Abstract
Acute myeloid leukemia (AML) is a challenging disease to treat with the majority of patients dying from their illness. While overall survival has been markedly prolonged in acute promyelocytic leukemia (APL), survival in younger adults with other subtypes of AML has only modestly improved over the last twenty years. Physicians who treat AML eagerly await drugs like Imatinib for chronic myeloid leukemia, Cladribine for hairy cell leukemia, and Rituximab for non-Hodgkin Lymphoma which have had an important impact on improving outcome. Recent research efforts have focused on refining traditional chemotherapeutic agents to make them more active in AML, targeting specific genetic mutations in myeloid leukemia cells, and utilizing novel agents such as Lenalidomide that have shown activity in other hematologic malignancies. Here, we focus on reviewing the recent literature on agents that may assume a role in clinical practice for patients with AML over the next five years.
Keywords: Acute Myeloid Leukemia, novel drugs, emerging agents
INTRODUCTION
In 2010, approximately 12,000 people in the United States will be diagnosed with acute myeloid leukemia (AML). Of those, 8,590 are predicted to die of their disease. Standard induction treatment for AML since the 1970s has been chemotherapy with an anthracycline and cytarabine with the goal of achieving a complete hematologic remission (CR). Following achievement of CR, further courses of chemotherapy or allogeneic hematopoietic cell transplantation (HCT) are undertaken to “consolidate” the remission and achieve long-term cure. Although overall survival (OS) among adults with AML has improved for patients under age 60 during the last 4 decades, the majority of such younger patients continue to die from their disease. In addition, as risk stratification of patients by cytogenetics and molecular genetics has been refined, it is clear that a subset of patients with poor-risk cytogenetic and molecular mutations have long-term OS of less than 10%. In addition, patients over age 60 have a distinctly poor prognosis; the 5-year survival is 10% or less. Much of the driving force behind the improved OS in AML among younger patients is attributable to more intensive consolidation chemotherapy and allogeneic HCT. Disappointingly, there has been essentially no improvement in the outcome of older adults during the last 4 decades. If any slight improvement has occurred, it is likely attributable to better supportive care: newer antimicrobials, and better anti-fungals. It is clear that new drugs are needed for AML.
Aside from the disappointing lack of highly effective new drugs for AML, the heterogeneity of the AML patient population makes drug development difficult; a drug that produces exciting results in a young patient may not work in an older patient. In addition AML is typically a disease of older adults many of whom have comorbid medical conditions that make them unable to tolerate treatment regimens with multiple toxicities. Finally, a drug that works in the “up front” setting may not show activity in the relapsed or refractory setting. In this review we outline new agents and novel formulations of old medications currently in early and late phase clinical trials.
CHEMOTHERAPY
Elacytarabine
Elacytarabine is an analogue of the traditional cytotoxic chemotherapeutic drug Cytarabine with a novel mechanism of cellular uptake. Traditional Cytarabine depends on Human Equilibrative Nucleoside Transporter 1 (hENT1) for cellular entry. This renders Cytarabine ineffective in entering the targeted myeloblast of AML if there is down regulation or absence of hENT1 on the cellular surface. Elacytarabine is a lipid conjugated derivative of Cytarabine that enters the cell independent of hENT1 cell surface expression. Once Elacytarabine enters the cell its lipid tail, which allowed cellular entry, is cleaved and the parent drug becomes active. In a multicenter phase II study, 61 patients with either refractory or relapsed AML (with duration of CR lasting less than 6 months) were given 2000mg/m2 of Elacytarabine as a continuous infusion on days 1–5 once every three weeks. Results were compared with historical controls. CR or CRp was attained in 9 out of the 61 patients for an overall remission rate of 15%. The most frequently reported adverse events of grade 3 or higher were hematologic toxicity, with thrombocytopenia, neutropenia, anemia and leukopenia. The duration of these adverse events was not reported. Thirty day all cause mortality was 13%1. A second phase II study of Elacytarabine in combination with Idarubicin for patients refractory to an initial induction regimen containing traditional cytarabine is currently accruing with the primary outcome being rates of complete remission. Secondary outcomes include disease-free survival (DFS) and event (death)-free survival (EFS).
Based on the results of the initial phase II study showing a 15% CR rate in a relapsed/refractory AML population, a large international randomized phase III clinical trial of Elacytarabine versus investigator’s choice in relapsed/refractory AML is currently accruing with the goal to enroll 350 patients. Primary outcome is OS at 6 months. The trial is estimated to be completed in March, 2012.
CPX-351 (Liposomal Carrier Containing Cytarabine and Daunorubicin)
An anthracycline and Cytarabine are traditionally given as initial induction chemotherapy for AML. Theoretically, this drug combination may act synergistically, additively, or antagonistically resulting in varying drug ratios in vivo and perhaps suboptimal intracellular drug concentrations. Because of this concern, CPX-351, a liposomal carrier containing Cytarabine and Daunorubicin in a fixed 5:1 ratio, was developed; each liposomal unit contains 1mg of Cytarabine and 0.44 mg of Daunorubicin. Pre-clinical in-vitro studies showed avoidance of antagonism [1]. In addition, in vivo mouse models showed that the 5:1 molar ratio was maintained in mouse plasma and bone marrow for greater than 24 hours and leukemia bearing mice survived longer than those mice given conventional Cytarabine and Daunorubicin [2]. Based on the pre-clinical data, a phase I study was undertaken in a relapsed/refractory AML patient population to identify the maximum tolerated dose, evaluate the persistence of the 5:1 molar ratio in vivo and determine the CR rate [3]. Forty-eight patients were enrolled in the study. The maximum tolerated dose was 101 units/m2 with no patients at this dose level experiencing severe drug-related toxicity. Of the 48 patients enrolled in the study, 26 died of progressive leukemia within the first 30 days of treatment. In all, nine CRs and one CRp were observed.
CPX-351 has also been evaluated as possible induction therapy for older adults with AML. In a phase IIB trial reported at the American Society of Hematology Meeting in 2010, patients between the ages of 60 and 75 were randomized in a 2:1 fashion to receive either standard induction chemotherapy (60mg/m2 of Daunorubicin for three days and 100mg/m2 of Cytarabine for seven days) or CPX-351, 100mg/m2 on days 1, 3 and 5. Primary endpoints were rates of CR and CRi while secondary endpoints were OS at one year, EFS, 30-day mortality and 60-day mortality2.
Compared to standard Daunorubicin and Cytarabine induction chemotherapy, CPX-351 increased the CR and CRp rate from 51.2% to 66.7%, largely attributable to increased CRp in the CPX-351 group. Higher response rates were particularly seen in patients greater than 70 years old (33.3% vs 64.3%) and in those with adverse cytogenetics (38.5% vs. 76.2%). More adverse events related to cytopenias (febrile neutropenia, ecchymoses and petechiae) were seen in the CPX-351 group. In addition the mean time to count recovery was longer in the CPX-351 group. Hazard ratio for OS was 0.83 in the CPX-351 group, but was not statistically significant (p = 0.24)3.
The results of a phase IIb open label randomized trial between CPX-351 and “Investigator’s choice” for AML in first relapse was reported in late 2011. Median overall survival was 8.3 months in the patients receiving CPX-351 versus 6.3 months in control patients4.
NEW CHEMOTHERAPY AGENTS
Clofarabine
The poor prognosis for older individuals (usually defined as those older than 60 years old) with AML is due to poorer risk cyto and molecular genetics, inherent resistance, and the inability of older adults with comorbidities to tolerate intensive chemotherapy. New chemotherapeutic agents with fewer side effects and reduced doses of older agents are being investigated in older patients.
Clofarabine is a second generation purine nucleoside analog designed to incorporate the qualities of Fludarabine and Cladribine without the dose-limiting neurotoxicity. A number of clinical trials in various phases of design are seeking to incorporate the use of Clofarabine in the treatment of both younger and older adults with AML both as single agent therapy and in combination with other drugs active in AML.
Initially, Kantarjian and colleagues, in a phase II clinical trial, evaluated the efficacy of clofarabine monotherapy in relapsed and refractory AML, MDS, ALL and CML in blastic phase [4] clofarabine was given at a dose of 40mg/m2 on days 1–5 every three to six weeks depending on response and count recovery. Patients who failed to achieve an objective response after two courses of induction with clofarabine were removed from the study. Those who did respond were allowed to continue with up to six cycles of consolidation given once every 28 days. 31 of the 62 patients enrolled had AML. Of those, 17 (55%) had a CR or CRp. Median overall survival for the entire cohort (including those with MDS, ALL and blastic phase CML) was slightly greater than 7 months.
Faderl and colleagues, sought to improve the results demonstrated by Kantarjian by combining clofarabine with other agents and in 2005 published the results of a phase I/II study of clofarabine in combination with intermediate dose cytarabine (1g/m2) in patients with relapsed or refractory AML, ALL, high risk MDS or CML in blast phase [6] Of the AML patients, only those in first relapse or with primary refractory disease (two cycles of previous chemotherapy without a remission) were allowed to enroll on the trial. Clofarabine was administered on days 2–6 and cytarabine was administered on days 1–5 on an every 3 to 5 week schedule (depending on response and count recovery). Clofarabine at a dose of 40mg/m2 was established as the maximum tolerated dose in combination with cytarabine, based on previous phase I studies using clofarabine as monotherapy. Of the 25 patients with AML, the overall response rate (CR and CRp) was 40%. Median survival of all patients was 5.5 months.
To follow up, Faderl and colleagues published the results of a phase II trial of clofarabine and intermediate dose cytarabine (1g/m2) administered to previously untreated adults older than age 50 with AML or high risk MDS with the same dosage schedule as described in their original paper. Of the 60 patients enrolled, 54 (90%) had AML. Among all patients the response rate (CR and CRp) was 60% and the median overall survival in the intention to treat population was 10.3 months. Those patients that achieved a CR had a median survival of 23.5 months [7].
Faderl subsequently conducted an adaptive randomized study of clofarabine, versus clofarabine and low dose, subcutaneous cytarabine in a population of patients older than 60 years of age who had never been treated with intensive chemotherapy [8]. The 70 patients enrolled on the trial were given clofarabine at a dose of 30mg/m2 on days 1–5, while in the combination arm, cytarabine was given at a dose of 20mg/m2 subcutaneously on days 1–14. Patients with complete remission or CRi were eligible for consolidation treatment. In addition, patients with partial response, stable disease or hematologic response after one cycle were allowed a second cycle of induction chemotherapy. If CR or CRi was achieved after the second cycle, these patients could also enter the consolidation phase of the trial. In consolidation, the monotherapy arm was given Clofarabine at a dose of 30mg/m2 on days 1–3 and 20mg/m2 of Cytarabine was administered on days 1–7. Sixteen patients were randomized to the Clofarabine monotherapy arm and 54 patients were randomized to the Clofarabine and Cytarabine combination arm. Overall response rate (CR + CRi) was 67% vs. 31% in the Clofarabine vs. combination arms respectively. Response rates were higher in those patients with normal cytogenetics and those that had no FLT-3 mutations. Median overall survival was 11.4 months on patients in the combination arm and 5.8 months for patients on the monotherapy arm.
The MD Anderson group proceeded to perform the Classic II study, a multicenter phase II trial of Clofarabine monotherapy in an untreated group of older adults with at least one of four poor prognostic factors: age > 70, ECOG performance status of 2, presence of an antecedent hematologic disorder or an intermediate or unfavorable karyotype (as defined by Bloomfield in 1998) [9] 30mg/m2 of Clofarabine was administered on days 1–5 of cycle 1. Those who had stable disease but no remission could receive a second cycle of induction with a dose of 20mg/m2 of Clofarabine [10]. Those with a documented CR or CRp after induction were allowed to receive a maximum of six subsequent cycles of consolidation chemotherapy with Clofarabine at a dose of 20mg/m2 given once every 28 days. The overall response rate (CR + CRp) was 46% in this patient population with unfavorable baseline characteristics. Median overall survival was 41 weeks for all patients, 59 weeks for patients with a CR or CRp and 72 weeks for patients who achieved a CR.
In two similar European studies, whose results were published together in 2010, older, untreated patients deemed unsuitable for intensive chemotherapy were given Clofarabine at a dose of 30mg/m2 on days 1–5 which was repeated every 28–42 days for four to six cycles. Of the 106 patients enrolled the overall response rate (CR + CRi) was 48%. With a median follow up of 31 months, the median overall survival was 19 weeks [11].
In the only phase III study of Clofarabine reported to date, Faderl and colleagues at the ASCO 2011 meeting randomized adults older than 55 years old with relapsed or refractory AML to Clofarabine 40mg/m2 and Cytarabine 1g/m2 or Cytarabine 1g/m2 with placebo5. Although overall response rate and event free survival were increased in the combination arm, disappointingly, this did not translate into a difference in overall survival.
mTOR Inhibition
The PI3K/AKT signaling pathway can be dysregulated in AML and contribute to the leukemic phenotype through activation of the mTOR kinase and its downstream effectors. In a phase I dose escalation trial published in 2009 patients with relapsed, refractory or secondary AML were given mitoxantrone, etoposide and cytarabine (MEC) concurrent with sirolimus, an mTOR inhibitor [12]. Of 27 subjects enrolled in the trial, 22% achieved a complete or partial remission. However, the synergy of MEC and sirolimus could not be confirmed in correlative laboratory studies.
Two studies reported at the American Society of Hematology annual meeting in 2010 sought to combine mTOR inhibitor with other agents. In a phase II trial, temsirolimus was combined with low dose clofarabine (20mg/m2) in patients over the age of 60 with relapsed or refractory AML. Of the 54 patients enrolled in the trial, 11 achieved a CR or CRi for a response rate of 21%6.
In a separate phase I b/II study everolimus was combined with azacitidine in patients (all ages) with relapsed or refractory AML. Of 20 evaluable patients, the overall response rate was 36%7. Currently two trials with mTOR inhibitors are actively recruiting patients with AML. The first seeks to combine everolimus with Midostaurin, while the second again seeks to combine MEC with sirolimus in patients with high risk AML.
Sapacitabine
Sapacitabine is a rationally designed prodrug of 2′-C-Cyano-2′-deoxy-B-D-arabino-pentofuranosylcytosine (CNADC), a nucleoside analogue. Based on promising pre-clinical data, a total of 47 patients with AML or MDS either in the relapsed/refractory setting or with never treated disease were enrolled in a phase I clinical trial using a classical 3+3 design. Of these patients, 17 percent had received no prior treatment and 23 percent had received four or more salvage therapies. Doses between 150 mg twice a day and 375mg twice a day were used for seven consecutive days for 3–4 weeks until the maximum tolerated dose was established. Ultimately the maximum tolerated dose recommended for a phase II study was 425mg orally twice a day on days one to three, weekly for two weeks, based on severe thrombocytopenia seen in one patient on a higher dose of 475mg twice a day [13].
Of the 47 patients evaluated, 13 achieved an objective response; four with a CR, two with a CRp, and seven with a CRi. Of these, 6 were in second salvage or beyond. In addition, two of the responders were receiving Sapacitabine as frontline therapy for their disease. The most common drug related toxicities were gastrointestinal, fatigue and cytopenias. Of the entire study group, the estimated four week mortality was 4%.
Based on these findings three trials are underway to establish the best clinical use of Sapacitabine. One of these is a phase III trial of patients aged 70 years and older with newly diagnosed AML who are not candidates for intensive cytotoxic chemotherapy. This three armed trial compares the use of Sapacitabine and Decitabine given in alternating cycles to Sapacitabine alone and Decitabine alone. The primary endpoint of the trial is overall survival and the anticipated completion date is March, 2014.
Vosaroxin
Vosaroxin is a novel quinolone derivative that acts as a topoisomerase II inhibitor that has been investigated in a phase Ib study in patients with relapsed or refractory leukemia (AML, ALL, high risk MDS or CML in blast crisis) [14]. 75 patients were enrolled of which 62 had a diagnosis of AML. Patients were placed into one of two study groups; in the first patients were given Vosaroxin once weekly for 3 weeks, while in the second group patients were treated twice weekly with Vosaroxin each given in a 28 day cycle. The most common dose limiting toxicity was stomatitis. 22% of patients achieved a morphologic leukemia free state (less than 5% bone marrow blasts). Five patients had a documented CR or CRp. Currently, a randomized phase III trial is underway evaluating the cytarabine and Vosaroxin versus cytarabine and placebo for replaced or refractory AML.
TARGETED THERAPIES
FLT-3 Inhibition
FMS–like tyrosine kinase three receptors (FLT-3 receptors) play a role during normal hematopoiesis in regulating normal differentiation and proliferation of cellular progenitors. FLT -3 mutations, either in the form of internal tandem duplications (in which between 3 and 60 amino acids are repeated in the juxtamembrane or tyrosine kinase domain) or single point mutations that most often occur in the activating loop allow growth factor independent growth of leukemic cells. Recent studies have demonstrated that patients harboring normal cytogenetics but FLT-3 mutations have a distinctly poor prognosis. In addition, the favorable prognosis of patients with normal cytogenetics but a nucleophosmin one mutation (NPM-1) is abrogated by a FLT-3 mutation.
Midostaurin
Because of the poor prognosis of FLT-3 mutant patients, development of FLT-3 mutant inhibitors are ongoing. Midostaurin (PKC412) is an inhibitor of FLT-3 as well as c-KIT, VEGFR-2 and PDGF. In a phase IIb trial, high risk MDS patients, patients with relapsed/refractory AML, or those ineligible to receive standard chemotherapy were assigned to receive single agent oral Midostaurin at a dose of 50 or 100 mg twice daily. Of note, patients harboring either FLT-3 mutant or FLT-3 wild-type were eligible to enroll in the study [15].
95 patients enrolled in the trial. 37% of the patients had FLT-3 mutations and of these FLT-3 mutants, 74 percent were internal tandem duplications. Outcomes included CRs, partial remissions, hematologic improvement and blast responses. No patient in either the FLT-3 mutant or wild type groups experienced a CR. One patient with a FLT-3 mutation experienced a partial remission. 46% of patients with FLT-3 mutations and 35% of patients with wild type FLT-3 achieved hematologic improvement as defined by the guidelines for improvement in myelodysplastic syndromes. Blast reductions occurred in 71% of patients with FLT-3 mutations and 42% of patients with wild type FLT-3. Of these, 49% of FLT-3 mutants and 33% of wild type FLT-3 achieved a 2-log reduction in blast count. Median survival estimates were 100 days in the mutant group and 159 days in the wild type population. The most common adverse event thought to be related to Midostaurin was moderate nausea and vomiting.
With no patients achieving a CR the results of this phase II study initially appeared disappointing. However, the population of relapsed/refractory patients studied has an extremely poor prognosis with conventional chemotherapy. In addition, responses with any single agent therapy and especially one that is not myelosuppressive chemotherapy is an achievement and suggests that FLT-3 inhibition in combination with traditional chemotherapeutic regimens may result in greater overall survival in the FLT-3 mutant population when used as initial treatment.
Based on the phase II trial, a phase III randomized, double blind intergroup trial of induction chemotherapy with Daunorubicin, Cytarabine and Midostaurin versus Daunorubicin and Cytarabine alone is underway in patients with FLT-3 mutations led by the Cancer and Leukemia Group B (CALGB – protocol C10603). In those patients achieving a CR, consolidation therapy with high-dose cytarabine with or without Midostaurin for up to four cycles is prescribed. The primary outcome is OS. A potential confounding factor in enrolling patients on this trial is that the standard for FLT-3 mutated AML is shifting from consolidation with high-dose cytarabine alone to referral for allogeneic HCT in first complete remission (CR1). Therefore, the placebo arm of this trial may be receiving consolidation treatment that is inferior to allogeneic HCT.
AC-220
Like Midostaurin, AC-220 is an oral receptor tyrosine kinase inhibitor with potent in vitro and in vivo activity in FLT-3 and KIT dependent tumor cell lines (Fig. 2). This is a so-called “second generation” FLT-3 inhibitor because of its greater inhibition of FLT-3 mutants than first generation agents such as Midostaurin. In a phase I trial, AC-220 was administered in relapsed/refractory AML patients regardless of FLT-3 mutation status8.
Fig. 2.
Structure of selected small molecules being investigated for use in AML.
In this heavily pretreated group of patients, 12% had a CR and 18% of patients had a partial response. 56% of FLT-3 internal tandem duplication patients were responders while 19% of patients with wild type FLT-3 responded. Of note 4 out of 6 patients with FLT-3 ITD responded at the 200mg dose. Based on the results of this phase I study a phase II international study is actively recruiting patients with AML in second relapse (less than 60 years old), first relapse (greater than 60 years old) or with refractory disease (all patients) to be given at a dose of 200mg continuously on a 28 day treatment cycle until disease progression. Unlike Midostaurin, single agent AC-220 was able to induce a CR in heavily pretreated patients, generating the exciting possibility of durable CRs in FLT-3 positive patients.
Sorafenib
Sorafenib is a multikinase inhibitor that is known to target kinases such as Raf, VEGF, PDGFR-B, KIT, FLT-3 and RET (Fig. 2). Given the poor outcome of patients with FLT-3 mutated normal karyotype AML, a number of studies have been undertaken seeking to utilize Sorafenib, either in combination with traditional cytotoxic chemotherapy or alone.
In a phase II trial, 51 patients with newly diagnosed AML were given induction therapy consisting of Idarubicin 12mg/m2 for three days, cytarabine 1.5g/m2 for four days and Sorafenib 400mg twice a day for the first seven days of induction. Consolidation consisted of Idarubicin and cytarabine (up to five cycles) with the addition of Sorafenib 400mg twice a day for up to 28 days, and after consolidation patients with a CR were maintained on 400mg of Sorafenib twice a day for up to a total of one year of Sorafenib therapy.
Of the 51 patients enrolled in the phase II portion of this study 15 had FLT-3 mutations and 38 (75%) achieved a CR. More interestingly 14 of 15 patients with FLT-3 mutations achieved a CR and one out of 15 achieved a CRp. At 62 weeks, 10 of the FLT-3 mutant patients had progressed while 5 remained in CR. By just more than 72 weeks, all of the FLT-3 patients had progressed. Correlative studies demonstrated that FLT-3 inhibition was achieved in those patients taking Sorafenib. The exact role of Sorafenib in the treatment of FLT-3 mutant AML continues to be studied in ongoing clinical trials [16].
In a separate phase II German multicenter study published in abstract form, Sorafenib or placebo was added to standard chemotherapy in patients over the age of 60. Patients were given up to two cycles of standard induction chemotherapy with daunorubicin and cytarabine and consolidated with two cycles of intermediate dose cytarabine. Between cycles patients were randomized to 400mg of Sorafenib twice a day or placebo for up to one year after the initiation of induction9.
In the final analysis, both EFS and OS were similar in both trial arms. Median EFS was 7 months in the placebo arm and 5 months in the Sorafenib arm and median OS was 15 months in the placebo arm and 13 months in the Sorafenib arm. Patients with wild type NPM1 had a non-significant trend towards decreased EFS in the Sorafenib arm. No CR, EFS or OS differences were noted in the 14.2% of patients with FLT-3 ITD mutations.
MIXED LINEAGE LEUKEMIA (MLL) TRANSLOCATION INHIBITORS
Dot 1L
Disrupter of telomeric silencing (DOT1) and its mammalian homolog Dot 1-like (Dot1l) is a histone methyltransferase strongly associated with leukemias that arise from translocations of the mixed lineage leukemia (MLL) gene. In a critical study, Dot1L was shown to be required for leukemic transformation in cells harboring MLL fusion proteins [17]. In addition, a recent study has demonstrated that Dot 1L is required for the transformation of MLL-AF9 translocated cells into a leukemic clone, making inhibition of a DOT1L an attractive therapeutic target for AML with the MLL translocations. Indeed, at least one pharmaceutical company (Epizyme) has developed a small molecule inhibitor of Dot1L. In a recently published paper, this inhibitor of DOT1L was shown to be effective in a mouse xenograft model of MLL rearranged leukemia [18]
Gemtuzumab Ozogamicin
Gemtuzumab Ozogamicin (GO) is an anti CD-33 monoclonal antibody linked to a cytotoxic agent that was initially given accelerated approval by the United States Food and Drug Administration for the treatment of patients over age 60 with relapsed AML. Subsequently a phase III study conducted by the Southwest Oncology Group (S0106) randomizing patients to Daunorubicin and Cytarabine with or without Gemtuzumab, showed no improvement in overall survival and an increase in deaths in the Gemtuzumab group during induction therapy. This led Pfizer, to withdraw GO from the US market in 2010.
Two clinical trials reported at the American Society of Hematology meeting in 2011 have revived interest in Gemtuzumab. In the first, patients aged 50–70 years old with previously untreated AML were randomized to receive induction chemotherapy with Daunorubicin 60mg/m2 on days 1–3 and Cytarabine 200mg/m2 on days 1–7 with or without fractionated GO on days 1,4 and 7 at a dose of 3mg/m2. Patients achieving a CR or CRp, received two courses of consolidation chemotherapy with Daunorubicin 60mg/m2/day on day 1 and Cytarabine 1g/m2/day on days 1–4 with or without GO 3mg/m2 on day 1. The primary study objective was event free survival and the secondary objective was overall survival10.
After two years of follow up, event free survival was 41.4% in the arm that received GO and 15.6% in the arm that did not receive GO. Median OS was 25.4 months in the GO arm and 15.3 months in the non-GO arm. After adjustment for cytogenetics and favorable genotype the GO arm was associated with increased event free survival but not overall survival. 3 cases of liver veno-occlusive disease were observed in the GO arm.
The results of the UK NCRI AML 16 randomized trial were also reported this year. In this study, older patients received induction chemotherapy with or without GO at a dose of 3mg/m2 on day 1 of induction. OS at 2 years was 35% in the GO arm and 29% in the non-GO arm. This survival benefit was most robust in those patients with de novo disease or intermediate cytogenetics11.
HYPOMETHYLATING AGENTS
Decitabine and Azacitidine
It has emerged that methylation of cytosine by DNA methyltransferases leads to silencing of tumor suppressor genes during hematopoiesis. This silencing is theorized to inhibit the normal differentiation of hematopoietic cells. Both Decitabine and Azacitidine are nucleoside analogues that induce hypomethylation of DNA to overcome the differentiation block present in myelodysplastic syndromes (MDS).
Because older adults with MDS generally tolerate Decitabine and Azacitidine, a number of studies have been reported seeking to use these hypomethylating agents in older adults with AML. In a phase II study, Decitabine was given to 55 patients older than age 60 at a dose of 20mg/m2 daily for five days on a four week cycle [19] Response was assessed by bone marrow aspirates collected after every cycle beginning with cycle 2.
In the intention to treat population 24% of patients had a morphologic CR and 15% achieved a cytogenetic CR. Median time to achieve a CR was 126 days or 4.5 cycles. Interestingly, two patients who presented with leukemia cutis had complete resolution of skin lesions. 29% of patients had stable disease or improved blast counts. Median overall survival in the intention to treat population was 7.7 months. Median survival for those who achieved a CR was 14 months.
A recent randomized phase III trial comparing decitabine 20mg/m2 on days 1–5, every 4 weeks versus “patient’s choice with physician’s advice” of supportive care or low dose cytarabine (20mg/m2). 485 patients were enrolled in the trial and 242 with equal randomization between the two arms. In the protocol specified final analysis a statistically non-significant trend favored overall survival in the Decitabine group (7.7 months vs. 5.0 months). Interestingly, in an updated unplanned overall survival analysis showed the same median overall survival that became statistically significant [20].
In a separate phase III trial, azacitidine was evaluated as treatment for patients with intermediate-II and high risk MDS. One third of the patients evaluated were classified as having refractory anemia with excess blasts in transformation (RAEB-t). This old classification of the FAB system includes patients with 20%–30% blasts in the marrow, a group now defined by the WHO classification scheme as having full blown AML. Because of this a subgroup analysis of this RAEB-t group was published in 2010 [21].
In this study 55patients with greater than 20% marrow blasts were assigned to azacitidine and 58 patients were randomly assigned to a conventional care regimen (CCR) that consisted of one of three options: best supportive care, low dose Cytarabine, or intensive chemotherapy.
After a median follow up of 20.1 months, median OS in the azacitidine group was 24.5 months versus 16 months for the CCR. This was largely driven by a significant difference in OS favoring azacitidine versus best supportive care. No significant difference was observed between azacitidine and low dose Cytarabine.
Currently a multicenter, international phase III clinical trial of Azacitidine vs. physician’s choice of treatment (standard induction chemotherapy, low dose cytarabine or best supportive care) in newly diagnosed patients over 65 is enrolling patients.
OTHER AGENTS WITH NOVEL MECHANISMS OF ACTION
Lenalidomide
Lenalidomide, a newer agent related to Thalidomide and used primarily in the treatment of plasma cell dyscrasias has been found to be particularly useful in patients with MDS and a 5q deletion. A phase II study sought to explore the safety and efficacy of high dose single agent Lenalidomide in previously untreated older patients with AML and deletion 5q. In this study, 37 patients older than 60 years of age were given Lenalidomide at a dose of 50mg per day for up to 28 days as induction therapy. Of these patients 51% had a prior diagnosis of MDS. Of the evaluable patients, five achieved CR, CRi or PR. All of these patients received the full course of induction therapy. Of these five, three relapsed, one died fifteen months after CR without relapse and one was alive 19 months after achieving a PR [22].
Unfortunately, toxicity was seen in those patients who received induction treatment. Only 14 of the patients initially enrolled were able to complete induction therapy and of these only 8 initiated maintenance Lenalidomide. Of the 23 patients who did not complete induction, three died secondary to adverse events thought to be possibly related to therapy, two died secondary to disease progression and two died secondary to adverse events not related to therapy.
In a separate phase II study, Fehringer, et. al. sought to evaluate the efficacy of Lenalidomide in older patients with AML without 5q abnormalities [23]. Patients 60 years of age or older who had not received prior AML therapy and did not harbor an isolated 5q abnormality were eligible for enrollment. Lenalidomide at 50mg/day was given for one 28 day cycle. If patients had a CR or CRi they were started on low dose maintenance Lenalidomide at 10mg per day for up to 12 cycles (each cycle lasting 28 days). Patients who failed to achieve a CR or CRi after high dose cycle 1 were allowed an additional high dose cycle. Patients with stable disease, CR or CRi after high dose cycle two, were eligible for low dose maintenance Lenalidomide at 10mg/day.
Thirty three patients were enrolled in the study. 70% had de novo AML and 30% had either AML transformed from MDS or secondary AML after receiving prior chemotherapy. Of the patients analyzed, 30% achieved a CR or CRi. Interestingly, only patients who had a presenting white blood count less than 10,000/ul and a peripheral blast count less than 1000/ul achieved a CR/CRi. Using statistical testing, the peripheral blast count, presenting white blood count, and bone marrow blast percentage were all predictive of response to Lenalidomide.
The median OS in the patients that received at least one dose of Lenalidomide was four months, but for the 19 patients who completed high dose Lenalidomide the median OS was 11 months. Patients who achieved a CR or CRi had a median OS of 16 months (Fig. 1) [23]. Almost all patients (91%) had a grade 3 or greater toxicity and 24% of patients needed to discontinue therapy because of an adverse event.
Fig. 1. Lenalidomide.
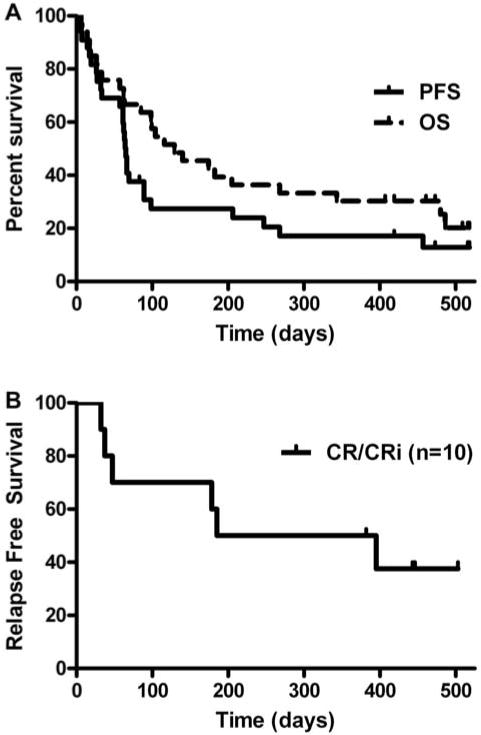
Kaplan-Meier survival curves. (A) Kaplan-Meier curves for OS and PFS in all patients who received at least one dose of lenalidomide (N = 33). Median OS estimated at 4 months (95% CI, 3–9 months), and median PFS estimated at 2 months (95% CI, 2–3 months). (B) Kaplan-Meier curves for relapse-free survival in patients who obtained a CR/CRi (n = 10). Median relapse-free survival for the CR/CRi patients was 10 months (95% CI, 2-NE(Fehniger T A et al. Blood 2011;117:1828–1833)
Currently a number of phase II trials are in progress to investigate the use of Lenalidomide, either in combination with traditional Daunorubicin and Cytarabine chemotherapy or as maintenance therapy for patients (often older than age 65) who are unable to tolerate traditional cytotoxic chemotherapy.
VORINOSTAT
Vorinostat is a novel histone deacetylase inhibitor that has been approved for use in the United States for cutaneous T-cell lymphoma. Its unique mechanism of action has prompted interest in its use for a wide range of hematologic malignancies. In a phase II trial, Vorinostat was given in combination with Idarubicin and Cytarabine for patients with AML and higher risk MDS. Patients who achieved a remission were allowed to receive up to five rounds of consolidation chemotherapy followed by maintenance therapy with single agent Vorinostat for up to 12 months.12
With a median follow up of 82 weeks, the median OS was 82 weeks and the median event free survival was 47 weeks. Gastrointestinal toxicity was common including diarrhea and nausea in 72% and 65% of patients respectively.
CONCLUSION
New therapeutic agents that improve OS for patients with AML, particularly those who are elderly, infirm or otherwise not candidates for intensive cytotoxic chemotherapy are desperately needed. Drugs that effectively target a specific protein product of a specific genetic mutation remain among the most promising in other leukemias and intensive efforts are being made to find a similar drug or group of drugs for AML. In addition the reformulation of old drugs, such as Elacytarabine and CPX-351, may lead to incremental benefits in OS for patients with AML (Table 1). Investigators continue to look for new molecular pathways that can be targeted to produce durable CRs and improve OS.
Table 1.
Summary of selected novel agents in clinical trials.
| Agent | Mechanism | Comments |
|---|---|---|
| Elacytarabine [1] | Elaidic Ester of Ara-C | Modest remission in advanced disease |
| CPX-351 [4,5] | Fixed ratio Dauno/Ara-C | High Response Rate |
| Clofarabine [7–10, 13, 14] | Nucleoside Analogue | Tolerated in older adults |
| Dot1L inhibitor [24,25] | Histone Methyltransferase | Encouraging Pre-Clinical data |
| Sorafenib [22,23] | Multi-Kinase Inhibitor | CR with single agent |
| AC 220 [21] | FLT-3 Inhibitor | CR with single agent |
| Sirolimus/Everolimus [15–17] | mTOR inhibitor | CR with single agent |
| Sapacitabine [18] | Nucleoside Analogue | CR with single agent |
Acknowledgments
Declared None.
ABBREVIATIONS
- AML
Acute myeloid leukemia
- APL
Acute promyelocytic leukemia
- CR
Complete hematologic remission
- CRi
Complete remission with incomplete count recovery
- OS
Overall survival
- DFS
Disease-free survival
- EFS
Event (death)-free survival
- HCT
Hematopoietic cell transplantation
- hENT1
Human Equilibrative Nucleoside Transporter 1
- CNADC
2′-C-Cyano-2′-deoxy-B-D-arabino-pentofuranosylcytosine
- GO
Gemtuzumab Ozogamicin
Footnotes
O’Brien, S.; Rizzieri, D. A.; Vey, N.; Ravandi, F.; Krug, U. O.; Sekeres, M. A.; Dennis, M.; Venditti, A.; Jacobsen, T. F.; Staudacher, K.; Nilsson, B. I.; Giles, F. J. A Phase II Multicentre Study with Elacytarabine as Second Salvage Therapy in Patients with AML. ASH Annual Meeting Abstracts, 2009, 114, 1042.
Lancet, J. E.; Cortes, J. E.; Hogge, D. E.; Tallman, M.; Kovacsovics, T.; Damon, L. E.; Ritchie, E.; Komrokji, R. S.; Louie, A. C.; Feldman, E. J. Phase 2B Randomized Study of CPX-351 Vs. Cytarabine (CYT) + Daunorubicin (DNR) (7+3 Regimen) In Newly Diagnosed AML Patients Aged 60–75. ASH Annual Meeting Abstracts, 2010, 116, 655.
Tyner, J. W.; Tardi, P.; Mayer, L.; Fletcher, L. B.; Spurgeon, S.; Kovacsovics, T.; Loriaux, M. M. Evaluation of CPX-351 (cytarabine:daunorubicin) Liposome Injection. Anti-Leukemic Activity Against Primary Patient Leukemia Cells. ASH Annual Meeting Abstracts, 2010, 116 (21), 2886-.
Cortes, Jorge E., Feldman, Eric J, Goldberg, Stuart L., Rizzieri, David A., Paulsen, Kim H., Louie, Arthur C., Kolitz, Jonathan E. CPX-351: A Randomized Phase 2b Study of CPX-351 v. Intensive Salvage Therapy in ‘65 Yo First Relapse AML Patients: Initial Efficacy and Safety Report. ASH Annual Meeting Abstracts 2011, 118, 254.
Faderl, S. Clofarabine plus cytarabine compared to cytarabine alone in older patients with relapsed or refractory (R/R) acute myelogenous leukemia (AML): Results from the phase III CLASSIC 1 trial. ASCO annual meeting, 2011.
Amadori, S.; Venditti, A.; Ammatuna, E.; Martelli, A. M.; Meloni, G.; Pane, F.; Martinelli, G.; Lunghi, M.; Pagano, L.; Cilloni, D.; Rizzoli, V.; Di Raimondo, F.; Fozza, C.; Annino, L.; Piciocchi, A.; La Sala, E.; Fazi, P.; Vignetti, M Temsirolimus, An mTOR Inhibitor, In Combination with Low-Dose Clofarabine in Older Patients with Advanced Acute Myeloid Leukemia: Results of a Phase 2 GIMEMA Study (AML-1107). ASH Annual Meeting Abstracts, 2010, 116, 510.
Wei, A. H.; Sadawarte, S.; Catalano, J.; Schwarer, A. P.; Avery, S.; Patil, S. S.; Spencer, A. Clinical Activity of Azacitidine In Combination with the Oral mTOR Inhibitor Everolimus (RAD001) In Relapsed and Refractory AML: Interim Analysis of a Phase Ib/II Study. ASH Annual Meeting Abstracts, 2010, 116, 3301.
Cortes, J.; Foran, J.; Ghirdaladze, D.; DeVetten, M. P.; Zodelava, M.; Holman, P.; Levis, M. J.; Kantarjian, H. M.; Borthakur, G.; James, J.; Zarringkar, P. P.; Gunawardane, R. N.; Armstrong, R. C.; Padre, N. M.; Wierenga, W.; Corringham, R.; Trikha, M. AC220, a Potent, Selective, Second Generation FLT3 Receptor Tyrosine Kinase (RTK) Inhibitor, in a First-in-Human (FIH) Phase 1 AML Study. ASH Annual Meeting Abstracts, 2009, 114, 636.
Serve, H.; Wagner, R.; Sauerland, C.; Brunnberg, U.; Krug, U.; Schaich, M.; Ottmann, O. G.; Duyster, J.; Wandt, H.; Herr, W.; Giagounidis, A. A. N.; Neubauer, A.; Reichle, A.; Aulitzky, W. E.; Noppeney, R.; Blau, I. W.; Kunzmann, V.; Schmitz, N.; Kreuzer, K.-A.; Kramer, A.; Brandts, C.; Steffen, B.; Heinecke, A.; Thiede, C.; Muller-Tidow, C.; Ehninger, G.; Berdel, W. E. Sorafenib In Combination with Standard Induction and Consolidation Therapy In Elderly AML Patients: Results From a Randomized, Placebo-Controlled Phase II Trial. ASH Annual Meeting Abstracts, 2010, 116, 333.
Castaigne, Sylvie, Pautas, Cecile, Terre, Christine, Raffoux, Emmanuel, Bordessoule, Dominique, Bastie, Jean-Noel, Legrand, Ollivier, Thomas, Xavier, Turlure, Pascal, Reman, Oumedaly, De Revel, Thierry, Gastaud, Lauris, Gardin, Claude, Sutton, Laurent, Marolleau, Jean Pierre, De Botton, Stephane, Hermine, Olivier, Plantier, Isabelle, Janvier, Maud, Dupriez, Brigitte, Simon, Marc, De Gunzburg, Noemie, Foucault-Haiat, Stephanie, Contentin, Nathalie, Berthon, Celine, Fenaux, Pierre, Henry, Estelle, Rousselot, Philippe, Preudhomme, Claude, Chevret, Sylvie, Dombret, Herve Fractionated Doses of Gemtuzumab Ozogamicin (GO) Combined to Standard Chemotherapy (CT) Improve Event-Free and Overall Survival in Newly-Diagnosed De Novo AML Patients Aged 50–70 Years Old: A Prospective Randomized Phase 3 Trial From the Acute Leukemia French Association (ALFA). ASH Annual Meeting Abstracts 2011, 118, 6.
Burnett, Alan K, Hills, Robert K, Hunter, Ann E., Milligan, Donald, Kell, William J., Wheatley, Keith, Yin, John L, Ali, Sahra, Kjeldsen, Lars, Bowen, David, Russell, Nigel H. The Addition of Gemtuzumab Ozogamicin to Intensive Chemotherapy in Older Patients with AML Produces a Significant Improvement in Overall Survival: Results of the UK NCRI AML16 Randomized Trial. ASH Annual Meeting Abstracts 2011, 118, 582.
Garcia-Manero, Guillermo, Tambaro, Francesco Paolo, Bekele, Nebiyou, Yang, Hui, Ravandi, Farhad, Jabbour, Elias, Borthakur, Gautam, Kadia, Tapan, Konopleva, Marina, Faderl, Stefan, Cortes, Jorge E., Brandt, Mark, Hu, Yumin, McCue, Deborah, Newsome, Willie Mae, Pierce, Sherry, DeLima, Marcos, Kantarjian, Hagop M. Final Report of a Phase II Trial of Vorinostat with Idarubicin and Cytarabine for Patients with Newly Diagnosed Acute Myelogenous Leukemia (AML) or Myelodysplastic Syndrome (MDS). ASH Annual Meeting Abstracts 2011, 118, 763.
CONFLICT OF INTEREST
Declared None.
References
- 1.Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, Ramsay EC, Bally MB, Janoff AS. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5:1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 2.Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, Harvie P, Bermudes D, Mayer L. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33:129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Feldman EJ, Lancet JE, Kolitz JE, Ritchie EK, Roboz GJ, List AF, Allen SL, Asatiani E, Mayer LD, Swenson C, Louie AC. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29:979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarjian H, Gandhi V, Cortes J, Verstovsek S, Du M, Garcia-Manero G, Giles F, Faderl S, O’Brien S, Jeha S, Davis J, Shaked Z, Craig A, Keating M, Plunkett W, Freireich EJ. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 5.Faderl S, Gandhi V, O’Brien S, Bonate P, Cortes J, Estey E, Beran M, Wierda W, Garcia-Manero G, Ferrajoli A, Estrov Z, Giles FJ, Du M, Kwari M, Keating M, Plunkett W, Kantarjian H. Results of a phase 1–2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105:940–947. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- 6.Faderl S, Verstovsek S, Cortes J, Ravandi F, Beran M, Garcia-Manero G, Ferrajoli A, Estrov Z, O’Brien S, Koller C, Giles FJ, Wierda W, Kwari M, Kantarjian HM. Clofarabine and cytarabine combination as induction therapy for acute myeloid leukemia (AML) in patients 50 years of age or older. Blood. 2006;108:45–51. doi: 10.1182/blood-2005-08-3294. [DOI] [PubMed] [Google Scholar]
- 7.Faderl S, Ravandi F, Huang X, Garcia-Manero G, Ferrajoli A, Estrov Z, Borthakur G, Verstovsek S, Thomas DA, Kwari M, Kantarjian HM. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloomfield CD, Lawrence D, Byrd JC, Carroll A, Pettenati MJ, Tantravahi R, Patil SR, Davey FR, Berg DT, Schiffer CA, Arthur DC, Mayer RJ. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58:4173–4179. [PubMed] [Google Scholar]
- 9.Kantarjian HM, Erba HP, Claxton D, Arellano M, Lyons RM, Kovascovics T, Gabrilove J, Craig M, Douer D, Maris M, Petersdorf S, Shami PJ, Yeager AM, Eckert S, Abichandani R, Faderl S. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28:549–555. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 10.Burnett AK, Russell NH, Kell J, Dennis M, Milligan D, Paolini S, Yin J, Culligan D, Johnston P, Murphy J, McMullin MF, Hunter A, Das-Gupta E, Clark R, Carr R, Hills RK. European development of clofarabine as treatment for older patients with acute myeloid leukemia considered unsuitable for intensive chemotherapy. J Clin Oncol. 2010;28:2389–2395. doi: 10.1200/JCO.2009.26.4242. [DOI] [PubMed] [Google Scholar]
- 11.Faderl S. Clofarabine plus cytarabine compared to cytarabine alone in older patients with relapsed or refractory (R/R) acute myelogenous leukemia (AML): Results from the phase III CLASSIC 1 trial. 2011 doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perl AE, Kasner MT, Tsai DE, Vogl DT, Loren AW, Schuster SJ, Porter DL, Stadtmauer EA, Goldstein SC, Frey NV, Nasta SD, Hexner EO, Dierov JK, Swider CR, Bagg A, Gewirtz AM, Carroll M, Luger SM. A phase I study of the mammalian target of rapamycin inhibitor sirolimus and mec chemotherapy in relapsed and refractory acute myelogenous leukemia. Clin Cancer Res. 2009;15:6732–6739. doi: 10.1158/1078-0432.CCR-09-0842. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, Garcia-Manero G, O’Brien S, Faderl S, Ravandi F, Westwood R, Green SR, Chiao JH, Boone PA, Cortes J, Plunkett W. Phase I clinical and pharmacokinetic study of oral sapacitabine in patients with acute leukemia and myelodysplastic syndrome. J Clin Oncol. 2010;28:285–291. doi: 10.1200/JCO.2009.25.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancet JE, Ravandi F, Ricklis RM, Cripe LD, Kantarjian HM, Giles FJ, List AF, Chen T, Allen RS, Fox JA, Michelson GC, Karp JE. A phase Ib study of vosaroxin, an anticancer quinolone derivative, in patients with relapsed or refractory acute leukemia. Leukemia. 2011 doi: 10.1038/leu.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C, Fox E, Ehninger G, Feldman EJ, Schiller GJ, Klimek VM, Nimer SD, Gilliland DG, Dutreix C, Huntsman-Labed A, Virkus J, Giles FJ. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28:4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, O’Brien S, Estrov Z, Borthakur G, Thomas D, Pierce SR, Brandt M, Byrd A, Bekele BN, Pratz K, Luthra R, Levis M, Andreeff M, Kantarjian HM. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang MJ, Wu H, Achille NJ, Reisenauer MR, Chou CW, Zeleznik-Le NJ, Hemenway CS, Zhang W. Histone H3 lysine 79 methyltransferase Dot1 is required for immortalization by MLL oncogenes. Cancer Res. 2010;70:10234–10242. doi: 10.1158/0008-5472.CAN-10-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daigle SR, Olhava EJ, Therkelsen CA, Majer CR, Sneeringer CJ, Song J, Johnston LD, Scott MP, Smith JJ, Xiao Y, Jin L, Kuntz KW, Chesworth R, Moyer MP, Bernt KM, Tseng JC, Kung AL, Armstrong SA, Copeland RA, Richon VM, Pollock RM. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell. 2011;20:53–65. doi: 10.1016/j.ccr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 20.Thomas XG. Results from a randomized phase III trial of decitabine versus supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed AML. J Clin Oncol. 29 doi: 10.1200/JCO.2011.38.9429. (suppl; abstr 6504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF, Dombret H, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 22.Sekeres MA, Gundacker H, Lancet J, Advani A, Petersdorf S, Liesveld J, Mulford D, Norwood T, Willman CL, Appelbaum FR, List AF. A phase 2 study of lenalidomide monotherapy in patients with deletion 5q acute myeloid leukemia: SWOG Study S0605. Blood. 2011;118:523–528. doi: 10.1182/blood-2011-02-337303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fehniger TA, Uy GL, Trinkaus K, Nelson AD, Demland J, Abboud CN, Cashen AF, Stockerl-Goldstein KE, Westervelt P, DiPersio JF, Vij R. A phase 2 study of high-dose lenalidomide as initial therapy for older patients with acute myeloid leukemia. Blood. 2011;117:1828–1833. doi: 10.1182/blood-2010-07-297143. [DOI] [PMC free article] [PubMed] [Google Scholar]


