Abstract
Background and Aims
Seed longevity, a fundamental plant trait for ex situ conservation and persistence in the soil of many species, varies across populations and generations that experience different climates. This study investigates the extent to which differences in seed longevity are due to genetic differences and/or modified by adaptive responses to environmental changes.
Methods
Seeds of two wild populations of Silene vulgaris from alpine (wA) and lowland (wL) locations and seeds originating from their cultivation in a lowland common garden for two generations (cA1, cL1, cA2 and cL2) were exposed to controlled ageing at 45 °C, 60 % relative humidity and regularly sampled for germination and relative mRNA quantification (SvHSP17.4 and SvNRPD12).
Key Results
The parental plant growth environment affected the longevity of seeds with high plasticity. Seeds of wL were significantly longer lived than those of wA. However, when alpine plants were grown in the common garden, longevity doubled for the first generation of seeds produced (cA1). Conversely, longevity was similar in all lowland seed lots and did not increase in the second generation of seeds produced from alpine plants grown in the common garden (cA2). Analysis of parental effects on mRNA seed provisioning indicated that the accumulation of gene transcripts involved in tolerance to heat stress was highest in wL, cL1 and cL2, followed by cA1, cA2 and wA.
Conclusions
Seed longevity has a genetic basis, but may show strong adaptive responses, which are associated with differential accumulation of mRNA via parental effects. Adaptive adjustments of seed longevity due to transgenerational plasticity may play a fundamental role in the survival and persistence of the species in the face of future environmental challenges. The results suggest that regeneration location may have important implications for the conservation of alpine plants held in seed banks.
Keywords: Seed longevity, ageing, alpine plants, adaptation, climate change, epigenetic, mRNA provisioning, Silene vulgaris subsp. vulgaris (Moenk) Garcke, Caryophyllaceae
INTRODUCTION
The longevity of seeds is an important plant trait, which allows the ex situ conservation of plant germplasm for tens or even hundreds of years (Walters et al., 2005). In orthodox seeds (sensu Roberts, 1973), longevity is primarily determined by the temperature and moisture content of the seeds during storage: seed longevity increases predictably with decreasing temperature and moisture content (Ellis and Roberts, 1980). Using this relationship, it is possible to accelerate the seed ageing processes to estimate seed life span and extrapolate to seed banking conditions (Ellis and Roberts, 1980) and also to infer indications of persistence in the soil seed bank (Long et al., 2008). Whether ageing processes under dry and partially hydrated conditions (e.g. under seed bank and controlled ageing, respectively) are driven by the same biochemical mechanisms is still an open question, since, in the former, seed longevity loss occurs at a rate too low to be investigated. Conversely, the significant positive correlation between seed persistence in the soil and resistance to artificial ageing found by Long et al. (2008) suggests that viability loss in nature may be controlled by mechanisms similar to those that occur under ageing experiments.
The study of ecological factors driving differences in seed longevity across species is crucial for understanding how this seed trait has evolved and may change in the future. Seed longevity has been found to vary considerably across species (Priestley et al., 1985), and such variation was reported to be reflected at plant family and/or genus levels (Walters et al., 2005). There are also significant variations in seed longevity related to traits such as seed structure and the climate at the species' origin (Probert et al., 2009). Mondoni et al. (2011) found that alpine plants have short-lived seeds compared with those from lowland populations of the same or related taxa, indicating that different selection pressures on seed resistance to ageing may have occurred. Subsequent observations showed that differences between alpine and lowland seed lots are reflected at the genetic level, by the rate of rearrangement of DNA and antioxidant responses during ageing (Donà et al., 2013). DNA damage occurring during seed ageing is a primary cause of genetic variation and genomic alteration. Seeds are particularly vulnerable to environmental stresses during maturation on the mother plant, during post-dispersal storage in the soil seed bank (persistence) and during the process of germination (Ventura et al., 2012). If repair mechanisms are not functional, genotoxic stresses resulting in the accumulation of DNA damage lead to seed death. However, currently, little information is available on the impact of environment on the tolerance to subsequent genotoxic stress (Kranner et al., 2010).
The fact that seeds of plants from cool, wet climates are shorter lived than those from warm, dry climates when exposed to controlled ageing at 45 °C, 60 % relative humidity (RH) (see Probert et al., 2009; Mondoni et al., 2011) may suggest a different ability to withstand ageing after dispersal too. In this regard, Donà et al. (2013) showed that partly hydrated seeds [at 60 % equilibrium RH (eRH)] of Silene acaulis from alpine locations were more vulnerable to oxidative damage, compared with seeds of S. vulgaris subsp. vulgaris from lowland locations. Furthermore, Mondoni et al. (2011) suggested that the lower resistance to ageing of alpine seeds compared with lowland counterparts could be related to the low average temperature of the post-dispersal environment in alpine locations (Körner, 1999). Seeds have low resistance to ageing under the warm, partially hydrated conditions of the controlled ageing test, but remain viable and form long-term persistent seed banks in their natural habitat (Schwienbacher et al., 2010) presumably because average temperatures are much lower and seeds are more or less fully hydrated. It follows that plants adapted to growing under much warmer and drier, or partially hydrated conditions will be under selection pressure to produce seeds with greater resistance to ageing in order to cope with a more stressful post-dispersal environment. Therefore, the results from controlled ageing tests are probably a better reflection of resistance to ageing under warm, dry post-dispersal conditions, rather than persistence in the soil seed bank.
Recent studies have revealed significant transgenerational changes in seed longevity associated with environment-induced effects (Kochanek et al., 2011), indicating that differences between collections could also be driven by plastic (adaptive) responses to the local environment or seasonality. During seed maturation, there is a tight connection between the stresses to which the parental plant is subjected and the phenotypic characters of the offspring, suggesting that a complex mechanism evolved to prepare the offspring for the dispersal environment (Herman and Sultan, 2011). This may be explained by the fact that translational activity during the early phases of germination predominantly involves a stored pool of mRNA, with the different provisioning of transcripts influencing the germinability of the embryo (Rajjou et al., 2012). Functionally adaptive transgenerational effects, elicited by environmental stress, are known to affect several plant traits in the offspring, including fruit and seed production (Whittle et al., 2009), biomass (Kou et al., 2011), germination and seedling growth (Meyer and Allen, 1999; Boyko et al., 2010). However, despite the growing interest, few studies have documented functionally adaptive transgenerational effects in seeds (Herman and Sultan, 2011) and even fewer have considered effects on seed longevity.
In the present study, to investigate the extent to which the measured differences of seed longevity between lowland and alpine seed lots are due to genetic differences and/or affected by adaptive responses to environmental changes, seeds of two wild populations of S. vulgaris subsp. vulgaris (Caryophyllaceae), originating from alpine and lowland locations, were transplanted into a common garden in a lowland site for two growing seasons. Wild populations of S. vulgaris have been shown to have variable seed longevity when exposed to controlled ageing (Mondoni et al., 2011), making it a useful candidate to investigate environmentally induced variation in seed longevity. The seeds produced from the common garden plants and those from the wild populations were used to determine how seed longevity responded to changes in the parental environment. In addition to observations of seed germination, parental provisioning of mRNA was used to interpret differences across the seed lots exposed to a controlled ageing protocol.
MATERIALS AND METHODS
Wild population seed collection
Silene vulgaris (Moenk) Garcke subsp. vulgaris (hereafter S. vulgaris) is a perennial species distributed throughout Eurasia and North Africa (Talavera, 1990). It displays a variety of ecotypes (Aeschimann and Bocquet, 1980) and occupies diverse habitats, but prefers disturbed areas including roadsides and cultivated ground. In Italy it can be found from 0 to 2800 m a.s.l. (Pignatti, 1982).
Seed collections were made at the time of natural dispersal, on 20 July 2010, from a lowland population (wL) of S. vulgaris growing spontaneously in the Ticino Natural Park (Po Plain; Lombardy; 63 m a.s.l.; 45°5′28″N, 09°07′03″E) and on 1 September 2010 from an alpine population (wA) of the Trento Dolomites (Val Giumella, Trentino; 2310 m asl; 46°26′20′'N, 11°44′25″E), in northern Italy. Thereafter, seeds were held at the Lombardy Seed Bank under international seed bank standard conditions of –20 °C after drying at 15 % RH, 15 °C (FAO/IPGRI, 1994) until use.
Plant growth and collection of later generations of seed
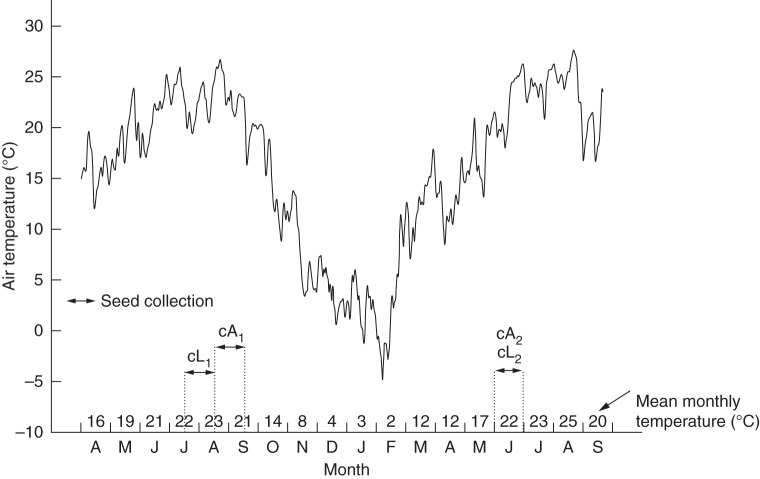
Plant growth experiments were carried out at the Botanic Garden of the University of Pavia (78 m a.s.l.; 45°11′7·2″N, 9°9′46·6″E), about 10 km from the wL site. Seeds used for plant growth were incubated for 3 d at 25 °C on 1 % water–agar medium to which 722 μmol gibberellic acid (GA3, Merck S.p.A.) was added, and then transferred to cell propagation trays containing a soil mix (perlite and peat, model Fertil: LIGHTter, Berco s.r.l., Bergamo, Italy). Trays were watered daily and maintained in a greenhouse (25 ± 3·5 °C, natural light) for about 2 months (April and May 2011), by which time all seeds had germinated, seedling roots had filled the cells and 2–4 mature leaves had emerged. At this time, lowland and alpine seedlings were transplanted into separate beds in a natural lowland environment, similar to that normally experienced by wL (Fig. 1).
Fig. 1.
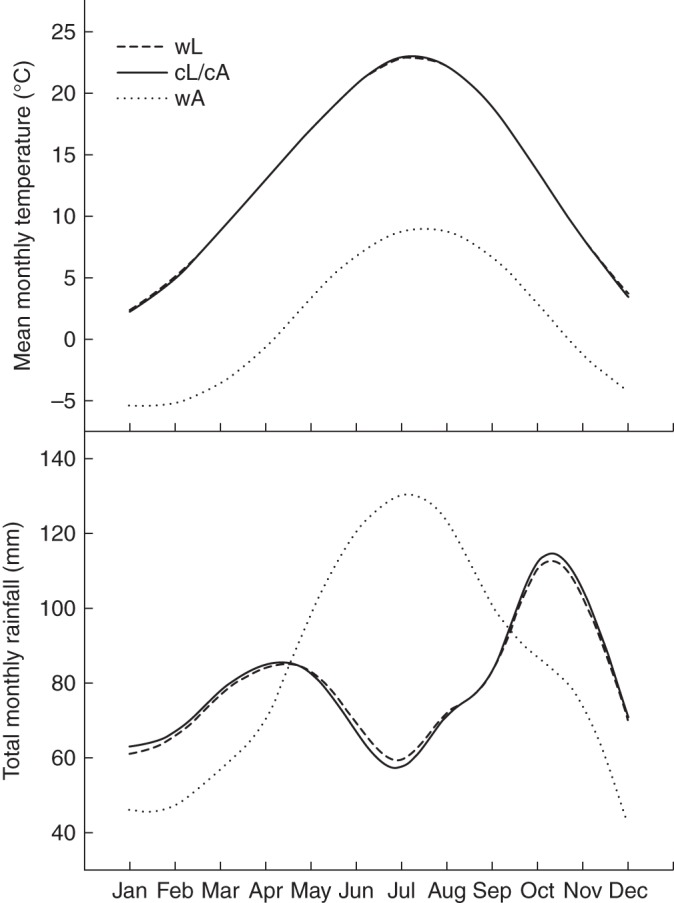
Mean monthly temperature and total monthly rainfall at the alpine (wA) and lowland (wL) wild population sites and at the common lowland growing site (cL/cA). Data were obtained by querying WORLDCLIM data (download version 1.4, http://www.worldclim.org/) at a maximum resolution of 30 arc-s (approx. 1 km) using the ‘extract values to point’ tool in ERSI ArcMap (version 9.1).
Silene vulgaris is normally pollinated by moths (Marsden-Jones and Turrill, 1957), although geitonogamy occurs as a result of haptogamy (flowers touching each other; Pettersson, 1992). Hence, to prevent cross-pollination between alpine and lowland plants, the beds were positioned approx. 35m apart and were covered with a mosquito net so that seeds were mainly produced by geitonogamy. In the first growing season (2011), flowering and subsequent seed-setting periods were about 1 month apart, with plants resulting from the wL seed lot (cL1) beginning to flower during the last week of June, while those resulting from the wA seed lot (cA1) began to flower in the last week of July (Fig. 2). Seed collection was carried out when the capsules started to split open, between mid-July and mid-August in the case of cL1 and between mid-August and mid-September in the case of cA1 (Fig. 2). In the second growing season (2012), the phenology of plants from cA1 and cL1 seed lots (producing seed lots cA2 and cL2, respectively) was synchronized, with flowering and fruiting occurring in May and June, respectively. In each case, after collection, seeds were dried (at 15 % RH, 15 °C) and frozen (–20 °C) until use. Air temperature at the level of the flowers/fruits was recorded at hourly intervals using Tiny Tag data loggers (Gemini Data Logger Ltd, Chichester, UK) throughout two growing seasons (April 2011–September 2012; Fig. 2).
Fig. 2.
Mean daily air temperature (°C) at the level of the flowers/fruits calculated from measurements made at hourly intervals at the flowerbeds from April 2011 to September 2012. Horizontal arrows indicate the period of seed collection from plants of the lowland (cL) and alpine (cA) genotypes cultivated in the flowerbeds. Also shown are the mean monthly temperatures.
Ageing test
Ageing experiments commenced in October 2010 for seeds collected from the two wild populations (wL and wA) and in October 2011 and September 2012 for seeds of the first and the second generations, respectively, of both ecotypes cultivated in the common lowland environment (cL1, cA1, cL2 and cA2); in each case the experiments were concluded after 3 months.
Seed longevity was determined using a standard rapid ageing protocol (Newton et al., 2009). To raise the moisture content of the seeds prior to ageing and to minimize the subsequent adjustment of moisture content when samples were transferred to the ageing conditions, for each seed lot, seven samples of 250 seeds each were rehydrated at 47 % RH, 20 °C in open glass vials or Petri dishes; the vials/dishes were placed over a non-saturated solution of LiCl (anhydrous, Laboratory Reagent Grade; Fisher Scientific UK Ltd, Leics, UK) in distilled water held in a sealed 300 × 300 × 130 mm electrical enclosure box (Ensto UK Ltd, Southampton, UK). At the end of the rehydration period (14 d), seed eRH was checked using a sample of the equilibrating seeds. The eRH was measured using a water activity measuring instrument which comprised a hygrometer sensor housed in an AW-DI0 water activity probe, used in conjunction with a HygroPalm 3 display unit (Rotronic Instruments UK Ltd, Crawley, UK). Once the test species was judged to have reached equilibrium, all samples were transferred to a second electrical enclosure box, over a non-saturated solution of LiCl at 60 % RH placed in a compact incubator (Binder FD53, VWR International) without light at 45 ± 2 °C. The RH generated by the LiCl in the box was checked at 4- to 6-week intervals by pipetting a sample of approx. 10 mL of solution and placing it into the sample chamber of the water activity-measuring instrument described above. The bulk solution was adjusted if necessary, usually by adding distilled water, stirring and allowing the solution to equilibrate before rechecking the RH (Hay et al., 2008). One sample of 250 seeds for each seed lot was removed after each of 0, 5, 10, 20, 30, 60 and 90 d for germination testing (50 seeds) and genetic analysis (200 seeds, approx. 100 mg).
Germination test
Seeds were sown on 1 % distilled water agar held in 90 mm diameter Petri dishes and placed in an LMS 250A cooled incubator (LMS Ltd, Sevenoaks, UK) at a temperature regime previously found to be optimal for germination of that accession (25/15 °C + GA3 at 722 μmol). Plates were checked weekly for germination and seeds were scored as germinated once the radicle had reached approx. 2 mm. At the completion of each germination test (6 weeks after sowing), ungerminated seeds were cut to confirm whether or not there were empty seeds. Ungerminated full seeds were assumed to have died or suffered severe damage during the ageing treatment, since there were no other constraints to germination. Visual and physical assessments (e.g. colour and hardness changes) further confirmed that ungerminated seeds were not viable.
RNA extraction and qRT-PCR analysis
RNA extraction from dry seeds was performed as described by Oñate-Sánchez and Vicente-Carbajosa (2008). The RNA extracted was quantified spectrophotometrically. The cDNA was synthesized starting from 1 μg of RNA for each sample using EuroRT reverse transcriptase (Euroclone, Italy) and random primers following the manufacturer's instructions. The reaction mix for the quantitative real-time PCR (qRT-PCR) analysis comprised the following: 2·5 ng of cDNA, 500 nm of each primer and 1 × Maxima SybrGreen Master Mix (Fermentas, Burlington, Canada). The qRT-PCR was performed on a Rotor-Gene 6000 apparatus (Corbett Robotics, Brisbane, Australia) using the following conditions: denaturation at 94 °C for 10 min, and cycling at 94 °C 10 s, 60 °C 60 s with fluorescence acquisition. To analyse the expression of the SvHSP17.4 (SiESTA accession SvH_FF3TYA401DZTFC) and SvNRPD12 (SiESTA accession SvH_FEYVE6F02JAVH3) genes, the oligonucleotide primers were designed using the Real Time PCR Primer Design program from GenScript. SvEF1a was used as reference gene as previously described (Donà et al., 2013).
The S. vulgaris homologues of arabidopsis Hsp17.4 (Heat Shock Protein 17·4) and NRPD12 (Non-catalytic subunit common to nuclear DNA-dependent RNA polymerases II, IV and V) were identified using the BLAST tool on the SiESTa database (Blavet et al., 2011). The raw data were analysed using the Rotor-Gene 6000 (Corbett Robotics) software and subjected to statistical analysis with Tukey's t-test coupled to analysis of variance (ANOVA).
Seed longevity analysis
A generalized linear model (GLM) with binomial error and probit link function was fitted to the survival data (numbers of seeds germinating, number of seeds sown) in GenStat Release 15 (VSN International Ltd, Oxford, UK) with storage period as an explanatory variate, thereby fitting the viability equation (Ellis and Roberts, 1980):
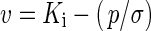 |
(1) |
where v is the viability (in normal equivalent deviates, NED) of the seed lot after p days in storage, Ki is the initial viability (NED) of the seed lot, and σ is the time (d) for viability to fall by 1 NED (i.e. the standard deviation of the normal distribution of seed deaths over time). Both seed lot source (alpine or lowland) and cultivation (wild, cultivated year 1, cultivated year 2) were included in the GLM as factors, thereby allowing Ki and/or σ to be constant/vary for the different seed lots. The full model was fitted twice (changing the order of the terms) with accumulated analysis of deviance. The effect of dropping terms was also considered, using an approximate F-test to assess significance.
RESULTS
Seed viability declined as the period of experimental storage increased, for all seed lots (Fig. 3). When fitting the probit GLM, in the accumulated analysis of deviance (changing the order of the cultivation and source terms), cultivation and the interaction of cultivation with source were not significant (P > 0·05), i.e. the differences in Ki were not significant. All slope terms were significant. Dropping intercept (Ki) terms from the model did not result in a significant increase in residual deviance (P = 0·091) and hence the final model fitted is that showing differences in the slope (σ–1) only (Fig. 3; Table 1).
Fig. 3.
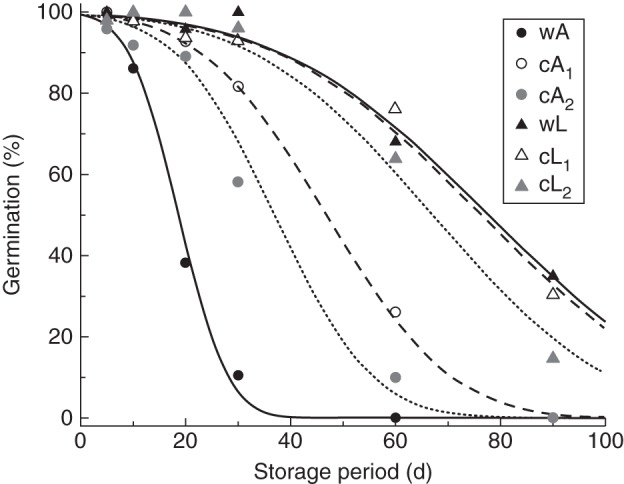
Survival curves fitted using a generalized linear model with binomial error and probit link function for seed lots collected from the lowland (wL) and alpine (wA) wild populations (black symbols, continuous lines) and for those resulting from their cultivation (cL and cA, respectively) in the common lowland environment in the fist (open symbols, broken lines) and in the second (grey symbols, dotted lines) generations (model parameters shown in Table 1).
Table 1.
Survival curve parameters for each seed lot determined using a generalized linear model with binomial error and probit link function
| Seed lot | Ki (NED) | s.e. | σ–1 (d–1) | s.e. | σ (d) | p50 (d) |
|---|---|---|---|---|---|---|
| wA | 2·50 | 0·102 | 0·134 | 0·0098 | 7·5 | 18·7 |
| cA1 | 0·053 | 0·0033 | 18·9 | 47·2 | ||
| cA2 | 0·068 | 0·0045 | 14·7 | 36·8 | ||
| wL | 0·032 | 0·0096 | 31·3 | 78·1 | ||
| cL1 | 0·033 | 0·0032 | 30·3 | 75·8 | ||
| cL2 | 0·037 | 0·0052 | 27·0 | 67·6 |
Seed lot source and cultivation were included as factors. Dropping terms from the model, estimates of Ki could be constrained to a single value for the different seed lots.
Ki, initial viability [normal equivalent deviates (NED)] of the seed lot; σ, time (d) for viability to fall by 1 NED; p50, time (d) for viability to fall to 50%.
There was wide variation in the estimate for σ (7·5–31·3 d) and hence in the time taken for viability to fall to 50 % (p50; 18·7–78·1 d). Seeds collected from the original lowland population (wL) and first-generation lowland population in the common garden (cL1) showed a slower rate of viability loss compared with seeds produced by the corresponding alpine origin population (wA) and cA1 (Table 1). Seeds collected from the first generation of the alpine population in the common garden (cA1) survived for much longer (p50 = 47·2 d), than seeds collected from the original alpine population (wA) (p50 = 18·7 d), but for far less time than seeds collected from seed lots originating from the lowland population (wL and/or cL) (p50 = 78·1 and 75·8 d, respectively). The longevity of the first-generation alpine population (cA1) was about midway between wL (or cL1) and wA (Fig. 3). Seeds collected from the second-generation alpine plants (cA2) showed higher longevity (p50 = 36·8 d) compared with the original alpine population (wA) (p50 = 18·7 d), but a faster rate of viability loss in comparison with cA1. The longevity of the seeds from the second-generation lowland population (cL2) was similar to that of the previous lowland generations (wL and cL1), except the rate of loss of viability was a little faster (σ value of 27·0 d compared with 31·3 and 30·3 for wL and cL, respectively).
Parental effects on mRNA seed provisioning
The qRT-PCR on dry seeds collected from the wild (wL and wA) and the cultivated (cL1, cL2, cA1 and cA2) populations showed significant differences between the wild lowland and alpine seed lots, and between the wild and cultivated seed lots (Fig. 4). The relative abundance of HSP17.4 transcript in the wA seeds was found to be significantly (P < 0·05) lower (by up to 0·27 ± 0·033 s.d.-fold) compared with wL. Moreover, in seeds of both the first and second alpine populations (cA1 and cA2), the HSP17.4 transcript expression was found to be significantly higher (2-fold) compared with the original alpine population (wA). Similarly, NRPD12 transcript expression in seeds from wA showed an 0·016 ± 0·003 s.d.-fold reduction in comparison with wL seeds. However, the same transcript was found to be more abundant in cA1 seeds (0·52 ± 0·13 s.d.-fold compared with wA) and then declined in cA2 (0·073 ± 0·026 s.d.-fold compared with wA).
Fig. 4.
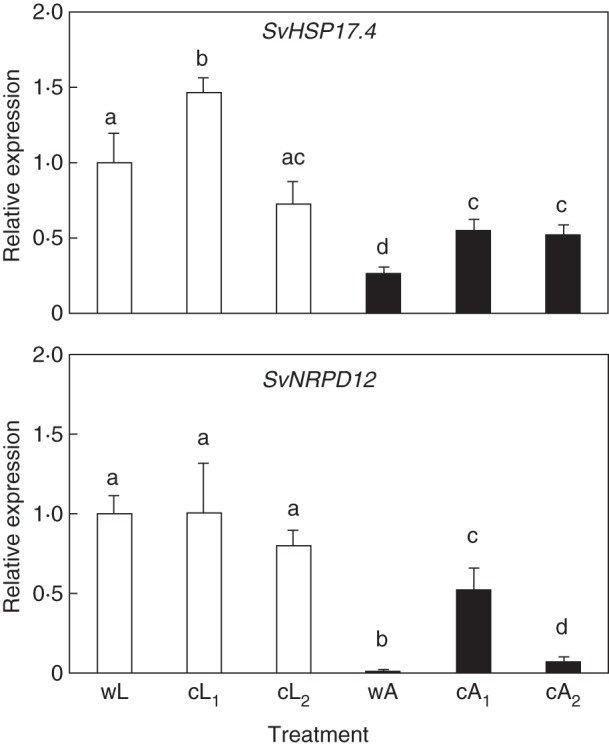
Expression profiles of the SvHSP17.14 and SvNRPD12 genes in S. vulgaris seeds, evaluated by qRT-PCR. Values are expressed as means ± s.d. of three independent experiments and normalized to the wL sample. Statistical significance was calculated using Tukey's test; the same letters indicate no significant differences (P > 0·05). wL, wild lowland; cL1, cultivated lowland first generation; cL2, cultivated lowland second generation; wA, wild alpine; cA1, cultivated alpine first generation; cA2, cultivated alpine second generation.
DISCUSSION
The possibility that controlled ageing at high humidity and temperature (60 % RH, 45 °C) may have ecological significance was first demonstrated by Long et al. (2008), who found significant relationships between seed life span under these conditions and persistence in the soil seed bank. Following this view, Mondoni et al. (2011) suggested that the reduced longevity of seeds of alpine plants exposed to controlled ageing was caused by low selection pressure for seed resistance to ageing, simply because seeds buried in the alpine soil are normally exposed to lower temperatures than seeds buried in the lowland soil, and therefore rates of ageing are expected to be lower. The results presented here have shown for the first time that seed longevity has a genetic basis, but may show strong adaptive responses to the local environment. Indeed, the longevity of seeds from the original lowland population (wL) was significantly higher (p50 = 78·1 d) than that of seeds from the original alpine population (wA) (p50 = 18·7 d); when an alpine seed lot is grown in a warmer lowland environment (hence under more detrimental conditions for seed viability after dispersal), seed longevity is greatly enhanced (p50 = 47·9). However, importantly, while seeds produced by the first generation of alpine plants in the lowland common garden had markedly increased longevity compared with those collected from the wild, they did show a faster rate of deterioration compared with the first-generation lowland seeds (Table 1), suggesting that seed longevity has a genetic basis and can be subject to natural selection. Supporting this, the longevity of seeds of the second alpine generation grown in the lowland location (cA2) was similar to that of those of the first generation (cA1), indicating that this trait could not adapt further to the local habitat. The near-identical survival curves for the original lowland population and its first generation in the common garden (Table 1; Fig. 3) confirmed that the growing environment of the common garden reliably reproduced the natural conditions of the lowland population.
The longevity of seeds is known to be affected by the temperature during seed development and maturation; Kochanek et al. (2010) found that elevated temperatures during seed maturation in Wahelenbergia tumidifructa led to increased seed longevity. Accordingly, Waterworth et al. (2010) have shown that prolonged exposure of Arabidopsis thaliana seeds to low temperatures resulted in reduced viability and vigour, due to increased oxidative stress under those conditions. Therefore, a plausible explanation for the observed transgenerational increase in longevity in seeds produced by alpine plants growing in a lowland location is that the warmer climate experienced during the reproductive period caused an increase in resistance to ageing.
Growth environment can also affect seed longevity via a parental effect; by growing plants of Plantago cunninghamii Decne. in different environmental conditions before seed set, Kochanek et al. (2011) found a highly plastic parental response, which passed to the progeny and changed seed longevity. Transgenerational plasticity driven by parental effects is known to play an important role in ensuring plants cope with environmental changes (Galloway and Etterson, 2007). In particular, parent individuals alter specific traits in their progeny in response to particular environmental stresses, to enhance offspring performance under those same stresses (Herman and Sultan, 2011). Adaptive maternal cues elicited by the local environment may indeed occur if they increase offspring performance (Mousseau and Fox, 1998). It is therefore possible that plants developing under warmer conditions produce longer lived seeds (see also Probert et al., 2009) because they have to withstand much harsher conditions to maintain viability after dispersal, compared with plants growing in cooler environments. Our results suggest that the offspring seed longevity of alpine plants growing in a lowland location functionally changed to cope with the new environment through a parental effect.
In this regard, plastic responses to environmental changes can be maternally transmitted to offspring via multiple mechanisms in the absence of DNA sequence variations (Herman and Sultan, 2011), such as seed provisioning (sensu Koller, 1972; Srivastava, 2002); mRNA, hormone and protein content of seeds (Roach and Wulff, 1987); and DNA methylation (epigenetic effects; Johannes et al., 2009). Here, we have shown that differential accumulation of SvHSP17·4 and SvNRPD12 mRNAs within seeds of populations coming from lowland and alpine environments depends on both their genetic background and parental effects. Indeed, while all alpine seed lots showed a lower level of both SvHSP17·4 and SvNRPD12 than their lowland counterparts, either the first (cA1) or the second (cA2) seed generation of the alpine genotype grown in the lowland showed greater accumulation of these mRNAs compared with the wild progenitor (wA), indicating that the parental growth environment significantly affected the provisioning of these transcripts. Moreover, the possibility that the observed increase in SvHSP17·4 is directly linked to enhanced resistance to ageing shown in the cA1 and cA2 seed lots cannot be ruled out, since HSP17·4 in A. thaliana seeds is strictly correlated to desiccation and stress tolerance (Wehmeyer and Vierling, 2000). Furthermore, the NRPD12 gene product is involved in the functional assembly of the transcription machinery, including all the RNA polymerase complexes (Ream et al., 2009), and therefore it is essential for a number of processes necessary for embryo development, such as transcription or gene silencing. Therefore, another explanation of the different seed longevity of cA1 and cA2 compared with that of the wild progenitor (wA) is that the increased SvNRPD12 accumulation contributed to a better seed quality in these seed lots, increasing their longevity. These observations suggest that a warmer parental growth environment leads to a better seed provisioning in S. vulgaris, through multiple effects, which promote an increased tolerance of the offspring seeds to heat stress.
The results reported here indicate that when seeds of alpine species are intended for long-term conservation in seed banks, their regeneration in a warm environment (e.g. a glasshouse) may allow the collection of seeds with an increased resistance to ageing. This may have important implications for the ex situ seed conservation of alpine plants, which are considered short lived in storage (Mondoni et al., 2011). Further research is, therefore, needed to understand the extent to which the results shown here for the alpine population of S. vulgaris may be typical for alpine species and perhaps short-lived species in general. Indeed, S. vulgaris is a euryoecious species, growing from lowland up to alpine meadows, mainly in anthropogenic and disturbed habitats. In such conditions, longer seed longevity may allow the species to produce a viable seed bank that buffers plant populations against unpredictable environmental changes (Thompson, 2000). Hence, the plasticity shown in seed longevity may be expressed in other key functional traits that along with genetic characteristics and phylogenetic history, would explain how S. vulgaris has such a large ecological amplitude. Conversely, other species with a solely alpine distribution may show less adaptive responses, but this remains to be investigated.
Plastic responses to environmental changes are common in sessile organisms such as plants (Nicotra et al., 2010). Among various environmental parameters, temperature has the greatest influence (Steadman et al., 2004; Qaderi et al., 2006). For instance, plastic changes in morphology, phenology and physiological processes have been documented in plants under the effect of climate change (Nicotra et al., 2010). In our study, the increased seed resistance to ageing shown by the alpine seed lots grown in the warmer lowland environment suggests that seed longevity might show strong adaptive responses in a future warmer climate. However, such an interesting possibility needs further investigation since temperature changes are unlikely to be as large as we have considered here (i.e. alpine vs. lowland climate). Adaptive adjustment due to transgenerational plasticity may indeed buffer a population against such evolutionary change, allowing existing genotypes to maintain fitness in the face of environmental challenges (Sultan, 2000). Seeds are considered the main source for upward alpine plant migration with global warming (Parolo and Rossi, 2008), and, therefore, their quality and longevity will probably play a fundamental role in the survival and persistence of these species.
ACKNOWLEDGEMENTS
This work was conducted in the framework of the project NEXT DATA, promoted and funded by Italian National Research Council (CNR). The authors would like to express their thanks to the Ev-K2-CNR committee.
LITERATURE CITED
- Aeschimann D, Bocquet G. Les types biologiques du Silene vulgaris s.l. (Caryophyllaceae) Candollea. 1980;35:451–496. [Google Scholar]
- Blavet N, Charif D, Oger-Desfeux C, Marais GA, Widmer A. Comparative high-throughput transcriptome sequencing and development of SiESTa, the Silene EST annotation database. BMC Genomics. 2011;26:12–376. doi: 10.1186/1471-2164-12-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A, Blevins T, Yao YL, et al. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of dicerlike proteins. PLoS One. 2010;5:e9514. doi: 10.1371/journal.pone.0009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donà M, Balestrazzi A, Mondoni A, et al. DNA profiling, telomere analysis and antioxidant properties as tools for monitoring ex situ seed longevity. Annals of Botany. 2013;111:987–998. doi: 10.1093/aob/mct058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RH, Roberts EH. Improved equations for the prediction of seed longevity. Annals of Botany. 1980;45:13–30. [Google Scholar]
- FAO/IPGRI . Genebank standards. Rome: Food and Agriculture Organization of the United Nations/International Plant Genetic Resource Institute; 1994. [Google Scholar]
- Galloway LF, Etterson JR. Transgenerational plasticity is adaptive in the wild. Science. 2007;318:1134–1136. doi: 10.1126/science.1148766. [DOI] [PubMed] [Google Scholar]
- Hay FR, Adams J, Manger K, Probert RJ. The use of non-saturated lithium chloride solution for experimental control of seed water content. Seed Science and Technology. 2008;36:737–746. [Google Scholar]
- Herman JJ, Sultan SE. Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Frontiers in Plant Science. 2011;102:1–9. doi: 10.3389/fpls.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes F, Porcher E, Teixeira F, et al. Assessing the impact of transgenerational epigenetic variation on complex traits. PLoS Genetics. 2009;5:e1000530. doi: 10.1371/journal.pgen.1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek J, Buckley JM, Probert RJ, Adkins SW, Steadman KJ. Pre-zygotic parental environment modulates seed longevity. Austral Ecology. 2010;35:837–848. [Google Scholar]
- Kochanek J, Steadman KJ, Probert RJ, Adkins SW. Parental effects modulate seed longevity: exploring parental and offspring phenotypes to elucidate pre-zygotic environmental influences. New Phytologist. 2011;191:223–233. doi: 10.1111/j.1469-8137.2011.03681.x. [DOI] [PubMed] [Google Scholar]
- Koller D. Environmental control of seed germination. In: Kozlowski TT, editor. Seed biology. New York: Academic Press; 1972. pp. 2–102. [Google Scholar]
- Kou HP, Li Y, Song XX, et al. Heritable alteration in DNA methylation induced by nitrogen-deficiency stress accompanies enhanced tolerance by progenies to the stress in rice (Oryza sativa L.) Journal of Plant Physiology. 2011;168:1685–1693. doi: 10.1016/j.jplph.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Körner C. Alpine plant life: functional plant ecology of high mountain ecosystems. Berlin: Springer-Verlag; 1999. [Google Scholar]
- Kranner I, Beckett RP, Minibayeva FV, Seal CE. What is stress? Concepts, definitions and applications in seed science. New Phytologist. 2010;188:655–673. doi: 10.1111/j.1469-8137.2010.03461.x. [DOI] [PubMed] [Google Scholar]
- Long RL, Panetta FD, Steadman KJ, et al. Seed persistence in the field may be predicted by laboratory controlled aging. Weed Science. 2008;56:523–528. [Google Scholar]
- Marsden-Jones EM, Turrill WB. The bladder champions. London: The Ray Society; 1957. [Google Scholar]
- Meyer SE, Allen PS. Ecological genetics of seed germination regulation in Bromus tectorum L. II. Reaction norms in response to a water stress gradient imposed during seed maturation. Oecologia. 1999;120:35–43. doi: 10.1007/s004420050830. [DOI] [PubMed] [Google Scholar]
- Mondoni A, Probert RJ, Rossi G, Vegini E, Hay FR. Seeds of alpine plants are short lived: implications for long-term conservation. Annals of Botany. 2011;107:171–179. doi: 10.1093/aob/mcq222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW. Maternal effects as adaptations. New York: Oxford University Press; 1998. [Google Scholar]
- Newton R, Hay F, Probert RJ. 2009. Protocol for comparative seed longevity testing. Technical Information Sheet_01, Royal Botanic Gardens Kew, UK http://www.kew.org/ucm/groups/public/documents/document/ppcont_014163.pdf. [accessed 2 September 2013] [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, et al. Plant phenotypic plasticity in a changing climate. Trends in Plant Science. 2010;15:684–692. doi: 10.1016/j.tplants.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Oñate-Sánchez L, Vicente-Carbajosa J. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res Notes. 2008;1:93. doi: 10.1186/1756-0500-1-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolo G, Rossi G. Upward migration of vascular plants following a climate warming trend in the Alps. Basic and Applied Ecology. 2008;9:100–107. [Google Scholar]
- Pettersson MW. Advantages of being a specialist female in gynodioecious Silene vulgaris (Caryophyllaceae) American Journal of Botany. 1992;79:1389–1395. [Google Scholar]
- Pignatti S. Flora d'Italia. Bologna: Edagricole; 1982. [Google Scholar]
- Priestley DA, Cullinan VI, Wolfe J. Differences in seed longevity at the species level. Plant, Cell and Environment. 1985;8:557–562. [Google Scholar]
- Probert RJ, Daws MI, Hay FR. Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Annals of Botany. 2009;104:57–69. doi: 10.1093/aob/mcp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaderi MM, Cavers PB, Hamill AS, Downs MP, Bernards MA. Maturation temperature regulates germinability and chemical constituents of Scotch thistle (Onopordum acanthium) cypselas. Canadian Journal of Botany. 2006;84:28–38. [Google Scholar]
- Rajjou L, Duval M, Gallardo K, et al. Seed germination and vigor. Annual Review of Plant Biology. 2012;63:507–533. doi: 10.1146/annurev-arplant-042811-105550. [DOI] [PubMed] [Google Scholar]
- Ream TS, Haag JR, Wierzbicki AT, et al. Subunit compositions of the RNA-silencing enzymes Pol IV and Pol V reveal their origins as specialized forms of RNA polymerase II. Molecular Cell. 2009;33:192–203. doi: 10.1016/j.molcel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach DA, Wulff RD. Maternal effects in plants. Annual Review of Ecology and Systematics. 1987;18:209–235. [Google Scholar]
- Roberts EH. Predicting the storage life of seeds. Seed Science and Technology. 1973;1:499–514. [Google Scholar]
- Schwienbacher E, Marcante S, Erschbamer B. Alpine species seed longevity in the soil in relation to seed size and shape – a 5-year burial experiment in the Central Alps. Flora. 2010;205:19–25. [Google Scholar]
- Srivastava LM. Plant growth and development. San Diego: Academic Press; 2002. [Google Scholar]
- Steadman KJ, Ellery AJ, Chapman R, Moore A, Turner NC. Maturation temperature and rainfall influence seed dormancy characteristics of annual ryegrass (Lolium rigidum) Australian Journal of Agricultural Research. 2004;55:1047–1057. [Google Scholar]
- Sultan SE. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science. 2000;5:537–542. doi: 10.1016/s1360-1385(00)01797-0. [DOI] [PubMed] [Google Scholar]
- Talavera S. Silene L. In: Castroviejo S, Laínz M, López González G, et al., editors. Flora Iberica. Vol. 2. Real Jardín Botánico, CSIC: Madrid; 1990. pp. 313–406. [Google Scholar]
- Thompson K. The functional ecology of soil seed banks. In: Fenner M, editor. Seeds: the ecology of regeration in plant communities. Wallingford, UK: CABI Publishing; 2000. pp. 215–235. [Google Scholar]
- Ventura L, Donà M, Macovei A, et al. Understanding the molecular pathways associated with seed vigor. Plant Physiology and Biochemistry. 2012;60:196–206. doi: 10.1016/j.plaphy.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Walters C, Wheeler LM, Grotenhuis JM. Longevity of seeds stored in a genebank: species characteristics. Seed Science Research. 2005;15:1–20. [Google Scholar]
- Waterworth WM, Masnavi G, Bhardwaj RM, Jiang Q, Bray CM, West CE. A plant DNA ligase is an important determinant of seed longevity. The Plant Journal. 2010;63:848–860. doi: 10.1111/j.1365-313X.2010.04285.x. [DOI] [PubMed] [Google Scholar]
- Wehmeyer N, Vierling E. The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiology. 2000;4:1099–1108. doi: 10.1104/pp.122.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle C, Otto S, Johnston M, Krochko J. Adaptive epigenetic memory of ancestral temperature regime in Arabidopsis thaliana. Botany. 2009;87:650–657. [Google Scholar]