Abstract
Background
Poly(ADP-ribose) polymerase-1 (PARP-1) is a nuclear chromatin-associated enzyme involved in several important cellular processes, particularly in the DNA repair system. PARP-1 rs1136410: C>T is among the most studied polymorphisms and likely involved in human carcinogenesis. However, results from previous studies are inconclusive. Thus, a meta-analysis was conducted to derive a more precise estimation of the effects of this enzyme.
Methodology and Principal Findings
A comprehensive search was conducted in the PubMed and EMBASE databases until December 9, 2013. A total of 39 studies with 16,783 cancer cases and 23,063 control subjects were included in the meta-analysis on the basis of the inclusion and exclusion criteria. No significant association between the PARP-1 Val762Ala polymorphism and cancer risk was found when all of the studies were pooled into the analysis (VA + AA vs. VV: OR = 1.03, 95% CI = 0.95–1.11). The subgroup analysis of cancer types revealed that the –762Ala allele was associated with increased risk of gastric, cervical, and lung cancers and a decreased risk of glioma. In addition, a significantly increased risk of cancer associated with the polymorphism was observed in Asian descendents (VA + AA vs. VV: OR = 1.17, 95% CI = 1.09–1.25; AA vs. VV: OR = 1.28, 95% CI = 1.08–1.51; VA vs. VV: OR = 1.12, 95% CI = 1.04–1.20; AA vs. VA + VV: OR = 1.09, 95% CI = 1.03–1.39). These results also indicated that a joint effect between PARP-1 Val762Ala and XRCC1 Arg399Gln could be involved in the risk of cancer development (OR = 3.53, 95% CI = 1.30–9.59).
Conclusion
The present meta-analysis provides evidence that the PARP-1 Val762Ala may be involved in cancer development at least in some ethnic groups (Asian) or some specific cancer types (gastric, cervical, and lung cancers, and glioma).
Introduction
The etiology and development of cancer are a result of complex interactions between genetic and environmental factors. Physical and chemical agents originated from either endogenous processes, such as cellular metabolism, or exogenous exposure, including ionizing radiation, tobacco smoke, and genotoxic chemicals, are responsible for oxidative cell DNA damage; when left unrepaired or incorrectly repaired, cell DNA damage may lead to mutations and genomic instability [1]. Base excision repair (BER) system repairs base damage and single-strand breaks caused by X-rays, oxygen radicals, and alkylating reagents. However, inherited defects in DNA repair pathways result in the accumulation of DNA damage, cell apoptosis, or unregulated cell growth and development of malignancy [2]–[4].
Poly(ADP-ribose) polymerase-1 (PARP-1), also called adenosine diphosphate ribosyl transferase, is one of the most important components of the BER system. PARP1 is a nuclear nick sensor enzyme that becomes activated in response to DNA breakage [5]. In general, PARP1 binds to the sites of DNA damage via the N-terminal DNA-binding domain and catalyzes the addition of poly(ADP-ribose) polymers from NAD+ to nuclear acceptor proteins, including histones, P53, and PARP-1 itself, thereby causing chrome relaxation and recruitment of other repair proteins (e.g., XRCC1, DNA-PK) into the damaged site [6], [7]. Therefore, PARP-1 is essential for the surveillance and maintenance of genome integrity and interaction with various proteins involved in multiple DNA repair pathways, including BER, SSBR (Single-strand break repair), and DSBR (DNA double-strand break repair). Moreover, PARP-1 is implicated in other molecular and cellular processes, such as gene transcription modulation, apoptosis decision, telomere maintenance, and chromatin remodeling [8], [9]. Evidence has suggested that the deficiency of PARP-1 results in DNA repair defects, genomic instability, failure of induction of cell death, and modulation of gene transcription, thereby contributing to carcinogenesis [10]–[12].
The human PARP1 gene, located on chromosome 1q41–42, is approximately 47.3 kb in length and consists of 23 exons. Numerous single nucleotide polymorphisms (SNPs), including 17 non-synonymous SNPs, have been identified in PARP-1; among these SNPs, rs1136410 at codon 762 in exon 17, a non-synonymous T→C polymorphism changing valine to alanine, is the most extensively investigated. This polymorphism is located in the sixth helix of the COOH-terminal NAD-binding region with all of the catalytic activities of the full-length enzyme. This amino acid change contributes to low poly(ADP-ribosyl)ation activities in a dosage-dependent manner, thereby impairing DNA repair and enhancing the susceptibility of variant allele carriers to damage caused by environmental carcinogens and cancer risk [5], [13]. Thus far, molecular epidemiological studies have indicated the genetic association of Val762Ala with the risk of many cancer types, including cancers of the breast, stomach, lung, cervix, brain, and colorectum, as well as other types of malignancies [14]–[19]. However, these studies have not yet produced consistent results. The discrepancies of the findings are partially attributed to the limited power of individual studies with small sample sizes and differences in the baseline characteristics of included patients. Although the PARP-1 Val762Ala polymorphism and susceptibility to cancers have been discussed [20], [21], all of the eligible studies have not been included, particularly case-control studies published in the past two years. Therefore, these meta-studies are disputed because of the limited number of included studies and relatively small sample size. The present meta-analysis aimed to update previous meta-analyses and derive a reliable conclusion regarding the effect of the V762A polymorphism on the function of PARP-1 in cancer. This meta-analysis also aimed to quantify the potential of heterogeneity between studies.
Materials and Methods
Literature search
Relevant publications were identified by conducting a literature search in PubMed and EMBASE databases using the following search terms: PARP-1 or ADPR, variant or polymorphism or SNP, and cancer or carcinoma or tumor. The last search was updated on December 9, 2013. The references of the identified studies and reviews were also screened to find additional eligible studies. If studies with overlapped subjects were reported, only the one with the most complete data was included in the meta-analysis. Search results were limited to studies published in English.
Inclusion and exclusion criteria
Studies were included in our meta-analysis if the following criteria were satisfied: (1) studies were designed as cohort or case-control; (2) studies investigated the association between PARP-1 Val762Ala polymorphism and cancer susceptibility; and (3) sufficient genotype data were provided to estimate the odds ratio (OR) and a corresponding 95% confidence interval (CI). Studies were excluded if the following criteria were satisfied: (1) case-only, case reports, or reviews; (2) duplicate of previous publications; (3) family-based studies; and (4) based on insufficient data for calculation.
Data extraction
Two investigators dependently reviewed the publications and obtained information according to a standard data form. The following data were extracted from each study: name of first author; year of publication; country or region of origin; ethnicity of the study population; cancer type; number of cases and controls; allele and genotype frequency; evidence of Hardy-Weinberg equilibrium (HWE) in controls; source of controls; and genotyping method. Disagreements between the two investigators were resolved by discussing the results with a third investigator.
Statistical analysis
The strength of the association between the PARP-1 Val762Ala polymorphism and the risk of cancer was measured by OR with 95% CI in five genetic models, including dominant model (VA + AA vs. VV), recessive model (AA vs. VA + VV), homozygous model (AA vs. VV), heterozygous model (VA vs. VV), and allele model (A vs. V). The significance of the pooled OR was determined by a Z-test, and P<0.05 was considered statistically significant. A statistical test to determine heterogeneity between studies was performed using Q-test and I2 test. In the Q-test, P>0.10 indicates the absence of heterogeneity. The pooled OR estimates of each study were calculated using the fixed-effect model, the Mantel-Haenszel method. Otherwise, a random-effect model, the Dersimonain and Laird method, was applied. The I2 test was used to quantify the effect of heterogeneity (ranges from 0% to 100%); The test represents the proportion of inter-study variability that can be attributed to heterogeneity rather than by chance. Subgroup analyses were also performed to evaluate the potential effects of ethnicity, cancer types, source of controls, and genotyping method. Sensitivity analysis was conducted by omitting each study to identify the effect of an individual study on the pooled OR. Publication bias was qualitatively detected using Begger's funnel plots, and Egger's linear regression test was performed to determine funnel plot asymmetry (P<0.05 was considered as statistically significant publication bias). All of the P values were two-tailed. Statistical analyses were performed using STATA version 11.0 (Stata Corporation, College Station, TX, USA).
Results
Characteristics of eligible studies
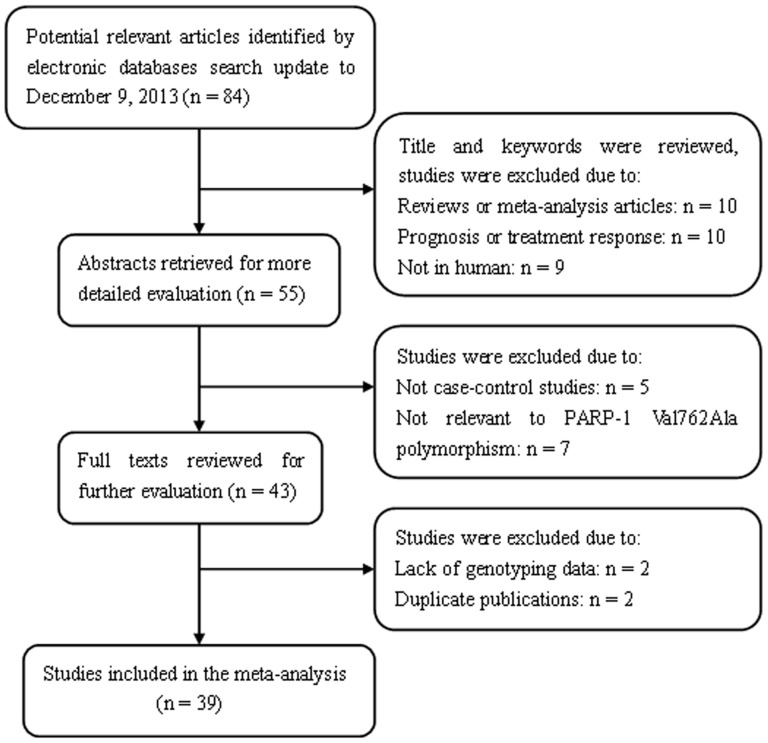
A total of 84 articles relevant to search keywords were identified after our literature search from PubMed and EMBASE was completed. According to the inclusion criteria, 45 studies were excluded. Among these studies, two were excluded because of a lack of genotyping data [22], [23]. The flow chart of the detailed steps of study selection is shown in Figure 1. A total of 39 case-control studies with 16,783 cancer cases and 23,063 control subjects were included in our meta-analysis. The main characteristics of the eligible studies are listed in Table 1. A total of 21 studies involved Caucasian populations and 18 focused on Asian populations. Among these studies, three focused on colorectal, lung, cervical, and bladder cancer, individually; and four described gastric, glioma, and breast cancer, individually. The distribution of the genotypes in the control subjects was in agreement with HWE except three studies [15], [24], [25].
Figure 1. Flow chart of literature search and study selection.
Table 1. Characteristics of eligible studies included in the meta-analysis.
| Name | Year | Country | Ethnicity | Cancer type | Sample size | Source | Controls in HWE | Genotyping method | |
| cases/controls | |||||||||
| Hosono [33] | 2013 | Japan | Asian | Endometrial | 91 | 261 | HB | Yes | TaqMan |
| Li [18] | 2013 | China | Asian | Colorectal | 451 | 626 | Mixed | Yes | PCR-RFLP |
| Roszak [17] | 2013 | Poland | Caucasian | Cervical | 446 | 491 | PB | Yes | PCR-HRM |
| Xue [34] | 2013 | China | Asian | Lung | 410 | 410 | HB | Yes | PCR-RFLP |
| Nakao [24] | 2012 | Japan | Asian | Pancreas | 185 | 1465 | HB | No | TaqMan |
| Pan [25] | 2012 | China | Asian | Gastric | 176 | 308 | Mixed | No | MassARRAY |
| Santonocito [19] | 2012 | Italy | Caucasian | Melanoma | 167 | 99 | NM | Yes | PCR-HRM |
| Santos [35] | 2012 | Potugal | Caucasian | Thyroid | 108 | 216 | HB | Yes | TaqMan |
| Wen [32] | 2012 | China | Asian | Gastric | 307 | 307 | Mixed | Yes | MassARRAY |
| Ye [36] | 2012 | China | Asian | Cervical | 539 | 800 | HB | Yes | MA-PCR |
| Yuan [37] | 2012 | China | Asian | Head and neck | 395 | 883 | PB | Yes | TaqMan |
| Zhang [38] | 2012 | China | Asian | Cervical | 80 | 176 | HB | Yes | SNPware 12plex assay |
| Yosunkaya [39] | 2010 | Turkey | Caucasian | Glioma | 119 | 180 | PB | Yes | PCR-RFLP |
| Gao [40] | 2010 | US | Caucasian | Prostate | 453 | 119 | HB | Yes | Sequence |
| Jin [41] | 2010 | Korea | Asian | NHL | 573 | 721 | PB | Yes | PCR-HRM |
| Rajaraman [42] | 2010 | US | Caucasian | Glioma | 340 | 463 | HB | Yes | TaqMan |
| Rajaraman | 2010 | US | Caucasian | Meningioma | 121 | 463 | HB | Yes | TaqMan |
| Rajaraman | 2010 | US | Caucasian | Acoustic neuroma | 65 | 463 | HB | Yes | TaqMan |
| Wang [43] | 2010 | China | Asian | Bladder | 234 | 253 | HB | Yes | PCR-RFLP |
| Liu [44] | 2009 | US | Caucasian | Glioma | 372 | 365 | PB | Yes | MassARRAY |
| McKean [45] | 2009 | US | Caucasian | Glioblastoma | 987 | 1935 | Mixed | Yes | MassARRAY |
| Zhang [46] | 2009 | China | Asian | Gastric | 236 | 320 | HB | Yes | PCR-RFLP |
| Chiang[47] | 2008 | China | Asian | Thyroid | 283 | 469 | HB | Yes | TaqMan |
| Smith[14] | 2008 | US | Caucasian | Breast | 314 | 397 | HB | Yes | MassARRAY |
| Berndt[48] | 2007 | US | Caucasian | Colorectal | 649 | 659 | NM | Yes | TaqMan |
| Cao[49] | 2007 | France | Caucasian | Breast | 83 | 100 | HB | Yes | Sequence |
| Figueroa[50] | 2007 | Spain | Caucasian | Bladder | 1138 | 1131 | HB | Yes | TaqMan |
| Li[51] | 2007 | US | Caucasian | Head and neck | 830 | 854 | HB | Yes | PCR-RFLP |
| Stern[52] | 2007 | Singapore | Asian | Colorectal | 307 | 1173 | PB | Yes | TaqMan |
| Landi[53] | 2006 | Multiple regions | Caucasian | Lung | 292 | 307 | HB | Yes | APEX |
| Li[54] | 2006 | US | Caucasian | Melanoma | 602 | 603 | HB | Yes | PCR-RFLP |
| Miao[15] | 2006 | China | Asian | Gastric | 500 | 1000 | PB | No | PCR-RFLP |
| Shen[55] | 2006 | US | Caucasian | NHL | 455 | 535 | PB | Yes | TaqMan |
| Wu[56] | 2006 | US | Caucasian | Bladder | 606 | 595 | HB | Yes | TaqMan |
| Zhai[57] | 2006 | China | Asian | Breast | 302 | 639 | HB | Yes | PCR-RFLP |
| Zhang[58] | 2006 | US | Caucasian | Breast | 1715 | 1371 | PB | Yes | TaqMan |
| Zhang[16] | 2005 | China | Asian | Lung | 1000 | 1000 | HB | Yes | PCR-RFLP |
| Hao[59] | 2004 | China | Asian | Esophageal | 414 | 479 | HB | Yes | PCR-RFLP |
| Lockett[5] | 2004 | US | Caucasian | Prostate | 438 | 427 | HB | Yes | MassARRAY |
PB: population-based; HB: hospital-based; HWE: Hardy-Winberg equilibrium. Genotyping method: PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism; MassARRAY: genotyping was performed using the Sequenom MassARRAY iPLEXTM platform2. MassARRAY Workstation version 3.3 software was used to process and analyze iPLEX SpectroCHIP bioarrays; PCR-HRM, PCR cycling and high resolution melting analysis was performed on the Rotor-Gene 6000TM. APEX: polymorphism was analyzed together for a given sample by a microarray technique based on the arrayed primer extension principle.
Quantitative synthesis
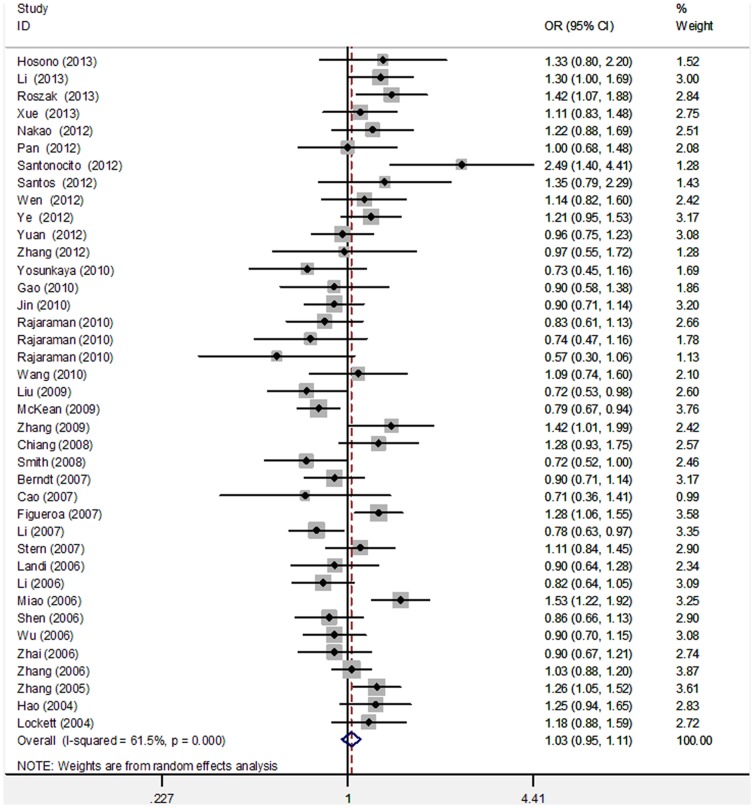
The meta-analysis findings of the correlation between PARP-1 V762A and cancer risk are summarized in Table 2. After the 39 studies were pooled into meta-analysis, no evidence of a significant association between V762A polymorphism and cancer risk was observed (dominant model: OR = 1.03, 95% CI = 0.95–1.11; recessive model: OR = 1.10, 95% CI = 0.97–1.26; homozygous model: OR = 1.13, 95% CI = 0.98–1.31; heterozygous model: OR = 1.02, 95% CI = 0.95–1.10; allele model: OR = 1.04, 95% CI = 0.97–1.11; Table 2; Figure 2). We excluded three studies with genotypic distribution in control subjects that deviated from HWE and found that the results did not significantly alter from the corresponding pooled OR (Table 2).
Table 2. Meta-analysis of the association between PARP-1 Val762Ala polymorphism and cancer risk.
| No. of subjects cases/controls | n | VA+AA vs. VV | AA vs. VA+VV | AA vs. VV | VA vs. VV | A vs. V | ||||||
| OR (95% CI) | Phet | OR (95% CI) | Phet | OR (95% CI) | Phet | OR (95% CI) | Phet | OR (95% CI) | Phet | |||
| Total | 16783/23063 | 39 | 1.03 (0.95–1.11) | 0.000 | 1.10 (0.97–1.26) | 0.000 | 1.13 (0.98–1.31) | 0.000 | 1.02 (0.95–1.10) | 0.001 | 1.04 (0.97–1.11) | 0.000 |
| Controls in HWE | 15922/20290 | 36 | 1.01 (0.94–1.09) | 0.000 | 1.09 (0.95–1.29) | 0.000 | 1.11 (0.96–1.28) | 0.000 | 1.00 (0.94–1.08) | 0.001 | 1.03 (0.96–1.10) | 0.000 |
| Ethnicities | ||||||||||||
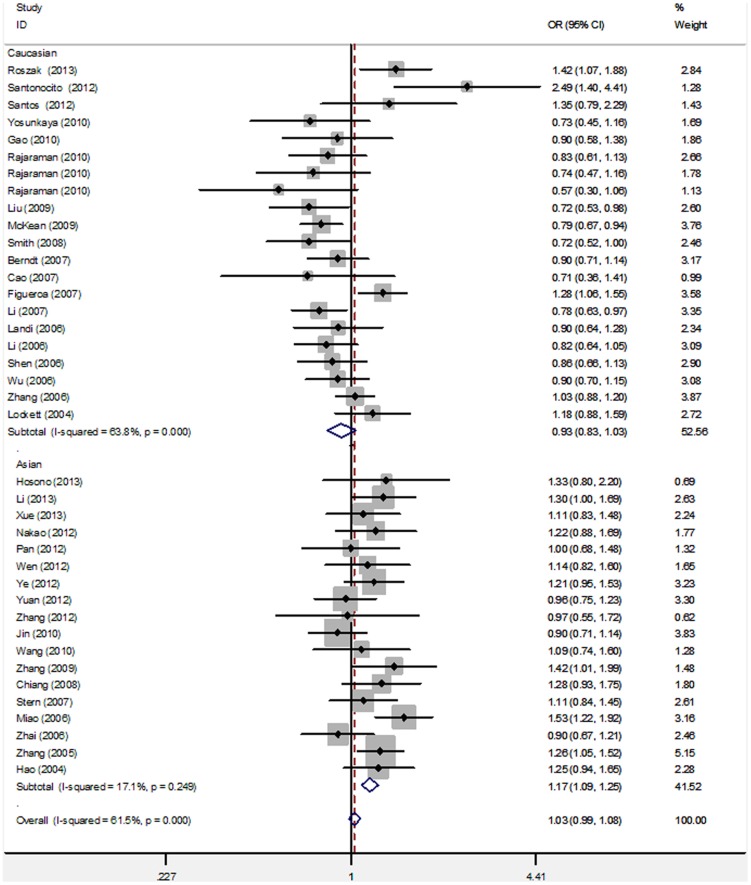
| Caucasian | 10300/11773 | 21 | 0.93 (0.83–1.03) | 0.000 | 0.95 (0.76–1.18) | 0.079 | 0.92 (0.78–1.08) | 0.111 | 0.94 (0.84–1.04) | 0.000 | 0.96 (0.87–1.05) | 0.000 |
| Asian | 6483/11290 | 18 | 1.17 (1.09–1.25)* | 0.249 | 1.09 (1.03–1.39)* | 0.000 | 1.28 (1.08–1.51)* | 0.000 | 1.12 (1.04–1.20)* | 0.805 | 1.12 (1.05–1.21)* | 0.001 |
| Cancer type | ||||||||||||
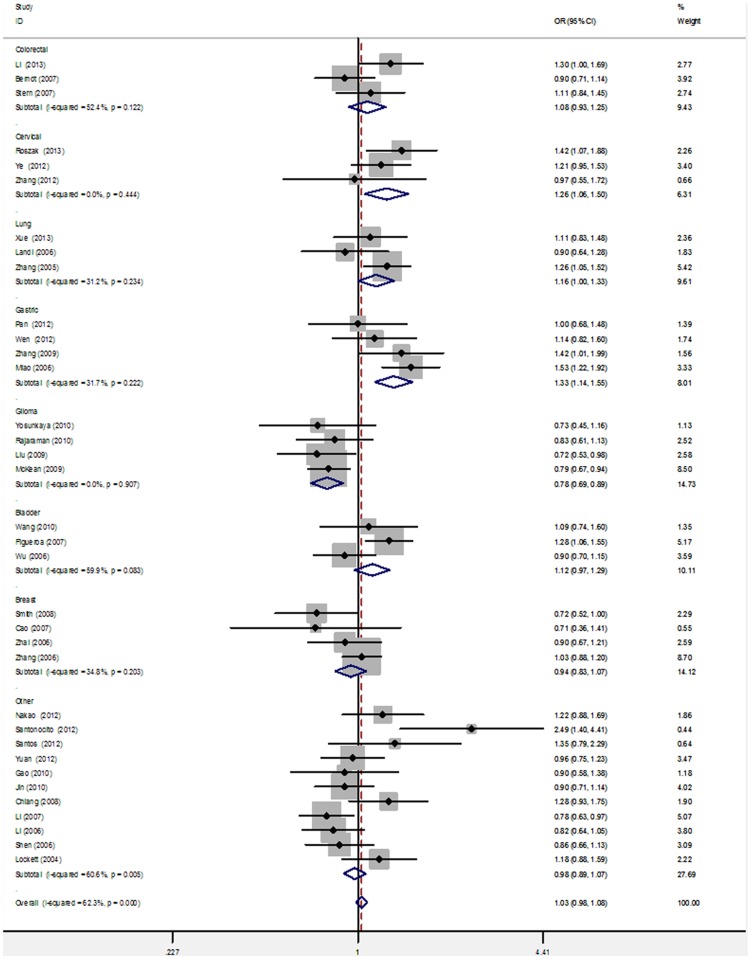
| Colorectal | 1407/2458 | 3 | 1.08 (0.93–1.25) | 0.122 | 1.14 (0.79–1.67) | 0.064 | 1.18 (0.76–1.85) | 0.039 | 1.05 (0.90–1.23) | 0.419 | 1.08 (0.88–1.31) | 0.032 |
| Cervical | 1065/1467 | 3 | 1.26 (1.06–1.50)* | 0.444 | 1.59 (0.82–3.07) | 0.011 | 1.68 (0.91–3.10) | 0.036 | 1.14 (0.95–1.36) | 0.252 | 1.31 (1.16–1.48)* | 0.201 |
| Lung | 1702/1717 | 3 | 1.16 (1.00–1.33)* | 0.234 | 1.32 (1.09–1.61)* | 0.487 | 1.42 (1.14–1.76)* | 0.326 | 1.10 (0.95–1.28) | 0.447 | 1.16 (1.05–1.28)* | 0.182 |
| Gastric | 1219/1935 | 4 | 1.33 (1.14–1.55)* | 0.222 | 1.22 (0.77–1.94) | 0.001 | 1.38 (0.84–2.26) | 0.002 | 1.28 (1.09–1.51)* | 0.742 | 1.19 (0.95–1.48) | 0.006 |
| Glioma | 1818/2943 | 4 | 0.78 (0.69–0.89)* | 0.907 | 1.06 (0.46–2.42) | 0.013 | 0.92 (0.48–1.78) | 0.071 | 0.79 (0.68–0.91)* | 0.302 | 0.84 (0.75–0.95)* | 0.414 |
| Bladder | 1978/1979 | 3 | 1.09 (0.86–1.39) | 0.083 | 0.96 (0.69–1.33) | 0.818 | 0.99 (0.70–1.40) | 0.850 | 1.10 (0.84–1.44) | 0.057 | 1.08 (0.96–1.22) | 0.159 |
| Breast | 2414/2507 | 4 | 0.94 (0.83–1.07) | 0.203 | 0.92 (0.71–1.19) | 0.852 | 0.89 (0.68–1.17) | 0.838 | 0.95 (0.84–1.08) | 0.176 | 0.95 (0.86–1.05) | 0.317 |
| Other | 4489/6391 | 11 | 1.02 (0.88–1.19) | 0.005 | 0.98 (0.78–1.22) | 0.075 | 0.99 (0.76–1.30) | 0.024 | 1.01 (0.89–1.15) | 0.048 | 1.02 (0.90–1.16) | 0.001 |
| Source of controls | ||||||||||||
| PB | 4882/6719 | 9 | 1.02 (0.87–1.19) | 0.001 | 1.17 (0.90–1.51) | 0.003 | 1.18 (0.88–1.59) | 0.001 | 1.03 (0.89–1.20) | 0.013 | 1.07 (0.94–1.22) | 0.000 |
| HB | 9164/12410 | 24 | 1.03 (0.93–1.13) | 0.002 | 1.12 (0.94–1.33) | 0.001 | 1.15 (0.95–1.39) | 0.001 | 1.01 (0.93–1.10) | 0.04 | 1.03 (0.95–1.12) | 0.000 |
| Mixed | 1921/3176 | 4 | 1.03 (0.79–1.34) | 0.01 | 0.98 (0.67–1.42) | 0.019 | 1.03 (0.69–1.55) | 0.02 | 1.03 (0.80–1.33) | 0.028 | 0.99 (0.80–1.23) | 0.002 |
| Genotyping method | ||||||||||||
| PCR-RFLP | 5098/6364 | 11 | 1.09 (0.93–1.27) | 0.000 | 1.29 (1.07–1.55)* | 0.008 | 1.34 (1.07–1.67)* | 0.002 | 1.03 (0.90–1.19) | 0.004 | 1.10 (0.98–1.24) | 0.000 |
| TaqMan | 6458/10147 | 14 | 1.02 (0.92–1.12) | 0.055 | 0.97 (0.85–1.12) | 0.866 | 1.0 (0.86–1.16) | 0.628 | 1.04 (0.96–1.12) | 0.118 | 1.00 (0.93–1.09) | 0.061 |
| MassARRAY | 2594/3739 | 6 | 0.89 (0.75–1.07) | 0.051 | 0.90 (0.71–1.13) | 0.134 | 0.93 (0.73–1.20) | 0.133 | 0.94 (0.76–1.15) | 0.063 | 0.93 (0.79–1.10) | 0.039 |
| Other | 2261/2330 | 6 | 1.16 (0.89–1.50) | 0.005 | 1.42 (0.73–2.74) | 0.000 | 1.44 (0.73–2.85) | 0.000 | 1.10 (0.87–1.40) | 0.031 | 1.18 (0.92–1.52) | 0.000 |
Phet: P-value of Q-test for heterogeneity test. The fixed-effects model was used when P-value for heterogeneity test >0.10; otherwise, the random-effects model was used.
*indicate significant difference.
Figure 2. Forest plot for pooled OR of association between the PARP-1 Val762Ala polymorphism and overall cancer risk under dominant model (VA+AA vs. VV).
Significant heterogeneity was observed among the overall 39 studies of the PARP-1 V762A polymorphism (e.g., dominant model: Q = 98.58 on 38 d.f., P = 0.000, I2 = 61.5%). To explore the source of heterogeneity, we performed stratified analyses on ethnicity, cancer type, source of controls, and genotyping method. In the subgroup analysis of ethnicity, PARP-1 V762A was significantly associated with an increased risk of cancer in Asian populations in all of the genetic models (e.g., dominant model: OR = 1.17, 95% CI = 1.09–1.25; Table 2; Figure 3). However, no significant association was found in Caucasian populations in any models (e.g., dominant model: OR = 0.93, 95% CI = 0.83–1.03; Table 2; Figure 3). The studies were further stratified on the basis of cancer type and the results showed that PARP-1 V762A polymorphism may be a risk factor of lung cancer in all of the genetic models except the heterozygous model (dominant model: OR = 1.16, 95% CI = 1.00–1.33; recessive model: OR = 1.32, 95% CI = 1.09–1.61; homozygous model = OR = 1.42, 95% CI: 1.14–1.76; heterozygous model = OR = 1.10, 95% CI = 0.95–1.28; allele model: dominant model: OR = 1.16, 95% CI = 1.05–1.28; Table 2; Figure 4). We also found significant correlation between the Ala carrier of PARP-1 V762A polymorphism and increased risk of cervical cancer (dominant model: OR = 1.26, 95% CI = 1.06–1.50; allele model: OR = 1.31, 95% CI = 1.16–1.48) and gastric cancer (dominant model: OR = 1.33, 95% CI = 1.14–1.55; heterozygous model: OR = 1.28, 95% CI = 1.09–1.51). By contrast, the PARP-1 V762A polymorphism was significantly associated with a decreased risk of glioma in three genetic models (Table 2; Figure 4). However, studies on colorectal, bladder, breast, and other cancer types have suggested null association (OR = 0.92–1.18; Table 2; Figure 4). Furthermore, V762A polymorphism was significantly associated with increased cancer risk in the subgroup of PCR-RFLP genotyping method (recessive model: OR = 1.29, 95% CI = 1.07–1.55; homozygous model: OR = 1.34, 95% CI = 1.07–1.67; Table 2). No significant associations were detected when the studies were stratified on the basis of the source of control subjects (Table 2).
Figure 3. Subgroup analysis by ethnicity of ORs for cancer risk associated with the PARP-1 Val762Ala polymorphism under dominant model (VA+AA vs. VV).
Figure 4. Subgroup analysis by cancer type of ORs for cancer risk associated with the PARP-1 Val762Ala polymorphism under dominant model (VA+AA vs. VV).
Considering that PARP-1 functionally interacts with XRCC1 in BER processes, we performed a gene-gene interaction analysis of the five studies that reported joint effects between PARP1 Val762Ala and XRCC1 Arg399Gln on cancer risks. In Table 3, a significant interaction between the pairwise-coding SNPs in XRCC1-PARP1 was found because subjects with the PARP1 Ala/Ala and XRCC1 Gln/Gln genotypes exhibited a higher risk of cancer compared with subjects carrying the PARP1 Val/Val and XRCC1 Arg/Arg genotypes (pooled OR = 3.53, 95% CI = 1.30–9.59).
Table 3. Pooled analysis of the interaction effects between PARP1 Val762Ala and XRCC1 Arg399Gln on overall cancer risk.
| XRCC1 Arg399Gln | PARP1 Val762Ala | No. of subjects cases/controls | OR (95% CI) | P | Phet |
| Arg/Arg | Val/Val | 282/536 | 1 | ||
| Either variant genotype | 1142/1668 | 1.32 (0.94–1.87) | 0.111 | 0.016 | |
| Both heterozygous genotype | 875/1097 | 1.62 (0.96–2.71) | 0.068 | 0.000 | |
| Gln/Gln | Ala/Ala | 67/52 | 3.53 (1.30–9.59)* | 0.014 | 0.067 |
Either variant genotype: an individual with any variant homozygote or heterozygote at one site and wild-type homozygote at the other site.
Both heterozygous genotype: an individual with heterozygote at both sites.
*indicate significant difference.
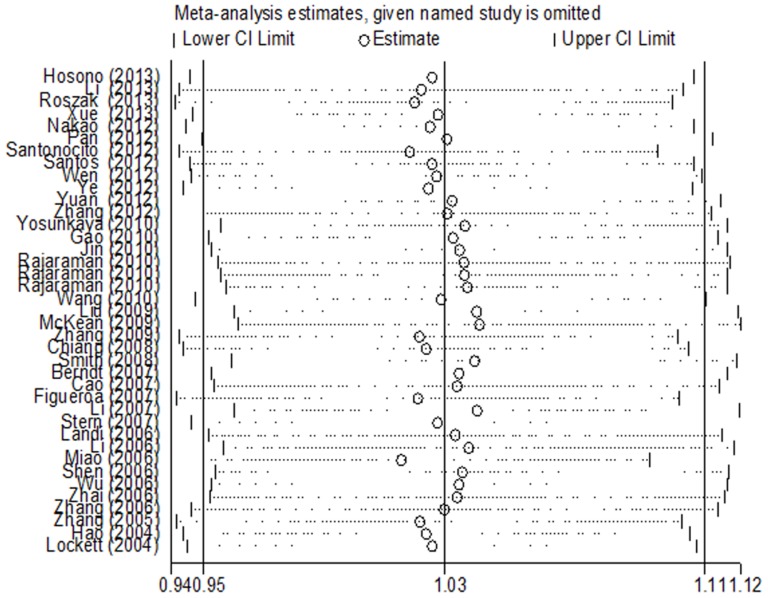
Sensitivity analysis
Sensitivity analysis was conducted to verify the effect of each study on the overall OR by repeating the meta-analysis, but any single study was omitted at each time. In Figure 5, no individual study affected the pooled OR qualitatively, indicating that the pooled results were statistically robust.
Figure 5. Sensitivity analysis of overall OR coefficients for dominant model (VA+AA vs. VV).
The analysis was conducted by omitting each study in turn. Meta-analysis random-effects estimates were used. The two ends of the dotted lines represent the 95%CI.
Publication bias
Begger's funnel plot and Egger's test were performed to evaluate the publication bias of the studies. The shape of the funnel plots showed that the dots were nearly symmetrically distributed predominantly in pseudo 95% confidence limits (dominant model, Figure 6). The results of Egger's test statistically confirmed the absence of publication bias in the dominant model (t = −0.11, P = 0.916).
Figure 6. Begger's funnel plot of publication bias for PARP-1 Val762Ala polymorphism with cancer risk under dominant model (VA+AA vs. VV).
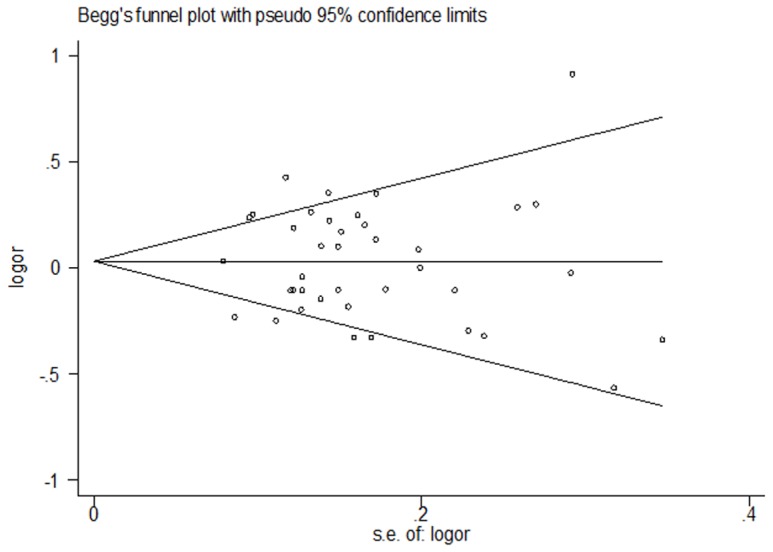
Each dot represents a separate study for the indicated association. Funnel plot of all 39 eligible studies P = 0.753, Egger's test P = 0.916.
Discussion
PARP-1, the first discovered member of the PARP family, is involved in various important molecular and cellular processes, including cellular stress response, cell cycle control, telomere maintenance, chromatin remodeling, and mitotic apparatus functions. This nuclear DNA binding protein also functions in DNA single-strand break repair. This protein specifically detects DNA strand breaks generated by different genotoxic agents, facilitates the formation of DNA repair complexes, such as BRCA1 or BRCA2, and activates regulatory enzymes, namely, ATM and ATR, involved in the cell cycle [26]. Gene polymorphisms may also influence the rate of gene transcription, the stability of mRNA, or the quantity and activity of the resulting protein [27]. Thus, variations in PARP-1 gene may affect DNA repair in normal populations and facilitate cancer development in normal or exposed individuals.
Thus far, approximately 1,066 single-nucleotide polymorphisms in the PARP-1 gene have been reported; among these polymorphisms, a T to C nucleotide transition results in Val762Ala substitution located in the C-terminal catalytic site and characterizes a commonly occurring PARP-1 polymorphism; this alteration is frequently investigated because of its association with cancer risk [28]. Several in vitro experiments have characterized the functional effect of this polymorphism on PARP1. For instance, Wang et al. [29] found that PARP-Ala762 displays approximately half of the activity of PARP-Val762 for both auto-poly(ADP-ribosyl)ation and trans-poly(ADP-ribosyl)ation of histone H1. Lockett et al. [5] also suggested that the PARP-1 Val762Ala polymorphism reduces the enzymatic activity of PARP1 in response to oxidative damage. Molecular epidemic studies have also been conducted to investigate the functional relevance of this variant with susceptibility to cancer. However, results remain inconsistent.
A total of 39 studies with 16,783 cancer cases and 23,063 controls were considered in the present meta-analysis. The results indicated no significant association of PARP-1 Val762Ala polymorphism with overall cancer risk. In the stratified analysis by ethnicity, the variant –762Ala allele was significantly associated with an increased cancer risk among Asian populations. By contrast, no significant correlation was detected among Caucasians. The discrepancy in ethnicity could be attributed to the evident difference in the minor allele frequency (MAF) of Val762Ala polymorphism in Asians and Caucasians in our meta-analysis (41.6% and 16.2%, respectively). This genetic polymorphism variance with ethnicity was consistent with those described in a previous study [30]. Significant risks were also found in subgroup analysis based on cancer types. Subjects with the variant Ala allele were more susceptible to cancers of the cervix, lung, and stomach, whereas the polymorphism was a potential protective factor against glioma in dominant, heterozygous, and allele models. PARP-1 variant genotypes may possibly be tissue specific because of high or low PARP-1 expression levels in different tumor tissues [12], [31]. Moreover, this result could be interpreted partially on the basis of the different functions of PARP-1 in different tumor types as a result of distinct mechanisms in terms of cancer susceptibility. In addition, stratified analysis by genotyping techniques indicated that studies involving PCR-RFLP assay likely acquired significant results in the overall comparison. This trend is possible because studies involving Asians mainly utilized PCR-RFLP. In studies involving Caucasians, Taqman and MassArray were the main genotyping techniques. Considering gene-gene interaction analysis, we found a significant joint effect of ERCC1 –399Gln and PARP-1–762Ala on increased cancer risk in a homozygous genetic model. However, this result should be carefully interpreted because of a relatively small sample size; moreover, this result should be confirmed by conducting further analysis of additional published studies.
Compared with two previous meta-analyses, our meta-analysis involved a remarkably larger number of studies (39 vs. 21 and 28) and provided a more comprehensive and reliable conclusion. Pooling the data from 39 studies, we reconfirmed the function of PARP-1 Val762Ala in increased cancer risk among Asian populations. Furthermore, cancer types in the study were more multifarious (seven types) and a significant association was found in cervical, lung, and gastric cancers, as well as glioma. In addition, the potential interaction effect of XRCC1 Arg399Gln on PARP-1 Val762Ala was also evaluated in the present analysis.
Some potential limitations of this study should also be considered. First, the pooled results were based on unadjusted estimates because not all of the studies provided adjusted ORs; when these studies revealed adjusted ORs, these ORs were not adjusted by the same confounders. Hence, a precise analysis should be performed if individual data, such as age, sex, BMI, and smoking and drinking status, were available. Second, several factors, such as gene-gene or gene-environment interaction, may influence gene-disease factor. The joint effect between PARP-1 Val762Ala and XRCC1 Arg399Gln genotypes on the risk of cancer was addressed in the present study. However, the lack of individual data from the included studies limited the further evaluation of other potential interactions, as in other genes and environment factors. For instance, only two studies have reported the combined effect of XRCC1 Arg194Trp and PARP-1 Val762Ala genotypes on the risk of cancer [25], [32]. Third, only articles written in English were included; as such, bias may be observed in our meta-analysis.
In conclusion, the present meta-analysis provided strong evidence of the association of PARP-1 Val762Ala with increased cancer risk among Asian populations. The same results were observed in the subgroups of gastric, cervical, and lung cancers, as well as in studies using PCR-RFLP genotyping method. Our findings suggested that the PARP-1 Val762Ala polymorphism may function in cancer development in an ethnicity- or cancer-specific manner. Well-designed epidemiological studies should be conducted by carefully matching cases and control subjects to verify our observations. Further studies may focus on the influence of gene-gene and gene-environment interactions on the association of cancer and PARP-1 Val762Ala polymorphism.
Supporting Information
PRISMA checklist.
(DOC)
Funding Statement
Research and Innovation Project for College Graduates of Jiangsu Province (No. CXZZ12_0588), the Natural Science Foundation of China (No. 81272504), the Innovation Team (No. LJ201123), Jiangsu Provincial Natural Science Fund (No. BK2011854), “333” Project of Jiangsu Province (No. BRA2012210), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) (No. JX10231801), the Key Academic Discipline of Jiangsu Province “Medical Aspects of Specific Environments”, and the Six Major Talent Peak Project of Jiangsu Province (No. 2013-WSN-040). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goode EL, Ulrich CM, Potter JD (2002) Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev 11: 1513–1530. [PubMed] [Google Scholar]
- 3. Wood RD, Mitchell M, Sgouros J, Lindahl T (2001) Human DNA repair genes. Science 291: 1284–1289. [DOI] [PubMed] [Google Scholar]
- 4. Hoeijmakers JH (2001) Genome maintenance mechanisms for preventing cancer. Nature 411: 366–374. [DOI] [PubMed] [Google Scholar]
- 5. Lockett KL, Hall MC, Xu J, Zheng SL, Berwick M, et al. (2004) The ADPRT V762A genetic variant contributes to prostate cancer susceptibility and deficient enzyme function. Cancer Res 64: 6344–6348. [DOI] [PubMed] [Google Scholar]
- 6. Caldecott KW, Aoufouchi S, Johnson P, Shall S (1996) XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly (ADP-ribose) polymerase, and DNA ligase III is a novel molecular 'nick-sensor' in vitro. Nucleic Acids Res 24: 4387–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El-Khamisy SF, Masutani M, Suzuki H, Caldecott KW (2003) A requirement for PARP-1 for the assembly or stability of XRCC1 nuclear foci at sites of oxidative DNA damage. Nucleic Acids Res 31: 5526–5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim MY, Zhang T, Kraus WL (2005) Poly(ADP-ribosyl)ation by PARP-1: 'PAR-laying' NAD+ into a nuclear signal. Genes Dev 19: 1951–1967. [DOI] [PubMed] [Google Scholar]
- 9. Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, et al. (2002) Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297: 259–263. [DOI] [PubMed] [Google Scholar]
- 10. Masutani M, Nakagama H, Sugimura T (2005) Poly(ADP-ribosyl)ation in relation to cancer and autoimmune disease. Cell Mol Life Sci 62: 769–783. [DOI] [PubMed] [Google Scholar]
- 11. Shiokawa M, Masutani M, Fujihara H, Ueki K, Nishikawa R, et al. (2005) Genetic alteration of poly(ADP-ribose) polymerase-1 in human germ cell tumors. Jpn J Clin Oncol 35: 97–102. [DOI] [PubMed] [Google Scholar]
- 12. Bieche I, de Murcia G, Lidereau R (1996) Poly(ADP-ribose) polymerase gene expression status and genomic instability in human breast cancer. Clin Cancer Res 2: 1163–1167. [PubMed] [Google Scholar]
- 13. Zaremba T, Ketzer P, Cole M, Coulthard S, Plummer ER, et al. (2009) Poly(ADP-ribose) polymerase-1 polymorphisms, expression and activity in selected human tumour cell lines. Br J Cancer 101: 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith TR, Levine EA, Freimanis RI, Akman SA, Allen GO, et al. (2008) Polygenic model of DNA repair genetic polymorphisms in human breast cancer risk. Carcinogenesis 29: 2132–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miao X, Zhang X, Zhang L, Guo Y, Hao B, et al. (2006) Adenosine diphosphate ribosyl transferase and x-ray repair cross-complementing 1 polymorphisms in gastric cardia cancer. Gastroenterology 131: 420–427. [DOI] [PubMed] [Google Scholar]
- 16. Zhang X, Miao X, Liang G, Hao B, Wang Y, et al. (2005) Polymorphisms in DNA base excision repair genes ADPRT and XRCC1 and risk of lung cancer. Cancer Res 65: 722–726. [PubMed] [Google Scholar]
- 17. Roszak A, Lianeri M, Sowinska A, Jagodzinski PP (2013) Involvement of PARP-1 Val762Ala polymorphism in the onset of cervical cancer in caucasian women. Mol Diagn Ther 17: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y, Li S, Wu Z, Hu F, Zhu L, et al. (2013) Polymorphisms in genes of APE1, PARP1, and XRCC1: risk and prognosis of colorectal cancer in a northeast Chinese population. Med Oncol 30: 505. [DOI] [PubMed] [Google Scholar]
- 19. Santonocito C, Scapaticci M, Penitente R, Paradisi A, Capizzi R, et al. (2012) Polymorphisms in base excision DNA repair genes and association with melanoma risk in a pilot study on Central-South Italian population. Clin Chim Acta 413: 1519–1524. [DOI] [PubMed] [Google Scholar]
- 20. Pabalan N, Francisco-Pabalan O, Jarjanazi H, Li H, Sung L, et al. (2012) Racial and tissue-specific cancer risk associated with PARP1 (ADPRT) Val762Ala polymorphism: a meta-analysis. Mol Biol Rep 39: 11061–11072. [DOI] [PubMed] [Google Scholar]
- 21. Yu H, Ma H, Yin M, Wei Q (2012) Association between PARP-1 V762A polymorphism and cancer susceptibility: a meta-analysis. Genet Epidemiol 36: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi JE, Park SH, Jeon HS, Kim KM, Lee GY, et al. (2003) No association between haplotypes of three variants (codon 81, 284, and 762) in poly(ADP-ribose) polymerase gene and risk of primary lung cancer. Cancer Epidemiol Biomarkers Prev 12: 947–949. [PubMed] [Google Scholar]
- 23. Haiman CA, Hsu C, de Bakker PI, Frasco M, Sheng X, et al. (2008) Comprehensive association testing of common genetic variation in DNA repair pathway genes in relationship with breast cancer risk in multiple populations. Hum Mol Genet 17: 825–834. [DOI] [PubMed] [Google Scholar]
- 24. Nakao M, Hosono S, Ito H, Watanabe M, Mizuno N, et al. (2012) Selected polymorphisms of base excision repair genes and pancreatic cancer risk in Japanese. J Epidemiol 22: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan XF, Xie Y, Loh M, Yang SJ, Wen YY, et al. (2012) Polymorphisms of XRCC1 and ADPRT genes and risk of noncardia gastric cancer in a Chinese population: a case-control study. Asian Pac J Cancer Prev 13: 5637–5642. [DOI] [PubMed] [Google Scholar]
- 26. Dent P (2013) PARP inhibitors are not all equal. Cancer Biol Ther 14: 873–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Huang L, Xu Y, Shi Z, Wang Y, et al. (2012) Association between survivin -31G > C promoter polymorphism and cancer risk: a meta-analysis. Eur J Hum Genet 20: 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cottet F, Blanche H, Verasdonck P, Le Gall I, Schachter F, et al. (2000) New polymorphisms in the human poly(ADP-ribose) polymerase-1 coding sequence: lack of association with longevity or with increased cellular poly(ADP-ribosyl)ation capacity. J Mol Med (Berl) 78: 431–440. [DOI] [PubMed] [Google Scholar]
- 29. Wang XG, Wang ZQ, Tong WM, Shen Y (2007) PARP1 Val762Ala polymorphism reduces enzymatic activity. Biochem Biophys Res Commun 354: 122–126. [DOI] [PubMed] [Google Scholar]
- 30. Jiang J, Zhang X, Yang H, Wang W (2009) Polymorphisms of DNA repair genes: ADPRT, XRCC1, and XPD and cancer risk in genetic epidemiology. Methods Mol Biol 471: 305–333. [DOI] [PubMed] [Google Scholar]
- 31. Ghabreau L, Roux JP, Frappart PO, Mathevet P, Patricot LM, et al. (2004) Poly(ADP-ribose) polymerase-1, a novel partner of progesterone receptors in endometrial cancer and its precursors. Int J Cancer 109: 317–321. [DOI] [PubMed] [Google Scholar]
- 32. Wen YY, Pan XF, Loh M, Tian Z, Yang SJ, et al. (2012) ADPRT Val762Ala and XRCC1 Arg194Trp polymorphisms and risk of gastric cancer in Sichuan of China. Asian Pac J Cancer Prev 13: 2139–2144. [DOI] [PubMed] [Google Scholar]
- 33. Hosono S, Matsuo K, Ito H, Oze I, Hirose K, et al. (2013) Polymorphisms in base excision repair genes are associated with endometrial cancer risk among postmenopausal Japanese women. Int J Gynecol Cancer 23: 1561–1568. [DOI] [PubMed] [Google Scholar]
- 34. Xue X, Yin Z, Lu Y, Zhang H, Yan Y, et al. (2013) The joint effect of hOGG1, APE1, and ADPRT polymorphisms and cooking oil fumes on the risk of lung adenocarcinoma in Chinese non-smoking females. PLoS One 8: e71157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santos LS, Branco SC, Silva SN, Azevedo AP, Gil OM, et al. (2012) Polymorphisms in base excision repair genes and thyroid cancer risk. Oncol Rep 28: 1859–1868. [DOI] [PubMed] [Google Scholar]
- 36. Ye F, Cheng Q, Hu Y, Zhang J, Chen H (2012) PARP-1 Val762Ala polymorphism is associated with risk of cervical carcinoma. PLoS One 7: e37446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yuan H, Li H, Ma H, Niu Y, Wu Y, et al. (2012) Genetic polymorphisms in key DNA repair genes and risk of head and neck cancer in a Chinese population. Exp Ther Med 3: 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang L, Ruan Z, Hong Q, Gong X, Hu Z, et al. (2012) Single nucleotide polymorphisms in DNA repair genes and risk of cervical cancer: A case-control study. Oncol Lett 3: 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yosunkaya E, Kucukyuruk B, Onaran I, Gurel CB, Uzan M, et al. (2010) Glioma risk associates with polymorphisms of DNA repair genes, XRCC1 and PARP1. Br J Neurosurg 24: 561–565. [DOI] [PubMed] [Google Scholar]
- 40. Gao R, Price DK, Dahut WL, Reed E, Figg WD (2010) Genetic polymorphisms in XRCC1 associated with radiation therapy in prostate cancer. Cancer Biol Ther 10: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jin XM, Kim HN, Lee IK, Park KS, Kim HJ, et al. (2010) PARP-1 Val762Ala polymorphism is associated with reduced risk of non-Hodgkin lymphoma in Korean males. BMC Med Genet 11: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rajaraman P, Hutchinson A, Wichner S, Black PM, Fine HA, et al. (2010) DNA repair gene polymorphisms and risk of adult meningioma, glioma, and acoustic neuroma. Neuro Oncol 12: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang M, Qin C, Zhu J, Yuan L, Fu G, et al. (2010) Genetic variants of XRCC1, APE1, and ADPRT genes and risk of bladder cancer. DNA Cell Biol 29: 303–311. [DOI] [PubMed] [Google Scholar]
- 44. Liu Y, Scheurer ME, El-Zein R, Cao Y, Do KA, et al. (2009) Association and interactions between DNA repair gene polymorphisms and adult glioma. Cancer Epidemiol Biomarkers Prev 18: 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McKean-Cowdin R, Barnholtz-Sloan J, Inskip PD, Ruder AM, Butler M, et al. (2009) Associations between polymorphisms in DNA repair genes and glioblastoma. Cancer Epidemiol Biomarkers Prev 18: 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Q, Li Y, Li X, Zhou W, Shi B, et al. (2009) PARP-1 Val762Ala polymorphism, CagA+ H. pylori infection and risk for gastric cancer in Han Chinese population. Mol Biol Rep 36: 1461–1467. [DOI] [PubMed] [Google Scholar]
- 47. Chiang FY, Wu CW, Hsiao PJ, Kuo WR, Lee KW, et al. (2008) Association between polymorphisms in DNA base excision repair genes XRCC1, APE1, and ADPRT and differentiated thyroid carcinoma. Clin Cancer Res 14: 5919–5924. [DOI] [PubMed] [Google Scholar]
- 48. Berndt SI, Huang WY, Fallin MD, Helzlsouer KJ, Platz EA, et al. (2007) Genetic variation in base excision repair genes and the prevalence of advanced colorectal adenoma. Cancer Res 67: 1395–1404. [DOI] [PubMed] [Google Scholar]
- 49. Cao WH, Wang X, Frappart L, Rigal D, Wang ZQ, et al. (2007) Analysis of genetic variants of the poly(ADP-ribose) polymerase-1 gene in breast cancer in French patients. Mutat Res 632: 20–28. [DOI] [PubMed] [Google Scholar]
- 50. Figueroa JD, Malats N, Real FX, Silverman D, Kogevinas M, et al. (2007) Genetic variation in the base excision repair pathway and bladder cancer risk. Hum Genet 121: 233–242. [DOI] [PubMed] [Google Scholar]
- 51. Li C, Hu Z, Lu J, Liu Z, Wang LE, et al. (2007) Genetic polymorphisms in DNA base-excision repair genes ADPRT, XRCC1, and APE1 and the risk of squamous cell carcinoma of the head and neck. Cancer 110: 867–875. [DOI] [PubMed] [Google Scholar]
- 52. Stern MC, Conti DV, Siegmund KD, Corral R, Yuan JM, et al. (2007) DNA repair single-nucleotide polymorphisms in colorectal cancer and their role as modifiers of the effect of cigarette smoking and alcohol in the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev 16: 2363–2372. [DOI] [PubMed] [Google Scholar]
- 53. Landi S, Gemignani F, Canzian F, Gaborieau V, Barale R, et al. (2006) DNA repair and cell cycle control genes and the risk of young-onset lung cancer. Cancer Res 66: 11062–11069. [DOI] [PubMed] [Google Scholar]
- 54. Li C, Liu Z, Wang LE, Strom SS, Lee JE, et al. (2006) Genetic variants of the ADPRT, XRCC1 and APE1 genes and risk of cutaneous melanoma. Carcinogenesis 27: 1894–1901. [DOI] [PubMed] [Google Scholar]
- 55. Shen M, Zheng T, Lan Q, Zhang Y, Zahm SH, et al. (2006) Polymorphisms in DNA repair genes and risk of non-Hodgkin lymphoma among women in Connecticut. Hum Genet 119: 659–668. [DOI] [PubMed] [Google Scholar]
- 56. Wu X, Gu J, Grossman HB, Amos CI, Etzel C, et al. (2006) Bladder cancer predisposition: a multigenic approach to DNA-repair and cell-cycle-control genes. Am J Hum Genet 78: 464–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhai X, Liu J, Hu Z, Wang S, Qing J, et al. (2006) Polymorphisms of ADPRT Val762Ala and XRCC1 Arg399Glu and risk of breast cancer in Chinese women: a case control analysis. Oncol Rep 15: 247–252. [PubMed] [Google Scholar]
- 58. Zhang Y, Newcomb PA, Egan KM, Titus-Ernstoff L, Chanock S, et al. (2006) Genetic polymorphisms in base-excision repair pathway genes and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 15: 353–358. [DOI] [PubMed] [Google Scholar]
- 59. Hao B, Wang H, Zhou K, Li Y, Chen X, et al. (2004) Identification of genetic variants in base excision repair pathway and their associations with risk of esophageal squamous cell carcinoma. Cancer Res 64: 4378–4384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)