Abstract
COX-derived prostanoids play multiple roles in inflammation and cancer. This review highlights research examining COX-2 and PGE2-dependent regulation of immune cell polarization and function within the tumor microenvironment, particularly as it pertains to breast cancer. Appreciating PGE2-mediated immunomodulation is important in understanding how tumors evade immune surveillance by reeducating infiltrating inflammatory and immune cells to support tumorigenesis. Elucidation of the multiple and complex influences exerted by tumor stromal components may lead to targeted therapies in breast and other cancers that restrain microenvironmental permissiveness and maintain natural defenses against malignancies.
Keywords: Breast cancer, COX-2, Macrophage polarization, Microenvironment, PGE2, Regulatory T lymphocytes, Myeloid-derived suppressor cells, Immune suppression
1. Introduction
Cyclooxygenase (COX) is the enzyme responsible for the conversion of arachidonic acid into the various prostanoids, a family of lipid mediators that have widespread and diverse biological function [1]. COX exists in two main isoforms, COX-1, which is predominantly constitutive and responsible for generation of prostanoids for “housekeeping functions”, and COX-2, the inducible isoform, which contributes prostanoids involved in a variety of growth and inflammatory events [1,2]. Synthesis of eicosanoids begins after the release of arachidonic acid (AA) from membrane phospholipids through the action of cytosolic phospholipase A2. COX-1/COX-2, also known as prostaglandin G/H synthase 1/2, converts AA into prostaglandin (PG) G2 and then reduces PGG2 to PGH2. PGH2 can be metabolized by the various PG synthases into PGD2, PGE2, PGF2α, PGI2, and thromboxane (TX) A2, which then act via distinct downstream G protein-coupled receptors.
A large body of work describing a link between inflammation and cancer [3] has generated intense interest in targeting COX enzymes, COX-2 in particular, for cancer therapy or chemoprevention. COX-2 is upregulated in 40% of breast cancers, with up to 84% increases in some studies [4]. Clinical studies have noted a reduced risk for breast, lung, prostate, and colon cancers after treatment with non-steroidal anti-inflammatory drugs (NSAIDs), which non-selectively inhibit COX-1 and COX-2, or with selective inhibition of COX-2 [5]. The beneficial effects of aspirin are less clear in part because many studies do not distinguish between consumption of low dose aspirin, whose effect is limited to inhibition of platelet COX-1 function, and higher doses that inhibit systemic function of both isozymes. In the Women’s Health Initiative observational study, chronic regular use of NSAIDs was associated with reduced risk of breast cancer but subgroup analysis revealed no effect of low dose aspirin (<100 mg) [6]. Similarly, the Women’s Health Study, a long term randomized trial, showed no effect of low dose aspirin every other day on breast cancer incidence [7]. Reduced risk of breast cancer death and distant recurrence, but not incidence of primary disease, was associated with regular aspirin use in the prospective observational Nurses’ Health Study but dose was not reported [8]. In contrast, in another recent study, lifetime aspirin use was associated with a 32% decreased risk of breast cancer, though, again, no information on dosage was collected [9]. Analysis of eight aspirin trials revealed reduced cancer death that was independent of dose across several common cancers although scant information was available in breast cancer [10].
Certain COX-2-derived products, particularly PGE2, are known to act via classical cancer signaling pathways in primary tumor cells to promote tumorigenesis. Recent evidence has shined a spotlight not only on the tumor cell itself, but the tumor microenvironment, or stroma, which surrounds the tumor. This is evidenced by Hanahan and Weinberg recently updating their landmark review of the hallmarks of cancer to include microenvironment specific components [11]. The microenvironment contains multiple resident and infiltrating cells, including immune cells, along with the growth factors and cytokines that they release. A supportive tumor microenvironment appears crucial for the development of a tumor as well as its transition to malignancy, and the characteristics of a pro-tumorigenic microenvironment has been well reviewed [12]. This review will focus on tumor evasion of immune surveillance, and how COX-2-derived PGE2 can modulate local immune responses in the tumor stroma to support progression and metastasis.
2. Metabolism and tumorigenic properties of PGE2
PGE2 makes up the majority of secreted prostaglandin in tumors and is thought to be the principal tumorigenic COX-2-derived product. This has been studied in a broad range of cancers, though perhaps most intensively in colorectal cancer [2]. PGE2 is generated through the conversion of PGH2 by microsomal PGE synthases (mPGES) 1 or 2, or cytosolic (c) PGES. Like COX-2, mPGES-1 is inducible and appears to be the dominant PGE2-generating enzyme in tumors [13]. Functional coupling of COX-2 and mPGES-1 has been reported [14] while the constitutive cPGES couples to COX-1 (mPGES-2 has yet to be well characterized). PGE2 acts through four distinct G-protein coupled receptors termed EP1, EP2, EP3, and EP4. Regulation of prostaglandin signaling relies not only on their synthesis, but also on their cellular transport and degradation. Solute carrier organic anion transporter 2A1 (SLCO2A1), also known as OAT2A1 or prostaglandin transporter, directs uptake of PGE2, PGD2, and PGF2α from the extracellular space into the cytosol. Once there, 15-hydroxyprostaglandin dehydrogenase (15-PGDH) catalyzes the initial step in prostanoid breakdown into their inactive 13,14-dihydro-15-keto-metabolites [15]. The multidrug resistance protein 4 (MRP4) can transport PGE2 and PGF2α from the intracellular to the extracellular space [16] and thus may contribute to elevated PGE2 levels and EP receptor activation. Coordinated regulation of these multiple steps in PGE2 biosynthesis, metabolism and function, ultimately determines the biological response.
The tumorigenic properties of PGE2 have been thoroughly reviewed elsewhere [2,4,17], including an in-depth analysis of how PGE2 contributes to the hallmarks of cancer [18,19] –self-sufficiency in growth signals, insensitivity to antigrowth signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, and tissue invasion/metastasis. Briefly, PGE2 enhances Wnt signaling through EP2-mediated suppression of glycogen synthase kinase (GSK) 3β [20]. Subsequent accumulation of the β-catenin/T cell factor 4 complex leads to transactivation of perixosome proliferator-activated receptor (PPAR) δ and upregulation of pro-oncogenic genes [21]. GSK3β suppression is mediated by activation of phosphoinositide 3-kinase and Akt. In addition, Gαs, which couples to EP2, complexes with Axin, dissociating it from the β-catenin destruction complex, leading to further enhancement of the Wnt signaling pathway [20]. PGE2 also promotes cell survival by induction of the anti-apoptotic protein Bcl-2 via Ras-MAPK signaling [22], an effect that is partially mediated by PGE2 transactivation of extracellular growth factor receptor [23]. Multiple studies have implicated PGE2 production in tumors or tumor cell lines in increased expression of vascular endothelial growth factor and its receptors [24,25], an effect that appears mediated by Gq-coupled EP3 [26,27] signaling through protumorigenic extracellular signaling-regulated kinase/c-Jun N-terminal kinases [28].
3. COX-2 in breast cancer
Animal and human studies report COX-2 overexpression in breast cancer [4,29–31], and strongly support a role for this enzyme in disease progression. Targeted overexpression of COX-2 gene in the mammary epithelium, via the mouse mammary tumor virus, was sufficient to induce mammary tumorigenesis in multiparous mice through a PGE2-EP2 pathway [32,33]. Further studies in this model revealed an upregulation of cytochrome P450 aromatase that was reversed following COX-2 inhibition with celecoxib [31]. COX-2 inhibition reduced tumorigenesis across a wide range of animal breast cancer models. These have been reviewed extensively [2,4]. Briefly, celecoxib and rofecoxib, both considered selective for COX-2 inhibition, suppressed mammary tumorigenesis in rats treated with 7,12-dimethylbenzanthracene and N-methyl-N-nitrosourea [34,35]. The same inhibitors reduced disease in HER2/neu- and Lewis lung carcinoma (3LL) xenograft-induced models [36,37]. In many studies the molecular mechanism of reduced tumorigenesis has not been defined, other than to note a reduction in PGE2 signaling on mitogenic and anti-apoptotic pathways. Reduced multiplicity and size of HER2/neu-driven mammary tumors in global COX-2 knock-out (KO) mice was attributed to a concurrent suppression in tumor angiogenesis [38], consistent with the reported contribution of COX-2-derived PGE2 to the angiogenic switch in mammary tumors that allows disease progression [39].
We have used Cre recombinase technology to target deletion of COX-2 expression selectively to the mammary epithelium. Significantly delayed tumorigenesis was observed independent of modified angiogenesis but coincident with a change in the number and phenotype of tumor infiltrating cells [40]. These, and other studies [41], indicate a wider role for COX-2 in control of tumor progression via regulation of the microenvironment.
4. Immune regulation of tumorigenesis
In the past decade, evidence has quickly mounted that genetic mutations in classical cancer signaling pathways of tumor epithelial cells cannot fully explain differences in phenotype and clinical development of tumors [42,43]. Indeed, cancer is increasingly considered a disease of the tissue and its progression depends on the supportive or suppressive nature of the microenvironment in the surrounding stroma. The microenvironment contains several different cell types, including fibroblasts, endothelial cells, and immune cells, along with all mediators they release. Cells involved in immune surveillance of tumors include T cells, antigen-presenting cells (APC) such as macrophages and dendritic cells (DC), mast cells, natural killer (NK) cells, myeloid-derived suppressor cells (MDSC), neutrophils, and others [12]. Immune regulation of tumorigenesis and metastasis has been reviewed extensively by others [12,42]. Leukocytic infiltration in tumors was originally considered an attempt by the immune system to reject the tumor. Indeed, this may be the original purpose of cells that have migrated to a tumor site. However, tumors can “hijack” this process by re-educating the immune response, leading to suppression of tumoricidal and immune competent phenotypes, as well as promotion of alternative immune cell functions, often important for normal development and wound healing, that support tumor growth [44,45]. A prominent example of alternative activation is the CD4+ T helper lymphocyte type 1 and type 2 (TH1 and TH2, respectively) paradigm, in which appropriately stimulated TH1 cells release cytokines that support cytotoxicity while TH2 cells contribute to adaptive immunity and humoral antibody production. TH1-derived cytokines can suppress TH2 cytokine responses, and vice versa. These complex differential responses by immune cells underscore the need to understand not only the tumor cells themselves, but also the immune responses occurring in the tumor stroma (Fig. 1).
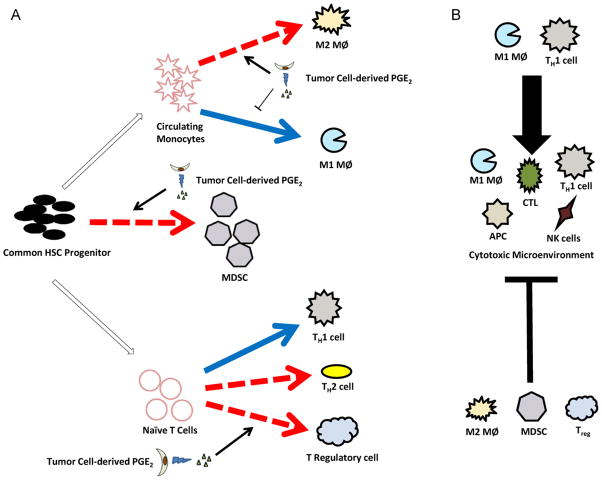
Fig. 1.
Schematic of how PGE2 may affect immune cell differentiation and function in tumors. (A) Immune cells of the microenvironment may be immunogenic (solid arrows) or immunosuppressive (dashed arrows). Tumor cell-derived PGE2 can affect the differentiation, infiltration, and maturation of several tumor-associated immune cells. PGE2 suppressed polarization of macrophages (MØ) to the M1 phenotype while enhancing M2 characteristics. PGE2 promotes differentiation to and function of MDSCs and is correlated with increased MDSC numbers. PGE2 has been implicated in increased differentiation and infiltration Treg which in turn suppress the maturation of other T cells. (B) These actions of PGE2 work to shift the tumor microenvironment into an immunosuppressive environment by suppressing maturation of cytotoxic cells that are essential for immune surveillance.
5. Effect of PGE2 on tumor-associated macrophages
Tumor-associated macrophages (TAM) represent the majority of tumor infiltrating leukocytes [46]. Macrophages are particularly relevant to the tumor microenvironment because, in addition to their role as modulators of angiogenesis, tumor cell migration, and matrix remodeling, they can support or suppress local immune responses thereby contributing to immune surveillance or escape. Macrophages express Toll-like receptors, mannose receptors, and scavenger receptors that can all activate classical innate immunity [47]. They also have adhesion receptors that allow interfacing of macrophages with other immune cells and major histocompatability complexes (MHC) for antigen-presenting functions. In addition, macrophages express a variety of cytokine receptors that can activate signal transducer and activator of transcription (STAT) signaling and induce further release of cytokines [48]. Through their direct and indirect effects on other immune cells, macrophages play a central role in the orchestration of the localized tumor immune response that can support or suppress tumor growth.
As early as the 1970s, macrophages stimulated with lipopolysaccharide (LPS), were shown to kill tumor cells in vitro [49]. These experiments supported the general notion that macrophage activation would be tumor suppressive, a hypothesis reinforced by reports of cytokine-induced release of reactive nitrogen and oxygen intermediates from macrophages [50]. In 1992, Gordon and colleagues coined the term “alternatively activated macrophages” after noting that stimulating macrophages with interleukin (IL)-4 inhibited pro-inflammatory cytokine production and restricted expression of MHC class II complexes [51]. These two unique polarization states have since been termed M1 (classically activated) and M2 (alternatively activated), so-named after the TH1/TH2 paradigm, and seem to represent two poles of macrophage function. Indeed, research within the past decade has revealed that M2 macrophages, which share many features of TAMs [52], support cell growth, tissue remodeling, and angiogenesis, and are considered to be pro-tumorigenic [53]. In addition, whereas M1 macrophages release type 1 cytokines, such as tumor necrosis factor (TNF) α and IL-6, that promote T lymphocyte differentiation to pro-immune CD4+ TH1 and cytotoxic T lymphocytes (CTL; CD8+ T lymphocytes), M2 macrophages release immunosuppressive cytokines such as IL-10 and TGFβ that result in T cell anergy. M1 and M2 macrophages also utilize distinct arginine-metabolizing enzymes that characterize their polarization state and contribute to their distinct functions: M1 macrophages express inducible nitric oxide synthase (iNOS), metabolizing L-arginine into nitric oxide thus contributing to M1-associated tumoricidal functions. M2 macrophages, in contrast, express arginase-1, which metabolizes L-arginine into L-ornithine and proline, benefiting cell growth and promoting extracellular matrix remodeling. Further, the markedly enhanced expression of arginase-I in M2 macrophages depletes the pool of local L-arginine that is necessary for normal CTL function [54].
PGE2 is typically associated with immunosuppression, restraint of type 1 cytokine production and M1 macrophage polarization, as well as enhanced expression of M2 markers. In early work, treatment of Lewis lung carcinoma (LLC) tumor bearing mice with indomethacin, a mixed COX-1/COX-2 inhibitor, attenuated tumor suppressive natural killer (NK) cells, an effect that was associated with macrophages and PGE2 [55]. Additional studies support a role for PGE2 in the control of macrophage phenotype and function. A recent report that COX-2 inhibition reversed tumor-mediated bone marrow-derived cell differentiation to APCs revealed an autocrine function of COX-2 derived mediators in regulation of myeloid cell phenotype [48]. Interestingly, suppression of the APC phenotype by tumor-derived factors was coincident with a decrease in bone-marrow cell expression of 15-PGDH/SLCO2A1 mRNA and an increase in MRP4 mRNA, suggesting a coordinated effort at multiple points in the COX-2-PGE2 metabolic pathway. However, inhibition of mPGES-1, the dominant source of macrophage PGE2 [56], did not recapitulate the phenotypic rescue observed with a COX-2 inhibitor, suggesting significant complexity in the biology, perhaps involving other COX-2-derived prostanoids [48]. STAT1/3 signaling in macrophages, a known cytokine regulatory pathway, was implicated in COX-2-mediated suppression of APC function. Suppressed function of other inflammatory/immune signaling pathways has been linked with COX-2 and PGE2. PGE2 can induce expression and homodimerization of the inhibitory p50 subunit of nuclear factor (NF)-κB, resulting in decreased NFκB activation and depressed M1 cytokine release in TAMs isolated from human ovarian cancers [57]. PGE2 also decreased elaboration of several type 1 cytokines, including IL-1β [58], TNFα, and IL-6 [59] in LPS-stimulated human peripheral blood monocytes via EP2/EP4-cAMP signaling, IL-8 in LPS-stimulated human alveolar macrophages [60], and IL-6 and TNFα in LPS-induced murine peritoneal macrophages, again through an EP2 and EP4 cAMP-mediated pathway [61]. PGE2 also decreased expression of iNOS after LPS stimulation in J774 cells [62].
Conversely, PGE2 is implicated in the promotion of M2 macrophage function, promoting expression of the M2 cytokine IL-10 and arginase-1 in LPS-stimulated murine peritoneal macrophages [61] and bone marrow-derived macrophages [63]. Enhanced PGE2 metabolism, via forced 15-PGDH overexpression, reduced secretion of IL-10, IL-13, and IL-6, with a coincident increase in F4/80+/CD11c+ APC and a decrease in F4/80+/CD11c−M2 macrophages in a xenograft model of colon cancer. Experiments in our lab examining the role of PGE2 in modulating M1 and M2 polarization reinforce these findings.
Together, these data support autocrine and paracrine effects of PGE2, and possibly other COX-2-derived prostanoids, in the restraint of M1, and/or promotion of M2, macrophage function. The studies outlined above describing COX-2 and PGE2 modulation of macrophage and APC functions reinforce the paradigm that PGE2 downregulates expression of M1 markers/cytokines (e.g. TNFα, IL-6, and iNOS) and functions (e.g. antigen-presenting ability and release of NO), while upregulating M2 markers/cytokines (IL-10 and arginase-1). Much of this work has been in in vitro or ex vivo models. Additional research is warranted to determine the contribution of COX-2 and PGE2 to regulation of M1/M2 polarization in vivo across the entire spectrum of tumor onset and progression. Indeed, while TAMs have many of the characteristics of M2 macrophages, they are a distinct population. In particular, the high expression levels of COX-2 in TAM diverges from the typical M2 phenotype in which COX-2 is suppressed, underscoring the complex influence of tumor-associated mediators and COX-2-derived prostanoids in paracrine and autocrine control of macrophages.
6. Effect of PGE2 on myeloid-derived suppressor cells
A growing appreciation for the role of MDSCs in tumor immunosuppression has developed over the past decade. Interest in MDSCs began in the 1980s when MDSCs were more commonly referred to as natural suppressor cells or bone marrow suppressor cells. MDSCs were originally identified as a subset of bone marrow cells that could inhibit T cell and NK proliferation that were characteristically distinguished from macrophages by their inability to adhere to nylon wool [64]. Since then, murine MDSCs have been defined as CD11b+, Gr-1+, F4/80+, CD11clow cells that express both arginase-1 and iNOS [65]. MDSCs include a variety of myeloid progenitor cells as well as modified myeloid-derived cells, including immature macrophages, DCs, and granulocytes (such as neutrophils).
MDSC regulation of the immune system, especially as it pertains to cancer, has been well reviewed [65]. In short, cytokine receptor activation can signal through the Janus kinase (JAK)-STAT pathway to regulate cell survival and activation. STAT3 is particularly important in tumor-dependent expansion of MDSCs, as STAT3 regulation of gene transcription can lead to Myc, Bcl-XL, and cyclin D1 transcription, enhancing MDSC survival. Leukocyte-derived type 1 and 2 cytokines signal primarily through STAT1 and STAT6, respectively, enhancing the immunosuppressive environment through upregulation of TGFβ, IL-10, arginase-1, and iNOS in MDSCs. Dual expression of both arginase-1 and iNOS differentiates MDSCs from other mature myeloid cells. Both of these enzymes use L-arginine as a substrate, and L-arginine depletion inhibits T cell proliferation through a variety of mechanisms [54]. In addition, NO production inhibits JAK–STAT signaling in T lymphocytes, inhibits antigen-presenting functions of MHC II expressing cells, including macrophages [66,67], and causes T cell apoptosis [68,69]. Importantly, reactive oxygen species (ROS) production through arginase-1 and iNOS can produce peroxynitrite, which is directly linked to insensitivity of CD8+ CTLs to antigen [70]. Given their multiple and potent immunosuppressive effects, MDSCs may provide a valuable target in host immune defense against tumorigenesis.
PGE2 has been strongly implicated in MDSC function with respect to chemoattraction of MDSCs to tumors sites as well as differentiation of non-bone marrow cells to MDSCs in the tumor microenvironment. MDSCs positive for both CD11b and Gr-1 derived from 4T1 mouse mammary tumors expressed all four EP receptors [71]. In addition, treatment of bone marrow cells with PGE2 or EP2 selective agonist (butaprost) increased differentiation to MDSC by 20% and 40%, respectively, while antagonism of EP1/EP2 or EP4 prevented differentiation into MDSC [71]. PGE2-induced elevation in MDSC was coincident with decreased T cell activation [71]. In addition, reduced 4T1-xenograft tumor growth in EP2−/− mice was coincident with decreased MDSC infiltration in tumors [71]. Another 4T1 injection model replicated these results, showing that PGE2 and TGFβ were both necessary for MDSC differentiation from bone marrow cells [72]. Further, PGE2 concentrations in tumor cell supernatants correlated very strongly with ability to induce MDSC differentiation in BM cells and MDSC differentiation was offset when PGE2-depleting antibody was added to the culture mixture [72].
In 3LL injection models of murine tumorigenesis, C57BL/6 MDSCs expressed similar levels of arginase-1 as those from severe combined immunodeficiency mice, indicating that arginase-1 expression may not be dependent on T cell-derived factors [73]. Instead, MDSCs cultured with tumor cells or conditioned medium showed a significant increase in arginase-1 expression. PGE2 was identified as a dominant soluble tumor-derived mediator driving arginase-1 expression via an EP4/cAMP signaling cascade [73]. In another 3LL tumor cell study, PGE2 released downstream of Fas ligation increased chemoattraction of MDSCs implicating COX-2 and PGE2 in Fas-dependent MDSC tumor infiltration [74]. COX-2 inhibition with celecoxib prolonged survival of 1,2 dimethylhydrazine-injected mice coincident with a decrease in Gr-1+, CD11b+ MDSCs and a decrease in iNOS and arginase-1 mRNA [75]. Celecoxib treatment in mesothelioma-injected mice also showed a decreased expansion of MDSCs coincident with decreased PGE2 concentrations and improved DC-based co-therapy [76]. Thus, the chemopreventative benefits associated with COX-2 inhibition likely involves repressed MDSC differentiation, infiltration, and/or function.
Intensive research on PGE2 dependent regulation of MDSC function tumors continues with a strong consensus building that PGE2 supports MDSC proliferation and consequent immunosuppression. While findings in vitro and in tumor xenograft models are promising, further in vivo modeling of PGE2 and MDSC interactions will determine the viability of MDSC-related targets in cancer prevention or treatment via the COX-2 pathways.
7. Effect of PGE2 on T regulatory cells
A subset of CD4+ T cells that suppress effector T cell functions have a crucial role in preventing self (vs. non-self) destruction and thus are implicated in a variety of autoimmune diseases [77]. This same subset of T cells may be involved in T cell anergy in tumorigenesis and modify the immune response to accept the tumor as self instead of marking it for destruction. Classification, development, and function of these regulatory T cells (Tregs), which is still ill-defined and the subject of much research, has been reviewed elsewhere [77,78]. CD4+CD25+FOXP3+ cells are generally accepted as a population of Treg, and strength of interaction with Treg MHC complexes appear crucial to Treg development. Treg suppressor function has been expanded to encompass many of the effector cells of the immune system, including TH cell subsets, CD8+ CTLs, macrophages, NK cells, and mast cells [79]. The exact mechanism of Treg suppression is still under intensive investigation although IL-10, TGFβ, and IL-35 suppressive cytokines are considered characteristic of Tregs, as are interactions between lymphocyte-activating gene 3 and MHC II complexes in DCs leading to suppressed DC maturation and limited APC functions [78]. Potential cancer immunotherapies targeted at Tregs include TGFβ depletion and stimulation of glucocorticoid-induced TNF receptor to induce T effector cell differentiation [79].
In gastric cancer patients, the proportion of CD4+CD25+FOXP3+ cells was increased in both the peripheral blood and tumor tissues as compared to normal healthy patients [80]. This correlated with stage of tumor progression, as well as concentration of PGE2, in ascites of gastric cancer patients but interestingly not with levels of TGFβ and IL-10, both of which are Treg factors. COX-2 expression also strongly correlated with FOXP3 expression by flow cytometry, implicating Tregs as PGE2-producing cells in the tumor microenvironment. FOXP3 protein levels were reduced using both a non-selective COX and COX-2 selective inhibitors, but not using EP2 or EP4 antagonists [80]. However, these antagonists did match COX-2 inhibitors in reversing suppressed proliferation of tumor-infiltrating T lymphocytes. These may indicate that PGE2 acting on receptors EP2 and EP4 are only partially responsible for Treg differentiation and infiltration and/or other COX-2-derived factors contribute to effector T cell suppression. In a separate study using CD4+CD25+ Tregs isolated from human peripheral blood, stimulation with anti-CD3/CD28 led to a large induction of PGE2, IL-10, and TGFβ. Addition of COX-2 inhibitor, anti-IL-10, or anti-TGFβ reversed Treg suppression of IFNγ-producing CD4+ T cells, a measure of effector T cell function. Concordant with the correlation COX-2 and FOXP3 expression [80,81], treatment with PGE2 dose-dependently increased FOXP3 expression in peripheral blood Tregs, as well as in colon cancer patients [82]. These studies emphasize a role for PGE2 mediating T cell proliferation in both an autocrine Treg → Treg and paracrine Treg → effector T cell manner.
In an injection model of non-small cell lung cancer, knockdown of COX-2 in tumor cells led to decreased Tregs at the tumor site, an effect that was mirrored with anti-PGE2 antibody or a selective COX-2 inhibitor [83]. In ex vivo studies, COX-2 inhibition increased effector T cell proliferation in co-cultures with murine Tregs. Robust upregulation of FOXP3 was seen when CD4+CD25+ cells were pulsed with PGE2, an effect that was partially reduced through the use of EP4−/− cells and completely ablated in EP2−/− cells [83]. These studies provide further evidence that COX-2-derived PGE2, acting through EP2 and EP4, increases Treg infiltration, maturation, and function, leading to immunosuppression and increased tumor cell survival.
In general, there is a consensus that PGE2 can enhance Treg proliferation and FOXP3 overexpression with consequent suppression of effector T cell proliferation. However, reports that PGE2 can instead suppress proliferation of Tregs [84,85] reveal the complexity of COX-2 and PGE2 in regulation of Treg differentiation, proliferation, and contribution to disease in the already immunosuppressive environment of a growing tumor.
8. Conclusion
The tumor immune microenvironment involves a remarkably complex interplay of cells and mediators that can drive a tumor towards limitless growth or imminent destruction. Multiple different cell types, and subtypes, exist in a milieu of growth factors, cytokines, oxidative species, and lipid mediators. Among these, PGE2 has emerged as a mediator that not only impacts classical oncogenic signaling pathways in tumor cells, but also contributes to shifting the tumor microenvironment towards immune suppression and evasion, promoting tumorigenesis. A substantial body of work indicates the immune suppressive role of PGE2, including the restraint of M1, and promotion of M2, macrophage phenotypes, augmentation of MDSC differentiation and infiltration, and support of Treg proliferation and function. These immunosuppressive leukocyte subtypes share common cytokine mediators, including TGFβ and IL-10. More importantly, each cell subtype can generate PGE2, providing an autocrine mechanism for prolonging and enhancing their own immunosuppressive phenotype. This emphasizes the importance of exploring the tumor microenvironment as a whole, rather than focusing on alterations in an individual subset of tumor-associated cells. There are several other types of immune cells that have not been discussed above, including B cells, mast cells, eosinophils, and neutrophils, which also appear to contribute to immune surveillance of tumorigenesis. Indeed, an N1/N2 tumor-associated neutrophil paradigm has recently been discussed [86]. Discrepancies between the immunosuppressive actions of PGE2 across in vitro or ex vivo experiments underscore the context-specific nature of the tumor microenvironment, a context that is unique to individual cancers and disease stages. Continuing research in COX-2-derived PGE2-based immunosuppression may yield appealing targeted immunotherapies to reclaim the immune system’s ability to destroy a cancer.
Acknowledgments
Part of the work described in this review was supported by a grant from the American Cancer Society (RSG-08-024-21 to E.M.S.). We acknowledge the technical assistance of Ms Victoire Ndong.
Abbreviations
- COX
cyclooxygenase
- PG
prostaglandin
- mPGES
microsomal prostaglandin E synthase
- 15-PGDH
15-hydroxyprostaglandin dehydrogenase
- SLCO2A1
solute carrier organic anion transporter 2A1
- APC
antigen-presenting cell
- DC
dendritic cell
- NK
natural killer
- MDSC
myeloid-derived suppressor cell
- TAM
tumor-associated macrophage
- CTL
cytotoxic T lymphocyte
- iNOS
inducible nitric oxide synthase
- Tregs
regulatory T cells
References
- 1.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50(Suppl):S423–8. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248(3):171–83. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 4.Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007;9(4):210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17(2):55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- 6.Harris RE, Chlebowski RT, Jackson RD, et al. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women’s Health Initiative. Cancer Res. 2003;63(18):6096–101. [PubMed] [Google Scholar]
- 7.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 8.Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28(9):1467–72. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasky TM, Bonner MR, Moysich KB, et al. Non-steroidal anti-inflammatory drug (NSAID) use and breast cancer risk in the Western New York Exposures and Breast Cancer (WEB) Study. Cancer Causes Control. 2010;21(9):1503–12. doi: 10.1007/s10552-010-9579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamei D, Murakami M, Nakatani Y, Ishikawa Y, Ishii T, Kudo I. Potential role of microsomal prostaglandin E synthase-1 in tumorigenesis. J Biol Chem. 2003;278(21):19396–405. doi: 10.1074/jbc.M213290200. [DOI] [PubMed] [Google Scholar]
- 14.Murakami M, Naraba H, Tanioka T, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275(42):32783–92. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- 15.Tai HH, Ensor CM, Tong M, Zhou H, Yan F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002;6(8–69):483–93. doi: 10.1016/s0090-6980(02)00050-3. [DOI] [PubMed] [Google Scholar]
- 16.Reid G, Wielinga P, Zelcer N, et al. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci USA. 2003;100(16):9244–9. doi: 10.1073/pnas.1033060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh-Ranger G, Salhab M, Mokbel K. The role of cyclooxygenase-2 in breast cancer: review. Breast Cancer Res Treat. 2008;109(2):189–98. doi: 10.1007/s10549-007-9641-5. [DOI] [PubMed] [Google Scholar]
- 18.Greenhough A, Smartt HJ, Moore AE, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30(3):377–86. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 20.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310(5753):1504–10. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Wang H, Shi Q, et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004;6(3):285–95. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58(2):362–6. [PubMed] [Google Scholar]
- 23.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8(3):289–93. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 24.Fujino H, Toyomura K, Chen XB, Regan JW, Murayama T. Prostaglandin E regulates cellular migration via induction of vascular endothelial growth factor receptor-1 in HCA-7 human colon cancer cells. Biochem Pharmacol. 2011;81(3):379–87. doi: 10.1016/j.bcp.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Cianchi F, Cortesini C, Bechi P, et al. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121(6):1339–47. doi: 10.1053/gast.2001.29691. [DOI] [PubMed] [Google Scholar]
- 26.Amano H, Hayashi I, Endo H, et al. Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med. 2003;197(2):221–32. doi: 10.1084/jem.20021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amano H, Ito Y, Suzuki T, et al. Roles of a prostaglandin E-type receptor, EP3, in upregulation of matrix metalloproteinase-9 and vascular endothelial growth factor during enhancement of tumor metastasis. Cancer Sci. 2009;100(12):2318–24. doi: 10.1111/j.1349-7006.2009.01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pai R, Szabo IL, Soreghan BA, Atay S, Kawanaka H, Tarnawski AS. PGE(2) stimulates VEGF expression in endothelial cells via ERK2/JNK1 signaling pathways. Biochem Biophys Res Commun. 2001;286(5):923–8. doi: 10.1006/bbrc.2001.5494. [DOI] [PubMed] [Google Scholar]
- 29.Nakatsugi S, Ohta T, Kawamori T, et al. Chemoprevention by nimesulide, a selective cyclooxygenase-2 inhibitor, of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary gland carcinogenesis in rats. Jpn J Cancer Res. 2000;91(9):886–92. doi: 10.1111/j.1349-7006.2000.tb01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamid R, Singh J, Reddy BS, Cohen LA. Inhibition by dietary menhaden oil of cyclooxygenase-1 and -2 in N-nitrosomethylurea-induced rat mammary tumors. Int J Oncol. 1999;14(3):523–8. doi: 10.3892/ijo.14.3.523. [DOI] [PubMed] [Google Scholar]
- 31.Subbaramaiah K, Howe LR, Port ER, et al. HER-2/neu status is a determinant of mammary aromatase activity in vivo: evidence for a cyclooxygenase-2-dependent mechanism. Cancer Res. 2006;66(10):5504–11. doi: 10.1158/0008-5472.CAN-05-4076. [DOI] [PubMed] [Google Scholar]
- 32.Chang SH, Ai Y, Breyer RM, Lane TF, Hla T. The prostaglandin E2 receptor EP2 is required for cyclooxygenase 2-mediated mammary hyperplasia. Cancer Res. 2005;65(11):4496–9. doi: 10.1158/0008-5472.CAN-05-0129. [DOI] [PubMed] [Google Scholar]
- 33.Liu CH, Chang SH, Narko K, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276(21):18563–9. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 34.Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60(8):2101–3. [PubMed] [Google Scholar]
- 35.Kubatka P, Ahlers I, Ahlersova E, et al. Chemoprevention of mammary carcinogenesis in female rats by rofecoxib. Cancer Lett. 2003;202(2):131–6. doi: 10.1016/j.canlet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Lanza-Jacoby S, Miller S, Flynn J, et al. The cyclooxygenase-2 inhibitor, celecoxib, prevents the development of mammary tumors in Her-2/neu mice. Cancer Epidemiol Biomarkers Prev. 2003;12(12):1486–91. [PubMed] [Google Scholar]
- 37.Qadri SS, Wang JH, Coffey JC, et al. Surgically induced accelerated local and distant tumor growth is significantly attenuated by selective COX-2 inhibition. Ann Thorac Surg. 2005;79(3):990–5. doi: 10.1016/j.athoracsur.2004.07.042. discussion-5. [DOI] [PubMed] [Google Scholar]
- 38.Howe LR, Chang SH, Tolle KC, et al. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005;65(21):10113–9. doi: 10.1158/0008-5472.CAN-05-1524. [DOI] [PubMed] [Google Scholar]
- 39.Chang SH, Liu CH, Conway R, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci USA. 2004;101(2):591–6. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markosyan N, Chen E, Ndong V, et al. Deletion of cyclooxygenase 2 in mouse mammary epithelial cells delays breast cancer onset through augmentation of type 1 immune responses in tumors. Carcinogenesis. 2011 doi: 10.1093/carcin/bgr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polyak K, Kalluri R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harb Perspect Biol. 2010;2(11):a003244. doi: 10.1101/cshperspect.a003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008;18(1):27–34. doi: 10.1016/j.gde.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolberg DS, Bissell MJ. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309(5968):552–6. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- 44.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 45.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26(3–4):373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 46.DeNardo DG, Barreto JB, Andreu P, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 48.Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal advance: tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE catabolism in myeloid cells. J Leukoc Biol. 2010;88(5):839–48. doi: 10.1189/jlb.1209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doe WF, Henson PM. Macrophage stimulation by bacterial lipopolysaccharides. I. Cytolytic effect on tumor target cells. J Exp Med. 1978;148(2):544–56. doi: 10.1084/jem.148.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141(7):2407–12. [PubMed] [Google Scholar]
- 51.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176(1):287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 53.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez PC, Quiceno DG, Ochoa AC. L-Arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109(4):1568–73. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young MR, Wheeler E, Newby M. Macrophage-mediated suppression of natural killer cell activity in mice bearing Lewis lung carcinoma. J Natl Cancer Inst. 1986;76(4):745–50. doi: 10.1093/jnci/76.4.745. [DOI] [PubMed] [Google Scholar]
- 56.Hui Y, Ricciotti E, Crichton I, et al. Targeted deletions of cyclooxygenase-2 and atherogenesis in mice. Circulation. 2010;121(24):2654–60. doi: 10.1161/CIRCULATIONAHA.109.910687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saccani A, Schioppa T, Porta C, et al. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66(23):11432–40. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- 58.Knudsen PJ, Dinarello CA, Strom TB. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J Immunol. 1986;137(10):3189–94. [PubMed] [Google Scholar]
- 59.Bailly S, Ferrua B, Fay M, Gougerot-Pocidalo MA. Differential regulation of IL 6, IL 1 A, IL 1 beta and TNF alpha production in LPS-stimulated human monocytes: role of cyclic AMP. Cytokine. 1990;2(3):205–10. doi: 10.1016/1043-4666(90)90017-n. [DOI] [PubMed] [Google Scholar]
- 60.Standiford TJ, Kunkel SL, Rolfe MW, Evanoff HL, Allen RM, Strieter RM. Regulation of human alveolar macrophage- and blood monocyte-derived interleukin-8 by prostaglandin E2 and dexamethasone. Am J Respir Cell Mol Biol. 1992;6(1):75–81. doi: 10.1165/ajrcmb/6.1.75. [DOI] [PubMed] [Google Scholar]
- 61.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med. 1994;180(6):2365–70. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D’Acquisto F, Sautebin L, Iuvone T, Di Rosa M, Carnuccio R. Prostaglandins prevent inducible nitric oxide synthase protein expression by inhibiting nuclear factor-kappaB activation in J774 macrophages. FEBS Lett. 1998;440(1–2):76–80. doi: 10.1016/s0014-5793(98)01407-0. [DOI] [PubMed] [Google Scholar]
- 63.Wu WK, Llewellyn OP, Bates DO, Nicholson LB, Dick AD. IL-10 regulation of macrophage VEGF production is dependent on macrophage polarisation and hypoxia. Immunobiology. 2010;215(9–10):796–803. doi: 10.1016/j.imbio.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 64.Young MR, Newby M, Wepsic HT. Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res. 1987;47(1):100–5. [PubMed] [Google Scholar]
- 65.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160(12):5729–34. [PubMed] [Google Scholar]
- 67.Harari O, Liao JK. Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Curr Pharm Des. 2004;10(8):893–8. doi: 10.2174/1381612043452893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rivoltini L, Carrabba M, Huber V, et al. Immunity to cancer: attack and escape in T lymphocyte–tumor cell interaction. Immunol Rev. 2002;188:97–113. doi: 10.1034/j.1600-065x.2002.18809.x. [DOI] [PubMed] [Google Scholar]
- 69.Bronte V, Serafini P, De Santo C, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170(1):270–8. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 70.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13(7):828–35. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67(9):4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 72.Xiang X, Poliakov A, Liu C, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–33. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez PC, Hernandez CP, Quiceno D, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202(7):931–9. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Liu Q, Zhang M, Yu Y, Liu X, Cao X. Fas signal promotes lung cancer growth by recruiting myeloid-derived suppressor cells via cancer cell-derived PGE2. J Immunol. 2009;182(6):3801–8. doi: 10.4049/jimmunol.0801548. [DOI] [PubMed] [Google Scholar]
- 75.Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int Immunopharmacol. 2007;7(2):140–51. doi: 10.1016/j.intimp.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 76.Veltman JD, Lambers ME, van Nimwegen M, et al. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010;10:464. doi: 10.1186/1471-2407-10-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 78.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11(2):119–30. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng LL, Wang X. Targeting Foxp3+ regulatory T cells-related immunosuppression for cancer immunotherapy. Chin Med J (Engl) 2010;123(22):3334–42. [PubMed] [Google Scholar]
- 80.Yuan XL, Chen L, Li MX, et al. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134(3):277–88. doi: 10.1016/j.clim.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Yokokawa J, Cereda V, Remondo C, et al. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14(4):1032–40. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 82.Yaqub S, Henjum K, Mahic M, et al. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008;57(6):813–21. doi: 10.1007/s00262-007-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma S, Yang SC, Zhu L, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65(12):5211–20. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 84.Lee BP, Juvet SC, Zhang L. Prostaglandin E2 signaling through E prostanoid receptor 2 impairs proliferative response of double negative regulatory T cells. Int Immunopharmacol. 2009;9(5):534–9. doi: 10.1016/j.intimp.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 85.Chen H, Qin J, Wei P, et al. Effects of leukotriene B4 and prostaglandin E2 on the differentiation of murine Foxp3+ T regulatory cells and Th17 cells. Prostaglandins Leukot Essent Fatty Acids. 2009;80(4):195–200. doi: 10.1016/j.plefa.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 86.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: N1 versus N2 TAN. Cancer Cell. 2009;16(3):183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]