Abstract
The frequency of superantigen production among Staphylococcus aureus isolates associated with endocarditis is not well defined. We tested 154 S. aureus isolates from definite infective endocarditis cases for the presence of staphylococcal enterotoxins A-E, H and TSST-1 by PCR, ELISA and using an HLA-DR3 transgenic mouse splenocyte proliferation assay. Sixty-three isolates (50.8%) tested positive for at least one superantigen gene, with 21 (16.9%) testing positive for more than two. tst (28.6%) was most common, followed by seb (27%), sea (22.2%), sed (20.6%), see (17.5%), and sec (11.1%). Of 41 methicillin-resistant S. aureus, 21 had superantigen genes, with sed being more frequently detected in this group compared to methicillin-susceptible S. aureus (P<0.05). Superantigen genes were not associated with mortality (P=0.81). 75% of PCR-positive isolates induced robust splenocyte proliferation. Overall, more than half of S. aureus isolates causing endocarditis carry superantigen genes of which most are functional.
Introduction
Staphylococcus aureus is a common cause of serious diseases including pneumonia, septicemia, infective endocarditis (IE) and toxic shock syndrome (Klevens, et al., 2007). S. aureus produces many virulence factors, among which are the superantigens (Becker, et al., 2003; Vojtov, Ross, & Novick, 2002). To date, several superantigen (SAg) genes have been identified, and globally, SAg genes have been found in over 70% of S. aureus isolates (Becker, et al., 2003; Varshney, et al., 2009). SAgs trigger a massive release of pro-inflammatory cytokines, and have been associated with septic shock and increased severity of infection (Bone, Grodzin, & Balk, 1997; Dinges, Orwin, & Schlievert, 2000; Ferry, et al., 2005). However, van Belkum et al. suggest that the impact of SAgs in septic shock and mortality is incompletely defined (van Belkum, et al., 2006).
Salgado-Pabón W et al., recently showed that staphylococcal enterotoxin C (SEC) plays a role in the pathogenesis of experimental rabbit S. aureus IE (Salgado-Pabon, et al., 2013). Specifically, it was demonstrated that SEC-production promotes initiation and establishment of vegetations and induces cytokine production by endothelial cells. Previous studies suggest that the ability of S. aureus to cause endocarditis is associated with genotype of the infecting strain (Fowler, et al., 2007; Gill, et al., 2011). Nienaber et al. reported that compared to S. aureus isolates associated with soft tissue infection, S. aureus isolates associated with IE were more likely to contain tst, sea, sed, see, and sei (Nienaber, et al., 2011). They studied 114 methicillin-susceptible S. aureus (MSSA) IE isolates, of which 26 were from North America, showing that tst, sea, seb, sec, sed, see, seg, seh, sei and sej were present in 94, 65, 4, 25, 21, 27, 70, 11, 90 and 4%, respectively. Aside from this study, which included only MSSA, a limited number of North American isolates, and did not examine SAg production, the prevalence of SAgs and their production among S. aureus associated with IE, particularly in the US, has not been well-defined. We analysed the prevalence of SAg genes and their association with outcomes in patients with S. aureus IE. We also assessed for SAg production from S. aureus grown planktonically and in the biofilm state. Finally, we evaluated the biological activity of SAgs produced by IE isolates using an in vitro murine splenocyte proliferation bioassay.
Methods
Bacterial isolates and patient data
One hundred twenty-four clinical S. aureus isolates collected randomly between 1997 and 2011 from patients with definitive diagnosed endocarditis who were admitted to Mayo Clinic in Rochester, MN were studied. Demographic characteristics, clinical presentations and outcomes were assessed by review of the medical records. Definitive S. aureus IE was defined according to the modified Duke Criteria (Li, et al., 2000). Septicemia, systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock were defined according to the criteria of American College of Chest Physicians and the Society of Critical Care Medicine Conference (Bone, et al., 1992). Septicemia was defined by the presence of organism in blood without SIRS, SIRS was defined by the presence of two or more of the following: Body temperature, >38 or <36°C; heart rate, >90 beats per minute; respiratory rate, >20 breaths per min; PaCO2 <32 mmHg); and abnormal leukocyte count (i.e., >12,000 or <4000 cells per mm3 or > 10% of immature neutrophils). Sepsis was defined by the presence of SIRS associated with infection, with severe sepsis defined as sepsis associated with transient hypotension, organ dysfunction, or hypoperfusion. Septic shock was defined as sepsis-induced hypotension despite adequate fluid resuscitation, with hypoperfusion or organ dysfunction. This study was approved by the Institutional Review Board at to Mayo Clinic in Rochester, MN.
Preparation of genomic DNA
Bacteria were grown overnight on sheep blood agar. Five to six colonies were suspended in 180 μL of buffer ATL solution (DNeasy blood & tissue kit; Qiagen, Hilden, Germany), 20 μL of proteinase K added, and the suspension incubated at 56°C for 30 minutes. DNA was extracted according to the manufacturer’s instructions with DNA elution in 100 μL molecular grade water.
Typing of SAg genes
Genes for staphylococcal enterotoxins A, B, C, D, E, H and TSST-1 were assayed using individual real-time PCR assays. The nucleotide sequences of the PCR primers are shown in Table 1. PCR was performed using the LightCycler® 1.0 instrument (Roche Applied Science, Indianapolis, IN) with FastStart SYBR Green Master kits (Roche Applied Science). The presence of PCR product was determined by melting curve analysis, each product having a characteristic melting temperature of the target DNA.
Table 1.
Primer sequences, amplification product size and cycling conditions for detection of superantigen genes by PCR
| Gene | Primer name | Oligonucleotide sequence (5′-3′) | Size of amplification product (base pairs) | PCR cycling1 conditions | Reference |
|---|---|---|---|---|---|
| sea | SEA-1 SEA-2 |
GCA GGG AAC AGC TTT AGG C GTT CTG TAG AAG TAT GAA ACA CG |
521 | 10 s, 95°C; 5 s,52°C; 27 s, 72°C | (Lovseth, Loncarevi c, & Berdal, 2004) |
| seb | SEB-1 SEB-2 |
TCG CAT CAA ACT GAC AAA CG GCA GGT ACT CTA TAA GTG CC |
478 | 10 s, 95°C; 5 s,56°C; 27 s, 72°C | (Johnson, et al., 1991) |
| sec | SA-U SEC-2 |
TGT ATG TAT GGA GGT GTA AC AAT TGT GTT TCT TTT ATT TTC ATA A |
102 | 10 s, 95°C; 10 s,50°C; 15 s, 72°C | (Letertre, Perelle, Dilasser, & Fach, 2003) |
| sed | SED-1 SED-2 |
CTA GTT TGG TAA TAT CTC CT TAA TGC TAT ATC TTA TAG GG |
317 | 10 s, 95°C; 5 s,54°C; 27 s, 72°C | (Johnson, et al., 1991) |
| see | SA-U SEE-2 |
TGT ATG TAT GGA GGT GTA AC GCC AAA GCT GTC TGA G |
213 | 10 s, 95°C; 10 s,50°C; 15 s, 72°C | (Letertre, et al., 2003) |
| seh | SA-U SEH-2 |
TGT ATG TAT GGA GGT GTA AC TCT CTA GGA GTT TTC ATA TC |
245 | 10 s, 95°C; 10 s,48°C; 10 s, 72°C | (Letertre, et al., 2003) |
| tst | TSST-1 TSST-2 |
GCT TGC GAC AAC TGC TAC AG TGG ATC CGT CAT TCA TTG TTA T |
559 | 10 s, 95°C; 5 s,52°C; 27 s, 72°C | (Lovseth, et al., 2004) |
30 cycles
Preparation of planktonic and biofilm supernatants with clinical endocarditis isolates
For planktonic cultures, S. aureus (108 CFU/ml) was grown at 37°C in trypticase soy broth (TSB) for 24 hours. Biofilms were pre-established on 1 cm diameter-Teflon discs by incubating the discs overnight with 106 CFU S. aureus/ml in TSB at 37°C. After 24 hours, the discs were rinsed in sterile saline (0.9% sodium chloride irrigation, Baxter Corp., Deerfield, IL) to remove planktonic cells, placed into 2 ml TSB containing 4 μg/ml vancomycin (to inhibit planktonic growth) and incubated for an additional 24 hours. The fluid surrounding the discs was collected and centrifuged at 4000 g for five minutes. The supernatants were passed through a 0.22 μm syringe filter (MILLEX®GP; Millipore, MA, USA). To determine the bacterial biofilm colony count, the biofilm-coated discs were placed into 1 ml saline and sonicated as previously described (del Pozo, Rouse, Mandrekar, Steckelberg, & Patel, 2009); ~106–108 CFU S. aureus/ml were recovered from each disc. In addition to the IE isolates, we studied isogenic strains of S. aureus that either express only SEB or do not express any SAg, S. aureus RN6734 containing pRN5543::seb (pRN7114), and RN6734 containing a derivative of the plasmid with a large 3′ deletion in seb, pRN5543::seb(b.2) (pRN7116), respectively [generous gifts from Richard Novick, New York University Medical Center, New York, NY (Vojtov, et al., 2002)]. These strains were grown planktonically in TSB with 20 μg/ml chloramphenicol.
ELISA
The presence of staphylococcal enterotoxins A, B, C, D and E in bacterial planktonic and biofilm supernatants was assessed using the TECRA™ Staph Enterotoxins ID kit (3M, St. Paul, Minnesota) following the manufacturer’s protocol. An OD405 <0.2 was considered negative.
T cells proliferation assay with HLA-DR3 transgenic mouse splenocytes
AE°.HLA-DR3 transgenic mice expressing functional HLA-DRA1*0101 and HLA-DRB1*0301 transgenes on a MHC class II-deficient background as well as mice devoid of all endogenous MHC class II molecules (AE°) were studied (Cheng, Smart, Hanson, & David, 2003; Rajagopalan, et al., 2003; Tilahun, Karau, Clark, Patel, & Rajagopalan, 2012). Naïve splenocytes from these mice were cultured in HEPES-buffered RPMI 1640 containing 5% fetal calf serum, serum supplement, and streptomycin and penicillin at 106 cells/ml in 100 μl volumes in 96-well round-bottomed tissue culture plates. Planktonic bacterial supernatants were added at 1 in 256 dilutions in RPMI 1640 culture medium and incubated at 37°C. After 48 hours, 1 μg of tritiated [3H] thymidine was added, incubated for additional 18 hours and the extent of proliferation determined by measuring incorporated radioactivity using standard procedures.
Statistical analysis
Statistical analysis was performed using SPSS software (version 19.0; SPSS, Chicago, IL, USA). Continuous scaled data were compared using the Student’s t- or Mann-Whitney U tests and categorical variables were compared using Chi-square or Fisher’s exact test.
Results
Study population
S. aureus clinical isolates from 124 patients with definitive IE were studied. Patient demographics are presented in Table 2. The mean patient age was 63.4±16.0 (range 20–95) years, and 72 (58.1%) were male. Twenty (16.1%) patients had prosthetic valve endocarditis and 41 (33.1%) isolates were resistant to methicillin. Seventeen patients (13.7%) had septic shock and 20 (16.1%) died as a result of S. aureus endocarditis.
Table 2.
Characteristics of subjects with Staphylococcus aureus endocarditis
| Characteristic | No. (%) (Total N=124) |
|---|---|
| Mean age in years (range) | 63.4±16.0 (20 – 95) |
| Gender | |
| Male | 72 (58.1) |
| Female | 52 (41.9) |
| No. with methicillin-resistant S. aureus | 41 (33.1) |
| No. with a prosthetic valve | 20 (16.1) |
| Severity of disease | |
| Septicemia | 30 (24.2) |
| Sepsis | 70 (56.5) |
| Severe sepsis | 7 (5.6) |
| Septic shock | 17 (13.7) |
| Endocarditis-related death | 20 (16.1) |
Distribution of SAg genes among S. aureus endocarditis isolates
Of the 124 isolates, 63 (50.8%) carried SAg genes (Table 3). Of the 63, 42 tested positive for just one of the seven SAg genes assessed and 21 (33.3%) had two or more SAg genes detected. tst (28.6%) was most common SAg gene detected, followed by seb (27%), sea (22.2%), sed (20.6%), see (17.5%), and sec (11.1%). tst was detected in 18 isolates, 13 (72.2%) of which carried genes for other SAgs (Table 3).
Table 3.
Correlation between the presence of superantigen genes in S. aureus endocarditis isolates and patient survival
| Gene | No (%) of S. aureus isolates with superantigen genes from patients with endocarditis
|
|||
|---|---|---|---|---|
| Total N=124 |
Alive N=104 |
Deceased N=20 |
P value | |
| Detection of gene(s) | 63 (50.8) | 52 (50.0) | 11 (55.0) | 0.81 |
| One superantigen | 42 (66.7) | 35 (67.3) | 7 (63.6) | |
| ≥2 Superantigens | 21 (33.3) | 17 (32.7) | 4 (36.4) | |
| Gene detected1 | ||||
| sea | 14 (22.2) | 10 (19.2) | 4 (36.4) | 0.24 |
| seb | 17 (27.0) | 16 (30.8) | 1 (9.1) | 0.30 |
| sec | 7 (11.1) | 5 (9.6) | 2 (18.2) | 0.32 |
| sed | 13 (20.6) | 10 (19.2) | 3 (27.3) | 0.44 |
| see | 11 (17.5) | 9 (17.3) | 2 (18.2) | 0.41 |
| seh | 7 (11.1) | 5 (9.6) | 2 (18.2) | 0.32 |
| tst | 18 (28.6) | 16 (30.8) | 2 (18.2) | 0.74 |
Distribution of individual superantigen genes among superantigen gene-positive S. aureus endocarditis isolates
Of the 124 S. aureus isolates, 17 (13.7%) were associated with septic shock of which nine were PCR-positive with four (44.4%) having a single detected SAg gene and five (55.6%) having two or more SAg genes detected. Among the SAg-positive S. aureus, although the septic shock-associated isolates had a trend towards having a higher prevalence of sea (44.4%), sec (22.2%), see (22.2%) and tst (33.3%), than those not associated with septic shock, these differences were not statistically significant (P=0.10, 0.25, 0.51 and 0.46, respectively). The overall prevalence of the seven SAg genes assessed did not differ between isolates with regards to the presence or absence of septic shock (Table 4).
Table 4.
Correlation between the presence of superantigen genes in S. aureus endocarditis isolates and the occurrence of septic shock
| Gene | No (%) of S. aureus isolates with superantigen genes from patients with endocarditis
|
||
|---|---|---|---|
| No. with septic shock (%) N=17 | No. without septic shock (%) N=107 | P value | |
| Detection of genes | 9 (52.9) | 54 (50.5) | 0.53 |
| One superantigen | 4 (44.4) | 38 (70.4) | |
| ≥2 Superantigens | 5 (55.6) | 16 (29.6) | |
| Gene detected1 | |||
| sea | 4 (44.4) | 10 (18.5) | 0.10 |
| seb | 2 (22.2) | 15 (27.8) | 0.58 |
| sec | 2 (22.2) | 5 (9.3) | 0.25 |
| sed | 1 (11.1) | 12 (22.2) | 0.44 |
| see | 2 (22.2) | 10 (18.5) | 0.51 |
| seh | 1 (11.1) | 6 (11.1) | 0.65 |
| tst | 3 (33.3) | 15 (27.8) | 0.46 |
Distribution of individual superantigen genes among superantigen gene-positive S. aureus endocarditis isolates
Among the total 124 isolates, 41 (33.1%) were methicillin-resistant S. aureus (MRSA) of which, 21 (51.2%) had SAg genes. Eighty-three of 124 (66.9%) were MSSA, with 42 (50.6%) having SAg genes (Table 5). Although the overall distribution of SAg genes between MRSA and MSSA was not different (P=1.00), MRSA were more often positive for sed than were MSSA isolates (52.4% versus 4.8%, P<0.05). Conversely, MSSA were more often positive for sea than were MRSA isolates (31% versus 4.8%, P<0.05). The overall presence of SAg genes among isolates associated with native and prosthetic valve IE was similar (50.9% and 50.0%, respectively, P=1.00, Table 5).
Table 5.
Correlation of methicillin susceptibility and valve type with the presence of superantigen genes in S. aureus endocarditis isolates
| Gene | Methicillin susceptibility | Valve type | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Susceptible (%) N=83 | Resistant (%) N=41 | P value | Native (%) N=104 | Prosthetic (%) N=20 | P value | |
| Detection of gene(s) | 42 (50.6) | 21 (51.2) | 1.00 | 53 (50.9) | 10 (50.0) | 1.00 |
| One superantigen | 23 (54.8) | 19 (90.5) | 33 (62.3) | 9 (90.0) | ||
| ≥2 Superantigens | 19 (42.2) | 2 (9.5) | 20 (37.7) | 1 (10.0) | ||
| Toxin detected1 | ||||||
| sea | 13 (31.0) | 1 (4.8) | 0.03 | 12 (22.6) | 2 (20.0) | 1.00 |
| seb | 14 (33.3) | 3 (14.3) | 0.18 | 16 (30.2) | 1 (10.0) | 0.30 |
| sec | 7 (16.7) | 0 | 0.94 | 7 (13.2) | 0 | 0.60 |
| sed | 2 (4.8) | 11 (52.4) | <0.01 | 11 (20.8) | 2 (20.0) | 1.00 |
| see | 10 (23.8) | 2 (9.5) | 0.33 | 10 (18.9) | 2 (20.0) | 1.00 |
| seh | 6 (14.3) | 1 (4.8) | 0.42 | 6 (11.3) | 1 (10.0) | 1.00 |
| tst | 14 (33.3) | 4 (19.0) | 0.42 | 15 (28.3) | 3 (30.0) | 1.00 |
Distribution of individual superantigen genes among superantigen gene-positive S. aureus endocarditis isolates
Detection of SAg in planktonic and biofilm supernatants using ELISA
Planktonic and biofilm culture supernatants from a convenience sample of 30 S. aureus isolates, 13 positive for SAg by PCR (sea to see, but not tst) and 17 negative for any of the SAgs assessed, were screened for the presence of the SAgs using ELISAs. Of the 13 planktonic supernatants from S. aureus isolates that were positive by PCR, 11 were also positive for the corresponding SAg by ELISA (Table 6). Only two of the planktonic supernatants from PCR-positive isolates (both positive for sed) were negative by ELISA (i.e., OD405<0.2). Interestingly, all 13 biofilm supernatants from the PCR-positive isolates were strongly positive by ELISA for their respective SAg, suggesting that biofilms produce more SAg compared to planktonically grown bacteria (mean±SE, 2.250±1.174 vs 0.663±0.602, respectively, P<0.05). For example, the OD405 values for the six SED-positive planktonic culture supernatants shown in Table 6 were 0.600±0.230 (mean±SE), whereas the OD405 values for the same isolates grown as biofilms was 2.605±0.523 (P<0.05). Moreover, under identical planktonic culture conditions, some isolates harbouring the same SAg gene produced different amounts of SAg. For example, among the three SEB-positive isolates (IDRL-4420, -4587 and -5494), only one (IDRL-4420) produced high amounts of SEB under planktonic culture conditions, whereas in the biofilm state, all three produced high levels of SEB. Similarly, not all SED-positive isolates produced equivalent amounts of SED; some produced high amounts of SAg (OD405 >1; IDRL-6728 and IDRL-6982), some produced intermediate amounts of SAg (OD405 0.3 to 1; IDRL-4576 and IDRL-6609), and some produced low amounts of SAg (OD405 <0.3; IDRL-6517 and IDRL-6613) when grown planktonically. Therefore, there appears to be variability in the amount of SAg produced between different isolates.
Table 6.
Comparison of superantigen detection by PCR and ELISA in S. aureus endocarditis isolates.
| Isolate | Gene detected by PCR | Superantigen detected by ELISA1
|
|
|---|---|---|---|
| Planktonic growth (OD405) | Biofilm growth (OD405) | ||
| 4297 | sea, see | SEA (0.747), SEE (0.189) | SEA (1.397), SEE (0.268) |
| 4319 | sea | SEA (0.564) | SEA (1.590) |
| 4578 | sea | SEA (0.517) | SEA (1.437) |
| 4420 | seb | SEB (2.281) | SEB (2.597) |
| 4587 | seb | SEB (0.334) | SEB (2.364) |
| 5494 | seb | SEB (0.334) | SEB (2.220) |
| 4320 | sec | SEC (0.716) | SEC (4.000) |
| 4576 | sed | SED (0.421) | SED (0.496) |
| 6517 | sed | Negative | SED (1.636) |
| 6609 | sed | SED (0.316) | SED (3.308) |
| 6613 | sed | Negative | SED (2.887) |
| 6728 | sed | SED (1.512) | SED (3.504) |
| 6982 | sed | SED (1.073) | SED (3.803) |
| 4288 | None | Negative | Negative |
| 4295 | None | Negative | Negative |
| 4329 | None | Negative | Negative |
| 4331 | None | Negative | Negative |
| 4334 | None | Negative | Negative |
| 4335 | None | Negative | Negative |
| 4339 | None | Negative | Negative |
| 4341 | None | Negative | Negative |
| 4372 | None | Negative | Negative |
| 4408 | None | Negative | Negative |
| 4410 | None | Negative | Negative |
| 4577 | None | Negative | Negative |
| 5417 | None | Negative | Negative |
| 5525 | None | Negative | Negative |
| 6512 | None | Negative | Negative |
| 6523 | None | Negative | Negative |
| 6528 | None | Negative | Negative |
OD405 <0.2 considered negative
T cell proliferation induced by superantigen
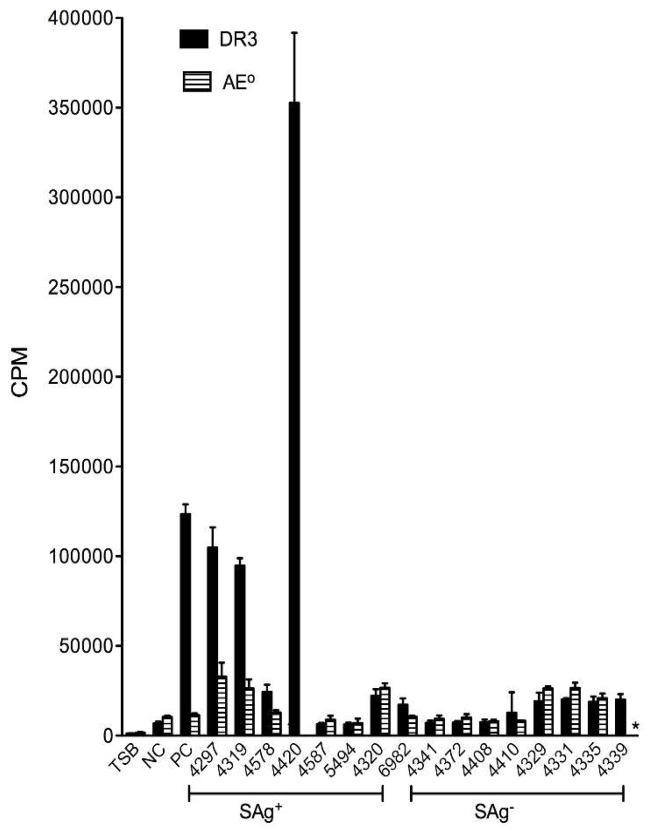
A convenience sample of eight SAg-positive and eight SAg-negative planktonic culture supernatants that had been tested by ELISA were examined for mitogenicity in an in vitro proliferation assay using splenocytes from AE° and HLA-DR3 transgenic mice. We have previously shown that HLA class II transgenic mice respond more robustly to staphylococcal SAgs than conventional (e.g., C57Bl/6) laboratory mice (Rajagopalan, et al., 2003; Tilahun, et al., 2012). Culture supernatants from the control SEB-positive strain induced robust proliferation in splenocytes from HLA-DR3 transgenic but not AE° mice (Figure 1). Since AE° mice lack MHC class II molecules, we conclude that SEB was responsible for mitogenicity of the culture supernatant. Supernatant from the isogenic SEB-negative control strain was not mitogenic to splenocytes, authenticating the SAg specificity of splenocyte proliferation assay. There was variation in the mitogenicity of culture supernatants among the IE isolates tested, with results generally correlating with those of ELISA. For example, of the three SEB-positive isolates tested (IDRL-4420, -4587 and -5494), planktonic cultures of IDRL-4420, which had highest amounts of SEB by ELISA also induced the most splenocyte proliferation. Mitogenicity of the three SEA-positive isolates (IDRL-4297, -4319 and -4578) also correlated with SEA levels detected by ELISA. IDRL-4297 was most mitogenic, possibly due to the additional presence of SEE, followed by IDRL-4319 and -4578. Supernatant from IDRL-4320 caused low but similar proliferation in DR3 and AE° splenocytes, indicating non-specific proliferation. While supernatant from the SED-positive isolate IDRL-6982 was not highly mitogenic, it induced more proliferation in splenocytes from DR3 compared to AE° mice (CPM of 17237±3574 versus 10561±580, respectively) suggesting SAg-dependent proliferation (Figure 1). None of the eight supernatants from SAg-negative isolates were differentially mitogenic to DR3 splenocytes, suggesting absence of any SAgs.
Figure 1.
Superantigencity of staphylococcal endocarditis isolates. A thymidine-based proliferation assay using splenocytes from AE° and HLA-DR3 transgenic mice was used to assess the biological activity of superantigens in bacterial culture supernatants. The following planktonic culture supernatants were diluted in RPMI (1 in 256 dilution) and tested: IDRL-4297, IDRL-4319 and IDRL-4578 (positive for sea and tst); IDRL-4420, IDRL-4587 and IDRL-5494 (positive for seb); IDRL-4320 (positive for sec and tst) and IDRL-6982 (positive for sed). The remainder of the supernatants were from isolates that tested negative for sea, seb, sec, sed, see, seh and tst by PCR. Planktonic culture supernatant of S. aureus RN6734 containing intact cloned seb pRN5543::seb (pRN7114) was used as a positive control (PC). Supernatant from S. aureus RN6734 containing seb with a large 3′ deletion, pRN5543::seb(b.2) (pRN7116) was used as a negative control (NC). TSB indicates splenocytes cultured with trypticase soy broth alone. Each bar represents the mean±standard error of triplicate wells. CPM, counts per minute. *Not done.
Discussion
S. aureus is one of the most frequent bacterial pathogens and causes several life-threatening infectious diseases. Staphylococcal SAgs are important virulence factors that influence a variety of diseases caused by S. aureus, including toxic shock syndrome, pneumonia and sepsis (Schlievert, et al., 2000). Recent studies have investigated the prevalence of SAg genes among clinical S. aureus strains isolated from different specimens, however there is a lack of a large and well-characterized population of IE isolates among them (Becker, et al., 2003; Campbell, et al., 2008; Holtfreter, et al., 2007; van Belkum, et al., 2006; Varshney, et al., 2009). Bannan et al. (Bannan, Visvanathan, & Zabriskie, 1999) suggested that SAgs amplify an inflammatory response through the adaptive immune system in the septic shock. While the role of SAgs in the pathogenesis of human IE is incompletely understood, based on experimental IE models it is likely that they contribute to the pathogenesis of IE in humans (Salgado-Pabon, et al., 2013). Therefore, it is important to establish the prevalence of SAgs in human IE and to establish associations between their presence and disease characteristics and outcome.
In the present study, 63 S. aureus isolates (50.8%) collected from patients with IE had at least one SAg gene which is lower than the 70–90% reported in studies of clinical isolates (Becker, et al., 2003; Desachy, et al., 2007; Ferry, et al., 2005; Nienaber, et al., 2011; Varshney, et al., 2009). Further, only 17 subjects (13.7%) had septic shock and just nine of those with septic shock (52.9%) were infected with isolates with SAg genes. Among the staphylococcal SAgs, tst is the most common SAg associated with staphylococcal toxic shock syndrome, whereas a higher rate of sea has been shown in patients with S. aureus-mediated septic shock (Dinges, et al., 2000; Ferry, et al., 2005; Peacock, et al., 2002). In the current study, although we noticed a trend toward higher prevalence of sea in isolates from endocarditis with septic shock, this finding was not statistically significant. Overall, the occurrence of septic shock per se was not correlated with the presence of SAg genes.
Similar to a Chinese study showing that MRSA more frequently carry sed than MSSA (Yu, et al., 2012), MRSA in our study was more often positive for sed than was MSSA (P <0.05). However, Yu et al. did not find any difference in the distribution of sea between MRSA and MSSA, while in our study, sea was more frequently present in MSSA than MRSA (P<0.05). This could be due to geographical differences and/or the type of infection (bacteremia versus IE). There was no difference in prevalence of SAgs and clinical outcomes between native and prosthetic valve IE.
The major strength of our study is the large size number of IE isolates, all associated with definite IE. However, a limitation is that we only analyzed seven classical staphylococcal SAg genes, sea through see and tst, while more than 20 different types SAgs (Proft & Fraser, 2003) have been described to date. Despite this limitation, the in vitro proliferation assay using splenocytes from AE° and HLA-DR3 transgenic mice should have detected any functional SAgs including those not targeted through our molecular studies.
Based on the semi-quantitative ELISA and the in vitro proliferation assay using splenocytes from AE° and HLA-DR3 transgenic mice, we investigated the functionality of SAgs and deduced their potential role in vivo. Several interesting observations emerged, which may have clinical relevance. First, the same isolate can produce different amounts of SAgs in planktonic and biofilm cultures. Even though biofilm cultures contained equal or lower numbers of bacteria compared to planktonic cultures, they yielded more biologically active SAgs. To our knowledge, this phenomenon has not been described. Since in IE S. aureus grows in a biofilms on the endocardium (Salgado-Pabon, et al., 2013), higher production of SAg during biofilm growth in vitro may have clinical significance in vivo. Also, when assessing for SAg production, it may be most fruitful to do so with bacteria grown in the biofilm state. Second, among isolates harbouring the same SAg gene, the amount of SAg produced varied. The reasons for this discrepancy warrant further investigation at a molecular level. A limitation of our study is that the T cell proliferation assay was performed on planktonic and not biofilm culture supernatants. Furthermore, while T cell proliferation is a sensitive bioassay for detecting SAgs, a few confounding factors need to be taken into consideration, including the binding affinity of SAgs for the HLA class II isotype (HLA-DR versus HLA-DQ), their preference for specific HLA class II alleles (e.g., HLA-DR2, HLA-DR3, HLA-DQ6 or HLA-DQ8) and the frequency of T cells bearing the specific TCR Vβ family (or families) reacting with individual SAgs. Therefore, although higher amounts of SAg may be present in culture supernatants, a lower affinity of the SAg to the specific HLA class II molecules expressed on the antigen presenting cells and lower frequency of T cells bearing the specific TCR Vβ family may result in lower thymidine incorporation.
In conclusion, our study showed that more than half of S. aureus isolates causing IE carry one of the seven SAg genes tested with tst (28.6%) and seb (27%) being the most common. While the prevalence of certain SAg genes was different between MRSA and MSSA, their presence was not associated with septic shock or death. The major strength of our study is the large sample size. However, given the presence of more than 20 different types SAg genes and their broader distribution among the isolates, both of which are beyond our control, establishing a meaningful association between the presence of SAg, the type of SAg present and clinical outcome is challenging. Nonetheless, this study provides data regarding the distribution of SAg genes in IE isolates, providing insight into the pathogenesis of staphylococcal IE. There is heterogeneity in the amount of SAg produced between isolates and under varied growth conditions, with more SAg production from biofilm than planktonic cells. This observation regarding SAg may be applicable to other staphylococcal exotoxins (e.g., Panton-Valentine leukocidin, hemolysins); use of both genetic and functional assays may be important when assaying exotoxin production by S. aureus.
Acknowledgments
This study was funded by NIH grants AI101172 to GR and AI68741 to GR and CSD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bannan J, Visvanathan K, Zabriskie JB. Structure and function of streptococcal and staphylococcal superantigens in septic shock. Infectious disease clinics of North America. 1999;13:387–96. ix. doi: 10.1016/s0891-5520(05)70081-7. [DOI] [PubMed] [Google Scholar]
- Becker K, Friedrich AW, Lubritz G, Weilert M, Peters G, Von Eiff C. Prevalence of genes encoding pyrogenic toxin superantigens and exfoliative toxins among strains of Staphylococcus aureus isolated from blood and nasal specimens. Journal of clinical microbiology. 2003;41:1434–9. doi: 10.1128/JCM.41.4.1434-1439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–43. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- Campbell SJ, Deshmukh HS, Nelson CL, Bae IG, Stryjewski ME, Federspiel JJ, et al. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. Journal of clinical microbiology. 2008;46:678–84. doi: 10.1128/JCM.01822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Smart M, Hanson J, David CS. Characterization of HLA DR2 and DQ8 transgenic mouse with a new engineered mouse class II deletion, which lacks all endogenous class II genes. Journal of autoimmunity. 2003;21:195–9. doi: 10.1016/s0896-8411(03)00120-3. [DOI] [PubMed] [Google Scholar]
- del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R. The electricidal effect: reduction of Staphylococcus and pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrobial agents and chemotherapy. 2009;53:41–5. doi: 10.1128/AAC.00680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desachy A, Lina G, Vignon P, Hashemzadeh A, Denis F, Etienne J, et al. Role of superantigenic strains in the prognosis of community-acquired methicillin-susceptible Staphylococcus aureus bacteraemia. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2007;13:1131–3. doi: 10.1111/j.1469-0691.2007.01810.x. [DOI] [PubMed] [Google Scholar]
- Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clinical Microbiology Reviews. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry T, Thomas D, Genestier AL, Bes M, Lina G, Vandenesch F, et al. Comparative prevalence of superantigen genes in Staphylococcus aureus isolates causing sepsis with and without septic shock. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005;41:771–7. doi: 10.1086/432798. [DOI] [PubMed] [Google Scholar]
- Fowler VG, Jr, Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, Archer GL, et al. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. The Journal of infectious diseases. 2007;196:738–47. doi: 10.1086/520088. [DOI] [PubMed] [Google Scholar]
- Gill SR, McIntyre LM, Nelson CL, Remortel B, Rude T, Reller LB, et al. Potential associations between severity of infection and the presence of virulence-associated genes in clinical strains of Staphylococcus aureus. PloS one. 2011;6:e18673. doi: 10.1371/journal.pone.0018673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtfreter S, Grumann D, Schmudde M, Nguyen HT, Eichler P, Strommenger B, et al. Clonal distribution of superantigen genes in clinical Staphylococcus aureus isolates. Journal of clinical microbiology. 2007;45:2669–80. doi: 10.1128/JCM.00204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA: the journal of the American Medical Association. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, Lamlertthon S, Park L, Rude TH, et al. Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. The Journal of infectious diseases. 2011;204:704–13. doi: 10.1093/infdis/jir389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock SJ, Moore CE, Justice A, Kantzanou M, Story L, Mackie K, et al. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infection and immunity. 2002;70:4987–96. doi: 10.1128/IAI.70.9.4987-4996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proft T, Fraser JD. Bacterial superantigens. Clinical and Experimental Immunology. 2003;133:299–306. doi: 10.1046/j.1365-2249.2003.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan G, Smart MK, Cheng S, Krco CJ, Johnson KL, David CS. Expression and function of HLA-DR3 and DQ8 in transgenic mice lacking functional H2-M. Tissue antigens. 2003;62:149–61. doi: 10.1034/j.1399-0039.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- Salgado-Pabon W, Breshears L, Spaulding AR, Merriman JA, Stach CS, Horswill AR, et al. Superantigens are critical for Staphylococcus aureus Infective endocarditis, sepsis, and acute kidney injury. mBio. 2013:4. doi: 10.1128/mBio.00494-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert PM, Jablonski LM, Roggiani M, Sadler I, Callantine S, Mitchell DT, et al. Pyrogenic toxin superantigen site specificity in toxic shock syndrome and food poisoning in animals. Infection and immunity. 2000;68:3630–4. doi: 10.1128/iai.68.6.3630-3634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilahun AY, Karau MJ, Clark CR, Patel R, Rajagopalan G. The impact of tacrolimus on the immunopathogenesis of staphylococcal enterotoxin-induced systemic inflammatory response syndrome and pneumonia. Microbes and infection/Institut Pasteur. 2012;14:528–36. doi: 10.1016/j.micinf.2012.01.001. [DOI] [PubMed] [Google Scholar]
- van Belkum A, Melles DC, Snijders SV, van Leeuwen WB, Wertheim HF, Nouwen JL, et al. Clonal distribution and differential occurrence of the enterotoxin gene cluster, egc, in carriage- versus bacteremia-associated isolates of Staphylococcus aureus. Journal of clinical microbiology. 2006;44:1555–7. doi: 10.1128/JCM.44.4.1555-1557.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney AK, Mediavilla JR, Robiou N, Guh A, Wang X, Gialanella P, et al. Diverse enterotoxin gene profiles among clonal complexes of Staphylococcus aureus isolates from the Bronx, New York. Applied and environmental microbiology. 2009;75:6839–49. doi: 10.1128/AEM.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtov N, Ross HF, Novick RP. Global repression of exotoxin synthesis by staphylococcal superantigens. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10102–7. doi: 10.1073/pnas.152152499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Li T, Huang X, Xie J, Xu Y, Tu J, et al. Virulence gene profiling and molecular characterization of hospital-acquired Staphylococcus aureus isolates associated with bloodstream infection. Diagnostic microbiology and infectious disease. 2012;74:363–8. doi: 10.1016/j.diagmicrobio.2012.08.015. [DOI] [PubMed] [Google Scholar]