Abstract
Background and Aims
Recently, molecular approaches have been used to investigate the phylogeny of subtribe Oncidiinae, resulting in the re-alignment of several of its genera. Here, a description is given of the structure of the floral elaiophores (oil glands) of four species formerly assigned to Oncidium Sw. Those of Vitekorchis excavata (Lindl.) Romowicz & Szlach., Cyrtochilum meirax (Rchb.f.) Dalström and a species of Oncidium displaying floral dimorphism, namely O. heteranthum Poepp. & Endl. var. album, are compared with that of Gomesa longipes (Lindl.) M.W. Chase & N.H. Williams, whose epithelial elaiophores are typical of many Oncidiinae, in order to extend our understanding of elaiophore diversity within this subtribe.
Methods
Floral elaiophore structure was examined and compared at anthesis for all four species using light microscopy, scanning electron microscopy, transmission electron microscopy and histochemistry.
Key Results
In all species investigated, with the exception of C. meirax, the floral elaiophore occurs on the labellar callus and is of the intermediate type, possessing both glabrous and trichomatous regions. By contrast, although all four species produce lipid secretions, C. meirax lacks an obvious elaiophore. In each case, the secretory tissue is represented by a single-layered epidermis of cuboidal cells (trichomatous and/or atrichomatous). Palisade cells are absent. The secretion may be wax- or oil-like and is usually produced by smooth endoplasmic reticulum (SER). However, in C. meirax, where rough endoplasmic reticulum (RER) predominates, oil accumulates as plastoglobuli within elaioplasts. These plastoglobuli are then discharged into the cytoplasm, forming oil bodies. In some species, oil usually accumulates within vesicles at the plasmalemma or in the periplasmic space before traversing the cell wall and accumulating beneath the cuticle, sometimes with distension of the latter. Gomesa longipes is unusual in its production of a heterogeneous secretion, whereas Vitekorchis excavata is equally remarkable for the protuberances found on the walls of its secretory cells.
Conclusions
Anatomically, the secretory tissues of all four species, despite currently being assigned to four different genera, are remarkably similar and indicative of homoplasy. This supports previous investigations of the floral elaiophore in Oncidiinae, which showed that the same elaiophore characters may be shared by different clades, but not always by species of the same genus. Consequently, elaiophores are considered to be of limited value in investigating the phylogeny of this subtribe. Furthermore, floral dimorphism does not greatly modify elaiophore structure in the fertile flowers of Oncidium heteranthum var. album. Based on the presence or absence of well-defined elaiophores, the nature of the secretion and the cell ultrastructure, it is likely that floral oil may be produced in Oncidiinae in one of two ways: by the ER (mainly SER) or by plastids, most notably elaioplasts. Once the oil is discharged into the cytoplasm as oil bodies or oil droplets, there is little difference between the subsequent stages of oil secretion; the oil traversing the cytoplasm (often vesicle-mediated) and cell wall before accumulating beneath the cuticle.
Keywords: Anatomy, floral elaiophore, lipids, micromorphology, oil glands, Oncidiinae, ultrastructure, Vitekorchis, Cyrtochilum, Oncidium, Gomesa, Orchidaceae
INTRODUCTION
A number of Oncidiinae (sensu Chase et al., 2003; Neubig et al., 2012) present floral food rewards in the form of oil (Chase et al., 2003; Chase, 2005; Neubig et al., 2012) produced in special epidermal glands or glandular hairs called epithelial and trichomal elaiophores, respectively (Vogel, 1974). Some, however, have intermediate elaiophores, with both glabrous and trichomatous secretory areas (Pacek et al., 2012; Gomiz et al., 2013). Their flowers are thought to mimic those of oil-secreting Malpighiaceae or sympatric species of Calceolaria L. (Calceolariaceae; Neubig et al., 2012, and references therein) and are pollinated by oil-gathering Centris, Paratetrapedia and Tetrapedia bees. The oil is fed to larvae or used for waterproofing larval cells (van der Pijl and Dodson, 1969; Buchmann, 1987; Dressler, 1990; Singer and Cocucci, 1999; van der Cingel, 2001; Torretta et al., 2011; Neubig et al., 2012, and references therein).
Detailed study of elaiophore structure did not commence until relatively recently. Nevertheless, to date, the structure of both the epithelial and trichomal elaiophores of several Oncidiinae genera have been investigated, including that of Gomesa R. Br., Lockhartia Hook., non-dimorphic Oncidium Sw., Trichocentrum Poepp. & Endl. and representatives of the former Ornithocephalinae Schltr. (Singer and Cocucci, 1999; Pacek and Stpiczyńska, 2007; Stpiczyńska et al., 2007, 2013; Stpiczyńska and Davies, 2008; Davies and Stpiczyńska, 2008, 2009; Aliscioni et al., 2009; Davies, 2009; Pansarin and Pansarin, 2011; Pacek et al., 2012; Blanco et al., 2013; Gomiz et al., 2013). Previous investigations found that the morphology and anatomical organization of the epithelial elaiophore of Gomesa and Oncidium are remarkably similar (Stpiczyńska et al., 2007, 2013; Pacek et al., 2007; Stpiczyńska and Davies, 2008; Aliscioni et al., 2009; Davies and Stpiczyńska, 2009; Gomiz et al., 2013), occurring on the callus or on each of the lateral lobes of the labellum, adjacent to the callus. Typically, the elaiophore consists of cuboidal or palisade-like, secretory, epidermal cells and one to several layers of isodiametric, sub-epidermal cells, beneath which occurs ground parenchyma supplied with vascular bundles and often containing idioblasts with raphides. A bi-layered or stratified cuticle is present, comprising an outer, lamellate and an inner, reticulate region, and this becomes distended as secreted material accumulates beneath its surface. Secretion-filled cavities often appear at anthesis in the outer, tangential cell wall, and these usually coincide with the position of radial walls. Often, concomitant with the development of these cavities, dissolution of the middle lamella and separation of cells result in the formation of intercellular spaces, including spaces formed alongside radial walls, in which accumulates secreted material that contains spherical profiles or cavities. The epidermal cell walls of the elaiophores of some Gomesa and Oncidium species are unusual in that they have peg-like protuberances, and these are also present in Zygostates grandiflora (Lindl.) Mansf. (Pacek et al., 2012). Trichocentrum cavendishianum (Bateman) M.W. Chase & N.H. Williams (Stpiczyńska et al., 2007) has similar elaiophore anatomy, comprising secretory, palisade-like epidermal cells and sub-epidermal tissue. Moreover, cuticular distension and cell wall cavities are also present in this species.
Generally, the floral oil of Oncidiinae is characterized by the presence of diacylglycerols in which the acetyl group is invariably located at position 1 of the glycerol moiety, and the fatty acid at position 2, with the long-chain fatty acid having either hydroxyl or acetoxy groups at positions 3 and 7 (Reis et al., 2000, 2003, 2006; Singer et al., 2006). The major component of the floral oil of Gomesa radicans (Rchb.f.) M.W. Chase & N.H. Williams [as Ornithophora radicans (Rchb.f.) Garay & Pabst] is (2S,3′R,7′R)-1-acetyl-2-(3′,7′-diacetoxy-eicosanoyl)-glycerol (Reis et al., 2003), and acylglycerols are thought also to occur in the floral oils of Gomesa loefgrenii (Cogn.) M.W. Chase & N.H. Williams (as Oncidium loefgrenii Cogn.) and other species of Gomesa (R. B. Singer, pers. comm., 2006), including Gomesa praetexta (Rchb.f.) M.W. Chase & N.H. Williams (as Oncidium enderianum auct.) and related taxa.
Molecular approaches have recently permitted the resolution of Oncidiinae into a number of strongly supported clades, together with the re-alignment of genera (Williams et al., 2001; Chase et al., 2009; Neubig et al., 2012). Thus, based mainly on the generic concepts of Chase (2009) and the molecular work of Neubig and co-workers (2012), Oncidiinae currently includes 61 genera containing approx. 1600 species. As a result, most species once considered to be members of Oncidium were transferred to the newly re-circumscribed genus Gomesa, whereas others, such as O. excavatum Lindl., were transferred to Vitekorchis Romowicz & Szlach. Although the anatomy of the epithelial elaiophores of both Gomesa and Oncidium has been thoroughly investigated, to date that of Cyrtochilum Kunth and Vitekorchis has not, nor has that of any Oncidium spp. displaying floral dimorphism.
Thus, the present investigation focuses on the floral elaiophore structure of members of Vitekorchis, Cyrtochilum and a species of Oncidium displaying floral dimorphism. Comparison with that of Gomesa and non-dimorphic species of Oncidium follows.
Gomesa, as currently circumscribed (Neubig et al., 2012), contains some 125 species, and at least another 23 generic concepts. It occurs from Argentina to Amazonian Peru, and has its centre of distribution in Brazil, especially the Mata Atlântica, where it largely replaces Oncidium. Despite its diverse floral morphology, most members of this genus are easily recognized by their fused lateral sepals.
By contrast, Oncidium, as currently circumscribed (Neubig et al., 2012), contains about 520 species including representatives of the previously recognized genera Odontoglossum Kunth, Sigmatostalix Rchb.f., Cochlioda Lindl., Symphyglossum Schltr., Mexicoa Garay and Miltonioides Brieger & Lückel, as well as Chamaeleorchis Senghas & Lückel, Collare-stuartense Senghas & Bockemühl and Heteranthocidium Szlach., Mytnik & Romowicz. It is generally absent from Brazil, and is distributed from Mexico and Florida, through the Caribbean, Central America south to Bolivia and Peru. The flowers of Oncidium s.s. are adapted for pollination by relatively large oil-collecting bees (e.g. Centris spp.) and a tabula infrastigmatica is present. This structure is a thickened pad on the column which the female bee grasps with its mandibles, thereby freeing its legs for gathering oil, and its presence is strongly correlated with that of an oil reward or an oil-deceit pollination system. It is considered to be a functional analogue to the clawed petal of a malpighiaceous flower. The floral elaiophores often occur on the lateral lobes of the lip. Some species display floral dimorphism and produce a branched inflorescence, the branches bearing a single, terminal fertile flower, but the proximal flowers are abortive and sterile, comprising perianth lobes and no real column. These sterile flowers possibly have an advertising role or function as osmophores for the entire inflorescence. The flowers of others, such as O. hyphaematicum Rchb.f., are said to be pollinated when attacked by territorial bees, a phenomenon known as pseudoantagonism or pseudotrespassing (van der Pijl and Dodson, 1969; Dressler, 1990; van der Cingel, 2002).
Vitekorchis is an Andean genus comprising six species, and is sister to Oncidium, having similar flowers and chromosome counts, but without strong bootstrap support (Neubig et al., 2012). Its members have large, ridged pseudobulbs, with numerous subtending leaves and massive inflorescences, the stipes being small relative to the pollinia.
Cyrtochilum, a genus of 120 species, is restricted to the high Andes of Colombia and Venezuela south to Peru. Cyrtochilum meirax (Rchb.f.) Dalström, one of the subjects of this paper, is the only species of Cyrtochilum to occur in the Caribbean (Dalström, 2001). Many species have long, vining inflorescences with large, showy flowers, sometimes with prominent elaiophores, but a few species are small with diminutive flowers. Cyrtochilum has dull pseudobulbs that are round or ovoid in cross-section and bear 2–4 apical leaves with 2–6 leaf-bearing sheaths and relatively thick roots, in contrast to Oncidium, which has glossy, ancipitous pseudobulbs and thin roots (Neubig et al., 2012). There are few reported observations of pollination for this genus.
The considerable similarity between the flowers of certain species of Gomesa and Oncidium is due to homoplasy, since floral traits in Oncidiinae are highly plastic and reflect evolutionary shifts in pollinators (Neubig et al., 2012).
Here, the elaiophore structure of Vitekorchis excavata, Cyrtochilum meirax (two genera for which elaiophore anatomy has not yet been examined in detail) and a species of Oncidium displaying floral dimorphism, O. heteranthum var. album (also not investigated to date), is compared with that of Gomesa longipes (considered to possess elaiophores typical of many Oncidiinae). Moreover, that of florally dimorphic O. heteranthum var. album is compared with that of non-dimorphic members of the genus in order to investigate whether floral dimorphism, and thus a change in pollination strategy, results in modification of elaiophore structure.
MATERIALS AND METHODS
Plants used in this study were obtained from the first author's collection. They include Gomesa longipes (Lindl.) M.W. Chase & N.H. Williams (accession no. KLD 201105), Vitekorchis excavata (Lindl.) Romowicz & Szlach. (accession no. KLD 200503), both sterile and fertile flowers of Oncidium heteranthum var. album Poepp. & Endl. (accession no. KLD 201107), together with flowers of Cyrtochilum meirax (Rchb.f.) Dalström (accession no. KLD 201201). Spirit-preserved voucher material was deposited at the herbarium of Warsaw Botanic Garden, Poland under the accession nos WABG 002577 (G. longipes), WABG 002578 (V. excavata), WABG 002579 (O. heteranthum var. album) and WABG 002580 (C. meirax). Abbreviations for authors of plant names follow Brummitt and Powell (1992) throughout.
Preliminary observations and localization of elaiophore tissue was performed on fresh, intact flowers by immersing them for 5–20 min in a saturated ethanolic solution of Sudan III.
Elaiophore tissue of flowers at anthesis was examined using light microscopy (LM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) as follows. Pieces of elaiophore tissue (approx. 1 mm3) were excised and fixed in 2·5 % (v/v) glutaraldehyde/4 % (v/v) formaldehyde in phosphate buffer (pH 7·4; 0·1 m) for 2 h at 4 °C, washed three times in phosphate buffer and post-fixed in 1·5 % (w/v) osmium tetroxide solution for 1·5 h at 0 °C. The fixed material was then dehydrated using a graded ethanol series, and infiltrated and embedded in LR White resin (LR White acrylic resin, medium grade, Sigma). Following polymerization at 60 °C, sections were cut at 70 nm for TEM using a Reichert Ultracut-S ultramicrotome and a glass knife, stained with uranyl acetate and lead citrate (Reynolds, 1963) and examined using a FEI Tecnai Spirit G2 or Tesla BS 540 transmission electron microscope, at an accelerating voltage of 90 kV.
Semi-thin sections (0·9–1·0 μm thick) were prepared for LM. These were stained for general histology with aqueous 1 % (w/v) methylene blue/1 % (w/v) azure II (1:1) (MB/AII) for 5–7 min.
Histochemical tests were used to detect the presence of lipids and starch in elaiophore tissue by treating the latter with a saturated ethanolic solution of Sudan III and aqueous IKI (iodine–potassium iodide) solution, respectively. Furthermore, a 10 % (w/v) aqueous solution of FeCl3 was used to test for catechol-type dihydroxyphenols (Gahan, 1984) and the periodic acid–Schiff (PAS) reaction to detect the presence of insoluble polysaccharides (Jensen, 1962). Micrometry and photomicrography of elaiophore tissue were accomplished using a Nikon Eclipse E200 (NIS-Elements AR software) for LM images and FEI Tecnai Spirit G2 (TEM Imaging & Analysis computer program) for TEM.
For SEM, fixed pieces of labellum were dehydrated and subjected to critical-point drying using liquid CO2. They were then sputter-coated with gold and examined using a Tescan Vega II LS scanning electron microscope, at an accelerating voltage of 10 kV.
RESULTS
Elaiophore characters for the four taxa are summarized in Table 1.
Table 1.
Comparison of floral secretory tissue in investigated taxa
| Character | Gomesa longipes (Lindl.) M.W. Chase & N.H. Williams | Vitekorchis excavata (Lindl.) Romowicz & Szlach | Oncidium heteranthum Poepp. & Endl. var. album | Cyrtochilum meirax (Rchb.f.) Dalström |
|---|---|---|---|---|
| Position and type of elaiophore | Callus Intermediate |
Callus Intermediate |
Callus Intermediate |
No obvious elaiophore Oil-like, but seemingly less viscid and more volatile |
| Appearance of secretion | Wax-like and heterogeneous | Wax-like | Oil-like | |
| Type of secretory tissue | Cuboidal epidermal cells, trichomes and papillae | Cuboidal epidermal cells, trichomes and papillae | Cuboidal epidermal cells, trichomes and papillae | Cuboidal epidermal cells |
| Secretory residue visible under SEM | + | + |
Fertile flower + Sterile flower – |
– |
| Type of epidermal cuticle | Smooth | Striate |
Fertile flower Striate Sterile flower Smooth |
Finely striate |
| Cuticular blisters | – | – |
Fertile flower + Sterile flower – |
– |
| Subcuticular accumulation of secretion | + | – | Fertile flower – | + |
| Cell wall protuberances | – | + | Fertile flower – | – |
| Secretion or vesicles present in wall or periplasmic space | + | + | Fertile flower + | – |
| Endoplasmic reticulum | Mainly SER | Mainly SER | Fertile flower Mainly RER | Mainly RER |
| Plastids | Amyloplasts with starch, plastoglobuli and poorly developed internal membranes |
Amyloplasts with starch, plastoglobuli and poorly developed internal membranes |
Fertile flower Amyloplasts with starch, plastoglobuli and poorly developed internal membranes, but also chromoplasts Sterile flower Amyloplasts as above |
Amyloplasts with starch, plastoglobuli and poorly developed internal membranes, but also oil-laden elaioplasts |
| Cytoplasmic lipid droplets | + | + |
Fertile flower + Sterile flower + |
+, but also irregularly shaped osmiophilic bodies |
| Intravacuolar membranes | Myelin-like figures present | Membranous enclaves, but highly organized myelin-like figures absent |
Fertile flower Membranous enclaves, but highly organized myelin-like figures absent Sterile flower Myelin-like figures absent |
Myelin-like figures absent |
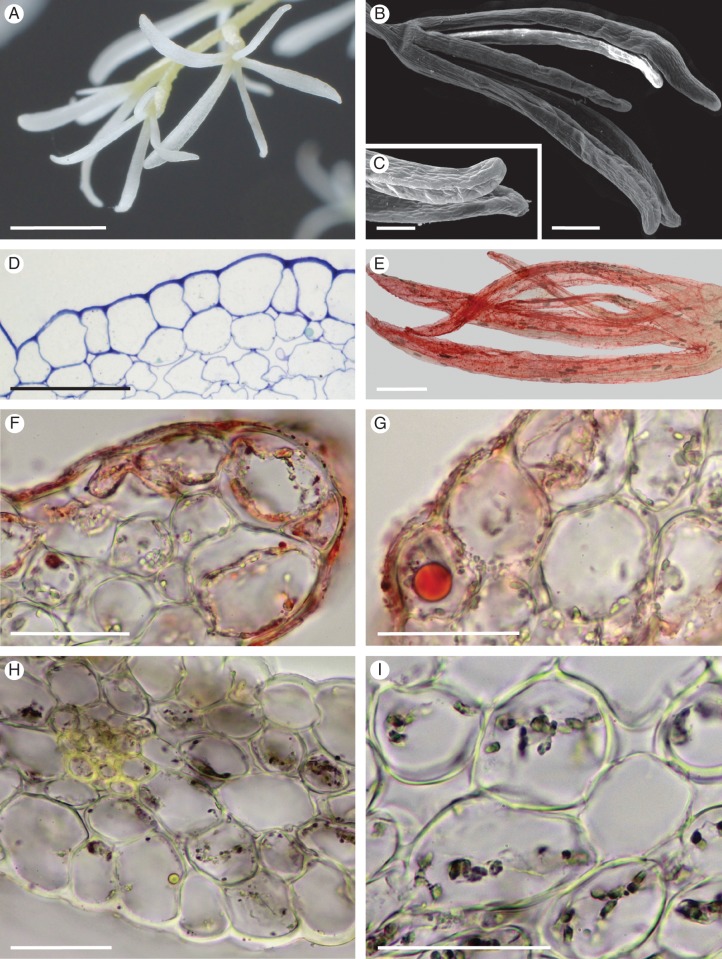
Gomesa longipes
The yellow and brown flowers of G. longipes are few and borne upon a simple, racemose inflorescence. They lack a scent perceptible to humans. The column is expanded basally to form a tabula infrastigmatica (Fig. 1A, C).The callus of the labellum is tuberculate (Fig. 1A–C). The elongate projections of the callus (Fig. 1B–D) have glabrous, cuboidal epidermal cells and between these projections occur unicellular papillae and trichomes of varying length (Figs 1E, I, J and 2A–D). A white, amorphous, wax-like deposit occurs on the callus, and both this substance and the callus region stained red with Sudan III, indicating the presence of lipids (Fig. 1C). Lipids present on the apices of the projections (Fig. 1D) and as small droplets on the surface of the cell walls of trichomes and papillae borne on the callus also stained red with Sudan III (Fig. 1E, F). Treatment with FeCl3 did not indicate the presence of phenolic compounds. Atrichomatous epidermal cells contain cytoplasm that stained intensely with MB/AII, amyloplasts and a basally located nucleus (Fig. 1G). The sub-epidermal or hypodermal tissue comprises a single layer of small parenchymatous cells and encloses the ground parenchyma whose cells have relatively large intercellular spaces (Fig. 1G, H). Large idioblasts with raphides are present in the ground parenchyma (Fig. 1G). Papillae and trichomes contain a large, centrally located vacuole and a narrow layer of parietal cytoplasm (Fig. 1I, J). These cells, together with the sub-epidermal parenchyma, also contain amyloplasts with starch grains (Fig. 1J). The tangential walls of the epidermal hairs and sub-epidermal cells are cellulosic, thick and perforated with primary pit-fields containing plasmodesmata, whereas the radial walls are much thinner (Fig. 1I, J). The cuticle overlying the cell walls of atrichomatous epidermal cells of the callus projections, papillae and trichomes lacks blisters, but secretory residues are present on all these structures (Figs 1G, I, 2B–D and 3A–C). This secretion also accumulates between the outer tangential wall of epidermal cells and the cuticle, is heterogeneous and is composed of darkly stained, electron-dense, osmiophilic material (Fig. 3A–C). Frequently, this substance also occurs adjacent to the plasmalemma (Fig. 3D) or between the structural elements of the cell wall (Fig. 3A–C, E, F). Arrays of smooth endoplasmic reticulum (SER) predominate in the cytoplasm, together with lipid bodies and mitochondria (Fig. 3A–D). Dictyosomes are occasionally seen. Vacuolar profiles vary in size from small vacuoles with membranous enclaves to large, centrally located vacuoles containing myelin-like figures (Fig. 3A–B, D, E). Plastids are mostly regularly oval in outline, have a mean diameter of 1·8 μm and contain poorly developed internal membranes, one to several starch grains, and plastoglobuli that usually occur close to the plastid envelope (Fig. 3F, G).
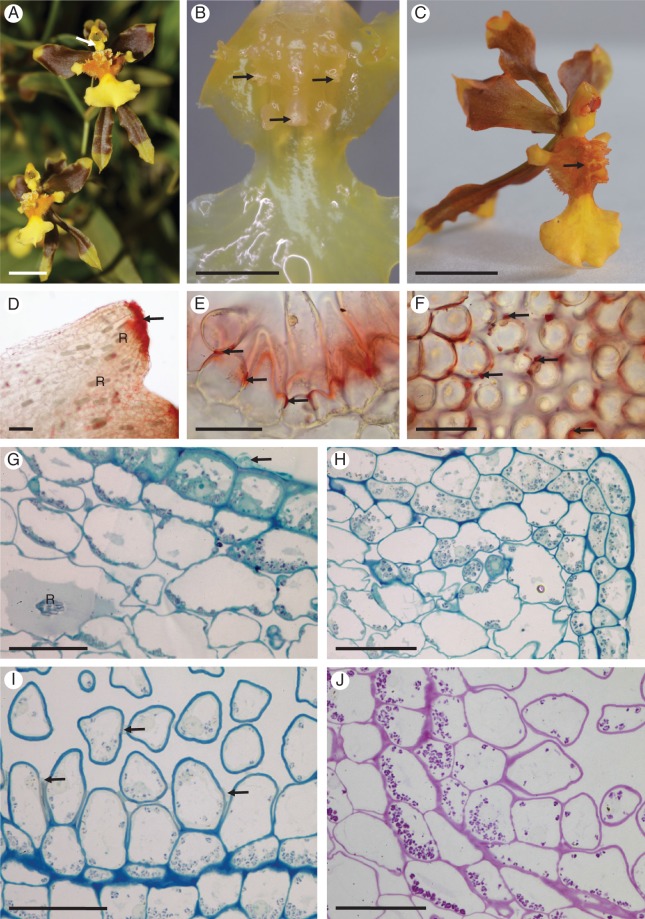
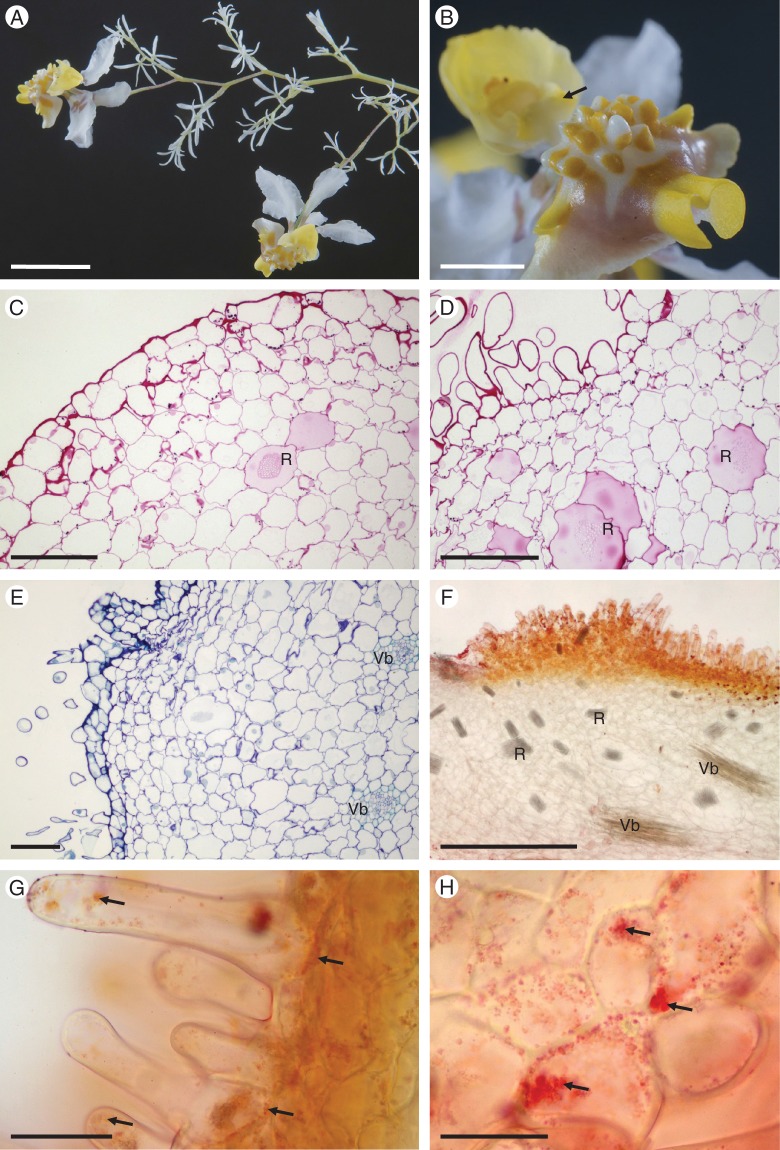
Fig. 1.
Gomesa longipes: habit and LM. (A) Habit of flower with pronounced callus and tabula infrastigmatica (arrow). (B) Callus showing projections (arrows). (C) Entire flower following immersion in alcoholic Sudan III solution clearly showing stained callus region (arrow). Note the pronounced tabula infrastigmatica. (D) Apical part of callus projection stains red for lipids (arrow) following treatment with Sudan III. Numerous idioblasts containing raphides occur in the ground parenchyma. (E) Lipid-rich material is also present towards the bases of trichomes (arrows). (F) Lipid droplets also occur on the surface of epidermal papillae (arrows). (G) Section of callus stained with MB/AII showing glabrous epidermis and residues of secreted material (arrow) on the callus surface. (H) Section of callus projection stained with MB/AII. Epidermal and ground parenchyma cells contain amyloplasts. (I, J) Section of callus epidermis with trichomes. Note the thick tangential cell walls of sub-epidermal parenchyma. (I) Secretory residues (arrows) following staining with MB/AII. (J) Starch grains within trichomatous and atrichomatous epidermal cells, together with ground parenchyma cells, following the PAS reaction. Scale bars = 1 cm, 3 mm, 1 cm, 100 μm, 50 μm, 50 μm, 50 μm, 50 μm, 50 μm, 50 μm, respectively. R, idioblasts with raphides.
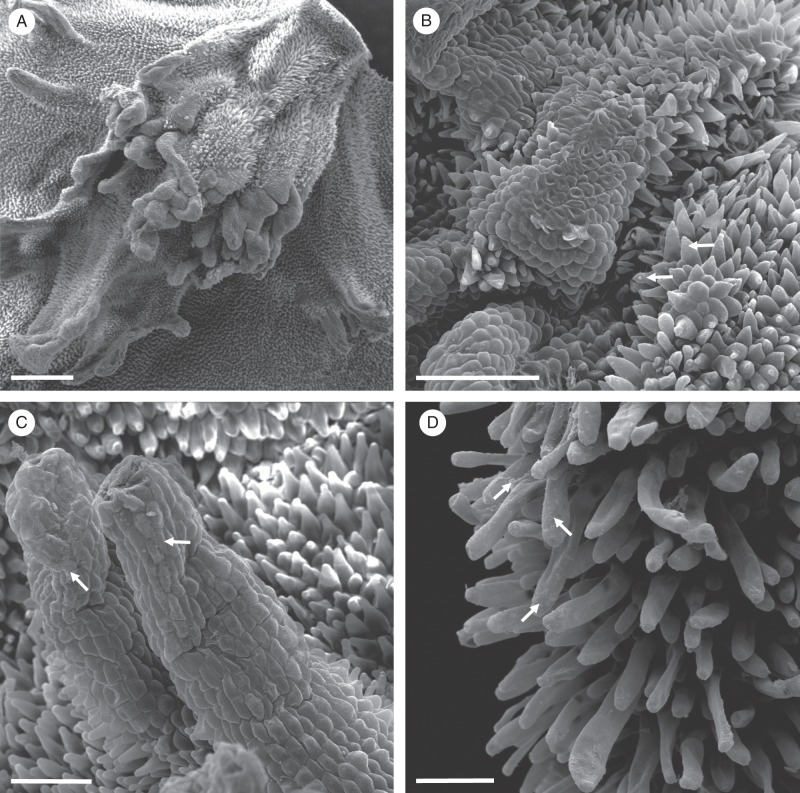
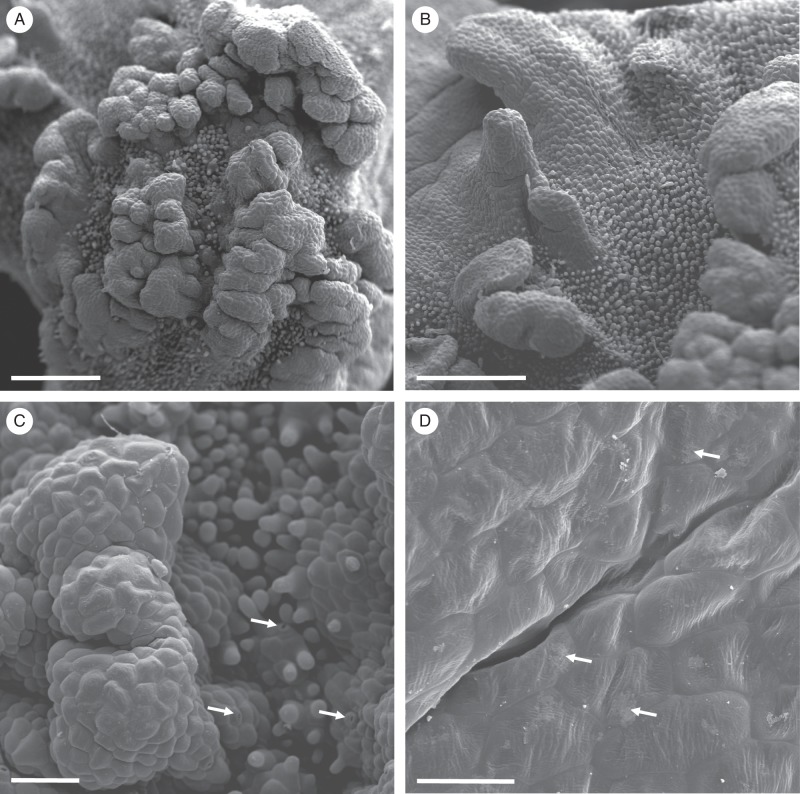
Fig. 2.
Gomesa longipes: SEM. (A) Surface of labellar callus showing projections, papillae and trichomes. (B) Conical papillae with residues of secretory material (arrows). (C) Secreted material (arrows) on the surface of the glabrous epidermis overlying the callus projection. (D) Secreted material on the surface of trichomes (arrows). Scale bars = 500 μm, 200 μm, 100 μm, 100 μm, respectively.
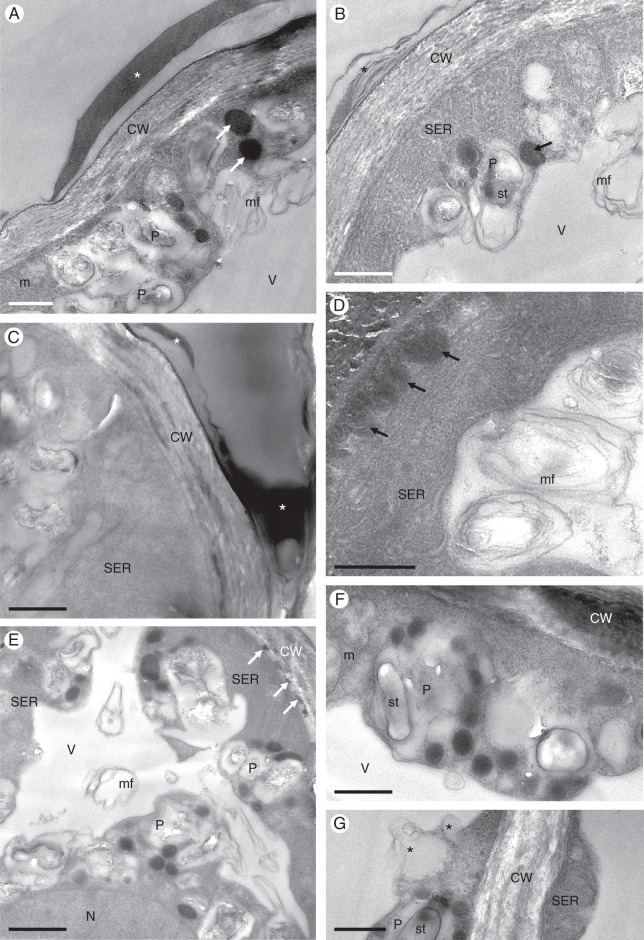
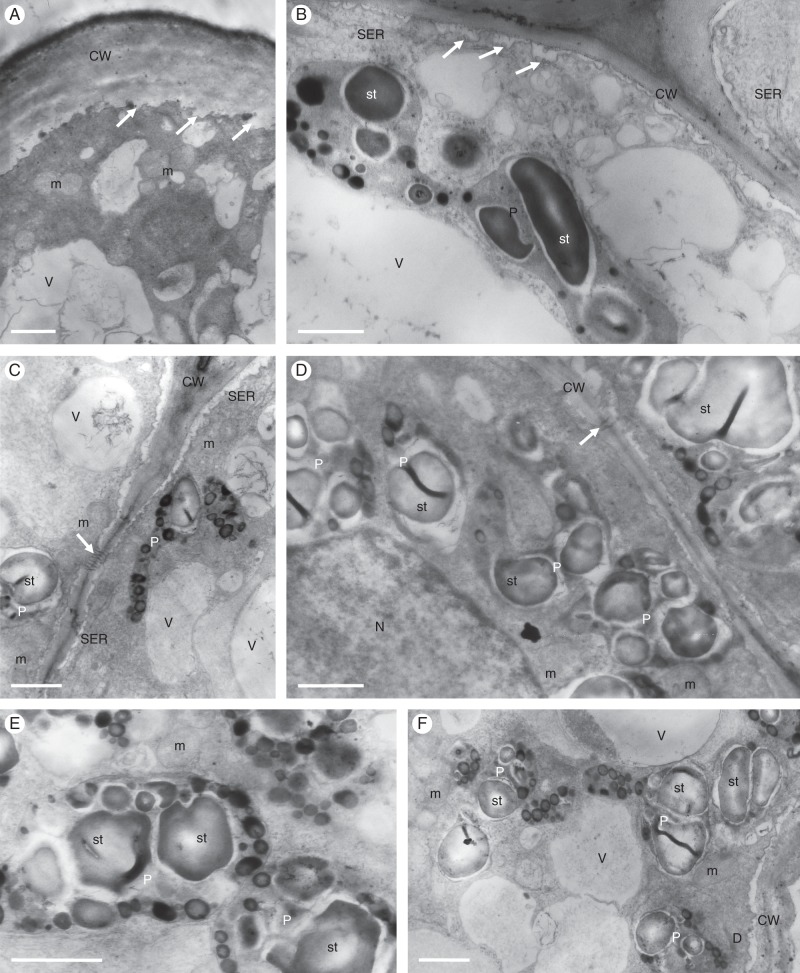
Fig. 3.
Gomesa longipes: TEM. (A) Osmiophilic secretion (asterisk) on the surface of epidermal cell wall of callus. Large, osmiophilic droplets (arrows) occur in the parietal cytoplasm. (B) Heterogeneous secretion (asterisk) on the epidermal cell wall surface. Abundant smooth endoplasmic reticulum profiles, plastids and osmiophilic droplets (arrow) occur in the cytoplasm, together with intravacuolar myelin-like figures. (C) Cell wall of basal part of trichome coated with secretion (asterisks). Smooth endoplasmic reticulum predominates in the cytoplasm. (D) Osmiophilic material accumulates beneath the cell wall (arrows). (E) Osmiophilic droplets occur in the cytoplasm and between the structural elements of the cell wall (arrows). (F) Plastids containing starch grains and plastoglobuli. (G) Details of plastid with few internal membranes, starch and plastoglobuli. Two vacuoles coalesce (asterisks), resulting in the initiation of myelin-like figure formation. Scale bars = 1 μm, 1 μm, 1 μm, 1 μm, 2 μm, 1 μm, 1 μm, respectively. CW, cell wall; m, mitochondrion; mf, myelin-like figure; N, nucleus; P, plastid; SER, smooth endoplasmic reticulum; st, starch; V, vacuole.
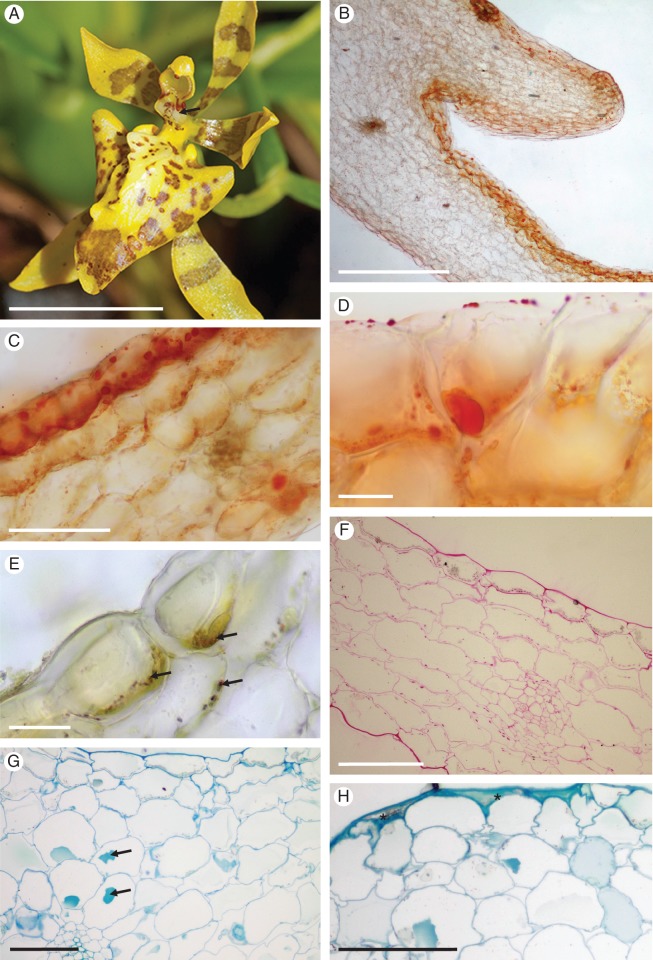
Vitekorchis excavata
The similarly coloured flowers of V. excavata are numerous, occur on a paniculate inflorescence, and the column is expanded basally to form a tabula infrastigmatica (Fig. 4A, B). The crested labellar callus bears rugae or projections (Fig. 4B) with glabrous epidermal cells and stomata. Elsewhere, papillae and unicellular trichomes are present (Figs 4C–H and 5A–C), but the former are the more common. White, amorphous, wax-like material occurs on the callus, and both this substance and the callus tissue stained red with Sudan III, indicating the presence of lipids (Fig. 4B). Lipid droplets were also visible within typical, cuboidal, atrichomatous epidermal cells, epidermal hairs, papillae and sub-epidermal parenchyma (Fig. 4F). None of the tissues examined stained with FeCl3. Idioblasts, often containing raphides, were frequently present in the ground parenchyma (Fig. 4C, D) and these stained only slightly with the PAS reaction (Fig. 4G, H). The walls of epidermal and sub-epidermal cells are thin and cellulosic (Fig. 4D–F), and a narrow layer of parietal cytoplasm is present (Fig. 4E). Several large vacuoles occur in both atrichomatous and trichomatous epidermal cells, and these contain anthocyanins (Fig. 4D). Epidermal and sub-epidermal cells, as well as the ground parenchyma, contain amyloplasts with numerous starch grains (Fig. 4G, H). Investigations by SEM revealed the presence of only traces of secretory residues on the callus surface (Fig. 5C, D). The cuticle overlying the epidermal cell walls is only slightly striate, and cuticular blisters or cracks are absent (Fig. 5D). No sub-cuticular accumulation of secretion was observed. The cytoplasm of atrichomatous, trichomatous and papillose epidermal cells mainly contain SER and numerous mitochondria, dictyosomes and vesicles of various sizes (Fig. 6A–F). Small vesicles frequently occur in the periplasmic space, and these are formed by folding of the plasmalemma (Fig. 6B, C). Furthermore, protuberances arise from the inner surface of the cell wall (Fig. 6A), and the epidermal cells are interconnected by numerous plasmodesmata (Fig. 6C, D). Vacuoles of various sizes contain inconspicuous membranous enclaves (Fig. 6A–C), but highly organized myelin-like structures are absent. Numerous, irregularly shaped plastids of mean diameter 2·5 μm contain a poorly developed system of internal membranes. Each contains one to several starch grains, and numerous plastoglobuli occur adjacent to the plastid envelope (Fig. 6B–F).
Fig. 4.
Vitekorchis excavata: habit and LM. (A, B) Flower prior to (A) and following treatment with Sudan III (B) showing distinctly stained, lipid-rich callus region (arrow). (C) Section of callus showing glabrous, abaxial and papillose, adaxial epidermis. Note that the callus projection lacks papillae. (D) Epidermal papillae contain intravacuolar anthocyanins, whereas large idioblasts occurring in the ground parenchyma contain raphides. (E) Plastids in parietal cytoplasm following staining with MB/AII. (F) Surface and intracellular lipids stained with Sudan III. These compounds occur in both epidermal papillae and sub-epidermal cells. (G, H) Starch grains present in glabrous epidermal cells, papillae and parenchyma cells stain with the PAS reaction. Note the weak staining of large idioblasts and vascular bundles near the abaxial epidermis (H). Scale bars = 1 cm, 1 cm, 500 μm, 50 μm, 20 μm, 50 μm, 100 μm, 100 μm, respectively. R, idioblasts with raphides; Vb, vascular bundle.
Fig. 5.
Vitekorchis excavata: SEM. (A, B) Papillose labellar callus bearing glabrous rugae (A) and projections (B). (C) Detail of glabrous epidermal cells overlying rugae. Unicellular, conical papillae and trichomes occur between the projections and rugae. Stomata are indicated by arrows. (D) Residues of secreted material (arrows) on the cuticular surface of glabrous epidermal cells. Scale bars = 600 μm, 500 μm, 100 μm, 40 μm, respectively.
Fig. 6.
Vitekorchis excavata: TEM. (A) Protuberances arising from the inner surface of the trichome cell wall (arrows), the dense cytoplasm containing mitochondria. (B) Plastids of trichomes with large starch grains. Folding of the plasmalemma (arrows) results in the formation of small vesicles within the periplasmic space. (C) Plastid of irregular shape with starch and numerous plastoglobuli, together with vacuoles containing inconspicuous membranous enclaves. Plasmodesmata interconnect adjoining epidermal cells (arrow). (D) Plastids containing starch and plastoglobuli. The cell walls of adjoining epidermal cells have irregular outlines and are perforated by primary pit-fields with plasmodesmata (arrow). (E) Plastid lacking well-developed internal membranes and containing plastoglobuli that are located adjacent to the plastid envelope. (F) Vacuoles with fine flocculent contents, but lacking myelin-like structures. Scale bars = 1 μm, 1 μm, 1 μm, 1 μm, 1 μm, 1 μm, respectively. CW, cell wall; D, dictyosome; m, mitochondrion; N, nucleus; P, plastid; SER, smooth endoplasmic reticulum; st, starch; V, vacuole.
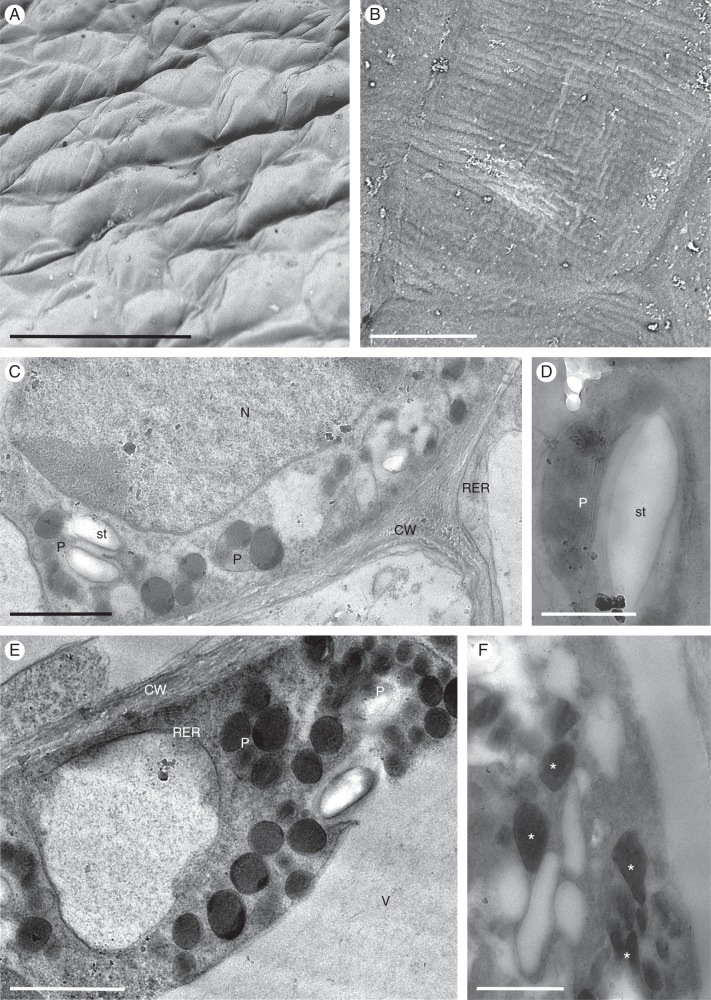
Oncidium heteranthum var. album
The dimorphic flowers of O. heteranthum var. album are paler than the type, numerous and borne on a paniculate inflorescence. A single fertile flower occurs terminally on first- and second-order inflorescence branches, whereas several sterile flowers, composed entirely of linear perianth lobes, arise from each second-order lateral branch (Fig. 7A). The flowers are subtended by lanceolate, concave bracts. The pedicels of both sterile and fertile flowers, together with the branches of the inflorescence, are verrucose, the swellings corresponding to the position of stomata. Branches of the inflorescence also bear occasional, short, robust, conical epidermal hairs. These, together with the contents of elongate, atrichomatous epidermal cells with convex, outer tangential walls, stain bright red on applying Sudan III, indicating the presence of lipid. Moreover, both the cuticle and a layer of colourless material present on the surface of the epidermal cells of the inflorescence also stain with this reagent. This species produces a floral fragrance resembling that of mown hay.
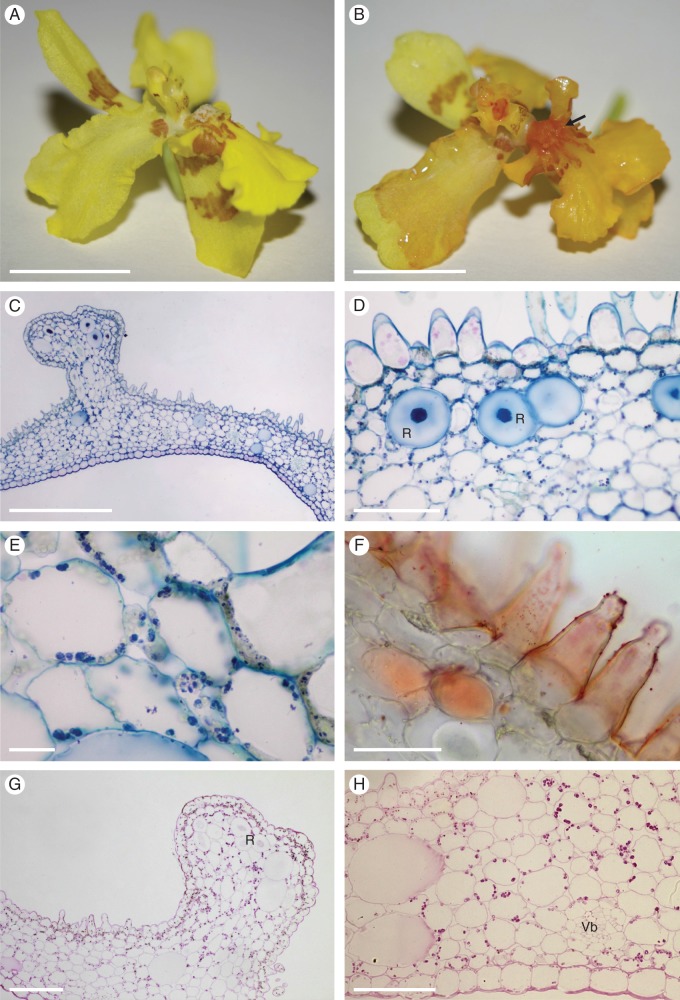
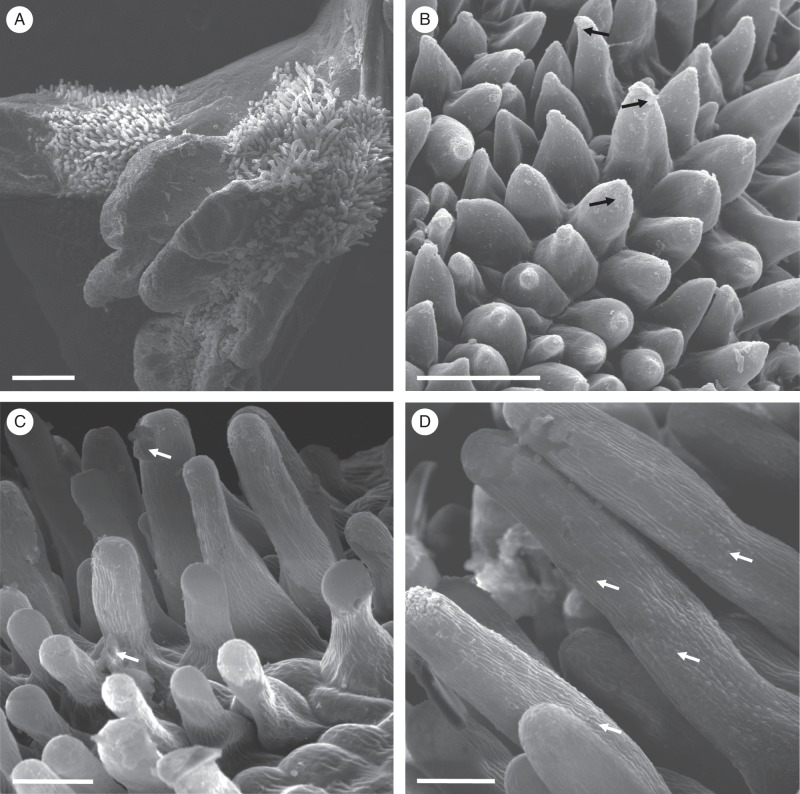
Fig. 7.
Oncidium heteranthum var. album: habit and LM. (A) Habit of paniculate inflorescence with branches bearing numerous sterile flowers and few terminal, fertile flowers. (B) Tuberculate callus of fertile flower with projections. Note the tabula infrastigmatica (arrow), which is located on the column. (C) Section of callus projection with overlying glabrous epidermis containing numerous stomata. Idioblasts containing raphides occur in the ground parenchyma. Small quantities of starch, mainly in the sub-epidermal parenchyma, stain with the PAS reaction. (D) Trichomes and papillae occur between the callus projections, whereas idioblasts and occasional starch grains occur in the ground parenchyma. (E) Section of tuberculate callus stained with MB/AII showing vascular bundles that supply the ground parenchyma. None of these vascular elements supplies the sub-epidermis. (F) Callus trichomes stain red with Sudan III, indicating the presence of lipid. Idioblasts with raphides, as well as vascular bundles occur in the ground parenchyma. (G) Small lipid droplets (arrows) occur in the trichomatous and atrichomatous epidermal cells, as well as the sub-epidermal cells. (H) Lipid droplets are also visible within glabrous epidermal cells (arrows). Scale bars = 1 cm, 3 mm, 100 μm, 100 μm, 100 μm, 500 μm, 50 μm, 50 μm, respectively. R, idioblasts with raphides; Vb, vascular bundle.
Fertile flower
The perianth lobes are mainly white, basally marked with pink-brown, and the labellum and callus are mainly yellow (Fig. 7A, B). The column of each fertile flower is expanded basally to form a tabula infrastigmatica (Fig. 7B). The tuberculate callus bears numerous projections whose epidermis, consisting of cuboidal cells, is mainly glabrous and bears stomata (Fig. 7B, C). However, the regions between these projections bear trichomes and papillae of various length (Figs 7D–G and 8A–D). Treatment with IKI and the PAS reaction revealed the presence of only small quantities of starch, mainly in the sub-epidermal parenchyma (Fig. 7C, D). Furthermore, the cellulosic tangential walls of the sub-epidermal parenchyma are irregularly thickened (Fig. 7C, E). Both atrichomatous and trichomatous epidermal cells usually contain a single, centrally located vacuole and parietal cytoplasm with occasional plastids. Following treatment with Sudan III, the glabrous epidermal cells of the callus projections, as well as the callus trichomes, stained red, indicating the presence of lipid (Fig. 7F–H). Callus cells did not stain with FeCl3, indicating the absence of phenolic compounds. Investigations by SEM (Fig. 8A–D) revealed the presence of secretory residues on the surface of cell walls of both papillae and trichomes (Fig. 8B, C). Furthermore, SEM of the striate cuticle revealed the presence of small, but numerous, blisters (Fig. 8D). Investigations by TEM did not indicate the presence of any cavities in the outer cell wall nor a lamellate cuticle (Fig. 9A). Furthermore, no sub-cuticular accumulation of secretion was observed. The granular cytoplasm contains numerous, relatively short profiles of endoplasmic reticulum [predominantly rough endoplasmic reticulum (RER)] and mitochondria (Fig. 9B–F). Moreover, the cytoplasm contains osmiophilic globules. Frequently, vesicles occur in the periplasmic space (Fig. 9B). Plastids contain one to several starch grains, numerous plastoglobuli and poorly developed internal membranes, but starchless plastids with an electron-dense stroma were also observed (Fig. 9B, E, F). Although occasional membranous, intravacuolar figures were observed, these were poorly developed and lacked myelin-like configurations. The perianth cells of fertile flowers contained relatively few lipid droplets. Even so, these flowers were distinctly fragrant.
Fig. 8.
Oncidium heteranthum var. album: fertile flower, SEM. (A) Part of callus showing projections with overlying glabrous epidermis. Between these projections occur unicellular trichomes and papillae. (B) Residues of secreted material (arrows) occur on the surface of the papillae. (C) Secreted material (arrows) is also present on the surface of trichome cell walls. (D) Striate cuticle of callus trichomes with small blisters (arrows). Scale bars = 500 μm, 50 μm, 50 μm, 20 μm, respectively.
Fig. 9.
Oncidium heteranthum var. album: fertile flower, TEM. (A) Outer cell wall of glabrous epidermal cell of callus with associated osmiophilic cuticle. (B) Granular cytoplasm of epidermal cell with vesicles present in the periplasmic space (arrows) and a plastid lacking both starch and plastoglobuli. (C) Parietal cytoplasm with mitochondria and short profiles of rough endoplasmic reticulum. The vacuoles generally lack myelin-like figures. (D) Granular cytoplasm of sub-epidermal cell containing mitochondria and rough endoplasmic reticulum. (E) Sub-epidermal cell with plastids containing plastoglobuli and starch. (F) The inner tangential walls of epidermal cells, adjacent to those of the sub-epidermal tissue are relatively thick. The cytoplasm contains both plastids and mitochondria. Scale bars = 1 μm, 200 nm, 1 μm, 1 μm, 1 μm, 2 μm, respectively. CW, cell wall; Is, intercellular space; m, mitochondrion; P, plastid; RER, rough endoplasmic reticulum; st, starch; V, vacuole.
Sterile flower
The sterile flowers are white, usually with five perianth lobes (Fig. 10A). The latter are more or less uniform in size and shape (Fig. 10B) and have a glabrous epidermis with a smooth cuticle that appears to lack striae and blisters, even when examined using SEM (Fig. 10C). Both epidermal and sub-epidermal cells have thin cell walls and prominent vacuoles (Fig. 10D). As in fertile flowers, TEM did not reveal the presence of intravacuolar myelin-like figures. Perianth lobes of sterile flowers stained pink to red with Sudan III (Fig. 10E), and numerous lipid droplets of various sizes were present, mainly in the epidermal cells (Fig. 10F, G). Furthermore, TEM showed that large, spherical, osmiophilic droplets occur in the cytoplasm. Amyloplasts with starch grains (Fig. 10H, I) were clearly visible, mainly in parenchyma cells, using LM, whereas TEM revealed that these organelles contain numerous plastoglobuli. Sterile flowers were not as strongly fragrant as fertile flowers.
Fig. 10.
Oncidium heteranthum var. album: sterile flowers, habit, LM and SEM. (A, B) The white, sterile flowers have five, simple perianth lobes of uniform size and shape. (C) The perianth lobes have a glabrous epidermis lacking a striate cuticle. (D) Section of perianth lobe stained with MB/AII showing glabrous epidermis with cuticle, together with mesophyll (ground parenchyma) showing well-developed, intercellular spaces. Both tissues have thin cell walls and prominent vacuoles. (E) The entire perianth stains red following treatment with Sudan III for lipids. (F) Lipids are mainly present in epidermal cells and on the surface of their walls. (G) In some cells, however, large lipid droplets may be formed. (H) Starch, stained here with IKI, is present within amyloplasts of ground parenchyma cells. (I) Detail of ground parenchyma. Cells of this tissue have thin walls, large vacuoles and contain amyloplasts with starch. Scale bars = 3 mm, 500 μm, 200 μm, 100 μm, 500 μm, 50 μm, 50 μm, 50 μm, 50 μm, respectively.
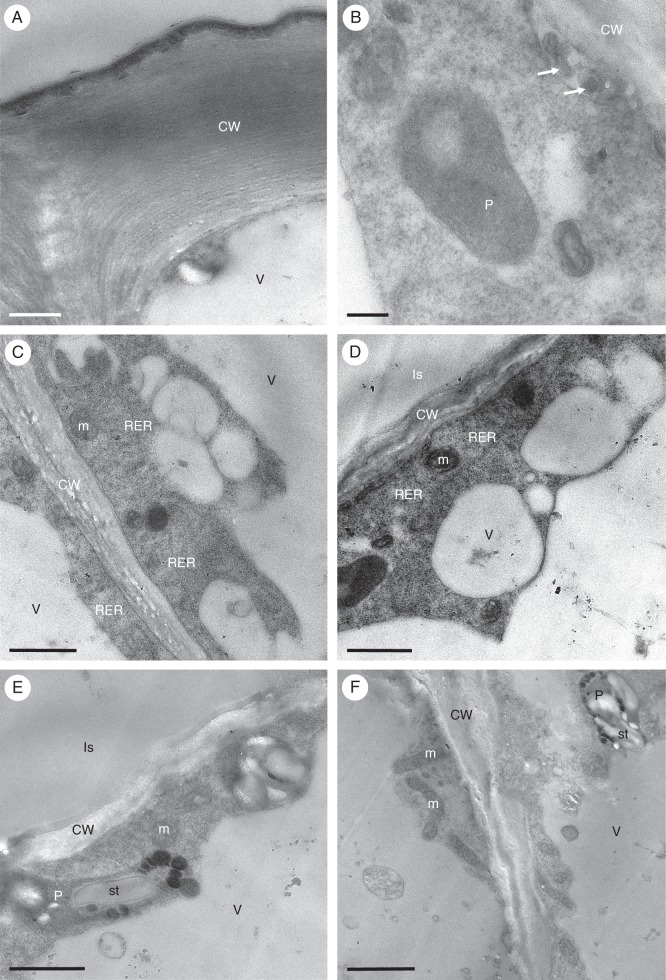
Cyrtochilum meirax
The sweetly, but faintly fragrant, yellow and brown flowers of C. meirax are few and occur on a simple, racemose inflorescence. Again, the column base is expanded and possibly functions as a tabula infrastigmatica (Fig. 11A). The perianth lobes are often glossy and the labellar callus simple and trilobed, neither crested nor tuberculate, lacking obvious secretory material upon its surface and associated with three keels that pass onto the mid-lobe of the labellum, with the labellum arranged almost perpendicular to the basal part of the column (Fig. 11A). Treatment of the entire flower with Sudan III for 20 min stained all the perianth lobes a very pale, uniform orange (with the exception of the small, inflated structures borne on the labellum adjacent to the base of the column, which stained slightly darker), but, without magnification, provided no unequivocal evidence for the presence of lipids or for the presence of a discrete elaiophore. However, when the flower was removed from the watchglass of stain, a relatively large droplet of liquid had collected at its base, beneath the ethanolic stain solution. The stain was carefully pipetted off and water added to the remaining droplet which clearly had an affinity for Sudan III and had stained pale red. The droplet was hydrophobic, immiscible with water and floated on its surface. Nevertheless, it became dispersed when a drop of dilute soap solution was added. These tests showed that the droplet probably comprised a lipid of relatively low molecular weight. Subsequent observations revealed the accumulation of small droplets of colourless liquid at the base of the column and on the inflated structures described above. These droplets also occurred on the tri-lobed callus and other parts of the labellum. However, they disappeared following treatment with ethanolic Sudan III, probably having dissolved in the ethanol. Hand-cut sections of the callus lobes stained with Sudan III revealed the presence of lipid droplets in the cuboidal epidermal cells and, to a lesser degree, in the sub-epidermal parenchyma cells (Fig. 11B–D). These cells also contain amyloplasts with starch grains (Fig. 11E, F). Staining with MB/AII revealed that the thin cell walls of the epidermal and sub-epidermal tissues are cellulosic. Furthermore, the contents of idioblasts present in the ground parenchyma became completely stained with these reagents, whereas the vacuolar contents of some parenchyma cells were only partly stained (Fig. 11G, H). Observations by SEM did not reveal the presence of blisters or secretory residues on the surface of the finely striate cuticle of the callus epidermis (Fig. 12A, B); however, in some sections, intensely stained material accumulated between the outer tangential wall of epidermal cells and the cuticle (Fig. 11H). The TEM of epidermal cells revealed the presence of parietal cytoplasm containing a large nucleus (Fig. 12C). The cytoplasm is both highly granular and vesiculate, and contains arrays of RER (Fig. 12C, E), but no vesicles accumulated in the periplasmic space. The plastids often contain poorly developed internal membranes and usually a single starch grain, together with numerous plastoglobuli (Fig. 12C–E). Some plastids, however, contain only plastoglobuli and these are thought to be elaioplasts. Osmiophilic bodies, not unlike plastoglobuli except for their less regular profile, also occur in the cytoplasm (Fig. 12F). Intravacuolar myelin-like figures are absent.
Fig. 11.
Cyrtochilum meirax: habit and LM. (A) Yellow and brown flowers are borne on a simple, racemose inflorescence. The tabula infrastigmatica is indicated by an arrow. (B) Section of labellum with glabrous callus stained with Sudan III. (C) Lipids accumulate mainly in the adaxial epidermal cells of the labellum. (D) Large lipid droplets stained with Sudan III are present both within epidermal cells and upon their surface. (E) Epidermal and sub-epidermal cells contain small quantities of starch (arrows) that stain with IKI. (F) Section of labellum following the PAS reaction showing thin cellulose walls of epidermal and ground parenchyma cells, and occasional starch grains. (G) Vacuolar contents of some parenchyma cells only partly stain with MB/AII (arrows). (H) The secreted material (asterisks) present between the outer tangential wall of epidermal cells and the cuticle stains intensely with MB/AII. Scale bars = 1 cm, 500 μm, 50 μm, 20 μm, 20 μm, 50 μm, 50 μm, 100 μm, respectively.
Fig. 12.
Cyrtochilum meirax: SEM and TEM. (A) Cuticular surface of epidermal cells of the callus lacking obvious secreted material. (B) Detail of the finely striate cuticle lacking blisters and cracks. (C) Highly granular and vesiculate parietal cytoplasm containing a large nucleus and plastids. (D) Plastid containing few internal membranes, as well as a starch grain and numerous plastoglobuli. (E) Large plastoglobuli may occur within plastids. (F) Osmiophilic bodies (asterisks), not unlike plastoglobuli, may also occur in the cytoplasm. Scale bars = 100 μm, 10 μm, 2 μm, 1 μm, 2 μm, 500 nm, respectively. CW, cell wall; N, nucleus; P, plastid; RER, rough endoplasmic reticulum; st, starch; V, vacuole.
DISCUSSION
The subjects of this study differ in a number of ways from the majority of oil-secreting Oncidiinae studied to date. Although, like many Oncidiinae, the floral elaiophore of G. longipes, V. excavata and O. heteranthum var. album occurs on the callus (Pacek et al., 2007; Stpiczyńska et al., 2007, 2013; Stpiczyńska and Davies, 2008; Aliscioni et al., 2009; Davies and Stpiczyńska, 2009; Gomiz et al., 2013), it is unusual in that it is of the intermediate, rather than the epithelial type, comprising both glabrous, cuboidal, epidermal cells and trichomes, like G. flexuosa (Sims) M.W. Chase & N.H. Williams (Gomiz et al., 2013). Oil-secreting palisade cells, commonly found in the epithelial elaiophores of many Oncidiinae, including Oncidium (Stpiczyńska et al., 2013), are absent. Even more remarkable is that flowers of C. meirax appear to lack well-defined elaiophores, and this range in secretory tissues (well-defined to diffuse secretory tissues) parallels that reported for species of resin-secreting Rhetinantha M.A. Blanco (subtribe Maxillariinae; Davies and Stpiczyńska, 2012). Furthermore, whereas the flowers of most oil-producing Oncidiinae studied to date, like O. heteranthum var. album, secrete a viscid liquid (Pacek et al., 2007; Stpiczyńska et al., 2007, 2013; Stpiczyńska and Davies, 2008; Aliscioni et al., 2009; Davies and Stpiczyńska, 2009; Gomiz et al., 2013), those of G. longipes and V. excavata produce a white, wax-like secretion, while, at the other extreme, the flowers of C. meirax produce a seemingly much more volatile secretion and, again, this range parallels that seen in species of Rhetinantha, where some species produce a wax-like secretion, whereas others produce a viscid, resinous secretion (Davies et al., 2003; Davies and Stpiczyńska, 2012).
Differences in the chemical composition of the floral secretion of C. meirax may enable it to be leached more easily from the flower by ethanol than that of other taxa, and this might explain the absence of secretory residues on the flower surface when viewed using SEM and the difficulty in successfully staining hand-cut sections with ethanolic Sudan III.
Another remarkable feature is that unlike most species of Gomesa investigated (Stpiczyńska et al., 2007, 2013; Stpiczyńska and Davies, 2008; Aliscioni et al., 2009; Gomiz et al., 2013), the secretion produced by G. longipes appears to be heterogeneous and, to date, such secretions have been reported only rarely, e.g. for Lockhartia, certain species of Oncidium (Oncidiinae) and the resin-secreting flowers of Rhetinantha divaricata (Barb.Rodr.) M.A. Blanco (as Maxillaria cf. notylioglossa Rchb.f.; Davies et al., 2003; Blanco et al., 2013; Stpiczyńska et al., 2013). However, unlike these, the secretion did not appear to contain spherical profiles or droplets.
Cuticular distension is widespread amongst oil-producing Oncidiinae as the secretion accumulates between the elaiophore cell wall and the cuticle, and is known to occur both in species having trichomal and intermediate elaiophores [e.g. Lockhartia spp. (Blanco et al., 2013), Ornithocephalus ciliatus (Pacek and Stpiczyńska, 2007), Ornithocephalus gladiatus, Phymatidium falcifolium, Zygostates grandiflora and Z. lunata (Pacek et al., 2012)], as well as those having epithelial elaiophores [e.g. Gomesa echinata (Barb. Rodr.) M.W. Chase & N.H. Williams (Stpiczyńska et al., 2013), Gomesa radicans (as Ornithophora radicans; Stpiczyńska and Davies, 2008), Gomesa ranifera (Lindl.) M.W. Chase & N.H. Williams (Stpiczyńska et al., 2013), Oncidium amazonicum (Schltr.) M.W. Chase & N.H. Williams (Stpiczyńska et al., 2013), Oncidium cheirophorum Rchb.f. (Pacek and Stpiczyńska, 2007), Oncidium oxyceras (Königer & J.G. Weinm.) M.W. Chase & N.H. Williams (Stpiczyńska et al., 2013), O. sotoanum R. Jiménez & Hágsater (as O. ornithorhynchum Kunth; Davies and Stpiczyńska, 2009) and Trichocentrum cavendishianum (Stpiczyńska et al., 2007)]. Rupture of the cuticle results in the discharge of the secretory product onto the cuticle surface. However, cuticular distension or blistering was not observed for any of the species investigated in the present study, except for fertile flowers of O. heteranthum var. album, the secretion passing directly through the cell wall and cuticle as lipid moieties. Indeed, osmiophilic lipid bodies were present in the outer tangential cell wall of G. longipes and in small vesicles within the periplasmic space of V. excavata and fertile flowers of O. heteranthum var. album.
A bi-layered or stratified cuticle, comprising an outer, lamellate and an inner, reticulate region of the type found in Gomesa echinata, G. ranifera, Oncidium amazonicum and O. oxyceras (Stpiczyńska et al., 2013) was absent from all four taxa.
Secretion-filled cavities, as described for epidermal walls of the elaiophores of Gomesa bifolia (Sims) M.W. Chase & N.H. Williams, G. echinata, G. loefgrenii, G. venusta (Drapiez) M.W. Chase & N.H. Williams (as Oncidium trulliferum Lindl.), Oncidium amazonicum, O. cheirophorum, O. oxyceras and Trichocentrum cavendishianum, were also absent, and, in this respect, the subjects of this study resemble G. flexuosa, G. radicans (as Ornithophora radicans), G. recurva R. Br., G. riograndensis (Cogn.) M.W. Chase & N.H. Williams, G. varicosa (Lindl.) M.W. Chase & N.H. Williams and O. sotoanum (as O. ornithorhynchum; Pacek et al., 2007; Stpiczyńska et al., 2007, 2013; Stpiczyńska and Davies, 2008; Aliscioni et al., 2009; Davies and Stpiczyńska, 2009; Gomiz et al., 2013).
Protuberances occur on the inner surface of the outer tangential wall of the callus epidermal cells of V. excavata. Such structures have also been reported for Gomesa ranifera, O. amazonicum, O. oxyceras (Stpiczyńska et al., 2013) and Zygostates grandiflora (Pacek et al., 2012), but not for any other species of Oncidium or Gomesa, as currently circumscribed. As yet, their function is not clear.
Anatomically, the floral elaiophores of Gomesa and non-florally dimorphic Oncidium are similar. Those of Gomesa may be epithelial or intermediate, with a secretory layer of either cuboidal or palisade cells and a single-layered or bi-layered cuticle comprising an outer, lamellate layer and an inner, reticulate layer. Likewise, Oncidium has epithelial elaiophores with a secretory layer of cuboidal or palisade cells and a single- or bi-layered cuticle (Pacek and Stpiczyńska, 2007; Stpiczyńska et al., 2013). The fertile flower of florally dimorphic O. heteranthum var. album, however, has an intermediate type of elaiophore. This elaiophore also has a secretory layer of cuboidal cells and a single-layered cuticle, and thus closely resembles that of non-florally dimorphic Oncidium spp., indicating that floral dimorphism does not greatly modify elaiophore structure. The sterile flowers of this species also secrete droplets along the perianth lobes that stain with Sudan III, and these may represent droplets of fragrance. By contrast, the perianth cells of fertile flowers of this species contain relatively few lipid droplets, but these flowers seem to be more fragrant to humans (though not necessarily to insect pollinators) than their sterile counterparts. The presence of lipid droplets in sterile flowers may yet support the hypothesis that the latter function as osmophores for the entire inflorescence.
The unusual secretion produced by C. meirax, the absence of a distinct elaiophore, the abundance, predominance and distribution of RER, and the presence of elaioplasts and of free, irregularly shaped osmiophilic bodies scattered throughout the cytoplasm all suggest that a different mode of secretion from that found in the other taxa investigated operates in this species. As in Rhetinantha divaricata (Davies et al., 2003), copious synthesis of oil bodies, initially as plastoglobuli, is thought to occur primarily within plastids that gradually become converted from amyloplasts into elaioplasts. Disruption of the plastid envelope allows the discharge of these oil bodies into the cytoplasm where they become more irregular in shape and eventually oil traverses the cell wall and cuticle as lipid moieties. It is probable that there is less involvement of the SER during both lipid synthesis and lipid transport in C. meirax than in the other species investigated, and free, osmiophilic bodies and elaioplasts were not encountered as frequently in these. The exact sequence of events, however, awaits autoradiographic investigation.
Myelin-like, membranous configurations are found mainly in vacuoles, where they are associated with invagination of the tonoplast. They are typical of rapidly differentiating plant tissues (Davies et al., 1992, and references therein), including those of elaiophores, and are thought to be associated with rapid membrane turnover, including the recycling of SER engaged in lipid synthesis. As such, differences in the degree of tissue differentiation, autolysis and associated membrane turnover may account for differences in their occurrence and their level of organization in elaiophore tissue (Davies et al., 1992; Stpiczyńska et al., 2007, 2013; Blanco et al., 2013).
Despite currently being assigned to four different genera, the subjects of the present study have floral elaiophores that are anatomically very similar. Indeed, notwithstanding their division into epithelial and trichomal types, elaiophore structure is remarkably uniform for all Oncidiinae taxa studied to date, and this is indicative of homoplasy. This further supports our previous investigations of Oncidiinae which showed that some elaiophore characters may be shared by different clades, but not always with species of the same genus. Consequently, this study endorses our previous assertion that elaiophores are of limited value in investigating the phylogeny of this subtribe (Stpiczyńska et al., 2013).
ACKNOWLEDGEMENTS
The authors are grateful to Magdalena Kamińska (University of Life Sciences, Lublin, Poland) for her assistance in the laboratory, and to Alan Gregg (Swansea Botanical Complex, Swansea, UK) for helping with the preparation of this paper. The study was partly financed by the Departmental Grant BST (to M.S.) from the Faculty of Biology, University of Warsaw.
LITERATURE CITED
- Aliscioni SS, Torretta JP, Bello ME, Galati BG. Elaiophores in Gomesa bifolia (Sims) M.W. Chase & N.H. Williams (Oncidiinae: Cymbidieae: Orchidaceae): structure and oil secretion. Annals of Botany. 2009;104:1141–1149. doi: 10.1093/aob/mcp199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco MA, Davies KL, Stpiczyńska M, Carlsward BS, Ionta GM, Gerlach G. Floral elaiophores in Lockhartia Hook. (Orchidaceae: Oncidiinae): their distribution, diversity and anatomy. Annals of Botany. 2013;112:1775–1791. doi: 10.1093/aob/mct232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummitt RK, Powell CE. Authors of plant names. Kew, UK: Royal Botanic Gardens; 1992. [Google Scholar]
- Buchmann SL. The ecology of oil flowers and their bees. Annual Review of Ecology and Systematics. 1987;18:343–369. [Google Scholar]
- Chase MW. Classification of Orchidaceae in the age of DNA data. Curtis's Botanical Magazine. 2005;22:2–7. [Google Scholar]
- Chase MW. Sub-tribe Oncidiinae. In: Pridgeon AM, Chase MW, Cribb PJ, Rasmussen FN, editors. Genera Orchidacearum Volume 5 Epidendroideae (Part 2) Oxford: Oxford University Press; 2009. pp. 211–394. [Google Scholar]
- Chase MW, Barrett RL, Cameron KN, Freudenstein JV. DNA data and Orchidaceae systematics: a new phylogenetic classification. In: Dixon KM, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications; 2003. pp. 69–89. [Google Scholar]
- Chase MW, Williams NH, de Faria AD, Neubig KM, Mario do Carmo E Amaral, Whitten WM. Floral convergence in Oncidiinae (Cymbidieae: Orchidaceae): an expanded concept of Gomesa and a new genus Nohawilliamsia. Annals of Botany. 2009;104:387–402. doi: 10.1093/aob/mcp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Cingel NA. An atlas of orchid pollination – America, Africa, Asia and Australia. Rotterdam, The Netherlands: A.A. Balkema; 2001. [Google Scholar]
- Dalström S. A synopsis of the genus Cyrtochilum (Orchidaceae: Oncidiinae): taxonomic re-evaluation and new combinations. Lindleyana. 2001;16:56–80. [Google Scholar]
- Davies KL. Food-hair form and diversification in orchids. In: Kull T, Arditti J, Wong SM, editors. Orchid biology – reviews and perspectives. Vol. X. Berlin: Springer Science + Business Media; 2009. pp. 159–184. [Google Scholar]
- Davies KL, Stpiczyńska M. The anatomical basis of floral, food-reward production in Orchidaceae. In: Teixeira da Silva JA, editor. Floriculture, Ornamental and plant biotechnology: advances and topical issues. Vol. 5. Isleworth, UK: Global Science Books; 2008. pp. 392–407. [Google Scholar]
- Davies KL, Stpiczyńska M. Comparative histology of floral elaiophores in the orchids Rudolfiella picta (Schltr.) Hoehne (Maxillariinae sensu lato) and Oncidium ornithorhynchum H.B.K. (Oncidiinae sensu lato) Annals of Botany. 2009;104:221–234. doi: 10.1093/aob/mcp119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Stpiczyńska M. Comparative labellar anatomy of resin-secreting and putative resin-mimic species of Maxillaria s.l. (Orchidaceae: Maxillariinae) Botanical Journal of the Linnean Society. 2012;170:405–435. [Google Scholar]
- Davies KL, Davies MS, Francis D. Vacuolar development in the root meristem of Festuca rubra L. New Phytologist. 1992;121:581–585. [Google Scholar]
- Davies KL, Turner MP, Gregg A. Lipoidal labellar secretions in Maxillaria Ruiz & Pav. (Orchidaceae) Annals of Botany. 2003;91:439–446. doi: 10.1093/aob/mcg038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler RL. The orchids – natural history and classification. London: Harvard University Press; 1990. [Google Scholar]
- Gahan PB. Plant histochemistry and cytochemistry: an introduction. London: Academic Press; 1984. [Google Scholar]
- Gomiz NE, Torretta JP, Aliscioni SS. Comparative anatomy of elaiophores and oil secretion in Gomesa R. Br. (Orchidaceae: Cymbidieae: Oncidiinae) Turkish Botanical Journal. 2013;37:859–871. [Google Scholar]
- Jensen WA. Botanical histochemistry: principles and practice. San Francisco: W.H. Freeman; 1962. [Google Scholar]
- Neubig KM, Whitten WM, Williams NH, et al. Generic recircumscriptions of Oncidiinae (Orchidaceae: Cymbidieae) based on maximum likelihood analysis of combined DNA datasets. Botanical Journal of the Linnean Society. 2012;168:117–146. [Google Scholar]
- Pacek A, Stpiczyńska M. The structure of elaiophores in Oncidium cheirophorum Rchb.f. and Ornithocephalus kruegeri Rchb.f. (Orchidaceae) Acta Agrobotanica. 2007;60:9–14. [Google Scholar]
- Pacek A, Stpiczyńska M, Davies KL, Szymczak G. Floral elaiophore structure in four representatives of the Ornithocephalus clade (Orchidaceae: Oncidiinae) Annals of Botany. 2012;110:809–820. doi: 10.1093/aob/mcs158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansarin ER, Pansarin LM. Reproductive biology of Trichocentrum pumilum: an orchid pollinated by oil-collecting bees. Plant Biology. 2011;13:576–581. doi: 10.1111/j.1438-8677.2010.00420.x. [DOI] [PubMed] [Google Scholar]
- van der Pijl L, Dodson CH. Orchid flowers: their pollination and evolution. Coral Gables, FL: University of Miami Press; 1969. [Google Scholar]
- Reis MG, de Faria AD, Bittrich V, Amaral MCE, Marsaioli AJ. The chemistry of flower rewards – Oncidium (Orchidaceae) Journal of the Brazilian Chemical Society. 2000;11:600–608. [Google Scholar]
- Reis MG, de Faria AD, Amaral MCE, Marsaioli AJ. Oncidinol – a novel diacylglycerol from Ornithophora radicans Barb. Rodr. (Orchidaceae) floral oil. Tetrahedron Letters. 2003;44:8519–8523. [Google Scholar]
- Reis MG, Singer RB, Gonçalves R, Marsaioli AJ. The chemical composition of Phymatidium delicatulum and P. tillandsioides (Orchidaceae) floral oils. Natural Product Communications. 2006;1:757–761. [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain for electron microscopy. Journal of Cell Biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RB, Cocucci AA. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana. 1999;14:47–56. [Google Scholar]
- Singer RB, Marsaioli AJ, Flach A, Reis MG. The ecology and chemistry of pollination in Brazilian orchids: recent advances. In: Teixeira da Silva JA, editor. Floriculture, ornamental and plant biotechnology. Vol. IV. Global Science Books; 2006. pp. 569–582. [Google Scholar]
- Stpiczyńska M, Davies KL. Elaiophore structure and oil secretion in flowers of Oncidium trulliferum Lindl. and Ornithophora radicans (Rchb.f.) Garay & Pabst (Oncidiinae: Orchidaceae) Annals of Botany. 2008;101:375–384. doi: 10.1093/aob/mcm297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczyńska M, Davies KL, Gregg A. Elaiophore diversity in three contrasting members of Oncidiinae Benth. (Orchidaceae) Botanical Journal of the Linnean Society. 2007;155:135–148. [Google Scholar]
- Stpiczyńska M, Davies KL, Pacek-Bieniek A, Kamińska M. Comparative anatomy of the floral elaiophore in representatives of the newly re-circumscribed Gomesa and Oncidium clades (Orchidaceae: Oncidiinae) Annals of Botany. 2013;112:839–854. doi: 10.1093/aob/mct149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torretta JP, Gomiz NE, Aliscioni SS, Bello ME. Biología reproductiva de Gomesa bifolia (Orchidaceae, Cymbidieae, Oncidiinae) Darwiniana. 2011;49:16–24. [Google Scholar]
- Vogel S. Ölblumen und ölsammelnde Bienen. Abhandlungen Akademie Wissenschaften Mathematisch-Naturwissenschaften Klasse Tropische und Subtropische Pflanzenwelt. 1974;7:1–267. [Google Scholar]
- Williams NH, Chase MW, Fulcher T, Whitten WM. Molecular systematics of the Oncidiinae based on evidence from four DNA sequence regions: expanded circumscriptions of Cyrtochilum, Erycina, Otoglossum, and Trichocentrum and a new genus (Orchidaceae) Lindleyana. 2001;16:113–139. [Google Scholar]