Abstract
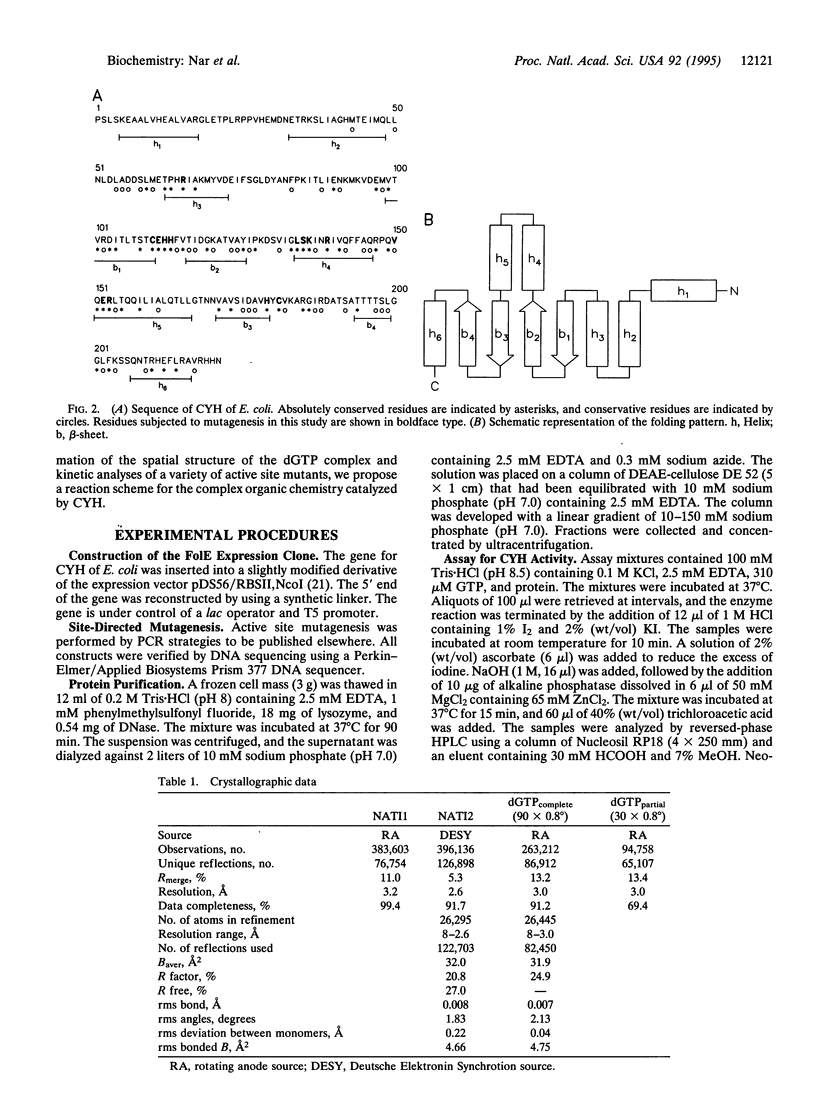
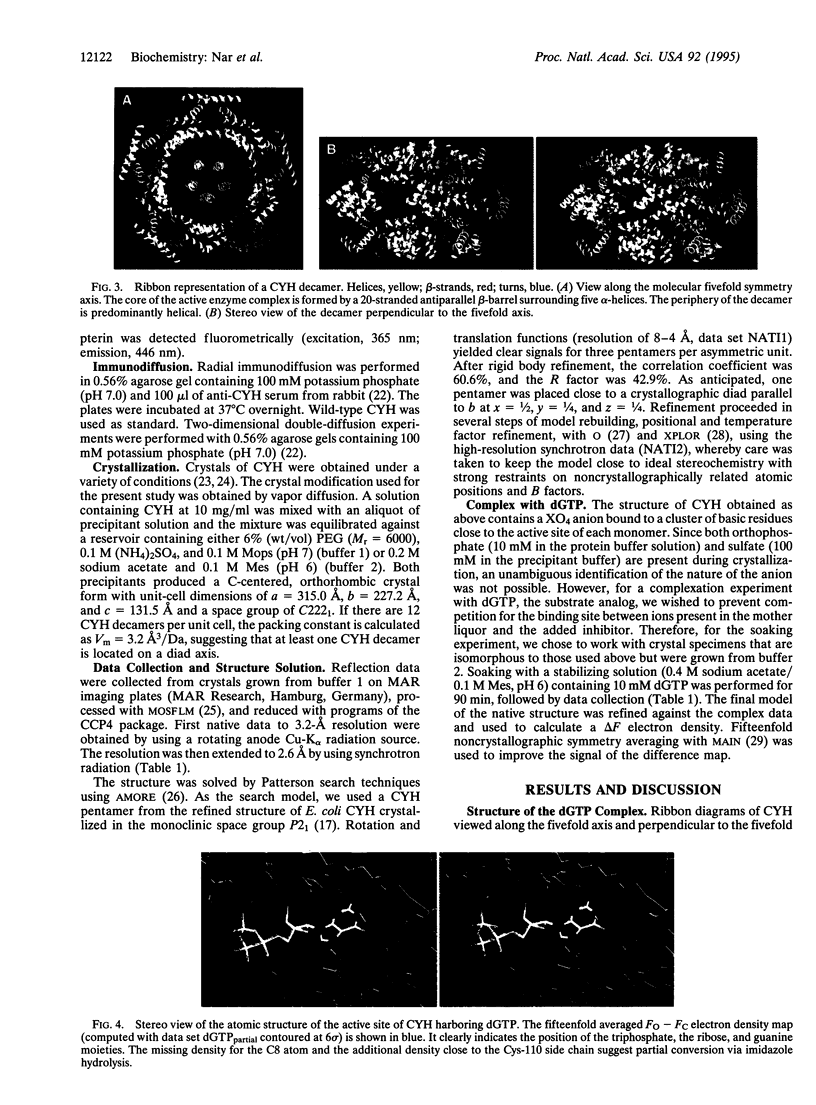
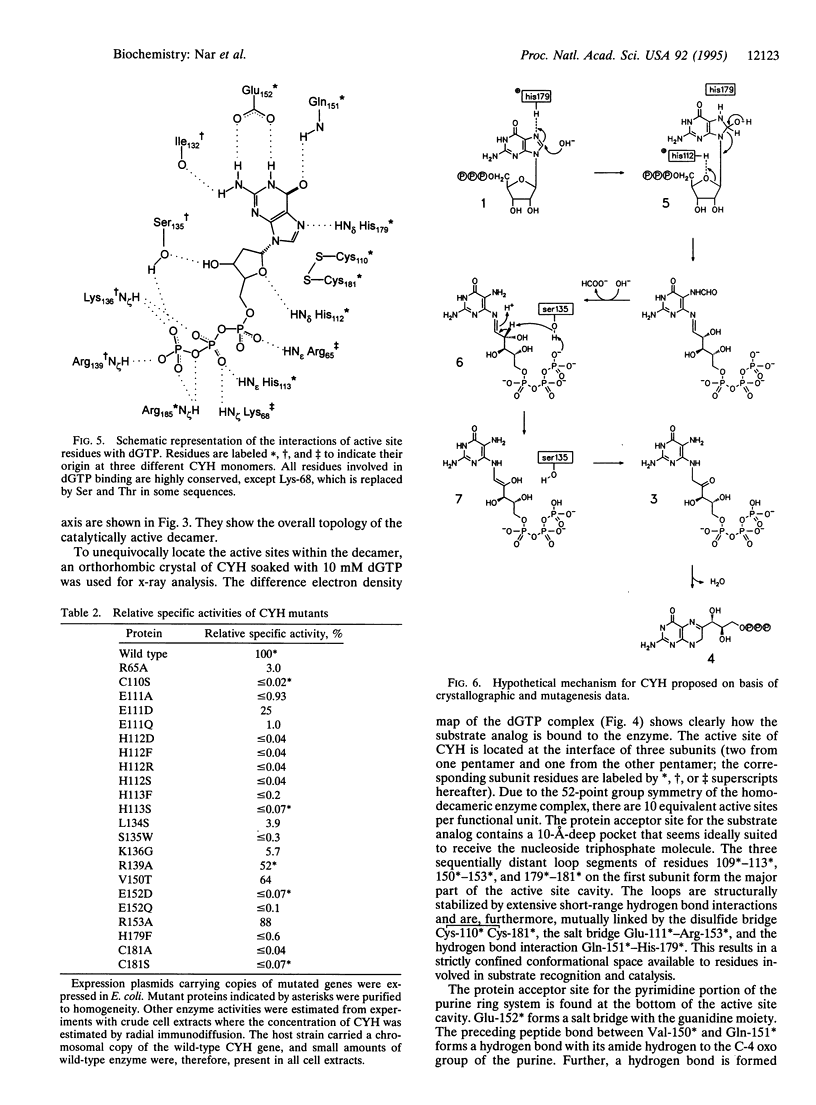
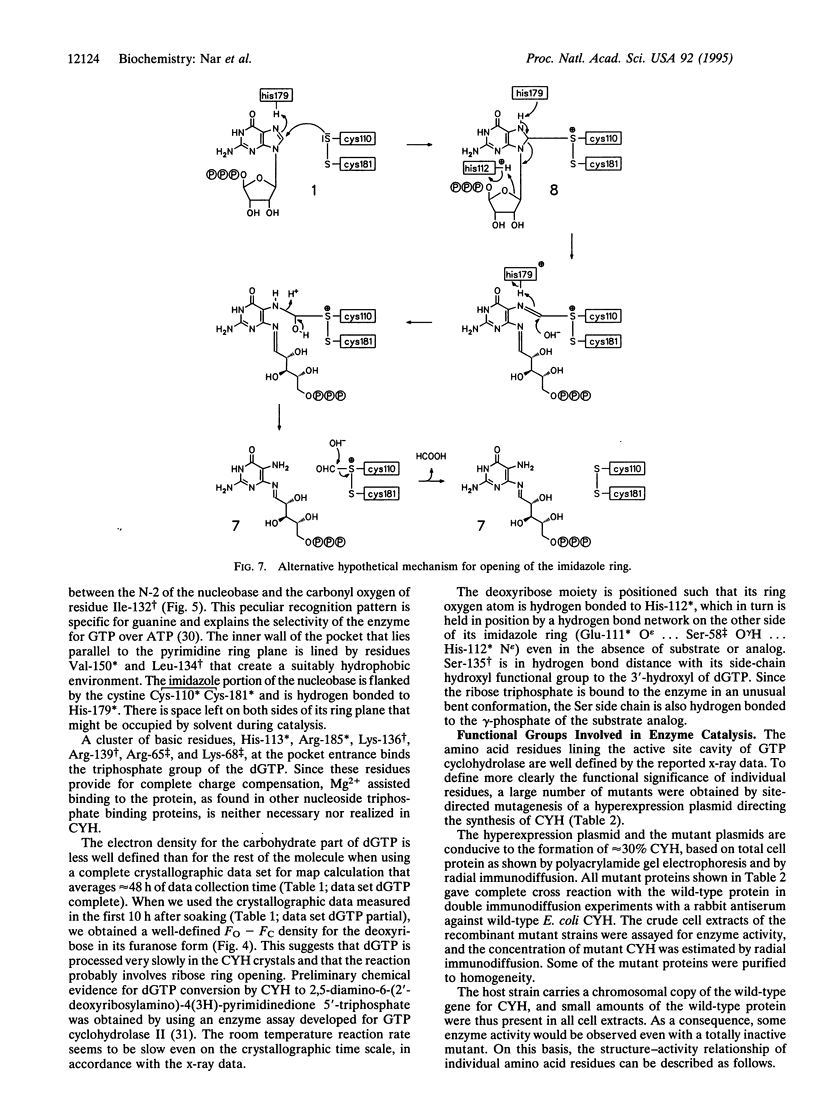
GTP cyclohydrolase I of Escherichia coli is a torus-shaped homodecamer with D5 symmetry and catalyzes a complex ring expansion reaction conducive to the formation of dihydroneopterin triphosphate from GTP. The x-ray structure of a complex of the enzyme with the substrate analog, dGTP, bound at the active site was determined at a resolution of 3 A. In the decamer, 10 equivalent active sites are present, each of which contains a 10-A deep pocket formed by surface areas of 3 adjacent subunits. The substrate forms a complex hydrogen bond network with the protein. Active site residues were modified by site-directed mutagenesis, and enzyme activities of the mutant proteins were measured. On this basis, a mechanism of the enzyme-catalyzed reaction is proposed. Cleavage of the imidazole ring is initiated by protonation of N7 by His-179 followed by the attack of water at C8 of the purine system. Cystine Cys-110 Cys-181 may be involved in this reaction step. Opening of the imidazole ring may be in concert with cleavage of the furanose ring to generate a Schiff's base from the glycoside. The gamma-phosphate of GTP may be involved in the subsequent Amadori rearrangement of the carbohydrate side chain by activating the hydroxyl group of Ser-135.
Full text
PDF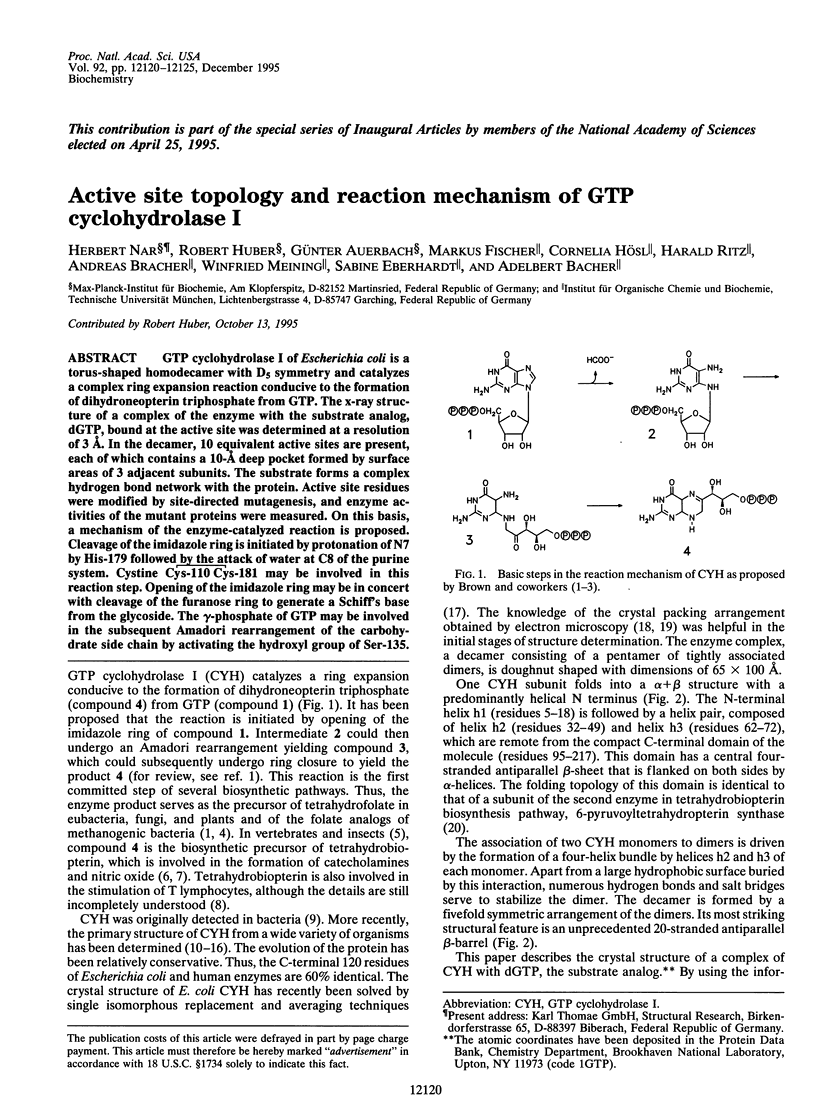

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babitzke P., Gollnick P., Yanofsky C. The mtrAB operon of Bacillus subtilis encodes GTP cyclohydrolase I (MtrA), an enzyme involved in folic acid biosynthesis, and MtrB, a regulator of tryptophan biosynthesis. J Bacteriol. 1992 Apr;174(7):2059–2064. doi: 10.1128/jb.174.7.2059-2064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg A. W., Brown G. M. The biosynthesis of folic acid. 8. Purification and properties of the enzyme that catalyzes the production of formate from carbon atom 8 of guanosine triphosphate. J Biol Chem. 1968 May 10;243(9):2349–2358. [PubMed] [Google Scholar]
- Burg A. W., Brown G. M. The biosynthesis of folic acid. VI. Enzymatic conversion of carbon atom 8 of guanosine triphosphate to formic acid. Biochim Biophys Acta. 1966 Mar 28;117(1):275–278. doi: 10.1016/0304-4165(66)90180-2. [DOI] [PubMed] [Google Scholar]
- FINK K., ADAMS W. S., PFLEIDERER W. A NEW URINARY PYRIMIDINE, 5-ACETYLAMINO-6-AMINO-3-METHYLURACIL. ITS ISOLATION, IDENTIFICATION, AND SYNTHESIS. J Biol Chem. 1964 Dec;239:4250–4256. [PubMed] [Google Scholar]
- Hatakeyama K., Inoue Y., Harada T., Kagamiyama H. Cloning and sequencing of cDNA encoding rat GTP cyclohydrolase I. The first enzyme of the tetrahydrobiopterin biosynthetic pathway. J Biol Chem. 1991 Jan 15;266(2):765–769. [PubMed] [Google Scholar]
- Katzenmeier G., Schmid C., Kellermann J., Lottspeich F., Bacher A. Biosynthesis of tetrahydrofolate. Sequence of GTP cyclohydrolase I from Escherichia coli. Biol Chem Hoppe Seyler. 1991 Nov;372(11):991–997. doi: 10.1515/bchm3.1991.372.2.991. [DOI] [PubMed] [Google Scholar]
- Kwon N. S., Nathan C. F., Stuehr D. J. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989 Dec 5;264(34):20496–20501. [PubMed] [Google Scholar]
- Lacks S. A., Greenberg B., Lopez P. A cluster of four genes encoding enzymes for five steps in the folate biosynthetic pathway of Streptococcus pneumoniae. J Bacteriol. 1995 Jan;177(1):66–74. doi: 10.1128/jb.177.1.66-74.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J. R., Krishnakumar S., O'Donnell J. M. Multiple mRNAs from the Punch locus of Drosophila melanogaster encode isoforms of GTP cyclohydrolase I with distinct N-terminal domains. J Biol Chem. 1993 Dec 25;268(36):27191–27197. [PubMed] [Google Scholar]
- Meining W., Bacher A., Bachmann L., Schmid C., Weinkauf S., Huber R., Nar H. Elucidation of crystal packing by X-ray diffraction and freeze-etching electron microscopy. Studies on GTP cyclohydrolase I of Escherichia coli. J Mol Biol. 1995 Oct 13;253(1):208–218. doi: 10.1006/jmbi.1995.0545. [DOI] [PubMed] [Google Scholar]
- Nar H., Huber R., Heizmann C. W., Thöny B., Bürgisser D. Three-dimensional structure of 6-pyruvoyl tetrahydropterin synthase, an enzyme involved in tetrahydrobiopterin biosynthesis. EMBO J. 1994 Mar 15;13(6):1255–1262. doi: 10.1002/j.1460-2075.1994.tb06377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nar H., Huber R., Meining W., Schmid C., Weinkauf S., Bacher A. Atomic structure of GTP cyclohydrolase I. Structure. 1995 May 15;3(5):459–466. doi: 10.1016/s0969-2126(01)00179-4. [DOI] [PubMed] [Google Scholar]
- Nichol C. A., Smith G. K., Duch D. S. Biosynthesis and metabolism of tetrahydrobiopterin and molybdopterin. Annu Rev Biochem. 1985;54:729–764. doi: 10.1146/annurev.bi.54.070185.003501. [DOI] [PubMed] [Google Scholar]
- Nomura T., Ichinose H., Sumi-Ichinose C., Nomura H., Hagino Y., Fujita K., Nagatsu T. Cloning and sequencing of cDNA encoding mouse GTP cyclohydrolase I. Biochem Biophys Res Commun. 1993 Mar 15;191(2):523–527. doi: 10.1006/bbrc.1993.1249. [DOI] [PubMed] [Google Scholar]
- Richter G., Ritz H., Katzenmeier G., Volk R., Kohnle A., Lottspeich F., Allendorf D., Bacher A. Biosynthesis of riboflavin: cloning, sequencing, mapping, and expression of the gene coding for GTP cyclohydrolase II in Escherichia coli. J Bacteriol. 1993 Jul;175(13):4045–4051. doi: 10.1128/jb.175.13.4045-4051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C., Ladenstein R., Luecke H., Huber R., Bacher A. Crystallization and preliminary crystallographic characterization of GTP cyclohydrolase I from Escherichia coli. J Mol Biol. 1992 Aug 20;226(4):1279–1281. doi: 10.1016/0022-2836(92)91067-y. [DOI] [PubMed] [Google Scholar]
- Schmid C., Meining W., Weinkauf S., Bachmann L., Ritz H., Eberhardt S., Gimbel W., Werner T., Lahm H. W., Nar H. Studies on GTP cyclohydrolase I of Escherichia coli. Adv Exp Med Biol. 1993;338:157–162. doi: 10.1007/978-1-4615-2960-6_30. [DOI] [PubMed] [Google Scholar]
- Tayeh M. A., Marletta M. A. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989 Nov 25;264(33):19654–19658. [PubMed] [Google Scholar]
- Togari A., Ichinose H., Matsumoto S., Fujita K., Nagatsu T. Multiple mRNA forms of human GTP cyclohydrolase I. Biochem Biophys Res Commun. 1992 Aug 31;187(1):359–365. doi: 10.1016/s0006-291x(05)81501-3. [DOI] [PubMed] [Google Scholar]
- Wolf W. A., Brown G. M. The biosynthesis of folic acid. X. Evidence for an Amadori rearrangement in the enzymatic formation of dihydroneopterin triphosphate from GTP. Biochim Biophys Acta. 1969 Dec 30;192(3):468–478. doi: 10.1016/0304-4165(69)90396-1. [DOI] [PubMed] [Google Scholar]
- Yim J. J., Brown G. M. Characteristics of guanosine triphosphate cyclohydrolase I purified from Escherichia coli. J Biol Chem. 1976 Aug 25;251(16):5087–5094. [PubMed] [Google Scholar]
- Ziegler I. Production of pteridines during hematopoiesis and T-lymphocyte proliferation: potential participation in the control of cytokine signal transmission. Med Res Rev. 1990 Jan-Mar;10(1):95–114. doi: 10.1002/med.2610100104. [DOI] [PubMed] [Google Scholar]





