Abstract
The Saccharomyces genus is the main yeast involved in wine fermentations to play a crucial role in the production and release of aromatic compounds. Despite the several studies done into the genome-wide expression analysis using DNA microarray technology in wine S. cerevisiae strains, this is the first to investigate other species of the Saccharomyces genus. This research work investigates the expression of the genes involved in flavor compound production in three different Saccharomyces species (S. cerevisiae, S. bayanus var. uvarum and S. kudriavzevii) under low (12°C) and moderate fermentation temperatures (28°C). The global genes analysis showed that 30% of genes appeared to be differently expressed in the three cryophilic strains if compared to the reference strain (mesophilic S. cerevisiae), suggesting a very close cold adaptation response. Remarkable differences in the gene expression level were observed when comparing the three species, S. cerevisiae, S. bayanus var. uvarum and S. kudriavzevii, which will result in different aroma profiles. Knowledge of these differences in the transcriptome can be a tool to help modulate aroma to create wines with the desired aromatic traits.
Introduction
Yeasts play a crucial role in the development of the so-called wine secondary aroma, with higher alcohols, acetate esters and ethyl esters being the main aromatic compounds that contribute to floral and fruity aroma [1]. Higher alcohols are synthesized from amino acids through transamination and decarboxylation reactions. These chemical reactions are carried out by amino acid permeases (codified by GAP1, BAP2, MEP2), transaminases (codified by BAT1, BAT2, ARO8, ARO9), decarboxylases (codified by PDC1, PDC5, PDC6, THI3, ARO10) and dehydrogenases (codified by ADH1-7, SFA1) [2]. Acetate esters are synthetised by a condensation between higher alcohols and acetyl-CoA.This reaction is mediated by acetyltransferases codified by genes ATF1 and ATF2 [3]. Ethyl esters are produced by condensation between ethanol and acyl-CoA, a reaction mediated by acyltransferases codified by genes EHT1, EEB1 and YMR210W [3]. Besides, the effect of the esterases codified by IAH1 and TIP1 must be taken into account in the final concentration of both acetate and ethyl esters in wine [3]. Saccharomyces yeasts can also participate in primary aroma release through glycosidases [4]. Examples of the genes codifying glycosidases and glucanases are BGL2, EXG1, SPR1 and the ORF YIR007W [5], [6].
During the winemaking process, ethanol, glycerol, acetic acid and acetaldehyde can be synthesised by yeasts. Ethanol decreases aroma perception by increasing the solubility of aromatic compounds in wine [7]. Acetic acid (volatile acidity) at a high concentration, as occurs in stuck and sluggish fermentations, confers wine an undesirable odor [8]. Acetaldehyde is obtained by pyruvate decarboxylation, and although it can be reduced to ethanol, a small quantity may remain and produce wine oxidation [8]. The genes codifying piruvate decarboxylases, aldehyde dehydrogenases and alcohol dehydrogenases are involved in the metabolism of acetaldehyde, acetic acid and ethanol.
The main yeasts responsible for wine production belong to Saccharomyces genus. S. cerevisiae is the most important species involved in winemaking, and closely related species Saccharomyces bayanus var. uvarum may also participate [9], [10]. From the oenological point of view, several properties of these Saccharomyces species differ. A comparison made between S. bayanus var. uvarum and S. cerevisiae revealed that the former is more cryotolerant and produces smaller acetic acid quantities [11]–[13]. Wines produced by S. bayanus var. uvarum strains have a higher aromatic intensity than those produced by S. cerevisiae [14], [15]. Specifically, S. bayanus var. uvarum generates larger amounts of 2-phenylethanol, 2-phenylethyl acetate and ethyl lactate [13], [16], [17]. In contrast, S. bayanus var. uvarum is less common and appears mainly in fermentations at low temperatures [18]. S. kudriavzevii has been isolated from decayed leaves in Japan [9], and recently from oak barks in Portugal [19] and Spain [20]. Although it is not involved in winemaking, S. kudriavzevii participates in the hybridization processes with other Saccharomyces species like S. cerevisiae or Saccharomyces bayanus var. uvarum [21]–[23].
Nowadays there is a winemaking trend which consists in lowering fermentation temperatures in order to improve the aromatic profile of wines. Previous studies have demonstrated that low-fermentation temperatures result in not only higher aroma retention, but also in reduced higher alcohols and volatile acidity, and in an increase of esters and fatty acids [1], [24], [25]. Other studies stress the importance of yeast species, or even strains, in aroma production [26]. However, low-fermentation temperatures have their disadvantages, including prolonged process duration and a higher risk of halted or sluggish fermentations [27]. As mentioned before, both S. kudriavzevii and S. bayanus var. uvarum are characterized as cryotolerant and they constitute a potential tool to carry out low-temperature fermentations efficiently [26]. After the genome sequence of S. cerevisiae was reported [28], many studies have been done on the genome-wide expression analysis using DNA microarrays to better understand winemaking processes [29], [30], temperature influence on growth or aroma production [31], [32], the genes involved in aroma production [33], a general or sugar stress response [34], [35], or the response to nitrogen depletion [36]. Despite several genome-wide expression studies in S. cerevisiae using DNA microarray technology, there is no equivalent information available on other species of the genus.
This research work focuses on the expression of the genes involved in the production of flavor compounds during winemaking in three different cryotolerant Saccharomyces strains of the species S. cerevisiae, S. bayanus var. uvarum and S. kudriavzevii at low and moderate fermentation temperatures.
Materials and Methods
Yeast strains
The yeast strains used in this study belong to different species from the genus Saccharomyces, and the commercial wine strains Lalvin T 73 and Fermol cryophile (S. cerevisiae), IFO 1802 (S. kudriavzevii) and CECT 12600 (S. bayanus var. uvarum var. bayanus).
Fermentation and aroma analysis
Fermentative compounds and aroma data can be found in Gamero et al., 2013 [26].
Comparative genomic hybridization (CGH)
S. cerevisiae strain S288C was used as a control for microarray hybridizations. Yeast strains were cultivated in 5 ml YPD (1% yeast extract, 2% peptone, 2% glucose), at 28°C for 24 h and DNA was isolated according to standard procedures [37].
The karyotyping experiments were carried out following the methodology proposed by [38]. All the experiments were performed using duplicate arrays, and Cy5-dCTP and Cy3-dCTP dye-swap assays were performed to reduce the dye-specific bias.
Array slides were scanned in an Axon GenePix 4100A scanner (Axon Instruments), and the images were analyzed using the GenePix Pro 6.0 software (Molecular Devices Corp., Union City, CA, USA). With the Acuity 4.0 software (Molecular Devices Corp., Union City, CA, USA), manually flagged bad spots were eliminated and the local background was subtracted before averaging the replicate features in the array. Log2 intensity ratios (M values) were then Median-normalized to correct for differences in the genomic DNA labeling efficiency between samples. The relative hybridization signal of each ORF was derived from the average of the two dye-swap hybridizations performed per strain. The normalized log2 ratio (M value) was considered a measure of the relative abundance of each ORF in relation to that of reference strain S288C. Deviations from the 1∶1 hybridization ratio were taken as being indicative of changes in the DNA copy number. Given that the variability usually observed between Saccharomyces genomes (either within laboratory strains or natural isolates) is much lower than this estimate, we interpreted the statistically significant depletions in the hybridization signal as ORF deletions.
The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE52446 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE52446).
Total RNA extraction and cDNA labeling with Cy3 and Cy5
Cells were collected by centrifugation (4000 rpm/min, 5 min) from two independent fermentations done at 12°C and 28°C at the beginning of stationary phase, and determined when 50% of the reducing sugars were consumed. The RNA extraction method was based on consecutive treatments with phenol-tris, phenol-chloroform (5∶1) and chloroform-isoamyl alcohol (24∶1), and a final precipitation with ethanol and sodium acetate [39]. RNA concentrations and purity were determined using a Nanodrop spectrophotometer ND-1000 (Nanodrop Technologies, Wilmington, DE, USA). RNA integrity was determined by electrophoresis in 1% agarose gel. 2–4 µg of total RNA from each sample was linearly amplified using the Low RNA Input Fluorescent Linear Amplification kit (Agilent Technologies, CA, USA). Then 2–3 ug of amplified cRNA were used as a template for cDNA synthesis. cDNA was marked indirectly with the “SuperScript Indirect cDNA Labeling System” (Invitrogen, San Diego, CA, USA). The fluorophores used were Cy3 and Cy5 mono-reactive Dye (Amersham GE Healthcare, Amersham, UK) and dye incorporation was monitored by a Nanodrop spectrophotometer.
Microarrays hybridization, washing and scanning
A mixture of 200 to 300 pmol of the two labeled samples was concentrated in a Concentrator Plus (Eppendorf, Hamburg, Germany). Competitive hybridization was performed on a Yeast 6.4K Array with PCR-amplified ORFs of yeast S288c strain (Microarray Centre, UHN, Toronto, Ontario, Canada) in AHC hybridization chambers (ArrayIt Corporation, CA, USA) at 42°C overnight. The prehybridization solution contained 3X SSC, 0.1% SDS and 0.1 mg/ml BSA. The hybridization solution contained 5X SSC, 0.1% SDS and 0.1 mg/ml of salmon DNA. Microarrays were washed manually with different solutions containing distinct SSC 20X and SDS 10% concentrations (Sol.1: 2X SSC-0.1% SDS; Sol.2: 0.1X SSC-0.1% SDS; Sol.3: 0.1 SSC; Sol4: 0.01X SSC). The signal intensities of Cy3 and Cy5 were acquired with an Axon GenePix 4100A scanner (Molecular Devices, CA, USA) using the GenePix Pro v.6.1 software at a resolution of 10 µm.
Microarray data analysis
Microarray data were derived from three independent experiments for cDNA hybridization. Raw data with global background subtraction were generated from GenePix pro 6.0. Analyses were done using the Acuity 4.0 software (Molecular Devices, CA, USA).Individual data sets were normalized to a log2 ratio value of 1. After normalization, data were filtered to remove the spots flagged as not found and were manually processed for print tip effect corrections. Only the spots with at least two replicates were considered. Finally, replicates were combined and their medians were calculated. The first cut-off was the selection of the genes presenting at least 2-fold log2 ratio values, according to the literature [31]–[36]. For these genes, a “GO terms” enrichment analysis was done using the GO Term Finder tool in the Saccharomyces Genome Database (http://www.yeastgenome.org/cgi-bin/GO/goTermFinder.pl). Regarding the statistics, a False Discovery Rate (FDR) analysis and a significance level of 99% (p value <0.01) were applied. Heat maps and hierarchical clustering were done using the Genesis software 1.7.6 (Graz University of Technology, Austria).
The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE30778 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE30778).
Results
A microarray analysis was carried out employing the RNA extracted from the cells harvested at the beginning of the stationary phase from the wine microfermentations done at 12°C and 28°C in Tempranillo must [26].
Global analysis of genes presenting changes in expression
The two more divergent species used in this study were S. cerevisiae and S. bayanus var. uvarum, which display approximately 80% identity of coding and 74% identity of non coding sequences [40]. Hybridization of cDNA from the three Saccharomyces species was achieved under heterologous hybridization conditions in the S. cerevisiae microarrays. The hybridization conditions were previously tested by employing DNA-DNA microarrays, which showed that most genes of S. bayanus var. uvarum CECT 12600 (99.5%) and S. kudriavzevii IFO 1802 (98.7%) hybridize perfectly in the arrays and under the conditions employed in this study. Among the genes related to aroma synthesis, only ILV5 and PDA1 of S. kudriavzevii and MUP3 of S. bayanus var. uvarum did not hybridize in the S. cerevisiae arrays employed in this study.
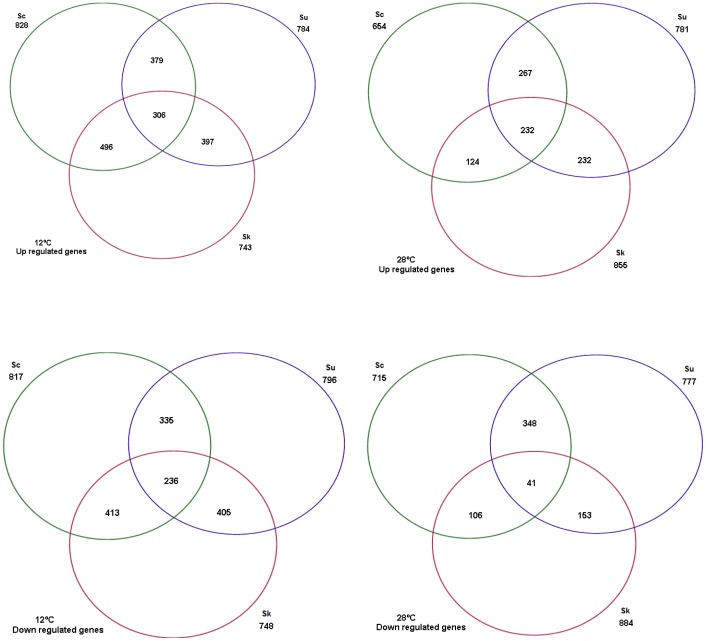
Gene expression was determined at the beginning of the stationary phase in the fermentations carried out at 12°C and 28°C. The gene expression profiles of the three cryophilic strains used in this study (S. cerevisiae Fermol cryophile, S. bayanus var. uvarum CECT 12600 and S. kudriavzevii IFO 1802) were compared to the gene expression of reference mesophilic strain Lalvin T73. Only those genes with a fold change in expression of over 2 (positive or negative) in relation to S. cerevisiae Lalvin T73 were taken into account for further analyses. Figure 1 shows the amount of the up- and down-regulated genes found in each species in relation to Lalvin T73 at both fermentation temperatures. Aproximately 30% of the genes of the three cryophilic strains were differently expressed at 12°C or 28°C if compared to mesophilic S. cerevisiae Lalvin T73. The first point that stands out is the large number of up- and down-regulated genes shared by the three cryophilic species at 12°C, 306 and 236, respectively. However, a comparison made of the up- and down-regulated genes at 28°C revealed that only 77 up-regulated and 41 down-regulated genes were shared by the three cryophilic strains.
Figure 1. Global genetic expression analyses at 12°C and 28°C.
Sc: commercial Saccharomyces cerevisiae Fermol Cryophile; Sb: Saccharomyces bayanus var. uvarum CECT 12600; Sk: Saccharomyces kudriavzevii IFO 1802.
Go terms show the metabolic functions in which a significant number of up- or down-regulated genes are involved. Go terms were done with the up- and down-regulated genes for each species at both temperatures (Tables 1 and 2). No significant Go terms were found in any cryophilic strain among the up-regulated genes at 12°C. Conversely, the common down-regulated functions at 12°C among the cryophilic strains were observed. These include several basic metabolic pathways, such as catalytic activity and oxidoreductase activity. It is worth noting that S. cerevisiae Fermol Cryophile showed down-regulated metabolic functions in relation to transmembrane transport activity. Furthermore, S. cerevisiae Fermol Cryophile and S. kudriavzevii presented down-regulated genes in most of the genes involved in aryl-alcohol dehydrogenase activity (Table 1).
Table 1. Go terms for the down regulated genes at 12°C.
| Fermol cryophile (Sc) | CECT 12600 (Su) | IFO 1802 (Sk) |
| 3735. Structural constituent of ribosome | 3824. Catalytic activity | 3824. Catalytic activity |
| 3824. Catalytic activity | 16491. Oxidoreductase activity | 4022. Alcohol dehydrogenase (NAD) activity |
| 5353. Fructose transmembrane transporter activity | 16614. Oxidoreductase activity, acting on CH-OH group of donors | 16614. Oxidoreductase activity, acting on CH-OH group of donors |
| 5355. Glucose transmembrane transporter activity | 16616. Oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor | 16616. Oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor |
| 15144. Carbohydrate transmembrane transporter activity | 16491. Oxidoreductase activity | |
| 15145. Monosaccharide transmembrane transporter activity | 18456. Aryl-alcohol dehydrogenase activity | |
| 15149. Hexose transmembrane transporter activity | ||
| 15578. Mannose transmembrane transporter activity | ||
| 16491. Oxidoreductase activity | ||
| 16614. Oxidoreductase activity, acting on CH-OH group of donors | ||
| 16616. Oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor | ||
| 18456. Aryl-alcohol dehydrogenase activity | ||
| 22857. Transmembrane transporter activity | ||
| 22891. Substrate-specific transmembrane transporter activity | ||
| 22892. Substrate-specific transporter activity | ||
| 51119. Sugar transmembrane transporter activity | ||
| 70011. Peptidase activity, acting on L-amino acid peptides |
GO terms obtained from Saccharomyces Genome Database http://www.yeastgenome.org/; Sc: Saccharomyces cerevisiae; Su: Saccharomyces uvarum; Sk: Saccharomyces kudriavzevii.
Table 2. Go terms for the up regulated genes at 28°C.
| Fermol cryophile (Sc) | CECT 12600 (Su) | IFO 1802 (Sk) |
| 3735. Structural constituent of ribosome | 3735. Structural constituent of ribosome | No significant GO terms |
| 3743.Translation initiation factor activity | 3824. Catalytic activity | |
| 5198. Structural molecule activity | 5198. Structural molecule activity | |
| 8135. Translation factor activity, nucleic acid binding | 8135. Translation factor activity, nucleic acid binding | |
| 15078. Hydrogen ion transmembrane transporter activity | 16491. Oxidoreductase activity | |
| 16491. Oxidoreductase activity |
GO terms obtained from Saccharomyces Genome Database http://www.yeastgenome.org/; Sc: Saccharomyces cerevisiae; Su: Saccharomyces uvarum; Sk: Saccharomyces kudriavzevii.
However, the common up-regulated functions at 28°C were observed between the S. cerevisiae Fermol Cryophile and S. bayanus var. uvarum strains, among which the structural functions of the ribosome, nucleic acid binding, translation factor activity and oxidoreductase activity were found. In addition, S. cerevisiae presented an up-regulation in transmembrane transport, and S. bayanus var. uvarum did so in catalytic activity (Table 2). No significant GO terms were found for S. kudriavzevii. Finally at 28°C, no significant Go terms were observed in any cryophilic strain among the down-regulated genes.
Analysis of the expression of genes related to aroma production
The expression level of the genes involved in amino acids, higher alcohols, acetate esters, ethyl esters, ethanol, acetaldehyde and acetate metabolism, and the enzymes involved in wine primary aroma release, appear in Figure 2.
Figure 2. Heat maps depicting the level of expression of the genes related to flavour formation at 12°C and 28°C.
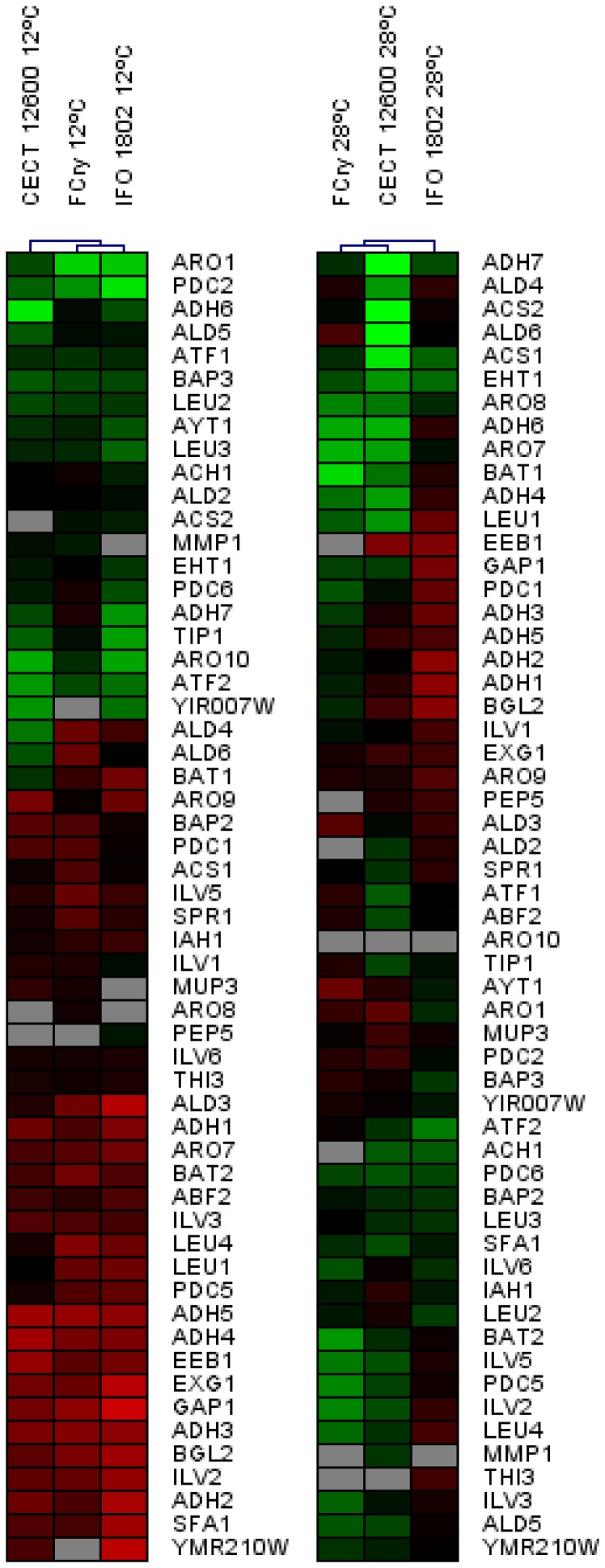
Green: up-regulation; red: down-regulation; black: no changes in expression; gray: no hybridization.
The hierarchical clustering of the gene expression at 12°C of the genes involved in aroma formation showed that the expression profiles of S. cerevisiae and S. kudriavzevii were closer than that of S. bayanus var. uvarum. Conversely, the expression profile of S. cerevisiae was similar to that of S. bayanus var. uvarum at 28°C, but differed from the gene expression profile of S. kudriavzevii.
According to gene expression, two groups of genes were clearly seen at 12°C (Figure 2): the genes up-regulated in the three species (green in the upper part of the heat map) and the genes down-regulated in the three species (red in the lower part of the heat map). By taking into account only the genes presenting an at least 2-fold change in expression in comparison with the reference strain, it is possible to divide the genes relating to aroma production into twelve different clusters.
In contrast, no clear groups were observed at 28°C. When bearing in mind only the genes presenting an at least 2-fold change in expression in comparison to the reference strain, it is possible to divide the genes relating to aroma production into eleven different clusters.
Analysis of the genes presenting the same changes in expression in S. cerevisiae, S. bayanus var. uvarum and S. kudriavzevii
In this part of the work, the objective was to compare the differences in gene expression of the three cryotolerant Saccharomyces strains of the species S. cerevisiae (Fermol cryophile), S. bayanus and S. kudriavzevii employing mesophilic S. cerevisiae Lalvin T.73 as a reference.
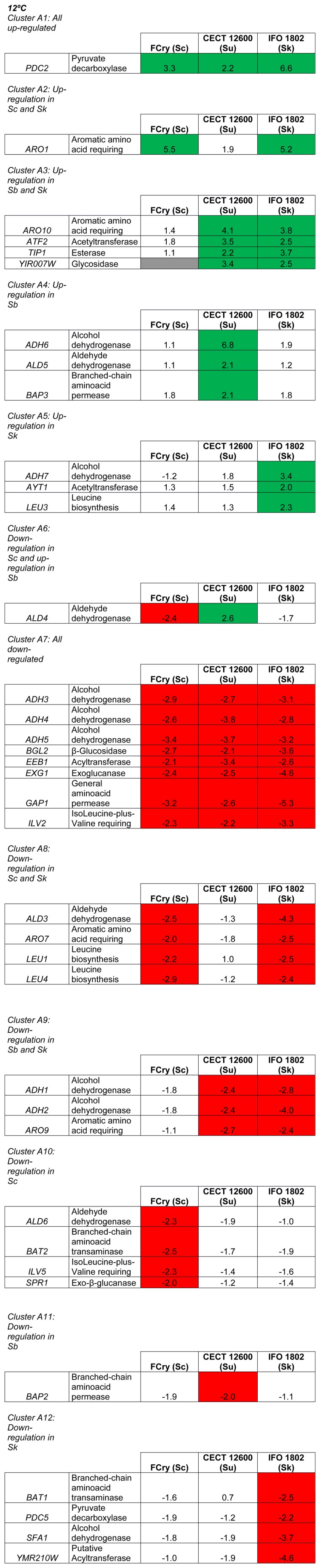
In the fermentations carried out at 12°C, only gene PDC2 (pyruvate decarboxylase) appeared to be up-regulated in the three strains included in this study (Figure 3, Cluster A1). Conversely, larger numbers of genes were down-regulated in the three species (Figure 3, Cluster A7). These genes were some alcohol dehydrogenases (ADH3-5), genes codifiying enzymes related to wine primary aroma release (BGL2 and EXG1), and some genes related to amino acids metabolism (the general amino acid permease codifyed by GAP1 and ILV2, involved in branched-chain amino acids synthesis). The metabolisms of amino acids and ethanol/acetaldehyde were also affected.
Figure 3. Clusters according to different changes in expression.
S. cerevisiae (Sc), S. bayanus var. uvarum (Su) and S. kudriavzevii (Sk) at 12°C.
In the fermentations carried out at 28°C (Figure 4), the three criotolerant strains showed no gene presenting the same changes in expression comparing to the reference strain.
Figure 4. Clusters according to different changes in expression.
S. cerevisiae (Sc), S. bayanus var. uvarum (Su) and S. kudriavzevii (Sk) at 28°C.
Analysis of the genes presenting the same changes in expression in S. cerevisiae and S. bayanus var. uvarum
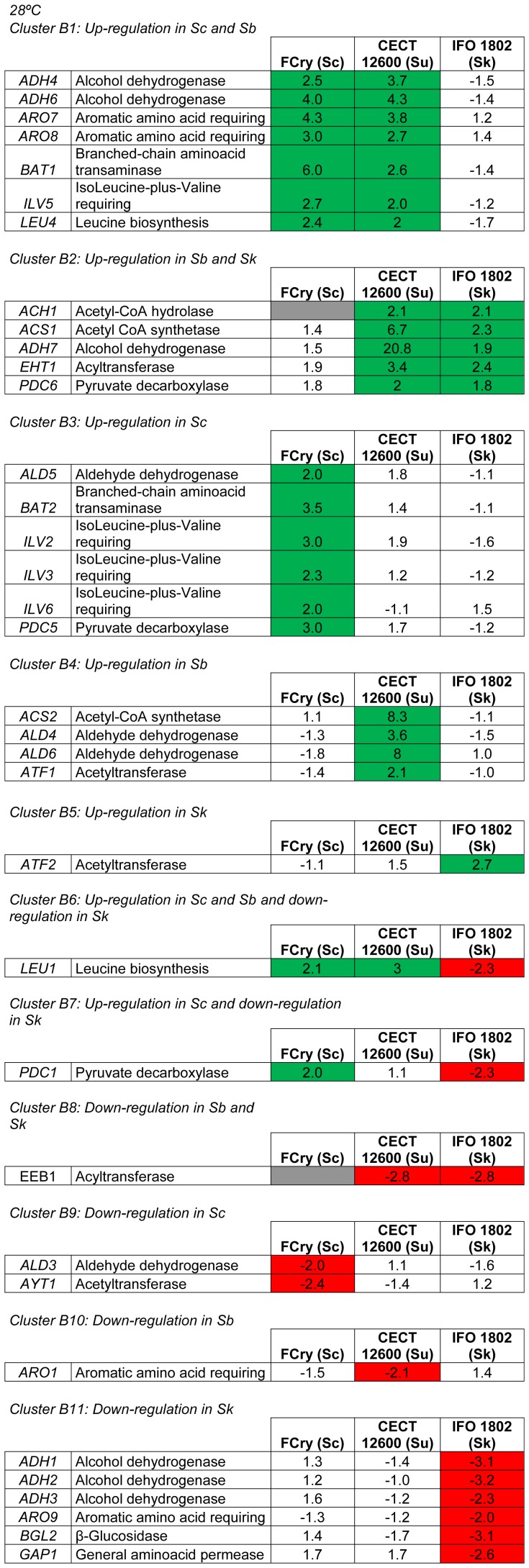
In the fermentations done at 12°C, S. cerevisiae and S. bayanus var. uvarum did had not have any gene that presenteding the same changes in expression if comparedwith respect to the reference strain. In the fermentations carried out at 28°C, several genes were up-regulated in S. cerevisiae and S. bayanus var. uvarum (Figure 4, Cluster B1). These genes codify alcohol dehydrogenases (ADH4, ADH6), a transaminase (BAT1), and also the enzymes involved in aromatic and branched-chain amino acids synthesis (ARO7, ARO8, ILV5, LEU4).
Analysis of the genes presenting the same changes in expression in S. cerevisiae and S. kudriavzevii
In the fermentations at 12°C (Figure 3), S. cerevisiae and S. kudriavzevii presented an up-regulation in gene ARO1, which is involved in the synthesis of aromatic amino acids (Figure 3, Cluster A2). At this temperature, these two species shared several down-regulated genes (Figure 3, Cluster A8), and these genes were ALD3, codifying an aldehyde dehydrogenase, and some genes involved in branched-chain and aromatic amino acids synthesis (ARO7, LEU1, LEU4). In the fermentations performed at 28°C (Figure 4), S. cerevisiae and S. kudriavzevii had no gene which presented the same changes in expression if compared to the reference strain.
Analysis of the genes presenting the same changes in expression in S. bayanus var. uvarum and S. kudriavzevii
In the fermentations at 12°C, S. bayanus var. uvarum and S. kudriavzevii (the two typical criotoleranst species), up-regulation was seen in some genes that presented different functions (Figure 3, Cluster A3), such as the synthesis of aromatic amino acids (ARO10), and acetyltransferase, esterase and glycosidase activities (ATF2, TIP1 and YIR007W, respectively).
However at the same temperature, it is possible to see in Cluster A9 (Figure 3) that S. bayanus var. uvarum and S. kudriavzevii presented a down-regulation in two genes codifying alcohol dehydrogenases (ADH1-2) and in one gene involved in the synthesis of aromatic amino acids (ARO9). In the fermentations done at 28°C, Cluster B2 (Figure 4) shows several up-regulated genes in both S. bayanus var. uvarum and S. kudriavzevii, such as ACH1 and ACS1 involved in the acetate synthesis and degradation, respectively, and the degradation of acetate in S. bayanus var. uvarum was more highly expressed than in S. kudriavzevii. In addition, the up-regulation of alcohol dehydrogenase gene ADH7, acyltransferase gene EHT1 and pyruvate decarboxylase gene PDC6 also occurred. The acyltransferase codified by EEB1 appeared to be down-regulated in both S. bayanus var. uvarum and S. kudriavzevii (Figure 4, Cluster B8). Finally, it is worth mentioning the high expression of PDC6.
Analysis of the genes presenting changes in expression exclusively in the cryotolerant S. cerevisiae strain.
Interestingly, in the fermentation at 12°C, no genes were exclusively up-regulated in S. cerevisiae (Fermol cryophile). Nevertheless, several genes showed a down-regulation of about 2–2.5 fold in this species (Figure 3 Cluster A10), such as aldehyde dehydrogenase gene ALD6 and some genes involved in the branched-chain amino acids metabolism (BAT2, ILV5). Besides, S. cerevisiae showed a down-regulation in exo-β-glucanase gene SPR1.
In the fermentation at 28°C, several genes appeared to be exclusively up-regulated in S. cerevisiae (Figure 4 Cluster B3), such as some of the genes involved in branched-chain amino acids metabolism (BAT2, ILV2-3, ILV6), and some genes related to acetaldehyde metabolism (ALD5 and PDC5). At 28°C, S. cerevisiae also presented two genes that were exclusively down-regulated (Figure 4, Cluster B9), aldehyde dehydrogenase gene ALD3 and acetyltransferase AYT1.
Analysis of the genes presenting changes in expression exclusively in S. bayanus var. uvarum
In the fermentation at 12°C, some genes appeared to be exclusively up-regulated in S. bayanus var. uvarum (Figure 3, Cluster A4), such as alcohol dehydrogenase gene ADH6 and branched-chain amino acid permease gene BAP3. In addition, the down-regulation of branched-chain amino acid permease gene BAP2 appeared (Figure 3, Cluster A11).
In the fermentation performed at 28°C, some genes were exclusively up-regulated in S. bayanus var. uvarum (Figure 4, Cluster B4), such as ACS2 and aldehyde dehydrogenase genes ALD4 and ALD6. Finally, S. bayanus var. uvarum only presented one gene that was exclusively down-regulated at 28°C (Figure 4, Cluster B10). This gene is involved in the synthesis of aromatic amino acids (ARO1).
Analysis of the genes presenting changes in expression exclusively in S. kudriavzevii
In the fermentation at 12°C, some genes were exclusively up-regulated in S. kudriavzevii (Figure 3, Cluster A5), such as alcohol dehydrogenase gene ADH7, gene LEU3 involved in leucine biosynthesis, and acetyltransferase gene AYT1. Several genes were exclusively down-regulated in S. kudriavzevii (Figure 3, Cluster A12), branched-chain amino acid transaminase gene BAT1 and alcohol dehydrogenase SFA1. The down-regulation of pyruvate decarboxylase gene PDC5, and in the putative acyltransferase codified by YMR210W, was also observed. In the fermentation at 28°C, only acetyltransferase gene ATF2 appeared to be exclusively up-regulated in S. kudriavzevii (Figure 4, Cluster B5). The down-regulation in some alcohol dehydrogenases (ADH1-3), ARO9, involved in aromatic amino acids metabolism, and general aminoacid permease gene GAP1 (Figure 4 cluster B11), were also observed. Finally, this species showed down-regulation in BGL2, which codified a β-glucosidase and was involved in primary aroma release (Figure 4, cluster B11).
Other genes presenting changes in expression
Other genes were found to be up- or down-regulated depending on the species. In the fermentations done at 12°C, aldehyde dehydrogenase gene ALD4 was down-regulated in S. cerevisiae (Fermol cryophile), but was up-regulated in S. bayanus var. uvarum, and no change in expression was observed for S. kudriavzevii (Figure 3, Cluster A6).
At 28°C, one gene involved in the biosynthesis of leucine (LEU1) was up-regulated in S. cerevisiae and S. bayanus var. uvarum, whereas this gene was down-regulated in S. kudriavzevii (Figure 4, Cluster B6). Finally, pyruvate decarboxylase gene PDC1 was up-regulated in S. cerevisiae and was down-regulated in S. kudriavzevii (Figure 4, Cluster B7).
Discussion
Functional genomic approaches, such as microarray technology, are powerful tools to analyze gene expression at the whole genome level, and provide a comprehensive view of yeast physiology [41]–[43]. However, yeast secondary metabolism is a complex network of biochemical pathways which, although well mapped from a biochemical viewpoint, is not well understood in terms of its physiological roles and genetic and biochemical regulation [34].
The genetic profile of the yeast used when carrying out the alcoholic fermentation, mainly of the Saccharomyces genus, is obviously important in the formation of the metabolites conferring specific flavors to wine [44]. Besides, other factors like temperature can influence the aromatic quality of wine. Several authors have observed that low-temperature fermentations lead to greater aroma retention, a drop in higher alcohols and volatile acidity, and to an increase in volatile esters [1], [24], [25], [27], [45]. However, other studies suggest that the way in which fermentation temperature affects the wine aroma profile is highly dependent on the strain that carries out the process [26].
The expression of the genes related to aroma production was determined at the beginning of the stationary phase since the most active period of aroma compound accumulation appears to occur in earlier fermentation stages [34], [44]. The species studied in the present research were selected for their remarkable aroma production during wine microfermentations in Tempranillo must at 12°C and 28°C, and also for their adaptation to ferment well at low temperature [26]. The use of S. cerevisiae microarrays to hybridize different Saccharomyces species did not pose a problem since the Saccharomyces species evaluated in this study presented high percentages of genetic homology, and heterologous hybridization conditions were employed to increase hybridization efficiency. Only the genes that presented changes in expression of at least 2-fold have been mentioned in this research work given their potential impact on aroma production during fermentation. The selection of this cut-off is common practice and has been used in several global analysis studies in the past to investigate gene expression [31], [33]–[36]. The global analysis of the genes showed that 30% of the genes appeared to be differently expressed in the cryophilic strains if compared to the mesophilic reference strain, and that the three cryophilic strains shared many similarities in gene expression at 12°C, suggesting a very close cold adaptation response. Conversely, the list of the shared genes presenting changes in expression at 28°C was very limited.
A previous study, which consisted in microfermentations in Tempranillo must with the same strains employed in this research work, showed that at 12°C, S. bayanus var. uvarum excelled in higher alcohol and acetate ester production, whereas S. cerevisiae did so in ethyl esters synthesis. In addition, both strains yielded a large amount of acetaldehyde. Yet at 28°C, the production of higher alcohols and acetate esters by S. cerevisiae was remarkable, as was the acetate ester and acetic acid synthesis carried out by S. kudriavzevii [26]. Finally, S. bayanus var. uvarum yielded a large amount of acetaldehyde at 28°C. A comparison made between the transcriptomic data obtained in this research work and the aforementioned chemical data indicates certain correlations. Higher alcohol levels produced by different species can be explained by gene expression at both temperatures, whereas it is not possible to correlate ester amounts with gene expression data in all cases. In these cases, differences might be due to differences in the enzyme activities involved in the corresponding pathway or other explanations can be hypothesized. For instance, alcohol dehydrogenases ALD4 and ALD5 (involved in acetaldehyde conversion into acetate) were up-regulated in the S. bayanus var. uvarum strain at 12°C. One possible explanation for the low acetate levels detected in the wines produced by this strain is that part of this compound is used for ethyl acetate production since this species produces the largest ethyl acetate amount at this temperature.
Some genes that perform the same enzymatic function might be more important in aroma formation than others, and must also be taken into account to analyze the correlation between chemical data and the transcriptome. For example, the S. bayanus var. uvarum and S. kudriavzevii strains at 28°C presented an up-regulation in acyltranferase EHT1 and a down-regulation in acyltranferase EEB1 (the genes involved in ethyl esters formation). The low ethyl ester production in the S. bayanus var. uvarum and S. kudriavzevii strains as compared to the reference strain suggests that acyltransferase EEB1 is more important in the production of these aromatic compounds than EHT1, which has been previously described [34]. Besides, both acyltransferases have been related to esterase activity [46]. Likewise, our data confirms that ADH4 has not a major role in the interconversion of ethanol and acetaldehyde as ADH1 is the main gene responsible for this transformation [47]. In higher alcohol synthesis, different families of amino acids are involved; branched-chain amino acids valine and leucine are necessary for isobutanol and isoamyl alcohol production, respectively, whereas aromatic amino acid phenylalanine is required for 2-phenylethanol synthesis. The up-regulation of the genes codifying the permeases, transaminases and other enzymatic activities involved in branched-chain amino acids metabolism was observed in the S. bayanus var. uvarum and S. cerevisiae strains at 28°C. Furthermore according to the chemical data, higher levels of isobutanol and/or isoamyl alcohol were found in S. cerevisiae strain (Fermol cryophile), at 28°C than in the reference strain [26]. However, no increase in any of these compounds was observed in the S. bayanus var. uvarum strain at this temperature [26], although this strain presented up-regulated alcohol dehydrogenases. The discrepancy found between the chemical data and the transcriptome in S. bayanus var. uvarum at 28°C may be due to the utilization of 2-phenylethanol to produce the corresponding acetate. The larger amount of this acetate detected in S. bayanus var. uvarum at 28°C if compared to the reference strain supports this hypothesis [26]. The relevant production of 2-phenylethanol and the corresponding acetate, phenylethyl acetate, is a typical trait of the S. bayanus var. uvarum species [16]–[18], [48]. Conversely, the down-regulation of the gene codifying enzymes involved in branched-chain amino acids metabolism in the S. cerevisiae strain at 12°C and the S. kudriavzevii strain at 28°C coincided with isobutanol and/or isoamyl alcohol production [26]. Furthermore, the up-regulation of the genes codifying transaminases and other enzymes relating to aromatic amino acids metabolism were found in S. cerevisiae and S. bayanus var. uvarum at 28°C, which correlated with high 2-phenylethanol production.
In our study, the best acetate ester producers at 12°C, S. bayanus var. uvarum and S. kudriavzevii [26], presented an up-regulation in acetyltransferase gene ATF2, whereas at 28°C, only in the case of S. kudriavzevii it was possible to find a correlation between genetic and phenotypic data. Previous studies have also struggled to find correlations when analyzing the correspondence between ATF1 and ATF2 and the acetate ester levels in fermentations conducted by S. cerevisiae at 13°C and 25°C [32]. Oher authors have observed that mutants and transformants, which overproduce certain higher alcohols, showed a clearly increased synthesis of the respective acetate esters [49], [50], whereas other works have affirmed that ester synthesis cannot be explained solely by higher alcohol availability since high oxygen and unsaturated fatty acid levels are known to increase fusel alcohol production, but to lower ester levels [51]–[53]. In our study, S. cerevisiae presented a down-regulation of acetyltransferase gene AYT1 at 28°C, but high levels of higher alcohols and acetate esters suggest that acetate ester synthesis might be more dependent on substrate availability than on the expression of acetyltransferase genes. Nevertheless, this was not the case of S. bayanus var. uvarum at 28°C because low levels of acetate esters were found despite the high production for higher alcohols found.
S. cerevisiae presented the highest production of higher alcohols and up-regulations in most genes relating to higher alcohol production.The highest acetate ester producer at 12°C was S. bayanus var. uvarum, which showed an up-regulation in acetyltransferase gene ATF2, and despite presenting an up-regulation of the esterase TIP1 gene, this esterase has been related only to ethyl esters. At 28°C, the main acetate ester producers were S. kudriavzevii and S. cerevisiae [26]. S. kudriavzevii presented an up-regulation in the ATF2 gene, whereas S. cerevisiae showed a down-regulation of one acetyltransferase gene, which has not been related to acetate ester synthesis.
In conclusion, the transcriptome analysis of the genes related to aroma production can provide us with an idea of the compounds that will be synthesized during the fermentation process, as previously stated by other authors for S. cerevisiae [34]. Remarkable differences in the gene expression level were observed when comparing the three species, S. cerevisiae, S. bayanus var. uvarum and S. kudriavzevii, which resulted in different aroma profiles. Knowledge of these differences in the transcriptome can be a tool to help modulate aroma in order to create wines that offer the desired aromatic traits.
Funding Statement
AGL2009-12673-CO2-01, AGL2012-39937-C02-01 and from the Spanish Government and PROMETEO grant (Project PROMETEO/2009/019) from the Generalitat Valenciana to A. Querol. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lambrechts MG, Pretorius IS (2000) Yeasts and its importance to wine aroma - A review. S Afr J Enol Vitic 21: 97–129. [Google Scholar]
- 2. Hazelwood LA, Daran JM, van Maris AJA, Pronk JT, Dickinson JR (2008) The Ehrlich Pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae . Metabol Appl Environ Microbiol 74: 2259–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saerens SMG, Delvaux FR, Verstrepen KJ, Thevelein JM (2010) Production and biological function of volatile esters in Saccharomyces cerevisiae . Microbial Biotechnol 3: 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delcroix A, Günata Z, Sapis JC, Salmon JM, Bayonove C (1994) Glycosidase activities of three enological yeast strains during winemaking: Effect on the terpenol content of Muscat wine. Am J Enol Vitic 45: 291–296. [Google Scholar]
- 5. Mrsa V, Klebl F, Tanner W (1993) Purification and Characterization of the Saccharomyces cerevisiae BGL2 Gene Product, a Cell Wall Endo β-1,3-glucanase. J Bacteriol 175: 2102–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt S, Raineri S, Witte S, Matern U, Martens S (2011) Identification of a glucosidase from Saccharomyces cerevisiae hydrolyzing flavonoid glucosides. Appl Environ Microbiol 77: 1751–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldner MC, Zamora MC, Di Leo, Lira P, Gianninoto H, et al. (2009) Effect of ethanol level in the perception of aroma attributes and the detection of volatile compounds in red wine. J Sensory Studies 24: 243–257. [Google Scholar]
- 8. Swiegers JH, Pretorius IS (2005) Yeast Modulation of Wine Flavor. Adv appl microbiol 57: 131–175. [DOI] [PubMed] [Google Scholar]
- 9. Naumov GI, Masneuf I, Naumova ES, Aigle M, Dubourdieu D (2000) Association of Saccharomyces bayanus var. uvarum var. bayanus with some French wines: genetic analysis of yeast populations. Res Microbiol 151: 683–691. [DOI] [PubMed] [Google Scholar]
- 10. Demuyter C, Lollier M, Legras JL, Le Jeune C (2004) Predominance of Saccharomyces bayanus var. uvarum during spontaneous alcoholic fermentation, for three consecutive years, in an Alsatian winery. J Appl Microbiol 97: 1140–1148. [DOI] [PubMed] [Google Scholar]
- 11. Giudici P, Caggia C, Pulvirenti A, Zambonelli C, Rainieri S (1998) Electrophoretic profile of hybrids between cryotolerant and non-cryotolerant Saccharomyces strains. Lett Appl Microbiol 27: 31–34. [DOI] [PubMed] [Google Scholar]
- 12. Belloch C, Orlic S, Barrio E, Querol A (2008) Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int J Food Microbiol 122: 188–195. [DOI] [PubMed] [Google Scholar]
- 13. Tosi E, Azzolini M, Guzzo F, Zapparoli G (2009) Evidence of different fermentation behaviours of two indigenous strains of Saccharomyces cerevisiae and Saccharomyces bayanus var. uvarum isolated from Amarone wine. J Appl Microbiol 107: 210–218. [DOI] [PubMed] [Google Scholar]
- 14. Coloretti F, Zambonelli C, Tini V (2006) Characterization of flocculent Saccharomyces interspecific hybrids for the production of sparkling wines. Food Microbiol 23: 672–676. [DOI] [PubMed] [Google Scholar]
- 15. Henschke PA, Kwiatkowski MJ, Fogarty MW, McWilliam SJ, Hoj PB, et al. (2000) The effect of Saccharomyces bayanus var. uvarum-mediated fermentation on the chemical composition and aroma profile of Chardonnay wine. Aust J Grape Wine Res 6: 190–196. [Google Scholar]
- 16. Antonelli A, Castellari L, Zambonelli C, Carnacini A (1999) Yeast influence on volatile composition of wines. J Agric Food Chem 47: 1139–1144. [DOI] [PubMed] [Google Scholar]
- 17. Masneuf I, Hansen J, Groth C, Piskur J, Dubourdieu D (1998) New hybrids between Saccharomyces sensu stricto yeast species found among wine and cider production strains. Appl Environ Microbiol 64: 3887–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masneuf-Pomarède I, Bely M, Marullo P, Lonvaud-Funel A, Dubourdieu D (2010) Reassessment of phenotypic traits for Saccharomyces bayanus var. uvarum wine yeast strains. Int J Food Microbiol 139: 79–86. [DOI] [PubMed] [Google Scholar]
- 19. Sampaio JP, Gonçalves P (2008) Natural populations of Saccharomyces kudriavzevii in Portugal are associated with oak bark and are sympatric with S. cerevisiae and S. paradoxus . Appl Environ Microbiol 74: 2144–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopes CA, Barrio E, Querol A (2010) Natural hybrids of S. cerevisiae x S. kudriavzevii share alleles with European wild populations of Saccharomyces kudriavzevii . FEMS Yeast Res 10(4): 412–421. [DOI] [PubMed] [Google Scholar]
- 21. Belloch C, Pérez-Torrado R, González SS, Pérez-Ortín JE, García-Martínez J, et al. (2009) Chimeric genomes of natural hybrids of Saccharomyces cerevisiae and Saccharomyces kudriavzevii . Appl Environ Microbiol 75: 2534–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. González SS, Barrio E, Gafner J, Querol A (2006) Natural hybrids from Saccharomyces bayanus var. uvarum, S. cerevisiae and S. kudriavzevii in wine fermentations. FEMS Yeast Res 6: 1221–1234. [DOI] [PubMed] [Google Scholar]
- 23. Sipiczki M (2008) Interspecies hybridization and recombination in Saccharomyces wine yeasts. FEMS Yeast Res 8: 996–1007. [DOI] [PubMed] [Google Scholar]
- 24. Torija MJ, Beltrán G, Novo M, Poblet M, Guillamón JM, et al. (2003) Effects of fermentation temperature and Saccharomyces species on the cell fatty acid composition and presence of volatile compounds in wine. Int J Food Microbiol 85: 127–136. [DOI] [PubMed] [Google Scholar]
- 25. Llauradó JM, Rozès N, Constantí M, Mas A (2005) Study of some Saccharomyces cerevisiae strains for winemaking after preadaptation at low temperatures. J Agric Food Chem 53: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 26. Gamero A, Tronchoni J, Querol A, Belloch C (2013) Oenological characterization of cryotolerant Saccharomyces species and hybrids at low and moderate fermentation temperature. J Appl Microbiol 114: 1405–1414. [DOI] [PubMed] [Google Scholar]
- 27. Bisson L (1999) Stuck and sluggish fermentations. Am J Enol Vitic 50: 107–109. [Google Scholar]
- 28. Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, et al. (1996) Life with 6000 genes. Science 274: 546–567. [DOI] [PubMed] [Google Scholar]
- 29. Rossignol T, Dulau L, Julien A, Blondin B (2003) Genome-wide monitoring of wine yeast gene expression during alcoholic fermentation. Yeast 20: 1369–1385. [DOI] [PubMed] [Google Scholar]
- 30. Varela C, Cárdenas J, Melo F, Agosin E (2005) Quantitative analysis of wine yeast gene expression profiles under winemaking conditions. Yeast 22: 369–383. [DOI] [PubMed] [Google Scholar]
- 31. Beltrán G, Novo M, Leberre V, Sokol S, Labourdette D, et al. (2006) Integration of transcriptomicand metabolic analyses for understanding the global responses of low-temperature winemaking fermentations. FEMS Yeast Res 6: 1167–1183. [DOI] [PubMed] [Google Scholar]
- 32. Pizarro FJ, Jewett MC, Nielsen J, Agosin E (2008) Growth temperature exerts differential physiological and transcriptional responses in laboratory and wine strains of Saccharomyces cerevisiae . Appl Environ Microbiol 74: 6358–6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rossouw D, Næs T, Bauer FF (2008) Linking gene regulation and the exo-metabolome: A comparative transcriptomics approach to identify genes that impact on the production of volatile aroma compounds in yeast. BMC Genomics 9: 530–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marks VD, Ho Sui SJ, Erasmus D, van der Merwe GK, Brumm J, et al. (2008) Dynamics of the yeast transcriptome during wine fermentation reveals a novel fermentation stress response. FEMS Yeast Res 8: 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Erasmus DJ, van der Merwe GK, van Vuuren HJJ (2003) Genome-wide expression analyses: Metabolic adaptation of Saccharomyces cerevisiae to high sugar stress. FEMS Yeast Res 3: 375–399. [DOI] [PubMed] [Google Scholar]
- 36. Backhus LE, De Risi J, Bisson LF (2001) Functional genomic analysis of a commercial wine strain of Saccharomyces cerevisiae under differing nitrogen conditions. FEMS Yeast Res 1: 111–125. [DOI] [PubMed] [Google Scholar]
- 37. Querol A, Barrio E, Huerta T, Ramón D (1992) Molecular monitoring of wine fermentations conducted by active dry yeast strains. Appl Environ Microbiol 58: 2948–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peris D, Lopes CA, Belloch C, Querol A, Barrio E (2012) Comparative genomics among Saccharomyces cerevisiae × Saccharomyces kudriavzevii natural hybrid strains isolated from wine and beer reveals different origins. BMC Genomics 13: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Dijken JP, Sche!ers WA (1986) Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol Rev 32: 199–224. [Google Scholar]
- 40. Kondo K, Kowalski LR, Inouye M (1992) Cold shock induction of yeast NSR1 protein and its role in pre-rRNA processing. J Biol Chem 267: 16259–16265. [PubMed] [Google Scholar]
- 41. Holstege FCP, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, et al. (1998) Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95: 717–728. [DOI] [PubMed] [Google Scholar]
- 42. Spellman PT, Sherlock G, Zhang MQ, Vishwanath RI, Anders K, et al. (1998) Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell 9: 3273–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vollbrecht D, Radler F (1973) Formation of higher alcohols by amino acid deficient mutants of Saccharomyces cerevisiae. The decomposition of amino acids to higher alcohols. Arch Mikrobiol 94: 351–358. [PubMed] [Google Scholar]
- 44. Lee S, Villa K, Patino H (1995) Yeast strain development for enhanced production of desirable alcohols/esters in beer. J Am Soc Brew Chem 53: 153–156. [Google Scholar]
- 45. Llauradó JM, Rozès N, Bobet R, Mas A, Constantí M (2002) Low temperature alcoholic fermentation in high sugar concentration grape must. J Food Sci 67: 268–273. [Google Scholar]
- 46. Yoshimoto H, Fukushige T, Yonezawa T (2001) Sone (2001) Genetic and physiological analysis of branched-chain alcohols and isoamyl acetate production in Saccharomyces cerevisiae . Appl Microbiol Biotechnol 59: 501–508. [DOI] [PubMed] [Google Scholar]
- 47. Saerens SMG, Delvaux F, Verstrepen KJ, Van Dijck P, Thevelein JM, et al. (2008) Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl Environ Microbiol 74: 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Antunovics Z, Csoma H, Sipiczki M (2003) Molecular and genetic analysis of the yeast flora of botrytized Tokaj wines. Bull de l’OIV 76: 380–397. [Google Scholar]
- 49. Verstrepen KJ, Derdelinckx G, Dufour JP, Winderickx J, Pretorius IS, et al. (2003) The Saccharomyces cerevisiae alcohol acetyl transferase gene ATF1 is a target of the cAMP/PKA and FGM nutrient-signalling pathways. FEMS Yeast Res 4: 285–296. [DOI] [PubMed] [Google Scholar]
- 50. Taylor GT, Thurston PA, Kirsop BH (1979) Influence of lipids derived from malt spent grains on yeast metabolism and fermentation. J Inst Brew 85: 219–227. [Google Scholar]
- 51. García-Martínez J, Aranda A, Pérez-Ortín JE (2004) Genomic run-on evaluates transcription technique rates for all yeast genes and indentifies gene regulatory mechanisms. Molecular Cell 15: 303–313. [DOI] [PubMed] [Google Scholar]
- 52. Aerny J (1997) Composés azotes des moûts et des vins. Rev Suisse Vitic Hort 28: 161–165. [Google Scholar]
- 53. Rojas V, Gil JV, Piñaga F, Manzanares P (2001) Studies on acetate ester production by non-Saccharomyces wine yeasts. Int J Food Microbiol 70: 283–289. [DOI] [PubMed] [Google Scholar]