Abstract
P2X receptors (P2XRs) are a family of cation-permeable ligand-gated ion channels activated by synaptically released extracellular ATP. The P2X4 subtype is abundantly expressed in the CNS and is sensitive to low intoxicating ethanol concentrations. Genetic meta-analyses identified the p2rx4 gene as a candidate gene for innate alcohol intake and/or preference. The current study used mice lacking the p2rx4 gene (knockout, KO) and wildtype (WT) C57BL/6 controls to test the hypothesis that P2X4Rs contribute to ethanol intake. The early acquisition and early maintenance phases of ethanol intake were measured with three different drinking procedures. Further, we tested the effects of ivermectin (IVM), a drug previously shown to reduce ethanol’s effects on P2X4Rs and to reduce ethanol intake and preference, for its ability to differentially alter stable ethanol intake in KO and WT mice. Depending on the procedure and the concentration of the ethanol solution, ethanol intake was transiently increased in P2X4R KO versus WT mice during the acquisition of 24-hr and limited access ethanol intake. IVM significantly reduced ethanol intake in P2X4R KO and WT mice, but the degree of reduction was 50% less in the P2X4R KO mice. Western blot analysis identified significant changes in -γ aminobutyric acidA receptor (GABAAR) α1 subunit expression in brain regions associated with the regulation of ethanol behaviors in P2X4R KO mice. These findings add to evidence that P2X4Rs contribute to ethanol intake and indicate that there is a complex interaction between P2X4Rs, ethanol, and other neurotransmitter receptor systems.
Keywords: P2X4 receptor, ethanol acquisition, p2rx4 gene, alcohol use disorders (AUDs)
Introduction
Ligand gated ion channels (LGICs) are widely held to play an important role in ethanol-induced behaviors and drinking [1–8]. Research in this area has focused on investigating the effects of ethanol on two large “superfamilies” of LGICs: 1) The nicotinic acetylcholine receptor superfamily (cys-loop) with members including nicotinic acetylcholine receptors (nAChRs), 5- hydroxytryptamine type 3 receptors (5-HT3Rs), γ-aminobutyric acid type-A receptors (GABAARs) and glycine receptors [9,10] and 2) The glutamate superfamily [11,12].
P2X receptors (P2XRs) constitute a third superfamily of LGICs that are becoming a focus of investigation in neuroscience and ethanol studies [13–17]. P2XRs are fast acting, cation-permeable ion channels that are gated by synaptically released extracellular adenosine 5′-triphosphate (ATP) [18–20]. In the central nervous system (CNS), ATP directly mediates fast excitatory synaptic transmission by acting on P2XRs located on postsynaptic membranes. In addition, ATP can modulate the actions of other neurotransmitters (e.g., GABA, glycine and glutamate), known to play important roles in ethanol drinking and other behaviors, by acting on P2XRs located on pre- and postsynaptic membranes [18,19,21–23].
Of the seven P2XR subtypes, P2X4Rs are the most abundantly expressed in the CNS ranging from neurons to microglia [24,25]. Several lines of evidence suggest that P2X4Rs can modulate a spectrum of the effects of ethanol. In vitro studies report that ethanol concentrations starting at approximately 5 mM modulate ATP-activated currents in neurons [26–30] and recombinant models [31–36]. This concentration of ethanol is well below the 17 mM (i.e., 0.08%) blood ethanol concentration (BEC) that is considered “legally intoxicated” in the U.S. In addition, P2X4Rs are located in brain regions that have been identified as neural substrates of alcohol [e.g., hippocampus, cerebellum, ventral tegmental area (VTA) and nucleus accumbens (NAc)] [37–40].
Recent studies implicate P2X4Rs in the regulation of multiple CNS functions, including neuropathic pain [41,42], neuroendocrine functions [43] and hippocampal plasticity [23,44,38]. In addition, P2X4Rs have been recently shown to modulate the function of other major ionotropic targets, such as GABAARs [45] and N-methyl-D-aspartate receptors (NMDARs) [23]. Many physiological and behavioral functions linked to P2X4Rs are also affected by ethanol. In addition, converging evidence suggests a possible role for P2X4Rs in the genetic vulnerability for increased ethanol intake. These investigations, using microarray techniques, found an inverse relationship between p2rx4 gene expression and innate ethanol consumption and preference in rodents. Kimpel et al. [46] examined gene expression in brain areas associated with reward in inbred alcohol preferring (iP) and non-preferring (iNP) rat lines and found that functional p2rx4 expression was significantly reduced in iP rats. Along similar lines, Tabakoff and colleagues [47] found lower levels of whole brain expression of p2rx4 mRNA in inbred rats that display a high ethanol-drinking phenotype compared to those with a lower ethanol-drinking phenotype. Furthermore, pre-treatment with ivermectin (IVM), a drug that antagonizes ethanol-mediated inhibition of recombinant P2X4Rs in vitro [36,48,49], significantly reduced two-bottle choice ethanol intake and operant ethanol self-administration in mice [50,49].
Collectively, the findings outlined above suggest that P2X4Rs contribute to ethanol intake and that there is an inverse relationship between P2X4R activity and ethanol intake. Yet, direct evidence is lacking. The present study tests these two related hypotheses using a gene knockout (KO) strategy in combination with three measures of ethanol intake and one measure of ethanol sensitivity. And, based on evidence for developmental compensations in KO mice [51,52] and for cross-talk between P2X4Rs and GABAARs [45], protein expression of the GABAAR α1 subunit was measured because this subunit is widely expressed throughout the brain and is suggested to contribute to some behavioral effects of ethanol [53–55].
Materials and Methods
Animals
The present study utilized male homozygous p2rx4 null (i.e., P2X4R KO) mice from a breeding colony that we established at University of Southern California (USC; Los Angeles, CA). Re-derivation of P2X4R heterozygous (HZ) mice was performed by the USC transgenic core using frozen HZ embryos obtained from a previously established P2X4R KO colony [38]. This effort resulted in 7 HZ mice, which were backcrossed with C57BL/6J mice to produce the 1st generation of offspring. HZ offspring were backcrossed every three generations with wildtype (WT) C57BL/6J mice that were purchased from Jackson Laboratory (Bar Harbor, ME). Quantitative real time polymerase chain reaction was used to genotype DNA extracted from tail biopsies by probing with primers specific for LacZ (5′GCGAACGCGAATGGTGCAGC 3′) and P2X4 (5′TCGCTCTCTGGGTCTGGGGC 3′). All studies were conducted with male mice that were at least 2 months and no more than 6 months of age. Animals of similar age were used within each test. We used C57BL/6 mice from our colony or from Jackson Laboratory as WT controls. Pilot studies indicated that C57BL/6 mice from these different sources did not differ significantly in ethanol or total fluid intake. Upon weaning, all animals were separated by sex and group housed at 4–5 per polycarbonate cage until testing. Prior to the start of the drinking procedures, mice were acclimated to individual housing and a reverse light-dark cycle (12/12h; lights off at 1300 h) for a minimum of one week with access to only water. Mice received ad libitum access to food and water bottles fitted with ball-bearing sippers. All handling and experimental procedures were performed in accordance with NIH guidelines and protocols that were approved by USC’s Institutional Animal Care and Use Committee.
Drugs
Ethanol solutions were prepared from USP grade ethyl alcohol (Gold Shield Chemical Company, Hayward, CA) (200 proof) diluted (v/v) in tap water for drinking or 0.9% sodium chloride for systemic intraperitoneal (i.p.) injection. Saccharin (Sigma-Aldrich, St. Louis, MO) was prepared as a 0.033% and 0.066% w/v solution in tap water. Drinking solutions were prepared and refreshed once per week, while solutions for systemic administration were prepared fresh on the day of the experiment.
Western Blot Protein Analysis of Brain Tissue
Mice were euthanized by CO2 inhalation. Whole brain and liver tissue were excised from mice for quantification of P2X4R subunit expression. Similar to methods described by Sim and colleagues [38], tissues were homogenized in ice-cold hypotonic homogenization buffer (10 mM Tris-HCl, pH 7.4, 1 mM MgCl2 and protease inhibitor cocktail). Sodium chloride was added to the final concentration of 155 mM and the homogenates cleared by centrifugation. Membranes were recovered from the supernatant by ultracentrifugation (45 minutes at 100,000 X g, 4°C). Pelleted membranes were solubilized by incubation in phosphate buffer solution (PBS) containing 1% Triton X100. Solubilized protein was cleared by centrifugation at 13,000 rpm at 4°C for 10 minutes, and the protein concentration was determined with a protein assay kit (BCA, Pierce Biotechnology, Rockford, IL). The cerebellum, prefrontal cortex, striatum (dorsal and ventral), midbrain, hippocampus, and amygdala were dissected for quantification of GABAAR α1 subunit expression. These brain regions were chosen, based on their involvement in the neurocircuitry of ethanol reward [56] and in measures of ethanol intoxication [57]. Brain tissues were homogenized using 500μl of homogenization buffer (50mM Tris-HCl (pH 8.0), 150mM NaCl, 1mM EDTA, 0.1% SDS, 1/100 protein inhibitor cocktail) and sonicated. Samples (50 μg/lane) were run on 10% SDS-PAGE and transferred to polyvinylidine difluoride (PVDF) membranes. The membranes were blocked in 5% dry milk and incubated overnight at 4°C with a rabbit anti-P2X4 primary antibody (1:2000 dilution, Alomone Labs, Israel) and rabbit anti-GABAAR α1 antibody (1:1000 dilution, Phosphosolutions, Aurora, CO). Protein bands were visualized using enhanced chemiluminescence (Bio-Rad Laboratories) after incubation with the respective secondary anti-rabbit and anti-mouse antibody (1:20000 dilution, Bio-Rad Laboratories, Irvine, CA).
24-hr Access, Two-Bottle Choice
The 24-hr access, two-bottle choice (i.e., preference) ethanol drinking model [58–62] is widely used to assess changes in drinking and preference and is considered a model of “social drinking” behavior in rodents since sustained elevated ethanol intake, BECs, and intoxication are not achieved [63]. We utilized a modification of the procedure described previously by Yoneyama et al. [62] and Yardley et al. [50] to investigate differences in the acquisition of ethanol drinking between WT and P2X4R KO mice. In this experiment, we assessed ethanol intake in male P2X4R KO mice, using C57BL/6J mice (Jackson Laboratories) as the WT controls. This was due, in part, to the limited number of WT animals that were available at time of the study. Briefly, mice had 24-hr access to a 25 mL bottle containing a 10% ethanol solution (10E) and another containing tap water for 4 consecutive days. Bottle volumes were measured in the morning on each day with positions switched every other day to avoid side preferences. Body weights were measured for each mouse to calculate the g/kg/24-hr intake of ethanol. The percent preference for ethanol was calculated as the volume of the ethanol solution consumed divided by the total fluid volume consumed (i.e., ethanol + water) x 100.
A separate group of male C57BL/6 and P2X4R KO mice were tested for saccharin intake using the same two-bottle choice paradigm used in the ethanol study. These animals had a choice between a 25 mL bottle containing 0.033% saccharin or tap water for a period of four consecutive days. This was followed by a choice between 0.066% saccharin solution and tap water for four consecutive days.
24-hr Access, Two-Bottle Choice with Ivermectin (IVM)
IVM is a well-tolerated, broad spectrum anti-parasitic medication in humans and animals that is becoming a recognized pharmacological tool for identifying the contribution of P2X4Rs in ATP-mediated processes [64,19,16,36]. We recently found that IVM significantly reduced alcohol intake and preference in a dose dependent manner in male and female C57BL/6 mice [50].
We used a modification of the 24-hr access, two-bottle choice procedure described above and previously described [50]. The study was conducted in a separate group of P2X4R KO mice and C57BL/6J mice (Jackson Laboratories) as the WT controls. Prior to the study start, KO mice had been used in a pilot experiment measuring intake of 20% v/v ethanol (20E), 10E, and 5% v/v ethanol (5E) solutions in a modified drinking-in-the-dark (DID) procedure [65,66]. For the DID procedure, on days 1–3, mice received a single bottle of an ethanol solution for 2 hours, beginning 3 hours into the circadian dark, with access to water the remainder of the time. On day 4, mice had access to the ethanol solution for 4 hours. The 4 day DID procedure was used each week, with water available the remainder of the week. Ethanol intake was measured over a total of 7 weeks: 3 weeks of 20E consumption, 1 week of 10E consumption, and 3 weeks of 5E consumption. During the last 2 weeks of the study, mice were administered saline injections prior to 5E access.
On the day following the final DID session, mice were acclimated to the 24-h two-bottle choice ethanol paradigm for 5 days prior to the initiation of saline injections. Baseline 10E intake was measured over 3 days of saline injections to ensure that 10E intake had stabilized (± 10% variability in the mean 10E intake over 3 consecutive days). On the next day, all mice then received a single injection of IVM (5 mg/kg). Injections were administered immediately prior to the period of 24-hr access to 10E versus tap water so that changes in drinking over 24-hr after IVM administration was measured.
Intermittent, Limited Access
The intermittent, limited access procedure was used to model high ethanol drinking, as described previously by our lab and others [67,50,68]. Briefly, each alcohol naïve WT and KO mouse was tested with two concentrations of ethanol (10% then 20%, 10E and 20E respectively) according to the following procedures. Mice were given access to one bottle containing an ethanol solution for six drinking sessions, followed by a one week wash out period between the two ethanol concentrations in which only tap water was available. Ethanol access periods were every other day for 4-hrs beginning three hours into the circadian dark. Drinking session days were on Monday, Wednesday, and Friday. Water was available continuously between ethanol sessions. Ethanol intake was determined by measuring bottle volumes immediately prior to and after the 4-hr drinking period. Body weights were also measured prior to each drinking session. Water intake over four hours was measured during the time frame of the ethanol drinking period for three drinking sessions prior to initiating the experiment.
Loss of Righting Reflex (LORR) Duration and Blood Ethanol Concentration at Return of Righting Reflex (BECRR)
Sensitivity to ethanol’s hypnotic effect in WT and KO mice was assessed by measuring the duration of LORR and then determining the blood ethanol concentration at return of righting reflex (BECRR) [69]. Mice were injected (i.p.) with 3.6 g/kg of ethanol and returned to the cage until they appeared ataxic. Each mouse was placed on its back in a V-shaped trough and the LORR latency and duration was measured. The time from injection to LORR and the time from LORR to return of righting reflex were recorded. Return of righting reflex was defined as the animal’s ability to right itself on all 4 paws, three times in 60 seconds.
Blood samples (20 μL) were obtained from a subset of mice via the retro-orbital sinus immediately upon return of righting reflex. Blood samples were processed by dilution in storage vials containing a matrix (500 μL) comprised of 4 mM n-propanol (Sigma-Aldrich, St. Louis, MO) in deionized water. BECRRs were analyzed by head-space gas chromatography on a 30 μL aliquot at Oregon Health & Science University, using routine procedures [70]. Six pairs of ethanol standards (0.1–4.0 mg/ml), which included n-propanol (internal standard), were run before the samples. Mice that did not lose their righting reflex in less than five minutes post-ethanol administration or those that had an LORR duration or BECRR greater than two standard deviations from the group mean were excluded from the analysis [71,72].
Statistical Analysis
All data were analyzed with GraphPad Prism software (San Diego, CA) and are presented as mean ± SEM. Protein expression of the GABAAR α1 subunit was normalized to beta-actin levels in each lane. Genotype effects on GABAAR α1 receptor subunit expression levels and averaged 24 hr intake data (g/kg, preference ratio, water intake and total fluid intake in mls) were analyzed by unpaired, two-tailed student’s t-test. Repeated-measures, two-way ANOVA was used to analyze the effect of genotype and day/drinking session on two-bottle choice, DID intake and intermittent, limited access ethanol intake as well as the effect of genotype and concentration on two-bottle choice saccharin intake. The IVM study was analyzed by calculating the percent reduction from baseline after IVM injection for each dependent variable (10E intake, 10E preference, water intake and fluid intake) and performing an unpaired student’s t-test to determine the effects of genotype (KO, WT). Genotype effects on LORR and BECRR data were analyzed by unpaired, two-tailed student’s t-test. Statistical significance was set at p<0.05.
RESULTS
We assessed the levels of P2X4R protein in the brain and liver of P2X4R KO and WT littermates as a first step in our studies. We detected P2X4R protein at the expected molecular weight (60 kDa) in the brain of WT mice, but this protein was absent in the KO mice (Figure 1a). Similarly, P2X4R protein was determined to be in the liver (Figure 1b) of WT mice and not in the KOs. These results confirm the lack of P2X4R protein in KO mice. Subsequently, we used PCR to confirm the P2X4R genotype of all mice.
Figure 1. Western blot analysis of P2X4R protein expression in P2X4R KO and WT mice.
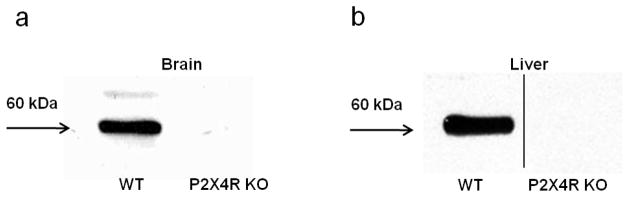
Confirmation of the presence of P2X4R protein in WT mice and absence in P2X4R KO mice in the (a) brain and (b) liver. 50 μg protein/lane.
GABAAR α1 subunit expression is altered in P2X4R KO mice in a brain-region specific manner
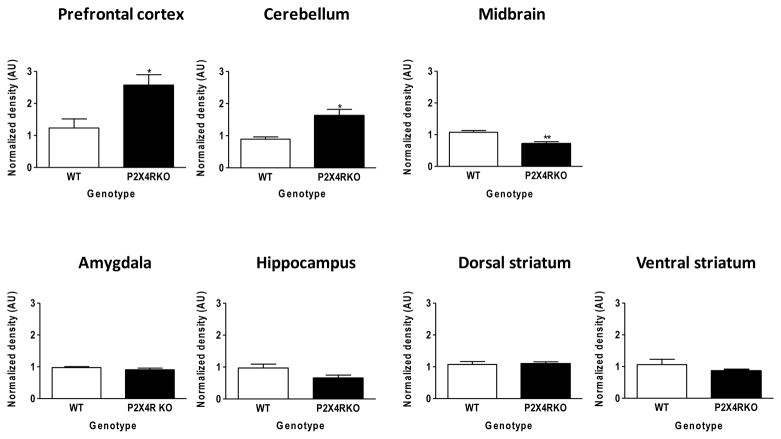
We next assessed the expression of GABAAR α1 subunit in discrete brain regions that were isolated from WT and P2X4R KO mice, since this subunit is widely expressed throughout the brain and is suggested to contribute to some behavioral effects of ethanol [53–55]. As depicted in Figure 2, we identified significant brain-regional alterations between WT and KO mice in GABAAR receptor expression of the α1 subunit. Specifically, there was a significant increase in α1 subunit expression in the prefrontal cortex (129%; P<0.05) and cerebellum (82%; P<0.05) and. Conversely, we found that GABAAR α1 subunit expression was significantly decreased in the midbrain (33%; P<0.01) of P2X4R KO compared to WT mice, whereas GABAAR α1 subunit expression in the amygdala, hippocampus, and striatum (dorsal and ventral) was similar in WT and KO mice.
Figure 2. Western blot analysis of GABAAR α1 subunit expression in P2X4R KO and WT mice.
Expression of the GABAAR α1 subunit was significantly increased in the prefrontal cortex and cerebellum and was significantly decreased in the midbrain of P2X4R KO mice (black bars) compared to WT controls (white bars). There were no genotype differences in expression of the GABAAR α1 subunit in the amygdala, hippocampus, dorsal and ventral striata. Values represent the mean ± SEM for 3–5 mice per genotype. Protein expression is normalized to beta-actin levels and is depicted as arbitrary units (AU); 50 μg protein/lane. *p<0.05; **p<0.01 compared to WT.
P2X4R KO mice demonstrate altered ethanol intake
Acquisition of 24-hr 10E and saccharin intake
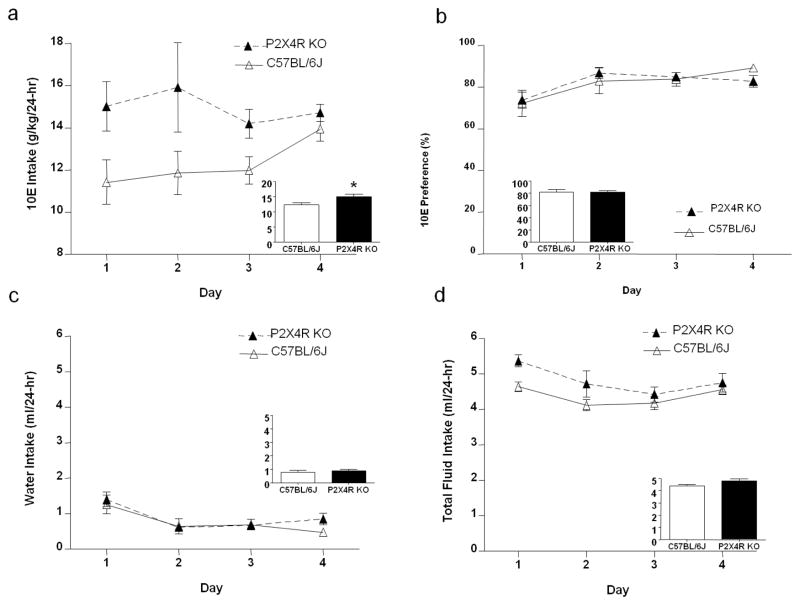
P2X4R KO and WT mice were provided 24-hr access to 10E and water as described in the methods. Deletion of the p2rx4 gene significantly increased averaged ethanol intake by 22%, when compared to WT controls (Figure 3a; inset). The repeated measures, two-way ANOVA across daily 10E consumption revealed a significant main effect of genotype on ethanol intake [F (1, 60) = 5.42, p<0.05] (i.e., KO > WT), with no effect of day or significant genotype x day interaction (Figure 3a). The 10E preference ratio did not differ significantly between P2X4R KO and WT controls (Figure 3b). Water intake was significantly affected by day [F (3, 60) = 12.50, p<0.001], but not by genotype (Figure 3c). Conversely, total fluid intake tended to be higher in KO versus WT mice [F (1, 60) = 4.13, p=0.056], and it was significantly affected by day [F (3, 60) = 7.42, p<0.001] (Figure 3d). There was no genotype x day interaction on any of these measures.
Figure 3. P2X4R genotype increases the early acquisition phase of 24-hr, two-bottle choice ethanol intake in male mice.
(a) P2X4R KO increases 10% ethanol (10E) intake compared to WT controls. (b) There was no significant effect of genotype on ethanol preference or (c) water intake, but there was (d) a trend for total fluid intake to be higher in P2X4R KO versus WT mice. The insets represent the average over the four consecutive days for WT (white bars) and P2X4R KO (black bars) mice. Values represent mean ± SEM for 12 WT and 10 KO mice. *p<0.05 compared to WT.
A separate group of P2X4R KO and WT mice were provided 24 hour access to saccharin solutions and water. Saccharin intake and preference for a 0.033% and 0.066% solution did not differ significantly between WT controls and P2X4R KO mice (Figure 4). The repeated measures, two-way ANOVA revealed a significant effect of saccharin concentration on intake [F (1, 16) = 337.10, p<0.001] and preference [F (1, 16) = 26.19, p<0.001], but no effect of genotype.
Figure 4. P2X4R genotype does not alter 24-hr, two-bottle choice saccharin intake and preference in male mice.
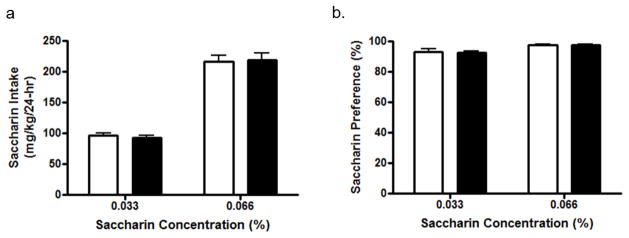
WT (white bars) and P2X4R KO (black bars) mice did not differ in their (a) intake and (b) preference for a 0.033% and 0.066% saccharin solution. Values represent mean ± SEM averages across 4 consecutive days of intake at each concentration for 6 WT and 12 KO mice.
Intermittent, 4-hr access to 10E and 20E solutions
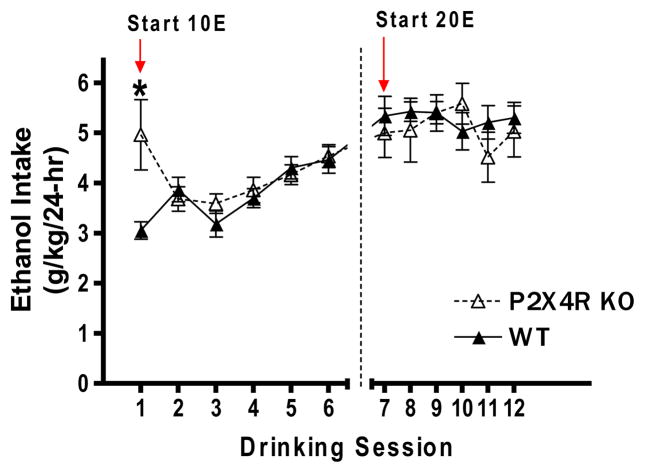
Male P2X4R KO and WT mice were exposed to an intermittent, limited (4-hr) access paradigm to determine if knocking out the p2rx4 gene altered ethanol consumption in a model of high ethanol intake. There was no significant difference in baseline total water intake between P2X4R KO (68.02 ± 4.759 ml/kg) versus WT (63.48 ± 4.583 ml/kg) mice measured during three drinking sessions prior to initiating the experiment. Intake of the 10E and 20E solutions was analyzed separately. Analysis of 10E (g/kg) intake revealed a significant interaction between genotype and drinking session [F (5, 100) = 4.98, p<0.001], a significant effect of drinking session [F (5, 100) = 4.96, p<0.001], with no main effect of genotype (Figure 6). Bonferroni post-hoc analysis identified a significant increase in 10E intake by KO mice on the first drinking session (p<0.01). There was no significant effect of genotype, drinking session, or genotype x drinking session interaction on 20E intake.
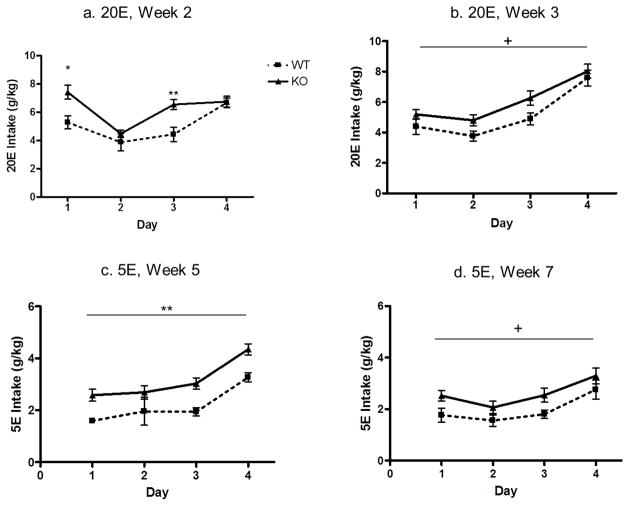
Figure 6.
Drinking-in-the-dark reveals transient higher intakes of 20% ethanol (20E) and 5% ethanol (5E) in P2X4R KO versus WT mice. (a) During Week 2, 20E intake was significantly higher in P2X4R KO mice versus WT controls on days 1 and 3; (b) During Week 3, there was a trend for 20E intake to remain higher in P2X4R KO versus WT mice; (c) During Week 5, consumption of 5E was significantly higher in P2X4R KO versus WT mice; (d) During Week 7, mice received a saline injection prior to 5E access, and there was a trend for 5E intake to remain higher in P2X4R KO versus WT mice. Values represent mean ± SEM for 12 P2X4R KO mice and 6 WT mice for weeks 1–5 and 6 P2X4R KO mice and 9 WT mice for week 6–7. +p<0.10; *p<0.05; **p<0.01. P2X4R KO mice are represented by black solid line; WT mice are represented by dotted line.
Drinking-in-the-dark (DID) procedure with 20E, 10E and 5E
Male P2X4R KO and WT mice were exposed to a modified DID procedure to determine if deletion of the p2rx4 gene altered ethanol consumption in a second model of high ethanol intake. Prior to initiating the alcohol studies, we conducted a DID study with water only. There was no significant difference in baseline DID water intake. The average 4-hr water intake for P2X4R KO mice on day 4 was 1.52 ± 0.098 ml, compared to WT mice that drank 1.42 ± 0.066 ml (p>0.05). Each week of ethanol intake was analyzed separately. Analysis of the first week of 20E intake (g/kg) revealed there was no significant difference between KO and WT (data not shown). However, analysis of the second week of 20E intake (g/kg) revealed a significant main effect of both day [F(3,48)=21.66; p<0.001] and genotype [F(1,48)=6.45; p<0.05] on 20E intake (Figure 6a). The interaction between time and genotype was also significant (F(3,48)=4.76; p<0.01). Post hoc t-tests revealed a significant increase in ethanol intake in KO mice on day 1 (t=2.748; p<0.05) and day 3 (t=3.432; p<0.01). There was a trend for 20E intake to remain higher in the KO versus WT mice during week 3 of the DID procedure (p = 0.09; Figure 6b). Consumption of 10E was measured during week 4 of the DID procedure, and there was no difference between KO and WT mice (not shown). Consumption of 5E was measured during weeks 5–7 of the DID procedure. For week 5, consumption of 5E was significantly higher in KO versus WT mice [F(1,48)=12.27; p<0.01]), and it was significantly influenced by day [F(3,48)=23.96; p<0.001]. There was no interaction between genotype and day (Figure 6c). For weeks 6 and 7, both KO and WT controls received daily saline injections 8 hours prior to alcohol access. There was no effect of genotype during week 6 (data not shown), but there was a trend for 5E intake to remain higher in the KO versus WT mice during week 7 (p=0.09; Figure 6d).
24-hr Access, Two-Bottle Choice and IVM
Following the completion of the DID study, the same P2X4R KO and WT mice were provided 24-hour access to 10E and water in the presence and absence of IVM to: 1) determine whether IVM would differentially alter 10E intake in WT versus P2X4R KO mice and 2) confirm the higher baseline 10E intake in saline-injected P2X4R KO versus WT mice (i.e., pre-IVM). Based on the results of our first study, a planned comparison confirmed that the 3-day averaged baseline 10E intake was significantly higher by 23% in P2X4R KO mice, when compared to WT controls (t=2.469, p<0.05; Figure 7a). IVM (5 mg/kg) decreased ethanol intake in WT mice by 40% vs. a 20% reduction in P2X4R KO mice; this percent reduction in 10E intake was significantly greater in WT compared to KO mice (t=3.150; p<0.01; Figure 7b). Similarly, IVM produced a significantly greater percent reduction in 10E preference in WT compared to KO mice (t=2.745; p<0.05; Figure 7c). There was no difference between WT and KO mice in the percent reduction of water intake or fluid intake following IVM pre-treatment.
Figure 7. IVM produces a 2 fold greater reduction of 24-hr, two-bottle choice ethanol drinking and preference in WT mice, when compared to P2X4R KO mice.
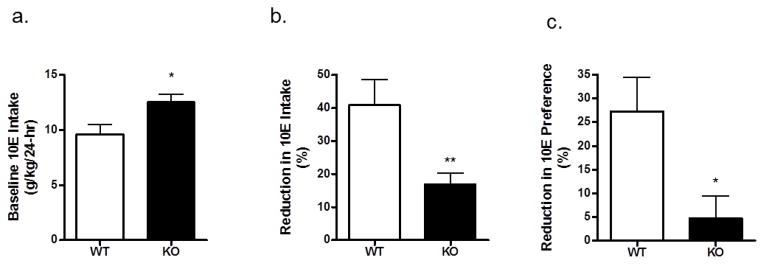
(a) Baseline 10% ethanol (10E) intake (averaged over 3 days) was significantly higher in P2X4R KO versus WT mice. (b) Following a 5 mg/kg dose of IVM, the percent reduction of 10E intake (versus baseline) was significantly greater in WT compared to KO mice. (c) IVM (5 mg/kg) also produced a greater percent reduction in 10E preference (versus baseline) in WT mice compared to P2X4R KO mice. Values represent mean ± SEM for 7 WT mice and 10 P2X4R KO mice. **p<0.01; ***p<0.001.
No change in sensitivity to a hypnotic dose of ethanol in P2X4R KO mice
We used LORR duration in combination with BECRR, measured in a subset of mice tested for LORR duration, to begin assessing whether a null mutation of P2X4Rs affected brain sensitivity to a hypnotic ethanol dose (3.6 g/kg). There was no significant difference between P2X4R KOs and WT controls in the latency to LORR (Figure 8a). While LORR duration was 27% longer in P2X4R KO versus WT mice (p<0.05; Figure 8b), BECRR did not differ in KO versus WT mice (Figure 8c).
Figure 8. P2X4R genotype does not influence sensitivity to the hypnotic effect of 3.6 g/kg ethanol.
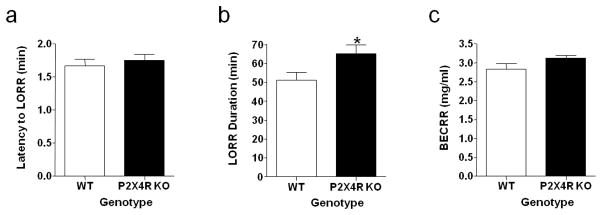
(a) There was no difference in latency to loss of righting reflex (LORR) in KO compared to WT controls. (b) LORR duration was increased in KO, when compared to WT mice. (c) Blood ethanol concentration at return of righting reflex (BECRR) did not differ in a subset of KO and WT mice that were tested for LORR duration. Values represent mean ± SEM for 24 WT and 25 KO mice (LORR) and 11 WT and 12 KO mice (BECRR). *p<0.05 compared to WT.
DISCUSSION
The current study tested the hypotheses that P2X4Rs contribute to ethanol intake and that there is an inverse relationship between P2X4R activity and ethanol consumption. With several different drinking procedures, there was a transient and modest increase in ethanol intake in male P2X4R KO versus WT mice, and the duration of the increase in ethanol intake differed, depending on the procedure and the concentration of the ethanol solution. The effect of genotype appeared to be specific for ethanol intake since KO and WT mice did not differ in saccharin intake or preference, nor did they differ in water intake. Collectively, these findings add to evidence that P2X4Rs contribute to aspects of ethanol intake.
Studies undertaken in our laboratory have demonstrated that P2X4Rs are inhibited by ethanol [34,73–75]. Specifically, mutational analyses have identified amino acid residues that play an important role in the action of ethanol [73,76] and IVM [36,48]. While these investigations begin to shed light on the mechanism of ethanol action on P2X4Rs, the findings are derived from isolated cellular preparations and thus do not provide insight into how ethanol affects P2X4R function in vivo or how these effects translate to behavioral change.
The present work provides two lines of evidence that P2X4Rs contribute to ethanol intake. Using the 24-hr, two-bottle choice paradigm, 10E intake was 22% higher in P2X4R KO versus WT mice during the initial acquisition of drinking. Interestingly, the strongest effect of the P2X4R KO on ethanol intake was during the first two days of 10E exposure (Figure 3a), suggesting a potential involvement of P2X4Rs in the initial acceptance of an ethanol solution or in the early acquisition phase of ethanol drinking. Notably, as presented in the IVM study, baseline 24-hr 10E intake was significantly higher (by 23%) in P2X4R KO versus WT mice, when measured after all animals had 7 weeks of prior ethanol intake in a DID procedure. Thus, there was an overall 22–23% increase in the acquisition phase of 24-hr 10E intake in P2X4R KO versus WT mice that was independent of ethanol drinking experience prior to the measurement of 24 hour 10E intake.
A slightly different pattern of results was found with two different models of high ethanol intake. With the intermittent, limited access procedure, deletion of P2X4Rs significantly increased 10E intake versus WT controls on the first exposure to the 10E solution (i.e., drinking session 1), but it did not have a significant effect on 10E intake in subsequent drinking sessions, nor was there an effect of genotype on consequent 20E intake with this procedure. However, with the DID procedure, there was a transient increase in 20E intake in KO versus WT mice, when this solution was offered during the second week of access (Figure 6a), with a trend for an overall increase in 20E during week 3 of access (Figure 6b). However, there was no effect of genotype on 10E intake during week 4 of the DID procedure. These findings suggest that the initial acceptance of 10E or 20E solutions can be influenced by P2X4R genotype. Interestingly, overall DID intake of 5E was significantly greater in P2X4R KO versus WT mice during week 5 and tended to be higher during week 7 of the DID procedure, suggesting that P2X4R genotype may exert a more persistent effect on intake of lower ethanol concentrations (i.e., 5E). Hence, with limited access ethanol procedures that are designed to model high ethanol intake, deletion of P2X4Rs may influence the acquisition and early maintenance phase of consumption of low ethanol concentrations (i.e., 5E) or primarily affect the acceptance and early drinking experience of higher ethanol concentrations (i.e, 10E and 20E).
The differences in the transient nature of the genotype-related changes in ethanol drinking also could be linked to the different drinking paradigms used. Notably, a recent study found that a single gene mutation in mice could disrupt the correlative relationship between two-bottle choice ethanol consumption and other drinking phenotypes, such as the DID model used in our study [77]. The results of the multivariate analyses that were conducted by Blednov and colleagues [77] support the conclusion that single gene mutations can disrupt the networks of multiple physiological systems that work in concert to regulate ethanol drinking behavior.
Typically, the link between specific receptor actions of a drug and the downstream behavioral cascade is investigated using selective pharmacological agonists and antagonists. At present, such tools are not available for P2X4Rs, but IVM has been used to help fill this gap. IVM is an positive allosteric modulator of several LGICs including GABAARs, glycine receptors, and nAChRs [78,79]. Within the P2X superfamily, IVM is selective for P2X4Rs and has been used to link these receptors to specific behavioral responses [17,38]. Further, in a recent NIAAA review, P2X4Rs were identified as a target of interest for the development of alcohol use disorder (AUD) medications, with IVM as a compound that acts on this target [16].
IVM can antagonize the inhibitory effects of ethanol by interacting at a site of ethanol action in the TM1-TM2 region of the receptor [36,48]. Recent investigations extended this work to mice where it was found that IVM significantly reduced ethanol intake in both male and female mice [50]. To begin to investigate the contribution of P2X4Rs to the IVM-induced reduction in ethanol intake, we determined whether P2X4R genotype would differentially alter the effect of IVM on ethanol intake. In agreement with previous studies [50,49], IVM significantly decreased 10E intake in WT controls. Importantly, the degree of reduction in ethanol intake by IVM was less in the KO mice when compared to the WT controls (Figure 7b), suggesting that at least a portion of IVM’s ability to reduce alcohol intake is linked to P2X4Rs [49]. Because IVM has been reported to exert effects on GABAAR, glycine receptors, and nAChRs [80–84], the results in the P2X4R KO mice are consistent with the idea that the IVM-induced reduction in ethanol intake reflects a combined effect of IVM on P2X4Rs as well as GABAARs and nAChRs [49]. Nonetheless, the collective findings suggest that deletion of P2X4Rs leads to a transient increase in ethanol intake and that stimulation of P2X4R function using a drug such as IVM reduces ethanol intake.
The current studies also provide some insight into the mechanistic complexity by which a reduction of P2X4R function might contribute to ethanol intake. LORR duration accompanied by the BECRR after an acute intoxicating dose of ethanol is a way by which to assess brain sensitivity, acute tolerance, or ethanol clearance [72]. We found that LORR duration was significantly longer in P2X4R KO versus WT mice, but that the KO mice regained their righting reflex at similar BECs as the WT controls. If the rate of ethanol metabolism and other pharmacokinetic factors were the same in KOs and controls, one would expect the KO mice to regain function with lower BECs. Thus, while there may be subtle changes in ethanol pharmacokinetics in the KO mice, the similar BECRR in KO and WT mice indicates that CNS sensitivity to a hypnotic ethanol dose does not differ in P2X4R KO mice and WT controls.
It is clear from the current studies that there is a complex interaction between the effects of the p2rx4 null mutation on ethanol intake, sensitivity, and metabolism. In contrast to an inverse relationship between sensitivity to ethanol intoxication and ethanol intake, sensitivity of the KO mice to ethanol intoxication was not associated with higher (albeit transient at times) ethanol intake. This result is consistent with the multivariate analysis conducted by Beldnov and colleagues (2012) where two bottle choice consumption of a 12% ethanol solution was not associated with LORR duration in either mutant or WT mice. A dose response assessment of LORR duration and other measures of ethanol intoxication is warranted in future studies to further investigate genotypic differences in sensitivity to ethanol intoxication.
In the present study we found differential changes in GABAAR α1 subunit expression in several brain regions including the cerebellum, prefrontal cortex, and midbrain of P2X4R KO mice, when compared with expression in WT controls. We focused on the GABAA receptor α1 subunit because it is ubiquitously expressed across multiple brain regions, and it has been demonstrated to play a role in several ethanol-induced behavioral effects [53–55], so we can draw some tentative conclusions from the present results. With regard to a measure of ethanol intoxication, increased α1 subunit expression in the cerebellum in the KO was not associated with a change in CNS sensitivity to a hypnotic ethanol dose. The remaining brain regions examined are important in the neurocircuitry of ethanol reward [56], but we did not observe a consistent pattern of changes in GABAAR α1 subunit expression in KO versus WT mice. This is not entirely surprising, when one considers that the potential impact of these changes in expression in the KO was a transient increase in ethanol intake. Nonetheless, the fact that α1 subunit expression in the KO was increased in the prefrontal cortex, decreased in the midbrain (which includes the VTA), and unchanged in the amygdala, hippocampus, and dorsal and ventral striatum indicated that deletion of the p2rx4 gene did not produce an overall compensatory increase in GABAAR α1 subunit expression in the brain.
The current findings, coupled with our recently published changes in NMDA and AMPA expression [85] illustrate how developmental compensations inherent in a KO model can alter the expression levels of many different receptor subtypes and receptor families. For example, in the prefrontal cortex, we found significant decrease in expression of GluN2A and GluN2B subunits in P2X4R KO mice as compared with WT mice. On the other hand, GluN1 subunit expression did not significantly differ. On the other hand, GluN1 subunit expression was significantly reduced in the hippocampus and in the cerebellum of P2X4KO mice, whereas hippocampal GluN2A and GluN2B subunit expression was similar between the KO and WT mice [85]. Further, the changes in GABAAR α1 subunit expression levels in the P2X4KO mice observed in the current study were consistent with reports by others indicating that P2X4Rs can modulate the function of major ionotropic targets, including GABAARs [45] and NMDARs [23]. This would suggest that there is some degree of communication between P2X4Rs and other LGICs in WT mice and that by eliminating the expression of the P2X4Rs, we have disrupted the normal cross-talk between signaling cascades that results in altered subunit expression.
CONCLUSION
The present findings using P2X4R KO mice add to the evidence that P2X4Rs contribute to ethanol intake. Thus, understanding the basic underlying neurobiological processes for ethanol intake in relation to P2X4R expression could provide key information regarding the development of new therapeutics for AUDs targeting this relatively new and unexplored family of LGICs.
Figure 5. P2X4R genotype produces a transient increase in intermittent, limited access ethanol intake.
Ethanol intake was measured for 6 drinking sessions at each concentration (10% ethanol, 10E and 20% ethanol, 20E). P2X4R KO mice drank more 10E on their first exposure (drinking session 1), when compared to WT controls. Values represent mean ± SEM for 12 WT and 10 KO mice. *p<0.05 compared to WT.
Acknowledgments
This work was conducted as part of fulfillment of the requirements for the Ph.D. degree in Molecular Pharmacology and Toxicology, University of Southern California (L.R.W.). We thank Miriam Fine for technical assistance and USC Undergraduates (Ayee Azah, Christina Minh, Vanessa Fimreite, and Megan Won) and STAR students for laboratorial assistance. We also want to thank Chris Snelling at OHSU for conducting the analysis of BECRRs. This work was supported (in part or in full) by the National Institutes of Health (NIH), NIAAA, Grants F31AA018926 (L.R.W), KO-1-AA017243 (L.A.), AA016981 (D.A.F), AA03972 (R.L.A.), AA13992 and AA022448 (D.L.D); the American Foundation for Pharmaceutical Education (M.Y.); the Dept of Veterans Affairs (D.A.F); USC Undergraduate Provosts Fellowship (D.L.D. and V.F.), USC Summer Undergraduate Research Fund (D.L.D. and C.M.); and the USC School of Pharmacy (R.L.A and D.L.D).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Deitrich R, Dunwiddie T, Harris RA, Erwin VG. Mechanism of action of ethanol: initial central nervous system actions. Pharmacol Rev. 1989;41:489–537. [PubMed] [Google Scholar]
- 2.Weight FF, Aguayo LG, White G, Lovinger DM, Peoples RW. GABA- and glutamate-gated ion channels as molecular sites of alcohol and anesthetic action. Adv Biochem Psychopharmacol. 1992;47:335–47. [PubMed] [Google Scholar]
- 3.Dildy-Mayfield JE, Mihic SJ, Liu Y, Deitrich RA, Harris RA. Actions of long chain alcohols on GABA A and glutamate receptors: relation to in vivo effects. Br J Pharmacol. 1996;118:378–84. doi: 10.1111/j.1476-5381.1996.tb15413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, et al. Sites of alcohol and volatile anaesthetic action on GABA A and glycine receptors. Nature. 1997;389:385–9. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 5.Harris RA. Ethanol actions on multiple ion channels: which are important? Alcoholism Clin Exp Res. 1999;23:1563–70. [PubMed] [Google Scholar]
- 6.Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–80. [PubMed] [Google Scholar]
- 7.Woodward JJ. Ethanol and NMDA receptor signaling. Crit Rev Neurobiol. 2000;14:69–89. doi: 10.1080/08913810008443548. [DOI] [PubMed] [Google Scholar]
- 8.Davies DL, Alkana RL. Direct evidence for a cause effect link between ethanol potentiation of GABA A receptor function and intoxication from hyperbaric studies in C57, LS and SS mice. Alcoholism Clin Exp Res. 2001;25:1098–106. [PubMed] [Google Scholar]
- 9.Betz H. Ligand-gated ion channels in the brain: the amino acid receptor superfamily. Neuron. 1990;5:383–92. doi: 10.1016/0896-6273(90)90077-s. [DOI] [PubMed] [Google Scholar]
- 10.Ortells MO, Lunt GG. Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci. 1995;18:121–7. doi: 10.1016/0166-2236(95)93887-4. [DOI] [PubMed] [Google Scholar]
- 11.Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- 12.Sommer B, Seeburg P. Glutamate receptor channels: novel properties and new clones. Trends Pharmacol Sci. 1992;13:291–6. doi: 10.1016/0165-6147(92)90088-n. [DOI] [PubMed] [Google Scholar]
- 13.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nature Reviews Drug Discovery. 2008;7:575–90. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 14.Gum RJ, Wakefield B, Jarvis MF. P2X receptor antagonists for pain management: examination of binding and physicochemical properties. Purinergic Signalling. 2012;8:41–56. doi: 10.1007/s11302-011-9272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asatryan L, Nam HW, Lee MR, Thakkar MM, Dar MS, et al. Implication of the purinergic system in alcohol use disorders. Alcoholism Clin Exp Res. 2011;35:584–94. doi: 10.1111/j.1530-0277.2010.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, et al. Medications development to treat alcohol dependence: a vision for the next decade. Addiction Biol. 2012;17:513–27. doi: 10.1111/j.1369-1600.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bortolato M, Yardley M, Khoja S, Godar SC, Asatryan L, et al. Pharmacological insights into the role of P2X4 receptors in behavioral regulation: lessons from ivermectin. Int J Neuropsychopharmacol. 2013;16:1059–70. doi: 10.1017/S1461145712000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chizh BA, Illes P. P2X receptors and Nociception. Pharmacol Rev. 2001;53:553–68. [PubMed] [Google Scholar]
- 19.Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Reviews Neurosci. 2001;2:165–74. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- 20.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–67. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 21.Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nature Neurosci. 1999;2:241–5. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- 22.Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, et al. Neuronal P2X 7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2001;21:7143–52. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baxter AW, Choi SJ, Sim JA, North RA. Role of P2X4 receptors in synaptic strengthening in mouse CA1 hippocampal neurons. Eur J Neurosci. 2011;34:213–20. doi: 10.1111/j.1460-9568.2011.07763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buell G, Lewis C, Collo G, North RA, Suprenant A. An antagonist insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- 25.Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor clonned from rat brain. Proc Natl Acad Sci USA. 1996;93:3684–8. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Peoples RW, Weight FF. Alcohol action on a neuronal membrane receptor: Evidence for a direct interaction with the receptor protein. Proc Natl Acad Sci USA. 1994;91:8200–4. doi: 10.1073/pnas.91.17.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Peoples RW, Weight FF. Ethanol-induced inhibition of a neuronal P2X purinoceptor by an allosteric mechanism. Br J Pharmacol. 1998;123:1–3. doi: 10.1038/sj.bjp.0701599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Peoples RW, Weight FF. Ethanol inhibits a neuronal ATP-gated ion channel. Mol Pharmacol. 1993;44:871–5. [PubMed] [Google Scholar]
- 29.Weight FF, Li C, Peoples RW. Alcohol action on membrane ion channels gated by extracellular ATP (P2X receptors) Neurochem Int. 1999;35:143–52. doi: 10.1016/s0197-0186(99)00056-x. [DOI] [PubMed] [Google Scholar]
- 30.Xiao C, Zhou C, Li K, Davies DL, Ye JH. Purinergic type 2 receptors at GABAergic synapses on ventral tegmental area dopamine neurons are targets for ethanol action. J Pharmacol Exp Ther. 2008;327:196–205. doi: 10.1124/jpet.108.139766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong K, Li C, Weight FF. Inhibition by ethanol of rat P2X 4 receptors expressed in Xenopus oocytes. Br J Pharmacol. 2000;130:1394–8. doi: 10.1038/sj.bjp.0703439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong KM, Li C, Weight FF. Differential modulation by short chain and long chain n -alcohols of rat P2X 4 receptors expressed in Xenopus oocytes. Alcoholism Clin Exp Res. 2001;25:7A. [Google Scholar]
- 33.Davies DL, Machu TK, Guo Y, Alkana RL. Ethanol sensitivity in ATP- gated P2X receptors is subunit dependent. Alcoholism Clin Exp Res. 2002;26:773–8. [PubMed] [Google Scholar]
- 34.Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, et al. Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacol. 2005;49:243–53. doi: 10.1016/j.neuropharm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Asatryan L, Popova M, Woodward JJ, King BF, Alkana RL, Davies DL. Roles of ectodomain and transmembrane regions in ethanol and agonist action in purinergic P2X2 and P2X3 receptors. Neuropharmacol. 2008;55:835–43. doi: 10.1016/j.neuropharm.2008.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asatryan L, Popova M, Perkins DI, Trudell JR, Alkana RL, Davies DL. Ivermectin antagonizes ethanol inhibition in P2X4 receptors. J Pharmacol Exp Ther. 2010;334:720–8. doi: 10.1124/jpet.110.167908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A. P2X receptors and synaptic plasticity. Neurosci. 2009;158:137–48. doi: 10.1016/j.neuroscience.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 38.Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, et al. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci. 2006;26:9006–9. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–46. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 40.McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacol. 2011;61:1097–108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuda M, Koizumi S, Kita A, Shigemoto Y, Ueno S, Inoue K. Mechanical allodynia caused by intraplantar injection of P2X receptor agonist in rats: involvement of heteromeric P2X2/3 receptor signaling in capsaicin-insensitive primary afferent neurons. J Neurosci. 2000;20:RC90. doi: 10.1523/JNEUROSCI.20-15-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–8. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zemkova H, Kucka M, Li S, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Characterization of purinergic P2X4 receptor channels expressed in anterior pituitary cells. Am J Physiol Endocrinol Metabol. 2010;298:E644–51. doi: 10.1152/ajpendo.00558.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorca RA, Rozas C, Loyola S, Moreira-Ramos S, Zeise ML, et al. Zinc enhances long-term potentiation through P2X receptor modulation in the hippocampal CA1 region. Eur J Neurosci. 2011;33:1175–85. doi: 10.1111/j.1460-9568.2010.07589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jo YH, Donier E, Martinez A, Garret M, Toulme E, Boue-Grabot E. Crosstalk between P2X4 and gamma-aminobutyric acid, type A receptors determines synaptic efficacy at a central synapse. J Biol Chem. 2011;286:19993–20004. doi: 10.1074/jbc.M111.231324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimpel MW, Strother WN, McClintick JN, Carr LG, Liang T, et al. Functional gene expression differences between inbred alcohol-preferring and -non-preferring rats in five brain regions. Alcohol. 2007;41:95–132. doi: 10.1016/j.alcohol.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tabakoff B, Saba L, Printz M, Flodman P, Hodgkinson C, et al. Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biol. 2009;7:70. doi: 10.1186/1741-7007-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popova M, Trudell J, Li K, Alkana R, Davies D, Asatryan L. Tryptophan 46 is a site for ethanol and ivermectin action in P2X4 receptors. Purinergic signalling. 2013 doi: 10.1007/s11302-013-9373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asatryan L, Yardley MM, Khoja S, Trudell JR, Huynh N, et al. Avermectins differentially affect ethanol intake and receptor function: Implications for developing new therapeutics for alcohol use disorders. Int J Neuropsychopharmacol. doi: 10.1017/S1461145713001703. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yardley M, Wyatt L, Khoja S, Asatryan L, Ramaker MJ, et al. Ivermectin reduces alcohol intake and preference in mice. Neuropharmacol. 2012;63:190–201. doi: 10.1016/j.neuropharm.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of gamma-aminobutyric acid type A receptor alpha1 subunit knockout mice. J Compar Neurol. 2006;495:408–21. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- 52.Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addiction Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- 53.Blednov YA, Walker D, Alva H, Creech K, Findlay G, Harris RA. GABAA receptor alpha 1 and beta 2 subunit null mutant mice: behavioral responses to ethanol. J Pharmacol Exp Ther. 2003;305:854–63. doi: 10.1124/jpet.103.049478. [DOI] [PubMed] [Google Scholar]
- 54.Kralic JE, Wheeler M, Renzi K, Ferguson C, O’Buckley TK, et al. Deletion of GABAA receptor alpha 1 subunit-containing receptors alters responses to ethanol and other anesthetics. J Pharmacol Exp Ther. 2003;305:600–7. doi: 10.1124/jpet.102.048124. [DOI] [PubMed] [Google Scholar]
- 55.June HL, Sr, Foster KL, Eiler WJ, 2nd, Goergen J, Cook JB, et al. Dopamine and benzodiazepine-dependent mechanisms regulate the EtOH-enhanced locomotor stimulation in the GABAA alpha1 subunit null mutant mice. Neuropsychopharmacol: Official Publication of the Am Col Neuropsychopharmacol. 2007;32:137–52. doi: 10.1038/sj.npp.1301097. [DOI] [PubMed] [Google Scholar]
- 56.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saeed Dar M. Co-modulation of acute ethanol-induced motor impairment by mouse cerebellar adenosinergic A1 and GABA(A) receptor systems. Brain Res Bulletin. 2006;71:287–95. doi: 10.1016/j.brainresbull.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 58.McClearn GE. The genetics of mouse behavior in novel situations. J Compar Physiolog Psychol. 1959;52:62–7. doi: 10.1037/h0044664. [DOI] [PubMed] [Google Scholar]
- 59.Rodgers DA. Research activities related to treatment of alcoholism. Comprehensive Psychiatry. 1966;7:57–67. doi: 10.1016/s0010-440x(66)80007-x. [DOI] [PubMed] [Google Scholar]
- 60.Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacol (Berl) 1993;112:503–10. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 61.Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–83. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- 62.Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–60. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blednov YA, Ozburn AR, Walker D, Ahmed S, Belknap JK, Harris RA. Hybrid mice as genetic models of high alchol consumption. Behav Genet. 2010;40:93–110. doi: 10.1007/s10519-009-9298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Priel A, Silberberg SD. Mechanism of Ivermectin Facilitation of Human P2X4 Receptor Channels. J Gen Physiol. 2004;123:281–93. doi: 10.1085/jgp.200308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol & Beh. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 67.Neasta J, Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci USA. 2010;107:20093–8. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcoholism Clin Exp Res. 2011;35:1938–47. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alkana RL, Finn DA, Bejanian M, Crabbe JC. Genetically determined differences in ethanol sensitivity influenced by body temperature during intoxication. Life Sci. 1988;43:1973–82. doi: 10.1016/0024-3205(88)90570-x. [DOI] [PubMed] [Google Scholar]
- 70.Finn DA, Snelling C, Fretwell AM, Tanchuck MA. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcoholism ClinExpRes. 2007;31:939–49. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 71.Parker CC, Ponicsan H, Spencer RL, Holmes A, Johnson TE. Restraint stress and exogenous corticosterone differentially alter sensitivity to the sedative-hypnotic effects of ethanol in inbred long-sleep and inbred short-sleep mice. Alcohol. 2008;42:477–85. doi: 10.1016/j.alcohol.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radcliffe RA, Floyd KL, Drahnak JA, Deitrich RA. Genetic dissociation between ethanol sensitivity and rapid tolerance in mouse and rat strains selectively bred for differential ethanol sensitivity. Alcoholism Clin Exp Res. 2005;29:1580–9. doi: 10.1097/01.alc.0000179208.05882.1f. [DOI] [PubMed] [Google Scholar]
- 73.Popova M, Asatryan L, Ostrovskaya O, Wyatt RL, Li K, et al. A point mutation in the ectodomain-transmembrane 2 interface eliminates the inhibitory effects of ethanol in P2X4 receptors. J Neurochem. 2010;112:307–17. doi: 10.1111/j.1471-4159.2009.06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ostrovskaya O, Asatryan L, Wyatt L, Popova M, Li K, et al. Ethanol is a fast channel inhibitor of purinergic P2X4 receptors. J Pharm Exp Ther. 2011;337:171–9. doi: 10.1124/jpet.110.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong K, Hu XQ, Stewart RR, Weight FF, Li C. The mechanism by which ethanol inhibits rat P2X4 receptors is altered by mutation of histidine 241. Br J Pharmacol. 2005;145:576–86. doi: 10.1038/sj.bjp.0706192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yi CL, Liu YW, Xiong KM, Stewart RR, Peoples RW, et al. Conserved extracellular cysteines differentially regulate the inhibitory effect of ethanol in rat P2X4 receptors. Biochem Biophys Res Commun. 2009;381:102–6. doi: 10.1016/j.bbrc.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 77.Blednov YA, Mayfield RD, Belknap J, Harris RA. Behavioral actions of alcohol: phenotypic relations from multivariate analysis of mutant mouse data. Genes Brain Behav. 2012;11:424–35. doi: 10.1111/j.1601-183X.2012.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khakh BS, Bao XR, Labarca C, Lester HA. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nature Neurosci. 1999;2:322–30. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- 79.Toulme E, Soto F, Garret M, Boue-Grabot E. Functional Properties of Internalization-Deficient P2X4 Receptors Reveal a Novel Mechanism of Ligand-Gated Channel Facilitation by Ivermectin. Mol Pharmacol. 2006;69:576–87. doi: 10.1124/mol.105.018812. [DOI] [PubMed] [Google Scholar]
- 80.Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, et al. Ivermectin: A positive allosteric effector of the alpha 7 meuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1998;53:283–94. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- 81.Dawson GR, Wafford KA, Smith A, Marshall GR, Bayley PJ, et al. Anticonvulsant and adverse effects of avermectin analogs in mice are mediated through the gamma-aminobutyric acid A receptor. J Pharmacol Exp Ther. 2000;295:1051–60. [PubMed] [Google Scholar]
- 82.Shan Q, Haddrill JL, Lynch JW. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem. 2001;276:12556–64. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- 83.Spinosa HS, Stilck SRAN, Bernardi MM. Possible anxiolytic effects of ivermectin in rats. Vet Res Commun. 2002;26:309–21. doi: 10.1023/a:1016094726033. [DOI] [PubMed] [Google Scholar]
- 84.Sattelle DB, Buckingham SD, Akamatsu M, Matsuda K, Pienaar I, et al. Comparative pharmacology and computational modelling yield insights into allosteric modulation of human alpha7 nicotinic acetylcholine receptors. Biochem Pharmacol. 2009;78:836–43. doi: 10.1016/j.bcp.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 85.Wyatt LR, Godar SC, Khoja S, Jakowec MW, Alkana RL, et al. Sociocommunicative and Sensorimotor Impairments in Male P2X4-Deficient Mice. Neuropsychopharmacol. 2013;38:1993–2002. doi: 10.1038/npp.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]