Abstract
Sensory neural hearing loss and vestibular dysfunction have become the most common forms of sensory defects, affecting millions of people worldwide. Developing effective therapies to restore hearing loss is challenging, owing to the limited regenerative capacity of the inner ear hair cells. With recent advances in understanding the developmental biology of mammalian and non-mammalian hair cells a variety of strategies have emerged to restore lost hair cells are being developed. Two predominant strategies have developed to restore hair cells: transfer of genes responsible for hair cell genesis and replacement of missing cells via transfer of stem cells. In this review article, we evaluate the use of several genes involved in hair cell regeneration, the advantages and disadvantages of the different viral vectors employed in inner ear gene delivery and the insights gained from the use of embryonic, adult and induced pluripotent stem cells in generating inner ear hair cells. Understanding the role of genes, vectors and stem cells in therapeutic strategies led us to explore potential solutions to overcome the limitations associated with their use in hair cell regeneration.
Keywords: sensory neural hearing loss, vestibular dysfunction, hair cell regeneration, viral vectors, gene delivery, stem cells
1. Introduction
Hearing loss has become one of the most common disabilities in the United States and can affect almost every age group. The number of people with hearing loss worldwide has been steadily increasing over recent years, reaching almost 49 million people in the US alone [1]. According to the National Institute on Deafness and Other Communication Disorders (NIDCD) 2010 statistics, approximately 17% of the American adult population experiences hearing loss and 3 out of every 1000 children are born deaf. The prevalence of hearing loss increases with age, as about 47% of adults over 75 years old have hearing impairment (NIDCD). Although hearing loss may not be life threatening, it can greatly influence the patient's quality of life, social interactions, and have a significant financial impact on society [1,2].
The ear is of tremendous importance in sensing the world around us. Aside from being the prime organ for the perception of sound, it also plays a crucial role in balance. The inner ear is a highly specialized sense organ with a complex structure and has been referred to as a labyrinth [3]. It contains hair cells arranged in a highly organized pattern and is innervated by sensory neurons. Acoustic energy, in the form of sound waves, is channeled into the ear canal where it strikes the tympanic membrane. As the energy hits the stapes located at the oval window, a pressure wave sets the cochlear fluid into motion. The hair bundles deflect as a result of the shearing force caused by the cochlear fluid and the stereocilia slide along one another. The movement on the stereocilia results in their depolarization and opens up gated calcium ion channels. This is followed by the release of neurotransmitters from the base of the hair cell. Primary auditory afferents then conduct the signal to the brainstem.
Sensorineural hearing loss (SNHL) involves damage to the cochlea (inner ear sensory hair cells) or the eighth nerve. It is irreversible and in most cases a hearing aid is required. Common causes for SNHL are aging, ototoxic drugs, noise induced trauma, inner ear concussion, and immune disorders [4,5,6]. Although only a small percentage of the cases can be treated medically and surgically, advances in molecular and stem cell therapies may provide tools to treat the irreparable damage of hair cells caused by SNHL [7].
Hair cells are sensory receptors located in the inner ear. They appear to be “hair-like” because of the numerous ciliary processes called stereocilia that extend from their surfaces. Hair cells are responsible for converting sound into electrical signals that are sent to the brain via the auditory nerve for processing. In the human cochlea, hair cell death can occur due to a variety of causes, such as age related deafness (presbycusis), a high dosage of ototoxic drugs (e.g., gentamycin, cisplatin, aminoglycosides), genetic disorders, infectious diseases, or high levels of noise exposure [3,8,9]. Patients exposed to high doses of aminoglycosides as a treatment regimen for bacterial infections often experience hair cell death. The aminoglycosides are known to induce cell death via activation of the intracellular caspase signaling pathway and trigger mitochondria to release cytochrome c into the cytoplasm, which consequently induces apoptosis via caspase-3 activation in hair cells. Noise induced cellular stress activates the JNK signaling pathway and causes neuronal cell death via necrosis. Necrotic hair cell death is less common than apoptotic cell death and is mainly induced by trauma or disease [3,9,10].
Over the past 30 years, several attempts have been made to understand the pathways involved in hair cell formation and death [11]. The process of hair cell regeneration was considered impossible to occur in higher vertebrates until two groups serendipitously discovered the amazing phenomenon in birds in the late 1980s [12]. At the same time, Jørgensen and Mathiesen showed that there were mitotic activity and continuous proliferation of hair cells in the vestibular epithelium of adult parakeets [13]. Ever since, there has been steady progress in understanding the mechanics and pathways of avian and mammalian (rat, mouse, guinea pig) hair cell regeneration [8,10,14,15,16,17,18,19,20,21]. Significant development in hair cell regeneration is outlined in the following reviews [7,22,23,24,25,26,27,28,29]. In this review, we explore how different genes, viral vectors and stem cell sources can be used in conjunction with each other to engineer inner ear hair cells and develop strategies in restoring the function of the inner ear.
2. Hair Cells, Supporting Cells and Their Function
2.1. Hair Cells
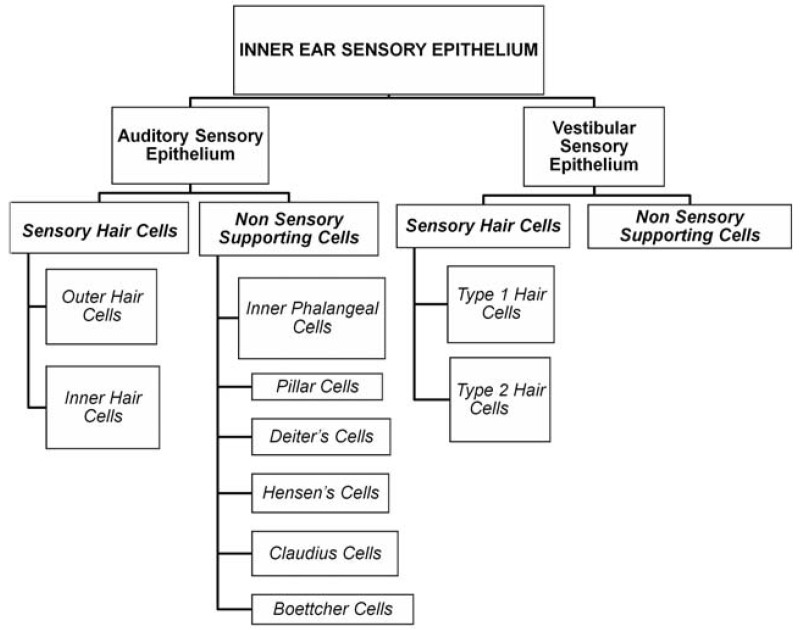
Hair cells are the mechanoreceptors in the inner ear that detect sound, head movements and orientation in space [9,30]. They are formed in the embryonic stages during the development of the inner ear. The otic ectoderm undergoes proliferation and gives rise to the sensory primordium. The proliferating sensory primordium precursors form the organ of corti from within the cochlear epithelium and patterns into an organized array of inner and outer hair cells separated from each other by non-sensory supporting cells. Hair cells are present in the sensory epithelium of both the auditory (inner and outer hair cells) and vestibular systems (Type 1 and Type 2 hair cells). All hair cells are surrounded by supporting cells and have physiological and morphological differences. Figure 1 shows the classification of inner ear sensory epithelium. Sensory epithelium in the inner ear is composed of both sensory and non-sensory cell types that have morphological and physiological differences. Table 1 gives the differences between inner and outer hair cells.
Figure 1.
Classification of inner ear sensory epithelium.
Table 1.
Differences between inner and outer hair cells.
| Factor | Inner hair cells | Outer hair cells | Ref. |
|---|---|---|---|
| Arrangement | Arranged in a single row | Arranged in three parallel rows | [31,32,33] |
| Shape | Round and small | Long and slim | [10] |
| Function | Transduce mechanical energy to neural signals | Appear to impact and regulate the sensitivity of the cochlea over a range of 32 dB | [10] |
| Effects | Sensory Neural Hearing loss | Alter properties of cochlear input to the brain | [34] |
| Approximate number | 3,000 to 3,500 | 9,000 to 12,000 | [32,33] |
2.1.1. Hair Cell Structure
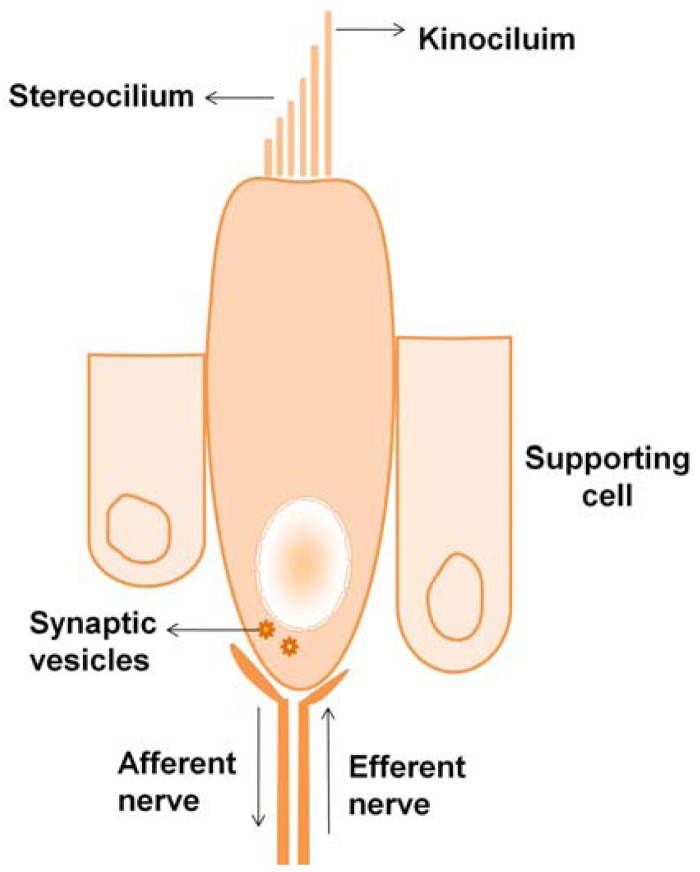
Hair cells are flask-shaped cells with extended processes at their apical ends called stereocilia. The stereocilia are made of actin filaments and actin bundling proteins—fimbrin and espin [35]. The vestibular system hair cells have one true cilium called the kinocilium that is thought to mark the polarity of the cell. Figure 2 shows the schematic representation of a type 2 vestibular hair cell, showing the flask-shaped hair cell supported by supporting cells at the base. The stereocilia at the top are connected by filamentous tip links that participate in transduction and ion exchange.
Figure 2.
Structure of a typical vestibular hair cell.
The kinocilium of cochlear hair cells in mammals degenerates a few weeks after birth [36,37,38,39,40]. Hair cells form synaptic connections from neurons at the basal end and terminate in the cochlear or vestibular nuclei of the brainstem. The term “mechanoreceptors” comes from their participation in transforming mechanical energy to electrical energy. In the auditory system, the mechanical energy occurs in the form of a wave and the hair cells are stimulated as the tectorial membrane shears against the organ of corti, whereas in the vestibular system, the mechanical energy is a result of displacement of hair cells due to the force of gravity or inertia.
2.1.2. Transduction in Hair Cells
The stereocilia are connected together by a filamentous tip link. The tips are connected to cation transduction channels that are involved in calcium and potassium ion exchange. Each hair cell has about 100 transduction channels; the hair cell's mechanical movement and ion exchange control the cell's membrane potential and provide the driving force for auditory or vestibular nerve excitation. Myosin motor proteins are activated by the calcium ions and play an important role in triggering the hair cell's adaptation to mechanical stimuli from stereocilia deflections [11].
2.2. Supporting Cells
Supporting cells are the non-sensory cells of the sensory epithelium and do not take part in sound transduction. They are located at the base of the hair cells and surround them, preventing contact between individual hair cells. The mammalian auditory system has two main types of supporting cells: the Deiter's cells support the outer hair cells at the base, and the pillar cells help in forming the reticular lamina, which isolates the stereocilia from their cell bodies. A few other supporting cells types include innerphalangeal cells, Hensen's cells, Claudius cells and Boettcher cells that are specialized cells and are not associated with hair cells [41]. Supporting cells of the vestibular system have yet to be studied in detail to understand their physiological and morphological differences [24,30]. Supporting cells are known to play an important role in avian hair cell regeneration by two different mechanisms: mitotic regeneration and transdifferentiation [42]. The former occurs when the supporting cell divides mitotically, stimulating one of them to differentiate into a hair cell, whereas the latter occurs when a supporting cell changes its gene expression and becomes a hair cell directly without dividing. Healthy supporting cell populations are vital for a number of hair cell regeneration strategies that rely on transdifferentiation to restore the damaged neuroeptithelium.
2.3. Summary
Hair cells reside in the auditory and vestibular systems and are responsible for mechanoelectrical transduction of sound. Ototoxic drugs or noise induced stress can damage hair cells, thus compromising inner ear function. Supporting cells located at the base of the hair cells have the capability to regenerate hair cells in the vestibular system by mitotic division or transdifferentiation, though supporting cell proliferation may not always occur to replace lost hair cells.
3. Essential Genes in Hair Cell Differentiation
It is important to identify and characterize genes that govern the ontogeny and differentiation of the cochlear epithelium; identifying such genes can lead to the design of several therapeutic approaches for sensory epithelial cell development and hair cell differentiation. A critical gene responsible for inner ear development is the Atonal gene—a protein belonging to the basic helix-loop-helix (bHLH) family of transcription factors that activates the E-box dependent transcription. Atoh1 has a unique auto regulatory enhancer element containing an E-box in the 3′ region of the gene [43]. Math1, also known as Atoh1, is the mouse homolog of the Drosophila melanogaster Atonal gene. The Math1 gene is essential for the differentiation of sensory hair cells from previously established sensory primordium and is limited to only a subpopulation of the non-sensory supporting cells, primarily the pillar cells [44,45]. Studies with embryonic Math1-null mice reported a failure to produce hair cells and deletion of Atoh1 using Pax2-Cre resulted in degeneration of cells in the organ of corti in mice [46], proving Math1 as a positive regulator in directing hair cell differentiation [47]. Gene delivery studies in guinea pigs, mice, and rats reported an over expression of Math1 in non-sensory cells, resulting in the production of ectopic immature hair cells outside the sensory epithelium via the transdifferentiation mechanism [16,44,48,49,50,51,52]. The non-sensory Math1 expressing cells attracted auditory nerve fibers and developed into mature hair cells [49,50].
The other homologues of the Atonal gene are Cath1 (chicken atonal homolog), Xath1 (Xenopus atonal homolog) and Hath1 (human atonal homolog), although Math1 is the most extensively studied and used transcription factor [53,54]. Studies with adenoviral expression of Hath1 in rats showed hair cell production without supporting cell proliferation [55].
Additional genes involved in the control of supporting cell fate include Hes1, Hes5, BETA2/Neurod1, Jagged2 and Notch Signaling [18,19]. Hes1 and Hes5 have been shown to influence supporting cell fate through negative regulation of Math1 [56,57]. Certain cell cycle kinases also influence inner ear development by regulating cell cycle and inhibiting hair cell differentiation (Refer Table 2). BETA2/Neurod1 gene has been shown to regulate the formation of sensory and neuronal ganglions in both cochlear and vestibular systems [58]. Table 2 gives a list of the different genes involved in hair cell differentiation.
Table 2.
Summary of different genes used in inner ear gene therapy.
| Gene | Role | Reference |
|---|---|---|
| Math1 | Also known as Atoh1. Primary gene responsible for hair cell differentiation. Other homologues include Hath1, Cath1 and Xath1. | [47,49,53,54,55] |
| Hes1 and Hes5 | Mammalian homologues of Hairy and Enhancer-of-split gene. Expressed in supporting cells and known to be negative regulators of Math1. However, a balance between Hes1/Hes5 is required to control the production of supernumerary hair cells and normal development of inner ear. | [56,57] |
| Sox2 | Responsible for development of inner ear sensory epithelium and is expressed in supporting cells and inner ear progenitors. Acts upstream of Math1 and maintains mitotic and transdifferentiation functions of supporting cells. | [59,60,61] |
| Jag2 | Member of the notch signaling pathway. Expressed in supporting cells of auditory and vestibular system. Required for the normal development of inner ear sensory organs. | [19,61,62] |
| BETA2/Neurod1 | Expressed in neurons and neural precursor cells. Promotes the formation of ganglion neurons in the cochlea. Absence of BETA2/NeuroD can compromise hair cell function. It is known to differentiate outer hair cells to inner ear hair cells and neurons to hair cells. | [58,63] |
| Rb1/Rbl2 | Required for hair cell quiescence and cell-cycle exit of embryonic mammalian hair cells but not for their early differentiation. Deletion of Rb1 from progenitor cells leads to aberrant hair cell and supporting cell. Deletion of Rbl2 results in extra row of hair cells and supporting cells in apical regions of the cochlea. | [64,65,66,67,68] |
| Cdkn1b and Cdkn2d | Cyclin-dependent kinase inhibitor. Expressed in sensory progenitors during the early embryonic development of the cochlea. Regulates cell cycle and inhibits hair cell differentiation. | [69,70,71] |
| MYCN | Member of the Myc family that regulates proliferation. Regulates the growth of the ear as a whole and promotes differentiation of certain sensory and non-sensory components. Absence of MYCN can lead to abnormal development of inner ear organs and disorganized neuronal innervations. | [72] |
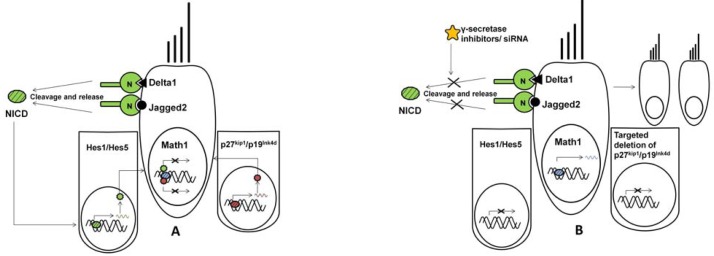
Figure 3 represents a schematic on the interaction of different genes and their contribution to positive and negative regulation of Math1 transcription factor in neonates and during the embryonic development of the cochlea. (A) Hair cells express hair cell-specific Math1 transcription factor and notch ligands—Delta1 and Jagged2. Notch receptor (N) binds to the ligands, cleaves with the help of γ-secretase and releases Notch Intracellular Domain (NICD). NICD enters the nucleus of supporting cells and activated Hes1/Hes5 transcription factors. Hes1/Hes5 proteins inhibit Math1 gene expression. Alternatively, expression of Cdkn1b (p27kip1) and Cdkn2d (p19Ink4d) in early progenitor supporting cells repress Math1 expression and maintain supporting cell fate. (B) In the presence of γ-secretase inhibitors, the notch receptor fails to cleave and release the NICD, thus inhibiting the activation of Hes1/Hes5 that would otherwise down regulate Math1 expression. Similarly, targeted deletion of p27kip1 and p19Ink4d genes allows ectopic expression of Math1 resulting in supernumerary hair cells. These pathways can be induced or inhibited via standard or molecular therapy and additionally can be used to control the differentiation of stem cells.
Figure 3.
Schematic on the interaction of different genes and their contribution to positive and negative regulation of Math1 transcription factor.
4. Gene Therapy and Stem Cell-Based Approaches for Treatment of Sensory Neural Hearing Loss
Current therapies for treating hearing loss involve the use of either hearing aids or cochlear implants. Cochlear implants are only available to patients with severe hair cell damage and profound loss of hearing ability. However, the implants are not absolutely efficient in restoring hearing; their performance varies from patient to patient and requires training to adapt to the device. With advances in regenerative medicine using stem cells and gene therapy, several new strategies have emerged with the hope of permanently curing deafness. Some of these strategies are discussed in the following paragraphs.
4.1. Gene Therapy in the Inner Ear
A key to modifying cell phenotype is developing an effective means of delivering genes to the inner ear. As discussed earlier, the Math1 gene is an essential gene in the differentiation of hair cells. Multiple approaches to deliver the Math1 gene into the inner ear have been evaluated. Most of the approaches involve the injection of viral or non-viral vectors into the inner ear canal to trigger endogenous cells in the organ of corti to differentiate. There are a number of other popular routes of vector administration for inner ear gene therapy that are well explained in the literature [73,74,75,76].
An ideal vector is one that would ensure patient safety and effective transformation of undifferentiated cells. The commonly used vectors are derived from adenovirus, adeno-associated virus (AAV), herpes virus and lentivirus. Adenoviral mediated Math1 and Hath1 gene delivery has shown promising results in vivo for regenerating inner ear hair cells in mammals [16,48,49,51,77]. Adenoviral vectors offer high transfection efficiency and have been extensively investigated in clinical trials for ocular disease and cystic fibrosis [78,79]. The prior experience with adenovectors in the clinic can make them more desirable for commercial and clinical applications. The efficacy of adenoviral vectors can be improved by modifying vector elements, such as using tissue specific promoters or deleting DNA sequences to eliminate production of harmful viral proteins. Adenoviral mediated vestibular hair cell regeneration has been reported to be more efficient with the glial fibrillary acidic protein (GFAP) promoter than the human cytomegalovirus (hCMV) and chicken β actin (cBA) promoters. Additionally, adenovectors with deleted E4 regions resulted in increased tolerability of the cells toward the vector [51,52,80]. In addition to delivering the Math1 gene, adenoviral vectors are used to deliver growth factors such as transforming growth factor-β1 (TGF-β1), glial cell-derived neurotrophic factor (GDNF), and B-cell lymphoma 2 (Bcl-2) to protect hair cells from sound trauma and ototoxic drugs [81,82]. Despite the success of adenoviruses in the effective delivery of Math1, short term gene expression and strong immune responses often result, but can be overcome by adeno-associated viruses. Studies show the capacity of adeno-associated viruses to deliver genes to inner ear blood vessels and certain auditory nerve fibers with negligible toxicity when compared to adenoviruses [76,83]. Herpes simplex viral vectors (HSV) derived from Herpes simplex type I can infect and replicate in non-dividing cells. Studies in cochlear gene therapy using HSV reported dispersed gene expression in the cochlea, limited to auditory and vestibular spiral ganglion neurons [84]. A limitation of HSV mediated gene delivery is that it requires the use of high viral stock volumes due to the difficulty of producing the vectors in high titers. In addition, HSV are reported to evoke strong inflammatory responses in guinea pigs [85]. Lentivirus is the best available viral vector in terms of transduction efficiency and transgene expression because of its ability to infect both proliferating and non-proliferating cells, including stem cells that are difficult to transduce. Research in lentiviral-mediated gene delivery in the guinea pig cochlea showed gene expression limited to the perilymphatic space in the cochlea. High gene expression was observed in ganglion neurons, glial cells, and supporting cells; however, the vector failed to infect sensory cells. Although lentiviral vectors offer long term gene expression in a variety of cell types, they are known to have a high risk of evoking a strong immune response and generating a replication-competent virus [86].
Currently, no ideal vector exists for use in inner ear gene delivery. However, adenovector based gene therapy is currently the most widely used form of gene therapy in the inner ear. Newer recombinant forms of adenoviruses carrying the Math1 gene have shown promising results in generating hair cells in both auditory and vestibular systems. Among all known viral vectors, adenovirus is widely used in delivering specific genes (Math1, GDNF, and Bcl-2) for regenerating and protecting hair cells. Table 3 lists the advantages and disadvantages of using different viral vectors in inner ear gene therapy.
Table 3.
Summary of different viral vectors used in inner ear gene therapy.
| Vector | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Adeno virus |
|
|
[49,51, 82,83,84,87,88] |
| Adeno-associated Virus |
|
|
[76,89,90,91,92] |
| Herpes Simplex Virus |
|
|
[84,85,93] |
| Lentivirus |
|
|
[86,94] |
The development and use of different viral vectors in inner ear gene therapy allows us to evaluate the effects of introducing specific genes and therapeutic molecules that can regenerate hair cells and prevent hair cell damage.
4.2. Stem Cell-Based Therapy for Inner Ear Hair Cell Regeneration
In situations where the loss of supporting cells may prevent transdifferentiation based regeneration strategies, hair cells may be replaced by stem cell therapy. In the last decade, there has been a considerable amount of attention directed toward stem cell-based therapies for treating diseases like Alzheimer's, Parkinson's and cardiovascular diseases. The success of stem cells in treating these diseases opened opportunities for researchers to explore the use of stem cells in treating hearing disabilities. Stem cell therapy is based on the concept that, upon transplantation, the undifferentiated stem cell has the capacity to respond and react to surrounding cell signals and differentiate into the appropriate cell type associated with the signal. Stem cells are a useful way of exploring the molecular pathways that underlie hair cell genesis. Some of the stem cell-based therapeutic strategies that have employed stem cells in the effort to cure hearing loss are listed below.
4.2.1. Embryonic Stem Cells
ESCs are pluripotent and capable of giving rise to cells from any of the three germ layers [95]. With respect to hair cell regeneration, it was reported that murine embryonic stem cells can generate inner ear progenitors in vitro [96]. These ESCs were allowed to form embryoid bodies and were cultured in the presence of epidermal growth factor (EGF), insulin-like growth factor 1 (IGF-1), and basic fibroblast growth factor (bFGF). The newly generated progenitor cells were reported to express markers characteristic of hair cell differentiation and hair cell specific markers. Additionally, the progenitor sensory cells had the capacity to integrate into sensory epithelial layers when injected into the developing inner ear of a chicken and to express hair bundle markers in vivo. Another study demonstrated the use of murine ESCs from transgenic Math1/nGFP mice [97]. The ESCs were differentiated using a step-by-step method toward the ectodermal lineage using otic-inducing growth factors. The generated otic progenitor cells had the capacity to develop into mechanosensitive sensory hair cells in vitro and demonstrated immature hair cell transduction currents [97]. ESCs have been reported to produce sensory auditory neurons and neural progenitors with the potential to restore auditory function by generating nerve connections to hair cells [98,99,100]. Embryoid bodies from murine ESCs co-cultured with hair cell explants showed neuron-like cells and positive staining for neurofilament [99]. Most of the embryonic stem cell studies have used murine stem cells; however, there have been attempts to differentiate human embryonic stem cells (hESCs) in the presence of growth factors such as neurotrophin-3 (NT-3), bFGF, BDNF, EGF and bone morphogenic protein 4 (BMP4). These differentiated cells expressed inner ear and synaptic markers [101,102,103]. hESCs have also been cultured to generate otic progenitors that were capable of differentiating into auditory sensory neurons [102].
Although these studies have demonstrated successful in vitro and in vivo generation of replacement hair cells and auditory neurons from ESCs, further investigations are crucial in developing treatment strategies for hearing loss because of the controversial and ethical issues linked to ESCs.
4.2.2. Adult Stem Cells
Promising results have been shown with bone marrow mesenchymal stem cells (BMSCs) in the field of hair cell regeneration. Mesenchymal stem cells derived from rat bone marrow have been reported to differentiate into inner ear progenitors and express sensory cell markers including myosin VIIa, espin, Brn3c, p27kip and jagged2 in vitro [104]. Additionally, the differentiated cells displayed morphological characteristics of hair cell stereociliary bundles. Studies have shown that BMSCs stimulated in the presence of growth factors were able to form neuronal progenitors, and after being transfected with the Math1 gene, were able to differentiate into inner ear sensory-like cells [104]. Adult stem cells also have the potential to deliver gene and therapeutic molecules to other parts of the inner ear. For example, Connexin 26, a protein present in cochlear gap junctions and supporting cells was expressed when bone marrow stromal cells were transplanted into the perilymphatic space of the mouse cochlea [105]. Bone marrow mesenchymal stem cells also have the potential to differentiate into auditory neurons in vitro and in vivo [106,107], demonstrating that a wide variety of inner ear cell types can be generated from stem cells.
Adult stem cells isolated from mouse macular organs have been shown to differentiate into hair cells when cultured with EFG and IGF-1 [108]. Adult stem cells isolated from olfactory neuroepithelium expressed hair cell markers and resembled hair cells phenotypically when co-cultured with cochlear cell supernatant [109].
Transplantation studies in mice with bone marrow-derived hematopoietic stem cells (BMHSCs) suggested the possibility of differentiation of BMHSCs into mesenchymal cells and fibrocytes in the adult inner ear [110]. The results with BMHSCs show their potential to attenuate cochlear injury by replacing mesenchymal cells and fibrocytes in the inner ear [110].
Adult stem cells from many tissues are now being used to investigate cures for various diseases. These cells are not concerned with any ethical issues and can enter clinical trials involving autologous transplantation therapies and used in bioengineered products. In treating inner ear disorders, bone marrow-derived stem cells have shown the most favorable results [105,106,110,111].
4.2.3. Induced Pluripotent Stem Cells
The discovery of generating induced pluripotent stem cells (iPSCs) introduced a new dimension to stem cell research. iPSCs are produced from fibroblasts, other somatic cells and adult stem cells, which are reprogrammed to express certain genes and maintain characteristic properties of embryonic stem cells. iPSCs opened up the possibility of generating and using patient-specific stem cells without immune rejection in vivo and, unlike hESCs, there are no controversial issues associated with their use. The most important factors in maintaining the pluripotency of ESC lines are Oct4, c-Myc, Klf4 and Sox2. Any adult stem cells forced to express the above four genes under ESC culture conditions can be reprogrammed into ESC-like cells [112]. Recently, it was demonstrated that human neural stem cells can be directly reprogrammed to iPSCs by just expressing Oct4 [113]. Like ESCs, most studies with iPSCs in hair cell regeneration are also of murine origin. A recent study showed generation of iPSCs from murine embryonic fibroblasts [97]. These fibroblasts were transduced with retroviruses to express Oct4, c-Myc, Klf4 and Sox2. The generated iPSCs were cultured in a medium containing otic-inducing FGF-3 and FGF-10 to produce otic progenitor cells. The generated otic progenitors differentiated into hair cell-like cells expressing hair cell markers. When co-cultured with fibroblast-like cells from embryonic chicken utricles, the differentiated cells developed hair bundle-like protrusions, responded to mechanical stimulation, and displayed transduction currents [97]. Another study explored the use of iPSCs for restoring auditory ganglion neurons. In vitro neuronal differentiation of iPSCs was induced by exposing them to stromal cell-derived inducing activity (SDIA). SDIA is a neural inducing activity demonstrated in stromal cells when they simultaneously produce inducing and inhibitory factors and was developed by Kawasaki et al. [114]. The differentiated cells were transplanted into the cochleas of mice. iPS cell-derived neurons projecting toward cochlear hair cells were observed 1 week after transplantation [115]. Although iPS cell techniques are novel, researchers have started to explore their potential in treating SNHL.
4.2.4. Summary of Stem Cell-Based Therapies
ESCs have high survival rates and migration capacity when implanted into the cochlea [116,117]. They migrated onto auditory neurons and exhibited neuronal differentiation. However, ESCs exhibited low integration into endogenous tissue and failed to differentiate completely at the implantation site [118,119]. There is also the risk of tumor formation and the risk of transmitting infections with ESCs because they use animal products during the culturing process. ESCs used in inner ear treatment require the use of immunosuppressive therapy or cloning to avoid graft-vs-host diseases. On the other hand, adult stem cells isolated from bone marrow can easily bypass the immune barriers and overcome the problem of immune rejection. BMSCs also have a high survival rate and can migrate into multiple regions in the cochlea and brain [106,120,121,122]. Although they can be stimulated to differentiate into a number of cell lineages, they differentiate more toward the mesoderm lineage and can be used to replace degenerated cochlear fibrocytes [123]. iPSCs do not have any ethical concerns with their use and are more patient specific, thus eliminating the risk of immune rejection. Their undifferentiated state allows them to migrate to regions surrounding the cochlea. However, one of the major concerns with iPSCs is the time required to produce the individual cell lines [124]. They also pose the risk of passing on the DNA from the genetically altered cell to future generations.
Some of the barriers that exist with the use of stem cells are the formation of tumors and graft-versus-host diseases. Another concern is the integration of stem cells in the inner ear. Owing to the spiral structure of the cochlea, it is challenging to direct the stem cells into the desired location and evaluate the integration of differentiated stem cells inside the cochlea. Further investigation with the use of different stem cell types and advancements in the existing approaches are necessary before they can enter the clinic and begin treating hearing disorders.
Nevertheless, these concerns bring opportunities for bioengineers and clinicians to engineer cells and vectors carrying a combination of genes and therapeutics and develop methods and devices to deliver therapeutics at appropriate locations. Recent advances in stem cell technology and gene based therapies provide the framework required for the development of potential treatment options.
5. Discussion
The success of current approaches unveils the possibility of using different viral vectors, genes, growth factors and stem cell types in regenerating inner ear hair cells. Nevertheless, more research is still required to overcome challenges involved before these approaches will be ready for clinical and commercial use. Although several studies have reported the generation of hair cell-like cells in vitro and in vivo (in animals), the outcome and characteristic properties of these cells must be further explored. A cell that simply resembles a hair cell morphologically and expresses hair cell markers would be immature without being able to respond to mechanical stimuli and transfer signals to the auditory neurons. A detailed investigation of their ultra structure and ability to connect to auditory neurons and produce transduction currents is essential. In terms of functional outcomes, crucial to any tissue engineering strategy, the mechanoelectrical transduction and maturity of transplanted hair cell-like cells can be evaluated by auditory brainstem response (ABR) and otoacoustic emissions tests (OAE) in animal studies.
Audiologists and scientists have come a long way toward reaching the goal for hair cell regeneration in mammals by developing inner ear gene therapy strategies in animal models. However, an improvement in the existing gene delivery techniques is required to suit clinical applications.
Some of the drawbacks associated with inner ear gene therapy are addressed below. For example, gene delivery studies have shown that over-expression of the Math1 gene led to the formation of ectopic hair cells [16,50]. Hair cells are extremely rare and the cochlea contains only about 14,000 hair cells that detect and amplify sound [103], and it is important to regulate the cell cycle for normal development of cochlear function. Studies in p27kip knockout mice resulted in production of supernumerary hair cells and later a massive degradation of the hair cells occurred, leading to a severe hearing impairment in these animals. This indicates the importance of precise control of cell cycle [69]. There are several pathways that control the expression of Math1 and drive a cell toward differentiating into a hair cell. Math1 inhibitors and down regulators can be used in addition to targeting the inner ear with only the Math1 gene. This approach can be used to produce an appropriate number of hair cells. The activation of a notch receptor can up regulate Hes and Hey genes that are potent inhibitors of Math1 [18,19,57]. Inclusion of notch receptor inhibitors in gene therapy can help in boosting Math1 gene expression. However, a balance of expression and inhibition between the Math1 gene and Math1 inhibitor is required for normal inner ear development and hearing.
Besides developing strategies to control the expression of the Math1 gene, the side effects caused by viral routes also need to be considered. Although viral methods have shown tremendous success in the animal model, there is always the risk of DNA mutations and cancer associated with viruses. Non-viral gene delivery techniques have recently gained attention due to the minimal risks associated in terms of clinical safety and reliability. Another drawback of Math1 gene therapy is that although it can be a potential cure for hearing loss caused by sound trauma or ototoxic damage, it may not cure hearing loss caused by genetic defects. Stem cell-based therapies in vivo have reported migration of these cells into different areas, and thus an ideal route for delivering cells to the right location in the cochlea is crucial to restoring hearing loss. The large majority of studies have used only animal models and animal stem cells. There is no doubt that these studies have played a major role in gathering valuable information about hair cell regeneration; however, the variation among species requires the need to explore the use of human stem cell lines like bone marrow derived mesenchymal and hematopoietic stem cells, umbilical cord blood and umbilical mesenchymal stromal stem cells, and induced pluripotent stem cells. Research in human stem cells lines will have more clinical relevance.
Gene delivery and stem cells are a potential cure to hearing loss; however, there are limitations that must be overcome. Gene therapies must consider using combinations of essential genes and cell cycle inhibitors to control the production of hair cells, and detailed quantification of hair cell functionality is necessary. Gene delivery vehicles with minimal risk of mutagenesis and immune response must also be developed. Finally, an ideal route to implant stem cells inside the cochlea would be essential for successful innervations of hair cells, thereby allowing better, if not pristine transmission of sound with the assistance of a cochlear implant.
References
- 1.Cotanche D., Kaiser C. Hair cell fate decisions in cochlear development and regeneration. Hear. Res. 2010;226:18–25. doi: 10.1016/j.heares.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oshima K., Suchert S., Blevins N., Heller S. Curing hearing loss: Patient expectations, health care practitioners, and basic science. J. Commun. Disord. 2010;43:311–318. doi: 10.1016/j.jcomdis.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodmer D. Protection, regeneration and replacement of hair cells in the cochlea: Implications for the future treatment of sensorineural hearing loss. Swiss Med. Wkly. 2008;138:708–712. doi: 10.4414/smw.2008.12260. [DOI] [PubMed] [Google Scholar]
- 4.Suter A.H., von Gierke H.E. Noise and public policy. Ear Hear. 1987;8:188–191. doi: 10.1097/00003446-198708000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Clark W.W., Bohne B.A. Effects of noise on hearing. J. Am. Med. Assoc. 1999;281:1658–1659. doi: 10.1001/jama.281.17.1658. [DOI] [PubMed] [Google Scholar]
- 6.Fligor B.J., Cox L.C. Output levels of commercially available portable compact disc players and the potential risk to hearing. Ear Hear. 2004;25:513–527. doi: 10.1097/00003446-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Pauley S., Kopecky B., Beisel K., Soukup G., Fritzsch B. Stem cells and molecular strategies to restore hearing. Panminerva Med. 2008;50:41–53. [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng A.G., Cunningham L.L., Rubel E.W. Mechanisms of hair cell death and protection. Curr. Opin. Otolaryngol. Head Neck Surg. 2005;13:343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- 9.Matsui J., Cotanche D. Sensory hair cell death and regeneration: Two halves of the same equation. Curr. Opin. Otolaryngol. Head Neck Surg. 2004;12:418–425. doi: 10.1097/01.moo.0000136873.56878.56. [DOI] [PubMed] [Google Scholar]
- 10.Kharlamova A., Aarts N. A review of past and present hair cell regeneration techniques. Contemp. Issues Commun. Sci. Disord. 2007;34:134–144. [Google Scholar]
- 11.Gillespie P., Müller U. Mechanotransduction by hair cells: Models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corwin J.T., Cotanche D.A. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 13.Jørgensen J., Mathiesen C. The avian inner ear. Naturwissenschaften. 1988;75:319–320. doi: 10.1007/BF00367330. [DOI] [PubMed] [Google Scholar]
- 14.Rubel E., Oesterle E., Weisleder P. Hair cell regeneration in the avian inner ear. Ciba Found. Symp. 1991;160:77–96. doi: 10.1002/9780470514122.ch5. [DOI] [PubMed] [Google Scholar]
- 15.Weisleder P., Rubel E. Hair cell regeneration in the avian vestibular epithelium. Exp. Neurol. 1992;115:2–6. doi: 10.1016/0014-4886(92)90211-8. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y., Chi F., Han Z., Yang J., Gao W., Li Y. New ectopic vestibular hair cell-like cells induced by Math1 gene transfer in postnatal rats. Brain Res. 2009;1276:31–38. doi: 10.1016/j.brainres.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Dooling R., Dent M. New studies on hair cell regeneration in birds. Acoust. Sci. Technol. 2001;22:93–99. [Google Scholar]
- 18.Wang G., Chatterjee I., Batts S., Wong H., Gong T., Gong S., Raphael Y. Notch signaling and Atoh1 expression during hair cell regeneration in the mouse utricle. Hear. Res. 2010;267:61–70. doi: 10.1016/j.heares.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zine A., van de Water T., de Ribaupierre F. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development. 2000;127:3373. doi: 10.1242/dev.127.15.3373. [DOI] [PubMed] [Google Scholar]
- 20.Corwin J. Regeneration in the auditory system. Exp. Neurol. 1992;115:7–12. doi: 10.1016/0014-4886(92)90212-9. [DOI] [PubMed] [Google Scholar]
- 21.Stone J., Rubel E. Cellular studies of auditory hair cell regeneration in birds. Proc. Natl. Acad. Sci. USA. 2000;97:11714–11721. doi: 10.1073/pnas.97.22.11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwan T., White P., Segil N. Development and regeneration of the inner ear. Ann. N. Y. Acad. Sci. 2009;1170:28–33. doi: 10.1111/j.1749-6632.2009.04484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edge A., Chen Z. Hair cell regeneration. Curr. Opin. Neurobiol. 2008;18:377–382. doi: 10.1016/j.conb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui J., Ryals B. Hair cell regeneration: An exciting phenomenon… But will restoring hearing and balance be possible? J. Rehabil. Res. Dev. 2005;42:187–196. doi: 10.1682/jrrd.2005.01.0008. [DOI] [PubMed] [Google Scholar]
- 25.Bermingham-McDonogh O., Rubel E. Hair cell regeneration: Winging our way towards a sound future. Curr. Opin. Neurobiol. 2003;13:119–126. doi: 10.1016/s0959-4388(03)00018-7. [DOI] [PubMed] [Google Scholar]
- 26.Brigande J., Heller S. Quo vadis, hair cell regeneration? Nat. Neurosci. 2009;12:679–685. doi: 10.1038/nn.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collado M., Burns J., Hu Z., Corwin J. Recent advances in hair cell regeneration research. Curr. Opin. Otolaryngol. Head Neck Surg. 2008;16:465–471. doi: 10.1097/MOO.0b013e32830f4ab5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beisel K., Hansen L., Soukup G., Fritzsch B. Regenerating cochlear hair cells: Quo vadis stem cell. Cell Tissue Res. 2008;333:373–379. doi: 10.1007/s00441-008-0639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H., Corrales C., Edge A., Heller S. Stem cells as therapy for hearing loss. Trends Mol. Med. 2004;10:309–315. doi: 10.1016/j.molmed.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Matsui J.I., Parker M.A., Ryals B.M., Cotanche D.A. Regeneration and replacement in the vertebrate inner ear. Drug Discov. Today. 2005;10:1307–1312. doi: 10.1016/S1359-6446(05)03577-4. [DOI] [PubMed] [Google Scholar]
- 31.Martin F., Clark J. Introduction to Audiology (with CD-ROM) Allyn & Bacon Inc.; Needham Heights, MA, USA: 2005. [Google Scholar]
- 32.Newby H., Popelka G. Audiology, Englewood Cliffs. Prentice-Hall; Upper Saddle River, NJ, USA: 1992. [Google Scholar]
- 33.Sataloff J. Hearing Loss. Lippincott; Philadephia, PA, USA: 1966. [Google Scholar]
- 34.Willott J.F. Aging and the Auditory System: Anatomy, Physiology and Psychophysics. Whurr Pub Ltd; London, UK: 1991. [Google Scholar]
- 35.LeMasurier M., Gillespie P. Hair-cell mechanotransduction and cochlear amplification. Neuron. 2005;48:403–415. doi: 10.1016/j.neuron.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Lindeman H., Ades H., Bredberg G., Engström H. The sensory hairs and the tectorial membrane in the development of the cat's organ of Corti: A scanning electron microscopic study. Acta Oto-Laryngol. 1971;72:229–242. doi: 10.3109/00016487109122478. [DOI] [PubMed] [Google Scholar]
- 37.Roberts W., Howard J., Hudspeth A. Hair cells: Transduction, tuning, and transmission in the inner ear. Annu. Rev. Cell Biol. 1988;4:63–92. doi: 10.1146/annurev.cb.04.110188.000431. [DOI] [PubMed] [Google Scholar]
- 38.Axelrod J.D. Basal bodies, kinocilia and planar cell polarity. Nat. Genet. 2008;40:10–11. doi: 10.1038/ng0108-10. [DOI] [PubMed] [Google Scholar]
- 39.Hudspeth A., Jacobs R. Stereocilia mediate transduction in vertebrate hair cells (auditory system/cilium/vestibular system) Proc. Natl. Acad. Sci. USA. 1979;76:1506–1509. doi: 10.1073/pnas.76.3.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moran D.T., Rowley J.C., Asher D.L. Calcium-binding sites on sensory processes in vertebrate hair cells. Proc. Natl. Acad. Sci. USA. 1981;78:3954–3958. doi: 10.1073/pnas.78.6.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White P., Doetzlhofer A., Lee Y., Groves A., Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 42.Stone J., Oesterle E., Rubel E. Recent insights into regeneration of auditory and vestibular hair cells. Curr. Opin. Neurol. 1998;11:17–24. doi: 10.1097/00019052-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Fritzsch B., Eberl D.F., Beisel K.W. The role of bHLH genes in ear development and evolution: Revisiting a 10-year-old hypothesis. Cell, Mol. Life Sci. 2010;67:3089–3099. doi: 10.1007/s00018-010-0403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen P., Johnson J., Zoghbi H., Segil H. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- 45.Matei V., Pauley S., Kaing S., Rowitch D., Beisel K., Morris K., Feng F., Jones K., Lee J., Fritzsch B. Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Dev. Dyn. 2005;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan N., Jahan I., Kersigo J., Kopecky B., Santi P., Johnson S., Schmitz H., Fritzsch B. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear. Res. 2010;275:66–80. doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bermingham N., Hassan B., Price S., Vollrath M., Ben-Arie N., Eatock R., Bellen H., Lysakowski A., Zoghbi H. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 48.Izumikawa M., Minoda R., Kawamoto K., Abrashkin K., Swiderski D., Dolan D., Brough D., Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 49.Kawamoto K., Ishimoto S.I., Minoda R., Brough D.E., Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J.Neurosci. 2003;23:4395–4440. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng J., Gao W. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 51.Baker K., Brough D., Staecker H. Repair of the vestibular system via adenovector delivery of Atoh1: A potential treatment for balance disorders. Adv. Otorhinolaryngol. 2009;66:52–63. doi: 10.1159/000218207. [DOI] [PubMed] [Google Scholar]
- 52.Staecker H., Praetorius M., Baker K., Brough D. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol. Neurotol. 2007;28:223–231. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- 53.Hong S., Huang A., Cao S. The current status and prospects of gene therapy for the inner ear. Hum. Gene Ther. 2011;22:1–12. doi: 10.1089/hum.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim P., Helms A.W., Johnson J.E., Zimmerman K. XATH-1, a vertebrate homolog of drosophila atonal, induces neuronal differentiation within ectodermal progenitors* 1. Dev. Biol. 1997;187:1–12. doi: 10.1006/dbio.1997.8572. [DOI] [PubMed] [Google Scholar]
- 55.Shou J., Zheng J., Gao W. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol. Cell. Neurosci. 2003;23:169–179. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 56.Zine A., Aubert A., Qiu J., Therianos S., Guillemot F., Kageyama R., de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J. Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng J., Shou J., Guillemot F., Kageyama R., Gao W. Hes1 is a negative regulator of inner ear hair cell differentiation. Development. 2000;127:4551–4560. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- 58.Liu M., Pereira F.A., Price S.D., Chu M., Shope C., Himes D., Eatock R.A., Brownell W.E., Lysakowski A., Tsai M.J. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiernan A.E., Pelling A.L., Leung K.K.H., Tang A.S.P., Bell D.M., Tease C., Lovell-Badge R., Steel K.P., Cheah K.S.E. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- 60.Oesterle E.C., Campbell S., Taylor R.R., Forge A., Hume C.R. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J. Assoc. Res. Otoaryngol. 2008;9:65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiernan A.E., Cordes R., Kopan R., Gossler A., Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132:4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- 62.Kiernan A.E., Xu J., Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006 doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jahan I., Pan N., Kersigo J., Fritzsch B. Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One. 2010 doi: 10.1371/journal.pone.0011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sage C., Huang M., Vollrath M.A., Brown M.C., Hinds P.W., Corey D.P., Vetter D.E., Chen Z.Y. Essential role of retinoblastoma protein in mammalian hair cell development and hearing. Natl. Acad. Sci. 2006;139:7345–7350. doi: 10.1073/pnas.0510631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sage C., Huang M., Karimi K., Gutierrez G., Vollrath M.A., Zhang D.S., García-Añoveros J., Hinds P.W., Corwin J.T., Corey D.P. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- 66.Bodson M., Breuskin I., Lefebvre P., Malgrange B. Hair cell progenitors: Identification and regulatory genes. Acta Oto-Laryngol. 2010;130:312–317. doi: 10.1080/00016480903121057. [DOI] [PubMed] [Google Scholar]
- 67.Rocha-Sanchez S.M., Scheetz L.R., Contreras M., Weston M.D., Korte M., McGee J.A., Walsh E.J. Mature mice lacking Rbl2/p130 gene have supernumerary inner ear hair cells and supporting cells. J. Neurosci. 2011;31:8883–8893. doi: 10.1523/JNEUROSCI.5821-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mantela J., Jiang Z., Ylikoski J., Fritzsch B., Zacksenhaus E., Pirvola U. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development. 2005;132:2377–2388. doi: 10.1242/dev.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen P., Segil N. p27 (Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 70.Chen P., Zindy F., Abdala C., Liu F., Li X., Roussel M.F., Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat. Cell Biol. 2003;5:422–426. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- 71.Löwenheim H., Furness D.N., Kil J., Zinn C., Gültig K., Fero M.L., Frost D., Gummer A.W., Roberts J.M., Rubel E.W. Gene disruption of p27Kip1 allows cell proliferation in the postnatal and adult organ of Corti. Proc. Natl. Acad. Sci. USA. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kopecky B., Santi P., Johnson S., Schmitz H., Fritzsch B. Conditional deletion of N Myc disrupts neurosensory and non sensory development of the ear. Dev. Dyn. 2011;240:1373–1390. doi: 10.1002/dvdy.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okano T., Nakagawa T., Kita T., Endo T., Ito J. Cell-gene delivery of brain-derived neurotrophic factor to the mouse inner ear. Mol. Ther. 2004;14:866–871. doi: 10.1016/j.ymthe.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 74.Oshima K., Shimamura M., Mizuno S., Tamai K., Doi K., Morishita R., Nakamura T., Kubo T., Kaneda Y. Intrathecal injection of HVJ-E containing HGF gene to cerebrospinal fluid can prevent and ameliorate hearing impairment in rats. FASEB J. 2004;18:212–214. doi: 10.1096/fj.03-0567fje. [DOI] [PubMed] [Google Scholar]
- 75.Rejali D., Lee V.A., Abrashkin K.A., Humayun N., Swiderski D.L., Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear. Res. 2007;228:180–187. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jero J., Mhatre A.N., Tseng C.J., Stern R.E., Coling D.E., Goldstein J.A., Hong K., Zheng W.W., Hoque A.T.M.S., Lalwani A.K. Cochlear gene delivery through an intact round window membrane in mouse. Hum. Gene Ther. 2001;12:539–548. doi: 10.1089/104303401300042465. [DOI] [PubMed] [Google Scholar]
- 77.Praetorius M., Brough D., Hsu C., Plinkert P., Pfannenstiel S., Staecker H. Adenoviral vectors for improved gene delivery to the inner ear. Hear. Res. 2009;248:31–38. doi: 10.1016/j.heares.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campochiaro P.A., Nguyen Q.D., Shah S.M., Klein M.L., Holz E., Frank R.N., Saperstein D.A., Gupta A., Stout J.T., Macko J. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: Results of a phase I clinical trial. Hum. Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 79.Alton E., Kitson C. Gene therapy for cystic fibrosis. Expert Opin. Invest. Drugs. 2000;9:1523–1535. doi: 10.1517/13543784.9.7.1523. [DOI] [PubMed] [Google Scholar]
- 80.Praetorius M., Hsu C., Baker K., Brough D., Plinkert P., Staecker H. Adenovector-mediated hair cell regeneration is affected by promoter type. Acta Oto-Laryngol. 2010;130:215–222. doi: 10.3109/00016480903019251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawamoto K., Yagi M., Stöver T., Kanzaki S., Raphael Y. Hearing and hair cells are protected by adenoviral gene therapy with TGF- 1 and GDNF. Mol. Ther. 2003;7:484–492. doi: 10.1016/s1525-0016(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 82.Pfannenstiel S., Praetorius M., Plinkert P., Brough D., Staecker H. Bcl-2 gene therapy prevents aminoglycoside-induced degeneration of auditory and vestibular hair cells. Audiol. Neurotol. 2009;14:254–266. doi: 10.1159/000192953. [DOI] [PubMed] [Google Scholar]
- 83.Luebke A.E., Foster P.K., Muller C.D., Peel A.L. Cochlear function and transgene expression in the guinea pig cochlea, using adenovirus- and adeno-associated virus-directed gene transfer. Hum. Gene Ther. 2001;12:773–781. doi: 10.1089/104303401750148702. [DOI] [PubMed] [Google Scholar]
- 84.Staecker H., Li D., O Malley B., van de Water T. Gene expression in the mammalian cochlea: A study of multiple vector systems. Acta Oto-Laryngol. 2001;121:157–163. doi: 10.1080/000164801300043307. [DOI] [PubMed] [Google Scholar]
- 85.Derby M.L., Sena-Esteves M., Breakefield X.O., Corey D.P. Gene transfer into the mammalian inner ear using HSV-1 and vaccinia virus vectors. Hear. Res. 1999;134:1–8. doi: 10.1016/s0378-5955(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 86.Han J.J., Mhatre A.N., Wareing M., Pettis R., Gao W.Q., Zufferey R.N., Trono D., Lalwani A.K. Transgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vector. Hum. Gene Ther. 1999;10:1867–1873. doi: 10.1089/10430349950017545. [DOI] [PubMed] [Google Scholar]
- 87.Holt J., Johns D., Wang S., Chen Z., Dunn R., Marban E., Corey D. Functional expression of exogenous proteins in mammalian sensory hair cells infected with adenoviral vectors. J. Neurophysiol. 1999;81:1881–1888. doi: 10.1152/jn.1999.81.4.1881. [DOI] [PubMed] [Google Scholar]
- 88.Weiss M.A., Frisancho J.C., Roessler B.J., Raphael Y. Viral-mediated gene transfer in the cochlea. Int. J. Dev. Neurosci. 1997;15:577–583. doi: 10.1016/s0736-5748(96)00112-8. [DOI] [PubMed] [Google Scholar]
- 89.Peter A.E.L.C.R., Poulsenb G.V.D.D.J. Adenoviral and AAV-mediated gene transfer to the inner ear: Role of serotype, promoter, and viral load on in vivo and in vitro infection efficiencies. Adv. Otorhinolaryngol. 2009;66:87–98. doi: 10.1159/000218209. [DOI] [PubMed] [Google Scholar]
- 90.Li Duan M., Bordet T., Mezzina M., Kahn A., Ulfendahl M. Adenoviral and adeno-associated viral vector mediated gene transfer in the guinea pig cochlea. Neuroreport. 2002;13:1295–1299. doi: 10.1097/00001756-200207190-00016. [DOI] [PubMed] [Google Scholar]
- 91.Lalwani A., Walsh B., Reilly P., Muzyczka N., Mhatre A. Development of in vivo gene therapy for hearing disorders: Introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther. 1996;3:588–592. [PubMed] [Google Scholar]
- 92.Kho S.T., Pettis R.M., Mhatre A.N., Lalwani A.K. Safety of adeno-associated virus as cochlear gene transfer vector: Analysis of distant spread beyond injected cochleae. Mol. Ther. 2000;2:368–373. doi: 10.1006/mthe.2000.0129. [DOI] [PubMed] [Google Scholar]
- 93.Glorioso J., DeLuca N., Fink D. Development and application of herpes simplex virus vectors for human gene therapy. Annu. Rev. Microbiol. 1995;49:675–710. doi: 10.1146/annurev.mi.49.100195.003331. [DOI] [PubMed] [Google Scholar]
- 94.Qian H., Hang X., Xu W., Zhu W., Cao H., Chen Y., Xu X., Wang M., Xie Y., Sun J. Lentivirus-modified human umbilical cord mesenchymal stem cells maintain their pluripotency. Biotechnol. Appl. Biochem. 2010;55:53–62. doi: 10.1042/BA20090210. [DOI] [PubMed] [Google Scholar]
- 95.Odorico J.S., Kaufman D.S., Thomson J.A. Multilineage differentiation from human embryonic stem cell lines. Stem cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 96.Li H., Roblin G., Liu H., Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oshima K., Shin K., Diensthuber M., Peng A., Ricci A., Heller S. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–716. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martinez-Monedero R., Edge A. Stem cells for the replacement of inner ear neurons and hair cells. Int. J. Dev. Biol. 2007;51:655–661. doi: 10.1387/ijdb.072372rm. [DOI] [PubMed] [Google Scholar]
- 99.Coleman B., Fallon J., Pettingill L., de Silva M., Shepherd R. Auditory hair cell explant co-cultures promote the differentiation of stem cells into bipolar neurons. Exp. Cell Res. 2007;313:232–243. doi: 10.1016/j.yexcr.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corrales C.E., Pan L., Li H., Liberman M.C., Heller S., Edge A.S.B. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: Growth of processes into the organ of corti. J. Neurobiol. 2006;66:1489–1500. doi: 10.1002/neu.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi F., Corrales C.E., Liberman M.C., Edge A.S.B. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur. J. Neurosci. 2007;26:3016–3023. doi: 10.1111/j.1460-9568.2007.05909.x. [DOI] [PubMed] [Google Scholar]
- 102.Chen W., Jongkamonwiwat N., Jonhson S. FGF signaling can induce the generation of cochlear progenitors from hESCs with the potential to differentiate into functional hair cell-like cells and neurons UK National Stem Cell Network Oxford. UK Natl. Stem Cell Netw. 2009;104:99–109. [Google Scholar]
- 103.Rivolta M.N., Li H., Heller S. Generation of inner ear cell types from embryonic stem cells. Methods Mol. Biol. 2006;330:71–92. doi: 10.1385/1-59745-036-7:71. [DOI] [PubMed] [Google Scholar]
- 104.Jeon S., Oshima K., Heller S., Edge A. Bone marrow mesenchymal stem cells are progenitors in vitro for inner ear hair cells. Mol. Cell. Neurosci. 2007;34:59–68. doi: 10.1016/j.mcn.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharif S., Nakagawa T., Ohno T., Matsumoto M., Kita T., Riazuddin S., Ito J. The potential use of bone marrow stromal cells for cochlear cell therapy. Neuroreport. 2007;18:351–354. doi: 10.1097/WNR.0b013e3280287a9a. [DOI] [PubMed] [Google Scholar]
- 106.Matsuoka A.J., Kondo T., Miyamoto R.T., Hashino E. In vivo and in vitro characterization of bone marrow derived stem cells in the cochlea. Laryngoscope. 2006;116:1363–1367. doi: 10.1097/01.mlg.0000225986.18790.75. [DOI] [PubMed] [Google Scholar]
- 107.Kondo T., Johnson S.A., Yoder M.C., Romand R., Hashino E. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc. Natl. Acad. Sci. USA. 2005;102:4789–4794. doi: 10.1073/pnas.0408239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li H., Liu H., Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat. Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- 109.Doyle K.L., Kazda A., Hort Y., McKay S.M., Oleskevich S. Differentiation of adult mouse olfactory precursor cells into hair cells in vitro. Stem Cells. 2007;25:621–627. doi: 10.1634/stemcells.2006-0390. [DOI] [PubMed] [Google Scholar]
- 110.Lang H., Ebihara Y., Schmiedt R., Minamiguchi H., Zhou D., Smythe N., Liu L., Ogawa M., Schulte B. Contribution of bone marrow hematopoietic stem cells to adult mouse inner ear: Mesenchymal cells and fibrocytes. J. Comp. Neurol. 2006;496:187–201. doi: 10.1002/cne.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Low C., Liou Y., Tang B. Neural differentiation and potential use of stem cells from the human umbilical cord for central nervous system transplantation therapy. J. Neurosci. Res. 2008;86:1670–1679. doi: 10.1002/jnr.21624. [DOI] [PubMed] [Google Scholar]
- 112.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 113.Kim J.B., Greber B., Araúzo-Bravo M.J., Meyer J., Park K.I., Zaehres H., Schöler H.R. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461:649–643. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 114.Kawasaki H., Mizuseki K., Nishikawa S., Kaneko S., Kuwana Y., Nakanishi S., Nishikawa S.I., Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 115.Nishimura K., Nakagawa T., Ono K., Ogita H., Sakamoto T., Yamamoto N., Okita K., Yamanaka S., Ito J. Transplantation of mouse induced pluripotent stem cells into the cochlea. Neuroreport. 2009;20:1250–1254. doi: 10.1097/WNR.0b013e32832ff287. [DOI] [PubMed] [Google Scholar]
- 116.Hildebrand M.S., Dahl H.H.M., Hardman J., Coleman B., Shepherd R.K., de Silva M.G. Survival of partially differentiated mouse embryonic stem cells in the scala media of the guinea pig cochlea. J. Assoc. Res. Otoaryngol. 2005;6:341–354. doi: 10.1007/s10162-005-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sekiya T., Kojima K., Matsumoto M., Kim T.S., Tamura T., Ito J. Cell transplantation to the auditory nerve and cochlear duct. Exp. Neurol. 2006;198:12–24. doi: 10.1016/j.expneurol.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 118.Hu Z., Ulfendahl M., Olivius N. Central migration of neuronal tissue and embryonic stem cells following transplantation along the adult auditory nerve. Brain Res. 2004;1026:68–73. doi: 10.1016/j.brainres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 119.Jongkamonwiwat N., Zine A., Rivolta N. Stem cell based therapy in the inner ear: Appropriate donor cell types and routes for transplantation. Curr. Drug Targets. 2010;11:888–897. doi: 10.2174/138945010791320836. [DOI] [PubMed] [Google Scholar]
- 120.Crain B.J., Tran S.D., Mezey E. Transplanted human bone marrow cells generate new brain cells. J. Neurol. Sci. 2005;233:121–123. doi: 10.1016/j.jns.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 121.Naito Y., Nakamura T., Nakagawa T., Iguchi F., Endo T., Fujino K., Kim T.S., Hiratsuka Y., Tamura T., Kanemaru S. Transplantation of bone marrow stromal cells into the cochlea of chinchillas. Neuroreport. 2004;15:1–4. doi: 10.1097/00001756-200401190-00001. [DOI] [PubMed] [Google Scholar]
- 122.Vlastarakos P., Nikolopoulos T., Tavoulari E., Papacharalambous G., Tzagaroulakis A., Dazert S. Sensory cell regeneration and stem cells: What we have already achieved in the management of deafness. Otol. Neurotol. 2008;29:758–768. doi: 10.1097/MAO.0b013e31817fdfad. [DOI] [PubMed] [Google Scholar]
- 123.Kamiya K., Fujinami Y., Hoya N., Okamoto Y., Kouike H., Komatsuzaki R., Kusano R., Nakagawa S., Satoh H., Fujii M. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am. J. Pathol. 2007;171:214–226. doi: 10.2353/ajpath.2007.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lanza R. Stem cell breakthrough: Don't forget ethics. Science. 2007;318:1917–1920. doi: 10.1126/science.318.5858.1865a. [DOI] [PubMed] [Google Scholar]