Abstract
Nearly every movement made by every animal is accompanied by a corollary discharge, a signal sent from the motor to sensory regions of its brain. Corollary discharges are a crucial mechanism by which the brain monitors its own actions, and there is some evidence that they are impaired in people with schizophrenia. Here, I briefly review this evidence and suggest that eye movements are a particularly valuable tool for assessing the role of corollary discharges in schizophrenia.
Keywords: Schizophrenia, Saccades, Vision, Corollary Discharge, Neurons
In his pioneering experiments on epileptic patients, Wilder Penfield found that electrical stimulation, when applied to the superior temporal gyrus of the human brain, often caused the subject to hear voices. The same stimulation applied to other brain regions elicited the appearance of shapes and colors, movement of the limbs, or the recall of memories. Penfield concluded a review of his successes in mapping the brain with a description of one consistent failure: “There is no place in the cerebral cortex where electrical stimulation will cause a patient to believe or to decide.” Penfield was referring to the fact that his patients were always aware that the electrode was causing them to hear, to move, or to remember; a typical response from a patient induced to vocalize during stimulation: “I didn’t make that sound. You [Penfield] pulled it out of me.”1
Clearly the electrical stimulation in Penfield’s experiments activated circuits normally dedicated to very specific functions. But this activation was not by itself sufficient to mimic natural brain function. Normally, the recall of a memory or the execution of a movement is accompanied by something akin to intention, which is represented separately within the brain. The neural responses that signal intention are often called corollary discharges.
Corollary discharges need not be associated with conscious intentions; indeed they are found throughout the animal kingdom, including in animals such as nematodes, which have very primitive nervous systems.2 In many cases, they originate from neurons that are fundamentally involved in controlling the movement of an effector, and they project to brain regions that are primarily sensory (Figure 1). Functionally, their role is straightforward: They inform sensory regions of the likely consequences of an impending movement. Thus, to take one of many possible examples, when a cricket elicits a chirp, motor structures in its brain cause contractions of muscles that rub the forewings together; these same structures send a well-timed corollary discharge that inhibits the auditory region, effectively turning off hearing in concert with the chirp. As a result the cricket is insensitive to its own chirping and more attuned to sounds emanating from external sources. Similar mechanisms are found in the bat’s echolocation system, the electrical organ function of certain fish, and the oculomotor system of primates.2
Figure 1.
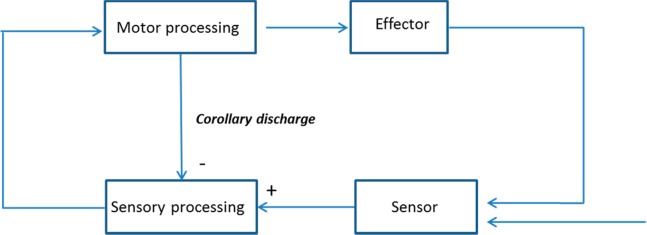
Canonical corollary discharge circuit. Neurons in brain regions dedicated to controlling specific effectors send copies of their motor commands to sensory regions. These corollary discharges allow sensory systems to monitor the activities of the motor system, providing for more effective sensory processing.
Corollary discharge mechanisms are such an integral part of the nervous system that one might wonder what happens when they fail to function properly. In such cases, one might expect deficits that are just as devastating as sensory agnosias or motor ataxias, but perhaps with subtler manifestations. One answer to this question is suggested by the mounting evidence for a corollary discharge dysfunction in schizophrenia.3 To make the link, consider the report of a person with schizophrenia, who described the onset of her symptoms as follows: “I increasingly heard voices... I concluded that other people were putting these ‘loud thoughts’ in my head.”.4 The situation is rather similar to the report of Penfield’s patients, who attributed specific aspects of their brain activity to external sources. The difference, of course, is that the corollary discharge was absent by design in Penfield’s experiments. The situation is far more complex in schizophrenia, but the reasoning is the same: An impairment in the corollary discharge associated with the generation of a thought will naturally lead to the notion that the thought has arisen through external sources, with the result being the delusions of mind control and thought insertion that are frequently observed in people with schizophrenia. Similar arguments apply to auditory hallucinations and other positive symptoms.
Early evidence for reduced corollary discharge function in people with schizophrenia came from experiments by Judith Ford and colleagues.5 They examined human auditory perception during speech, which involves a corollary discharge similar to that of the cricket described above. During speech, people are generally only dimly aware of the sounds of their own voices, and there is good evidence that the motor systems responsible for producing vocalizations send a corollary discharge to inhibit concurrent auditory processing. Ford and colleagues examined a cortical correlate of this inhibition, by recording event-related potentials associated with audition, in people with schizophrenia and in healthy controls. The key manipulation was to compare the magnitude of the responses to active speech (when the corollary discharge was presumably active) and passive listening to the same speech (when the corollary discharge was absent).
If the corollary discharge carries out the hypothesized function, then the auditory response during passive listening should be greater than that during active speech. Indeed, this is precisely what was found in both cohorts of subjects. However, the attenuation was significantly smaller in people with schizophrenia, whose potentials were more similar in the active and passive listening conditions. The results thus supported the theory that corollary discharge activity is less effective in schizophrenia; similar results have subsequently been found in other sensory domains as well.
One particularly promising avenue for investigating corollary discharge signals involves the brain systems responsible for planning and executing eye movements. Humans and other animals typically move their eyes several times per second, using ballistic movements known as saccades. Each saccade serves the important function of pointing the high-resolution foveal region of the retina at a specific point in space. As such, they facilitate our ability to explore our environments. However, they also introduce additional complexity, as the retinal locations of objects shift with each saccade. Consequently the brain needs to know where the eye is pointing in order to make sense of its surroundings. That is, an object may jump from the left visual field to the right during the execution of a saccade, even though it maintains the same position in absolute space.
Because of extensive work in monkeys, the neural circuits responsible for controlling eye movements are among the best understood aspects of the primate brain. Recent research in this area has revealed a detailed picture of the corollary discharge associated with saccades:2 Each saccade triggers a corollary discharge that arises initially in the superior colliculus, a brainstem nucleus and a key part of the oculomotor system. From the colliculus, the corollary discharge propagates through the medial dorsal nucleus of the thalamus to the frontal eye fields, after which it is distributed to visual structures in the temporal and parietal lobes.
This pathway has been shown to be crucial for keeping track of where one’s eye is pointing, especially during sequences of saccades. When it is inactivated by pharmacological blockade, animals consistently make errors in estimating their own eye positions following a saccade. This suggests that corollary discharges contribute to the ability to monitor one’s own eye movements, and consequently to keep track of the locations of visual objects during sequences of saccades.
Building on these findings, recent psychophysical work has examined the saccade-related corollary discharge in people with schizophrenia.6 Subjects were asked to make a saccade to a visual target and then to indicate the perceived position of a second object presented briefly around the time of the saccade. This task, like the monkey experiments described above, is often used as a probe of corollary discharge function, because it requires subjects to have access to an internal estimate of their own eye position. The results showed that people with schizophrenia were less accurate than age-matched controls in performing the task: They tended to make larger errors than controls in localizing the positions of objects presented around the time of each saccade. Additional analysis showed that the pattern of errors could be accounted for under the assumption that the patients with schizophrenia had a noisier corollary discharge signal. Interestingly, the noisiness of the signal was correlated with the magnitude of self-reported schizophrenia symptoms in the patient cohort.
This last result strongly supports Feinberg’s corollary discharge theory of schizophrenia, but it raises the key question of why the corollary discharge dysfunction should be so widespread across different systems within individual brains. That is, why should patients who experience auditory hallucinations or disturbances of thought be impaired in their ability to monitor their eye positions? This is an area of active investigation, with proposals including synaptic level dysfunctions3 and abnormal development of white matter fasciculi.7 The convergence of results from animal studies, human psychophysics, and computer modeling suggests the eye movement system as particularly fertile ground for making progress in this area.
Some of the work described in this paper was supported by grants from NSERC (341534-12) and NARSAD to the author.
The authors declare no competing financial interest.
References
- Penfield W. (1974) The mind and the highest brain-mechanism. Am. Scholar 443, 237–246. [Google Scholar]
- Crapse T. B.; Sommer M. A. (2008) Corollary discharge across the animal kingdom. Nat. Rev. Neurosci. 9, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I.; Guazzelli M. (1999) Schizophrenia—a disorder of the corollary discharge systems that integrate the motor systems of thought with the sensory systems of consciousness. Br J. Psychiatry 174, 196–204. [DOI] [PubMed] [Google Scholar]
- Bockes Z. (1985) First person account: ″freedom″ means knowing you have a choice. Schizophr. Bull. 11, 487–489. [DOI] [PubMed] [Google Scholar]
- Ford J. M.; Mathalon D. H.; Heinks T.; Kalba S.; Faustman W. O.; Roth W. T. (2001) Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am. J. Psychiatry 158, 2069–2071. [DOI] [PubMed] [Google Scholar]
- Richard A.; Churan J.; Whitford V.; O’Driscoll G. A.; Titone D.; Pack C. C. (2014) Perisaccadic perception of visual space in people with schizophrenia. J. Neurosci. 34, 4760–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford T. J.; Ford J. M.; Mathalon D. H.; Kubicki M.; Shenton M. E. (2012) Schizophrenia, myelination, and delayed corollary discharges: a hypothesis. Schizophr. Bull. 38, 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]


