Abstract
Background and Aims
The order Piperales has the highest diversity of growth forms among the earliest angiosperm lineages, including trees, shrubs, climbers and herbs. However, within the perianth-bearing Piperales (Asarum, Saruma, Lactoris, Hydnora, Prosopanche, Thottea and Aristolochia), climbing species only occur in the most species-rich genus Aristolochia. This study traces anatomical and morphological traits among these lineages, to detect trends in growth form evolution and developmental processes.
Methods
Transverse stem sections of different developmental stages of representatives of Asarum, Saruma, Lactoris, Hydnora, Thottea and Aristolochia were compared and anatomical traits were linked to growth form evolution. Biomechanical properties of representative climbers were determined in three-point bending tests and are discussed based on the anatomical observations. Growth form evolution of the perianth-bearing Piperales was reconstructed by ancestral character state reconstruction using Mesquite.
Key Results
While species of Asarum and Saruma are exclusively herbaceous, species of the remaining genera show a higher diversity of growth habit and anatomy. This growth form diversity is accompanied by a more complex stem anatomy and appropriate biomechanical properties. The ancestral growth form of the perianth-bearing Piperales is reconstructed with either a shrub-like or herbaceous character state, while the following three backbone nodes in the reconstruction show a shrub-like character state. Accordingly, the climbing habit most probably evolved in the ancestor of Aristolochia.
Conclusions
Since the ancestor of the perianth-bearing Piperales has been reconstructed with a herb- or shrub-like habit, it is proposed that the climbing habit is a derived growth form, which evolved with the diversification of Aristolochia, and might have been a key feature for its diversification. Observed anatomical synapomorphies, such as the perivascular fibres in Lactoris, Thottea and Aristolochia, support the phylogenetic relationship of several lineages within the perianth-bearing Piperales. In addition, the hypothesis that the vegetative organs of the holoparasitic Hydnoraceae are most probably rhizomes is confirmed.
Keywords: Aristolochia, Thottea, Lactoris, Hydnora, Asarum, Saruma, growth form, anatomy, biomechanics, secondary woodiness, heterochrony, perianth-bearing Piperales
INTRODUCTION
Piperales are known for their unique diversity of growth forms among the earliest angiosperm lineages (Isnard et al., 2012). Most recent molecular phylogenetic studies have sub-divided Piperales into a perianth-less clade (Piperaceae and Saururaceae) and a perianth-bearing clade (Aristolochiaceae, Lactoridaceae and Hydnoraceae) (Naumann et al., 2013). Within the latter, the following genera are included: Asarum, Saruma, Lactoris, Hydnora, Prosopanche, Thottea and Aristolochia. The most species-rich genus, Aristolochia, is well known for its large number of climbing species (approx. 350 species), with few species described as herbaceous (including geophytes) or shrub-like (e.g. Pfeifer, 1966; Isnard et al., 2012; Wagner et al., 2012). Herbaceous or shrub-like forms are characteristic of the remaining perianth-bearing Piperales genera (Isnard et al., 2012).
Climbers have evolved multiple times independently in many plant families (Gentry, 1991), and the climbing habit has been posited as a key innovation in angiosperms (Gianoli, 2004). While it has been shown that many climbing taxa are more diverse than their non-climbing sister groups (Gianoli, 2004), there is evidence that the majority of climbing species belong to only a small number of families (Gentry, 1991). This indicates that, even though the climbing habit has appeared numerous times in plant evolutionary history, this growth habit has only been successful in a few lineages (Gentry, 1991; Ibarra-Manríquez and Martínez-Ramos, 2002). The high growth form diversity and the dominance of climbing species makes Aristolochia and its relatives an interesting model for comparative studies concerning the evolution of the climbing habit as well as transitions between growth forms. Transitions from one plant growth habit to another, for example from an upright self-supporting one to a more flexible climbing one, require changes in the stem properties, including changes in tissue properties and/or tissue distribution.
In general, climbing plants are widely distributed; they contribute significantly to the diversity of tropical habitats and play important roles in gap succession and gap formation (Schnitzer and Bongers, 2002). For example, climbing plants can locally contribute up to 25–40 % of the total number of woody species, as well as up to a quarter of the total above-ground biomass in tropical forests (Jacobs, 1988; Gentry, 1991; Appanah et al., 1993; Gerwing and Lopes Farias, 2000; Pérez-Salicrup et al., 2001; Bongers et al., 2002; Schnitzer and Bongers, 2002). In addition, up to 50 % of larger tropical trees have climbers in their crowns (Gentry, 1991; Ingwell et al., 2010; Campanello et al., 2012). Although climbing plants have fascinated evolutionary biologists since the time of Charles Darwin, little is known about their functionality and evolutionary pathways favouring growth form shifts towards the climbing habit. In addition to their ecological importance, climbers also show a fascinating diversity of derived anatomical, morphological and physiological characteristics (Isnard and Silk, 2009). The specialization towards a limited capacity for upright growth results in a dependency on adjacent support, which requires mechanisms of attachment by ‘searcher’ shoots, as well as strategies to climb up and deal with movements and failures (tree falls). The reduced need for climbers to carry their own weight led to a general reduction in secondary thickening/growth, increased internode lengths and a potentially rapid colonization into the forest canopy (Putz and Holbrook, 1991). Thus, the relationship of leaf mass to transverse stem area is relatively high compared with trees, shrubs or herbaceous plants (Ewers, 1985), and therefore requires effective strategies for water transport and stem integrity under mechanical stress. In many climbers, vessel diameter is much larger compared with closely related self-supporting plants, which contributes to an effective xylem flux (Ewers et al., 1991). However, water flow can even be maintained while the stem is twisted several times (Putz and Holbrook, 1991). In addition, many eudicot climbers are characterized by anomalous secondary growth (i.e. cambial variants), such as successive cambia, interxylary phloem, lobed or flattened stems and increased phloem proliferation (Carlquist, 1991), which further lead to mechanical and physiological properties potentially unique to climbers. However, cambial variants have not been observed in climbers of early-diverging angiosperm lineages; instead, these climbers are often characterized by, for example, wide parenchymatous rays and large vessels (Carlquist, 1999, 2001; Feild et al., 2003; Isnard et al., 2012; Wagner et al., 2012; Feild and Isnard, 2013).
The genus Aristolochia consists of about 450 species in three subgenera: Aristolochia, Siphisia (previously erroneously considered as subgenus Isotrema) and Pararistolochia (Wanke et al., 2006). The subgenus Aristolochia contains approx. 350 species of mostly woody tropical climbers. However, herbaceous forms, including species from the Mediterranean region, are also known (Neinhuis et al., 2005). The second largest subgenus Siphisia (approx. 70 species) occurs in Asia as well as in North and Central America, and contains herbs, climbers and shrub-like species. Besides the shrubs, Siphisia contains two types of climbers characterized by different anatomical and biomechanical properties (Wagner et al., 2012, here Siphisia is named Isotrema). To avoid confusion when referring to the results of our recent publications, we use here the name Isotrema for the clade properly named Siphisia. The smallest subgenus Pararistolochia (approx. 35 species), distributed in tropical Australasia and Africa, contains exclusively climbers. The perianth-bearing relatives of Aristolochia are either shrubs (Thottea, approx. 44 species; Lactoris, monotypic), perennial herbs (Asarum, approx. 85 species; Saruma, monotypic) or holoparasites (Prosopanche and Hydnora), the latter with a highly modified sub-terranean bauplan (e.g. González and Stevenson, 2000; Neinhuis et al., 2005; Ohi-Toma et al., 2006; Wanke et al., 2006, 2007; Tennakoon et al., 2007; Oelschlägel et al., 2011; Naumann et al., 2013; Yao, 2013).
By comparing anatomy and growth forms within Aristolochia and its perianth-bearing relatives, we address the following questions. Which anatomical stem traits and trends in development occur in Aristolochia and its relatives? How do the observed traits and biomechanical properties influence growth habits within Aristolochia? What are the potential evolutionary constraints and developmental processes linked to the appearance of climbers, including transitions in growth forms among herbs, shrubs and climbers?
MATERIALS AND METHODS
Plant material and taxon sampling
In order to trace anatomical trends within the perianth-bearing Piperales, we sampled representatively among all main clades within the order (Table 1). Biomechanical properties of representative climbers of Aristolochia subgenera Aristolochia and Pararistolochia were determined and compared with biomechanical properties of climbers and shrubs of the subgenus Isotrema from Wagner et al. (2012). In addition, biomechanical data of herbs, climbers and shrubs of Thottea, Saruma, and Asarum from Isnard et al. (2012) are included. Lactoris, Prosopanche and Hydnora have not been included in the biomechanical analyses, since material was either unavailable (Lactoris) or in the case of Hydnoraceae was not amenable to mechanical tests because of their highly modified and entirely sub-terranean plant body. Plant material for biomechanics was obtained from the Botanical Gardens of Dresden and Bonn, Germany, or directly from fieldwork in Mexico, and was complemented for anatomical investigation with material obtained from herbaria.
Table 1.
Number of species assigned to each taxonomic unit of Aristolochiaceae and relatives, used for ancestral character state reconstruction, and representative species used for anatomical and/or biomechanical investigations; clades according to Wanke et al. (2006)
| Genus/subgenus | Clade | Number of species and growth form | Representatives sampled | Origin of material (BG with Garden accession, and/or field origin with voucher number |
|---|---|---|---|---|
| Aristolochia subgenus Pararistolochia | 35 climbers | A. promissa Mast. | BG Dresden (013523-14) | |
| A. triactina Hook.f. | BG Dresden (013524-15) | |||
| Aristolochia subgenus Isotrema | Endodeca | 2 herbs | A. serpentaria L. | Neinhuis 112 (DR); BG Dresden |
| As/NA clade | 55 climbers | A. tomentosa* Sims | BG Dresden (006146-17) | |
| A. westlandii Hemsl.* | Oelschlägel 022 (DR); BG Dresden | |||
| CA clade | 5 shrubs, 10 climbers | A. malacophylla Standl.* | Samain et al. 2009-282 (MEXU, DR); Mexico, Chiapas, Quixjob | |
| A. chiapensis J.F. Ortega & R.V. Ortega* | Samain et al. 2009-288 (MEXU, DR); Mexico, Chiapas, Ocosingo | |||
| A. asclepiadifolia Brandegee* | Isnard et al. 04 (MEXU, DR); Mexico, Veracruz, Puente Nacional | |||
| A. veracruzana J.F. Ortega* | Samain et al. 2009-295 (MEXU, DR); Mexico, Veracruz, San Andres Tuxtla | |||
| A. arborea Linden* | Samain et al. 2009-292 (MEXU, DR); Mexico, Tabasco, Teapa | |||
| A. kalebii Beutelsp.* | Isnard et al. 03 (MEXU, DR); Mexico, Chiapas, La Sepultura | |||
| Aristolochia subgenus Aristolochia | Thyrsicae | 30 climbers | A. ovalifolia Duch. | Isnard et al. 03 (MEXU, DR); Mexico, Veracruz, Estación Los Tuxtlas |
| A. grandiflora complex | 3 climbers | A. grandiflora Sw. | Isnard et al. 01 (MEXU); Mexico, Veracruz, San Andres Tuxtla; BG Dresden | |
| Aristolochia s.s. | Including 35 climbing and herbaceous species of Podanthemum and 80 climbing and herbaceous Mediterranean and Asian species | A. baetica L. (climber) | BG Dresden (s.n.) | |
| A. rotunda L. (herb) | BG Dresden (0012234-12) | |||
| A. clematitis L. (herb) | BG Dresden (002034-09) | |||
| ‘Howardia p.p.’ | 122 climbers | A. gigantea Mart. | BG Dresden (003818) | |
| A. leuconeura Linden | BG Dresden (015090-15) | |||
| A. fimbriata Cham. & Schltdl. | BG Dresden (0018700-16) | |||
| Pentandrae | 27 herbs, 18 climbers (including the species of the informal A. lindneri group) | A. lindneri A. Berger (herb) | BG Dresden (013979-29) | |
| Lactoris | 1 shrub | L. fernandeziana Phil. | Chile, Herbario Jardin Botanico Nacional Viña del Mar (1263) | |
| Asarum | 85 herbs | A. canadense L. | BG Dresden (009331-16) | |
| A. cardiophyllum Franch. | BG Dresden (016393-22) | |||
| Saruma | 1 herb | S. henryi Oliv. | BG Dresden (015115-13) | |
| Thottea | 44 shrubs | T. siliquosa (Lam.) Ding Hou | Pradeep s.n. (TBGT) | |
| Hydnora | Approx. 7 holoparasites | H. visseri Bolin, E. Maass & Musselman | Namibia, Gondwana Canyon Park | |
| H. longicollis Welw. | Namibia, Uis |
Species indicated with asterisks are studied in detail in Wagner et al. (2012).
As, Asian; NA, North American; CA, Central American; p.p., pro parte; BG, Botanical Garden.
Anatomy
Sections of different developmental stages (young, apical stems and mature stems with a significant degree of secondary growth) were used to compare the anatomy of all species studied. Sections were either hand-cut or prepared with a vibratome (HM 650 V, Thermo Scientific®, Dreieich, Germany; HYRAX V 50, Zeiss, Jena, Germany), adjusting the settings (speed, amplitude, frequency and section thickness) according to the sample. Cell content and tannins (Hydnora only) were bleached with 2·8 % NaClO. Sections were subsequently stained with carmine-green solution or phloroglucinol-HCl, following the methods used in Wagner et al. (2012), or with Astra blue-safranin in some sections of Hydnora. Photographs of sections were taken with a digital camera (DP71, Olympus, Tokyo, Japan; ProgRes SpeedXT core 5, Jenoptik, Jena, Germany) fitted to a light microscope (BX51, Olympus, Tokyo, Japan; Jenalumar, Zeiss, Jena, Germany) at high magnification and a dissecting microscope (SZX9, Olympus, Tokyo, Japan; SMT4, Askania, Rathenow, Germany) at low magnification, using the software Image-Pro Plus 7·0 (Media Cybernetics).
Biomechanics
Determination of Young's modulus provides a measure for the inherent stiffness of the plant stem as measured in bending, which in the case of biological materials is interpreted as bulk moduli of a complex structure (Structural Young's modulus) rather than of a homogeneous material. Three-point-bending tests, as well as calculation of Structural Young's modulus (hereafter termed simply Young's modulus) were carried out according to Wagner et al. (2012). Measurements in the field were conducted using a portable bending device (In-Spec 2200, Instron, Norwood, MA, USA). Greenhouse material was measured with a bench-top mechanical measuring device (BZ 2·5/TS1S, Zwick/Roell, Ulm, Germany). The trend in biomechanics during the plants development, i.e. increasing or decreasing values of Young's modulus, is used to compare different growth forms (Speck et al., 2003; Lahaye et al., 2005; Rowe et al., 2006).
Ancestral character state reconstruction in species-rich lineages
To detect trends in growth form evolution among genera, we applied ancestral character state reconstruction with Mesquite 2·75 (Maddison and Maddison, 2011). For reconstructing the evolution of character states using phylogenies, fully resolved and supported topologies, including most if not all species belonging to the clade of interest, are preferred (Salisbury and Kim, 2001). However, this is only possible when species-poor lineages are investigated. To avoid a potential bias by sampling only a few species belonging to a clade of up to 350 species such as in the subgenus Aristolochia, we assigned the number of known species to the clades to which they belong, based on morphology and molecular phylogenies (Table 1).
While the relationships of the subgenera within Aristolochia are well supported in previous studies, the relationship of clades within the subgenus Aristolochia remains unresolved. To be able to apply a character mapping, we randomly resolved the polytomic node 500 times using Mesquite 2·75 (Maddison and Maddison, 2011; Maddison et al., 2011). With this approach, we were able to verify whether the ancestral character state for the genus Aristolochia depends on the relationships between lineages within the subgenus Aristolochia. Species relationships within a defined clade can be disregarded because we were interested in ancestral nodes of the respective clade and nodes towards the root of the phylogeny. We then traced character evolution for the 500 trees with the randomly resolved node within Aristolochia, using the likelihood approach under the Markov k-state 1 parameter model (Mk1), implemented in Mesquite 2·75 (Maddison and Maddison, 2011). Frequencies of the likelihood approach were then averaged across all trees (Maddison and Maddison, 2006).
We scored discrete character states for the trait growth form (shrub, climber or herb) to each species, based on information from the literature (Kelly, 1998; Hsu, 2005; Wanke et al., 2006; Hallé et al., 2007; González et al., 2010, 2014; Oelschlägel et al., 2011; Wagner et al., 2012) or on our own observations. The state ‘shrub’ is assigned to self-supporting, sometimes leaning, upright species, with above-ground stems showing conspicuous secondary growth. The state ‘climber’ refers to all species, with above-ground stems that twine around a support. Other modes of climbing have not been reported for Aristolochiaceae. The terms ‘herb’ and ‘herbaceous’ are applied here for relatively small erect or creeping, non-climbing species, whose aerial stems are limited in diameter and height and often lack a periderm, but may show limited secondary growth. For species of Hydnoraceae, we used the state ‘underground parasitic’, as their habit differs from that of all remaining species of the perianth-bearing Piperales.
RESULTS
Anatomical traits
Primary organization
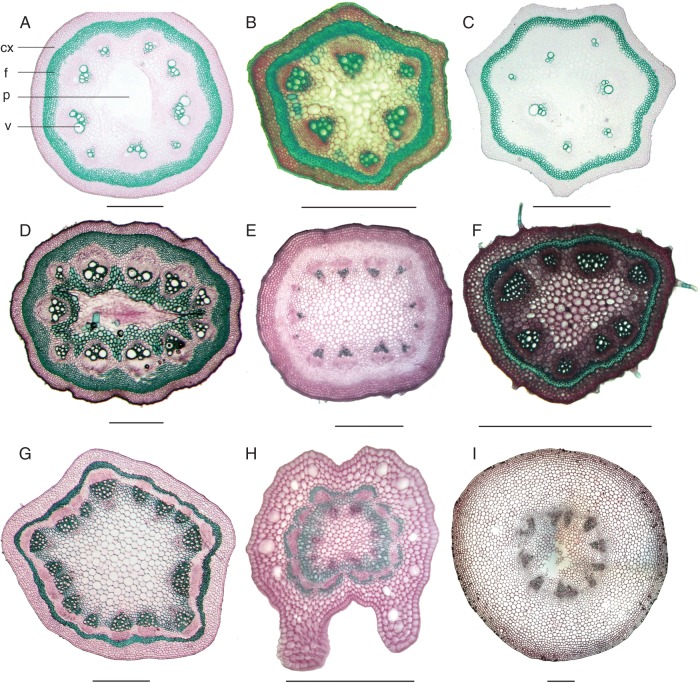
Young apical shoots of Saruma, Asarum, Aristolochia, Thottea, Lactoris and Hydnora all shared a ring of open collateral vascular bundles, enclosing a parenchymatous pith (Fig. 1). Young stems of Saruma, Asarum and Hydnora had a thick parenchymatous primary cortex, while shoots of Aristolochia and Thottea were further characterized by a continuous ring of perivascular fibres, which became lignified early during development. Perivascular fibres were also present in Lactoris fernandeziana and Saruma henryi, but, instead of building a continuous ring, they formed patches located peripheral to the vascular bundles (Fig. 1H).
Fig. 1.
Transverse sections of young apical parts of representative species of the perianth-bearing Piperales: (A) Aristolochia gigantea (climber); (B) A. leuconeura (climber); (C) A. grandiflora (climber); (D) A. ovalifolia (climber); (E) A. triactina (climber); (F) A. serpentaria (herb); as well as (G) Thottea siliquosa (shrub); (H) Lactoris fernandeziana (shrub); and (I) Asarum cardiophyllum (herb). In E, the hypodermal fibres are still unlignified. Sections are stained with carmine-green, colouring lignin-containing tissues in green and purely cellulosic tissues in pink; cx, cortex; f, fibre ring; p, pith; v, vessel. Scale bars = 500 μm.
Vascular cambium
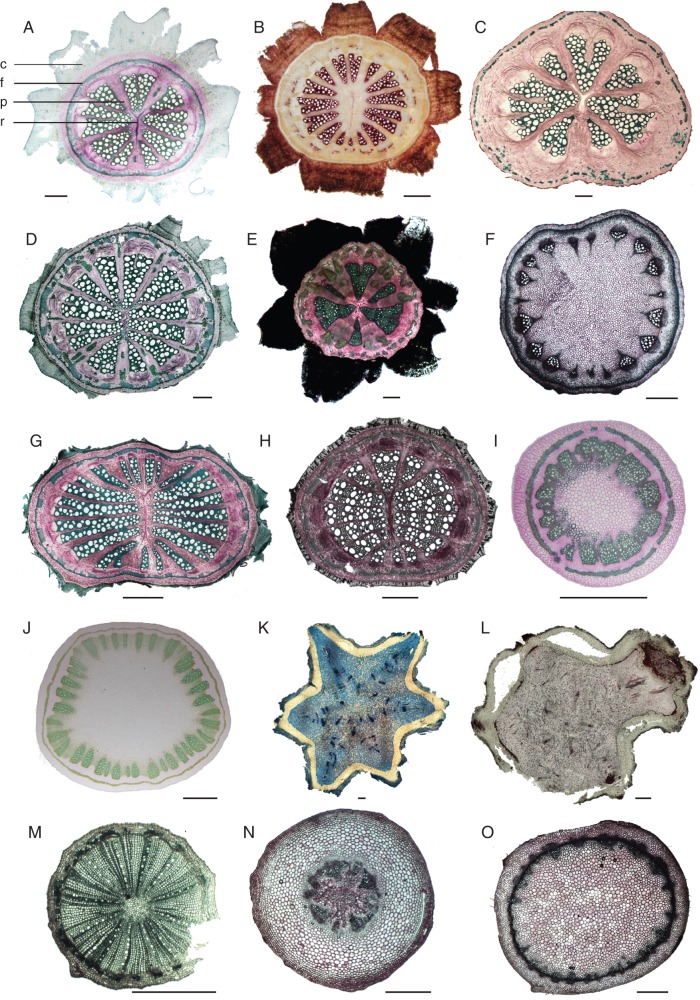
In species of Saruma and Asarum, a limited amount of vascular cambial activity was observed, with only very few secondarily derived cells added to the primary tissue (Figs 2N, O and 3A–C). Stems of Thottea (Figs 2J and 3I), Aristolochia (Figs 2A–I and 3E–H) and Lactoris (Figs 2M and 3D) showed diverse cambial activities, ranging from limited to conspicuous amounts of secondary tissues. While all species mentioned above formed a continuous cylinder of meristematic cells, cambial activity in Hydnora was very limited and restricted to the vascular areas (Figs 2K, L and 3J).
Fig. 2.
Transverse sections of mature proximal parts of representative species of the perianth-bearing Piperales: (A) Aristolochia gigantea (climber); (B) A. leuconeura (climber); (C) A. grandiflora (climber); (D) A. ovalifolia (climber); (E) A. baetica (climber); (F) A. clematitis (herb); (G) A. triactina (climber); (H) A. tomentosa (climber); (I) A. serpentaria (herb); as well as (J) Thottea siliquosa (shrub); (K) Hydnora visseri (underground parasite); (L) H. longicollis (underground parasite); (M) Lactoris fernandeziana (shrub); (N) Asarum cardiophyllum (herb); and (O) Saruma henryi (herb). Sections A, C–J and L–O are stained with carmine-green, section B is stained with phloroglucinol-HCl (lignified tissues in red) and section K is stained with Astra blue-Safranin (non-lignified tissues in blue, lignified tissues in dark red); c, cork; f, fibre ring; p, pith; r, wood ray. Scale bars = 1000 μm.
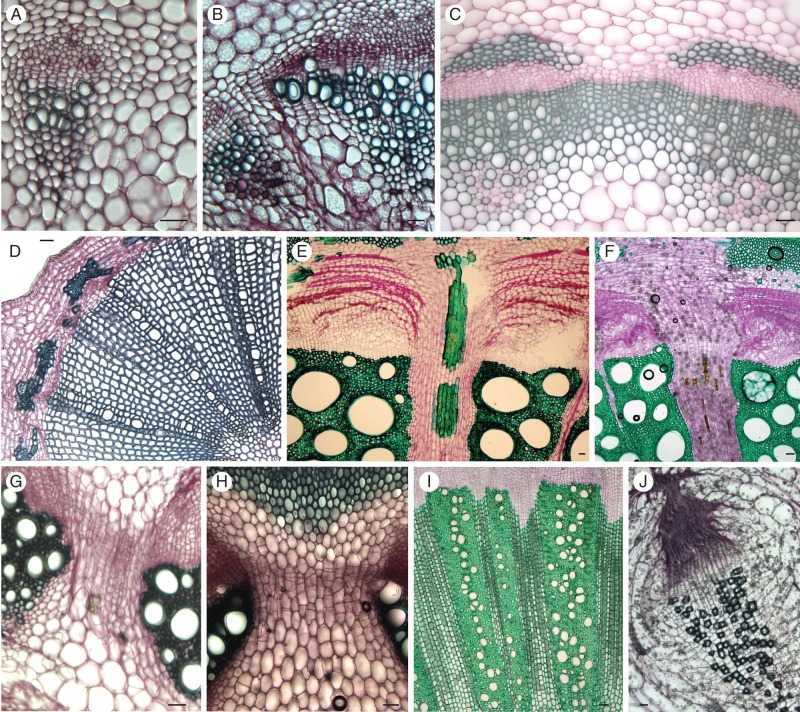
Fig. 3.
Details of transverse sections showing the degree of secondary growth in (A, B) Asarum cardiophyllum; (C) Saruma henryi; (D) Lactoris fernandeziana; (E), Aristolochia ovalifolia; (F) A. triactina; (G) A. lindneri; (H) A. clematitis; (I) Thottea siliquosa; and (J) Hydnora longicollis. Sections are stained with carmine-green. Scale bars = 50 μm.
Secondary vascular tissues
Interfascicular cambial activity in Asarum was initiated adjacent to vascular bundles (Fig. 3A), and subsequently a closed cylinder of meristematic cells is formed (Fig. 3B). Fusiform initials give rise to a few narrow vessels and axial parenchyma cells in the xylem, and a few secondary phloem cells (Fig. 3B). No wood fibres or tracheids were observed in the secondary xylem; vessels were only interspersed with axial parenchyma (Fig. 3A, B). Ray initials added a few parenchyma cells to the radial system of the stem, separating the vascular bundles. Stem diameter remained relatively constant along the stem. The vascular cambium in Saruma added a small amount of secondary xylem and phloem to the primary tissues (Fig. 3C). The narrow vessels were only produced in the vascular areas, while there were ray-like areas with fusiform, lignified parenchyma cells in between them (Fig. 3C). The size of the parenchymatous pith of Saruma increased towards the base, resulting in an increasing stem diameter.
Wide lignified rays and a circular transverse section characterized the mature stem of Lactoris, which mainly consisted of the wood cylinder (Figs 2M and 3D). Narrow vessels, axial parenchyma and wood fibres were located between the lignified wood rays, which were about ten or more cells wide. Moreover, the wood ray cells and the wood fibres of the vascular areas were very similar with respect to size and cell wall thickness (Fig. 3D). The parenchymatous pith was relatively small.
Secondary tissues of climbers in Aristolochia subgenera Aristolochia and Pararistolochia were characterized by thin-walled parenchymatous wood ray cells and wide vessels (Figs 2A–E, G and 3E, F), such as observed in the Asian/North American climbers of the subgenus Isotrema (Fig. 2H). In some climbers of Aristolochia (e.g. A. ovalifolia and A. baetica), sclereid patches were detected in the parenchymatous wood rays (Fig. 3E). All climbers of the subgenera Aristolochia (Fig. 2A–E) and Pararistolochia (Fig. 2G), as well as Asian/North American Isotrema (Fig. 2H) share a bilateral symmetry of the vascular system and a compressed pith in mature stages. As a result, those species have a noticeably flattened stem especially in the subgenus Pararistolochia (Fig. 2G), whereas that of the remaining species is more or less elliptical (Fig. 2A–D, G, H). In some species, such as Aristolochia tomentosa and A. ovalifolia, the symmetry of the stem was radial at the most basal parts of the plants (Fig. 4), and the stem even showed a non-compressed pith and partial lignification of the ray parenchyma (Fig. 4A). The Central American species of subgenus Isotrema are characterized by a radial symmetry even in younger stages, as well as by lignified wood rays, smaller vessels and a non-compressed parenchymatous pith (Wagner et al., 2012). Basal aerial stems of herbaceous species of the genus Aristolochia, such as A. clematitis (Figs 2F and 3H), showed a very limited activity of the fascicular cambium and even less of the interfascicular cambium. Secondary shoots of the creeping Aristolochia lindneri, as well as the herbaceous climber A. fimbriata, were characterized by a high proportion of parenchyma and parenchymatous thin-walled wood rays (Fig. 3G), similar to most of the climbers of the genus Aristolochia. Moreover, the secondary growth continuously compressed the pith, compensating for the increase in girth by secondary growth. Secondary growth of Aristolochia serpentaria produced a ring of secondary wood at the stem base with lignified wood rays (Fig. 2I). All Thottea species possessed a limited to conspicuous amount of secondary xylem and phloem (S. Isnard, unpubl. res.), with lignified wood rays, relatively small vessels interspersed with wood fibres, axial parenchyma and large piths (Fig. 3J).
Fig. 4.
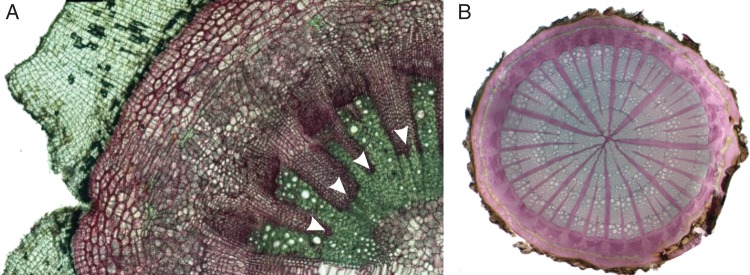
Transverse sections of old basal stem segments of (A) Aristolochia ovalifolia (diameter approx. 8 mm), showing the non-compressed pith, the radial symmetry and partially lignified ray tissue (arrows in the rays pointing to the lignified portions); lignified rays cells are coloured in green, such as the vascular tissue, whereas non-lignified cells are coloured in pink; and (B) A. tomentosa, with radial symmetry (diameter approx. 11 mm). Sections are stained with carmine-green.
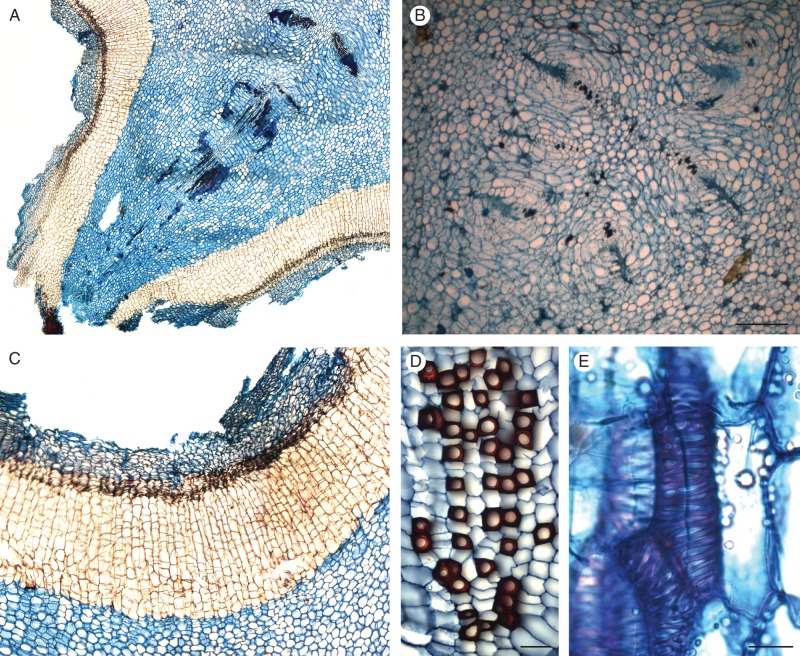
The vegetative structures of Hydnora longicollis were five-angular (Fig. 2L), while those of H. visseri were 4–6 sided (Fig. 2K). The edges of these sides formed ridges along the stem bearing lateral appendages, called ‘bumps’, which form small rounded extensions in H. longicollis, but more elongated ones in H. visseri. Vascular bundles in the central ring alternated with the outer lines of bumps, while groups of smaller vascular bundles, showing the xylem turned towards each other, were located below the ridges (Figs 2K, L and 5A, B). Vascular bundles were embedded in a large amount of tannin- and starch-containing parenchyma; a thick periderm covered the structures (Fig. 5C). The xylem consisted of vessels surrounded by axial parenchyma (Fig. 5D, E), while phloem cells appeared compressed (Fig. 5A). Fibres or tracheids have not been found. A limited amount of fascicular cambial activity could be observed. The vascular system of active ‘bumps’ originated from the vascular traces within the rows (Fig. 5A).
Fig. 5.
Details of anatomy of Hydnora. (A) Transverse section of an active bump of H. visseri, showing the developing vascular system of a new branch, originating from the vascular traces within the ridge; (B) transverse section of six vascular traces within a bump of H. visseri (scale bar = 500 μm); (C) transverse section of the periderm of H. visseri, including the primary cortical tissues; (D) transverse section of the xylem of H. longicollis, showing the lignified vessels (dark red) and the surrounding axial parenchyma (scale bar = 50 μm); and (E) tangential view of vessel elements of H. visseri (scale bar = 25 μm). Sections are stained with Astra blue-safranin.
Perivascular fibres
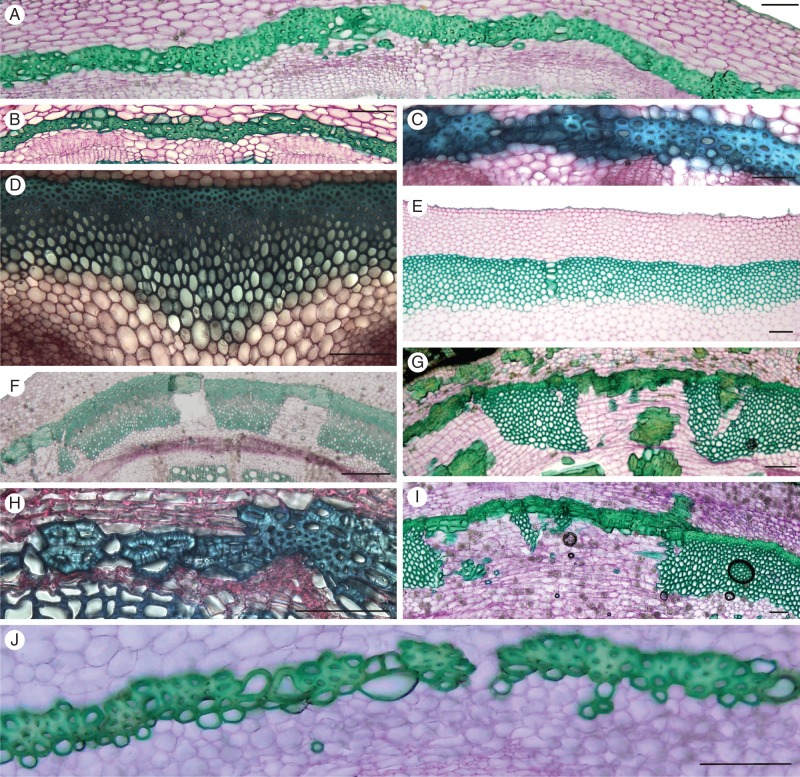
Older stems of Saruma developed thin fibre caps composed of relatively thin-walled lignified fibres, peripheral to the vascular bundles (Fig. 3C). The continuous ring of sclerenchymatous perivascular fibres observed in all species of Thottea and Aristolochia differed in the number of cell layers as well as in cell wall thickness (Fig. 6A–G, I, J). In most of the climbing species (Fig. 6E–G, I) the primary ring consisted of many more cell layers than in the shrubby species (Fig. 6A, B, H, J). Moreover, the sclerenchyma ring in climbers often shows an increase in cell wall thickness towards the periphery (Fig. 6E–G, I). The sclerenchyma ring was disrupted apparently due to secondary growth (Fig. 6). All species of Aristolochia and Thottea showed the gap filling of the fibre ring described as the self-repair mechanism by Busch et al. (2010). In most of the climbing species, only the outer layers of the primary ring were repaired, resulting in an uneven thickness of the ring in older stems (Figs 2A, D, G, H and 6F, G, I). The continuity of the ring was retained up to relatively large diameters of the stem, though eventually becoming more and more separated by gaps in very old basal plant segments of several species (Fig. 2C, E). In some species, such as Aristolochia leuconeura, the fibre ring becomes sloughed due to a cork cambium developing beneath the ring (Fig. 2B). The fibres could be observed in the periderm in older stems. Perivascular fibre caps of Lactoris were only one or two cells thick and were located peripheral to the vascular bundles (Figs 3D and 6H). Patches of sclereids, reported by Carlquist (1990), could also be observed in older stems (Fig. 6H). They consisted of two types of cells, the primary sclerenchymatous perivascular fibres, and sclereids originating from secondary differentiation of parenchyma cells. In Asarum and Hydnora, no lignified perivascular fibres were formed at any developmental stage.
Fig. 6.
Transverse sections of the perivascular fibres of (A) Aristolochia arborea; (B) A. kalebii; (C) A. serpentaria; (D) A. clematitis; (E) A. grandiflora; (F) A. westlandii; (G) A. malacophylla; (H) Lactoris fernandeziana; (I) Aristolochia triactina; and (J) Thottea siliquosa. Sections are stained with carmine-green. Scale bars = 100 μm.
Periderm
Saruma, Asarum and Lactoris have no active cork cambium (Fig. 2M–O). In Aristolochia, a cork cambium is active, but the periderm differed considerably in thickness: some species, such as A. ovalifolia (Fig. 2D) and A. leuconeura (Fig. 2B), produced a thick periderm, whereas that of the subgenus Pararistolochia (Fig. 2G) was much thinner. Finally, some species, such as A. grandiflora (Fig. 2C), virtually lack a periderm. In Thottea, cork cambial activity was highly variable (S. Isnard, unpubl. res.), while Hydnora species produced a thick layer of periderm (Figs 2K, L and 5A, C).
Mechanical traits within Aristolochia
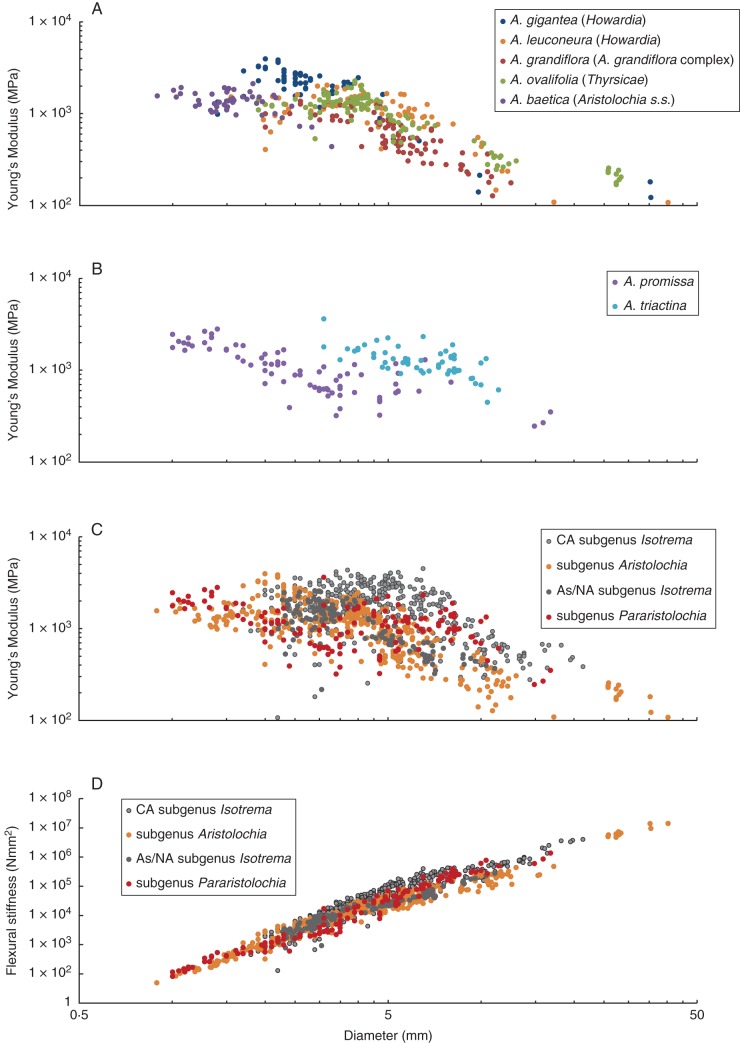
All studied climbers share an overall pattern in mechanical properties: a decreasing Young's modulus from young stems with small diameters to older stems with larger diameters (Fig. 7A–C). The values among individual species, however, differed slightly. In subgenus Aristolochia (Fig. 7A), the Asian/North American subgenus Isotrema (Fig. 7C) as well as Aristolochia promissa (subgenus Pararistolochia; Fig. 7B), a rapid and continuous decrease of Young's moduli is observed. Depending on the species, values drop from 2000–4000 MPa in axes from 1–3 mm to around 100–200 MPa in stems with diameters >20 mm. One exception is A. baetica, which does not develop larger stem diameters. Aristolochia triactina, a member of subgenus Pararistolochia (Fig. 7B), as well as the Central American species of Isotrema (Fig. 7C), showed a less rapid decrease, with maximum values of 4500 MPa in stems with diameters up to 8 mm. Young's moduli of older stems of these species are in a range comparable with the remaining ones. Flexural stiffness increased in all species with increasing diameter (Fig. 7D). Accordingly, values of Asian/North American Isotrema were in general slightly lower than those of the Mexican/Central American Isotrema species, but overlapped with the species of the subgenus Aristolochia; values of Pararistolochia were in between those of these two groups (Fig. 7D).
Fig. 7.
Biomechanical data of bending measurements for climbers of the genus Aristolochia. Young's modulus for representative species of (A) subgenus Aristolochia; (B) subgenus Pararistolochia. (C) Comparison of Young's modulus of respective clades. (D) Comparison of flexural stiffness of respective clades. Grey data are presented in detail in Wagner et al. (2012).
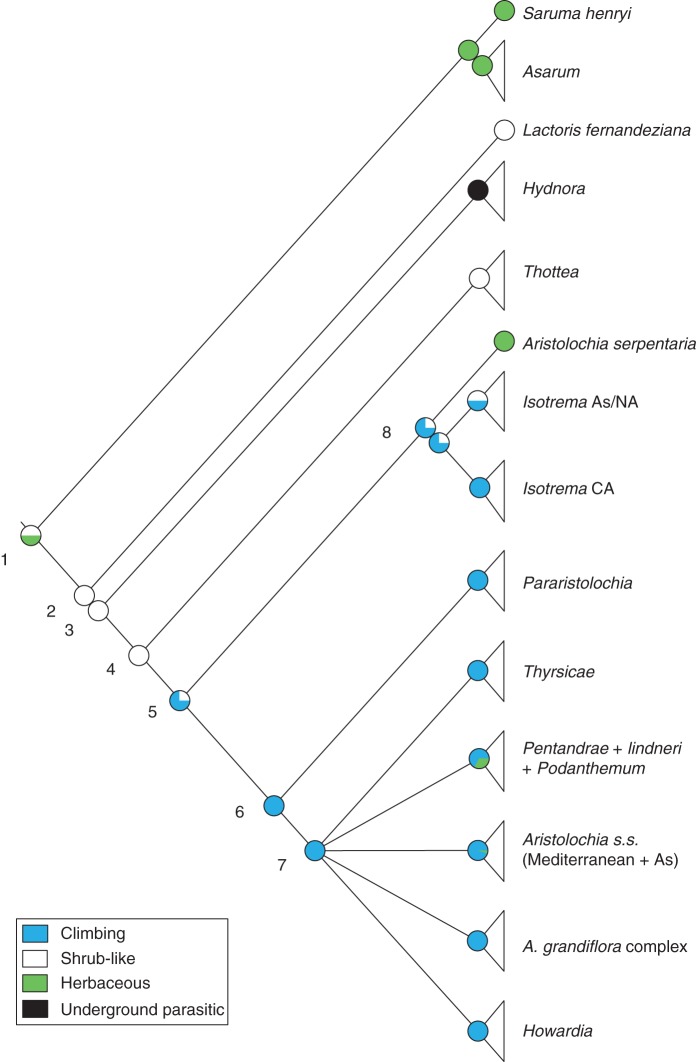
Ancestral character state reconstruction
The growth forms of the common ancestors of the main clades (Fig. 8, nodes 1–6) were reconstructed consistently, independent of the relationships among the lineages of subgenus Aristolochia. Accordingly, the climbing habit most probably evolved in the ancestors of the genus Aristolochia (Fig. 8, node 5). Less probably, the climbing habit had evolved at least twice in parallel: once in the subgenus Isotrema (Fig. 8, node 8), and once among the ancestors of the subgenera Pararistolochia and Aristolochia (Fig. 8, node 6). The ancestor of (Lactoris + (Hydnora + (Thottea + Aristolochia))), as well as the ancestor of (Thottea + Aristolochia), have been reconstructed as shrub-like (Fig. 8, nodes 2 and 4). The herbaceous habit in Aristolochia serpentaria, as well as the herbaceous lineages within the subgenus Aristolochia, has to be regarded as parallel evolution (Fig. 8), in addition to the herbaceous habit of the clade (Asarum + Saruma).
Fig. 8.
Ancestral character state reconstruction of the perianth-bearing Piperales. Reconstruction based on expected species number per clade (see Table 1); triangles represent the respective number of species in each clade; character state frequencies for the 500 randomly modified trees are depicted in pie chart diagrams at each node. Nodes are numbered for easier reference to the text. CA, Central America; NA, North America; As, Asia.
DISCUSSION
Trends in anatomy – pronounced secondary growth
Based on anatomical observations, the following trends in secondary tissue development have been detected in the perianth-bearing Piperales lineages. (1) There is a trend towards increased activity of vascular and cork cambium in the genus Aristolochia, resulting in larger stem diameters with specific traits of secondary tissues, compression of the pith parenchyma and a well-developed periderm. Furthermore, Aristolochia species tend to develop wide wood rays, which are lignified in some lineages. (2) In Hydnoraceae, despite the limited activity of vascular cambium, a pronounced periderm develops (see also Schimper, 1880). (3) In Thottea, a trend towards increased activity of the vascular and cork cambia is apparent. Early diverging suffrutescent species with extremely wide wood rays, such as Thottea tomentosa, show only a limited amount of secondary growth, while larger shrub-like species, such as T. sivarajanii, produce a thick wood cylinder with smaller rays (S. Isnard, unpubl. res.).
Trends in anatomy – a continuous cortical fibre ring
While either patches of fibres or a continuous fibre ring in the cortex characterizes Saruma, Lactoris, Aristolochia and Thottea, they are absent in Asarum and Hydnora (Figs 2, 5 and 6). The closed fibre ring found in stems of Aristolochia and Thottea, and its repair mechanism (Busch et al., 2010), are a synapomorphy for Aristolochioideae. Some evidence is found that Lactoris shares this character, even though the fibre ring is not completely closed. In mature stems, some of the adjacent parenchyma cells differentiate into sclereids, similar to the process in Aristolochia and Thottea. Such sclerenchymatous fibres and subsequently sclerified parenchyma cells in the periphery of the stem have not been observed in any other lineage and support the relationship of Lactoris with (Aristolochia + Thottea) based on molecular phylogenies. The lack of cortical fibres in Hydnoraceae supports the assumption of the highly modified morphology of these plants. Our results show that there is a trend towards the development of a continuous fibre ring among perianth-bearing Piperales, from lineages without any cortical fibres (e.g. Asarum), to species with only some fibres (e.g. Lactoris), to species with a primarily closed fibre ring (e.g. Thottea and Aristolochia).
Trends in growth habit and mechanical properties
While species of the genus Asarum are exclusively creeping, perennial herbs, with no capacity for upright growth (Cain and Damman, 1997; Kelly, 1998; Isnard et al., 2012), Saruma is a perennial caulescent herb, up to 1 m in height (Wagner, 1907; Dickison, 1996; Isnard et al., 2012). Lactoris is a small shrub, reaching a height from 0·5 to 1 m and stem diameter of up to 1 cm; plants are densely ramified and younger branches are filiform (Philippi, 1865; Engler, 1887; Stuessy et al., 1998). Thottea species are mainly self-supporting shrubs while Aristolochia are mainly non-self-supporting climbers. This implies an evolutionary trend from small herbaceous plants, potentially similar to Asarum and Saruma, to shrub-like species, like Thottea, and ending with predominantly climbing species, in Aristolochia. Within Aristolochia, further evolutionary trends towards (1) a shrub-like habit in subgenus Isotrema and (2) the appearance of herbaceous lineages (Fig. 8) are apparent. In having a shrub-like or herbaceous ancestor (Fig. 8, node 1), the perianth-bearing Piperales potentially differs from other climber-dominated groups, such as the subfamily Secamonideae of Apocynaceae, the genus Adenia of Passifloraceae as well as Menispermaceae and the tribe Morindeae of Rubiaceae. All the latter have been reconstructed with lianascence as the plesiomorphic state (Lahaye et al., 2005; Hearn, 2006, 2009; Ortiz et al., 2007; Razafimandimbison et al., 2012).
Several clades within Aristolochia subgenus Aristolochia, such as Aristolochia sensu stricto and Pentandrae, contain both climbing and herbaceous species. We suggest that the herbaceous habit has evolved independently within these lineages, which is supported by shared characters between climbers and herbs, such as the low number of vascular bundles and the non-lignified, thin-walled ray cells. On the other hand, shrub-like species of Lactoris, Thottea and Aristolochia share lignified wood rays and the non-compressed pith, as well as a congruent architectural organization assigned to the Mangenot model (Isnard et al., 2012).
While stems of Saruma have a very low stiffness (Isnard et al., 2012), the shrub-like and climbing species reach much higher values (Fig. 7; Isnard et al., 2012; Wagner et al., 2012; S. Isnard, unpubl. res.). In Thottea, a trend towards a typical shrub-like pattern of Young's modulus, from small suffrutescent species to larger shrubs (S. Isnard, unpubl. res.) is evident. The values and pattern of the latter are comparable with those of shrubs in subgenus Isotrema (Isnard et al., 2012; Wagner et al., 2012), implying a common developmental origin of the traits underlying the biomechanical properties typical of shrubs with relatively higher values of stiffness towards the base of the stem. The partitioning of the vascular system into relatively numerous bundles in stems of Saruma and Thottea might be a link between these species, and may serve as evidence for the retention of ancestral characters in Central American Isotrema.
All climbing Aristolochia species share a biomechanical pattern characteristic for climbers, which is a decreasing Young's modulus from younger apical towards older basal stem segments. Individual values, however, show a characteristic range of Young's moduli differing from climbers of other families, such as Euphorbiaceae (Ménard et al., 2009) and Convolvulaceae (Speck et al., 2003; Rowe et al., 2006). Climbers among Aristolochia lineages, in addition, show relatively species-specific values of Young's modulus (Fig. 7).
The role of heterochrony and evidence for secondary woodiness
The anatomical features of shrubs in Aristolochia are very probably influenced by heterochrony, for example a changed developmental rate in meristem activity (Wagner et al., 2012). The existence of partial ray lignification in basal parts of some Aristolochia climbers (e.g. A. ovalifolia) provides more evidence for the contribution of heterochrony during evolution. According to Carlquist (2009) ‘… the ease of transformations of growth must be attributed to heterochrony in the form of gene action dictating more cambial activity or less of it’. If the lignification of ray tissue in basal parts may be found in additional species of Aristolochia, e.g. to increase mechanical stiffness, the extended lignification of ray tissue from the base to the apex, as observed in the Central American Isotrema species, possibly indicates a shift in expression of the responsible genes. In addition, large rays, as observed for some Aristolochia species, might be indicative for heterochronic processes (Carlquist, 2013).
Large rays, as observed for some Aristolochia species, might be indicative of paedomorphism and secondary woodiness (Carlquist, 2013). Lactoris fernandeziana seems to resemble taxa such as Artemisia, Cyrtandra and Plantago in terms of ray development, which have been suggested to have evolved secondary woodiness. During early developmental stages these species are rayless, but develop rays later on (Carlquist, 2013), similar to L. fernandeziana (Carlquist, 1964). Lactoris, Thottea and the shrubs within the subgenus Isotrema share a distinct wood anatomy with small vessels and a high portion of lignified tissues such as lignified rays. Given the paedomorphic features found in Piperales and a putative herbaceous ancestor of perianth-bearing Piperales, the shrub-like species of Lactoris, Thottea and the subgenus Isotrema would consequently have evolved secondary woodiness. Secondary woodiness describes the increase in woodiness during the evolution of a group of plants, resulting in a tree, or a rosette tree or a large shrub (Carlquist, 1974). Ancestral plant lineages might be herbaceous, with some woodiness at the stem base, or they might be small shrubs. Most shrubs of Aristolochia and Thottea, as well as Lactoris grow in environments with little pronounced climatic fluctuation throughout the year, such as tropical forests and oceanic islands. These climatically isolated habitats are suggested to be optimal conditions for the occurrence of secondary woodiness (Carlquist, 1974, 2013).
Combining anatomy, biomechanics and growth habit
The externally placed cortical fibres in Lactoris, Thottea and Aristolochia undoubtedly contribute to the stiffness of the axis, especially in young stages of growth, where mechanical properties are not yet affected by the secondary tissues. Bending-resistant apical axes represent an important stage in the life of climbers, which allows plants to search for and locate a suitable support on which to climb (Caballé, 1993; Rowe and Speck, 1996; Speck and Rowe, 1999; Isnard et al., 2003; Rowe et al., 2006). In the case of climbing Aristolochia, plants start to twine around the support as soon as they contact it. After attachment occurs, stem stiffness starts to decrease continuously, which means that the relatively narrow climbing stems will bend and twist easily rather than break in the event of tree falls, branch falls or simple swaying of the supporting trees in the wind. The similar anatomy in all young Aristolochia stems is very probably one of the reasons why the difference in mechanical properties between climbers and shrubs is not as distinct in young developmental stages as compared with older stages. In the latter, the values differ mainly due to the varying amounts of parenchymatous vs. lignified tissues. Moreover, the biomechanical properties are influenced by the amount of periderm, by the size of the pith and by traits of the secondary tissues, such as vessel diameter, wood fibre content in the xylem and wood ray size. Our studies revealed that all investigated Aristolochia species are characterized by wide rays, but differ in ray length in the axial direction among growth forms: while rays are extremely long in climbers, the rays are relatively short in the shrub-like species. This potentially contributes to the different biomechanical properties of later developmental stages in shrubs and lianas where long parenchymatous rays are more flexible compared with lignified short and at least partially lignified rays. This pattern has also been found in comparative measurements in torsion (L. Hesse and S. Wagner, unpubl. res.). Wide, non-lignified ray tissue has been suggested to be of value in surviving high torque (twisting) forces (Carlquist, 2012). The higher separation of soft and stiff tissues might be advantageous under high torque and possibly bending forces. Under large torsional or bending moments, the parenchyma will probably be compressed, which reduces the stresses in neighbouring conductive and mechanical tissues comprising vessels and fibres. Besides the high amount of parenchymatous ray tissue, the presence of apotracheal axial parenchyma might also be beneficial in plant stems, which need to be flexible (Carlquist, 1993). Thin-walled cells in a compound of mainly stiff wood fibres may serve as a preferentially deformable zone when shear forces act on the secondary xylem. Long-term twisted stems of Aristolochia maxima have been shown to develop split xylem regions (Fisher and Ewers, 1991). Experiments on stems of A. ovalifolia resulted in similar findings (split secondary xylem segments, Fig. 9A). The ability to adjust tissue arrangements within the stem to avoid critical stem damage is important for climbing plants. The repair mechanism of the cortical fibre ring (Fig. 6), as well as the sclereid patches observed in the rays of A. ovalifolia and A. baetica (Fig. 2D, E), also suggest an ability to respond to acting loads: soft parenchymatous cells, which receive compression or tension forces due to secondary growth, are able to differentiate into sclereids. The sclerified patches in the rays most probably prevent the remaining non-lignified ray cells from further compression, while the repair mechanism in the fibre ring maintains the continuous ring of stiffening tissue, and thus both processes contribute to maintain the functionality of the stem. Moreover, the wide rays of Aristolochia have been found to contribute to a rapid healing of damaged vascular tissues (Fisher and Ewers, 1991).
Fig. 9.
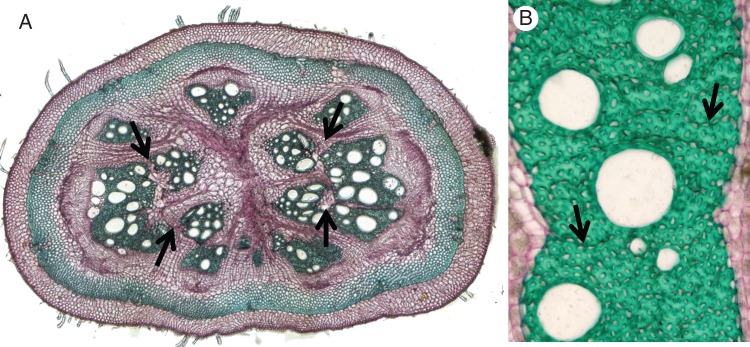
Transverse section of Aristolochia ovalifolia. (A) Reaction to mechanical forces: xylem segments have entirely split along regions of axial parenchyma (arrows). (B) Unloaded and unfissured xylem segment with strands of axial parenchyma (arrows). Sections are stained with carmine-green.
Hydnora – aligning a lineage with a highly modified bauplan
The holoparasitic Hydnoraceae has recently been placed in between Lactoris and (Aristolochia + Thottea) (Naumann et al., 2013). This highly modified, exclusively holoparasitic lineage among Piperales has fascinated botanists since the 19th century (e.g. Chatin, 1856; Meyer, 1833; Brown, 1844; Solms, 1889). Studies on morphology and anatomy have been carried out so far on Hydnora africana (Chatin, 1856; Schimper, 1880; Solms, 1889), H. triceps (Chatin, 1856; Tennakoon et al., 2007), H. abyssinica and Prosopanche americana (synonym of P. burmeisteri) (Schimper, 1880). Although the family has a highly derived sub-terranean morphology, these studies revealed several traits typically found in angiosperm stems, such as a central ring of collateral vascular bundles surrounding a conspicuous pith, and an active vascular cambium. The central ring of vascular bundles alternates with groups of vascular bundles subtending each other. All species develop a thick periderm. Schimper (1880) reported strands of fibres as well as mucilage ducts in the vegetative structures. Moreover, it has been observed that the appendages called ‘bumps’, which are located at the ridges of the vegetative body, either remain dormant or differentiate into haustoria, lateral ramifications or flower buds (Schimper, 1880; Tennakoon et al., 2007). These authors also assumed that smaller vascular bundles observed within the ridges might be vascular traces provisioning the bumps. We detected similar anatomical characteristics in Hydnora longicollis and H. visseri. Collateral vascular bundles with an active fascicular cambium, a distinct parenchymatous pith, a well-developed periderm and groups of smaller vascular bundles alternating with the bundles in the central ring were confirmed (Figs 3K, L and 5). The latter have not been described for H. africana (Solms, 1889), but have for P. americana (synonym of P. burmeisteri) (Schimper, 1880). In addition, we observed that smaller vascular bundles lead towards active bumps, similar to leaf traces or branch traces (Fig. 5A). These characters confirm the hypothesis of Tennakoon et al. (2007) that the vegetative organs of Hydnoraceae are likely to be rhizomes with otherwise typical stem traits.
Herbaceousness in Piperales
All studied species described as herbaceous are perennial and emerge from an underground organ after dormancy. Even if at least some species appear to be herbaceous, none of them truly lacks an active vascular cambium (Fig. 2). The secondary growth is restricted either to the basal part of the plant stem (e.g. Saruma and Aristolochia serpentaria), to the vascular bundle (e.g. Asarum) or to the underground organ [e.g. the rhizome of Aristolochia clematitis (Schweingruber et al., 2011) and Asarum]. Strictly speaking, only plants with green, non-woody aerial stems with limited diameter and height, and without the ability to produce secondary tissues from a bifacial vascular cambium, such as most monocots, should be called ‘herbs’ (Eiten, 1991; Rowe and Paul-Victor, 2012). Even the small sized Aristolochia serpentaria exhibits a wood cylinder with dense wood and lignified rays, similar to the shrubs in Thottea and the other species of Aristolochia subgenus Isotrema. Saruma also develops a relatively high amount of secondary tissues in basal parts of the stems. Thus, a more precise definition and usage of the terms ‘herb’ and ‘herbaceous’ is advisable.
Conclusions
A large number of species of Piperales exhibit a climbing habit. However, within the perianth-bearing Piperales, climbing species only occur in the most species-rich genus Aristolochia. The remaining genera contain considerably fewer species, all of them with a herb- or shrub-like habit, except Hydnora. Since the ancestor of the clade has been reconstructed with a herb- or shrub-like habit, we propose that the climbing habit is a derived growth form, which either evolved with the diversification of Aristolochia or might have been a key feature for its diversification. This study contributes to the increasing compilation of knowledge about climbing plants, which are relatively little studied compared with plants of greater economic value such as trees, by studying the anatomy and biomechanics of a basal angiosperm lineage. The rising dominance of lianas in tropical forests, potentially driven by, for example, increasing forest disturbance and turnover, changes in land use and fragmentation, and cumulative CO2 concentrations (Schnitzer and Bongers, 2011), and the resulting influences on the forest communities, demands a better understanding of how plant lineages shift between growth forms.
ACKNOWLEDGEMENTS
We thank the authorities of Mexico for collection permits (SGPA/DGGFS/712/2486/09 and SGPA/DGGFS/712/0424/10), H. Flores, H. Ochotorena, (both UNAM), C. Granados (GENT), J. Rivera (GEOBICOM, S.C.), G. Ortiz (Universidad Autónoma Chapingo, Centro Regional Universitario del Sureste, Teapa, Tabasco) and T. Stuessy (Vienna) for assistance with fieldwork, material and/or logistics, and S. Muster for support in experimental work. Many thanks to the Botanical Garden of Dresden and the herbarium of Viña del Mar, Chile for providing material. We thank S. Carlquist, an anonymous reviewer and the editorial board for comments and suggestions on improving this manuscript. This work was supported by the German Science Foundation (DFG, grant no. NE681/11–1) and the German Academic Exchange Service (DAAD PPP France). The first author had a scholarship from the European Centre of Emerging Materials and Processes (ECEMP).
LITERATURE CITED
- Appanah S, Gentry AH, LaFrankie JV. Liana diversity and species richness of Malaysian rain forests. Journal of Tropical Forest Science. 1993;6:116–123. [Google Scholar]
- Brown R. XXIII. Description of the female flower and fruit of Rafflesia arnoldi, with remarks on its affinities; and an illustration of the structure of Hydnora africana. Transactions of the Linnean Society of London. 1844;19:221–247. [Google Scholar]
- Bongers F, Schnitzer SA, Traore D. The importance of lianas and consequences for forest management in West Africa. BioTerre. 2002. pp. 59–70. Special edition.
- Busch S, Seidel R, Speck O, Speck T. Morphological aspects of self-repair of lesions caused by internal growth stresses in stems of Aristolochia macrophylla and Aristolochia ringens. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2113–2120. doi: 10.1098/rspb.2010.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballé G. Liana structure, function and selection: a comparative study of xylem cylinders of tropical rainforest species in Africa and America. Botanical Journal of the Linnean Society. 1993;113:41–60. [Google Scholar]
- Cain ML, Damman H. Clonal growth and ramet performance in the woodland herb, Asarum canadense. Journal of Ecology. 1997;85:883–897. [Google Scholar]
- Campanello PI, Villagra M, Garibaldi JF, Ritter LJ, Araujo JJ, Goldstein G. Liana abundance, tree crown infestation, and tree regeneration ten years after liana cutting in a subtropical forest. Forest Ecology and Management. 2012;284:213–221. [Google Scholar]
- Carlquist S. Morphology and relationships of Lactoridaceae. Aliso. 1964;5:421–435. [Google Scholar]
- Carlquist S. Island biology. New York: Columbia University Press; 1974. [Google Scholar]
- Carlquist S. Wood anatomy and relationships of Lactoridaceae. American Journal of Botany. 1990;77:1498–1504. [Google Scholar]
- Carlquist S. Anatomy of vine and liana stems: a review and synthesis. In: Putz FE, Mooney HA, editors. The biology of vines. Cambridge: Cambridge University Press; 1991. pp. 53–71. [Google Scholar]
- Carlquist S. Wood and bark anatomy of Aristolochiaceae; systematic and habital correlations. IAWA Journal. 1993;14:341–357. [Google Scholar]
- Carlquist S. Wood and bark anatomy of Schisandraceae: implications for phylogeny, habit, and vessel evolution. Aliso. 1999;18:45–55. [Google Scholar]
- Carlquist S. Observations on the vegetative anatomy of Austrobaileya: habital, organographic and phylogenetic conclusions. Botanical Journal of the Linnean Society. 2001;135:1–11. [Google Scholar]
- Carlquist S. Xylem heterochrony: an unappreciated key to angiosperm origin and diversifications. Botanical Journal of the Linnean Society. 2009;161:26–65. [Google Scholar]
- Carlquist S. How wood evolves: a new synthesis. Botany. 2012;90:901–940. [Google Scholar]
- Carlquist S. More woodiness/less woodiness: evolutionary avenues, ontogenetic mechanisms. International Journal of Plant Science. 2013;174:964–991. [Google Scholar]
- Chatin GA. Paris: Baillière; 1856. Anatomie comparée des végétaux: comprenant 1. les plantes aquatiques, 2. les plantes aériennes, 3. les plantes parasites, 4. les plantes terrestres, Vol. 2; pp. 507–517. [Google Scholar]
- Dickison WC. Stem and leaf anatomy of Saruma henryi Oliv., including observations on raylessness in the Aristolochiaceae. Bulletin of the Torrey Botanical Club. 1996;123:261–267. [Google Scholar]
- Eiten G. What is a herb? (with examples from the tropical ‘Savanna’ of Brazil and the humid temperate zone of Poland) Veröffentlichungen des Geobotanischen Institutes ETH, Stiftung Rübel, Zürich. 1991;106:288–304. [Google Scholar]
- Engler A. Über die Familie der Lactoridaceae. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie. 1887;8:53–56. [Google Scholar]
- Ewers FW. Xylem structure and water conduction in conifer trees, dicot trees, and lianas. IAWA Bulletin. 1985;6:309–317. [Google Scholar]
- Ewers FW, Fisher JB, Fichtner K. Water flux and xylem structure of vines. In: Putz FE, Mooney HA, editors. The biology of vines. Cambridge: Cambridge University Press; 1991. pp. 127–160. [Google Scholar]
- Feild TS, Isnard S. Climbing habit and ecophysiology of Schisandra glabra (Schisandraceae) – implications for the early evolution of angiosperm lianescence. International Journal of Plant Sciences. 2013;174:1121–1133. [Google Scholar]
- Feild TS, Franks PJ, Sage TL. Ecophysiological shade adaptation in the basal angiosperm, Austrobaileya scandens (Austrobaileyaceae) International Journal of Plant Sciences. 2003;164:313–324. [Google Scholar]
- Fisher JB, Ewers FW. Structural responses to stem injury in vines. In: Putz FE, Mooney HA, editors. The biology of vines. Cambridge: Cambridge University Press; 1991. pp. 99–124. [Google Scholar]
- Gentry AH. The distribution and evolution of climbing plants. In: Putz FE, Mooney HA, editors. The biology of vines. Cambridge: Cambridge University Press; 1991. pp. 3–49. [Google Scholar]
- Gerwing JJ, Lopes Farias L. Integrating liana abundance and forest stature into an estimate of total aboveground biomass for an eastern Amazonian forest. Journal of Tropical Ecology. 2000;16:327–335. [Google Scholar]
- Gianoli E. Evolution of a climbing habit promotes diversification in flowering plants. Proceedings of the Royal Society B: Biological Sciences. 2004;271:2011–2015. doi: 10.1098/rspb.2004.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González F, Stevenson DW. Perianth development and systematics of Aristolochia. Flora (Jena) 2000;195:370–391. [Google Scholar]
- González F, Esquivel HE, Murcia GA, Pabón-Mora N. Aristolochia pentandra (Aristolochiaceae) in Columbia: biogeographic implications and proposed synapomorphies between the pantandrous species of Aristolochia and its South American sister group. Revista de la Academia Colombiana de Ciencias. 2010;XXXIV:467–478. [Google Scholar]
- González F, Wagner ST, Salomo K, Symmank L, Samain M-S, Isnard S, Rowe NP, Neinhuis C, Wanke S. Present trans-Pacific disjunct distribution of Aristolochia subgenus Isotrema (Aristolochiaceae) was shaped by dispersal, vicariance and extinction. Journal of Biogeography. 2014;41:380–391. [Google Scholar]
- Hallé F, Danton P, Perrier C. Architectures de plantes de l'Île Robinson Crusoe, archipel Juan Fernández, Chili. Adansonia. 2007;29:333–350. [Google Scholar]
- Hearn DJ. Adenia (Passifloraceae) and its adaptive radiation: phylogeny and growth form diversification. Systematic Botany. 2006;31:805–821. [Google Scholar]
- Hearn DJ. Developmental patterns in anatomy are shared among separate evolutionary origins of stem succulent and storage root-bearing growth habits in Adenia (Passifloraceae) American Journal of Botany. 2009;96:1941–1956. doi: 10.3732/ajb.0800203. [DOI] [PubMed] [Google Scholar]
- Hsu E. 536. Saruma henryi. Curtis's Botanical Magazine. 2005;22:200–204. [Google Scholar]
- Ibarra-Manríquez G, Martínez-Ramos M. Landscape variation of liana communities in a Neotropical rain forest. Plant Ecology. 2002;160:91–112. [Google Scholar]
- Ingwell LL, Wright SJ, Becklund KK, Hubbell SP, Schnitzer SA. The impact of lianas on 10 years of tree growth and mortality on Barro Colorado Island, Panama. Journal of Ecology. 2010;98:879–887. [Google Scholar]
- Isnard S, Silk WK. Moving with climbing plants from Charles Darwin's time into the 21st century. American Journal of Botany. 2009;96:1205–1221. doi: 10.3732/ajb.0900045. [DOI] [PubMed] [Google Scholar]
- Isnard S, Speck T, Rowe NP. Mechanical architecture and development in Clematis: implications for canalised evolution of growth forms. New Phytologist. 2003;158:543–559. doi: 10.1046/j.1469-8137.2003.00771.x. [DOI] [PubMed] [Google Scholar]
- Isnard S, Prosperi J, Wanke S, et al. Growth form evolution in Piperales and its relevance for understanding angiosperm diversification: an integrative approach combining plant architecture, anatomy, and biomechanics. International Journal of Plant Sciences. 2012;173:610–639. [Google Scholar]
- Jacobs M. The tropical rain forest: a first encounter. Berlin: Springer; 1988. [Google Scholar]
- Kelly LM. Phylogenetic relationships in Asarum (Aristolochiaceae) based on morphology and ITS sequences. American Journal of Botany. 1998;85:1454–1467. [PubMed] [Google Scholar]
- Lahaye R, Civeyrel L, Speck T, Rowe NP. Evolution of shrub-like growth forms in the lianoid subfamily Secamonoideae (Apocynaceae s.l.) of Madagascar: phylogeny, biomechanics, and development. American Journal of Botany. 2005;92:1381–1396. doi: 10.3732/ajb.92.8.1381. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. StochChar: a package of Mesquite modules for stochastic models of character evolution. 2006. Version 1.1.
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. 2011. Version 2.75.
- Maddison WP, Maddison DR, Midford P. Tree Farm package for Mesquite. 2011. Version 2.75.
- Ménard L, McKey D, Rowe NP. Developmental plasticity and biomechanics of treelets and lianas in Manihot aff. quinquepartita (Euphorbiaceae): a branch-angle climber of French Guiana. Annals of Botany. 2009;103:1249–1259. doi: 10.1093/aob/mcp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. De Hydnora. Kaiserlich Leopoldinisch-Carolinische Akademie der Naturforscher. 1833;16:771–788. [Google Scholar]
- Naumann J, Salomo K, Der JP, et al. Single-copy nuclear genes place haustorial Hydnoraceae within Piperales and reveal a Cretaceous origin of multiple parasitic angiosperm lineages. PLoS One. 2013;8:e79204. doi: 10.1371/journal.pone.0079204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neinhuis C, Wanke S, Hilu KW, Müller K, Borsch T. Phylogeny of Aristolochiaceae based on parsimony, likelihood, and Bayesian analyses of trnL-trnF sequences. Plant Systematics and Evolution. 2005;250:7–26. [Google Scholar]
- Oelschlägel B, Wagner S, Salomo K, et al. Implications from molecular phylogenetic data for systematics, biogeography and growth form evolution of the genus Thottea Rottb. (Aristolochiaceae) The Gardens' Bulletin, Singapore. 2011;63:259–275. [Google Scholar]
- Ohi-Toma T, Sugawara T, Murata H, Wanke S, Neinhuis C, Murata J. Molecular phylogeny of Aristolochia sensu lato (Aristolochiaceae) based on sequences of rbcL, matK, and phyA genes, with special reference to differentiation of chromosome numbers. Systematic Botany. 2006;31:481–492. [Google Scholar]
- Ortiz Rdel C, Kellogg EA, Werff HV. Molecular phylogeny of the moonseed family (Menispermaceae): implications for morphological diversification. American Journal of Botany. 2007;94:1425–1438. doi: 10.3732/ajb.94.8.1425. [DOI] [PubMed] [Google Scholar]
- Pérez-Salicrup DR, Sork VL, Putz FE. Lianas and trees in a liana forest of Amazonian Bolivia. Biotropica. 2001;33:34–47. [Google Scholar]
- Pfeifer HW. Revision of the North and Central American hexandrous species of Aristolochia (Aristolochiaceae) Annals of the Missouri Botanical Garden. 1966;53:115–196. [Google Scholar]
- Philippi RA. Ueber zwei neue Pflanzen-Gattungen. Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien. 1865;15:517–524. [Google Scholar]
- Putz FE, Holbrook NM. Biomechanical studies of vines. In: Putz FE, Mooney HA, editors. The biology of vines. Cambridge: Cambridge University Press; 1991. pp. 73–97. [Google Scholar]
- Razafimandimbison SG, Ekman S, McDowell TD, Bremer B. Evolution of growth habit, inflorescence architecture, flower size, and fruit type in Rubiaceae: its ecological and evolutionary implications. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0040851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe NP, Paul-Victor C. Herbs and secondary woodiness – keeping up the cambial habit. New Phytologist. 2012;193:3–5. doi: 10.1111/j.1469-8137.2011.03980.x. [DOI] [PubMed] [Google Scholar]
- Rowe NP, Speck T. Biomechanical characteristics of the ontogeny and growth habit of the tropical liana Condylocarpon guianense (Apocynaceae) International Journal of Plant Sciences. 1996;157:406–417. [Google Scholar]
- Rowe NP, Isnard S, Gallenmüller F, Speck T. Diversity of mechanical architectures in climbing plants: an ecological perspective. In: Herrel A, Speck T, Rowe NP, editors. Ecology and biomechanics – a mechanical approach to the ecology of animals and plants. Boca Raton, FL: CRC Press; 2006. pp. 35–59. [Google Scholar]
- Salisbury BA, Kim J. Ancestral state estimation and taxon sampling density. Systematic Biology. 2001;50:557–564. [PubMed] [Google Scholar]
- Schimper AFW. Die Vegetationsorgane von Prosopanche Burmeisteri. Halle: Niemeyer; 1880. [Google Scholar]
- Schnitzer SA, Bongers F. The ecology of lianas and their role in forests. Trends in Ecology and Evolution. 2002;17:223–230. [Google Scholar]
- Schnitzer SA, Bongers F. Increasing liana abundance and biomass in tropical forests: emerging patterns and putative mechanisms. Ecology Letters. 2011;14:397–406. doi: 10.1111/j.1461-0248.2011.01590.x. [DOI] [PubMed] [Google Scholar]
- Schweingruber FH, Börner A, Schulze E-D. Atlas of stem anatomy in herbs, shrubs und trees. Berlin: Springer; 2011. [Google Scholar]
- Solms H. Hydnoraceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien nebst ihren Gattungen und wichtigeren Arten insbesondere den Nutzpflanzen. III. Teil. 1. Abteilung. Leipzig: Wilhem Engelmann; 1889. pp. 282–285. [Google Scholar]
- Speck T, Rowe NP. A quantitative approach for analytically defining size, growth form and habit in living and fossil plants. In: Kurmann MH, Hemsley AR, editors. The evolution of plant architecture. Kew: Royal Botanic Gardens; 1999. pp. 447–479. [Google Scholar]
- Speck T, Rowe NP, Civeyrel L, Classen-Bockhoff R, Neinhuis C, Spatz C. The potential of plant biomechanics in functional biology and systematics. In: Stuessy TF, Mayer V, Hörandl E, editors. Deep morphology: toward a renaissance of morphology in plant systematics. Königstein: Koeltz Scientific Books; 2003. pp. 241–271. [Google Scholar]
- Stuessy TF, Crawford DJ, Anderson GJ, Jensen RJ. Systematics, biogeography and conservation of Lactoridaceae. Perspectives in Plant Ecology, Evolution and Systematics. 1998;1:267–290. [Google Scholar]
- Tennakoon KU, Bolin JF, Musselman LJ, Maass E. Structural attributes of the hypogeous holoparasite Hydnora triceps Drège & Meyer (Hydnoraceae) American Journal of Botany. 2007;94:1439–1449. doi: 10.3732/ajb.94.9.1439. [DOI] [PubMed] [Google Scholar]
- Wagner R. Zur Kenntnis des Saruma Henryi Oliv. Österreichische Botanische Zeitschrift. 1907;57:265–271. [Google Scholar]
- Wagner ST, Isnard S, Rowe NP, Samain M-S, Neinhuis C, Wanke S. Escaping the lianoid habit: evolution of shrub-like growth forms in Aristolochia subgenus Isotrema (Aristolochiaceae) American Journal of Botany. 2012;99:1609–1629. doi: 10.3732/ajb.1200244. [DOI] [PubMed] [Google Scholar]
- Wanke S, González F, Neinhuis C. Systematics of pipevines: combining morphological and fast-evolving molecular characters to investigate the relationships within subfamily Aristolochioideae (Aristolochiaceae) International Journal of Plant Sciences. 2006;167:1215–1227. [Google Scholar]
- Wanke S, Jaramillo MA, Borsch T, Samain MS, Quandt D, Neinhuis C. Evolution of Piperales – matK gene and trnK intron sequence data reveal lineage specific resolution contrast. Molecular Phylogenetics and Evolution. 2007;42:477–497. doi: 10.1016/j.ympev.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Yao TL. Nine new species of Thottea (Aristolochiaceae) in Peninsular Malaysia and Singapore, with two taxa in Peninsular Malaysia redefined and a taxon lectotypified. Blumea. 2013;58:245–262. [Google Scholar]