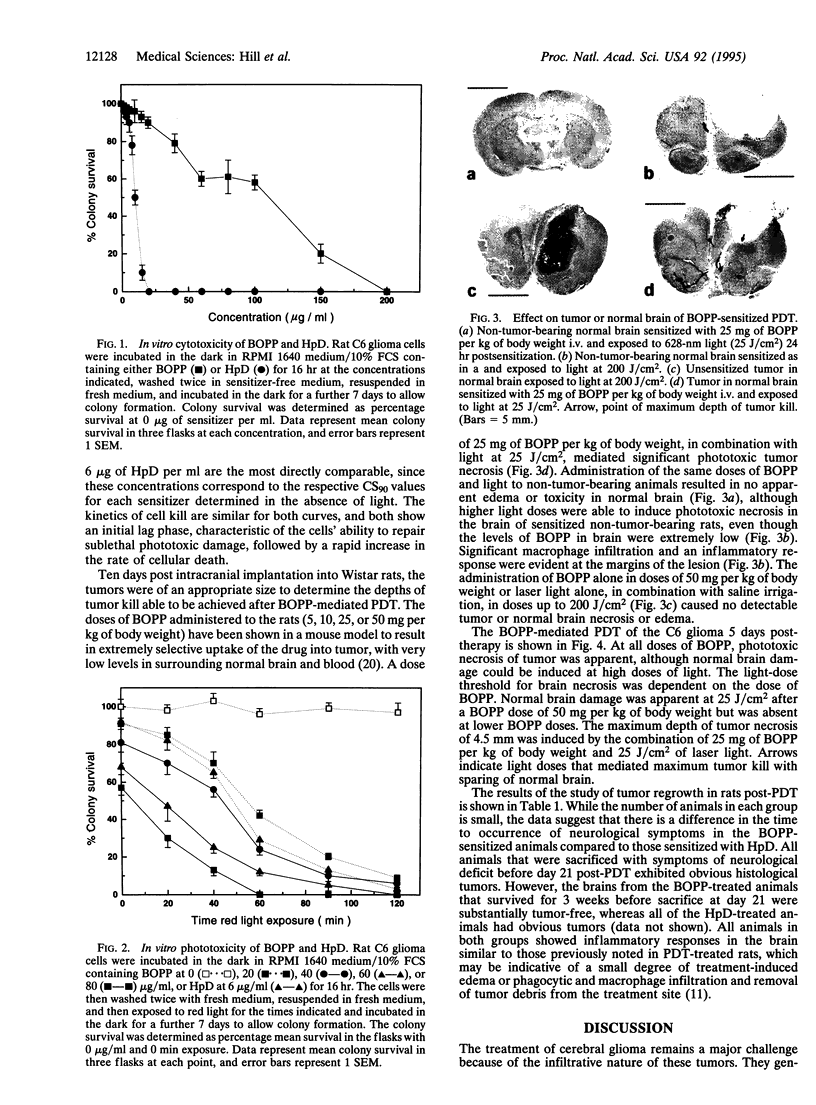
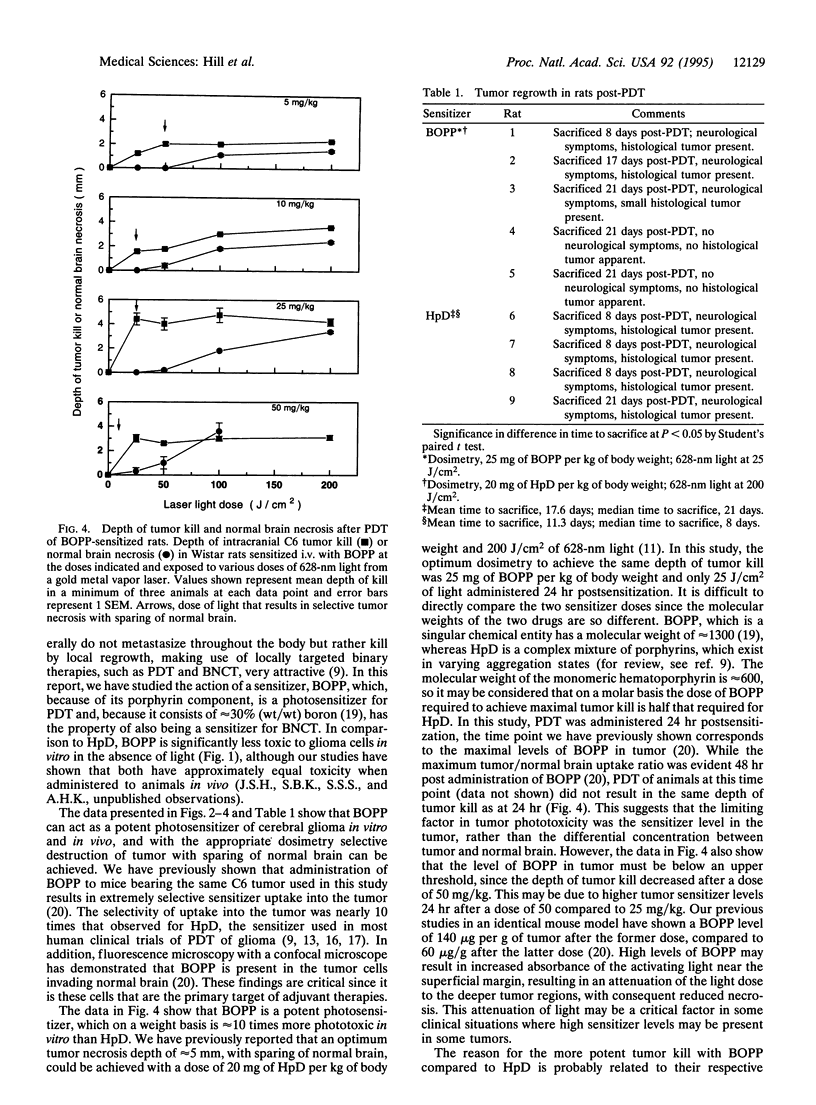
Abstract
The prognosis for patients with the high-grade cerebral glioma glioblastoma multiforme is poor. The median survival for primary tumors is < 12 months, with most recurring at the site of the original tumor, indicating that a more aggressive local therapy is required to eradicate the unresectable "nests" of tumor cells invading into adjacent brain. Two adjuvant therapies with the potential to destroy these cells are porphyrin-sensitized photodynamic therapy (PDT) and boron-sensitized boron neutron capture therapy (BNCT). The ability of a boronated porphyrin, 2,4-(alpha, beta-dihydroxyethyl) deuteroporphyrin IX tetrakiscarborane carboxylate ester (BOPP), to act as a photosensitizing agent was investigated in vitro with the C6 rat glioma cell line and in vivo with C6 cells grown as an intracerebral tumor after implantation into Wistar rats. These studies determined the doses of BOPP and light required to achieve maximal cell kill in vitro and selective tumor kill in vivo. The data show that BOPP is more dose effective in vivo by a factor of 10 than the current clinically used photosensitizer hematoporphyrin derivative and suggest that BOPP may have potential as a dual PDT/BNCT sensitizer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barba D., Saris S. C., Holder C., Rosenberg S. A., Oldfield E. H. Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J Neurosurg. 1989 Feb;70(2):175–182. doi: 10.3171/jns.1989.70.2.0175. [DOI] [PubMed] [Google Scholar]
- Boggan J. E., Bolger C., Edwards M. S. Effect of hematoporphyrin derivative photoradiation therapy on survival in the rat 9L gliosarcoma brain-tumor model. J Neurosurg. 1985 Dec;63(6):917–921. doi: 10.3171/jns.1985.63.6.0917. [DOI] [PubMed] [Google Scholar]
- Gabel D., Foster S., Fairchild R. G. The Monte Carlo simulation of the biological effect of the 10B(n, alpha)7Li reaction in cells and tissue and its implication for boron neutron capture therapy. Radiat Res. 1987 Jul;111(1):14–25. [PubMed] [Google Scholar]
- Gumerlock M. K., Belshe B. D., Madsen R., Watts C. Osmotic blood-brain barrier disruption and chemotherapy in the treatment of high grade malignant glioma: patient series and literature review. J Neurooncol. 1992 Jan;12(1):33–46. doi: 10.1007/BF00172455. [DOI] [PubMed] [Google Scholar]
- Gutin P. H., Leibel S. A., Wara W. M., Choucair A., Levin V. A., Philips T. L., Silver P., Da Silva V., Edwards M. S., Davis R. L. Recurrent malignant gliomas: survival following interstitial brachytherapy with high-activity iodine-125 sources. J Neurosurg. 1987 Dec;67(6):864–873. doi: 10.3171/jns.1987.67.6.0864. [DOI] [PubMed] [Google Scholar]
- Hanzlik C. A., Knox R. S., Gibson S. L., Hilf R. Picosecond fluorescence of R3230AC mammary carcinoma mitochondria after treatment with hematoporphyrin derivative and Photofrin II in vivo. Photochem Photobiol. 1989 Jul;50(1):45–53. doi: 10.1111/j.1751-1097.1989.tb04128.x. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Dougherty T. J. How does photodynamic therapy work? Photochem Photobiol. 1992 Jan;55(1):145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- Hill J. S., Kahl S. B., Kaye A. H., Stylli S. S., Koo M. S., Gonzales M. F., Vardaxis N. J., Johnson C. I. Selective tumor uptake of a boronated porphyrin in an animal model of cerebral glioma. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1785–1789. doi: 10.1073/pnas.89.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. S., Kaye A. H., Sawyer W. H., Morstyn G., Megison P. D., Stylli S. S. Selective uptake of hematoporphyrin derivative into human cerebral glioma. Neurosurgery. 1990 Feb;26(2):248–254. doi: 10.1097/00006123-199002000-00011. [DOI] [PubMed] [Google Scholar]
- Kaye A. H., Morstyn G., Apuzzo M. L. Photoradiation therapy and its potential in the management of neurological tumors. J Neurosurg. 1988 Jul;69(1):1–14. doi: 10.3171/jns.1988.69.1.0001. [DOI] [PubMed] [Google Scholar]
- Kaye A. H., Morstyn G., Ashcroft R. G. Uptake and retention of hematoporphyrin derivative in an in vivo/in vitro model of cerebral glioma. Neurosurgery. 1985 Dec;17(6):883–890. doi: 10.1227/00006123-198512000-00002. [DOI] [PubMed] [Google Scholar]
- Kaye A. H., Morstyn G. Photoradiation therapy causing selective tumor kill in a rat glioma model. Neurosurgery. 1987 Mar;20(3):408–415. doi: 10.1227/00006123-198703000-00009. [DOI] [PubMed] [Google Scholar]
- Laws E. R., Jr, Cortese D. A., Kinsey J. H., Eagan R. T., Anderson R. E. Photoradiation therapy in the treatment of malignant brain tumors: a phase I (feasibility) study. Neurosurgery. 1981 Dec;9(6):672–678. doi: 10.1227/00006123-198112000-00010. [DOI] [PubMed] [Google Scholar]
- Muller P. J., Wilson B. C. Photodynamic therapy of malignant brain tumours. Can J Neurol Sci. 1990 May;17(2):193–198. doi: 10.1017/s0317167100030444. [DOI] [PubMed] [Google Scholar]
- Oldfield E. H., Ram Z., Culver K. W., Blaese R. M., DeVroom H. L., Anderson W. F. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther. 1993 Feb;4(1):39–69. doi: 10.1089/hum.1993.4.1-39. [DOI] [PubMed] [Google Scholar]
- Powers S. K., Beckman W. C., Jr, Brown J. T., Kolpack L. C. Interstitial laser photochemotherapy of rhodamine-123-sensitized rat glioma. J Neurosurg. 1987 Dec;67(6):889–894. doi: 10.3171/jns.1987.67.6.0889. [DOI] [PubMed] [Google Scholar]
- Sandeman D. R., Bradford R., Buxton P., Bown S. G., Thomas D. G. Selective necrosis of malignant gliomas in mice using photodynamic therapy. Br J Cancer. 1987 Jun;55(6):647–649. doi: 10.1038/bjc.1987.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. D., Alexander E., Jr, Hunt W. E., MacCarty C. S., Mahaley M. S., Jr, Mealey J., Jr, Norrell H. A., Owens G., Ransohoff J., Wilson C. B. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978 Sep;49(3):333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Green S. B., Byar D. P., Alexander E., Jr, Batzdorf U., Brooks W. H., Hunt W. E., MacCarty C. S., Mahaley M. S., Jr, Mealey J., Jr Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980 Dec 4;303(23):1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- Wilson C. B. Current concepts in cancer: brain tumors. N Engl J Med. 1979 Jun 28;300(26):1469–1471. doi: 10.1056/NEJM197906283002605. [DOI] [PubMed] [Google Scholar]
- Woodburn K. W., Vardaxis N. J., Hill J. S., Kaye A. H., Phillips D. R. Subcellular localization of porphyrins using confocal laser scanning microscopy. Photochem Photobiol. 1991 Nov;54(5):725–732. doi: 10.1111/j.1751-1097.1991.tb02081.x. [DOI] [PubMed] [Google Scholar]