Abstract
Importance
Care of patients with malignant bowel obstruction caused by peritoneal metastases may present an ethical dilemma for surgeons when nonoperative management fails.
Objective
To characterize outcomes of palliative surgery for malignant bowel obstruction from peritoneal carcinomatosis to guide decision making about surgery and postoperative interventions for patients with terminal illness.
Evidence Review
We searched PubMed, EMBASE, Cochrane Library, Web of Knowledge, CINAHL Plus, and Google Scholar, and performed manual searches of selected journals from inception to August 30, 2012 with no filters, limits, or language restrictions. We used database-specific combinations of intestinal obstruction, malignant, surgery or surgical, and palliat*. We included studies reporting outcomes after palliative surgery for malignant bowel obstruction from peritoneal carcinomatosis from any primary malignancy and excluded case studies, curative surgery, isolated percutaneous procedures, stenting for intraluminal lesions, and studies in which benign and malignant obstructions could not be distinguished. We assessed quality with the Newcastle-Ottawa Scale.
Findings
We screened 2347 unique articles, selected 108 articles for full-text review, and included 17 studies. Surgery was able to palliate obstructive symptoms for 32 to 100% of patients, enable resumption of a diet for 45 to 75% of patients, and facilitate discharge to home in 34–87% of patients. Mortality was high (6–32%), and serious complications are common (7–44%). Frequent re-obstructions (6–47%), readmissions (38–74%), and re-operations (2–15%) occur. Survival was limited (median 26–237 days), and hospitalization for surgery consumed a substantial portion of the patient’s remaining life (11–61%).
Conclusions and Relevance
Although palliative surgery can benefit patients, it comes at the cost of high mortality and substantial hospitalization relative to the patient’s remaining survival time. Preoperatively, surgeons should present realistic goals and limitations of surgery. For patients choosing surgery, clarifying preferences for aggressive postoperative interventions preoperatively is critical given the high complication rate and limited survival after surgery for malignant bowel obstruction.
Introduction
Malignant bowel obstruction (MBO) is a common pre-terminal event for patients with advanced cancer, with an incidence as high as 28% in gastrointestinal cancer and 51% in ovarian cancer1,2. Patients with MBO are unable to eat, experience severe pain, and develop intractable nausea and vomiting – symptoms that incite considerable distress for patients and their families3,4. Treatment options include supportive care with nasogastric drainage, pain control, antiemetics, antisecretory medications, and corticosteroids; endoscopically placed stents; percutaneous endoscopic gastrostomy tubes; or palliative surgery to relieve symptoms5. Palliative operations can be successful for patients with MBO from intraluminal or localized tumors, but are less effective for patients with MBO from carcinomatosis5–7. However, patients with MBO from peritoneal metastases can develop distressing symptoms despite maximal medical treatment and present the surgeon with an ethical dilemma.
Surgical decision making in this setting is particularly difficult; an operation may provide relief of intolerable symptoms for a patient, but patients with MBO due to peritoneal metastases may have only weeks or months to live2,8 and are often poor surgical candidates because of malnutrition and underlying disease5,6. Patients with terminal illness may prefer to avoid burdensome treatments near the end of life9–11. Additionally, frail patients may agree to an initial operation to alleviate severe symptoms, but then choose to forgo aggressive treatments in the postoperative period12. Surgical decision making for MBO is further complicated by a lack of high quality data. Information regarding palliative outcomes including quality of life, functional outcomes, or patient distress is sparse.
We performed a systematic review of the literature to determine the effects of palliative surgery for MBO associated with peritoneal metastases on quality of life, successful palliation, postoperative mortality, complications, and survival to help surgeons and patients make decisions about surgery that are in line with the patient’s goals and values. This information may have particular value for surgeons and for patients who choose to have surgery, as it can facilitate preoperative discussion about the patient preferences for aggressive postoperative treatments.
Methods
We performed a systematic review according to guidelines outlined in the Cochrane Collaboration Handbook13. Before starting our literature search and data collection, we designed a protocol based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement14 and Meta-analysis of Observational Studies in Epidemiology guideline15.
Data Sources and Search Strategy
We searched PubMed, EMBASE, the Cochrane Library, Web of Knowledge, Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus, and Google Scholar from inception to August 30, 2012. We used database-specific combinations of the following index terms and text words: intestinal obstruction, malignant, surgery or surgical, and palliat*. To avoid studies focused on endoscopic stenting of obstructive intraluminal lesions, we designed our search to omit articles with the terms “stent” or “stenting” in the title. We used an English-only filter for our search of Google Scholar to obtain a manageable collection of results, but we did not use any filters or limits for the remaining databases. Details of the search strategy for each database are described in the Supplement (eTable). In addition, we performed a hand search of the tables of contents of Annals of Surgical Oncology, Palliative Medicine, Journal of Pain and Symptom Management, and Gynecologic Oncology from inception of each journal through August 30, 2012. These searches were supplemented with manual review of references from review articles retrieved from the primary database search. We used EndNote X5 and EndNote Web (Thomson Reuters, New York, NY) to organize references.
Inclusion and Exclusion Criteria
According to our protocol, we included original research describing outcomes of open or laparoscopic surgery for bowel obstruction from peritoneal carcinomatosis while excluding treatment of intraluminal lesions. Outcomes of interest included survival, postoperative mortality, postoperative complications (specifically rates of wound infection, wound dehiscence, enterocutaneous fistula, anastomotic leak, deep vein thrombosis/pulmonary embolus, bleeding complications, gastrointestinal bleeding, myocardial infarction, sepsis, or other complications), hospital length of stay, intensive care unit length of stay, postoperative use of life supporting interventions such as mechanical ventilation or cardiopulmonary resuscitation, additional procedures or operations, conversations about goals of care, pain control, control of nausea and vomiting, ability to tolerate a diet, freedom from nasogastric tube drainage, discharge disposition including hospice, incidence of re-obstruction, and patient-reported quality of life measures using validated instruments.
We excluded articles that did not report baseline characteristics of the study group, studies that did not separate surgical outcomes for benign obstructions from those for malignant obstructions, reports including operations with curative rather than palliative intent, series that reported only obstructions amenable to stenting (i.e. large bowel or gastroduodenal obstructions) or reported only percutaneous procedures, and case studies that included fewer than five patients. We also excluded reviews, editorials, conference proceedings, and articles that were not peer reviewed.
Study Selection and Data Extraction
One reviewer (TJPO) evaluated titles and abstracts of all identified articles to develop a subset for full-text review. We obtained translations of articles in French and Chinese which were identified by their abstracts in English as potentially relevant for full text review. Two independent reviewers (TJPO and CP) applied the inclusion and exclusion criteria from our protocol to the full-text articles to identify articles for review. We adjudicated discrepancies between the reviewers’ assessments through collaborative discussion. Because included studies covered a broad time-span, we did not contact study authors to obtain additional data. One reviewer (TJPO) extracted data from included studies according to the criteria defined in the protocol.
Data Synthesis
Fifteen of 17 studies included in this review were of low methodological quality with significant risk of bias and heterogeneity. As such, we were unable to perform statistical meta-analysis on the extracted data. Instead, we performed descriptive synthesis of the outcomes reported for studies that met our inclusion criteria.
Quality Assessment
We used the Newcastle-Ottawa Scale for cohort studies16 to assess the quality of studies included in this review. None to nine stars are awarded for the methodological quality of case selection, comparability of cohorts, and measurement of outcomes. Six or more stars are considered high methodological quality16,17. We also assessed confounding factors and risks of bias that were not addressed by study design or data analysis using the Cochrane Collaboration Handbook classification of bias13.
Results
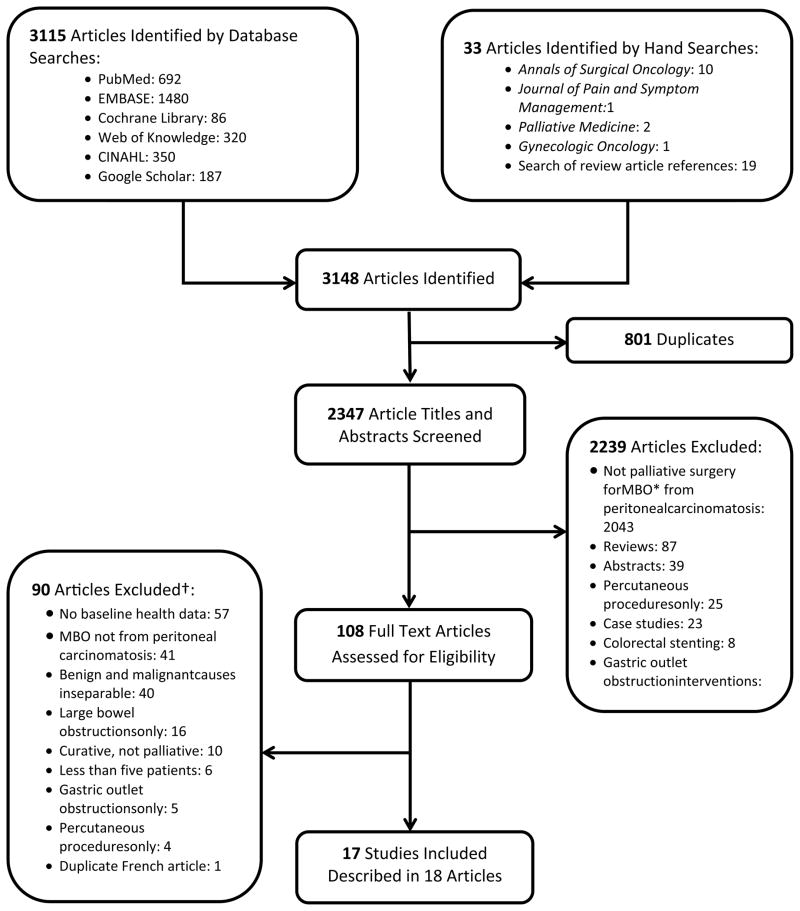
We identified 3115 articles through database retrieval and located an additional 33 titles by hand for a total of 3148 articles. Of these, 801 were duplicates, leaving 2347 unique articles. After screening titles and abstracts, we excluded 2239 articles that did not fit our inclusion criteria. We reviewed the full text versions of the remaining 108 articles and excluded an additional 90 articles. Two articles reported the results of a single study, so we extracted data from these articles as if they were one study. Our final cohort contained 18 articles describing 17 studies (Figure).
Figure 1.
Flowchart of literature search strategy.
Study and Patient Characteristics
We found 17 studies published between 1982 and 201218–35, including 11 retrospective single-institution case series18–20,22–26,30,31,34,35, three retrospective single-institution cohort studies21,28,29, two retrospective multi-center cohort studies27,32, and one prospective single institution cohort study33 (Table 1). Of the six cohort studies, one compared two distinct operative interventions (exploratory laparotomy alone compared with major intestinal surgery)28, three compared surgical patients to patients managed by gastric drainage21,27,29, one compared surgical patients to patients treated with octreotide32, and one compared surgical patients to patients managed with either percutaneous endoscopic gastrostomy (PEG) tubes or colonic stents for extrinsic intestinal obstruction from intra-abdominal tumor33.
Table 1.
Outcomes after palliative surgery for malignant bowel obstruction from peritoneal carcinomatosis.
| Study Name | N | % Female |
Median Age Years (Range) |
Primary Cancer |
Palliative Outcomes |
Post- operative Mortality |
Post-operative Complications |
Adverse Outcomes |
Survival in Days Median [Mean] (Range) |
Length of Stay in Days Median [Mean] (Range) |
|---|---|---|---|---|---|---|---|---|---|---|
| McCarthy 198618 (United States) | 12 | 83 | NR | Ovarian:7 CRCa: 2 GIb: 3 |
Diet 75% (9/12) |
25% (3/12) | 25% (3/12) Woundc: 3 |
NRd | NR [NR] (3–1080) | NR |
| Turnbull 198919 (United States) | 89 | 39 | 53 (19–90) | CRC: 59 GI: 25 HPBe: 6 |
Symptoms relieved/ Diet 74% (66/89) |
13% (12/89) | 44% (38/89) Wound: 6 Bowelf: 7 Infectiousg: 1 Systemich: 1i |
Re-obstruction with readmission 38% (25/66) Operations 15% (13/89) |
98 [135] (1–913) | 25 [31] (5–94) |
| Lau 199320 (Hong Kong) | 30 | 43 | 63j (23–84) | CRC: 30 | Symptoms relieved 63% (19/30) Home 57% (17/30) |
17% (5/30) | 27% (8/30) Wound: 2 Bowel: 1 Infectious: 3 Systemic: 2 |
Re-obstruction with readmission 47% (8/17) Operation 13% (4/30) |
Grp 1k 192 [210] (24–452) Grp 2l 26 [28] (15–52) |
NR |
| Van Ooijen 199321 (the Netherlands)m | 59 | 93 |
Grp 1n: 55.7j (26–72) Grp 2o: 53.2j (26–72) |
GYNp: 46 CRC: 8 HPB: 1 Otherq: 2 |
Symptoms relieved 76% (41/54) Home 34% (20/59) |
NR | NR | Re-obstruction 15% (7/46) |
Grp 1r 154 [366s] (29–1086) Grp 2 36 [58s] (3–151) |
Grp 2 22 [25s] (3–53) |
| Blair 200122 (United States) | 63 | 40 | 58 (25–83) | CRC: 31 GI: 13 HPB: 5 Other: 14 |
Diet 45% (29/63) |
21% (13/63) | 44% (28/63) Infectious: 15 Non-infectious: 13 |
Readmission 74% (39/53) |
Actuarial mean: 90 | NR |
| Legendre 200123 (Belgium) | 109t | 72t | 62t (32–88) | GYN: 37 CRC: 34 GI: 12 Other: 26 |
Home with diet 61% (45/73)u |
NRu | NRu | NRu | 58 [NR] (NR)u | NRu |
| Abbas 200624, 200725 (New Zealand) | 79 | 57 | 62 (19–91) | CRC: 31 GYN: 19 GI: 3 Other: 25 |
NR | 10% (8/79) | 35% (28/79) | NR | 150 [NR] (NR) | NR |
| Piver 198226 (United States) | 60 | 100 | 52 (31–81) | Ovarian: 60 | NR | 17% (10/60) | 31% (19/60) Bowel: 9 Infectious: 2 Systemic: 3 Miscellaneousv:4i, |
NR | 75 [250s] (<30–810) | NR |
| Lund 198927 (Denmark)w | 25 | 100 | 56 (24–71) | Ovarian: 25 | Symptoms relieved 32% (8/25) |
32% (8/25) | 32% (8/25) Wound: 6 Systemic: 2 |
Re-obstruction 38% (3/8) |
68 [273s] (7–919) | NR |
| Rubin 198928 (United States) | 52 | 100 | 53.8j (25–82) | Ovarian: 52 | Diet 65% (34/52) Home 87% (45/52) |
17% (9/52) | 15% (8/52) Bowel: 6 Infectious: 2 |
Operations 4% (2/52) |
NR [174] (0.5–1110) | NR |
| Bais 199529 (Netherlands)w | 19 | 100 | 53 (29–74) | Ovarian: 19 | Diet 68% (13/19) |
11% (2/19) | 32% (6/19) Wound: 2 Infectious: 4 |
Re-obstruction 21% (4/19) Operations 5% (1/19) |
109 [260s] (15–775) | NR [24] (NR) |
| Jong 199530 (Canada) | 53 | 100 | NR | Ovarian: 53 | Symptoms relieved 68% (36/53) Home 68% (36/53) |
32%x (17/53) | NR | Re-obstruction 40% (21/53) |
87.5 [271s] (5–892) | NR |
| Pothuri 200331 (United States) | 64 | 100 | 57j (29–79) | Ovarian: 64 | Diet 58% (37/64) |
6% (4/64) | 22% (15/68)y Bowel: 5 Infectious: 8 Systemic: 2 |
Re-obstruction 6% (4/64) Operations 6% (4/64) |
237 [NR] (NR) |
Bowel resectionz NR [14.3] (5–59) Ex lap onlyz NR [12.5] (5–31) |
| Mangili 200532 (Italy)w | 27 | 100 | 54.8j (31–77) | Ovarian: 47 | Diet 59% (16/27) |
22% (6/27) | 33% (9/27) Wound: 4 Bowel: 2 Systemic: 3 |
Operations 0% (0/27) |
NR [79] (9–350)aa | NR |
| Chi 200933 (United States)w | 14 | 100 | 54 (22–81)m | Ovarian: 14 | Symptoms relieved 100% (14/14) |
NR | 7% (1/14) | Re-obstruction 60 daysr 29% (4/14) 90 daysr 36% (5/14) |
191 [339s] (33–902) | 11 [16s] (1–43)bb |
| Kim 2009 (Korea)34 | 23 | 100 | <50 years old: 10 ≥50 years old: 13 |
Ovarian: 23 | Diet 48% (11/23) |
NR | 13% (3/23) Wound: 1 Bowel: 1 Systemic: 1 |
NR | 61% alive at median follow up of 3 months | NR |
| Kolomainen 201235 (England) | 90 | 100 | 57 (25–85) | Ovarian: 90 | Diet 66% (59/90) |
18% (16/90) | 27% (24/90) Wound: 9 Bowel: 13 Infectious: 1 Systemic: 5 Miscellaneous: 1i |
Re-obstruction 17% (10/59) Operations 2% (2/90) |
90.5 [599s] (<1–2190) | NR |
Colorectal cancer
GI = carcinoid, esophageal, gastric, duodenal, periampullary, small bowel cancers, tumors of unknown GI origin
Wound = wound infection, wound dehiscence, reoperation to close laparotomy, wound breakdown, parastomal abscess
Not reported
HPB = pancreatic, bile duct, gallbladder
Bowel = enterocutaneous fistulae, enteroperitoneal fistula, enterovaginal fistula, anastomotic leak, early obstruction, leakage from colostomy, high output stoma, stoma retraction
Infectious = intra-abdominal abscess, pulmonary infection/pneumonia, sepsis, bacteremia, urosepsis, line sepsis, fever, cystitis, neutropenic fever with hypotension, bacterial peritonitis
Systemic = Myocardial infarction, intra-abdominal hemorrhage, aspiration, pulmonary embolus, deep vein thrombosis, cardiovascular failure, heart failure, atrial fibrillation, pulmonary edema, pneumothorax, hypercalcemia
Total does not equal the sum of subgroups because either all complications were not reported or selected patients had multiple complications.
Mean
Group 1 = Patients with return of bowel function
Group 2 = Patients without return of bowel function
Three groups included in this study: two operative groups and one control group. For selected columns only the operative groups are reported. See Table 3 for a further discussion of this study.
Group 1 = 20 patients deemed appropriate for surgery with good prognostic features (no palpable masses or ascites, or patients with obstruction as the first sign of malignancy)
Group 2 = 20 patients deemed appropriate for surgery with poor prognostic features (palpable masses and/or ascites)
GYN = ovarian, cervical, uterine, and endometrial cancer, gynecologic malignancies of unspecified type
Other = kidney, bladder, adrenal, breast, GIST, melanoma, prostate, lung, unknown primary, other non-gynecologic visceral cancers, and sarcoma
Time to death or re-obstruction
Estimated
Entire cohort, which includes patients with MBO from peritoneal carcinomatosis or local recurrence
Only patients with MBO from peritoneal carcinomatosis
Miscellaneous = short gut with diarrhea, antibiotic nephrotoxicity, malignant ureteral obstruction, prolonged ileus
This study reported two groups, a surgical group and a control group. Only the results of the surgical group are reported in this table. See Table 3.
60-day postoperative mortality
68 operations were performed on 64 patients
The first group is patients who were able to undergo corrective surgery for MBO. The second group is patients who were found to have such extensive peritoneal carcinomatosis that only exploratory laparotomy was performed.
Overall mean survival for combined surgical and control groups
Entire cohort, which includes patients treated with operative or endoscopic interventions
The 17 studies included a total of 868 patients, of whom 77% were female. Median reported age was 52 to 63 years (range 19 – 90). Several types of surgical interventions were performed, including creation of an ostomy (colostomy, ileostomy, or jejunostomy), intestinal resection and/or bypass (enteroenterostomy, enterocolostomy, or colocolostomy), lysis of malignant adhesions, open or percutaneous placement of gastrostomy tube or long jejunal tube, and exploratory laparotomy without additional intervention for cases where carcinomatosis was too extensive. No study used a pre-specified protocol for operative intervention; the operations performed were determined by intra-operative findings18–35.
Patients had a variety of primary malignancies including colorectal, gastric, gynecologic (ovarian, cervical, uterine, endometrial, and unspecified gynecological malignancies), melanoma, breast, pancreaticobiliary, gallbladder, small bowel, gastrointestinal stromal tumor, kidney, bladder, lung, prostate, esophageal, duodenal, periampullary, carcinoid, adrenal, extremity sarcoma, other non-gynecologic visceral malignancies, tumors of unknown gastrointestinal origin, and unknown primary malignant neoplasms. Ten studies described only patients with ovarian cancer26–35.
Outcomes of Palliative Surgery
Studies demonstrated benefits from palliative surgery for MBO, including relief of obstructive symptoms, ability to tolerate a diet, and discharge to home. Obstructive symptoms were relieved or diet was resumed after surgery in 32–100% of patients18–21,27,30,32,33. Patients were able to tolerate a diet postoperatively in 45–75% of cases18,19,22,23,28,29,31,32,34,35, and 34–87% of patients were discharged to home20,21,23,28,30. Other measures of palliation such as validated quality of life metrics or measures of patient distress, were not reported by any studies. Furthermore, markers of quality end-of-life care such as goals-of-care meetings or discussions of do-not-resuscitate status were not reported in any studies.
Thirty-day postoperative mortality was high, with rates ranging from 6–32%18–20,22,24–32,35. The incidence of serious complications was 7–44%. These complications included enterocutaneous fistula, wound infection, wound dehiscence, early obstruction, high output ostomy, myocardial infarction/cardiovascular failure, deep vein thrombosis/pulmonary embolus, pulmonary infection/pneumonia, anastomotic leak, and infection18–20,22,24–29,31–35.
Re-obstruction occurred in 6–47% of patients19–21,27,29–31,33,35, and the duration of symptom relief after palliative surgery was short, with only 32–71% of patients remaining symptom-free or tolerating a diet 60 days postoperatively27,29–31,33–35. One study reported a 74% all-cause readmission rate22, and others reported readmission rates of 38–47% for recurrent bowel obstruction19,20. Few patients (2–15%) underwent additional operations to address complications or re-obstruction19,20,28,29,31,35. However, one study19 reported that only 46% of patients with repeat surgery were able to return home postoperatively, and complications and mortality were frequent in this group (46% and 23%, respectively).
Median survival time after diagnosis of MBO was 26–273 days19–27,29–31,33,35 and was related to prognostic features. Two studies compared survival for patients with favorable indicators (no ascites or palpable masses, return of bowel function postoperatively) to patients with poor prognostic features (ascites, palpable masses, or continued obstruction postoperatively). Median survival was 154–192 days with favorable prognostic features, whereas patients with poor prognostic features survived only 26–36 days20,21.
Mean hospital length of stay (LOS) for initial treatment of MBO, including a preoperative trial of conservative management, was 12.5–31 days and ranged from 1–94 days19,21,29,31,33. Time spent in the hospital relative to remaining life was considerable. Two studies19,29 reported that approximately one-fourth (22–26%) of the patient’s remaining life was spent in the hospital, and another reported 11%31. For the patients with poor prognostic features (ascites and/or palpable masses) reported by van Ooijen and colleagues21, 61% of the patients’ remaining life was spent in the hospital. Patients who were readmitted also spent significant time in the hospital, with median ranging from 22–41 days19,22.
Comparative Studies
Five studies compared outcomes between palliative surgery and non-surgical treatments for MBO21,27,29,32,33 (Table 2). Non-operative alternatives included gastrostomy tube (either open or percutaneous)21,33, endoscopically-placed intraluminal stents for extrinsic compression of the bowel from intra-abdominal metastases33, nasogastric drainage27,29, and octreotide32. In four of five studies27,29,32,33, surgery more effectively relieved obstructive symptoms or enabled patients to tolerate a diet, with rates of palliation ranging from 32–100% in the surgical group and 0–75% in the groups with non-operative treatment. Van Ooijen et al21 compared three groups: surgical patients with favorable prognostic features (no ascites or palpable masses), surgical patients with poor prognostic features (ascites and/or palpable masses), and patients with poor prognostic features who were not operative candidates and were treated with gastrostomy tube alone. They found high rates of palliation in patients with favorable prognostic features who had surgery and in patients with poor prognostic features treated with gastrostomy tubes (85% and 90%, respectively). However, only 43 % of patients with poor prognostic features who were treated with surgery achieved palliation.
Table 2.
Comparison of outcomes after palliative surgery or non-operative treatment for malignant bowel obstruction from peritoneal carcinomatosis.
| Study | N | Mean Age in Years (Range) | Palliative Outcomes Achieved (%)a | 30-Day Mortality (%) | Postoperative Complications (%) | Median Days to Death (Range) | P-value (Χ2 test) |
|---|---|---|---|---|---|---|---|
|
Van Ooijen 1993b,21
| |||||||
| Surgical Group 1c | 20 | 55.7 (26–72) | 85 | NRe | NR | 154 (29–1086)f | <0.001g |
| Surgical Group 2d | 20 | 53.2 (26–72) | 43 | NR | NR | 36 (3–151) | |
|
| |||||||
| Gastrostomy Tube | 25 | 53.1 (26–72) | 90 | NR | NR | 33 (8–163) | |
|
| |||||||
|
Chi 200933
| |||||||
| Surgery | 14 | NR | 100 | NR | 7 | 191 (33–902) | NR |
|
| |||||||
| Endoscopic Treatmenth | 12 | NR | 75 | NR | 25 | 78 (18–284) | |
|
| |||||||
|
Lund 198927
| |||||||
| Surgery | 25 | 56i (24–71) | 32 | 32 | 32 | 68 (7–919) | 0.3 |
|
| |||||||
| Nasogastric Drainage | 16 | 59i (49–73) | 0 | 35 | NR | 30 (7–842) | |
|
| |||||||
|
Bais 199529
| |||||||
| Surgery | 19 | 53i (29–74) | 68 | 11 | 32 | 109 (15–775) | NR |
|
| |||||||
| Nasogastric Drainage | 12 | 61i (29–74) | 17 | 83j | NR | 37 (13–157) | |
|
| |||||||
|
Mangili 200532
| |||||||
| Surgery | 27 | 54.8 (31–77) | 59 | 22 | 33 | NR | <0.001l |
|
| |||||||
| Octreotidek | 20 | 59.6 (31–77) | 30 | NR | 5 | NR | |
Relief of obstructive symptoms or ability to tolerate a diet
Includes patients with ovarian, colorectal, cervical, endometrial, gallbladder, and breast cancer. All other studies only include patients with ovarian cancer.
Patients appropriate for surgery with no palpable masses or ascites, or patients with obstruction as the first sign of malignancy.
Patients appropriate for surgery with palpable masses and/or ascites.
Not reported
Median time to death or intervention for re-obstruction
Group 1 compared to gastrostomy tube group, log-rank test.
Either percutaneous endoscopic gastrostomy (PEG) tube or endoscopically-placed colonic stent
Median
60-day mortality
Octreotide subcutaneous bolus or intravenous infusion starting at 0.3 mg daily until symptoms controlled; max dose 0.9 mg daily, median dose 0.6 mg daily.
Overall survival was calculated using Kaplan-Meier survival curves, and significance assessed by log-rank test.
Four of five studies reported improved survival with surgery compared with non-operative treatment21,29,32,33. Median survival after surgery ranged from 109–191 days versus 33–78 days for non-operative treatments21,29,33. Van Ooijen et al21 reported that patients with poor prognostic features who underwent surgery had a median survival comparable to that of patients with similar features who received only gastrostomy tubes(36 and 33 days, respectively). In contrast, surgical patients with favorable prognostic features had a median survival of 154 days.
Quality of Included Studies and Risk of Bias
The methodological quality of the included studies is summarized in Table 3. Twelve studies18–26,30,31,34,35 received two to three stars, indicating low methodological quality, and five studies27–29,32,33 received five to six stars, indicating moderate to high methodological quality.
Table 3.
Quality of included studies as assessed by Newcastle-Ottawa Scale (NOS) and type of bias identified.
| Study | Design | NOS | Types of Bias (Confounders) |
|---|---|---|---|
| McCarthy18 | Retrospective case series | *** |
|
| Turnbull19 | Retrospective case series | *** |
|
| Lau20 | Retrospective case series | ** |
|
| Van Ooijen21 | Retrospective cohort study | *** |
|
| Blair22 | Retrospective case series | *** |
|
| Legendre23 | Retrospective case series | ** |
|
| Abbas24,25 | Retrospective case series | *** |
|
| Piver26 | Retrospective case series | ** |
|
| Lund27 | Retrospective cohort study | ****** |
|
| Rubin28 | Retrospective cohort study | ****** |
|
| Bais29 | Retrospective cohort study | ***** |
|
| Jong30 | Retrospective case series | *** |
|
| Pothuri31 | Retrospective case series | *** |
|
| Mangili32 | Retrospective cohort study | ***** |
|
| Chi33 | Prospective cohort study | ***** |
|
| Kim34 | Retrospective case series | ** |
|
| Kolomainen35 | Retrospective case series | ** |
|
We identified multiple sources of bias. Selection bias, in which the baseline characteristics of groups are systematically different, was common. Most studies did not report or define the selection strategy for surgical intervention. Five studies20,27,29,31,32 described criteria for surgery and selected healthier patients for operative intervention. Additionally, three studies19,20,35 included a mix of patients who had urgent or elective surgery for MBO, but two of these studies19,20 did not adjust for this significant covariate36–39.
Patients received treatment with several oncologic interventions before and after treatment of MBO, introducing a risk of performance bias. Additionally, six of 1521,22,26,32,34,35 studies did not report length of follow up, had very short follow up, or lost a high percentage of patients to follow up, raising the concern for attrition bias. Finally, considerable variation in the reported outcomes (a source of detection bias) complicates comparison of the impact of surgery between studies.
Discussion
Palliative surgery for MBO from peritoneal carcinomatosis can provide relief from obstructive symptoms and enable patients to resume eating as well as return home. However, these benefits come at a cost; mortality and complication rates are high, and re-obstruction requiring readmission and additional procedures is common. Survival is short, and a substantial proportion of the patient’s remaining life may be spent in the hospital recovering from surgery and associated complications. This information can help surgeons and patients navigate the preference-sensitive and value-laden decisions surrounding palliative surgery.
For surgeons, these data can be used to facilitate frank discussion about whether palliative surgery is in line with patient preferences and goals of care. First, surgeons can inform patients about the probability of real symptomatic relief with surgery for at least a short time. However, these potential benefits should be presented along with the high probability of serious complications including the high rate of re-obstruction and the substantial duration of hospitalization associated with surgery. Additionally, although palliation might be achieved for a short time, the effects of surgery on quality of life are not well understood40–44. Second, surgeons routinely treat postoperative complications aggressively with burdensome treatments12,45–47 that patients with terminal illness are unlikely to want9–11. Because surgery for MBO has substantial morbidity, surgeons should preoperatively explore patients’ preferences for limiting aggressive treatments in the event of a postoperative complication. Surgeons often struggle when shifting focus from cure to comfort postoperatively47–49, but in the setting of palliative surgery, comfort is the primary goal. As such, preoperative clarification of desired “rescue” interventions can be used to inform difficult treatment decisions for patients, families, and surgeons if complications occur.
For patients, these data illustrate what palliative surgery can realistically accomplish in the setting of MBO. Surgery entails substantial risks for short-lived benefits, and survival is limited. Patients with incurable cancer often hope for considerable benefit and even cure from palliative interventions50–52. Data on the likelihood of such benefits can direct patients to anticipate more realistic postoperative outcomes. Patients also should be informed that, although palliative surgery can provide symptomatic relief, this benefit comes at the cost of spending a substantial proportion of their remaining life in the hospital recovering from surgery, even if the postoperative course is uncomplicated. As such, surgery may conflict with the patient’s goal of spending as much of his/her remaining life as possible at home with loved ones53.
For policy-makers, palliative operations contribute to a high proportion of overall mortality54–56, and as such, a metric that values the use of palliative surgery without penalizing surgeons for associated mortality is required. With increasing focus on outcomes profiling, surgeons and institutions risk penalties for their mortality rates which can be impacted by palliative operations. For example, at Memorial Sloan Kettering Cancer Center, palliative operations (6% of total cases) represent 36% of the institution’s 30-day operative mortality55. Quality assessment programs, such as the American College of Surgeons’ National Surgical Quality Improvement Program, adjust for underlying patient comorbidity but do not have strategies to identify operations performed with palliative intent or capture the palliative benefits offered by surgery aimed at comfort care. Identification of palliative operations and application of standard quality metrics for palliative care is needed to ensure that patients receive the care they desire and avoid aggressive postoperative interventions that conflict with their goals44,57–59. Informed patients should be able to choose palliative surgery to control intolerable symptoms, but they should not be subjected to undesired postoperative treatments. This should not be scored as a failure to attempt rescue12, but rather as a success in eliciting and honoring patient preferences for end-of-life care.
Our study has some limitations. The patients included in this review received treatment between 1977 and 2008. Significant changes in cancer treatment and palliative care occurred over this span of 31 years, including the evolution of effective medical management for MBO in 198560. In addition, patients described in these studies had a mix of primary malignancies. Different cancers have divergent behavior and variable response to treatment that can impact outcomes such as survival and rates of re-obstruction. However, all patients included in the present review had reached the common end point of MBO from peritoneal involvement and underwent palliative intervention. Additionally, it may not be possible to determine with certainty the cause of a bowel obstruction in patients with advanced cancer, particularly if they have had prior operations. This uncertainty can limit the usefulness of these data for a particular patient. This review is also limited by the poor reporting of palliative outcomes after surgery. Quality of life assessments after palliative surgery were rarely initiated, and studies that included quality of life measures were limited by a lack of pertinent assessment tools41,42. Research to adapt metrics of palliative care quality61 and quality of life assessment57 for surgical patients is needed to inform surgical decision making and surgical treatment for patients with terminal illness.
The information presented in this review can help surgeons and patients with difficult decisions for patients with terminal cancer. Palliative surgery for MBO can provide benefits, but patients risk serious complications, high rates of re-obstruction, and long hospitalizations. Surgeons can use these data to guide decisions about the role of surgery in the setting of incurable cancer and to advance preoperative discussions by determining patient preferences about burdensome postoperative treatments with unclear benefits. Palliative surgery can be valuable to patients; however, surgeons who provide this treatment should not be penalized for providing comfort for the terminally ill.
Supplementary Material
Acknowledgments
Authors’ sources of support:
Terrah Paul Olson is supported by the National Institutes of Health (T32 CA090217). Margaret Schwarze is supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021. These funding sources were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Authors’ potential conflicts of interest:
Terrah Paul Olson, Carolyn Pinkerton, Karen Brasel, and Margaret Schwarze have no conflicts of interest or relevant financial interests, activities, relationships, or affiliations to disclose.
Portions of this project were presented as a poster at Digestive Diseases Week in Orlando, FL, on May 20, 2013.
Supplementary information on the individual search strategies by database is included in an online-only table (eTable 1).
Author contributions:
Study conception and design: Terrah Paul Olson, Karen Brasel, Margaret Schwarze
Acquisition of data: Terrah Paul Olson, Carolyn Pinkerton
Analysis and interpretation of data: Terrah Paul Olson, Carolyn Pinkerton, Margaret Schwarze
Drafting of manuscript: Terrah Paul Olson, Margaret Schwarze
Critical revision of manuscript for important intellectual content: Carolyn Pinkerton, Karen Brasel
Statistical analysis: Terrah Paul Olson
Obtained funding: Margaret Schwarze
Supervision: Margaret Schwarze
Terrah Paul Olson and Margaret Schwarze had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Feuer DDJ, Broadley KE. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev. 2000;3(CD002764):1–29. doi: 10.1002/14651858.CD002764. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty A, Selby D, Gardiner K, Myers J, Moravan V, Wright F. Malignant bowel obstruction: natural history of a heterogeneous patient population followed prospectively over two years. J Pain Symptom Manage. 2011;41(2):412–420. doi: 10.1016/j.jpainsymman.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Gwilliam B, Bailey C. The nature of terminal malignant bowel obstruction and its impact on patients with advanced cancer. Int J Palliat Nurs. 2001;7(10):474–481. doi: 10.12968/ijpn.2001.7.10.9904. [DOI] [PubMed] [Google Scholar]
- 4.Borneman T, Chu DZJ, Wagman L, et al. Concerns of family caregivers of patients with cancer facing palliative surgery for advanced malignancies. Onc Nurs Forum. 2003;30(6):997–1005. doi: 10.1188/03.ONF.997-1005. [DOI] [PubMed] [Google Scholar]
- 5.Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer. 2008;44(8):1105–1115. doi: 10.1016/j.ejca.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Helyer L, Easson AM. Surgical approaches to malignant bowel obstruction. J Support Oncol. 2008;6(3):105–113. [PubMed] [Google Scholar]
- 7.Ripamonti CI, Twycross R, Baines M, et al. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer. 2001;9(4):223–233. doi: 10.1007/s005200000198. [DOI] [PubMed] [Google Scholar]
- 8.Soriano A, Davis MP. Malignant bowel obstruction: individualized treatment near the end of life. Cleve Clin J Med. 2011;78(3):197–206. doi: 10.3949/ccjm.78a.10052. [DOI] [PubMed] [Google Scholar]
- 9.Weeks JC, Cook F, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279(21):1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 10.Teno JM, Fisher ES, Hamel M, Coppola K, Dawson NV. Medical care inconsistent with patients’ treatment goals: association with 1-year Medicare resource use and survival. J Am Geriatr Soc. 2002;50(3):496–500. doi: 10.1046/j.1532-5415.2002.50116.x. [DOI] [PubMed] [Google Scholar]
- 11.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 12.Scarborough JE, Pappas TN, Bennett KM, Lagoo-Deenadayalan Failure-to-pursue rescue: explaining excess mortality in elderly emergency general surgical patients with preexisting “do-not-resuscitate” orders. Ann Surg. 2012;256(3):453–461. doi: 10.1097/SLA.0b013e31826578fb. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O’Connell D, et al. [Accessed December 10, 2012];The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 17.Shabanzadeh DM, Sorensen LT. Laparoscopic surgery compared with open surgery decreases surgical site infection in obese patients: a systematic review and meta-analysis. Ann Surg. 2012;256(6):934–945. doi: 10.1097/SLA.0b013e318269a46b. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy JD. A strategy for intestinal obstruction of peritoneal carcinomatosis. Arch Surg. 1986;121(9):1081–1082. doi: 10.1001/archsurg.1986.01400090113020. [DOI] [PubMed] [Google Scholar]
- 19.Turnbull AD, Guerra J, Starnes HF. Results of surgery for obstructing carcinomatosis of gastrointestinal, pancreatic, or biliary origin. J Clin Oncol. 1989;7(3):381–386. doi: 10.1200/JCO.1989.7.3.381. [DOI] [PubMed] [Google Scholar]
- 20.Lau PW, Lorentz TG. Results of surgery for malignant bowel obstruction in advanced, unresectable, recurrent colorectal cancer. Dis Colon Rectum. 1993;36(1):61–64. doi: 10.1007/BF02050303. [DOI] [PubMed] [Google Scholar]
- 21.van Ooijen B, van der Burg MEL, Planting ASTh, Siersema PD, Wiggers T. Surgical treatment or gastric drainage only for intestinal obstruction in patients with carcinoma of the ovary or peritoneal carcinomatosis of other origin. Surg Gynecol Obstet. 1993;176(5):469–474. [PubMed] [Google Scholar]
- 22.Blair SL, Chu DZJ, Schwarz RE. Outcome of palliative operations for malignant bowel obstruction in patients with peritoneal carcinomatosis from nongynecological cancer. Ann Surg Oncol. 2001;8(8):632–637. doi: 10.1007/s10434-001-0632-1. [DOI] [PubMed] [Google Scholar]
- 23.Legendre H, Vanhuyse F, Caroli-Bosc F-X, Pector J-C. Survival and quality of life after palliative surgery for neoplastic gastrointestinal obstruction. Eur J Surg Oncol. 2001;27(4):364–367. doi: 10.1053/ejso.2001.1120. [DOI] [PubMed] [Google Scholar]
- 24.Abbas SM, Merrie AE. Palliative small bowel surgery in patients with history of malignancy. Int J Cancer Res. 2006;2(1):42–46. [Google Scholar]
- 25.Abbas SM, Merrie AE. Resection of peritoneal metastases causing malignant small bowel obstruction. World J Surg Oncol. 2007;5(1):122–125. doi: 10.1186/1477-7819-5-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piver MS, Barlow JJ, Shashikant BL, Frank A. Survival after ovarian cancer induced intestinal obstruction. Gynecol Oncol. 1982;13(1):44–49. doi: 10.1016/0090-8258(82)90007-5. [DOI] [PubMed] [Google Scholar]
- 27.Lund B, Hansen M, Lundvall F, Nielsen NC, Sørensen BL, Hansen HH. Intestinal obstruction in patients with advanced carcinoma of the ovaries treated with combination chemotherapy. Surg Gynecol Obstet. 1989;169(3):213–218. [PubMed] [Google Scholar]
- 28.Rubin SC, Hoskins WJ, Benjamin I, Lewis JL. Palliative surgery for intestinal obstruction in advanced ovarian cancer. Gynecol Oncol. 34(1):16–19. doi: 10.1016/0090-8258(89)90097-8. [DOI] [PubMed] [Google Scholar]
- 29.Bais JMJ, Schilthuis MS, Slors JFM, Lammes FB. Intestinal obstruction in patients with advanced ovarian cancer. Int J Gynecol Cancer. 1995;5(5):346–350. doi: 10.1046/j.1525-1438.1995.05050346.x. [DOI] [PubMed] [Google Scholar]
- 30.Jong P, Sturgeon J, Jamieson CG. Benefit of palliative surgery for bowel obstruction in advanced ovarian cancer. Can J Surg. 1995;38(5):454–457. [PubMed] [Google Scholar]
- 31.Pothuri B, Vaidya A, Aghajanian C, Venkatraman E, Barakat RR, Chi DS. Palliative surgery for bowel obstruction in recurrent ovarian cancer: an updated series. Gynecol Oncol. 2003;89(2):306–313. doi: 10.1016/s0090-8258(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 32.Mangili G, Aletti G, Frigerio L, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer. 2005;15(5):830–835. doi: 10.1111/j.1525-1438.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- 33.Chi DS, Phaëton R, Miner TJ, et al. A prospective outcomes analysis of palliative procedures performed for malignant intestinal obstruction due to recurrent ovarian cancer. Oncologist. 2009;14(8):835–839. doi: 10.1634/theoncologist.2009-0057. [DOI] [PubMed] [Google Scholar]
- 34.Kim K, Kang SB, Kim MJ, et al. Factors associated with successful palliation and improved survival in patients with malignant bowel obstruction caused by ovarian cancer. J Women’s Med. 2009;2(2):54–58. [Google Scholar]
- 35.Kolomainen DF, Daponte A, Barton DPJ, et al. Outcomes of surgical management of bowel obstruction in relapsed epithelial ovarian cancer (EOC) Gynecol Oncol. 2012;125(1):31–36. doi: 10.1016/j.ygyno.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Diggs JC, Xu F, Diaz M, Cooper GS, Koroukian SM. Failure to screen: predictors and burdens of emergency colorectal cancer resection. Am J Manag Care. 2007;13(3):157–164. [PubMed] [Google Scholar]
- 37.McArdle CS, Hole DJ. Emergency presentation of colorectal cancer is associated with poor 5-year survival. Br J Surg. 2004;91(5):605–609. doi: 10.1002/bjs.4456. [DOI] [PubMed] [Google Scholar]
- 38.Patel SS, Patel MS, Goldfarb M, et al. Elective versus emergency surgery for ulcerative colitis: a National Surgical Quality Improvement Program analysis. Am J Surg. 2013;205(3):333–338. doi: 10.1016/j.amjsurg.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Tekkis PP, Kinsman R, Thompson MR, et al. The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg. 2004;240(1):76–81. doi: 10.1097/01.sla.0000130723.81866.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miner TJ, Jaques DP, Shriver CD. A prospective evaluation of patients undergoing surgery for the palliation of an advanced malignancy. Ann Surg Oncol. 2002;9(7):696–703. doi: 10.1007/BF02574487. [DOI] [PubMed] [Google Scholar]
- 41.Badgwell B, Krouse R, Cormier J, Guevara C, Klimberg VS, Ferrell B. Frequent and early death limits quality of life assessment in patients with advanced malignancies evaluated for palliative surgical intervention. Ann Surg Oncol. 2012;19(12):3651–3658. doi: 10.1245/s10434-012-2420-5. [DOI] [PubMed] [Google Scholar]
- 42.Selby D, Wright F, Stilos K, et al. Room for improvement? a quality-of-life assessment in patients with malignant bowel obstruction. Palliat Med. 2010;24(1):38–45. doi: 10.1177/0269216309346544. [DOI] [PubMed] [Google Scholar]
- 43.Podnos YD, Juarez G, Pameijer C, Uman G, Ferrel BR, Wagman LD. Surgical palliation of advanced gastrointestinal tumors. J Palliat Med. 2007;10(4):871–876. doi: 10.1089/jpm.2006.0174. [DOI] [PubMed] [Google Scholar]
- 44.Podnos YD, Juarez G, Pameijer C, Choi K, Ferrell BR, Wagman LD. Impact of surgical palliation on quality of life in patients with advanced malignancy: results of the Decisions and Outcomes in Palliative Surgery (DOPS) trial. Ann Surg Oncol. 2007;14(2):922–928. doi: 10.1245/s10434-006-9238-y. [DOI] [PubMed] [Google Scholar]
- 45.Schwarze ML, Bradley CT, Brasel KJ. Surgical “buy-in”: the contractual relationship between surgeons and patients that influences decisions regarding life-supporting therapy. Crit Care Med. 2010;38(3):843–848. doi: 10.1097/CCM.0b013e3181cc466b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarze ML, Redmann AJ, Alexander GC, Brasel KJ. Surgeons expect patients to buy-in to postoperative life support preoperatively: results of a national survey. Crit Care Med. 2013;41(1):1–8. doi: 10.1097/CCM.0b013e31826a4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cassell J, Buchman TG, Streat S, Stewart RM. Surgeons, intensivists, and the covenant of care: administrative models and values affecting care at the end of life – updates. Crit Care Med. 2003;31(5):1551–1557. [PubMed] [Google Scholar]
- 48.Schwarze ML, Redmann AJ, Brasel KJ, Alexander GC. The role of surgeon error in withdrawal of postoperative life support. Ann Surg. 2012;256(1):10–15. doi: 10.1097/SLA.0b013e3182580de5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penkoske PA, Buchman TG. The relationship between the surgeon and the intesivist in the surgical intensive care unit. Surg Clin North Am. 2006;86(6):1351–1357. doi: 10.1016/j.suc.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Ferrell BR, Chu DZJ, Wagman L, et al. Patient and surgeon decision making regarding surgery for advanced cancer. Oncol Nurs Forum. 2003;30(6):E106–E114. doi: 10.1188/03.ONF.E106-E114. [DOI] [PubMed] [Google Scholar]
- 51.Krouse RS, McCahill LE, Easson AM, Dunn GP. When the sun can set on an upoperated bowel obstruction: management of malignant bowel obstruction. J Am Coll Surg. 2002;195(1):117–128. doi: 10.1016/s1072-7515(02)01223-1. [DOI] [PubMed] [Google Scholar]
- 52.Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616–1625. doi: 10.1056/NEJMoa1204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J Palliat Med. 2000;3(3):287–300. doi: 10.1089/jpm.2000.3.287. [DOI] [PubMed] [Google Scholar]
- 54.Krouse RS, Nelson RA, Ferrell BR, et al. Surgical palliation at a cancer center: incidence and outcomes. Arch Surg. 2001;136(7):773–778. doi: 10.1001/archsurg.136.7.773. [DOI] [PubMed] [Google Scholar]
- 55.Miner TJ, Brennan MF, Jaques DP. A prospective, symptom related, outcomes analysis of 1022 palliative procedures for advanced cancer. Ann Surg. 2004;240(4):719–727. doi: 10.1097/01.sla.0000141707.09312.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCahill LE, Krouse RS, Chu DZJ, et al. Indications and use of palliative surgery – results of Society of Surgical Oncology survey. Ann Surg Oncol. 2002;9(1):104–112. doi: 10.1245/aso.2002.9.1.104. [DOI] [PubMed] [Google Scholar]
- 57.McCahill LE, Smith DD, Borneman T, et al. A prospective evaluation of palliative outcomes for surgery of advanced malignancies. Ann Surg Oncol. 2003;10(6):654–663. doi: 10.1245/aso.2003.06.011. [DOI] [PubMed] [Google Scholar]
- 58.McCahill LE, Krouse RS, Chu DZJ, et al. Decision making in palliative surgery. J Am Coll Surg. 2002;195(3):411–422. doi: 10.1016/s1072-7515(02)01306-6. [DOI] [PubMed] [Google Scholar]
- 59.Miner TJ, Jaques DP, Tavat-Motamen H, Shriver CD. Decision making on surgical palliation based on patient outcome data. Am J Surg. 1999;177(2):150–154. doi: 10.1016/s0002-9610(98)00323-7. [DOI] [PubMed] [Google Scholar]
- 60.Baines M, Oliver DJ, Carter RL. Medical management of intestinal obstruction in patients with advanced malignant disease. A clinical and pathological study. Lancet. 1985;2(8462):990–993. doi: 10.1016/s0140-6736(85)90534-3. [DOI] [PubMed] [Google Scholar]
- 61.Pasman HR, Brandt HE, Deliens L, Francke AL. Quality indicators for palliative care: a systematic review. J Pain Symptom Manage. 2009;38(1):145–156. doi: 10.1016/j.jpainsymman.2008.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.