Abstract
The array of therapeutic options available to clinicians for treating retinal disease is expanding. With these advances comes the need for better understanding of the etiology of these diseases on a cellular level as well as improved non-invasive tools for identifying the best candidates for given therapies and monitoring the efficacy of those therapies. While spectral domain optical coherence tomography (SD-OCT) offers a widely available tool for clinicians to assay the living retina, it suffers from poor lateral resolution due to the eye’s monochromatic aberrations. Adaptive optics (AO) is a technique to compensate for the eye’s aberrations and provide nearly diffraction-limited resolution. The result is the ability to visualize the living retina with cellular resolution. While AO is unquestionably a powerful research tool, many clinicians remain undecided on the clinical potential of AO imaging – putting many at a crossroads with respect to adoption of this technology. This review will briefly summarize the current state of AO retinal imaging, discuss current as well as future clinical applications of AO retinal imaging, and finally provide some discussion of research needs to facilitate more widespread clinical use.
Keywords: retinal imaging, adaptive optics, retinal degeneration, photoreceptor
Introduction
The human retina is a uniquely accessible tissue, and is routinely imaged in clinical practice using a number of different modalities such as optical coherence tomography (OCT), ultrasound, scanning laser ophthalmoscopy (SLO), and conventional fundus imaging. The ability to directly visualize the living retina provides an implicit advantage in diagnosing and monitoring retinal disease, however it is becoming appreciated that by the time pathology is visible with these imaging tools, significant cellular damage has often already occurred. Adaptive optics (AO) enables correction of the eye’s monochromatic aberrations,1 and as a result provides nearly diffraction-limited imaging when combined with any one of the above imaging modalities. The improved resolution provides a more sensitive tool with which to study retinal disease. However, while the use of AO imaging in clinical research has increased, nearly all peer-reviewed studies on retinal disease have used custom-built AO imaging systems housed in research labs. These systems are expensive and complex, and as such, AO imaging has not been viewed as a clinically viable tool until quite recently, with at least four companies having developed AO prototypes at the time of this minireview (Boston Micromachines Corporation, Canon, Inc., Imagine Eyes, and Physical Sciences, Inc.). The availability of commercial systems, coupled with the mounting examples of clinical utility from research-grade systems has dramatically increased the interest in AO imaging among ophthalmologists and optometrists. With potential widespread clinical adoption of this technology on the horizon, we provide here an overview of the current state of AO retinal imaging, summarize recent clinical applications of AO retinal imaging and highlight possible future applications, and finally discuss challenges facing widespread clinical adoption. This invited minireview was prompted by the extensive amount of AO work presented at the ARVO 2012 Annual Meeting in Fort Lauderdale, Florida, USA; as such we have included many of these in the current review to capture the rapid growth of this research area.
Retinal Structures Visible With AO Imaging Tools
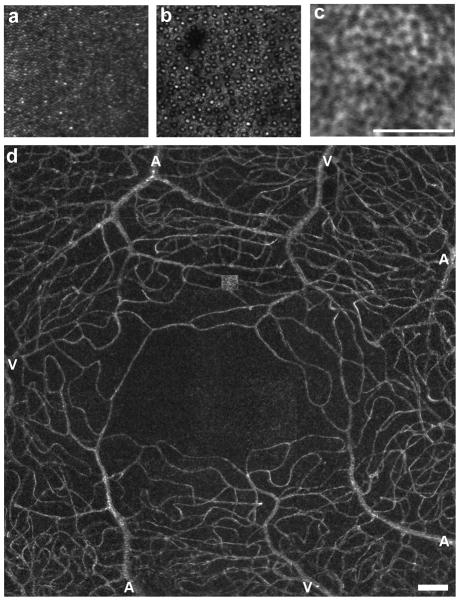
AO has been successfully integrated with the three major ophthalmic imaging technologies – fundus cameras,1–4 SLO,5–7 and OCT.8–11 Each modality offers different advantages, and it remains uncertain whether one will prove to have more utility in the clinical arena than the others. Current imaging systems are able to resolve numerous structural aspects of the living human retina. Photoreceptors have been the primary target for many groups, with it now possible to resolve even the smallest photoreceptor cells in the retina – rods and foveal cones (Figure 1a,b).12 Beyond the photoreceptors, there are reports on visualizing the retinal pigment epithelium (RPE) in the normal human retina using either reflectance in patients with retinal diseases,13 or by taking advantage of the intrinsic autofluorescence of the RPE in the normal retina (Figure 1c),14 though there are light safety issues to consider with the autofluorescence technique.15, 16 As shown in Figure 1d, it is also possible to examine the retinal vasculature (including the smallest foveal capillaries) and to noninvasively measure blood velocity.11, 17–22 The retinal nerve fiber layer (RNFL) and lamina cribrosa can also be visualized with AO imaging tools (Figure 2).23–27 While the images shown in Figure 2 are derived from AOSLO instruments, many of these same structures have also been visualized with AO-OCT imaging systems.9, 11 Although AO-OCT has not been applied to many clinical studies, it does offer some technical advantages over AOSLO that may make it a more desirable modality in the future, including much better axial resolution than AOSLO and increased sensitivity to weak reflections.
Figure 1.
Reflectance images of the photoreceptor mosaic (a, b). Panel a is an image of the parafoveal cone mosaic, with the foveal center at the lower right corner of the image; image courtesy of Jacque Duncan, MD, University of California San Francisco. Panel b is an image of the perifoveal rod and cone mosaic, with the rods being the smaller structures amongst the coarser cone mosaic.48 Panel c is an autofluorescence image of the RPE mosaic taken at approximately 10 deg superior from a 26 year old male (550 nm excitation wavelength). Panel d is a standard error map showing the complete foveal microvascular network in a 35 year old male. Image courtesy of Stephen Burns and Toco Chui, Indiana University.96 Arterioles and venules are labeled as “A” and “V”, respectively. Scale bars are 100 μm.
Figure 2.
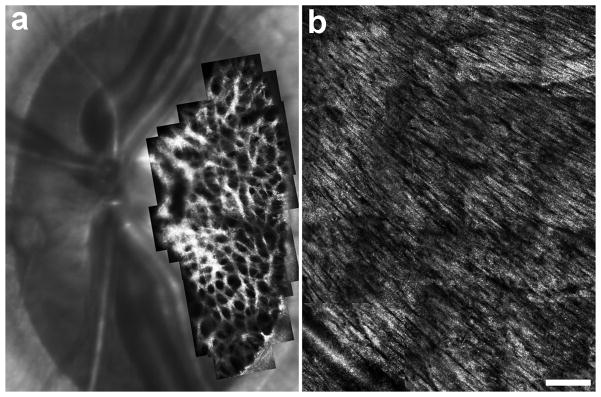
Imaging the lamina cribrosa (a) and retinal nerve fiber layer (b). Image in (a) is a montage from a 5-year old normal rhesus macaque monkey, courtesy of Jason Porter, PhD and Kevin Ivers at the University of Houston College of Optometry. The image in (b) is a montage of the nerve fiber layer in a 62-year-old male with normal vision. Scale bar is 200 μm.
Clinical Applications of AO Retinal Imaging
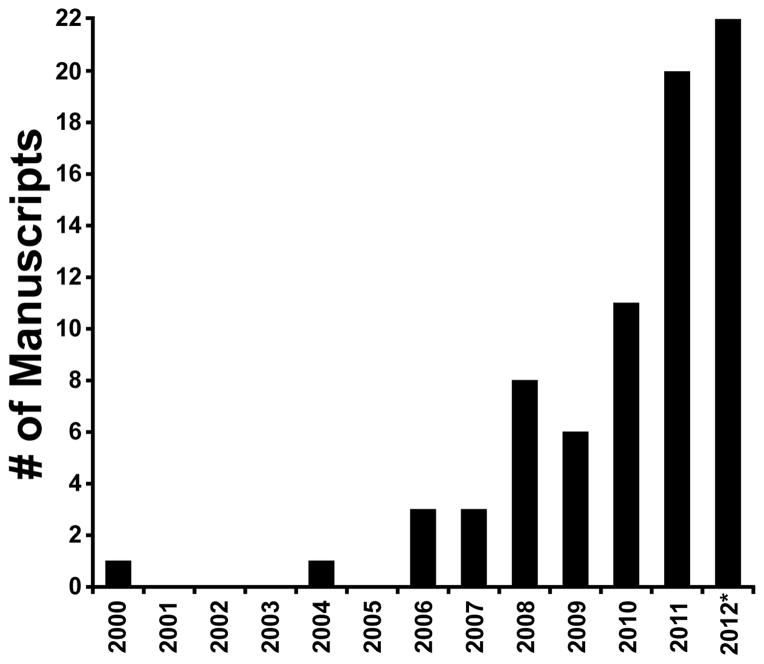
As shown in Figure 3, the number of publications using AO retinal imaging in a clinical setting has increased dramatically in recent years. As such, an exhaustive survey of the clinical applications of AO imaging is beyond the scope of this minireview, so we highlight here four examples with high clinical relevance.
Figure 3.
Number of peer-reviewed manuscripts by year that have used adaptive optics systems to image the retina in one or more patients. A literature search (February 1, 2013) found 75 such papers.9, 33, 37, 43–45, 49, 58, 62, 76, 77, 79, 88–90, 95, 97–155 *=2012 includes papers from the first few weeks of 2013. We did not include papers that only referenced normal subjects.
Inherited Retinal Degenerations
Inherited retinal degenerations affect about 1:2,000 – 1:7,000 people worldwide, and are characterized by progressive loss of vision.28, 29 While there are currently no effective treatments by which the course of these disorders can be altered, a number of therapeutic options are under development (e.g., stem cells, gene therapy, neuroprotective drugs, retinal prosthesis).30–32 The successful application of these approaches requires an improved understanding of the cellular pathogenesis of these degenerative diseases on an individualized basis – an ideal application for non-invasive AO imaging tools.
The first published application of AO to image human retinal pathology was in 2000, where images from a single patient with a cone–rod dystrophy were presented.33 Not surprisingly, subsequent early clinical applications also focused on inherited retinal degenerations. This was due to the fact that photoreceptors, the primary cell type affected in these conditions, are easily visualized with AO-based imaging tools due to their strong waveguiding behavior. In fact, in eyes of good optical quality, it is possible to resolve cone photoreceptors without the use of AO.34–36 Nevertheless, the ability to document and track longitudinally the integrity of the photoreceptor mosaic takes on vital importance as therapies for these conditions emerge. For example, in patients with retinitis pigmentosa (RP) receiving ciliary neurotrophic factor, AOSLO images revealed a relative preservation of cone structure despite an absence of significant functional improvement,37 suggesting that AO-based imaging tools could provide sensitive anatomical outcome measures for clinical trials, providing more immediate feedback as to whether the therapeutic intervention has positively impacted retinal structure. It was suggested by Ratnam et al. at ARVO 2012 that structural changes in RP can be detected with AOSLO even prior to functional changes on standard clinical tests of vision.38
Another way that AO imaging may prove useful in treating inherited retinal degenerations is in the selection of patients for specific trials. In both dog and mouse models of achromatopsia (ACHM),39–42 it was shown that cone function could be restored using a gene therapy approach. Translation of this specific approach to human trials requires that cone cells remain, else there are no cells whose function can be restored. AO imaging of patients with ACHM revealed a varying degree of retained cone structure,43 and though it is unclear how this impacts calculation of the risk:benefit ratio, one could imagine that such detailed information about the degree of residual cone structure in these patients could help set expectations as to the anticipated degree of functional recovery.
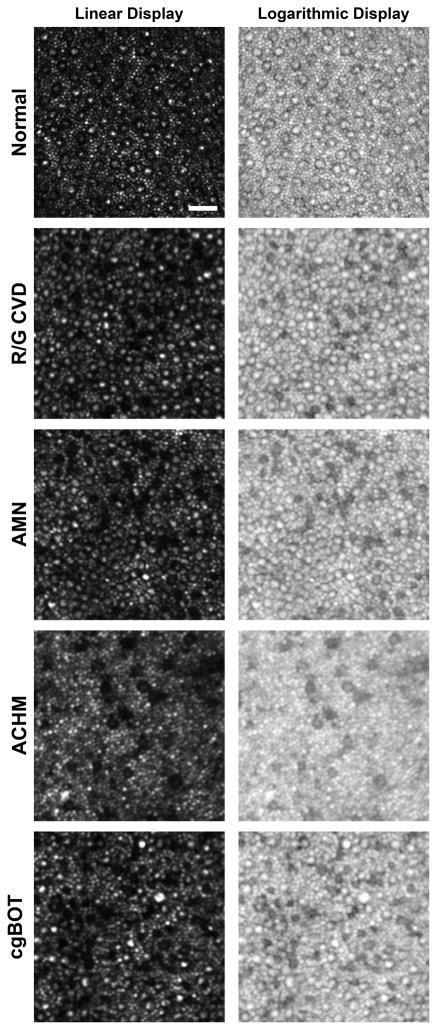
In patients with ACHM, the remaining cones are sparse and have diminished reflectivity43 (Figure 4). In patients with certain opsin mutations, we found that a subset of cones (presumably those expressing the mutant opsin) had similarly altered waveguiding44 (Figure 4). Interestingly, we see a similar cone phenotype in patients with acute macular neuroretinopathy (AMN)45 and closed-globe blunt ocular trauma (Figure 4). While the underlying structural defect is likely different in these cases, photoreceptor reflectivity appears to represent an optical biomarker of photoreceptor integrity, and might prove useful in examining photoreceptor integrity in other conditions. Similarly, rod and cone photoreceptors have been shown to vary in their intensity over time, and developing methods to reliably quantify this temporal variability may be useful in probing the health of individual photoreceptors.46–49 Needed are further studies exploring the reflective behavior of photoreceptors in retinal degenerative diseases to better understand the physiological origin of the variability and thus its potential as a biomarker.
Figure 4.
Non-waveguiding cones in retinal disease. Shown are perifoveal images (linear and logarithmic display) of the photoreceptor mosaic for a subject with normal vision and 4 subjects with various retinal disorders. Normal cones at this eccentricity (~10 degrees from fixation) have a bright reflective center surrounded by a dark ring, where the extent of the dark area represents the inner segment diameter. Numerous cones devoid of the central reflective profile can be seen in a subject with red-green color blindness due to an LIAVA opsin mutation (R/G CVD),44 a subject with acute macular neuroretinopathy (AMN),45 a subject with achromatopsia (ACHM),43 and a subject with vision loss as a result of closed-globe blunt ocular trauma (cg-BOT). This altered reflectivity profile may indicate altered outer segment morphology. Scale bar is 20 μm.
Glaucoma
Glaucoma is the leading cause of irreversible, preventable blindness worldwide, with some 11 million glaucoma sufferers worldwide being bilaterally blind from the disease.50, 51 Primary open angle glaucoma (POAG) is a chronic disease characterized by progressive loss of retinal ganglion cells that leads to structural damage of the inner retinal layers, as shown by progressive local or diffuse thinning of the retinal nerve fiber layer (RNFL).52 Axonal tissue loss in the RNFL has been reported to be one of the earliest detectable glaucomatous changes, preceding morphologic changes of the optic nerve head (ONH), as well as functional loss, as shown by progressive visual field (VF) defects. The temporal sequence of glaucomatous structural/functional damage suggests that micro-structural changes of the RNFL/ONH structures could allow for earlier detection of the disease.53–55 As AO imaging tools can visualize both the RNFL and lamina cribrosa with high resolution,23–27 we anticipate that application of AO imaging to glaucoma will increase in the coming years. Visualization of the RNFL is a particularly attractive target for AO-OCT, as it is possible to examine individual bundles of nerve fibers in cross section across the retina.24
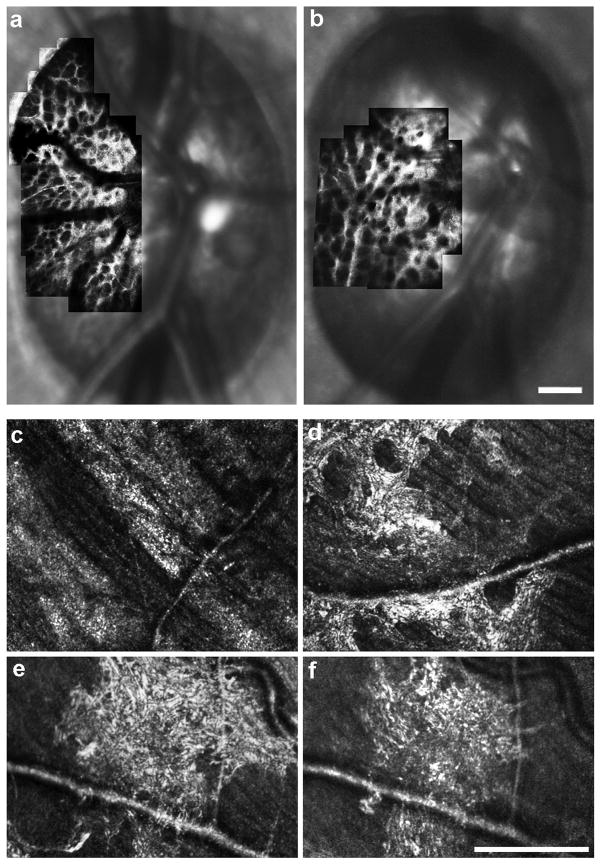
Previous applications of AO imaging to glaucoma examined retinal structures other than the RNFL. In experimentally induced primate models of glaucoma, AO imaging has shown altered morphology of the lamina cribrosa in glaucomatous versus fellow control eyes.56 Ivers et al. also demonstrated at ARVO 2012 the ability to examine the structure of the lamina cribrosa longitudinally in these models (Figure 5a,b),57 providing a powerful tool with which to monitor structural changes in response to experimentally-induced increases in intraocular pressure or even in response to therapeutic intervention. In human patients with glaucoma, Choi et al. reported a loss in cone density along with expected thinning of the inner retina; dark areas in the cone mosaic were found at the same retinal locations with reduced visual sensitivity (measured via visual field testing).58 Similar degradation of the photoreceptor mosaic has been reported histologically,59, 60 but whether this represents an early or late feature of the disease remains unclear. While the involvement of the photoreceptors in the disease process remains controversial, AO-based imaging tools provide a direct means to resolve the issue. Finally, recent work using AOSLO revealed numerous reflective structures in the inner retina in patients with primary open angle glaucoma (Figure 5d–f).61 It is unclear what the significance of these and other structures is, but they are nonetheless dynamic and are prevalent in glaucomatous eyes.61 While analysis of lamina pore geometry is relatively straightforward,25, 62, 63 the interpretation and quantification of these unique hyperreflective structures is difficult and represents a major challenge facing the application of this technology to a heterogeneous disease like glaucoma.
Figure 5.
AOSLO imaging in glaucoma. Panel (a) shows an image of the anterior lamina cribrosa surface from the eye of a normal rhesus macaque prior to induction of experimental glaucoma, and panel (b) shows the laminar surface of the same eye at an early stage of experimental glaucoma showing an alteration to laminar beam and pore geometry. Images courtesy of Jason Porter, PhD and Kevin Ivers at the University of Houston College of Optometry. In human patients with glaucoma, drastic changes in reflectivity and striation in the peripapillary NFL corresponding to what would be clinically described as arcuate defects can be seen (c). Panels (d–f) illustrate hyper-reflective structures residing near the RNFL.61 These features were observed over areas of reduced visual sensitivity, and were not visible on SD-OCT or fundus photography. A four-month follow-up in one patient revealed significant changes in both the extent and appearance of the hyper-reflective patterns (e, f). Scale bars are 200 μm.
Diabetic retinopathy
Diabetic retinopathy (DR) is a frequently occurring complication of diabetes mellitus (DM). According to the World Health Organization, DM is responsible for about 12% of new cases of blindness between the ages of 45 and 74 years in the developed world.64, 65 DR can be classified as non-proliferative (NPDR) or proliferative (PDR), and NPDR is further graded as mild, moderate and severe according to the Early Treatment Diabetic Retinopathy Study (ETDRS) severity scale. DR consists of a microangiopathy that induces pathological changes of the vascular structures and the blood rheological properties as a consequence of chronic hyperglycemia.66–68 Recently, DR has been postulated to be a multifactorial disease involving the retinal neuronal cells.69–73 The neurodegenerative change consists of apoptosis of several populations, including photoreceptors, bipolar and ganglion cells. This functional and structural impairment of neural tissue has been theorized to participate in the generation of the earliest morphological alterations of the vascular structures.69–71, 73, 74
The earliest clinical pathological changes associated with DR occur to the microvascular structures. Fluorescein angiography (FA) is usually performed to assess the integrity of the blood retinal barrier as the amount of fluorescein leakage is related to the dysfunction of the retinal vascular endothelium, though it requires injection of a fluorescent dye that can lead to unintended systemic complications.75 AOSLO imaging has recently demonstrated non-invasive images of the retinal microvascular damages in patients with DM without the need of contrast enhancing agents, by detecting microaneurysms, increased foveal avascular zone (FAZ) size and dropout of capillaries at the edge of the FAZ.76–79 Tam et al. evaluated the parafoveal capillary network in 15 patients with type 2 diabetes and no retinopathy. They showed a higher tortuosity of the arterovenous channels (26% higher) in eyes of patients with diabetes and no DR than in healthy controls.76 In a follow up study, longitudinal assessment of the capillaries near the FAZ showed microaneurysm formation and disappearance as well as the de novo formation of tiny capillary bends similar in appearance to intraretinal microvascular abnormalities.77 Phan et al. showed, in both mild and moderate NPDR eyes, decreased capillary density and blood flow stasis.78 Non-invasive assessment of the capillary network is also possible using AO-OCT11, 80 or even high-speed SD-OCT without AO,81 thus DR would be an ideal application for such imaging efforts.
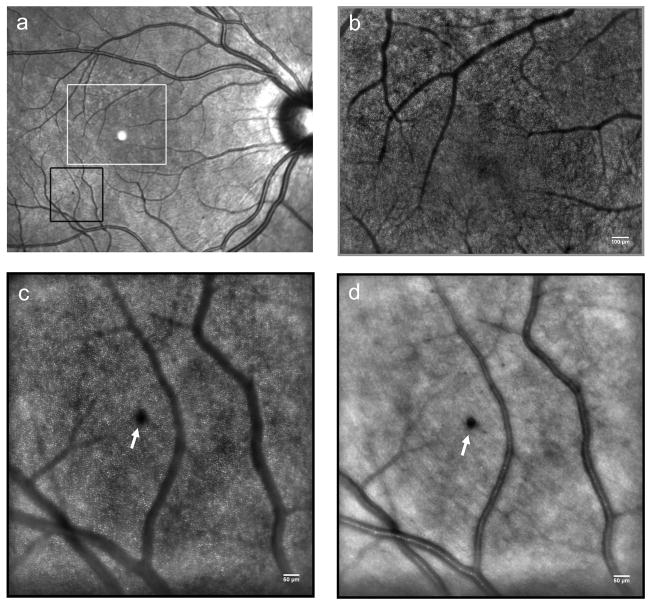
Recently, cone photoreceptor involvement in DR has also been examined with AO imaging tools. Two studies at the 2012 ARVO meeting reported disruption of the cone photoreceptor mosaic in patients with type 1 diabetes,82, 83 (and see Figure 6) though additional studies are required to understand how this involvement is related to the microvasculature aspects of the disease.
Figure 6.
Panel (a) shows a wide field fundus image from the left eye of a patient with NPDR. The white box indicates the area of the retina shown in panel (b) and the black box indicates the area of the retina displayed in panels (c) and (d). (b) Adaptive optics retinal imaging allows for a more detailed visualization of the capillary network around the FAZ. Scale bar is 100 μm. High-resolution images of the photoreceptor layer (c) and the overlying structures of the inner retina (d) can be acquired at exactly the same location with AO ophthalmoscopy. A micro-haemorrhage (white arrow) can be clearly focused on the retinal nerve fiber layer. The cone photoreceptors underlying the haemorrhage cannot be resolved (masking effect). (c) and (d) Scale bars represent 50 μm.
Age-related Macular Degeneration (AMD)
AMD is the leading cause of blindness in the elderly across the developed world. AMD is a multifactorial disease, involving ocular, systemic and genetic risk factors. Several patho-biological pathways have been implicated in the pathogenesis of AMD, including senescence (with lipofuscin accumulation in the RPE), choroidal ischemia and oxidative damage.84, 85 There are two clinical types of AMD, “dry” and “wet”. In the early asymptomatic stages of both types of AMD, which is asymptomatic, insoluble extracellular aggregates called drusen accumulate in the outer retina. The late stage of dry AMD, which is also known as geographic atrophy (GA), is characterized by scattered or confluent areas of degeneration of RPE cells and the overlying retinal photoreceptors, which rely on the RPE for trophic support. The other late stage form of AMD, the wet form (10–15%), is typified by choroidal neo-vascularization (CNV), wherein newly immature blood vessels grow toward the outer retina from the underlying choroid, often leaking fluid below or within the retina.86, 87
In the early stages of AMD, the ability to predict the rate of progression is currently limited. By monitoring drusen over time, en face and axial AO imaging tools could be used to monitor drusen progression and assess their direct effect on the overlying photoreceptor mosaic. For example, in a patient with early onset large colloid drusen, preservation of cones over the drusen was observed,88 consistent with observations in a patient with basal laminar drusen.89 In a study of early AMD with AO, Boretsky et al. identified several small drusen deposits that were not observed with wide field fundus imaging or SD-OCT in early AMD.90 They also investigated large coalescent drusen and areas of GA in advanced stages of dry AMD, showing a significant decrease in visible photoreceptor density. A 30% decrease in cone counts was found (at 5–7 degrees eccentricity) in eyes with later stages in comparison with eyes with earlier stages of AMD progression. Two groups at the 2012 ARVO meeting demonstrated the capability of AO imaging to visualize disruptions to the photoreceptor mosaic even outside the clinically visible GA lesions and to track the progression of the GA lesions over time.91, 92 Given the prevalence of AMD, it seems likely that this will be one of the more active growth areas in clinical AO imaging.
Where Do We Go From Here?
Recent years have seen an expanding interest in AO imaging for clinical applications, evident by the increasing number of presentations and publications utilizing this technology in clinical populations. As commercial prototypes become more widespread, it is worth highlighting a couple of areas of need to propel AO from a predominantly research “toy” to an invaluable tool in the clinical arsenal.
First, while there has been some work on characterizing the properties of normal photoreceptor mosaic, larger databases are desperately needed. Impeding construction of a reference database has been the lack of convergence on the methods used to quantify the mosaic. While density and spacing are commonly used, the way in which they are measured varies from group to group. Moreover, there are very few studies examining the reliability and repeatability of methods used to quantify the mosaic.93, 94 Such data is critical to the utilization of any particular method for assessing departures from normality. In addition, metrics that characterize mosaic geometry hold promise for detecting more subtle changes to the mosaic,82, 95 thus a reference database that incorporates multiple metrics is most desirable.
Second, as new structures become accessible (beyond photoreceptors), new analytical tools will be needed to describe these in quantitative terms, and will require the same validation studies as cone spacing or density does. Quantifying blood flow, determining capillary density, measuring RNFL bundles, measuring lamina cribrosa pore size, and assessing intrinsic RPE autofluorescence are all possible with existing AO technology and the reference/normative data for these assays are even more lacking than those for the cone mosaic. Resolving these issues requires putting this technology in the hands of more clinician scientists. The activation energy (cost and expertise) remains quite high to access AO technology; making it more widely available will help accelerate the maturation of the clinical applications of the technology. Commercialization of robust, easy-to-use devices is necessary to assist with expanding access. Moreover, there are multiple versions of AO retinal imaging tools (flood, SLO, & OCT) in use, and all have advantages and disadvantages. There have not been any systematic studies examining the same diseases with the various modalities, and as such the relative information provided by each modality is not clear, and would certainly vary on a disease-by-disease basis.
In summary, while there are certainly challenges to the clinical application of AO retinal imaging, the rapid growth in just the past couple of years suggests these can soon be overcome. Moreover, the current opportunities are many, with expansion of AO imaging into clinical trials among the most exciting. This could potentially enable targeting of treatments to specific patients or specific retinal areas, and allow for more sensitive evaluation of treatment response.37 In addition, the numerous areas of specific need in a relatively small field have already spawned a number of collaborative relationships to resolve them. The AO community remains a uniquely collegial field, and for this reason, the future appears very bright for AO as a clinical tool.
Acknowledgments
Funding: The writing of this manuscript was supported in part by Foundation Fighting Blindness, NIH grants R01EY017607, P30EY001931, UL1RR031973, an unrestricted departmental grant from Research to Prevent Blindness, and the National Framework Program for Research and Innovation (PON grant n. 0100110; M. Lombardo). A. Dubra is the recipient of a Career Development Award from Research to Prevent Blindness.
We thank Yusufu Sulai and John Flatter for their assistance.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Liang J, Williams DR, Miller D. Supernormal vision and high-resolution retinal imaging through adaptive optics. J Opt Soc Am A. 1997;14:2884–2892. doi: 10.1364/josaa.14.002884. [DOI] [PubMed] [Google Scholar]
- 2.Rha J, Jonnal RS, Thorn KE, Qu J, Zhang Y, Miller DT. Adaptive optics flood-illumination camera for high speed retinal imaging. Opt Express. 2006;14:4552–4569. doi: 10.1364/oe.14.004552. [DOI] [PubMed] [Google Scholar]
- 3.Dees EW, Dubra A, Baraas RC. Variability in parafoveal cone mosaic in normal trichromatic individuals. Biomed Opt Express. 2011;2:1351–1358. doi: 10.1364/BOE.2.001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedggood P, Metha A. Variability in bleach kinetics and amount of photopigment between individual foveal cones. Invest Ophth Vis Sci. 2012;53:3673–3681. doi: 10.1167/iovs.11-8796. [DOI] [PubMed] [Google Scholar]
- 5.Roorda A, Romero-Borja F, Donnelly WJ, Queener H, Hebert TJ, Campbell MCW. Adaptive optics scanning laser ophthalmoscopy. Opt Express. 2002;10:405–412. doi: 10.1364/oe.10.000405. [DOI] [PubMed] [Google Scholar]
- 6.Burns SA, Tumbar R, Elsner AE, Ferguson D, Hammer DX. Large-field-of-view, modular, stabilized, adaptive-optics-based scanning laser ophthalmoscope. J Opt Soc Am A. 2007;24:1313–1326. doi: 10.1364/josaa.24.001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubra A, Sulai Y. Reflective afocal broadband adaptive optics scanning ophthalmoscope. Biomed Opt Express. 2011;2:1757–1768. doi: 10.1364/BOE.2.001757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Rha JT, Jonnal RS, Miller DT. Adaptive optics parallel spectral domain optical coherence tomography for imaging the living retina. Opt Express. 2005;13:4792–4811. doi: 10.1364/opex.13.004792. [DOI] [PubMed] [Google Scholar]
- 9.Torti C, Považay B, Hofer B, Unterhuber A, Carroll J, Ahnelt PK, et al. Adaptive optics optical coherence tomography at 120,000 depth scans/s for non-invasive cellular phenotyping of the living human retina. Opt Express. 2009;17:19382–19400. doi: 10.1364/OE.17.019382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zawadzki RJ, Choi SS, Fuller AR, Evans JW, Hamann B, Werner JS. Cellular resolution volumetric in vivo retinal imaging with adaptive optics-optical coherence tomography. Opt Express. 2009;17:4084–4094. doi: 10.1364/oe.17.004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Kocaoglu OP, Cense B, Bruestle J, Jonnal RS, Gao W, et al. Imaging retinal capillaries using ultrahigh-resolution optical coherence tomography and adaptive optics. Invest Ophth Vis Sci. 2011;52:6292–6299. doi: 10.1167/iovs.10-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubra A, Sulai Y, Norris JL, Cooper RF, Dubis AM, Williams DR, et al. Non-invasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomed Opt Express. 2011;2:1864–1876. doi: 10.1364/BOE.2.001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roorda A, Zhang Y, Duncan JL. High-resolution in vivo imaging of the RPE mosaic in eyes with retinal disease. Invest Ophth Vis Sci. 2007;48:2297–2303. doi: 10.1167/iovs.06-1450. [DOI] [PubMed] [Google Scholar]
- 14.Morgan JIW, Dubra A, Wolfe R, Merigan WH, Williams DR. In vivo autofluorescence imaging of the human and Macaque retinal pigment epithelial cell mosaic. Invest Ophth Vis Sci. 2009;50:1350–1359. doi: 10.1167/iovs.08-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan JI, Hunter JJ, Masella B, Wolfe R, Gray DC, Merigan WH, et al. Light-induced retinal changes observed with high-resolution autofluorescence imaging of the retinal pigment epithelium. Invest Ophth Vis Sci. 2008;49:3715–3729. doi: 10.1167/iovs.07-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan JI, Hunter JJ, Merigan WH, Williams DR. The reduction of retinal autofluorescence caused by light exposure. Invest Ophth Vis Sci. 2009;50:6015–6022. doi: 10.1167/iovs.09-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns SA, Zhangyi Z, Chui TYP, Song H, AEE, Malinovsky VE. Imaging the inner retina using adaptive optics. Invest Ophth Vis Sci. 2008;49:E-Abstract 4512. [Google Scholar]
- 18.Martin JA, Roorda A. Pulsatility of parafoveal capillary leukocytes. Exp Eye Res. 2009;88:3563–3560. doi: 10.1016/j.exer.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam J, Martin JA, Roorda A. Non-invasive visualization and analysis of parafoveal capillaries in humans. Invest Ophth Vis Sci. 2010;51:1691–1698. doi: 10.1167/iovs.09-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong Z, Song H, Chui TY, Petrig BL, Burns SA. Noninvasive measurements and analysis of blood velocity profiles in human retinal vessels. Invest Ophth Vis Sci. 2011;52:4151–4157. doi: 10.1167/iovs.10-6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tam J, Tiruveedhula P, Roorda A. Characterization of single-file flow through human retinal parafoveal capillaries using an adaptive optics scanning laser ophthalmoscope. Biomed Opt Express. 2011;2:781–793. doi: 10.1364/BOE.2.000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popovic Z, Knutsson P, Thaung J, Owner-Peterson M, Sjöstrand J. Noninvasive imaging of human foveal capillary network using dual-conjugate adaptve optics. Invest Ophth Vis Sci. 2011;52:2649–2655. doi: 10.1167/iovs.10-6054. [DOI] [PubMed] [Google Scholar]
- 23.Scoles D, Gray DC, Hunter JJ, Wolfe R, Gee BP, Geng Y, et al. In-vivo imaging of retinal nerve fiber layer vasculature: imaging - histology comparison. BMC Ophthalmology. 2009;9:9. doi: 10.1186/1471-2415-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocaoglu OP, Cense B, Jonnal RS, Wang Q, Lee S, Gao W, et al. Imaging retinal nerve fiber bundles using optical coherence tomography with adaptive optics. Vision Res. 2011;51:1835–1844. doi: 10.1016/j.visres.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivers KM, Li C, Patel N, Sredar N, Luo X, Quenner H, et al. Reproducibility of measuring lamina cribrosa pore geometry in human and nonhuman primates with in vivo adaptive optics imaging. Invest Ophth Vis Sci. 2011;52:5473–5480. doi: 10.1167/iovs.11-7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang G, Qi X, Chui TY, Zhong Z, Burns SA. A clinical planning module for adaptive optics SLO imaging. Optom Vis Sci. 2012;89:593–601. doi: 10.1097/OPX.0b013e318253e081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takayama K, Ooto S, Hangai M, Arakawa N, Oshima S, Shibata N, et al. High-resolution imaging of the retinal nerve fiber layer in normal eyes using adaptive optics scanning laser ophthalmoscopy. PloS one. 2012;7:e33158. doi: 10.1371/journal.pone.0033158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fletcher EL. Mechanisms of photoreceptor death during retinal degeneration. Optom Vis Sci. 2010;87:269–275. doi: 10.1097/OPX.0b013e3181c9132b. [DOI] [PubMed] [Google Scholar]
- 29.Fasiuddin A. Inherited retinal degenerations. Int Ophthalmol Clin. 2010;50:45–56. doi: 10.1097/IIO.0b013e3181f1287b. [DOI] [PubMed] [Google Scholar]
- 30.Stone EM. Progress toward effective treatments for human photoreceptor degenerations. Curr Opin Genet Dev. 2009;19:283–289. doi: 10.1016/j.gde.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y, Enzmann V, Ildstad ST. Stem cell-based therapeutic applications in retinal degenerative diseases. Stem Cell Rev. 2011;7:434–445. doi: 10.1007/s12015-010-9192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrar GJ, Millington-Ward S, Chadderton N, Humphries P, Kenna PF. Gene-based therapies for dominantly inherited retinopathies. Gene Therapy. 2012;19:137–144. doi: 10.1038/gt.2011.172. [DOI] [PubMed] [Google Scholar]
- 33.Roorda A. Adaptive optics ophthalmoscopy. J Refract Surg. 2000;16:S602–S607. doi: 10.3928/1081-597X-20000901-23. [DOI] [PubMed] [Google Scholar]
- 34.Miller DT, Williams DR, Morris GM, Liang J. Images of cone photoreceptors in the living human eye. Vision Res. 1996;36:1067–1079. doi: 10.1016/0042-6989(95)00225-1. [DOI] [PubMed] [Google Scholar]
- 35.Wade AR, Fitzke FW. In vivo imaging of the human cone-photoreceptor mosaic using a confocal laser scanning ophthalmoscope. Lasers and Light in Ophthalmology. 1998;8:129–136. [Google Scholar]
- 36.Pircher M, Kroisamer JS, Felberer F, Sattmann H, Götzinger E, Hitzenberger CK. Temporal changes of human cone photoreceptors observed in vivo with SLO/OCT. Biomed Opt Express. 2010;2:100–112. doi: 10.1364/BOE.2.000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talcott KE, Ratnam K, Sundquist S, Lucero AS, Lujan BJ, Tao W, et al. Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Invest Ophth Vis Sci. 2011;52:2219–2226. doi: 10.1167/iovs.10-6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratnam K, Carroll J, Porco T, Duncan JL, Roorda A. Visual acuity and foveal sensitivity are not reliable measures of cone density at the fovea. Invest Ophth Vis Sci. 2012;53:E-Abstract: 4646. [Google Scholar]
- 39.Alexander JJ, Umino Y, Everhart D, Chang B, Min SH, Li Q, et al. Restoration of cone vision in a mouse model of achromatopsia. Nat Med. 2007;13:685–687. doi: 10.1038/nm1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komáromy AM, Alexander JJ, Chiodo VA, Hauswirth WW, Acland GM, Aguirre GD. Cone-directed gene therapy with rAAV leads to restoration of cone function in a canine model of achromatopsia. Invest Ophth Vis Sci. 2007;48:E-Abstract 4614. [Google Scholar]
- 41.Komáromy A, Alexander JJ, Rowlan JS, Garcia MM, Chiodo VA, Kaya A, et al. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet. 2010;19:2581–2593. doi: 10.1093/hmg/ddq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carvalho LS, Xu J, Pearson R, Smith AJ, Bainbridge JW, Morris LM, et al. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum Mol Genet. 2011;20:3161–3175. doi: 10.1093/hmg/ddr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genead MA, Fishman GA, Rha J, Dubis AM, Bonci DM, Dubra A, et al. Photoreceptor structure and function in patients with congenital achromatopsia. Invest Ophth Vis Sci. 2011;52:7298–7308. doi: 10.1167/iovs.11-7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carroll J, Neitz M, Hofer H, Neitz J, Williams DR. Functional photoreceptor loss revealed with adaptive optics: An alternate cause for color blindness. Proc Natl Acad Sci USA. 2004;101:8461–8466. doi: 10.1073/pnas.0401440101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen SO, Cooper RF, Dubra A, Carroll J, Weinberg DV. Selective cone photoreceptor injury in acute macular neuroretinopathy. Retina. 2012 doi: 10.1097/IAE.0b013e31828cd03a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jonnal RS, Besecker JR, Derby JC, Kocaoglu OP, Cense B, Gao W, et al. Imaging outer segment renewal in living human cone photoreceptors. Opt Express. 2010;18:5257–5270. doi: 10.1364/OE.18.005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jonnal RS, Rha J, Zhang Y, Cense B, Gao W, Miller DT. Functional imaging of single cone photoreceptors using an adaptive optics flood illumnation camera. Invest Ophth Vis Sci. 2007;48:E-abstract 1955. [Google Scholar]
- 48.Cooper RF, Dubis AM, Pavaskar A, Rha J, Dubra A, Carroll J. Spatial and temporal variation of rod photoreceptor reflectance in the human retina. Biomed Opt Express. 2011;2:2577–2589. doi: 10.1364/BOE.2.002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Godara P, Cooper RF, Sergouniotis PI, Diederichs MA, Streb MR, Genead MA, et al. Assessing retinal structure in complete congenital stationary night blindness and Oguchi disease. Am J Ophthalmol. 2012;154:987–1001. doi: 10.1016/j.ajo.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotecha A, Fernandes S, Bunce C, Franks WA. Avoidable sight loss from glaucoma: is it unavoidable? Br J Ophthalmol. 2012;96:816–820. doi: 10.1136/bjophthalmol-2012-301499. [DOI] [PubMed] [Google Scholar]
- 52.Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 53.Alencar LM, Zangwill LM, Weinreb RN, Bowd C, Sample PA, Girkin CA, et al. A comparison of rates of change in neuroretinal rim area and retinal nerve fiber layer thickness in progressive glaucoma. Invest Ophth Vis Sci. 2010:51. doi: 10.1167/iovs.09-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mansouri K, Leite MT, Medeiros FA, Leung CK, Weinreb RN. Assessment of rates of structural change in glaucoma using imaging technologies. Eye (London) 2011;25:269–277. doi: 10.1038/eye.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim TC, Chattopadhyay S, Acharya UR. A survey and comparative study on the instruments for glaucoma detection. Med Eng Phys. 2012;34:129–139. doi: 10.1016/j.medengphy.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 56.Vilupuru AS, Rangaswamy NV, Frishman LJ, Smith EL, Harwerth RS, Roorda A. Adaptive optics scanning laser ophthalmoscopy for in vivo imaging of lamina cribrosa. J Opt Soc Am A. 2007;24:1417–1425. doi: 10.1364/josaa.24.001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ivers KM, Sredar N, Patel NB, Rajagopalan L, Queener H, Harwerth RS, et al. High-resolution Longitudinal Examination Of The Lamina Cribrosa And Optic Nerve Head In Living Non-human Primates With Experimental Glaucoma. Invest Ophth Vis Sci. 2012;53:E-Abstract: 3697. [Google Scholar]
- 58.Choi SS, Zawadzki RJ, Lim MC, Brandt JD, Keltner JL, Doble N, et al. Evidence of outer retinal changes in glaucoma patients as revealed by ultrahigh-resolution in vivo retinal imaging. Br J Ophthalmol. 2011;95:131–141. doi: 10.1136/bjo.2010.183756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panda S, Jonas JB. Decreased photoreceptor count in human eyes with secondary angle-closure glaucoma. Invest Ophth Vis Sci. 1992;33:2532–2536. [PubMed] [Google Scholar]
- 60.Nork TM, Ver Hoeve JN, Poulsen GL, Nickells RW, Davis MD, Weber AJ, et al. Swelling and loss of photoreceptors in chronic human and experimental glaucomas. Arch Ophthalmol. 2000;118:235–245. doi: 10.1001/archopht.118.2.235. [DOI] [PubMed] [Google Scholar]
- 61.Scoles DH, Sulai YN, Manguikian AD, Shareef S, Dubra A. Reflectance adaptive optics nerve fiber layer imaging in primary open angle glaucoma. Invest Ophth Vis Sci. 2012;53:E-Abstract: 6957. [Google Scholar]
- 62.Akagi T, Hangai M, Takayama K, Nonaka A, Ooto S, Yoshimura N. In vivo imaging of lamina cribrosa pores by adaptive optics scanning laser ophthalmoscopy. Invest Ophth Vis Sci. 2012;53:4111–4119. doi: 10.1167/iovs.11-7536. [DOI] [PubMed] [Google Scholar]
- 63.Sredar N, Ivers KM, Queener H, Zouridakis G, Porter J. 3D modeling to characterize lamina cribrosa pore geometry using in vivo images from normal And glaucomatous eyes. Invest Ophth Vis Sci. 2012;53:E-Abstract: 815. [Google Scholar]
- 64.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116:497–503. doi: 10.1016/j.ophtha.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scully T. Diabetes in numbers. Nature. 2012;485:S2–S3. doi: 10.1038/485s2a. [DOI] [PubMed] [Google Scholar]
- 66.Cunha-Vaz JG. Pathophysiology of diabetic retinopathy. Br J Ophthalmol. 1978:62. doi: 10.1136/bjo.62.6.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kern TS, Engerman RL. Vascular lesions in diabetes are distributed non-uniformly within the retina. Exp Eye Res. 1995;60:545–549. doi: 10.1016/s0014-4835(05)80069-7. [DOI] [PubMed] [Google Scholar]
- 68.Moore J, Bagley S, Ireland G, McLeod D, Boulton ME. Three dimensional analysis of microaneurysms in the human diabetic retina. Journal of Anatomy. 1999;194:89–110. doi: 10.1046/j.1469-7580.1999.19410089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lieth E, Gardner TW, Barber AJ, Antonetti DA. Retinal neurodegeneration: early pathology in diabetes. Clin Exp Ophthalmol. 2000;28:3–8. doi: 10.1046/j.1442-9071.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 70.Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- 71.Fletcher EL, Phipps JA, Wilkinson-Berka JL. Dysfunction of retinal neurons and glia during diabetes. Clin Exp Optom. 2005;88:132–145. doi: 10.1111/j.1444-0938.2005.tb06686.x. [DOI] [PubMed] [Google Scholar]
- 72.Bearse MAJ, Adams AJ, Han Y, Schneck ME, Ng J, Bronson-Castain K, et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006;25:425–448. doi: 10.1016/j.preteyeres.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verma A, Rani PK, Raman R, Pal SS, Laxmi G, Gupta M, et al. Is neuronal dysfunction on early sign of diabetic retinopathy? Microperimetry and Spectral Domain Optical Coherence Tomography (SD-OCT) study in individuals with diabetes, but no diabetic retinopathy. Eye. 2009;23:1824–1830. doi: 10.1038/eye.2009.184. [DOI] [PubMed] [Google Scholar]
- 74.Van Dijk HW, Kok PH, Garvin M, Sonka M, De Vries JH, Michels RP, et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophth Vis Sci. 2009;50:3404–3409. doi: 10.1167/iovs.08-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W, et al. Fluorescein angiography complication survey. Ophthalmology. 1986;93:611–617. doi: 10.1016/s0161-6420(86)33697-2. [DOI] [PubMed] [Google Scholar]
- 76.Tam J, Dhamdhere KP, Tiruveedhula P, Manzanera S, Barez S, Bearse MA, Jr, et al. Disruption of the retinal parafoveal capillary newtork in type 2 diabetes before the onset of diabetic retinopathy. Invest Ophth Vis Sci. 2011;52:9257–9266. doi: 10.1167/iovs.11-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tam J, Dhamdhere KP, Tiruveedhula P, Lujan BJ, Johnson RN, Bearse MAJ, et al. Subclinical capillary changes in non-proliferative diabetic retinopathy. Optom Vis Sci. 2012;89:E692–703. doi: 10.1097/OPX.0b013e3182548b07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Phan A-DT, Elsner AE, Chui TY, VanNasdale DA, Clark CA, Malinovsky VE, et al. In Vivo Microvascular Changes in Diabetic Patients without Clinically Severe Diabetic Retinopathy. Invest Ophth Vis Sci. 2012;53:E-Abstract: 6964. [Google Scholar]
- 79.Lombardo M, Parravano M, Serrao S, Ducoli P, Stirpe M, Lombardo G. Analysis of retinal capillaries in patients with type 1 diabetes and non proliferative diabetic retinopathy using adaptive optics imaging. Retina. 2013 doi: 10.1097/IAE.0b013e3182899326. in press. [DOI] [PubMed] [Google Scholar]
- 80.Hammer DX, Iftimia NV, Ferguson RD, Bigelow CE, Ustun TE, Barnaby AM, et al. Foveal fine structure in retinopathy of prematurity: An adaptive optics fourier domain optical coherence tomography study. Invest Ophth Vis Sci. 2008;49:2061–2070. doi: 10.1167/iovs.07-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmoll T, Singh ASG, Blatter C, Schriefl S, Ahlers C, Schmidt-Erfurth U, et al. Imaging of the parafoveal capillary network and its integrity analysis using fractal dimension. Biomed Opt Express. 2011;2:1159–1168. doi: 10.1364/BOE2.001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun JK, Prager S, Radwan S, Ramsey DJ, Silva PS, Kwak H, et al. Photoreceptor mosaic changes in diabetic eye disease assessed by adaptive optics scanning laser ophthalmoscopy (AOSLO) Invest Ophth Vis Sci. 2012;53:E-Abstract 4647. [Google Scholar]
- 83.Parravano M, Lombardo M, Lombardo G, Boccassini B, Lioi S, Varano M. In Vivo Investigation of the Retinal Microscopy in Patients with Type 1 Diabetes Mellitus. Invest Ophth Vis Sci. 2012;53:E-Abstract: 5657. [Google Scholar]
- 84.Grisanti S, Tatar O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog Retin Eye Res. 2008;27:372–390. doi: 10.1016/j.preteyeres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 85.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 88.Querques G, Massamba N, Guigui B, Lea Q, Lamory B, Soubrane G, et al. In vivo evaluation of photoreceptor mosaic in early onset large colloid drusen using adaptive optics. Acta Ophthalmologica. 2012;90:e327–328. doi: 10.1111/j.1755-3768.2011.02228.x. [DOI] [PubMed] [Google Scholar]
- 89.Godara P, Siebe C, Rha J, Michaelides M, Carroll J. Assessing the photoreceptor mosaic over drusen using adaptive optics and SD-OCT. Ophthalmic Surg Lasers Imaging. 2010;41:S104–S108. doi: 10.3928/15428877-20101031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boretsky A, Khan F, Burnett G, Hammer DX, Ferguson RD, van Kuijk F, et al. In vivo imaging of photoreceptor disruption associated with age-related macular degeneration: A pilot study. Lasers Surg Med. 2012;44:603–610. doi: 10.1002/lsm.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossi EA, Williams DR, Dubra A, Song H, Folwell MA, Latchney LR, et al. Photoreceptor and RPE disruptions observed outside clinically visible geographic atrophy lesions in the living eye with fluorescence adaptive optics scanning laser ophthalmoscopy (FAOSLO) Invest Ophth Vis Sci. 2012;53:E-Abstract: 5599. [Google Scholar]
- 92.Nakashima K, Ullern M, Benchaboune M, Sahel J-A, Paques M. Adaptive optics imaging of geographic atrophy. Invest Ophth Vis Sci. 2012;53:E-Abstract: 2052. doi: 10.1167/iovs.12-10672. [DOI] [PubMed] [Google Scholar]
- 93.Garrioch R, Langlo C, Dubis AM, Cooper RF, Dubra A, Carroll J. Repeatability of in vivo parafoveal cone density and spacing measurements. Optom Vis Sci. 2012;89:632–643. doi: 10.1097/OPX.0b013e3182540562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lombardo M, Lombardo G, Schiano Lomoriello D, Ducoli P, Stirpe M, Serrao S. Interocular symmetry of parafoveal photoreceptor cone density. Retina. 2013 doi: 10.1097/IAE.0b013e3182807642. in press. [DOI] [PubMed] [Google Scholar]
- 95.Baraas RC, Carroll J, Gunther KL, Chung M, Williams DR, Foster DH, et al. Adaptive optics retinal imaging reveals S-cone dystrophy in tritan color-vision deficiency. J Opt Soc Am A. 2007;24:1438–1446. doi: 10.1364/josaa.24.001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chui TYP, Zhong Z, Song H, Burns SA. Foveal avascular zone and its relationship to foveal pit shape. Optom Vis Sci. 2012;89:602–661. doi: 10.1097/OPX.0b013e3182504227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Venkateswaran K, Romero-Borja F, Roorda A. Design of an adaptive optics scanning laser ophthalmoscope. In: Porter J, Queener H, Lin JH, Thorn K, Awwal A, editors. Adaptive optics for vision science. Hoboken: Wiley-Interscience; 2006. pp. 417–446. [Google Scholar]
- 98.Wolfing JI, Chung M, Carroll J, Roorda A, Williams DR. High-resolution retinal imaging of cone–rod dystrophy. Ophthalmology. 2006;113:1014–1019. doi: 10.1016/j.ophtha.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 99.Choi SS, Doble N, Hardy JL, Jones SM, Keltner JL, Olivier SS, et al. In vivo imaging of the photoreceptor mosaic in retinal dystrophies and correlations with visual function. Invest Ophth Vis Sci. 2006;47:2080–2092. doi: 10.1167/iovs.05-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duncan JL, Zhang Y, Gandhi J, Nakanishi C, Othman M, Branham KEH, et al. High-resolution imaging with adaptive optics in patients with inherited retinal degeneration. Invest Ophth Vis Sci. 2007;48:3283–3291. doi: 10.1167/iovs.06-1422. [DOI] [PubMed] [Google Scholar]
- 101.Kitaguchi Y, Bessho K, Yamaguchi T, Nakazawa N, Mihashi T, Fujikado T. In vivo measurements of cone photoreceptor spacing in myopic eyes from images obtained by an adaptive optics fundus camera. Japanese Journal of Ophthalmology. 2007;51:456–461. doi: 10.1007/s10384-007-0477-7. [DOI] [PubMed] [Google Scholar]
- 102.Bessho K, Fujikado T, Mihashi T, Yamaguchi T, Nakazawa N, Tano Y. Photoreceptor images of normal eyes and of eyes with macular dystrophy obtained in vivo with an adaptive optics fundus camera. Jap J Ophthalmol. 2008;52:380–385. doi: 10.1007/s10384-008-0575-1. [DOI] [PubMed] [Google Scholar]
- 103.Carroll J, Choi SS, Williams DR. In vivo imaging of the photoreceptor mosaic of a rod monochromat. Vision Res. 2008;48:2564–2568. doi: 10.1016/j.visres.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi SS, Zawadzki RJ, Greiner MA, Werner JS, Keltner JL. Fourier-domain optical coherence tomography and adaptive optics reveal nerve fiber layer loss and photoreceptor changes in a patient with optic nerve drusen. J Neuroophthalmol. 2008;28:120–125. doi: 10.1097/WNO.0b013e318175c6f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi SS, Zawadzki RJ, Keltner JL, Werner JS. Changes in cellular structures revealed by ultra-high resolution retinal imaging in optic neuropathies. Invest Ophth Vis Sci. 2008;49:2103–2119. doi: 10.1167/iovs.07-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chui TYP, Song HX, Burns SA. Individual variations in human cone photoreceptor packing density: Variations with refractive error. Invest Ophth Vis Sci. 2008;49:4679–4687. doi: 10.1167/iovs.08-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joeres S, Jones SM, Chen DC, Silva D, Olivier S, Fawzi A, et al. Retinal imaging with adaptive optics scanning laser ophthalmoscopy in unexplained central ring scotoma. Arch Ophthalmol. 2008;126:543–547. doi: 10.1001/archophthalmol.2007.33. [DOI] [PubMed] [Google Scholar]
- 108.Kitaguchi Y, Fujikado T, Bessho K, Sakaguchi H, Gomi F, Yamaguchi T, et al. Adaptive optics fundus camera to examine localized changes in the photoreceptor layer of the fovea. Ophthalmology. 2008;115:1771–1777. doi: 10.1016/j.ophtha.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 109.Marmor MF, Choi SS, Zawadzki RJ, Werner JS. Visual insignificance of the foveal pit: reassessment of foveal hypoplasia as fovea plana. Arch Ophthalmol. 2008;126:907–913. doi: 10.1001/archopht.126.7.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carroll J, Baraas RC, Wagner-Schuman M, Rha J, Siebe CA, Sloan C, et al. Cone photoreceptor mosaic disruption associated with Cys203Arg mutation in the M-cone opsin. Proc Natl Acad Sci USA. 2009;106:20948–20953. doi: 10.1073/pnas.0910128106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chui TY, Thibos LN, Bradley A, Burns SA. The mechanisms of vision loss associated with a cotton wool spot. Vision Res. 2009;49:2826–2834. doi: 10.1016/j.visres.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kitaguchi Y, Fujikado T, Kusaka S, Yamaguchi T, Mihashi T, Tano Y. Imaging of titanium: sapphire laser retinal injury by adaptive optics fundus imaging and Fourier-domain optical coherence tomography. Am J Ophthalmol. 2009;148:97–104. e102. doi: 10.1016/j.ajo.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 113.Stepien KE, Han DP, Schell J, Godara P, Rha J, Carroll J. Spectral-domain optical coherence tomography and adaptive optics may detect hydroxychloroquine retinal toxicity before symptomatic vision loss. Trans Am Ophthalmol Soc. 2009;107:28–34. [PMC free article] [PubMed] [Google Scholar]
- 114.Yoon MK, Roorda A, Zhang Y, Nakanishi C, Wong LJ, Zhang Q, et al. Adaptive optics scanning laser ophthalmoscopy images in a family with the mitochondrial DNA T8993C mutation. Invest Ophth Vis Sci. 2009;50:1838–1847. doi: 10.1167/iovs.08-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carroll J, Rossi EA, Porter J, Neitz J, Roorda A, Williams D, et al. Deletion of the X-linked opsin gene array locus control region (LCR) results in disruption of the cone mosaic. Vision Res. 2010;50:1989–1999. doi: 10.1016/j.visres.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen YF, Roorda A, Duncan JL. Advances in imaging of Stargardt disease. Adv Exp Med Biol. 2010;664:333–340. doi: 10.1007/978-1-4419-1399-9_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Godara P, Rha J, Tait DM, McAllister J, Dubis A, Carroll J, et al. Unusual adaptive optics findings in a patient with bilateral maculopathy. Arch Ophthalmol. 2010;128:253–254. doi: 10.1001/archophthalmol.2009.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li KY, Tiruveedhula P, Roorda A. Intersubject variability of foveal cone photoreceptor density in relation to eye length. Invest Ophth Vis Sci. 2010;51:6858–6867. doi: 10.1167/iovs.10-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Massamba N, Querques G, Lamory B, Querques L, Souied E, Soubrane G. In vivo evaluation of photoreceptor mosaic in type 2 idiopathic macular telangiectasia using adaptive optics. Acta Ophthalmologica. 2011;89:e601–603. doi: 10.1111/j.1755-3768.2010.02000.x. [DOI] [PubMed] [Google Scholar]
- 120.McAllister JT, Dubis AM, Tait DM, Ostler S, Rha J, Stepien KE, et al. Arrested development: High-resolution imaging of foveal morphology in albinism. Vision Res. 2010;50:810–817. doi: 10.1016/j.visres.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ooto S, Hangai M, Sakamoto A, Tsujikawa A, Yamashiro K, Ojima Y, et al. High-resolution imaging of resolved central serous chorioretinopathy using adaptive optics scanning laser ophthalmoscopy. Ophthalmology. 2010;117:1800–1809. doi: 10.1016/j.ophtha.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 122.Rha J, Dubis AM, Wagner-Schuman M, Tait DM, Godara P, Schroeder B, et al. Spectral domain optical coherence tomography and adaptive optics: Imaging photoreceptor layer morphology to interpret preclinical phenotypes. Adv Exp Med Biol. 2010;664:309–316. doi: 10.1007/978-1-4419-1399-9_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Telander DG, Choi SS, Zawadzki RJ, Berger N, Keltner JL, Werner JS. Microstructural abnormalities revealed by high resolution imaging systems in central macular arteriovenous malformation. Ophthalmic Surg Lasers Imaging. 2010;9:1–4. doi: 10.3928/15428877-20100215-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wagner-Schuman M, Neitz J, Rha J, Williams DR, Neitz M, Carroll J. Color-deficient cone mosaics associated with Xq28 opsin mutations: a stop codon versus gene deletions. Vision Res. 2010;50:2396–2402. doi: 10.1016/j.visres.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Audo I, El Sanharawi M, Vignal-Clermont C, Villa A, Morin A, Conrath J, et al. Foveal damage in habitual poppers users. Arch Ophthalmol. 2011;129:703–708. doi: 10.1001/archophthalmol.2011.6. [DOI] [PubMed] [Google Scholar]
- 126.Chen Y, Ratnam K, Sundquist SM, Lujan B, Ayyagari R, Gudiseva VH, et al. Cone photoreceptor abnormalities correlate with vision loss in patients with Stargardt disease. Invest Ophth Vis Sci. 2011;52:3281–3292. doi: 10.1167/iovs.10-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duncan JL, Ratnam K, Birch DG, Sundquist SM, Lucero AS, Zhang Y, et al. Abnormal cone structure in foveal schisis cavities in X-linked retinoschisis from mutations in exon 6 of the RS1 gene. Invest Ophth Vis Sci. 2011;52:9614–9623. doi: 10.1167/iovs.11-8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duncan JL, Talcott KE, Ratnam K, Sundquist SM, Lucero AS, Day S, et al. Cone structure in retinal degeneration associated with mutations in the peripherin/RDS gene. Invest Ophth Vis Sci. 2011;52:1557–1566. doi: 10.1167/iovs.10-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gelfand JM, Duncan JL, Racine CA, Gillum LA, Chin CT, Zhang Y, et al. Heterogeneous patterns of tissue injury in NARP syndrome. J Neurol. 2011;258:440–448. doi: 10.1007/s00415-010-5775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kitaguchi Y, Kusaka S, Yamaguchi T, Mihashi T, Fujikado T. Detection of photoreceptor disruption by adaptive optics fundus imaging and Fourier-domain optical coherence tomography in eyes with occult macular dystrophy. Clin Ophthalmol. 2011;5:345–351. doi: 10.2147/OPTH.S17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Merino D, Duncan JL, Tiruveedhula P, Roorda A. Observation of cone and rod photoreceptors in normal subjects and patients using a new generation adaptive optics scanning laser ophthalmoscope. Biomed Opt Express. 2011;2:2189–2201. doi: 10.1364/BOE.2.002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Michaelides M, Rha J, Dees E, Baraas RC, Wagner-Schuman ML, Mollon JD, et al. Integrity of the cone photoreceptor mosaic in oligocone trichromacy. Invest Ophth Vis Sci. 2011;52:4757–4764. doi: 10.1167/iovs.10-6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ooto S, Hangai M, Takayama K, Arakawa N, Tsujikawa A, Koizumi H, et al. High-resolution photoreceptor imaging in idiopathic macular telangiectasia type 2 using adaptive optics scanning laser ophthalmoscopy. Invest Ophth Vis Sci. 2011;52:5541–5550. doi: 10.1167/iovs.11-7251. [DOI] [PubMed] [Google Scholar]
- 134.Ooto S, Hangai M, Takayama K, Sakamoto A, Tsujikawa A, Oshima S, et al. High-resolution imaging of the photoreceptor layer in epiretinal membrane using adaptive optics scanning laser ophthalmoscopy. Ophthalmology. 2011;118:873–881. doi: 10.1016/j.ophtha.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 135.Ooto S, Hangai M, Yoshimura N. Photoreceptor restoration in unilateral acute idiopathic maculopathy on adaptive optics scanning laser ophthalmoscopy. Arch Ophthalmol. 2011;129:1633–1635. doi: 10.1001/archophthalmol.2011.345. [DOI] [PubMed] [Google Scholar]
- 136.Rossi EA, Chung M, Dubra A, Hunter JJ, Merigan WH, Williams DR. Imaging retinal mosaics in the living eye. Eye. 2011;25:301–308. doi: 10.1038/eye.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sallo FB, Leung I, Chung M, Wolf-Schnurrbusch UE, Dubra A, Williams DR, et al. Retinal crystals in type 2 idiopathic macular telangiectasia. Ophthalmology. 2011;118:2461–2467. doi: 10.1016/j.ophtha.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sarda V, Nakashima K, Wolff B, Sahel JA, Paques M. Topography of patchy retinal whitening during acute perfused retinal vein occlusion by optical coherence tomography and adaptive optics fundus imaging. Eur J Ophthalmol. 2011;21:653–656. doi: 10.5301/EJO.2011.6374. [DOI] [PubMed] [Google Scholar]
- 139.Song H, Chui TY, Zhong Z, Elsner AE, Burns SA. Variation in cone photoreceptor packing density with retinal eccentricity and age. Invest Ophth Vis Sci. 2011;52:7376–7384. doi: 10.1167/iovs.11-7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Werner JS, Keltner JL, Zawadzki RJ, Choi SS. Outer retinal abnormalities associated with inner retinal pathology in nonglaucomatous and glaucomatous optic neuropathies. Eye. 2011;25:279–289. doi: 10.1038/eye.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Carroll J, Dubra A, Gardner JC, Mizrahi-Meissonnier L, Cooper RF, Dubis AM, et al. The effect of cone opsin mutations on retinal structure and the integrity of the photoreceptor mosaic. Invest Ophth Vis Sci. 2012;53:8006–8015. doi: 10.1167/iovs.12-11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Duncan JL, Roorda A, Navani M, Vishweswaraiah S, Syed R, Soudry S, et al. Identification of a novel mutation in the CDHR1 gene in a family with recessive retinal degeneration. Arch Ophthalmol. 2012;130:1301–1308. doi: 10.1001/archophthalmol.2012.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Godara P, Wagner-Schuman ML, Rha J, Connor TB, Stepien K, Carroll J. Imaging the photoreceptor mosaic with adaptive optics: beyond counting cones. Adv Exp Med Biol. 2012;723:451–458. doi: 10.1007/978-1-4614-0631-0_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mkrtchyan M, Lujan BJ, Merino D, Roorda A, Duncan JL. Outer retinal structure in patients with acute zonal occult outer retinopathy. Am J Ophthalmol. 2012;153:757–768. doi: 10.1016/j.ajo.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ooto S, Hangai M, Takayama K, Ueda-Arakawa N, Hanebuchi M, Yoshimura N. Photoreceptor damage and foveal sensitivity in surgically closed macular holes: An adaptive optics scanning laser ophthalmoscopy study. Am J Ophthalmol. 2012;154:174–186. doi: 10.1016/j.ajo.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 146.Ratnam K, Västinsalo H, Roorda A, Sankila E-MK, Duncan JL. Cone structure in patients with Usher syndrome type III and mutations in the Clarin 1 gene. JAMA Ophthalmol. 2013;131:67–74. doi: 10.1001/2013.jamaophthalmol.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stepien KE, Martinez WM, Dubis AM, Cooper RF, Dubra A, Carroll J. Subclinical photoreceptor disruption in response to severe head trauma. Arch Ophthalmol. 2012;130:400–402. doi: 10.1001/archopthalmol.2011.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lombardo M, Serrao S, Devaney N, Parravano M, Lombardo G. Adaptive optics tchnology for high-resolution retinal imaging. Sensors (Basel) 2012;13:334–366. doi: 10.3390/s130100334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tojo N, Nakamura T, Fuchizawa C, Oiwake T, Hayashi A. Adaptive optics fundus images of cone photoreceptors in the macula of patients with retinitis pigmentosa. Clin Ophthalmol. 2013;7:203–210. doi: 10.2147/OPTH.S39879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Takayama K, Ooto S, Hangai M, Ueda-Arakawa N, Yoshida S, Akagi T, et al. High-resolution imaging of retinal nerve fiber bundles in glaucoma using adaptive optics scanning laser ophthalmoscopy. Am J Ophthalmol. 2013 doi: 10.1016/j.ajo.2012.11.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 151.Syed R, Sundquist SM, Ratnam K, Zayit-Soudry S, Zhang Y, Crawford JB, et al. High-resolution images of retinal structure in patients with choroideremia. Invest Ophth Vis Sci. 2013;54:950–961. doi: 10.1167/iovs.12-10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McClements M, Davies WI, Michaelides M, Carroll J, Rha J, Mollon JD, et al. X-linked cone dystrophy and colour vision deficiency arising from a missense mutation in a hybrid L/M cone opsin gene. Vision Res. 2013 doi: 10.1016/j.visres.2012.12.012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vincent A, Wright T, Garcia-Sanchez Y, Kisilak M, Campbell MCW, Wastall C, et al. Phenotypic characteristics including in vivo cone photoreceptor mosaic in KCNV2-related “Cone dystrophy with supernormal rod electroretinogram”. Invest Ophth Vis Sci. 2013;54:898–908. doi: 10.1167/iovs.12-10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kay DB, Land ME, Cooper RF, Dubis AM, Godara P, Dubra A, et al. Outer retinal structure in Best vitelliform macular dystrophy. JAMA Ophthalmol. 2013 doi: 10.1001/jamaophthalmol.2013.387. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vincent AL, Carroll J, Fishman GA, Sauer A, Sharp D, Summerfelt P, et al. Rhodopsin F45L allele does not cause autosomal dominant retinitis pigmentosa in a large Caucasian family. Trans Vis Sci Tech. 2013 doi: 10.1167/tvst.2.2.4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]