by acting on the BC/LA musculature (Rand and Breedlove, 1995). In castrated males, SNB motoneurons projecting to testosterone-implanted BC/LA muscles have significantly longer SNB dendritic lengths than those projecting to muscles on the contralateral side implanted with hydroxyflutamide. Based on these findings, it was suggested that the androgenic regulation of SNB motoneuron morphology may be due to some retrograde trophic factor whose effects are mediated by androgenic action at the target musculature (Rand and Breedlove, 1995).
One candidate trophic factor that is known to interact with androgens in the SNB neuromuscular system and may serve as a key retrograde signal influencing SNB morphology is brain-derived neurotrophic factor (BDNF). BDNF is a member of the neurotrophin family and promotes motoneuron survival and axon outgrowth (Kishino et al., 1997). Previous work has shown combinatorial treatment effects of testosterone and BDNF (Yang and Arnold, 2000a,b; Yang et al., 2004). In SNB motoneurons, treatment with testosterone and BDNF has an interactive effect on the regulation of androgen receptor expression (Yang and Arnold, 2000b) as well as dendritic length (Yang et al., 2004). Combined treatment with both testosterone and BDNF is more effective than treatment with either compound alone in the maintenance of androgen receptor immunoreactivity in axotomized SNB motoneurons (Yang and Arnold, 2000b). Similarly, although treatment with either compound alone is ineffective, dendritic lengths in axotomized SNB motoneurons are supported by combined treatment with both testosterone and BDNF (Yang et al., 2004).
Several possible explanations of these interactive effects have been suggested. For example, BDNF and its high-affinity receptor, trkB, are present in SNB motoneurons (Osborne et al., 2007; Ottem et al., 2007; Verhovshek et al., 2010b) and expression of BDNF and trkB mRNA and protein in SNB motoneurons is regulated by androgens (Osborne et al., 2007; Ottem et al., 2007; Verhovshek et al., 2010b). After castration, both BDNF and trkB mRNA and protein levels are decreased, and this decrease can be restored or prevented with systemic testosterone treatment.
While the androgenic regulation of BDNF and trkB receptors in SNB motoneurons provides a potential explanation for the interactive effects of testosterone and BDNF on SNB morphology, the site of action for this androgenic regulation is not known. As previously mentioned, SNB motoneurons express androgen receptors (Matsumoto et al., 1996), and thus it is possible that androgens could act directly at the motoneuron to regulate BDNF production. However, the BC muscles also express androgen receptors (Monks et al., 2004, 2006), implicating the BC muscle as another potential site for the regulation of BDNF in SNB motoneurons. In support of this idea, dendritic morphology of SNB motoneurons can be regulated by androgenic action at the BC musculature (Rand and Breedlove, 1995). Furthermore, BDNF is expressed in the BC musculature (Verhovshek et al., 2010b), and BDNF can be retrogradely transported from skeletal muscle to spinal motoneurons (DiStefano et al., 1992; Koliatsos et al., 1993). Therefore, to determine if the target musculature is a site of action for the androgenic regulation of BDNF in SNB motoneurons, we used an implant designed to deliver testosterone or the androgen receptor blocker hydroxyflutamide directly to the BC muscle, and then examined BDNF protein expression in SNB motoneurons.
MATERIALS AND METHODS
Implants
A small Silastic implant was used to deliver a highly localized treatment of testosterone or hydroxyflutamide directly to the BC muscle. The implants, similar to those used by Rand and Breedlove (1992, 1995), were constructed using a small piece of Tygon tubing (3.2 mm outer diameter, 1.6 mm inner diameter, 5 mm long) that was heat-sealed at one end. The tubing was filled either with a 1:40 mix of testosterone (4-androsten-17β-ol-3-one; Steraloids; Newport, RI):cholesterol (5-cholenic acid-3β-ol, Sigma, St. Louis, MO), the androgen receptor blocker hydroxyflutamide (2-hydroxyflutamide, LKT Laboratories, St. Paul, MN), or left blank. The Tygon tubes were then placed in a mold and embedded in liquid Silastic that encased the tubing and formed flaps used for suturing the implant to the BC muscle (Fig. 1). This type of implant has previously been shown to influence SNB dendritic morphology and BC muscle weight when sutured directly to the muscle (Rand and Breedlove, 1992, 1995). The dose of testosterone was chosen based on pilot studies and was sufficient to support SNB dendritic morphology in castrated animals in which the implant was sutured directly to the BC muscle but not when implanted interscapularly. Similarly, the dose of hydroxyflutamide was chosen based on its ability to reduce SNB dendritic length when sutured directly to at the BC muscle in gonadally intact males but not when placed interscapularly. Importantly, using this approach we are able to deliver an effective treatment with testosterone or hydroxyflutamide directly to the BC muscle, but do not provide a dose large enough to produce systemic effects of the hormone or drug. Thus, any effects we observe on BDNF labeling in the spinal cord in the muscle-implanted groups can be directly attributed to the effects of the hormone or drug treatment at the target muscle.
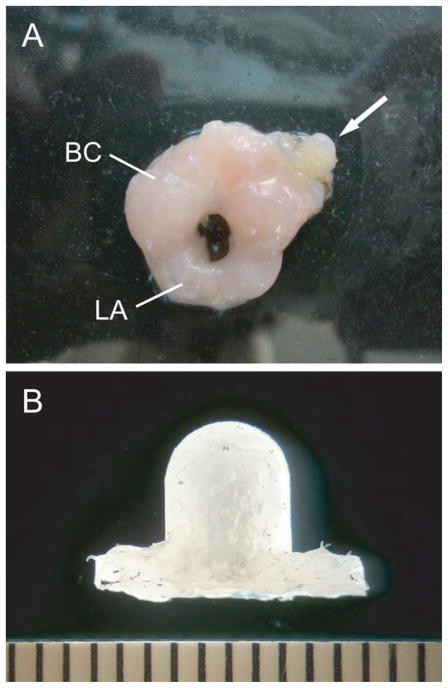
Figure 1.
(A) BC/LA muscle with a Silastic implant (arrow) sutured to the left BC muscle which delivered a highly localized treatment with a testosterone:cholesterol mixture (1:40) or hydroxyflutamide, or was left blank. (B) Higher magnification view of a Silastic muscle implant. Scale bar is in mm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Animals
Adult male, Sprague Dawley rats obtained from Harlan (approximately 100 days old; Indianapolis, IN) were maintained on a 12:12 hr light:dark cycle, with ad libitum access to food and water. Animals were anesthetized with isoflurane, castrated, and immediately had either a testosterone (n = 6) or a blank (n = 6) implant sutured directly to the left half of the BC muscle; another group of castrated males had a testosterone implant placed interscapularly (n = 6) and served as controls to rule out systemic effects of the testosterone treatment. In addition, two groups of gonadally intact males had a hydroxyflutamide implant either sutured directly to the left half of the BC muscle (n = 6) or placed interscapularly (n = 6). All procedures were carried out in accordance with the Indiana University Animal Care and Use Guidelines.
Four weeks after hormonal manipulations, a period sufficient to observe hormone-mediated morphological changes in motoneurons (Kurz et al., 1986; Verhovshek et al., 2010a), animals were weighed, given an overdose of urethane (0.25 g/100 g body weight) and perfused transcardially with phosphate-buffered saline (pH 7.2) followed by cold 4% paraformaldehyde in 0.1 M sodium phosphate (pH 7.2). The L5-S1 segments of the spinal cord were post-fixed in the same fixative for 1 hr and then transferred to sucrose phosphate buffer (30% w/v, pH 7.4) overnight for cryoprotection. The following day, spinal cords were sectioned horizontally into three alternate series at 30 μm and stored in 0.1 M phosphate buffer. To identify sections containing SNB motoneurons, one series of sections was immediately mounted on gelatin-coated slides and stained with neutral red. These sections were then examined under light microscopy for the presence of SNB motoneurons, and the appropriate matching sections in the alternate series were selected for immunohistochemical processing. The BC/LA muscle complex was also removed after perfusion and weighed.
Immunohistochemistry
To control for potential processing differences, sections from animals from all experimental groups were processed simultaneously in the same solutions through all steps. Sections were rinsed with 1% bovine serum albumin and 0.1% Triton X-100 in 0.1 M sodium phosphate and incubated in a 0.5% hydrogen peroxide blocking solution for 30 min to inhibit endogenous peroxidase activity. Sections were then incubated overnight at 4°C in rabbit anti-BDNF receptor primary antibody (1:1000 dilution, AB1779; Millipore, Temecula, CA) followed by incubation in biotinylated goat anti-rabbit secondary antibody (1:200; Vector Laboratories, Burlingame, CA). Antibody signal amplification was achieved via an ABC reaction (Vector Standard Elite Kit, Vector Laboratories) and visualized with 0.03% DAB and 0.003% H2O2 for 7 min. Sections were then mounted onto chrome-alum gelatin coated slides, counterstained with neutral red, and coverslipped. Control sections incubated without primary antibody were generated and showed virtually no immunostaining.
Densitometry
We used a previously described semiquantitative method to assign cells to categories of intense, moderate, or light immunostaining (Osborne et al., 2007; Verhovshek et al. 2010b). The SNB is a discrete nucleus located medially in the L5–S1 segments of the spinal lumbar spinal cord, which allows for reliable identification of SNB motoneurons based on location and morphology. SNB motoneurons were thus identified by their characteristic location, and all large, multipolar somata in the half of the nucleus ipsilateral to the muscle implant were assessed, regardless of the density of immunostaining. After outlining the perimeter of individual motoneuron somata, the optical density (OD) of immunolabeling in each soma was measured using a computer-based morphometry system (Stereo Investigator, MBF Bioscience, Williston, VT) as average luminosity per pixel (in 256 gray levels, where black = 0 and white = 256; a mean of approximately 6400 pixels per soma was assessed) at a final magnification of 1480× under bright-field illumination. To control for differences in immunostaining across sections and animals, OD measures within each section were expressed relative to immunostaining in an area (approximately 100 μm2) of the lateral funiculus, ipsilateral to the muscle implant. Although spinal cord white matter contains BDNF (Dreyfus et al., 1999), BDNF expression is greater in spinal cord gray matter compared with white matter (Scarisbrick et al., 1999). More importantly, the intensity of BDNF immunolabeling in the white matter does not vary with hormone condition (Verhovshek et al., 2010b) and thus provided a reliable reference measure of local immunostaining. Immunostaining of motoneuron somata was then categorized as being either intense (having a relative OD of at least 1 standard deviation below the mean of castrated males that had a testosterone implant sutured to the left BC), moderate, or light (having a relative OD of at least 1 standard deviation above the mean of castrated males that had a testosterone implant sutured to the left BC). Importantly, this method does not attempt to assess the actual amount of BDNF in motoneurons but rather simply provides a reliable method for categorizing cells by immunostaining intensity for subsequent frequency analyses.
Statistics
Analyses of variance (ANOVA) followed by appropriate planned comparisons (Fisher’s protected LSD) were used in cases of true interval data (e.g., muscle weight, cell number). For cases where the data were ordinal in nature (density of immunolabeling), analyses consisted of the nonparametric Kruskal-Wallis ANOVA by ranks. Frequency of immunolabeling was analyzed using a two-way ANOVA with repeated measures (group by label, with label as the repeated factor). For presentation purposes, digital light micrographs were obtained using an MDS 290 digital camera system (Eastman Kodak Company, Rochester, NY). Brightness and contrast of these images were adjusted in Adobe Photoshop (Adobe Systems, Inc., San Jose, CA).
RESULTS
BC/LA Muscle Weight
Body weights of animals differed across treatment groups [F(4,25) = 3.92, p < 0.05]. Body weights of castrated males that had either testosterone (380.67 ± 10.21 g; mean ± SEM) or blank (384.33 ± 13.23 g) implants at the BC muscle or interscapular testosterone implants (386.00 ± 11.77 g) were all similar, as were the body weights of gonadally intact males that had interscapular hydroxyflutamide implants (417.00 ± 5.77 g; LSDs, all n.s.). However, gonadally intact males with a hydroxyflutamide implants at the BC muscle (436.67 ± 18.11 g) had body weights that were on average 13.8% heavier than those of the castrate groups (LSDs, p <0.05). In order to rule out any potential confound of overall body weight on individual muscle weight, BC/LA muscle weights were corrected for body weight by dividing each individual animal’s BC/LA muscle weight by their body weight, and multiplying the result by 100.
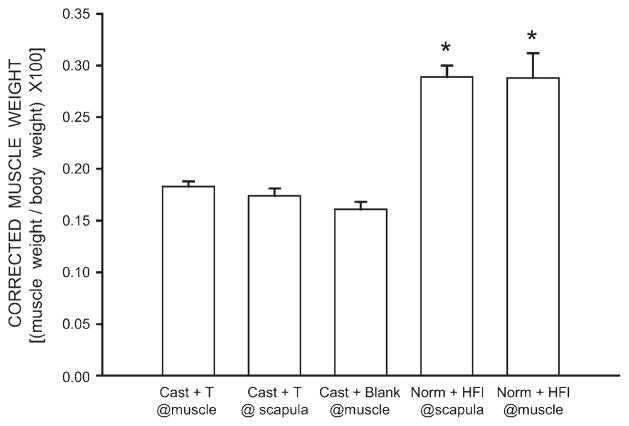
After correcting for the differences in body weight, differences in BC/LA muscle weight across groups remained [F(4,25) = 24.78, p < 0.0001]. The average corrected BC/LA muscle weight for both gonadally intact, hydroxyflutamide groups (0.29 ± 0.02 g) were typical (corrected BC/LA muscle weight for gonadally intact males = 0.33 ± 0.01 g) and did not differ from each other (LSDs, n.s.). While the corrected BC/LA muscle weights of both of the hydroxyflutamide treatment groups were similar to those of gonadally intact males, they were on average 66% larger than those of all of the castrate treatment groups (LSDs, p <0.0001; Fig. 2). The average corrected BC/LA muscle weights (0.17 ± 0.01 g) of all castrated groups did not differ from each other (LSDs, n.s., Fig. 2).
Figure 2.
Weights of BC/LA muscles expressed relative to individual body weights of castrated males with testosterone (T) implants at the BC muscle, castrated males with interscapular testosterone implants, castrated males with blank implants at the BC muscle, gonadally intact males with interscapular hydroxyflutamide (HFl) implants, and gonadally intact males with HFl implants at the BC muscle. Bar heights represent means ± SEM for six animals per group. *Significantly different from all castrate groups.
Immunohistochemistry
Mean (± SEM) OD of the white matter samples was 109.56 (± 4.37) and did not differ across hormonal condition [Kruskal-Wallis H(4) = 5.48, n.s.]. Immunostaining for BDNF resulted in pronounced labeling in SNB motoneurons (Fig. 3). The optical density of an average of 92.82 ± 13.15 somata was assessed over an average of 5.83 ± 0.61 sections per animal; the number of motoneurons sampled did not differ across groups [F(4,25) = 0.54, n.s.].
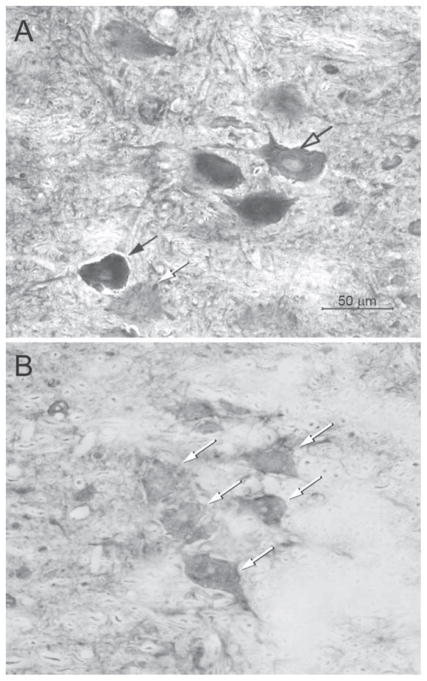
Figure 3.
Digital light micrographs of horizontal sections of regions of the spinal cord containing BDNF immunolabeled SNB motoneurons from castrated males with a testosterone implant (A) or a blank implant (B) at the BC muscle. Intensely (black arrow), moderately (open arrow), and lightly (white arrows) immunostained somata are indicated. Scale bar = 50 μm.
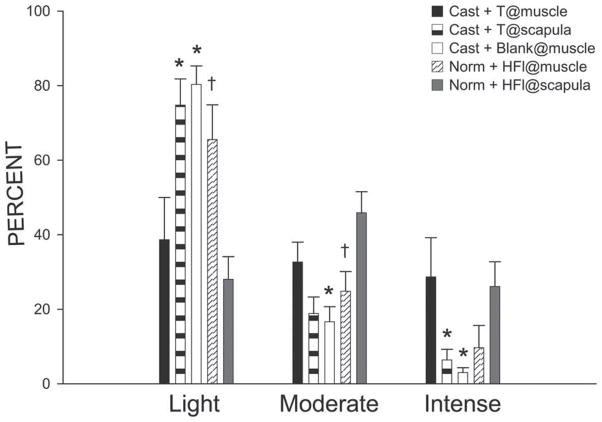
In castrated males that had testosterone implants at the BC muscle, the mean of the relative ODs of SNB motoneuron somata immunolabeled for BDNF was 0.64 with a SD of 0.08. Intensely immunostaining somata comprised 28.7% of the population; moderately immunostaining somata comprised 32.7% of the population; lightly immunostaining somata comprised the remaining 38.6% (Fig. 4). These percentages of BDNF immunostaining somata in each labeling intensity category for castrated males that had testosterone implants at the BC muscle were similar to the percentages reported previously for gonadally intact males (intense = 34.3%, moderate = 38.1%, light = 27.3%; Verhovshek et al., 2010b). BDNF immunolabeling in SNB motoneurons was sensitive to treatment condition [F(8,50) = 6.21, p <0.0001]. The frequency of intensely immunolabeled SNB motoneuron somata in castrated animals that had interscapular testosterone implants was decreased 77.7% compared with castrated males that had testosterone implants at the BC muscle (LSD, p <0.02); intensely immunolabeled SNB somata comprised only 6.4% of the population (Fig. 4). The frequency of moderately immunolabeled SNB somata was also decreased 42.3%, now only comprising 18.9% of the population, but this difference failed to reach significance (LSD, n.s.; Fig. 4). These decreases in the frequency of intensely and moderately immunolabeled somata were countered by increases in the frequency of lightly immunolabeled somata. In castrated males that had interscapular testosterone implants, the frequency of lightly immunolabeled SNB somata increased by 93.4% compared with castrated males that had testosterone implants at the BC muscle (LSD, p <0.005), and now comprised 74.8% of the SNB population (Fig. 4). Similar to castrated males that had interscapular testosterone implants, the frequency of intensely immunolabeled SNB motoneuron somata in castrates with blank implants at the BC muscle was decreased by 89.5% (LSD, p <0.01) compared with castrates with testosterone implants at the BC muscle; intensely immunolabeled SNB somata now comprised only 3.0% of the population (Fig. 4). The frequency of moderately immunolabeled SNB somata also differed across groups and in castrates with blank implants at the BC muscle was decreased 49% (LSD, p <.05) compared with castrates with testosterone implants at the BC muscle; moderately immunolabeled SNB somata now only comprised 16.7% of the population (Fig. 4). These decreases in the frequency of intensely and moderately immunolabeled somata were countered by a change in the frequency of lightly immunolabeled somata. In castrated males that had blank implants at the BC muscle, the frequency of lightly immunolabeled SNB somata increased by 108% compared with castrated animals that had testosterone implants at the BC muscle (LSD, p <0.05), and now comprised 80.3% of the SNB population (Fig. 4).
Figure 4.
Bar graph of the number of lightly, moderately, and intensely immunostained SNB motoneuron somata after immunolabeling for BDNF in castrated males with testosterone (T) implants at the BC muscle (filled bars), castrated males with interscapular testosterone implants (lightly shaded bars), castrated males with blank implants at the BC muscle (open bars), gonadally intact males with interscapular hydroxyflutamide implants (striped bars), and gonadally intact males with hydroxyflutamide implants at the BC muscle (darkly shaded bars). Bar heights represent means ± SEM. *Significantly different from castrated males with testosterone implants at the BC muscle; †Significantly different from gonadally intact males with interscapular hydroxyflutamide implants.
Similar to castrated males that had blank implants at the BC muscle, the frequency of intensely immunolabeled SNB motoneuron somata in gonadally intact males with hydroxyflutamide implants at the BC muscle was decreased 66.3% (LSD, p <0.05) compared with castrates with testosterone implants at the BC muscle; intensely immunolabeled SNB somata now comprised only 9.7% of the population (Fig. 4). The frequency of moderately immunolabeled SNB somata was also decreased 24.1% compared with castrates with testosterone implants at the BC muscle and now comprised 24.8% of the population, however, this difference failed to reach significance (LSD, n.s.; Fig. 4). These decreases in the frequencies of intensely and moderately immunolabeled somata were countered by a change in the frequency of lightly immunolabeled somata. In gonadally intact males that had hydroxyflutamide implants at the BC muscle, the frequency of lightly immunolabeled somata increased by 69.5% compared with castrated animals that had testosterone implants at the BC muscle (LSD, p <0.03), and now comprised 65.5% of the population (Fig 4).
On the other hand, immunolabeling for BDNF in SNB motoneuron somata in gonadally intact males with interscapular hydroxyflutamide implants did not differ from immunolabeling observed in castrated males that had testosterone implants at the BC muscle. The frequencies of intensely (26.1%), moderately (45.87%), and lightly (28.02%) immunostaining somata in gonadally intact males with interscapular hydroxyflutamide implants were similar to those of castrated males with testosterone implants at the BC muscle [F(2,20) = 0.77, n.s.; Fig. 4]. However, immunolabeling for BDNF in SNB motoneuron somata in gonadally intact animals that had interscapular hydroxyflutamide implants was different from immunolabeling observed in gonadally intact animals that had hydroxyflutamide implants at the BC muscle [F(2,20) = 8.05, p < 0.003]. In rats with hydroxyflutamide implants at the BC muscle, the frequency of intensely immunolabeled SNB somata was decreased 63.0% relative to that of rats with interscapular hydroxyflutamide implants; however, this difference failed to reach significance (LSD, n.s.; Fig. 4). The frequency of moderately immunolabeled SNB somata in gonadally intact animals with hydroxyflutamide implants at the BC muscle was also decreased 45.9% compared with gonadally intact animals with interscapular hydroxyflutamide implants (LSD, p <0.007). These decreases in intensely and moderately immunolabeled SNB somata were countered by a change in the frequency of lightly immunolabeled somata. In gonadally intact animals that had hydroxyflutamide implants at the BC muscle, the frequency of lightly immunolabeled SNB somata increased by 134% compared with that of gonadally intact animals that had interscapular hydroxyflutamide implants (LSD, p <0.03; Fig. 4).
DISCUSSION
Previously, we demonstrated that androgens regulate BDNF protein in SNB motoneurons (Verhovshek et al., 2010b). In the current experiment, we extend those findings to identify the BC musculature as a critical site of action for the androgenic regulation of BDNF protein in SNB motoneurons: BDNF immunolabeling in SNB motoneurons in castrated adult males was maintained by delivering testosterone directly to the BC muscle. The same implant placed interscapularly in castrated animals was ineffective in supporting BDNF immunolabeling in SNB motoneurons. The frequency of intensely and moderately BDNF-immunostaining SNB motoneurons was significantly decreased in castrated males with interscapular testosterone implants compared with castrates that had a testosterone implant at the BC muscle. The decrease in intensely and moderately BDNF-immunostaining SNB motoneurons was countered by an increase in the frequency of lightly BDNF-immunostaining SNB motoneurons. On the other hand, BDNF immunolabeling in castrated animals with interscapular testosterone implants was similar to that of castrated animals with blank implants at the BC muscle; the frequencies of intensely, moderately and lightly BDNF immunostaining SNB motoneurons did not differ across groups.
While informative, these results only demonstrate that local application of testosterone to the BC muscle is sufficient to regulate BDNF protein in SNB motoneurons. In order to confirm that the BC muscle is a critical site of action for the regulation of BDNF in SNB motoneurons, we further demonstrated that BDNF protein was decreased by blocking androgenic action locally at the BC muscle. BDNF immunolabeling in gonadally intact adult males treated with the androgen receptor blocker hydroxyflutamide at the BC muscle was decreased compared with that of gonadally intact animals with interscapular hydroxyflutamide implants or when compared with castrated animals with testosterone implants at the BC muscle. The frequencies of intensely and moderately BDNF-immunostained SNB motoneurons were significantly decreased in castrates with hydroxyflutamide implants at the BC muscle compared with animals that had interscapular hydroxyflutamide implants or castrates with testosterone implants at the BC muscle. The decrease in intensely and moderately BDNF-immunostained SNB motoneurons was countered by an increase in the frequency of lightly BDNF-immunostained SNB motoneurons. Furthermore, BDNF immunolabeling in gonadally intact males that had hydroxyflutamide implants at the BC muscle was similar to that of castrated animals that had either blank implants at the BC muscle or testosterone implants placed interscapularly; the frequencies of intensely, moderately and lightly BDNF-immunostaining SNB motoneurons were all similar. Taken together, these results demonstrate that androgen activity at the BC muscle is both necessary and sufficient for the androgenic maintenance of BDNF in SNB motoneurons.
Muscle weight data were collected to confirm the efficacy of the hormonal manipulations as well as to rule out any abnormal anabolic effects on the BC/LA muscle. As expected, BC/LA muscle weight was significantly decreased after castration (Wainman and Shipounoff, 1941). BC/LA muscle weights in all castrate groups were significantly reduced compared with those of both of the gonadally intact groups. Local delivery of testosterone directly to the BC muscle in castrated males did not increase BC/LA muscle weight. Examination of BC/LA muscle weights in the two hydroxyflutamide treatment groups also did not reveal any differences. Gonadally intact males that had hydroxyflutamide implants at the BC muscle had BC/LA muscle weights that were similar to those of gonadally intact animals that had hydroxyflutamide implants placed interscapularly. This finding is similar to the BC/LA muscle weights observed in the castrated groups. These results demonstrate that gonad removal was successful in reducing the level of circulating androgens and further, that the maintenance of BDNF immunolabeling in SNB motoneurons of castrated animals by local application of testosterone to the BC muscle was not due to a general anabolic effect on the BC/LA muscle complex. These data also suggest that separate mechanisms exist for the androgenic regulation of BDNF in SNB motoneurons versus BC/LA muscle weight.
Interestingly, the lack of an effect of either testosterone or hydroxyflutamide muscle implants on BC/LA muscle weight in our current results differs from those reported by Rand and Breedlove (1992, 1995). This disparity most likely reflects differences in experimental design across the studies. For example, in contrast to our results, Rand and Breedlove (1992) found that treatment of one side of the BC/LA complex with testosterone in castrated males produced heavier muscle weights compared with those of the blank implanted side. Our lack of an effect could simply reflect the substantially lower dosage of testosterone used in the current study (we used a 1:40 mixture of testosterone and cholesterol while Rand and Breedlove used pure testosterone). We also failed to find an effect of hydroxyflutamide treatment on BC/LA weight, again in contrast with the results of Rand and Breedlove (1992, 1995). Importantly, our hydroxyflutamide treatments were done in gonadally intact males, whereas the effects of hydroxyflutamide on the weight of the BC/LA complex reported by Rand and Breedlove (1992, 1995) were observed in castrated males with either testosterone or blank implants placed contralateral to the hydroxyflutamide implant. Thus differences in the hormonal milieu across the studies could underlie the discrepant findings.
Despite the lack of an effect on BC/LA muscle weight, the effects of testosterone treatment (in the castrate groups) and hydroxyflutamide treatment (in the gonadally intact groups) on BDNF immunolabeling in SNB motoneurons differed from the effects on BC/LA muscle weight. A similar dissociation between androgenic effects on BDNF expression in spinal motoneurons and skeletal muscle has been previously observed. BDNF immunolabeling was decreased after castration in motoneurons innervating the quadriceps muscles, but muscle weight was unaffected (Verhovshek et al., 2010b). Furthermore, treatment of castrates with testosterone maintained BDNF immunolabeling in quadriceps motoneurons at levels similar to those of gonadally intact males, but again had no effect on muscle weight (Verhovshek et al., 2010b). In addition, differential androgenic sensitivities across the various components of the SNB neuromuscular system have previously been demonstrated (Verhovshek et al., 2010a). For example, treatment of adult males with dihydrotestosterone fully supports SNB dendritic arbor after castration, however, it can only partially support BC/LA muscle weight. The current data suggest that different thresholds exist for the androgenic regulation of BDNF in SNB motoneurons versus the BC/LA muscle weight. It is possible that the reason we did not observe any local effects of hydroxyflutamide or testosterone treatment on BC/LA muscle weight but did observe changes in BDNF immunolabeling in SNB motoneurons is that the regulation of BDNF protein in SNB motoneurons may be more sensitive to androgenic manipulations.
It is important to note that the hydroxyflutamide-treated animals were gonadally intact and presumably had normal circulating levels of serum testosterone. Perhaps the relatively small dose of hydroxyflutamide that was delivered to the BC muscle was insufficient to block the anabolic actions of gonadally-released testosterone on BC/LA muscle weight. In any event, local blockade of androgen receptors at the BC muscle was effective in reducing BDNF immunolabeling in SNB motoneurons, similar to what has previously been reported for SNB dendritic length (Rand and Breedlove, 1995). In addition, the concentration of testosterone used in the muscle implants was exceedingly low (1:40 mixture of testosterone:cholesterol in 5 mm of tubing). In contrast, the previous experiments examining androgen and BDNF interactions in the SNB (Yang et al., 2004; Osborne et al., 2007; Verhovshek et al., 2010b) used implants (45 mm lengths of undiluted testosterone) that produce serum testosterone levels that approximate the daily average level of gonadally intact males (Smith et al., 1977). Although serum testosterone levels in testosterone-implanted castrated males were not measured in the current experiment, it can be reasonably assumed that they were far lower than those produced by 45 mm implants of undiluted testosterone intended to result in systemic effects. Despite its small size, when delivered in a highly localized manner this dose of testosterone effectively maintained BDNF protein expression in SNB motoneurons of castrated adult males, indicating an extreme androgen sensitivity of this feature of SNB motoneurons.
Recent work from our laboratory has bolstered the idea that the target musculature is a critical mediator of androgenic effects in spinal motoneurons. For example, androgenic sensitivity is conferred to spinal motoneurons with low androgen responsiveness by overexpressing androgen receptors exclusively in their skeletal muscle fibers (Huguenard et al., 2011). Unlike in SNB motoneurons, dendritic morphology of motoneurons innervating the quadriceps muscles does not change after castration in wild-type rats. However, dendritic arbor is reduced after castration in rats that overexpress androgen receptor in their skeletal muscles. This castration-induced reduction in dendritic arbor can be prevented or restored with testosterone treatment, similar to what is observed for SNB motoneurons (Kurz et al., 1986). In addition, a previous study by Rand and Breedlove (1995) suggests that the BC musculature is the critical site of action for the androgenic regulation of SNB dendritic morphology. The results in the current experiment are in agreement with these findings and support the idea that the target musculature is a critical site mediating androgenic effects on SNB motoneurons. Further, these results identify the androgenic regulation of BDNF via the BC muscle as a likely mediator of those effects.
One caveat in interpreting the current results is that although we specifically applied testosterone to the BC muscle, it is possible that these changes in BDNF immunolabeling in SNB motoneurons could be estrogenic in nature. Testosterone is converted to its two primary metabolites dihydrotestosterone and estradiol by the steroidogenic enzymes 5α-reductase and aromatase, respectively. Both 5α-reductase and aromatase are present in skeletal muscle (Bartsch et al., 1983; Pasupuleti and Horton, 1990; Larionov et al., 2003), and skeletal muscle has been identified as an extragonadal source of estrogens and androgens (Matsumine et al., 1986). Although the data from the hydroxyflutamide-treated animals argue against this possibility, further studies with estrogens or non-aromatizable androgens can address this question. Additionally, although our data support a local action of androgen at the target musculature in the regulation of BDNF in SNB motoneurons, we do not know the specific cellular nature of that target. For example, androgens could be acting at muscle fibers, satellite cells, fibroblasts, terminal Schwann cells or even the motor nerve terminals, and examples of hormone effects on motoneurons via different cell types in the neuromuscular periphery have been observed (e.g., Huguenard et al., 2011; Rudolph and Sengelaub, 2013). Determining which of these cellular sites are involved will clearly be important in understanding the mechanism(s) regulating the androgenic regulation of BDNF in SNB motoneurons.
BDNF expression is regulated through a calcium-dependent signaling pathway, involving CRE and CREB (Shieh et al., 1998; Tao et al., 1998; Tao et al., 2002), both of which can be activated by testosterone (Aarnisalo et al., 1998; Auger et al., 2001). Thus, it is possible that in the current experiment we have activated (via testosterone) or blocked (via hydroxyflutamide) a signaling pathway critical for BDNF production. Furthermore, our findings demonstrate that androgenic action at the BC muscle is necessary for the regulation of BDNF in SNB motoneurons. However, the source of BDNF that was measured in SNB motoneurons in the current experiment is not known. It is possible that androgens act at the BC muscle to upregulate a retrograde signal, which then results in increased production of BDNF in the motoneuron. An in situ hybridization experiment examining BDNF mRNA expression in SNB motoneurons using the same experimental design could address this question directly. For example, if BDNF mRNA expression is increased in SNB motoneurons after local testosterone treatment at the BC muscle, it would provide evidence that a source of BDNF protein measured in the current experiment is the motoneuron. Alternatively, given that BDNF is retrogradely transported from skeletal muscle to spinal motoneurons (DiStefano et al., 1992; Koliatsos et al., 1993), it is also possible that the source of the BDNF that was measured in SNB motoneurons is the BC muscle. However, we have previously demonstrated that BDNF concentrations are sharply increased in the BC muscle after removal of androgens via castration but can be maintained at normal levels in castrated males treated with testosterone (Verhovshek et al., 2010b). When taken together with our current findings, these data pose an apparent paradox: BDNF protein is increased in the BC musculature by androgen removal but increased in SNB motoneurons by androgen treatment.
We have previously argued that this apparent paradox may reflect post-castration changes in the retrograde transport of BDNF to the SNB motoneurons (Verhovshek et al., 2010b). The retrograde transport of BDNF is activity dependent (Watson et al., 1999), and castration decreases activity in the SNB neuromuscular system (Holmes and Sachs, 1992; Fargo et al., 2003). Thus, this decrease in activity could potentially result in a decrease in retrograde transport and a concomitant accumulation of BDNF in the BC muscle. Consistent with this idea, after ligation of the sciatic nerve, accumulation of BDNF is observed in the nerve distal to the ligation (Tonra et al., 1998). In addition, activity-dependent retrograde transport of BDNF involves the binding of BDNF to trkB receptors to form a receptor-ligand complex that is then rapidly transported in vesicles to the cell body (Watson et al., 1999; Bhattacharyya et al., 2002). After castration, trkB protein is down-regulated in SNB motoneurons (Osborne et al., 2007), and if trkB is similarly down-regulated in SNB axons, it could also account for a decrease in the retrograde transport of BDNF. Testosterone treatment of castrated males maintains axonal transport times (Frolkis et al., 1990) and trkB protein expression (Osborne et al., 2007) in spinal motoneurons. If the source of BDNF in SNB somata is the BC musculature, the decrease in BDNF after local hydroxyflutamide treatment and increase in BDNF after local testosterone treatment suggests that the retrograde transport of BDNF in the SNB neuromuscular system is influenced by androgenic manipulations and specifically, by androgenic action at the BC muscle. Further studies can address the effects of gonadal steroids on the expression and retrograde transport of BDNF in the SNB neuromuscular system.
CONCLUSION
The current experiment demonstrates that BDNF protein expression in SNB motoneurons can be regulated by androgenic action in the BC musculature. Furthermore, these results demonstrate that androgenic action in the BC musculature is both necessary and sufficient for the regulation of BDNF in the SNB. Taken together with our previous findings (Verhovshek et al., 2010b), these results support the idea that androgens influence motoneuron morphology by acting at the target musculature to regulate target-derived neurotrophic signals that are then retrogradely transported to the motoneurons to exert their effects.
Furthermore, our results implicate BDNF as such a candidate neurotrophic signal (Yang et al., 2004), and its production, axonal transport, and androgenic regulation could also be relevant for a variety of injury paradigms. For example, treatment with testosterone is protective in surviving motoneurons after partial depletion (Fargo and Sengelaub, 2004a,b; Little et al., 2009) and after spinal cord injury (Byers et al., 2012), perhaps through regulation of BDNF via the target musculature. Testosterone and BDNF are critical in maintaining motoneuron morphology after nerve injury, and the effectiveness of application of BDNF to the cut nerve is consistent with a peripheral source and the importance of axonal transport (Yang et al., 2004). Therefore, our demonstration that the target musculature is a critical site of action for androgenic regulation of BDNF has potentially important implications not only for adult maintenance of motoneuron morphology and function but also for neurotherapeutic or protective actions after motoneuron injury or disease (Fargo et al., 2009).
Acknowledgments
Contract grant sponsor: NIH-NICHD; contract grant number: HD35315 (to D.R.S.).
The authors thank Lauren Rudolph and Dr. Cara Wellman for their comments on this manuscript.
References
- Aarnisalo P, Palvimo J, Janne O. CREB-binding protein in androgen receptor-mediated signaling. Proc Natl Acad Sci USA. 1998;95:2122–2127. doi: 10.1073/pnas.95.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews TJ, Thrasivoulou C, Nesbit W, Cowen T. Target-specific differences in the dendritic morphology and neuropeptide content of neurons in the rat SCG during development and aging. J Comp Neurol. 1996;368:33–44. doi: 10.1002/(SICI)1096-9861(19960422)368:1<33::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Araki I, Harada Y, Kuno M. Target-dependent hormonal control of neuron size in the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1991;11:3025–3033. doi: 10.1523/JNEUROSCI.11-10-03025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger A, Hexter D, McCarthy M. Sex differences in the phosphorylation of cAMP response element binding protein (CREB) in neonatal rat brain. Brain Res. 2001;890:110–117. doi: 10.1016/s0006-8993(00)03151-6. [DOI] [PubMed] [Google Scholar]
- Bartsch W, Knabbe C, Voigt K-D. Regulation and compartmentalization of androgens in rat prostate and muscle. J Steroid Biochem. 1983;19:929–937. doi: 10.1016/0022-4731(83)90036-5. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Watson F, Pomeroy S, Zhang Y, Stiles C, Segal R. High-resolution imaging demonstrates dynein-based vesicular transport of activated trk receptors. J Neurobiol. 2002;51:302–312. doi: 10.1002/neu.10062. [DOI] [PubMed] [Google Scholar]
- Brannstrom T, Kellerth JO. Changes in synaptology of adult cat spinal alpha-motoneurons after axotomy. Exp Brain Res. 1998;118:1–13. doi: 10.1007/s002210050249. [DOI] [PubMed] [Google Scholar]
- Breedlove S, Arnold A. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Byers J, Huguenard A, Kuruppu D, Liu N-K, Xu X-M, Sengelaub D. Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. J Comp Neurol. 2012;520:2683–2696. doi: 10.1002/cne.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz RR, Pastor AM, Delgado-García JM. Influence of the postsynaptic target on the functional properties of neurons in the adult mammalian central nervous system. Rev Neurosci. 1996;7:115–149. doi: 10.1515/revneuro.1996.7.2.115. [DOI] [PubMed] [Google Scholar]
- DiStefano P, Friedman C, Radziejewski C, Alexander C, Boland P, Schick C, Lindsay R, Wiegand S. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- Dreyfus CF, Dai X, Lercher L, Racey B, Freidman W, Black IB. Expression of neurotrophins in the adult spinal cord in vivo. J Neurosci Res. 1999;56:1–7. doi: 10.1002/(SICI)1097-4547(19990401)56:1<1::AID-JNR1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Fargo K, Sengelaub D. Exogenous testosterone prevents motoneuron atrophy induced by contralateral motoneuron depletion. J Neurobiol. 2004a;60:348–359. doi: 10.1002/neu.20027. [DOI] [PubMed] [Google Scholar]
- Fargo K, Sengelaub D. Testosterone manipulation protects motoneurons from dendritic atrophy after contralateral motoneuron depletion. J Comp Neurol. 2004b;469:96–106. doi: 10.1002/cne.10991. [DOI] [PubMed] [Google Scholar]
- Fargo K, Foster A, Harty M, Sengelaub D. Estrogen alters the excitability but not morphology of a sexually dimorphic neuromuscular system in adult rats. J Neurobiol. 2003;56:66–77. doi: 10.1002/neu.10224. [DOI] [PubMed] [Google Scholar]
- Fargo K, Foecking E, Jones K, Sengelaub D. Neuroprotective actions of androgens on motoneurons. Front Neuroendocrinol. 2009;30:130–141. doi: 10.1016/j.yfrne.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolkis V, Tanin S, Marcinko V, Muradian K. Effects of hormones on the fast axoplasmic transport of substances in ventral horns of the spinal cord in rats of different ages. Arch Gerentol. 1990;11:33–41. doi: 10.1016/0167-4943(90)90054-a. [DOI] [PubMed] [Google Scholar]
- Hamburger V. The development and innervation of transplanted limb primordia of chick embryos. J Exp Zool. 1939;80:347–389. [Google Scholar]
- Hamburger V, Levi-Montalcini R. Proliferation, differentiation and degeneration in the spinal ganglia of the chick embryo under normal and experimental conditions. J Exp Zool. 1949;111:457–501. doi: 10.1002/jez.1401110308. [DOI] [PubMed] [Google Scholar]
- Holmes G, Sachs B. Erectile function and bulbospongiosus EMG activity in estrogen-maintained castrated rats vary with behavioral context. Horm Behav. 1992;26:406–419. doi: 10.1016/0018-506x(92)90010-s. [DOI] [PubMed] [Google Scholar]
- Huguenard A, Fernando S, Monks D, Sengelaub D. Overexpression of androgen receptors in target musculature confers androgen sensitivity to motoneuron dendrites. Endocrinology. 2011;152:639–650. doi: 10.1210/en.2010-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino A, Ishige Y, Tatsuno T, Nakayama C, Noguchi H. BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp Neurol. 1997;144:273–286. doi: 10.1006/exnr.1996.6367. [DOI] [PubMed] [Google Scholar]
- Koliatsos V, Clatterbuck R, Winslow J, Cayouette M, Price D. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- Kuno M. Target dependence of motoneuronal survival: The current status. Neurosci Res. 1990;9:155–172. doi: 10.1016/0168-0102(90)90001-u. [DOI] [PubMed] [Google Scholar]
- Kurz E, Sengelaub D, Arnold A. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- Larionov A, Vasyliev D, Mason J, Howie A, Berstein L, Miller W. Aromatase in skeletal muscle. J Steroid Biochem Mol Biol. 2003;84:485–492. doi: 10.1016/s0960-0760(03)00059-1. [DOI] [PubMed] [Google Scholar]
- Leedy M, Beatiie M, Bresnahan J. Testosterone induced plasticity of synaptic inputs to adult mammalian motoneurons. Brain Res. 1987;424:386–390. doi: 10.1016/0006-8993(87)91484-3. [DOI] [PubMed] [Google Scholar]
- Little C, Coons K, Sengelaub D. Neuroprotective effects of testosterone on morphology and function of somatic motoneurons following the death of neighboring motoneurons. J Comp Neurol. 2009;512:359–372. doi: 10.1002/cne.21885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubischer J, Arnold A. Evidence for target regulation of the development of androgen sensitivity in rat spinal motoneurons. Dev Neurosci. 1995a;17:106–117. doi: 10.1159/000111279. [DOI] [PubMed] [Google Scholar]
- Lubischer J, Arnold A. Axotomy transiently down-regulates androgen receptors in motoneurons of the spinal nucleus of the bulbocavernosus. Brain Res. 1995b;694:61–68. doi: 10.1016/0006-8993(95)00766-j. [DOI] [PubMed] [Google Scholar]
- Matsumine H, Hirato K, Yanaihara T, Tamada T, Yoshida M. Aromatization by skeletal muscle. J Clin Endocrinol Metab. 1986;63:717–720. doi: 10.1210/jcem-63-3-717. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Hyodo S. Androgenic regulation of expression of B-tubulin messenger ribonucleic acid in motoneurons of the spinal nucleus of the bulbocavernosus. J Neuroendocrinol. 1993;5:357–363. doi: 10.1111/j.1365-2826.1993.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Prins G. Androgenic regulation of androgen receptor immunoreactivity in motoneurons of the spinal nucleus of the bulbocavernosus of male rats. J Neuroendocrinol. 1996;8:553–559. doi: 10.1046/j.1365-2826.1996.04899.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arnold A, Zampighi G, Micevych P. Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. J Neurosci. 1988;8:4177–4183. doi: 10.1523/JNEUROSCI.08-11-04177.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain DL, Hoke VB. The role of the cytoskeleton in cell body enlargement, increased nuclear eccentricity and chromatolysis in axotomized spinal motor neurons. Bio Med Central Neurosci. 2005;6:19. doi: 10.1186/1471-2202-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna K, Nadelhaft I. Organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- Monks D, O’Bryant E, Jordan C. Androgen receptor immunoreactivity in skeletal muscle: Enrichment at the neuromuscular junction. J Comp Neurol. 2004;473:59–72. doi: 10.1002/cne.20088. [DOI] [PubMed] [Google Scholar]
- Monks D, Kopachik W, Breedlove S, Jordan C. Anabolic responsiveness of skeletal meucle correlates with androgen receptor protein but not mRNA. Canadian J Physiol Pharmacol. 2006;84:272–277. doi: 10.1139/y05-157. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Osborne M, Verhovshek T, Sengelaub D. Androgen regulates trkB immunolabeling in spinal motoneurons. J Neurosci Res. 2007;85:303–309. doi: 10.1002/jnr.21122. [DOI] [PubMed] [Google Scholar]
- Ottem E, Beck L, Jordan C, Breedlove S. Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology. 2007;148:3655–3665. doi: 10.1210/en.2007-0308. [DOI] [PubMed] [Google Scholar]
- Pasupuleti V, Horton R. Metabolism of 5 alpha-reduced androgens by various tissues of the male rat. J Androl. 1990;11:161–167. [PubMed] [Google Scholar]
- Rand M, Breedlove S. Androgen locally regulates rat bulbocavernosus and levator ani size. J Neurobiol. 1992;23:17–30. doi: 10.1002/neu.480230104. [DOI] [PubMed] [Google Scholar]
- Rand M, Breedlove S. Androgens alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci. 1995;15:4408–4416. doi: 10.1523/JNEUROSCI.15-06-04408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph LM, Sengelaub DR. Critical period for estrogen-dependent motoneuron dendrite growth is coincident with ERα expression in target musculature. Dev Neurobiol. 2013;73:72–84. doi: 10.1002/dneu.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs B. Role of striated penile muscle in penile reflexes, copulation and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- Scarisbrick I, Isackson P, Windebank A. Differential expression of brain-derived neurotrophic factor, neurotrophin-3 and neurotrophin-4/5 in adult rat spinal cord: regulation by the glutamate receptor agonist kainic acid. J Neurosci. 1999;18:7757–7769. doi: 10.1523/JNEUROSCI.19-18-07757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh P, Hu S, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Smith ER, Damassa DA, Davidson JM. Hormone administration: Peripheral and intracranial implants. In: Meyer RD, editor. Methods in Psychobiology. New York: Academic Press; 1977. pp. 259–279. [Google Scholar]
- Sumner BEH, Watson WE. Retraction and expansion of the dendritic tree of motor neurones [sic] of adult rats induced in vivo. Nature. 1971;233:273–275. doi: 10.1038/233273a0. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold D, Shaywitz A, Greenberg M. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Tao X, West A, Chen W, Corfas G, Greenberg M. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002;33:383–395. doi: 10.1016/s0896-6273(01)00561-x. [DOI] [PubMed] [Google Scholar]
- Tonra J, Curtis R, Wong V, Cliffer K, Park J, Timmes A, Nguyen T, Lindsay RM, Acheson A, DiStefano P. Axotomy up-regulates the anterograde transport and expression of brain-derived neurotrophic factor by sensory neurons. J Neurosci. 1998;18:4374–4383. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng GF, Hu ME. Axotomy induces retraction of the dendritic arbor of adult rat rubrospinal neurons. Acta Anat (Basel) 1996;155:184–193. doi: 10.1159/000147803. [DOI] [PubMed] [Google Scholar]
- Tseng GF, Wang YJ, Hu ME. Axotomy affects the retrograde labeling of cervical and lumbar-cord-projecting rubrospinal neurons differently. Anat Embryol (Berl) 1996;194:457–464. doi: 10.1007/BF00185993. [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Buckley K, Sergent M, Sengelaub D. Testosterone Metabolites differentially maintain adult morphology in a sexually dimorphic neuromuscular system. Dev Neurobiology. 2010a;70:206–221. doi: 10.1002/dneu.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhovshek T, Cai Y, Osborne M, Sengelaub D. Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology. 2010b;151:253–261. doi: 10.1210/en.2009-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyvodic JT. Peripheral target regulation of dendritic geometry in the rat superior cervical ganglion. J Neurosci. 1989;9:1997–2010. doi: 10.1523/JNEUROSCI.09-06-01997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainman P, Shipounoff G. The effects of castration and testosterone propionate on the striated musculature in the rat. Endocrinology. 1941;29:975–978. [Google Scholar]
- Watson F, Heerssen H, Moheban D, Lin M, Sauvageot C, Bhattacharyya A, Pomeroy S, Segal R. Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. J Neurosci. 1999;19:7889–7900. doi: 10.1523/JNEUROSCI.19-18-07889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Arnold A. Interaction of BDNF and testosterone in the regulation of adult perineal motoneurons. J Neurobiol. 2000a;44:308–319. doi: 10.1002/1097-4695(20000905)44:3<308::aid-neu2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Yang L, Arnold A. BDNF regulation of androgen receptor expression in axotomized SNB motoneurons of adult male rats. Brain Res. 2000b;852:127–139. doi: 10.1016/s0006-8993(99)02225-8. [DOI] [PubMed] [Google Scholar]
- Yang L, Verhovshek T, Sengelaub D. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145:161–168. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]
- Yawo H. Changes in the dendritic geometry of mouse superior cervical ganglion cells following postganglionic axotomy. J Neurosci. 1987;7:3703–3711. doi: 10.1523/JNEUROSCI.07-11-03703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Donnelly DF, Song XJ, Lamotte RH. Axotomy increases the excitability of dorsal root ganglion cells with unmyelinated axons. J Neurophysiol. 1997;78:2790–2794. doi: 10.1152/jn.1997.78.5.2790. [DOI] [PubMed] [Google Scholar]