Abstract
Background and Aims
Submergence and de-submergence are common phenomena encountered by riparian plants due to water level fluctuations, but little is known about the role of physiological integration in clonal plants (resource sharing between interconnected ramets) in their adaptation to such events. Using Alternanthera philoxeroides (alligator weed) as an example, this study tested the hypotheses that physiological integration will improve growth and photosynthetic capacity of submerged ramets during submergence and will promote their recovery following de-submergence.
Methods
Connected clones of A. philoxeroides, each consisting of two ramet systems and a stolon internode connecting them, were grown under control (both ramet systems untreated), half-submerged (one ramet system submerged and the other not submerged), fully submerged (both ramet systems submerged), half-shaded (one ramet system shaded and the other not shaded) and full-shaded (both ramet systems shaded) conditions for 30 d and then de-submerged/de-shaded for 20 d. The submerged plants were also shaded to very low light intensities, mimicking typical conditions in turbid floodwater.
Key Results
After 30 d of submergence, connections between submerged and non-submerged ramets significantly increased growth and carbohydrate accumulation of the submerged ramets, but decreased the growth of the non-submerged ramets. After 20 d of de-submergence, connections did not significantly affect the growth of either de-submerged or non-submerged ramets, but de-submerged ramets had high soluble sugar concentrations, suggesting high metabolic activities. The shift from significant effects of integration on both submerged and non-submerged ramets during the submergence period to little effect during the de-submergence period was due to the quick recovery of growth and photosynthesis. The effects of physiological integration were not found to be any stronger under submergence/de-submergence than under shading/de-shading.
Conclusions
The results indicate that it is not just the beneficial effects of physiological integration that are crucial to the survival of riparian clonal plants during periods of submergence, but also the ability to recover growth and photosynthesis rapidly after de-submergence, which thus allows them to spread.
Keywords: Alternanthera philoxeroides, alligator weed, Amaranthaceae, amphibious plant, clonal plant ecology, physiological integration, flooding, resource sharing, submergence, riparia
INTRODUCTION
In riparian ecosystems, submergence and de-submergence are common phenomena encountered by plants due to water level fluctuations (Lopez and Kursar, 2007). Both submergence and the subsequent de-submergence can greatly impair growth and physiological behaviours of plants (Sarkar et al., 2001; Mommer et al., 2004; Colmer and Voesenek, 2009; Luo et al., 2011; Striker, 2012). Submergence dramatically decreases gas exchange in plants due to reduced light intensity and slow O2 and CO2 exchange rates, leading to carbohydrate starvation and energy crisis (Mommer et al., 2004; Colmer and Voesenek, 2009). When floodwater recedes and plants are re-exposed to the atmosphere, tissue injuries suffered underwater can be intensified (Sarkar et al., 2006). A sudden increase in O2 upon de-submergence exacerbates the conditions unless plants can cope with excessive formation of reactive oxygen species and toxic oxidative products in different cells and organs (Sarkar et al., 2001; Blokhina et al., 2003; Luo et al., 2012). Direct sunlight also threatens leaves accustomed to low-light underwater environments, causing photoinhibition to the photosynthetic apparatus (Osmond, 1994).
Submergence-tolerant amphibious plants are able to alleviate some adverse impacts of submergence by changing the morphology and anatomy of shoots and roots, adjusting metabolic pathways or upregulating antioxidant defence mechanisms (Blokhina et al., 2003; Mommer et al., 2004; Colmer and Pedersen, 2008; Colmer and Voesenek, 2009). The capacity for fast growth recovery after de-submergence is also essential for flooding tolerance in plants (Chen et al., 2011; Striker, 2012). Quick adjustments of photosynthesis, antioxidative defence and carbohydrate accumulation are considered to be crucial for growth recovery of plants that deplete carbohydrate reserves during submergence (Panda et al., 2008; Hattori et al., 2009; Qin et al., 2013; Voesenek and Bailey-Serres, 2013). For those that can maintain high chlorophyll contents, efficiency of photosynthetic apparatus, antioxidative capacities and carbohydrate reserves throughout submergence, rapid hydrolysis of the retained carbohydrate also enables rapid start of regrowth (Fukao et al., 2006; Hattori et al., 2009; Striker, 2012; Voesenek and Bailey-Serres, 2013).
Many amphibious plants can spread by clonal growth and span from terrestrial to aquatic habitats or from aquatic to terrestrial habitats (Amsberry et al., 2000; Pennings and Callaway, 2000). A distinguishing feature of clonal growth is physiological integration, i.e. the ability to translocate substances such as photosynthates, water and mineral nutrients between interconnected individuals (ramets) of the same clone (Alpert, 1999; Janecek et al., 2004, 2008; Roiloa and Retuerto, 2006; Roiloa et al., 2013). Studies conducted on clonal plants in terrestrial environments generally show that physiological integration can greatly enhance growth and photosynthesis of ramets growing in adverse conditions when they are connected to ramets growing in benign conditions (van Kleunen et al., 2000; Alpert et al., 2003; Roiloa et al., 2007; Song et al., 2013). Besides, some wetland clonal plants can have O2 movements through their gas spaces in e.g. rhizomes, shoots or roots, enabling internal O2 transport between ramets (Colmer, 2003; van Bodegom et al., 2008; Armstrong and Armstrong, 2009; Yang et al., 2014), so that ramets underwater may import O2 from their connected ramets on land through physiological integration. Thereby, when ramets of amphibious plants suffer from injuries caused by complete submergence, interconnected ramets of the same clone grown in terrestrial conditions may increase translocation of essential resources to those growing under more adverse conditions (de Kroon et al., 1996, 1998; Alpert, 1999; Pennings and Callaway, 2000; Colmer, 2003; Armstrong and Armstrong, 2009). When floodwater is drained, recovery of de-submerged ramets suffering from submergence and post-submergence injuries may still rely on support from interconnected ramets or fast recovery of their own photosynthetic capacities, or both (Blokhina et al., 2003; Sarkar et al., 2006). However, little is known about whether and how physiological integration can help amphibious clonal plants cope with submergence and subsequent de-submergence in riparian habitats. We hypothesized that physiological integration can positively affect the responses of amphibious clonal plants to submergence and de-submergence.
To test the hypothesis, we subjected one part of or whole clonal fragments of an invasive, amphibious clonal plant, Alternanthera philoxeroides, to submergence and then de-submergence. We examined how growth, photosynthesis and carbohydrate accumulation of A. philoxeroides responded to submergence and subsequent de-submergence, and how these responses were modified by physiological integration. The submergence treatment was conducted under very low light intensities, mimicking the typical conditions in turbid floodwater. Specifically, we tested (1) whether physiological integration will improve the growth and photosynthetic capacity of submerged ramets during submergence, and (2) whether physiological integration will promote the recovery of de-submerged ramets during their subsequent recovery. Additionally, some of the plants were subjected to low-light stress to distinguish the responses induced by submergence from those induced by low light. Previous studies have showed that the effects of physiological integration could be greater in more stressful conditions (Friedman and Alpert, 1991; van Kleunen et al., 2000; Liu et al., 2009). Because submerged plants additionally suffered from hypoxia stress and de-submerged plants additionally suffered from post-hypoxia injuries (Blokhina et al., 2003; Colmer and Voesenek, 2009), we therefore expected (3) that the effects of integration would be greater in submerged conditions than in shaded conditions and in de-submerged conditions than in de-shaded conditions.
MATERIALS AND METHODS
Plant species
Alternanthera philoxeroides (Amaranthaceae) is an amphibious weed that occurs frequently in riparian regions in China and can establish in aquatic, semi-aquatic and terrestrial environments (Geng et al., 2007). It is native to South America, but highly invasive in many countries, including China. This species can spread quickly by clonal growth and produces hollow, creeping stolons. It can spread from dry areas to moist areas and vice versa, and can quickly elongate its shoots to regain contact with the atmosphere when submerged in deep water (Luo et al., 2011). Each stolon node is capable of bearing roots and two opposite leaves.
Experimental material
Plants of A. philoxeroides were collected from five locations at least 10 m apart in each of two wetlands in Taizhou, Zhejiang Province, China. In China, genetic variation of A. philoxeroides is very low and likely from the same genet (Xu et al., 2003). Thus, we mixed plants from the different locations and cultivated them vegetatively in a greenhouse of the Forest Science Company of Beijing Forestry University. Clonal fragments of A. philoxeroides, each consisting of two adjacent ramet systems (a group of interconnected ramets) and a connecting stolon internode, were at the same developmental stage and propagated from stolon cuttings. The relatively older ramet system, proximal to the mother ramet, is referred to as the ‘basal ramet system’ or the ‘basal ramets’ and the younger one, distal to the mother ramet, as the ‘apical ramet system’ or the ‘apical ramets’. The apical and basal ramets were grown in two adjoining containers (40 × 20 × 60 cm, length × width × height) divided by a board. The walls of the containers were covered with black plastic sheets to prevent light penetration. A hole 3 cm in diameter was made in the board 14 cm from the base, allowing the connecting stolon to be positioned through this hole. To avoid water penetration through the hole between two adjoining containers, the stolons were enclosed in a rubber stopper and placed in the hole. The containers were filled with a 1:1:1 (v:v:v) mixture of washed river sand, peat moss (Pindstrup Substrate, Pindstrup Mosebrug AS, Denmark) and surface soil collected from the riverside of Gui River, Yan Qin, Beijing, China, containing 0·48 g kg−1 total nitrogen, 0·65 g kg−1 total phosphorus and 7·83 g kg−1 total organic carbon.
Plants were grown for a month in the greenhouse and watered daily. The air temperature and relative humidity in the greenhouse ranged between 27 and 36 °C and between 40 and 60 %, respectively, during the cultivation and experimental periods. The photosynthetically active radiation measured at plant level at noon was 300–500 μmol photons m–2 s–1.
Experimental design
In July 2011, 66 plants (pairs of ramet systems) of similar size and age were selected for the experiment. Each pair consisted of an apical ramet system (i.e. a stem of several nodes), a basal ramet system and a stolon internode connecting them. Six of them were randomly selected and dried to measure initial dry mass (0·93 ± 0·10 g, mean ± s.e.), and the remaining 60 plants were randomly subjected to five treatments, resulting in five treatments × six replicates × two harvests. The five treatments were as follows: (1) control (both basal and apical ramets were kept un-treated in a soil water-saturated condition); (2) half-shaded (the basal ramets were kept in the soil water-saturated condition and covered with a neutral shading cloth during days 0–30, whereas the apical ramets were kept un-shaded in the soil water-saturated condition); (3) full-shaded (both basal and apical ramets were kept in the soil water-saturated condition and covered with a neutral shading cloth during days 0–30); (4) half-submerged (the basal ramets were submerged under 45-cm deep water and covered with a neutral shading cloth during days 0–30, whereas the apical ramets were kept un-submerged in the soil water-saturated condition); and (5) full-submerged (both basal and apical ramets were submerged under 45-cm deep water and covered with a neutral shading cloth during days 0–30). The water used for submergence was tap water and no extra minerals were added to the water during the treatments. The photosynthetically active radiation reaching the shaded and submerged ramets was <10 μmol photons m–2 s–1, mimicking the very low light intensities under deep or turbid floodwater. The shading cloth was kept about 1 cm below the water surface to ensure that the submerged plants remained completely underwater throughout the treatments, which simulated the conditions of deep submergence. After 30 d of different treatments (on day 30), half of the plants in each treatment were randomly selected and harvested. For the other half, the shading cloths were removed and floodwater was drained. The shaded and submerged ramets were brought back to the control conditions. Subsequently, recovery (after de-submergence/de-shading) was monitored for 20 d (until day 50) under the soil water-saturated condition (control condition).
Growth analyses
At harvest (days 30 and 50), the number of leaves, stolon length and the number of branches of the apical and basal ramets were measured separately. Then, the apical and basal ramets were separated into stolons, leaves and roots, dried at 80 °C for 72 h, and weighed.
Chlorophyll a fluorescence measurements
On the date of each harvest, the maximum and minimum fluorescences in dark-adapted leaves (Fm and F0, respectively) were measured after 30 min of dark adaptation using leaf clips. Measurements were conducted on the youngest fully expanded leaves (i.e. mature leaves closest to the growth zone of the shoot) of both apical and basal ramets by using a portable modulated fluorometer (PAM-2500, Heinz Walz, Germany) at 0900–1000 h. The intensity and duration of the saturation pulse applied to determine Fm were 3500 μmol photons m–2 s–1 and 1 s, respectively. We calculated the maximal quantum yield of photosystem II (Fv/Fm) as (Fm – F0)/Fm (Maxwell and Johnson, 2000).
Gas exchange measurements
Leaf gas exchange was measured in the greenhouse at 0900–1100 h on days 30 and 50 using a Li-6400 portable photosynthesis system (Li-Cor Biosciences, Lincoln, NE, USA). The leaves used for gas exchange measurements were those opposite to the leaves used for determination of Fv/Fm. Net photosynthetic rate (Pn), intercellular CO2 concentration (Ci) and transpiration rate (E) were measured at a CO2 concentration of 400 μmol mol–1 and a photo flux density of 800 μmol m–2 s–1 (the light saturation point of de-submerged leaves of this species obtained from a preliminary experiment). The leaf area used for the measurements was measured by scanning.
Pigment determinations
Concentrations of chlorophylls (Chl) a + b and carotenoids (Car) x + c (xanthophylls and carotenes) were measured on the same leaves as those used for gas exchange measurements. Leaf discs (0·89 cm2) were sampled from the mid-lamina area using a hole punch immediately after the measurements of gas exchange. The concentrations of Chl a + b and Car x + c in leaves were determined according to Wellburn (1994).
Non-structural carbohydrate measurements
The dried leaves, stolons and roots of apical and basal ramets were thoroughly milled, and about 15 mg of milled powder of each plant part was used for analysis. Soluble sugars and non-soluble sugars were analysed using the perchloric acid/anthrone method (Morris, 1948). This method has proved to be robust and has been used repeatedly to analyse carbohydrate in reserve organs (Klimes and Klimesova, 2001; Olano et al., 2006). The soluble sugars were extracted from dried material with 0·5 mL of 80 % ethanol at 80 °C for 30 min. The extract was then centrifuged at 15 493 g for 3 min and the supernatant was collected. This process was repeated twice; 1 mL of 80 % ethanol was used the last time. The combined supernatant was added with 80 % ethanol to the volume of 2 mL and mixed thoroughly. Then, 30 μL of the supernatant was mixed with 70 μL water, 0·15 mL anthrone reagent (1 g anthrone ethyl dissolved in 50 mL ethyl acetate) and 1·5 mL concentrated sulphuric acid, and heated at 100 °C for 15 min. Soluble sugar concentration was determined by measuring the absorbance at 630 nm in a spectrophotometer, and subtracting the absorbance value of sample blanks. These values were then regressed against readings from a set of standard solutions of glucose. The non-soluble sugars were extracted after hydrolyzing the residue with 1 mL of perchloric acid (35 %) for 2 h at 60 °C. The extract was then centrifuged at 15 493 g for 3 min, and the supernatant was used for determinations. Solubilized carbohydrate was analysed using the anthrone reaction with the method previously described for soluble sugars. In this case, the readings were regressed against another set of standard glucose solutions. The concentrations of soluble sugars and non-soluble sugars were calculated as glucose and expressed relative to dry mass of the samples (mg g–1 dry mass) and the contents of these sugars were given as sugar concentration × total dry mass (g) (Supplementary Data Fig. S1).
Statistical analysis
Before analyses, the data on growth, chlorophyll a fluorescence, gas exchange, pigment and carbohydrate were checked for normality and homogeneity variance. Repeated-measures ANOVA was used to test the overall effects of treatment on measured variables, with ramet position along the stolon (apical ramets versus basal ramets) as the repeated factor (Potvin et al., 1990; Huber and Hutchings, 1997). Subsequently, five planned contrasts were used for comparisons in both apical and basal ramets. For both apical and basal ramets, contrast 1 compared the control and full-shaded treatments, contrast 2 compared the control and full-submerged treatments, and contrast 3 compared the full-shaded and full-submerged treatments. These three contrasts were used to test the effects of shading and submergence. For the apical ramets, contrast 4 compared the control and half-shaded treatments, and contrast 5 compared the control and half-submerged treatments. These two contrasts were used to investigate the costs to the apical ramets in the half-shaded and half-submerged treatments. For basal ramets, contrast 4 compared the half-shaded and full-shaded treatments, and contrast 5 compared the half-submerged and full-submerged treatments. These two contrasts were used to examine the benefits to the basal ramets in the half-shaded and half-submerged treatments. Then, we determined whether the effects of physiological integration differed between the half-shaded and half-submerged treatments. Similar analyses were conducted for the data on day 50. To increase normality and homogeneity of variances, data on leaf numbers and concentrations and contents of non-structural carbohydrate of both apical and basal ramets were transformed to the square root before analysis. All analyses were done using SPSS 16·0 (SPSS, Chicago, IL, USA). Effects were considered to be significant if P < 0·05.
RESULTS
Growth responses of apical and basal ramets
Compared with the control, the full-shaded and full-submerged treatments significantly decreased all growth measures (dry mass, leaf number, stolon length and branch number) of both apical and basal ramets of A. philoxeroides on both day 30 (after submergence/shading) and day 50 (after de-submergence/de-shading; Fig. 1; Supplementary Data Table S1).
Fig. 1.
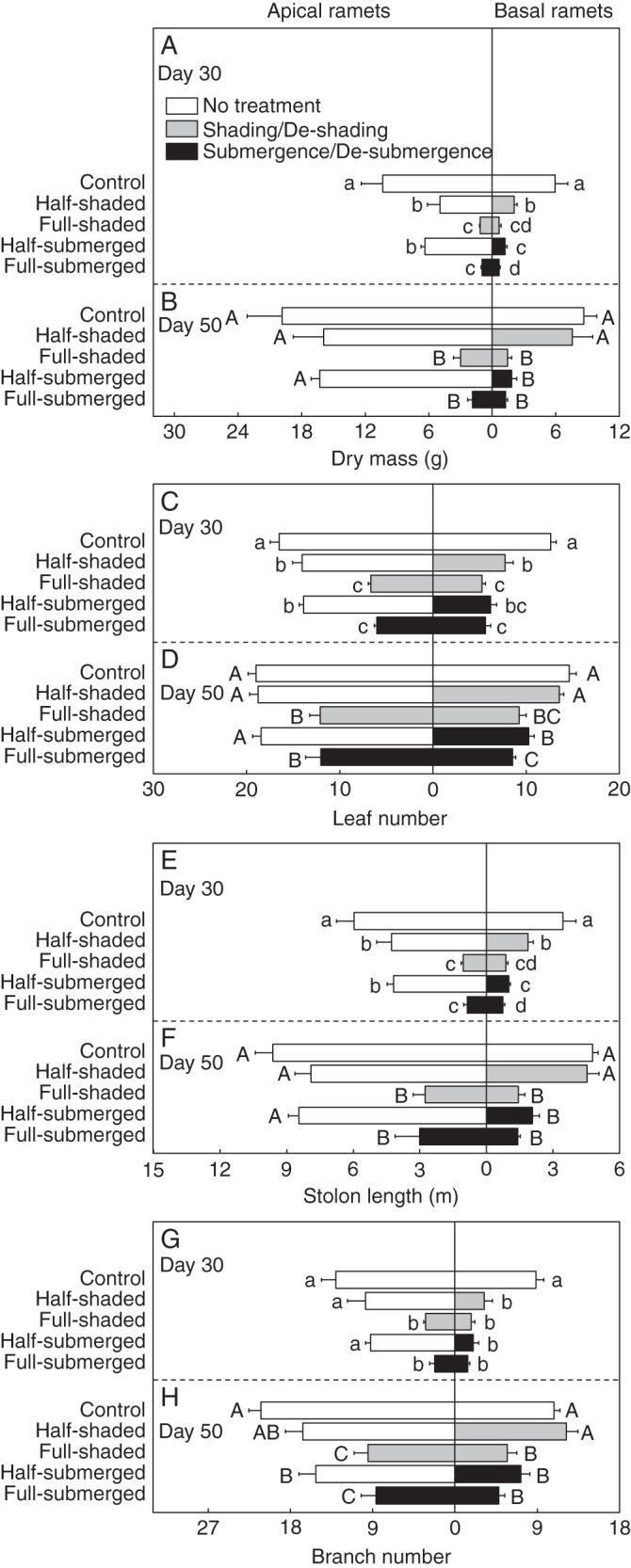
(A, B) Dry mass, (C, D) leaf number, (E, F) stolon length and (G, H) branch number of apical and basal ramets of A. philoxeroides after shading or submergence (day 30, upper graphs) and after de-shading or de-submergence (day 50, lower graphs). Data are mean values (± s.e., n = 6). Within each day, means sharing the same letter are not significantly different at P = 0·05.
After the 30-d submergence/shading (on day 30), for the apical (untreated) ramets, dry mass, leaf number and stolon length were significantly smaller in the half-shaded and half-submerged treatments than in the control (Fig. 1A, C, E; Supplementary Data Table S1). For the basal (treated) ramets, dry mass, leaf number and stolon length were higher in the half-shaded treatment than in the full-shaded treatment, and dry mass and stolon length were higher in the half-submerged treatment than in the full-submerged treatment (Fig. 1A, C, E). Notably, dry mass and stolon length of the basal ramets were higher in the half-shaded treatment than in the half-submerged treatment (Fig. 1A, E).
After 20 d of de-submergence/de-shading (on day 50), dry mass, leaf number or stolon length of the apical (un-treated) ramets did not differ among the control, half-shaded and half-submerged treatments (Fig. 1B, D and F). For the basal (de-treated) ramets, all four growth measures were significantly greater in the half-shaded than in the full-shaded treatment, whereas none of the four growth measures except leaf number differed significantly between the half-submerged and the full-submerged treatment (Fig. 1B, D, F, H). Remarkably, all growth measures of the basal ramets were much higher in the half-shaded treatment than in the half-submerged treatment (Fig. 1B, D, F, H).
Growth responses of clonal fragments
After submergence/shading (on day 30), all four growth measures of the clonal fragments (apical ramets plus basal ramets) were largest in the control, smallest in the full-shaded and full-submerged treatments and intermediate in the half-shaded and half-submerged treatments (Fig. 2A, C, E, G).
Fig. 2.
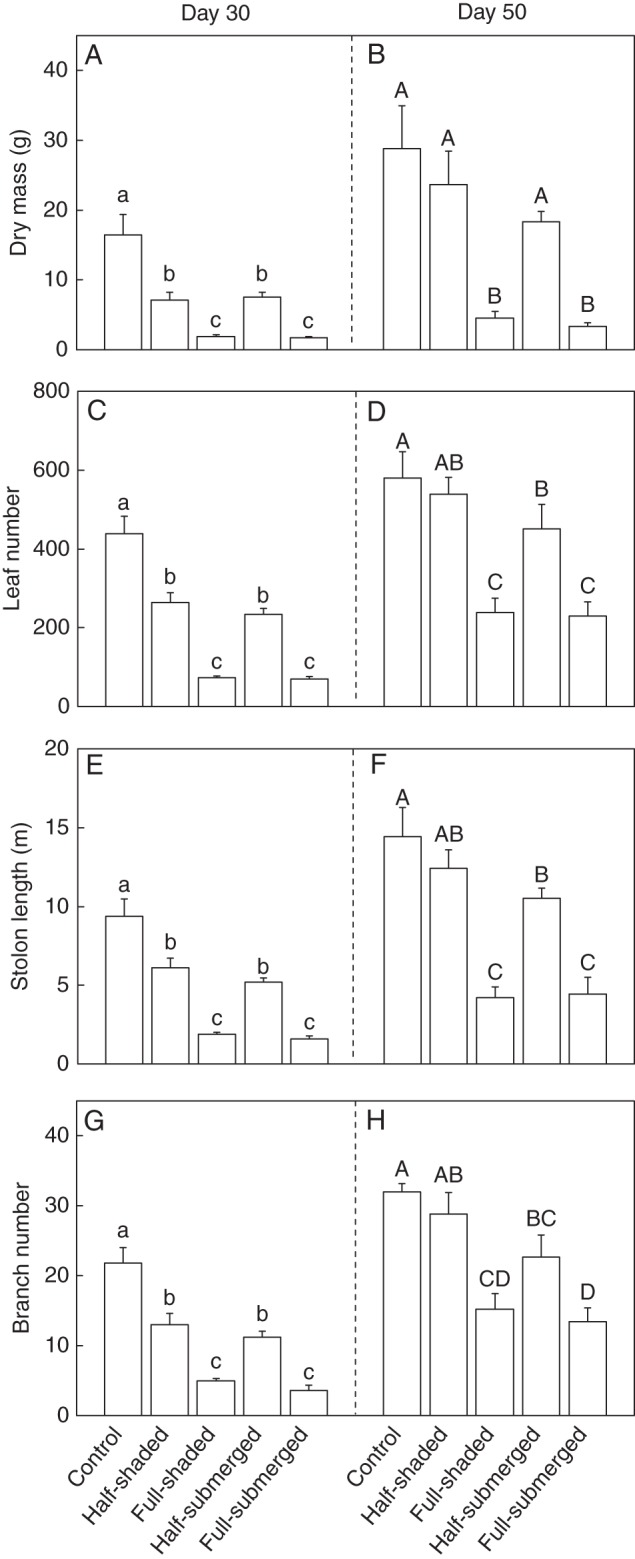
(A, B) Dry mass, (C, D) leaf number, (E, F) stolon length and (G, H) branch number of clonal fragments of A. philoxeroides after shading or submergence (day 30, left graphs) and after de-shading or de-submergence (day 50, right graphs). Data are mean values (± s.e., n = 6). Within each day, means sharing the same letter are not significantly different at P = 0·05.
After de-submergence/de-shading (on day 50), all growth measures of the clonal fragments were also higher in the control and half-shaded treatments than in the full-shaded treatment, and in the control and half-submerged treatments than in the full-submerged treatment (Fig. 2B, D, F, H). However, none of the growth measures differed between the control and half-shaded treatment, and dry mass did not differ between the control and half-submerged treatments.
Photosynthetic responses of apical and basal ramets
After submergence/shading (on day 30), both full-submerged and full-shaded treatments significantly decreased Fv/Fm and Pn of both apical and basal ramets compared with the control, with a stronger decrease in the full-submerged treatment (Fig. 3A, C; Supplementary Data Table S1). After de-submergence/de-shading (on day 50), these photosynthetic measures of both apical and basal ramets in the full-shaded and full-submerged treatments recovered to the control level (Fig. 3).
Fig. 3.
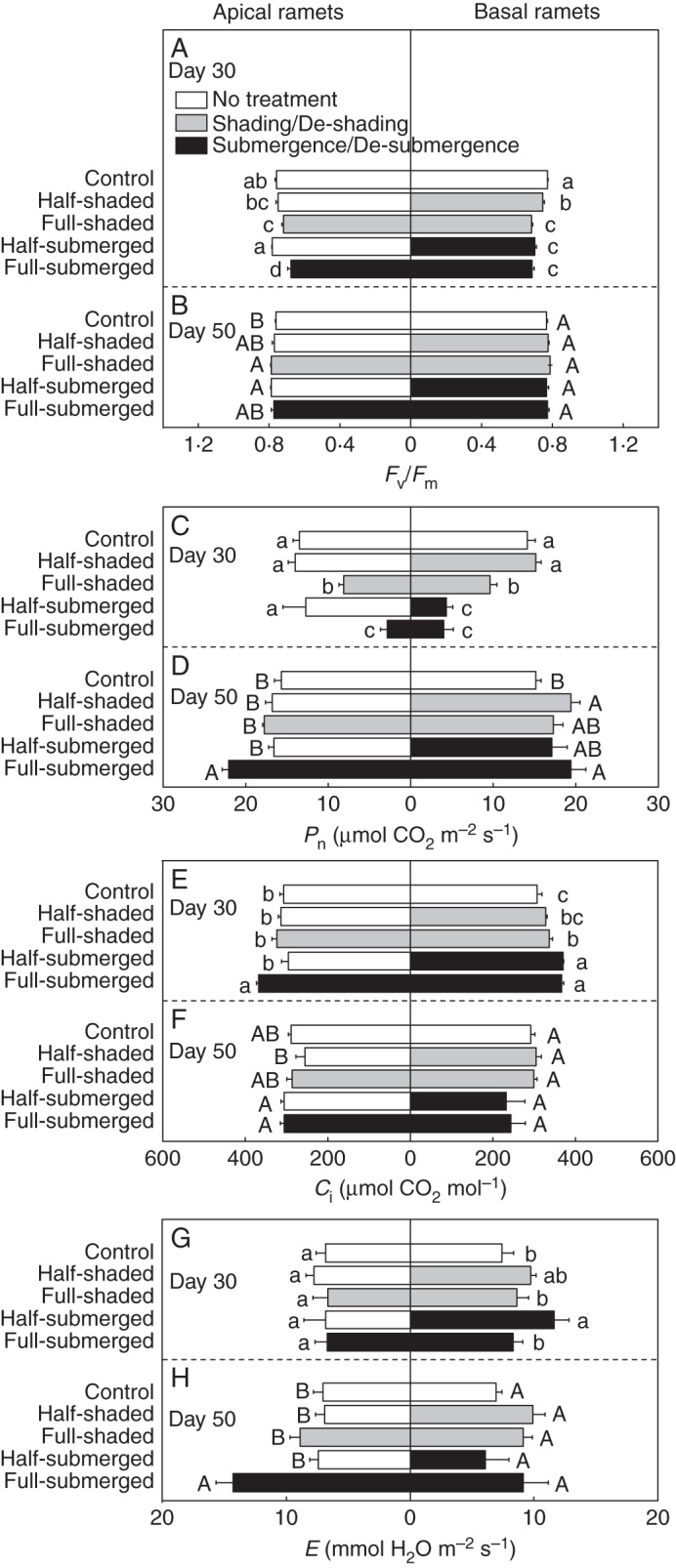
(A, B) Maximal quantum yield of photosystem II (Fv/Fm), (C, D) net photosynthetic rate (Pn), (E, F) intercellular CO2 concentration (Ci) and (G, H) transpiration rate (E) of apical and basal ramets of A. philoxeroides after shading or submergence (day 30, upper graphs) and after de-shading or de-submergence (day 50, lower graphs). The leaves used for measurements were the youngest fully expanded leaves. Data are mean values (± s.e., n = 6). Within each day, means sharing the same letter are not significantly different at P = 0·05.
After submergence/shading, for the apical ramets, Fv/Fm and Pn in the control did not differ from that in the half-shaded and half-submerged treatments (Fig. 3A, C; Supplementary Data Table S1). For the basal ramets, Fv/Fm and Pn were significantly higher in the half-shaded treatment than in the full-shaded, half-submerged and full-submerged treatments (Fig. 3A, C).
After de-submergence/de-shading, for the apical ramets, Fv/Fm and Pn in the half-shaded and half-submerged treatments reached the control level (Fig. 3B, D). For the basal ramets, neither Fv/Fm nor Pn differed significantly between the half-shaded and full-shaded treatments or between the half-submerged and full-submerged treatments.
Compared with the control, the other four treatments did not significantly decrease Ci or E of the apical or basal ramets on day 30 or 50 (Fig. 3E–H).
Pigment concentrations of apical and basal ramets
Compared with the control, the full-shaded and full-submerged treatments did not significantly decrease leaf pigment concentrations in the apical or basal ramets after submergence/shading (on day 30; Fig. 4A, C; Supplementary Data Table S1), but decreased them after de-submergence/de-shading (on day 50; Fig. 4B, D).
Fig. 4.
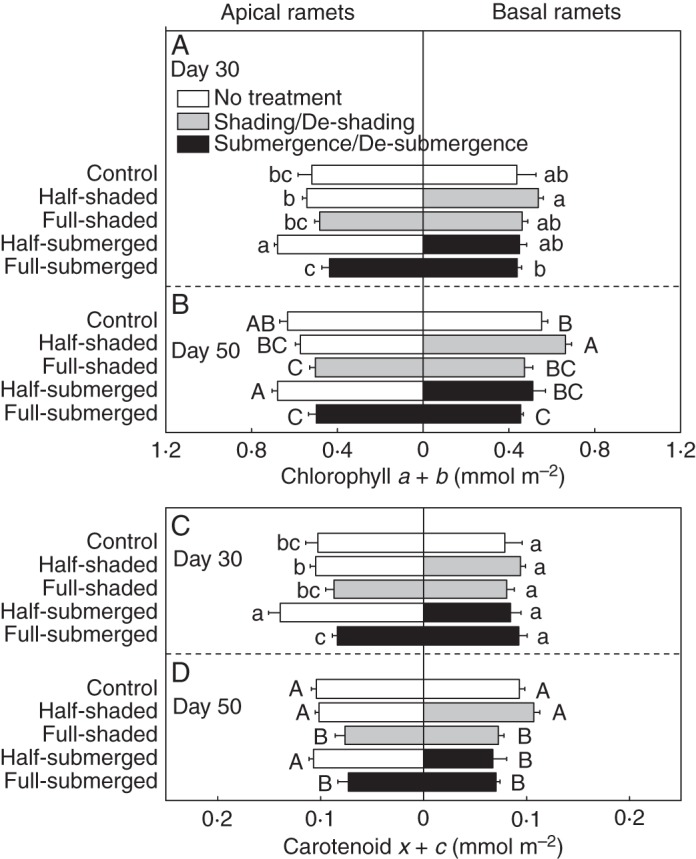
(A, B) Chlorophyll a + b and (C, D) carotenoid x + c concentrations of apical and basal leaves of A. philoxeroides after shading or submergence (day 30, upper graphs) and after de-shading or de-submergence (day 50, lower graphs). The ratios of area to dry mass were 426 and 506 cm2 g–1 dry mass for the control and treated leaves, respectively, and 426 and 478 cm2 g–1 dry mass for the control and de-treated leaves. Data are mean values (± s.e., n = 6). Within each day, means sharing the same letter are not significantly different at P = 0·05.
After submergence/shading, for the apical ramets, the half-shaded and half-submerged treatments did not decrease leaf pigment concentrations compared with the control (Fig. 4A, C). For the basal ramets, the pigment concentrations did not differ between the half-shaded and full-shaded treatments or between the half-submerged and full-submerged treatments (Fig. 4A, C).
After de-submergence/de-shading, for the apical ramets, leaf pigment concentrations did not decrease in the half-shaded or half-submerged treatment compared with the control (Fig. 4B, D). For the basal ramets, leaf pigment concentrations were higher in the half-shaded treatment than in the full-shaded, half-submerged and full-submerged treatments (Fig. 4B, D).
Non-structural carbohydrate concentrations of apical and basal ramets
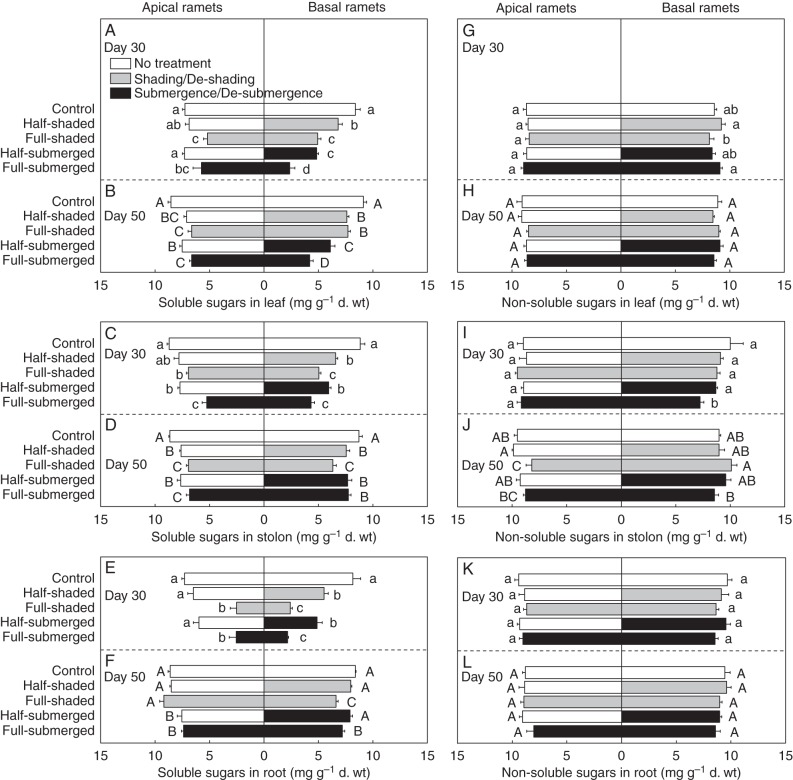
Compared with the control, the full-shaded and full-submerged treatments significantly decreased soluble sugar concentrations of leaves, stolons and roots in both apical and basal ramets on both day 30 (after submergence/shading) and day 50 (after de-submergence/de-shading; Fig. 5A–F; Supplementary Data Table S1). However, non-soluble sugar concentrations decreased only in the stolons of the basal ramets in the full-submerged treatment on day 30 and in the stolons of the apical ramets in the full-shaded treatment on day 50 (Fig. 5G–L).
Fig. 5.
Concentrations of soluble sugars (A–F) and non-soluble sugars (G–L) in leaves, stolons and roots of apical and basal ramets of A. philoxeroides after shading or submergence (day 30, upper graphs) and after de-shading or de-submergence (day 50, lower graphs). Sugar concentrations are given in equivalent amounts of glucose. The ratios of area to dry mass were 426 and 506 cm2 g–1 dry mass for the control and treated leaves, respectively, and 426 and 478 cm2 g–1 dry mass for the control and de-treated leaves. Data are mean values (± s.e., n = 4). Within each day, means sharing the same letter are not significantly different at P = 0·05.
After submergence/shading, for the apical ramets, neither the half-shaded nor the half-submerged treatment significantly decreased soluble sugar concentrations or non-soluble sugar concentrations compared with the control, except for soluble sugar concentrations in the stolons in the half-submerged treatment (Fig. 5A, C, E, G, I, K; Supplementary Data Table S1). For the basal ramets, soluble sugar concentrations in leaves, stolons and roots were higher in the half-shaded/half-submerged treatment than in the full-shaded/full-submerged treatment (Fig. 5A, C, E). Non-soluble sugar concentrations in leaves were higher in the half-shaded treatment than in the full-shaded treatment, and those in stolons were higher in the half-submerged treatment than in the full-submerged treatment (Fig. 5G, I).
After de-submergence/de-shading, for the apical ramets, soluble sugar concentrations in leaves, stolons and roots decreased in the half-shaded and half-submerged treatments compared with the control, except for soluble sugar concentrations in roots in the half-shaded treatment (Fig. 5B, D, F). For the basal ramets, soluble sugar concentrations in leaves, stolons and roots were higher in the half-shaded/half-submerged treatment than in the full-shaded/full-submerged treatment, except for soluble sugar concentrations in leaves in the half-shaded and full-shaded treatments and in stolons in the half-submerged and full-submerged treatments (Fig. 5B, D, F). The half-shaded and half-submerged treatments had little effect on non-soluble sugar concentrations (Fig. 5H, J, L).
DISCUSSION
Effects of physiological integration during submergence
Submergence greatly suppressed the growth, photosynthetic capacities and carbohydrate accumulation of A. philoxeroides (Figs 1, 3 and 5), agreeing with previous findings (Sarkar et al., 2001; Panda et al., 2008; Luo et al., 2011; Yu et al., 2012). On the other hand, when submerged ramets were connected with un-submerged ones, their growth and carbohydrate accumulation increased significantly compared with their counterpart ramets in the full-submerged treatment (Figs 1 and 5; Supplementary Data Fig. S1). These results support our first hypothesis, and suggest that physiological integration can enhance tolerance of ramets to submergence under floodwater of very low light intensity (van Kleunen et al., 2000; Alpert et al., 2003; Roiloa et al., 2007). Plants of A. philoxeroides are characterized by enhanced stem elongation, a typical feature of the escape strategy, upon submergence (Luo et al., 2011). Rapid stem elongation, together with high porosity in shoots and roots, may improve light environment and O2 and CO2 availability of A. philoxeroides during submergence, but it will lead to carbohydrate depletion when shoots fail to grow out of water (Panda et al., 2008; Hattori et al., 2009; Luo et al., 2011; Yu et al., 2012; Voesenek and Bailey-Serres, 2013). As natural floodwater can be deep and very turbid (Sarkar et al., 2006), A. philoxeroides may not be able to escape out of floodwater when completely submerged. To simulate the typical conditions in turbid floodwater, in our submerged treatments, the shade cloths were fixed at about 1 cm below the water surface, so that the submerged ramets were completely underwater throughout the treatments. Under such conditions, translocation of photosynthates and O2 via stolon internodes from un-submerged ramets may greatly benefit the performance of submerged ones (Alpert, 1999; Colmer, 2003; Janecek et al., 2004, 2008; van Bodegom et al., 2008; Roiloa et al., 2013; Yang et al., 2014).
However, connections with the submerged ramets conferred significant costs on the un-submerged ones: translocation of resources significantly reduced growth and carbohydrate contents of the un-submerged apical ramets (Fig. 1; Supple-mentary Data Fig. S1). Compared with other studies, resource translocation between interconnected ramets does not negatively affect the performance of un-stressed ramets (van Kleunen and Stuefer, 1999; Song et al., 2013). In the present study, rapid stolon elongation and high carbohydrate consumption in the basal (submerged) ramets of A. philoxeroides may have required high costs of physiological integration in the apical (un-submerged) ramets (Fig. 5; de Kroon et al., 1996, 1998; Alpert, 1999; van Kleunen et al., 2000). On the other hand, we found that the photosynthetic capacities and pigment concentrations of the apical (un-submerged) ramets were as high as those in the control, indicating a high capacity for photoassimilation (Figs 3 and 4). The results further suggest that the translocation of photosynthates from apical (un-submerged) ramets might greatly alleviate carbohydrate starvation in the basal (submerged) ramets under conditions of very low light intensity.
Effects of physiological integration after de-submergence
After 20 d of de-submergence, the growth and carbohydrate contents of the apical (un-submerged) ramets in the half-submerged treatment recovered to the levels of the control (Fig. 1; Supplementary Data Fig. S1). These results indicate that there were no significant costs of physiological integration in the un-submerged ramets, or that the effect of physiological integration was very weak, i.e. little resource translocation between the de-submerged basal ramets and the un-submerged apical ramets. In the basal (de-submerged) ramets, none of the four growth measures except leaf number differed between the half-submerged and the full-submerged treatment (Fig. 1), indicating that physiological integration was rather weak. Notably, soluble sugar concentrations in leaves and roots in the de-submerged ramets were higher in the half-submerged treatment than in the full-submerged treatment (Fig. 5). As soluble sugar provides energy and materials for rapid growth and structural/functional maturation, the higher concentrations of soluble sugar in de-submerged ramets in the half-submerged treatment suggests high metabolic activities in these organs (Panda et al., 2008; Yu et al., 2012; Qin et al., 2013). The results negate the second hypothesis and suggest that the de-submerged ramets of A. philoxeroides in the half-submerged treatment could be self-supported or plants may reallocate more dry mass in the un-submerged ramets to maintain the performance of the whole clone during recovery after de-submergence (de Kroon et al., 2005).
Physiological integration can greatly improve survival and growth of stressed ramets (Alpert, 1999; Alpert et al., 2003; Roiloa and Retuerto, 2006). Such effects can be suppressed under conditions of severe abiotic stress, homogeneous distribution of resources, abundant resources, stolon disconnection or ramet ageing (Alpert, 1996; Xiao et al., 2010; Li et al., 2011). In the case of A. philoxeroides, the leaf growth and photosynthetic capacities of the de-submerged ramets in the half-submerged treatment quickly recovered after de-submergence; notably, the net photosynthetic rate of the de-submerged ramets was comparable with that of the un-submerged ramets at the end of recovery (Figs 1 and 3). Correspondingly, soluble sugar concentrations in the de-submerged ramets largely increased during the 20 d of recovery (Fig. 5). These results are largely in agreement with the findings in our previous studies, in which A. philoxeroides could quickly restore photosynthesis and carbohydrate accumulation to resume growth within a few days following de-submergence (Luo et al., 2011). In the present study, the newly synthesized photoassimilates of the de-submerged ramets might be sufficient for their growth recovery after de-submergence. Therefore, abundant resources may be a reason for the independence of de-submerged basal ramets in A. philoxeroides after de-submergence. Post-submergence injuries could be another possible reason for suppression of integration, i.e. toxicity of reactive oxygen species products (Blokhina et al., 2003; Fukao et al., 2006; Colmer and Voesenek, 2009; Striker, 2012; Voesenek and Bailey-Serres, 2013). Besides, stolon wilting induced by low hydraulic conductivity of de-submerged plants can also impair the translocation of resources between ramets (Holbrook and Zwieniecki, 2003).
At the level of clonal fragments, although there were rather weak beneficial effects of integration, the increase in dry mass in the half-submerged treatment was much higher than that in the control during the recovery, i.e. 2·44- versus 1·75-fold (Fig. 2A, B). The greatly increased growth in the half-submerged treatment largely benefited from a quick recovery of the un-submerged apical ramets (Figs 1 and 2). Thus, once physiological integration between interconnected ramets is suppressed during recovery, A. philoxeroides may invest more in growth and clonal reproduction in the un-submerged apical ramets to improve resource capture for whole clonal fragments (de Kroon et al., 2005). The capacity for fast growth recovery of un-submerged ramets after de-submergence may be of critical importance for the spreading of whole clonal fragments in zones of water level fluctuation (de Kroon et al., 1996, 2005; Amsberry et al., 2000; Sarkar et al., 2001; Panda et al., 2008; Striker, 2012; Qin et al., 2013).
Different effects of physiological integration between (de-)submergence and (de-)shading
Our previous work has shown that growth and photosynthetic responses of A. philoxeroides during submergence (low light + low O2) were determined mainly by low light availability, whereas post-submergence recovery of growth was delayed by both low light and low O2 (Luo et al., 2011). Low light intensity and low O2 are therefore important limiting factors for the performance of plants in both submerged and de-submerged conditions (Mommer et al., 2004; Colmer and Voesenek, 2009).
Previous studies have found beneficial effects of physiological integration on ramets suffering from shading (Stuefer et al., 1994; Alpert, 1999; van Kleunen et al., 2000), but no studies have tested whether the beneficial effects of integration will be enhanced or attenuated by submergence. In the present study, the beneficial effects of integration on shaded ramets of A. philoxeroides were observed during shading as well as the subsequent de-shading (Figs 1 and 3; Supplementary Data Fig. S1). Compared with the half-shaded treatment, the benefits of integration for growth, photosynthetic capacities and carbohydrate accumulation were significantly lower in the half-submerged treatment, indicating that the effects of physiological integration are weaker under submerged conditions. Notably, the differences in the effects of physiological integration on submerged and shaded ramets became even larger during subsequent recovery, i.e. severe suppression of growth and carbohydrate accumulation by de-submergence, not by de-shading. The results thus negate the third hypothesis and suggest that the effects of integration are less important for submerged ramets compared with shaded ramets shortly after the treatment. Evidence that the effects of integration were decreased by stronger abiotic stress remains limited (but see Bullock et al., 1994; Li et al., 2011). Photosynthates, water and mineral nutrients are transported between ramets via phloem or xylem, and internal O2 diffusion is effected by transport via gas spaces along stolon internodes. Thus, lipid peroxidation of submerged organs and stolon wilting of de-submerged plants, which may happen shortly after de-submergence, may strongly impair translocation between ramets (Blokhina et al., 2003; Holbrook and Zwieniecki, 2003; Colmer and Voesenek, 2009; Striker, 2012), but these phenomena were not observed in the shaded/de-shaded plants.
Conclusions
In conclusion, the shift from significant effects of physiological integration on both submerged and un-submerged ramets during submergence to little effect of integration during de-submergence might be due to quick recovery of growth and photosynthesis. The effects of integration were not stronger under submergence/de-submergence than under shading/de-shading. Therefore, not only the beneficial effects of physiological integration but also rapid de-submergence recovery of growth and photosynthesis are crucial in enabling amphibious clonal plants to survive submergence and spread rapidly after de-submergence. More detailed studies on photosynthate translocation and allocation in submerged (shaded) and un-submerged (un-shaded) ramets and internal O2 transport along stolons are warranted.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Qian Zhang, Chun-Xiang Liu, Tan-Feng Yin and Bi-Cheng Dong for their assistance during plant cultivation and harvesting and Professor Tim Colmer and two anonymous referees for providing valuable comments. This research was supported by the Fundamental Research Funds for the Central Universities (grant TD-JC-2013-1), the Program for New Century Excellent Talents in University (grant NECT-10-0234), and the National Natural Science Foundation of China (grant 31200314).
LITERATURE CITED
- Alpert P. Nutrient sharing in natural clonal fragments of Fragaria chiloensis. Journal of Ecology. 1996;84:395–406. [Google Scholar]
- Alpert P. Clonal integration in Fragaria chiloensis differs between populations: ramets from grassland are selfish. Oecologia. 1999;120:69–76. doi: 10.1007/s004420050834. [DOI] [PubMed] [Google Scholar]
- Alpert P, Holzapfel C, Slominski C. Differences in performance between genotypes of Fragaria chiloensis with different degrees of resource sharing. Journal of Ecology. 2003;91:27–35. [Google Scholar]
- Amsberry L, Baker MA, Ewanchuk PJ, Bertness MD. Clonal integration and the expansion of Phragmites australis. Ecological Applications. 2000;10:1110–1118. [Google Scholar]
- Armstrong J, Armstrong W. Record rates of pressurized gas-flow in the great horsetail, Equisetum telmateia. Were Carboniferous calamites similarly aerated? New Phytologist. 2009;184:202–215. doi: 10.1111/j.1469-8137.2009.02907.x. [DOI] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bodegom PM, Sorrell BK, Oosthoek A, Bakker C, Aerts R. Separating the effects of partial submergence and soil oxygen demand on plant physiology. Ecology. 2008;89:193–204. doi: 10.1890/07-0390.1. [DOI] [PubMed] [Google Scholar]
- Bullock JM, Mortimer AM, Begon M. Physiological integration among tillers of Holcus lanatus: age-dependence and responses to clipping and competition. New Phytologist. 1994;128:737–747. [Google Scholar]
- Chen X, Visser EJW, de Kroon H, Pierik R, Voesenek LACJ, Huber H. Fitness consequences of natural variation in flooding-induced shoot elongation in Rumex palustris. New Phytologist. 2011;190:409–420. doi: 10.1111/j.1469-8137.2010.03639.x. [DOI] [PubMed] [Google Scholar]
- Colmer TD. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment. 2003;26:17–36. [Google Scholar]
- Colmer TD, Pedersen O. Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytologist. 2008;177:918–926. doi: 10.1111/j.1469-8137.2007.02318.x. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Voesenek L. Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology. 2009;36:665–681. doi: 10.1071/FP09144. [DOI] [PubMed] [Google Scholar]
- Friedman D, Alpert P. Reciprocal transport between ramets increases growth of Fragaria chiloensis when light and nitrogen occur in separate patches but only if patches are rich. Oecologia. 1991;86:76–80. doi: 10.1007/BF00317392. [DOI] [PubMed] [Google Scholar]
- Fukao T, Xu KN, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell. 2006;18:2021–2034. doi: 10.1105/tpc.106.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng YP, Pan XY, Xu CY, Zhang WJ, Li B, Chen JK. Plasticity and ontogenetic drift of biomass allocation in response to above- and below-ground resource availabilities in perennial herbs: a case study of Alternanthera philoxeroides. Ecological Research. 2007;22:255–260. [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–1031. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- Holbrook NM, Zwieniecki MA. Plant biology: water gate. Nature. 2003;425:361–361. doi: 10.1038/425361a. [DOI] [PubMed] [Google Scholar]
- Huber H, Hutchings MJ. Differential response to shading in orthotropic and plagiotropic shoots of the clonal herb Glechoma hirsuta. Oecologia. 1997;112:485–491. doi: 10.1007/s004420050336. [DOI] [PubMed] [Google Scholar]
- Janecek S, Janeckova P, Leps J. Influence of soil heterogeneity and competition on growth features of three meadow species. Flora. 2004;199:3–11. [Google Scholar]
- Janecek S, Kantorova J, Bartos M, Klimesova J. Integration in the clonal plant Eriophorum angustifolium: an experiment with a three-member-clonal system in a patchy environment. Evolutionary Ecology. 2008;22:325–336. [Google Scholar]
- van Kleunen M, Stuefer JF. Quantifying the effects of reciprocal assimilate and water translocation in a clonal plant by the use of steam-girdling. Oikos. 1999;85:135–145. [Google Scholar]
- van Kleunen M, Fischer M, Schmid B. Clonal integration in Ranunculus reptans: by-product or adaptation? Journal of Evolutionary Biology. 2000;13:237–248. [Google Scholar]
- Klimes L, Klimesova J. The effects of mowing and fertilization on carbohydrate reserves and regrowth of grasses: do they promote plant coexistence in species-rich meadows? Evolutionary Ecology. 2001;15:363–382. [Google Scholar]
- de Kroon H, Fransen B, van Rheenen JWA, van Dijk A, Kreulen R. High levels of inter-ramet water translocation in two rhizomatous Carex species, as quantified by deuterium labelling. Oecologia. 1996;106:73–84. doi: 10.1007/BF00334409. [DOI] [PubMed] [Google Scholar]
- de Kroon H, Huber H, Stuefer JF, van Groenendael JM. A modular concept of phenotypic plasticity in plants. New Phytologist. 2005;166:73–82. doi: 10.1111/j.1469-8137.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- de Kroon H, van der Zalm E, van Rheenen JWA, van Dijk A, Kreulen R. The interaction between water and nitrogen translocation in a rhizomatous sedge (Carex flacca) Oecologia. 1998;116:38–49. doi: 10.1007/s004420050561. [DOI] [PubMed] [Google Scholar]
- Li JJ, Peng PH, He WM. Physical connection decreases benefits of clonal integration in Alternanthera philoxeroides under three warming scenarios. Plant Biology. 2011;14:265–270. doi: 10.1111/j.1438-8677.2011.00500.x. [DOI] [PubMed] [Google Scholar]
- Liu H-D, Yu F-H, He W-M, Chu Y, Dong M. Clonal integration improves compensatory growth in heavily grazed ramet populations of two inland-dune grasses. Flora. 2009;204:298–305. [Google Scholar]
- Lopez OR, Kursar TA. Interannual variation in rainfall, drought stress and seedling mortality may mediate monodominance in tropical flooded forests. Oecologia. 2007;154:35–43. doi: 10.1007/s00442-007-0821-0. [DOI] [PubMed] [Google Scholar]
- Luo F-L, Nagel KA, Scharr H, Zeng B, Schurr U, Matsubara S. Recovery dynamics of growth, photosynthesis and carbohydrate accumulation after de-submergence: a comparison between two wetland plants showing escape and quiescence strategies. Annals of Botany. 2011;107:49–63. doi: 10.1093/aob/mcq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F-L, Thiele B, Janzik I, Zeng B, Schurr U, Matsubara S. De-submergence responses of antioxidative defense systems in two wetland plants having escape and quiescence strategies. Journal of Plant Physiology. 2012;169:1680–1689. doi: 10.1016/j.jplph.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Mommer L, Pedersen O, Visser EJW. Acclimation of a terrestrial plant to submergence facilitates gas exchange under water. Plant, Cell & Environment. 2004;27:1281–1287. [Google Scholar]
- Morris DL. Quantitative determination of carbohydrates with Dreywood's anthrone reagent. Science. 1948;107:254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Olano JM, Menges ES, Martinez E. Carbohydrate storage in five resprouting Florida scrub plants across a fire chronosequence. New Phytologist. 2006;170:99–105. doi: 10.1111/j.1469-8137.2005.01634.x. [DOI] [PubMed] [Google Scholar]
- Osmond CB. What is photoinhibition? Some insights from comparisons of shade and sun plants. In: Baker NR, Bowyer JR, editors. Photoinhibition of photosynthesis—from molecular mechanisms to the field. Oxford: BIOS Scientific Publishers; 1994. pp. 1–24. [Google Scholar]
- Panda D, Sharma SG, Sarkar RK. Chlorophyll fluorescence parameters, CO2 photosynthetic rate and regeneration capacity as a result of complete submergence and subsequent re-emergence in rice (Oryza sativa L.) Aquatic Botany. 2008;88:127–133. [Google Scholar]
- Pennings SC, Callaway RM. The advantages of clonal integration under different ecological conditions: a community-wide test. Ecology. 2000;81:709–716. [Google Scholar]
- Potvin C, Lechowicz MJ, Tardif S. The statistical analysis of ecophysiological response curves obtained from experiments involving repeated measures. Ecology. 1990;71:1389–1400. [Google Scholar]
- Qin XY, Li F, Chen XS, Xie YH. Growth responses and non-structural carbohydrates in three wetland macrophyte species following submergence and de-submergence. Acta Physiologiae Plantarum. 2013;35:2069–2074. [Google Scholar]
- Roiloa SR, Retuerto R. Physiological integration ameliorates effects of serpentine soils in the clonal herb Fragaria vesca. Physiologia Plantarum. 2006;128:662–676. [Google Scholar]
- Roiloa SR, Alpert P, Tharayil N, Hancock G, Bhowmik PC. Greater capacity for division of labour in clones of Fragaria chiloensis from patchier habitats. Journal of Ecology. 2007;95:397–405. [Google Scholar]
- Roiloa SR, Rodríguez-Echeverría S, Freitas H, Retuerto R. Developmentally-programmed division of labour in the clonal invader Carpobrotus edulis. Biological Invasions. 2013;15:1895–1905. [Google Scholar]
- Sarkar RK, Das S, Ravi I. Changes in certain antioxidative enzymes and growth parameters as a result of complete submergence and subsequent re-aeration of rice cultivars differing in submergence tolerance. Journal of Agronomy and Crop Science. 2001;187:69–74. [Google Scholar]
- Sarkar RK, Reddy JN, Sharma SG, Ismail AM. Physiological basis of submergence tolerance in rice and implications for crop improvement. Current Science. 2006;91:899–906. [Google Scholar]
- Song YB, Yu FH, Keser LH, et al. United we stand, divided we fall: a meta-analysis of experiments on clonal integration and its relationship to invasiveness. Oecologia. 2013;171:317–327. doi: 10.1007/s00442-012-2430-9. [DOI] [PubMed] [Google Scholar]
- Striker GG. Time is on our side: the importance of considering a recovery period when assessing flooding tolerance in plants. Ecological Research. 2012;27:983–987. [Google Scholar]
- Stuefer JF, During HJ, de Kroon H. High benefits of clonal integration in two stoloniferous species in response to heterogeneous light environments. Journal of Ecology. 1994;82:511–518. [Google Scholar]
- Voesenek LACJ, Bailey-Serres J. Flooding tolerance: O2 sensing and survival strategies. Current Opinion in Plant Biology. 2013;16:647–653. doi: 10.1016/j.pbi.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrometers of different resolution. Journal of Plant Physiology. 1994;144:307–313. [Google Scholar]
- Xiao Y, Tang JB, Qing H, et al. Clonal integration enhances flood tolerance of Spartina alterniflora daughter ramets. Aquatic Botany. 2010;92:9–13. [Google Scholar]
- Xu CY, Zhang WJ, Fu CZ, Lu BU. Genetic diversity of alligator weed in China by RAPD analysis. Biodiversity and Conservation. 2003;12:637–645. [Google Scholar]
- Yang J, Tam N-Y, Ye Z. Root porosity, radial oxygen loss and iron plaque on roots of wetland plants in relation to zinc tolerance and accumulation. Plant and Soil. 2014;374:815–828. [Google Scholar]
- Yu XQ, Luo N, Yan JP, Tang JC, Liu SW, Jiang YW. Differential growth response and carbohydrate metabolism of global collection of perennial ryegrass accessions to submergence and recovery following de-submergence. Journal of Plant Physiology. 2012;169:1040–1049. doi: 10.1016/j.jplph.2012.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.