Abstract
This study examined neurofunctional correlates of reading by modulating semantic, lexical, and orthographic attributes of letter strings. It compared the spatio-temporal activity patterns elicited by real words (RW), pseudowords, orthographically regular, pronounceable nonwords (PN) that carry no meaning, and orthographically illegal, nonpronounceable nonwords (NN). A double-duty lexical decision paradigm instructed participants to detect RW while ignoring nonwords and to additionally respond to words that refer to animals (AW). Healthy social drinkers (N=22) participated in both alcohol (0.6 g/kg ethanol for men, 0.55 g/kg for women) and placebo conditions in a counterbalanced design. Whole-head MEG signals were analyzed with an anatomically-constrained MEG method. Simultaneously acquired ERPs confirm previous evidence. Spatio-temporal MEG estimates to RW and PN are consistent with the highly replicable left-lateralized ventral visual processing stream. However, the PN elicit weaker activity than other stimuli starting at ~230 ms and extending to the M400 (magnetic equivalent of N400) in the left lateral temporal area, indicating their reduced access to lexicosemantic stores. In contrast, the NN uniquely engage the right hemisphere during the M400. Increased demands on lexicosemantic access imposed by AW result in greater activity in the left temporal cortex starting at ~230 ms and persisting through the M400 and response preparation stages. Alcohol intoxication strongly attenuates early visual responses occipito-temporally overall. Subsequently, alcohol selectively affects the left prefrontal cortex as a function of orthographic and semantic dimensions, suggesting that it modulates the dynamics of the lexicosemantic processing in a top-down manner, by increasing difficulty of semantic retrieval.
Keywords: reading, anatomically-constrained MEG, ERP, N400, lexical decision, pseudowords
1. Introduction
Reading comprehension emerges from dynamic interactions among cortical areas comprised by a broadly generic neurofunctional system that is engaged by letter strings differing in semantic, lexical and orthographic attributes. This multidimensional process has been investigated extensively with neuroimaging methods such as event-related potentials (ERPs) and functional magnetic resonance imaging (fMRI) that draw on their respective advantages in temporal dynamics vs. spatial mapping.
A huge body of ERP literature (Kutas and Federmeier, 2011) has explored a negative deflection peaking at ~400 ms (N400) which is sensitive to low-level lexical and orthographic factors, as well as semantic, mnemonic, and contextual aspects when reading single words, sentences, and discourse-level text (Hagoort and van Berkum, 2007; Halgren, 1990; Kutas and Federmeier, 2000; Osterhout and Holcomb, 1995). The N400 is modulated by a broad range of influences and may reflect an attempt to access lexicosemantic networks and integrate potentially meaningful stimuli into the current context (Bentin et al., 1985; Federmeier and Laszlo, 2009; Halgren, 1990; Holcomb, 1993; Kutas and Federmeier, 2000; Van Petten and Luka, 2006), see (Kutas and Federmeier, 2011) for an extensive discussion. The N400 amplitude is commonly greater to pseudowords, orthographically regular, pronounceable nonwords (PN) that carry no meaning (e.g. blont), as compared to real words (RW) (Bentin et al., 1985; Holcomb et al., 2002; Smith and Halgren, 1987; Ziegler et al., 1997). Under exceptional circumstances the N400 can also be elicited by orthographically illegal, nonpronounceable nonwords (NN, e.g. kdzv) (Laszlo et al., 2012). However, the neural basis of these effects is not well understood, prompting questions such as: to what degree are real words and pseudowords subserved by the same neurofunctional network; at which spatiotemporal processing stage does the divergence take place; are orthographically legal nonwords (PN) and illegal letter strings (NN) processed by different neural networks; are there hemispheric laterality differences in subserving different types of letter strings? Intracranial EEG evidence is limited in that regard (Nobre and McCarthy, 1995), but it suggests that, whereas the NN do not elicit the N400, the N400 generated by PN in the anterior temporal lobe is somewhat smaller than the negativity to real words. MEG evidence obtained with the equivalent current dipole modeling approach indicates that the RW and PN elicit stronger left-lateralized N400 magnetic equivalent (M400) than NN in the superior temporal cortex (Vartiainen et al., 2011). Functional MRI studies generally report that PN and RW activate the same left-lateralized fronto-temporal network, commonly with greater prefrontal activation to PW than RW (Clark and Wagner, 2003; Gold and Buckner, 2002; Mechelli et al., 2005). Evidence on NN is less clear but it appears that whereas the RW and PW elicit a strongly left-lateralized activity, the NN tend to evoke bilateral activation (Henson et al., 2002; Tagamets et al., 2000; Vigneau et al., 2005), but see (Vinckier et al., 2007). However, even though the fMRI method is an excellent mapping tool, it cannot provide insight into the real time processing dynamics, making the correspondence between the observed activation patterns and the N400 ambiguous.
Studies in healthy social drinkers indicate that acute alcohol intoxication impairs cognitive control (Field et al., 2010; Finn, 2000). Neuroimaging evidence indicates that the anterior cingulate cortex (ACC), as a central node subserving conflict processing, is uniquely sensitive to alcohol intoxication in conflict-inducing tasks such as the Stroop task (Kovacevic et al., 2012; Marinkovic et al., 2012a). In a recent companion paper, we have reported that alcohol attenuates event-related theta power estimated to ACC during response conflict in a double-duty lexical decision task (Marinkovic et al., 2012b). Moreover, in agreement with their sensitivity to memory processes (Klimesch et al., 2001), theta oscillations seem to reflect lexicosemantic retrieval of word meaning as they are greater to RW in comparison to meaningless but orthographically legal PN. Acute alcohol intoxication attenuates theta power to RW but not PN, suggesting that it selectively affects lexicosemantic processing (Marinkovic et al., 2012b). This is consistent with behavioral evidence of alcohol effects on semantic memory access (Acheson et al., 1998; Maylor et al., 1990). There have been no reports, however, on whether N400 deflections to letter strings differing in meaning and orthographic legality are dissociated under alcohol intoxication. A single previous ERP study that examined acute alcohol effects on language processing used only real words in a priming paradigm (Marinkovic et al., 2004b). The reported alcohol-induced attenuation of the posterior N180 is suggestive of deficient prelexical processing whereas an amplified N400 may indicate increased difficulty in accessing lexicosemantic representations of real words. Whether real words and different types of nonwords would be differentially affected by alcohol remains an outstanding question, along with the need to gain more precise insight into the neural basis of such effects.
The principal objective of the current study was to examine spatiotemporal stages of processing of words and nonwords as a function of acute alcohol intoxication. To that end, we employed an anatomically-constrained MEG method that combines distributed source modeling of the MEG signal with structural MRI. The resulting "brain movies", statistical parametric maps of estimated cortical activity across time, provide estimates of the anatomical distribution of the underlying neural networks in a time-sensitive manner (Dale et al., 2000; Dhond et al., 2001; Marinkovic et al., 2003). Scalp EEG was recorded simultaneously with MEG from a limited montage for comparison purposes. Healthy social drinkers (N = 22) participated in a lexical decision task under both alcohol and placebo conditions in a counterbalanced design. They were asked to detect real words while ignoring nonwords consisting of both PN and NN (Fig. 1). In this double-duty paradigm, response conflict was induced by an additional requirement to respond to all real words that also referred to animals (AW). The design made it possible to investigate effects of meaning, orthographic regularity, and decision conflict on the underlying neural dynamics. This approach can reveal what type of information is accessed at different stages of reading and could contribute to the long-standing discussion of the models of reading (Coltheart et al., 2001; Harm and Seidenberg, 2004). Furthermore, pharmacological manipulation of alcohol could facilitate parsing different aspects of word/nonword processing and indicate whether lexicosemantic access is selectively vulnerable to the effects of intoxication.
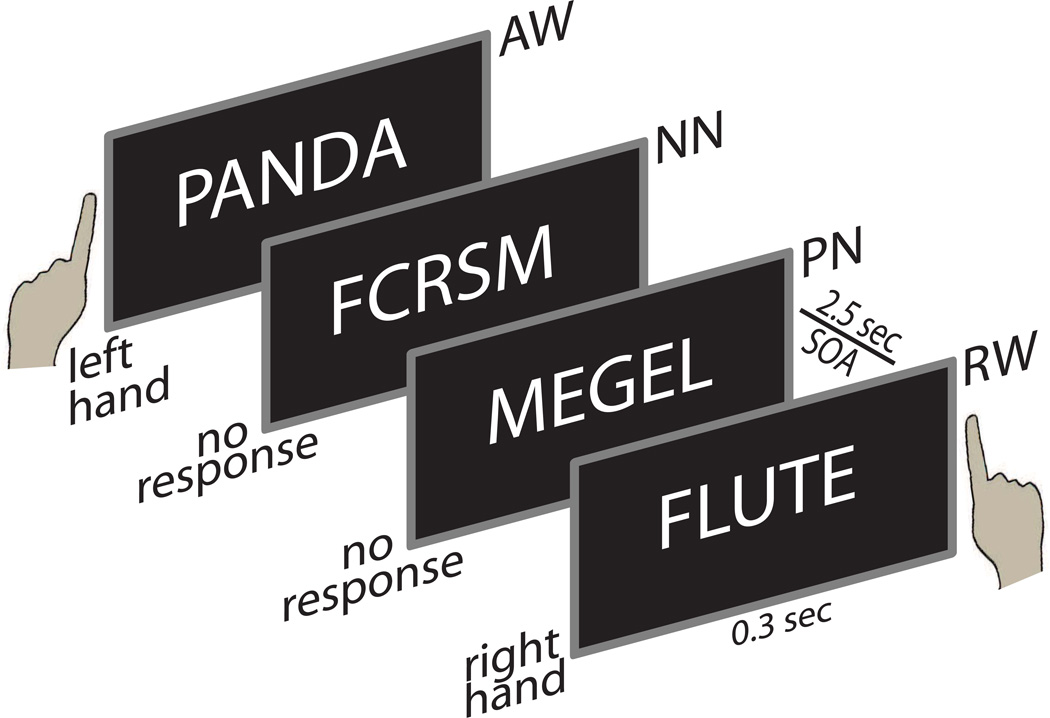
Fig. 1.
Examples of letter string types included in the double-duty lexical decision task. Participants are asked to respond to all real words (RW) with their right hand and to ignore pseudowords (pronounceable nonwords, PN) and nonpronounceable nonwords (NN). Response conflict is induced by the requirement to respond to words that refer to animals (AW) with left hand. Each stimulus is presented for 300 ms and is followed by a fixation string (xxxx) with total trial duration of 2.5 sec.
2. Results
2.1. Behavioral data
2.1.1. Task performance
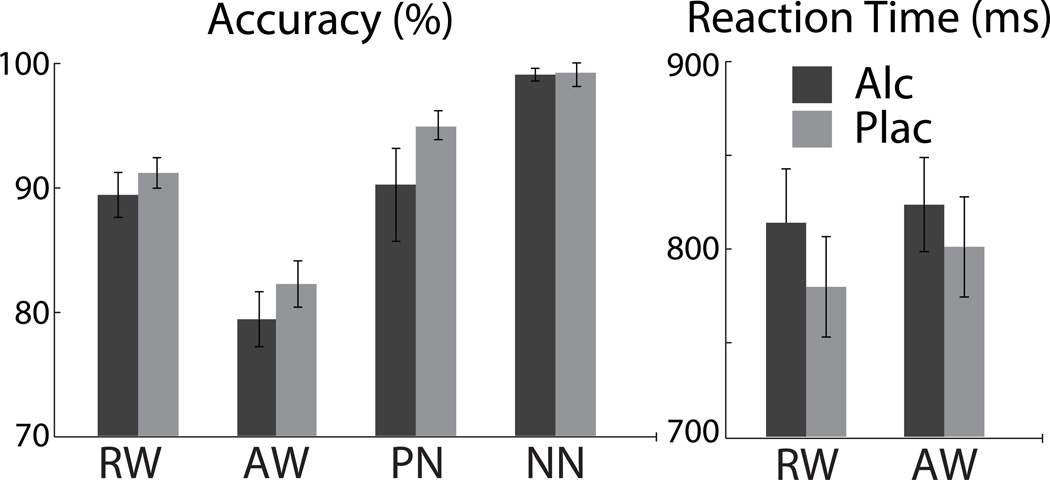
Performance accuracy differed across the four letter string types as indicated by the main effect of condition, F[3,63] = 20.0, p < 0.00001), with percent accuracy means (± SD) as follows: RW: 91.1 (± 7.2), AW: 83.2 (± 9.5), PN: 93.0% (± 13.0), and NN: 98.4% (± 3.5), Fig. 2. Accuracy was the lowest to AW, F[1,21] = 55.7, p < 0.00001 and the highest to NN as compared to other conditions, F[1,21] = 56.8, p < 0.00001. Subjects tended to be slower to respond to AW than to RW, F[1,21] = 3.9, p =0.06, with means (± SD) of 812 ± 118 ms and 797 ± 130 ms. Alcohol intoxication did not affect performance accuracy. However, the AW accuracy correlated negatively with blood alcohol levels measured just before the task, r = −0.56, p < 0.01, indicating that alcohol particularly affects demands on cognitive control. Alcohol intoxication increased RTs only marginally overall as indicated by a main effect of beverage, F[1,21] = 3.2, p < 0.1, with means (± SD) of 820 ± 125 ms and 791 ± 124 ms for alcohol and placebo sessions respectively.
Fig. 2.
Performance measures (means ± s.e.m.) reflected in percent accuracy and reaction times. Response accuracy was the lowest for AW and the highest for NN as compared to other conditions. Alcohol intoxication did not affect accuracy, but it marginally increased RTs overall.
2.1.2. Mood ratings and post-experimental questionnaire
Subjects rated their momentary moods and feelings with Biphasic Alcohol Effects Scale (BAES, Martin et al., 1993) on three occasions: prior to drinking (at baseline), immediately before, on ascending, and immediately after the task, on the descending limb of the breath alcohol concentration curve (BrAC). Overall, participants felt more stimulated before than after the task, F[1,21] = 15.6, p < 0.001. When asked to estimate how "high" they felt, participants' ratings were higher under alcohol than placebo on the ascending BrAC limb, F[1,21] = 10.1, p < 0.01.
On the scale from 1 (easy) to 5 (difficult), subjects rated the task as being moderately easy (2.3 ± 1.1) with no effects of alcohol on the perceived difficulty. Using Likert scales from 1 (certain that the beverage does not contain alcohol) to 5 (certain that it contains alcohol), subjects rated the beverage content as 4.9 ± 0.2 under alcohol and 1.5 ± 0.9 under placebo, Wilcoxon z = 4.2, p < 0.001. On the scale from 1 (not at all) to 5 (very much), participants reported feeling moderately intoxicated (2.9 ± 0.7) under alcohol, but not at all intoxicated under placebo (1.0 ± 0.2), Wilcoxon z = 4.1, p < 0.001. Subjects estimated that they were given 2.4 ± 0.8 "drinks" in alcohol session and 0.1 ± 0.4 "drinks" in placebo session. This estimate was slightly below the actual average amount of 2.8 standard drinks defined as 1.5 fl oz of vodka.
2.2. Spatiotemporal aMEG estimates
2.2.1. Effects of the letter string type
Group average maps of estimated activity to the four letter strings and across three successive time windows under placebo are displayed in Fig. 3. An alternative insight into the temporal dynamics of the activity estimated to the principal ROIs is provided by group average time courses shown in Fig. 4.
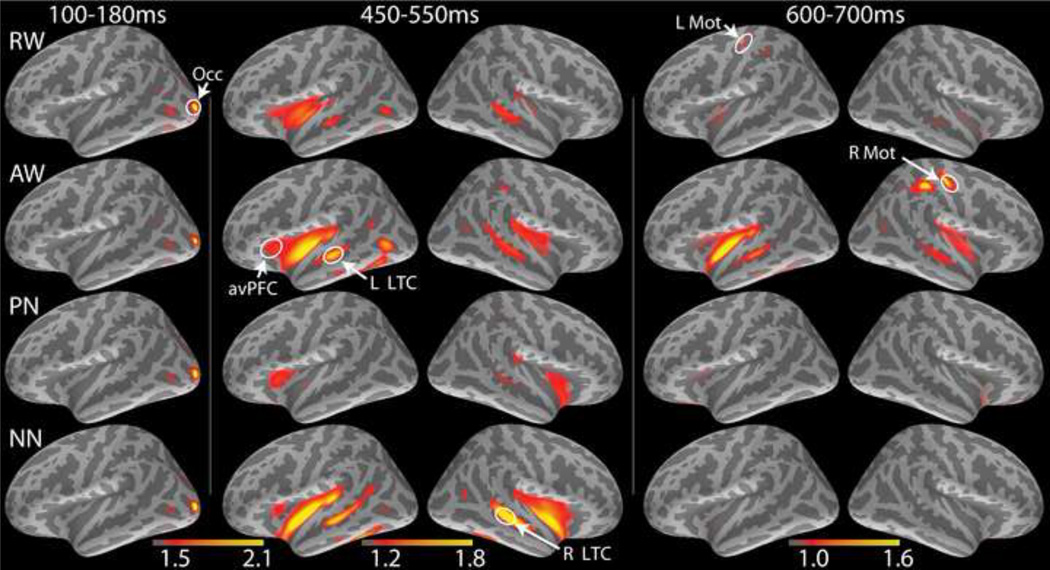
Fig. 3.
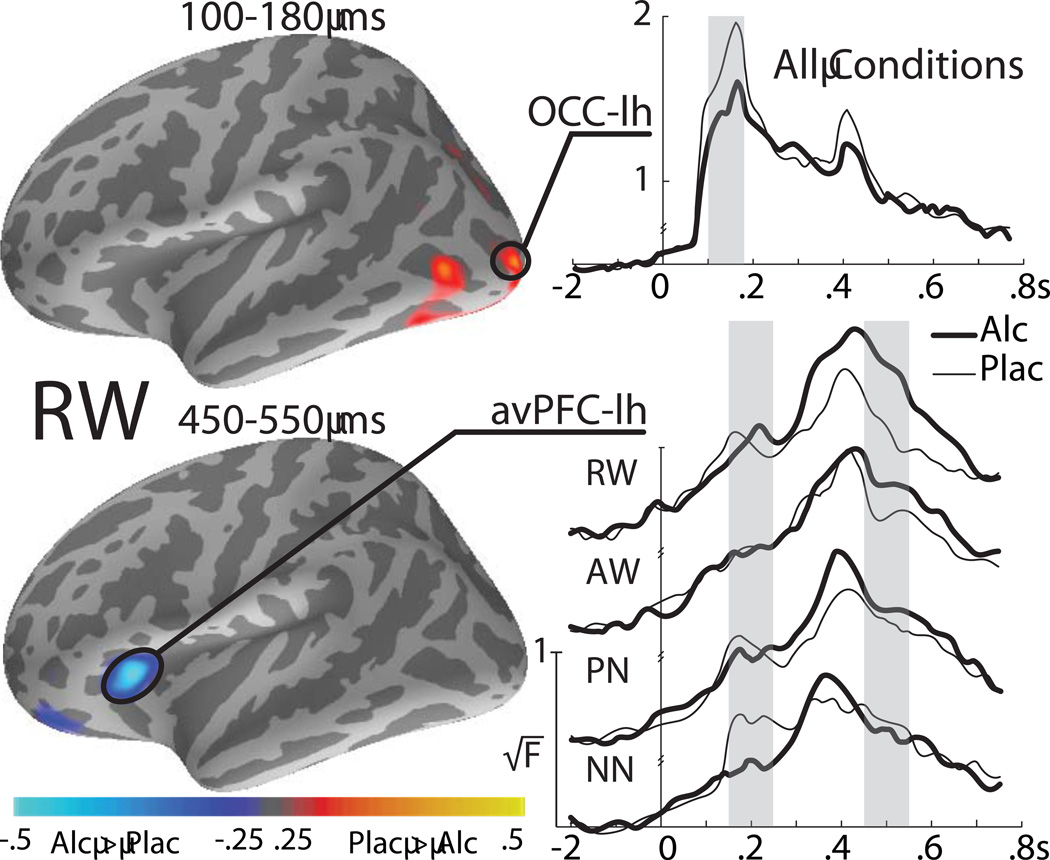
Effects of the letter string type on MEG source estimates: group average dynamic statistical parametric maps (dSPMs) of estimated responses to the four conditions across time under placebo. Subsequent to the early visual response (100 – 180 ms), activity spreads via the left-lateralized ventral visual stream encompassing frontotemporal areas. Activity during the 450 – 550 ms in the left LTC is the strongest to AW and weakest to PN. In contrast, the activity in the right LTC is uniquely sensitive to NN. Response preparation is reflected in the enhanced activity to RW and AW in the left and the right hand motor areas respectively. The maps reflect group averages of individual dSPM values (√F). Occ: occipital area; LTC: lateral temporal cortex; avPFC: anteroventral prefrontal cortex; Mot: hand motor area. RW: real words; AW: animal words; PN: pronounceable nonwords (pseudowords); NN: nonpronounceable nonwords.
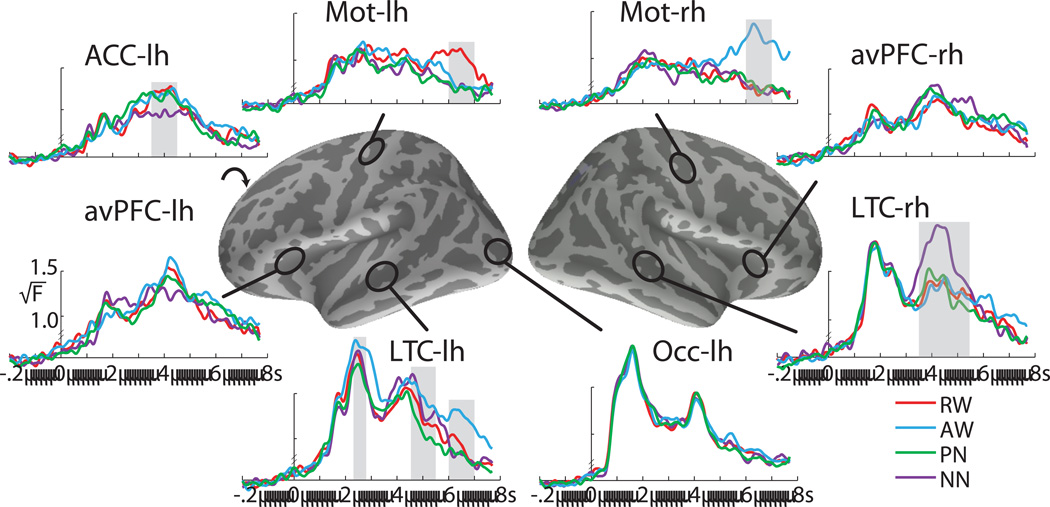
Fig. 4.
Group average time courses of the estimated MEG dipole strength for the four stimulus conditions for selected ROIs in both hemispheres. Vertical gray bars indicate the time windows during which condition-specific effects were observed under placebo. The estimated source amplitude is expressed as the group average (√F). Occ: occipital area; LTC: lateral temporal cortex; avPFC: anteroventral prefrontal cortex; ACC: anterior cingulate cortex; Mot: hand motor area. RW: real words; AW: animal words; PN: pronounceable nonwords (pseudowords); NN: nonpronounceable nonwords.
All four letter string types elicit identical early visual responses in the occipital cortex. Subsequently, activity spreads anteriorly via the left-lateralized ventral visual stream, in agreement with previous studies (Dhond et al., 2001; Dhond et al., 2003; Halgren et al., 2002; Marinkovic et al., 2003; Marinkovic, 2004). Following an early (~200 ms) condition- and beverage-sensitive activity in the left avPFC, a large amplitude activity peaks in the left lateral temporal cortex (LTC) by ~250 ms in a manner differentiated by letter string types. The magnetic equivalent of the N400 (M400) peaks at ~440 ms in the left LTC and avPFC with a similar profile across conditions except for the ACC where NN elicit the weakest activity. In the immediately following time window (450 – 550 ms) PN evoke the weakest activity in the left LTC. At the same time, the activity to NN is strongly right-lateralized to the LTC. Finally, the left and the right motor areas are selectively active to RW and AW respectively at ~650 ms and reflect motor preparation and execution (Figs 3 and 4). Repeated measures ANOVAs were carried out across ROIs to examine effects of letter string type and beverage. Strict standards were applied for statistical significance, with Bonferroni-corrected p-values reported for all condition and beverage comparisons.
The earliest main effect of letter string type under placebo is evident in the left LTC in the 230–280 ms time window, F[3,63] = 4.8, p < 0.01 (Fig. 4). It is due to the strongest activity to AW, F[1,21] = 10.0, p < 0.05 and the weakest activity to PN compared to all other letter strings, F[1,21] = 13.7, p < 0.01, suggesting that the LTC may be engaged in lexical access at this stage. Immediately preceding the M400 peak, during the 350–450 ms latency window, the main effect of stimulus type in the ACC, F[3,63] = 3.4, p < 0.05, is due to a weaker activation to NN than other conditions, F[1,21] = 14.0, p < 0.01, consistent with its role in detecting incongruity. However, during the subsequent time interval (450–550 ms), the main effect in the left LTC, F[3,63] = 4.2, p < 0.01, is due to a weaker activity to PN than other stimuli, F[1,21] = 13.2, p < 0.01. In the right hemisphere, the strongest and condition specific activity can be observed during the entire M400 time window (350–550 ms), as indicated by the main effect of condition, F[3,63] = 9.7, p < 0.0001. The right LTC is selectively sensitive to NN, which elicit a much stronger activity than other stimuli, F[1,21] = 20.0, p < 0.001. Response preparation (600–700 ms) is reflected in the enhanced activity to AW and RW in the hand motor areas bilaterally (Figs 3 and 4). During this stage, RW elicit greater activity than all other stimuli in the left motor area, F[1,21] = 26.4, p < 0.0001. Conversely, the AW evoke stronger activity in the right motor area than other stimuli, F[1,21] = 8.9, p < 0.05. At this time, the left LTC, F[1,21] = 17.4, p < 0.001, and the right ACC, F[1,21] = 14.8, p < 0.01 were also more active on AW trials, likely contributing to response preparation (Kovacevic et al., 2012).
2.2.2. Effects of alcohol intoxication
Overall, the present findings replicate and extend the results of our ERP study (Marinkovic et al., 2004b), showing that alcohol attenuates early sensory activity and augments M400 in the left prefrontal cortex (Fig. 5). Main effect of beverage is observed in the occipital cortex in the initial time window (100–180 ms), F[1,21] = 11.9, p < 0.01, indicating that alcohol decreases early activity overall. A surprisingly early effect of alcohol is also seen in the left avPFC in the form of a stimulus type×beverage interaction, F[3,63] = 4.6, p < 0.01 during the 150–250 ms time window. The activity to NN is decreased by intoxication, F[1,21] = 7.4, p = 0.05, causing its magnitude to be weaker than to other stimuli under alcohol, F[1,21] = 9.5, p < 0.05. In the left LTC in the subsequent latency window, condition-specific effects under alcohol replicate those observed under placebo, with AW eliciting the strongest and PN the weakest activity magnitude. Also replicated is the observation of the strongest activity to NN in the right LTC during the M400 interval. The only effect specific to alcohol intoxication during the 450–550 ms time-window is seen in the left avPFC (Fig. 5). The main effect of stimulus type under alcohol, F[3,63] = 3.5, p < 0.05, is due to greater activity to RW compared to other stimuli, F[1,21] = 10.8, p < 0.05. Compared to placebo, alcohol tends to increase the RW activity in the left avPFC at this time, F[1,21] = 6.8, p < 0.07. There are no alcohol-specific effects on the motor preparation stage during the 600–700 ms time window.
Fig. 5.
Effects of alcohol intoxication on MEG estimates: group average dSPMs of the placebo - alcohol differences (left column) and average time courses of the estimated dipole strength in the left occipital and avPFC ROIs (right panel). Alcohol intoxication attenuates early visual activity overall in the occipitotemporal cortex. In the left avPFC alcohol attenuates activity to NN during an early latency window and it augments later activity to real words. Gray bars denote time windows during which there were significant effects of alcohol on activity estimates. Occ: occipital area; avPFC: anteroventral prefrontal cortex.
2.3. Scalp ERPs
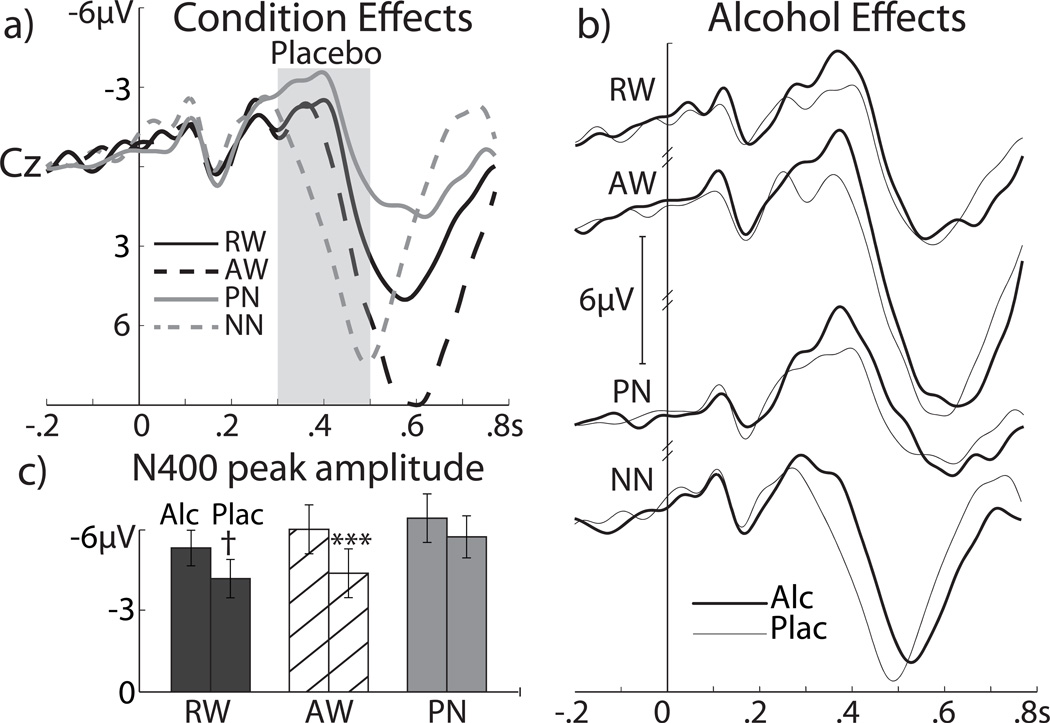
Fig. 6a shows grand average ERPs to the four letter string conditions under placebo. The waveforms start to diverge at ~300 ms with the negativity to NN peaking earlier (at 296 ms) than that to other letter strings (at 368 ms), F[1,19] = 41.2, p < 0.0001. Consistent with previous reports, PN evoke the largest negativity under placebo in the N400 time window, F[1,19] = 8.2, p < 0.01. The main effect of beverage, F[1,19] = 8.8, p < 0.01 is due to a greater negativity under alcohol as compared to placebo (Fig. 6b). This alcohol-induced enhancement of the N400 is particularly significant for the AW, F[1,19] = 19.8, p < 0.001, and is only a weak trend for the RW, F[1,19] = 3.8, p < 0.07. Alcohol also affects the N400 latency as a function of the letter string type. The beverage×condition interaction for the N400 latency, F[3,57] = 3.7, p < 0.05 is primarily due to longer latencies under alcohol for the PN, F[1,19] = 11.5, p < 0.05, compared to other conditions. The late positivity peaks at ~570 ms for all conditions. Alcohol intoxication affects only NN as the late positivity peaks earlier under placebo (~500 ms) than alcohol (~540), F[1,19] = 12.1, p < 0.05. The amplitude of the late positivity is the largest to AW (10.1 µV) and smallest to PN (3.5 µV) but is not affected by intoxication.
Fig. 6.
Effects of the letter string type and alcohol intoxication on scalp ERPs at Cz. Panel a): grand average ERP waveforms to the four letter strings under placebo. NN do not evoke the N400, with peak latency of 296 ms. In contrast, the PN evoke the largest N400 amplitude. Panel B: grand average waveforms obtained under alcohol and placebo are superimposed for the four conditions. Panel C: bar graphs of the N400 peak amplitudes elicited by the RW, AW, and PN conditions show that alcohol increases the N400 amplitude to AW and marginally to RW only. Shading of the bar graphs corresponds to the lines in a). Alcohol and placebo bars for each condition are positioned on the left and right respectively. Negative is up.
3. Discussion
The overall spatiotemporal pattern of activity to real words and pseudowords observed in this study is consistent with the highly replicable left-lateralized visual word processing activity that starts in the occipital cortex, advances along the ventral visual stream and engages lateral temporal and anteroventral prefrontal cortices (Dale et al., 2000; Dhond et al., 2001; Dhond et al., 2003; Halgren et al., 2002; Marinkovic et al., 2003). In contrast, orthographically illegal letter strings (NN) are subserved by a partially different neural network with distinctive contributions from the right temporal cortex. Confirming and extending our previous ERP findings (Marinkovic et al., 2004b), alcohol intoxication attenuates early sensory processing overall and amplifies activity during later lexicosemantic stage particularly to meaningful words.
3.1. Effects of meaning, orthographic regularity, and response conflict
The early visual response peak (at ~100 ms) is equivalent across all letter string types (Figs 3 and 4). Subsequently elicited processing stages, however, are differentially sensitive to lexicosemantic and pharmacological manipulation, especially in the lateral temporal (LTC) and anteroventral prefrontal (avPFC) cortices across both hemispheres. The spatiotemporal characteristics of these effects are described in greater detail here below.
3.1.1. Pseudowords (pronounceable nonwords, PN)
Our lexical decision paradigm employed only low frequency real words, assuring that responses were not made based on high familiarity with commonly used words. Therefore, PN served as good lures as they resemble real words but are not associated with any semantic representation. This is confirmed by performance accuracy of 91% and 93% for the RW and PN respectively. Only trials with correct responses were included in the analysis. The pattern of activity to PN is highly overlapping with that to real words. However, starting in the 230–280 ms window, the PN evoke weaker and the imperative AW elicit greater activity in the left LTC compared to other letter string types. This condition-based differentiation is partially consistent with an ERP study using rapid word presentation paradigm with the AW serving as targets (Martin-Loeches et al., 2001). In that study, ERP dipole modeling suggested a left ventral temporal source at ~250–300 ms with the strongest activity evoked by AW, followed by RW and PN, with the weakest activity to NN. These findings are consistent with proposals that the activity at around 250 ms may represent orthographic analysis and lexical access (Grainger and Holcomb, 2009; Martin-Loeches et al., 2001). Reliable repetition priming effects are also observed at this latency in the inferior temporal cortex, indicating engagement of the memory system (Halgren et al., 1994a; Halgren et al., 2006; Marinkovic et al., 2003). During the subsequent lexicosemantic processing stage reflected in the later part of the M400 time window (450–550 ms), the PN continue to elicit the weakest response in the left LTC (Figs 3 and 4). This observation is in contrast with the ERPs acquired simultaneously with MEG showing that the PN evoke the largest N400 amplitude of all other letter string types (Fig. 6). This ERP finding replicates numerous other reports of greater N400 to PN than real words (Bentin et al., 1985; Bentin et al., 1999; Holcomb et al., 2002; Smith and Halgren, 1987; Ziegler et al., 1997). MEG studies using equivalent current dipole modeling similarly find that PN elicit activity that is comparable or greater than that to RW at 400 ms in the posterior temporal cortex bilaterally (Vartiainen et al., 2011; Wydell et al., 2003). Such evidence has led to suggestions that the N400 reflects an attempt to access lexicosemantic stores, with the larger N400 to PN attributed to a more extensive search for word meaning triggered by pronounceable and hence potentially meaningful letter strings (Deacon et al., 2004; Federmeier and Laszlo, 2009; Halgren, 1990; Rugg and Nagy, 1987; Ziegler et al., 1997). fMRI studies often report greater activity to PN than to RW in the avPFC (Binder et al., 2005; Clark and Wagner, 2003; Gold and Buckner, 2002; Mechelli et al., 2005; Paulesu et al., 2000). Our aMEG estimates support the view that PN and real words are subserved by a common neural network (Glosser et al., 1998; Seidenberg and McClelland, 1989), as both real words and PN engage left-dominant lateral temporal and avPFC areas in a sustained manner from ~200 until ~550 ms. However, the consistently weaker activity to PN in the left LTC contradicts the ERP evidence of larger N400 to PN and argues against the interpretation of a more extensive search for meaning (Halgren, 1990; Ziegler et al., 1997). Instead, the present results corroborate the role of the LTC area in lexicosemantic retrieval whereby pseudowords elicit weaker activity because they carry no meaning (Marinkovic et al., 2012b). Such a view is in accordance with intracranial EEG studies (Nobre and McCarthy, 1995) and also with lesion evidence of impaired semantic comprehension at the single-word level after left temporal lobe lesions (Dronkers et al., 2004; Hart and Gordon, 1990). Gradients of temporal atrophy are associated with graded semantic deficits in semantic dementia patients (Mummery et al., 2000), confirming the role of the left LTC in semantic processing.
3.1.2. Orthographically illegal, nonpronounceable nonwords (NN)
Given their orthographic irregularity, the NN preclude phonological encoding and semantic search. However, our results indicate that they are processed within the same ventral visual stream as other letter strings until about ~300 ms, at which time they evoke the weakest activity in the ACC (Fig. 4). This finding is consistent with the ACC involvement in monitoring for discrepancies (Carter and van Veen, 2007), which was engaged by the double-duty nature of the task imposing demands on cognitive control. Indeed, in our previous aMEG studies, the ACC activity was weakest to those conditions that were least relevant to the task (Kovacevic et al., 2012; Marinkovic et al., 2011). In the immediately ensuing M400 time window, the activity to NN diverges dramatically from real words and pseudowords as they selectively elicit the M400 in the right LTC (Figs 3 and 4). The neuroimaging evidence on NN is rather limited, but it indicates that whereas the RW and PN evoke a strongly left-lateralized activity, the activity to NN tends to be more bilateral (Henson et al., 2002; Tagamets et al., 2000; Vigneau et al., 2005), consistent with the current findings. Right-lateralized activity is dominant to more unusual stimuli such as unfamiliar or false fonts in fMRI studies (Seghier and Price, 2011; Vinckier et al., 2007) as well as ERP and MEG investigations (Bentin et al., 1999; Proverbio et al., 2006; Vartiainen et al., 2011). Our ERP data clearly show that only meaningful or potentially meaningful stimuli elicit N400 (Fig. 6). The NN evoke an earlier negativity than words or pseudowords peaking at ~296 ms, in agreement with other studies (Bentin et al., 1999; Proverbio et al., 2004; Ziegler et al., 1997). Taken together, the evidence suggests that the NN are processed by the left-lateralized ventral visual stream along with other letter strings, but during the M400 latency window they uniquely engage the right hemisphere in a manner similar to unfamiliar fonts (Seghier and Price, 2011).
3.1.3. Response conflict (AW), response preparation and execution to RW and AW
The double-duty lexical decision paradigm instructed participants to respond to all real words (RW) with their right hand but to use their left hand when presented with words denoting animals (AW), which resulted in response conflict. In the companion paper analyzing theta oscillations (Marinkovic et al., 2012b), the AW evoked stronger event-related theta power in the ACC than other stimuli, in agreement with the essential contribution of ACC to theta generation (Cohen et al., 2008; Wang et al., 2005) and to response conflict (Carter and van Veen, 2007). In contrast, the event-related fields analyzed in the present study did not show such sensitivity to response conflict in the ACC. Overall, the RW and AW elicited the same spatiotemporal pattern but the left LTC was especially sensitive to AW which evoked the strongest response starting at ~230 ms and during the response preparation and execution stage. This could indicate increased demands on lexicosemantic access and retrieval. Even though only correct trials were included in the analysis, the increased demands could have resulted in part from the greater difficulty of the AW condition. This is indicated by the lowest performance accuracy and a tendency for slower RTs on AW trials. Latency of the earliest activity differentiating AW and RW starting at ~230 ms is consistent with other evidence obtained in a perceptual decision making task that manipulated difficulty. In an EEG study by Philiastides and colleagues (2006), participants were asked to categorize images of faces and cars that were degraded parametrically, making decisions progressively more difficult. Amplitude of a component at ~220 ms latency was highly correlated with the degree of categorization difficulty. The authors speculate that this component represents a top-down influence and that it could reflect recruitment of the attentional resources needed during making a difficult decision. Even though the underlying mechanism of this effect is not clear, it could be related to attention-induced increase in the gain of neural responses (Reynolds and Chelazzi, 2004) which is reflected in greater activation in the human visual and higher-level cognitive areas (Datta and DeYoe, 2009; Eldar et al., 2013; Sprague and Serences, 2013). This is consistent with suggestions that temporal lobe activity at this latency is modulated by increased demands on lexicosemantic access and by engagement of the memory system (Halgren et al., 2006; Martin-Loeches et al., 2001). Our results are also consistent with the idea that visual object attribute representations and the associated conceptual knowledge may be stored in the temporal lobe (Binder et al., 2009; Martin et al., 1996; Tranel et al., 2003). Intracranial electrophysiological recordings indicate that category-specific responses to animal words indeed evoke larger focal responses than objects in the anteroventral temporal lobe during these latencies (Chan et al., 2011). This type of evidence confirms that category-specific lexical access may preliminarily take place during the first pass of activity at ~230 ms.
Stronger activity to AW persists during the response preparation and execution time period in the LTC areas bilaterally (Figs 3 and 4). Concurrent with activity in the hand motor regions, the temporal cortex may contribute to semantic access and selection to ensure appropriate motor response, especially given the increased difficulty on AW trials. Left and right hand motor areas were selectively activated by the RW and AW respectively (Figs 3 and 4) during response preparation and execution, after ~600 ms. Temporal and motor areas may be engaged as a network in the service of maintaining the representation of imperative stimuli during response selection and execution. In addition, the AW elicited stronger activity in the right ACC during the response stage, indicating the ACC involvement in response control. Indeed, evidence obtained with the Stroop task indicates that the ACC is the only region showing differential activity during motor preparation as a function of induced conflict (Kovacevic et al., 2012). Its engagement in motor execution is made possible by its direct connections with the motor system (Picard and Strick, 1996) in the context of its top-down integrative role (Barbas, 2000; Ridderinkhof et al., 2004).
3.2. Alcohol intoxication affects early sensory and late lexicosemantic processing stages
Early visual responses estimated to the occipital cortex were strongly attenuated by alcohol intoxication across all stimulus types (Fig. 5). Different lines of evidence confirm the sensitivity of the visual system to moderate alcohol intoxication. fMRI studies show decreased activation of the visual areas under alcohol (Calhoun et al., 2004; Levin et al., 1998) and the strongest decrease in regional metabolism under alcohol is observed in occipital cortex (Wang et al., 2000). Furthermore, alcohol attenuates visual evoked potential amplitudes and contrast sensitivity (Marinkovic et al., 2004b; Pearson and Timney, 1998; Weschke and Niedeggen, 2012). Together with the present results, this evidence strongly indicates that moderate alcohol intoxication affects early sensory activity during visual stimulation. Indeed, single-unit recordings confirm reduced responsiveness and orientation selectivity of neurons in the primary visual cortex under alcohol (Chen et al., 2010). The depressant effects of alcohol are similarly observed in the thalamus (Hetzler et al., 1983), consistent with its inhibitory influence on glutamatergic thalamocortical projections (Nevo and Hamon, 1995). Alcohol enhances inhibitory effects of GABA (Moriguchi et al., 2007) which plays an important role in visual cell tuning (Ben-Yishai et al., 1995) and the spread of excitation in response to visual stimuli (Olivas et al., 2012). Therefore, the observed alcohol-induced reduction of the early visual activity may result from its direct modulation of the excitatory and inhibitory neurotransmitter systems involved in visual activity (Vengeliene et al., 2008).
The earliest condition-specific effect of alcohol can be seen in the left avPFC during the 150 – 250 ms time window (Fig. 5) where it attenuates activity to NN only. As a result, the activity to NN is weaker than to other stimuli under alcohol. Early latency prefrontal activity may represent top-down influence, changing the dynamics of the subsequent lexicosemantic access and retrieval during the M400. We have previously reported sensitivity of the left avPFC to inverse repetition priming (i.e. showing stronger activity to repeated words), (Marinkovic et al., 2003) suggesting involvement of the avPFC in the "early pass" processing stage. Alcohol affects prefrontal areas at this latency especially during conflict processing (Kovacevic et al., 2012). The current results suggest that alcohol intoxication interferes with the top-down influence on lexicosemantic processing.
In the left avPFC alcohol amplifies the M400 only to RW (Fig. 5) which may reflect greater difficulty of semantic access and retrieval under intoxication (Marinkovic et al., 2012b). Indeed, greater M400 is associated with longer RTs to RW under alcohol, r = 0.43, p < 0.05, but not under placebo, r = 0.28, n.s. in avPFC. Similarly, even though alcohol does not significantly reduce RW accuracy at a group average level (Fig 2), beverage-related differential avPFC activity during M400 is negatively correlated with differential accuracy to RW, r = −0.54, p < 0.01. In other words, worse performance accuracy under alcohol as compared to placebo is associated with greater alcohol-induced avPFC M400 activity. This alcohol-induced activity increase is evident only for RW and not for AW. A possible reason is that the difficulty of semantic access and retrieval was difficult overall on the AW trials under both beverage conditions. Overall, these findings confirm and extend the results of our ERP study (Marinkovic et al., 2004b) in which the early visual activity was attenuated and the N400 enhanced during a word recognition task. Whereas only real words were used in that study, the present experiment manipulated orthographic and semantic dimensions as well. Our results show that both the amplitude and the latency of the ERP N400 are affected by alcohol intoxication as a function of orthographic regularity and meaning. Alcohol increases the amplitude of the N400, particularly for the two real word conditions (Fig. 6), suggesting increased lexicosemantic access and retrieval. The N400 peak amplitude is negatively correlated with performance accuracy for the RW and AW under alcohol, r = −0.55, p < 0.01, but not placebo, r = −0.18, ns. Furthermore, alcohol increases the N400 latency to PN. Taken together, these results strongly suggest that acute alcohol intoxication selectively increases difficulty of semantic processing and corroborate behavioral evidence of alcohol-induced impairment of semantic memory access (Acheson et al., 1998; Maylor et al., 1990). Apart from our ERP study (Marinkovic et al., 2004b) we are not aware of any other electrophysiological investigation of the effects of acute alcohol effects on word processing. Deficits in semantic processing in alcohol-dependent individuals are also insufficiently investigated in contrast to well documented impairments in the executive domain (Moselhy et al., 2001; Oscar-Berman and Marinkovic, 2007; Sullivan and Pfefferbaum, 2005). Even though some evidence suggests that verbal skills are relatively well preserved in individuals with alcohol dependence (Oscar-Berman and Schendan, 2000; Parsons, 1987), ERP studies indicate deficits in semantic processing. Studies of abstinent long-term alcoholics indicate reduced N400 amplitudes, suggesting that such individuals do not benefit from priming induced by sentential or single-word paradigms (Ceballos et al., 2005; Nixon et al., 2002; Roopesh et al., 2010). Furthermore, similar priming deficiencies found in individuals with a family history of alcoholism (Roopesh et al., 2009) suggest a genetic component (Almasy et al., 2001). However, these studies investigated a limited number of conditions. The present paradigm, if employed in cohorts of long-term alcoholics or individuals at risk, could help illuminate the nature of the observed semantic deficits. Together with the studies of chronic alcoholics and individuals at risk, the effects of acute alcohol intoxication observed in this study indicate sensitivity of the neurofunctional system subserving lexicosemantic functions. Future studies will be needed to further parse out the effects of alcohol neurotoxicity, genetic susceptibility, and pharmacological influences on verbal processing.
3.3. Source aMEG estimates vs scalp ERP: functional profiles
Even though simultaneously recorded MEG and EEG reflect the same primary currents (Hamalainen et al., 1993), they differ in biophysical properties including large differences in leadfields, signal cancellation, propagation, sensitivity to source orientation and distance to sensors (Ahlfors et al., 2010a; Cuffin, 1990; Irimia et al., 2012; Liu et al., 2002; Marinkovic et al., 2004a). As a consequence, the MEG and EEG reveal different aspects of the underlying neural generators especially when these generators are distributed (Dehghani et al., 2010a; Dehghani et al., 2010b). Direct comparison is further precluded by methodological differences between source aMEG estimates and scalp ERPs. Nonetheless, a brief discussion of the most pertinent observations is in order. Our ERP findings replicate other evidence obtained in similar studies. More specifically, PN evoke larger N400 than other types of letter strings (Bentin et al., 1999; Holcomb et al., 2002). If a greater N400 to pronounceable and thus potentially meaningful PN indicates a more extensive search, this finding is compatible with proposals that the N400 reflects an attempt to access lexicosemantic stores (Deacon et al., 2004; Federmeier and Laszlo, 2009; Halgren, 1990; Rugg and Nagy, 1987; Ziegler et al., 1997). In contrast, the regionally differentiated aMEG estimates suggest that the activity to PN is weaker in the left LTC across successive processing stages. This is compatible with the view that the PN are less effective at the lexicosemantic access and retrieval as subserved by the left LTC (Marinkovic et al., 2012b). Unlike real words and pseudowords, the NN do not elicit an N400 but only an earlier N300 deflection under placebo (Bentin et al., 1999; Laszlo and Federmeier, 2009). In contrast, no such peak latency differences are obvious from the aMEG estimates. Instead, they indicate that the NN processing is subserved by a different neurofunctional system as they uniquely elicit right-lateralized activity in temporal cortex during the M400, similar to unfamiliar stimuli (Seghier and Price, 2011). Therefore, estimates of dipole strength to different conditions are sensitive to regional differences and they do not map directly to ERPs. Direct comparisons between results obtained with different neuroimaging methods are difficult for many reasons, even when the signals are simultaneously acquired, as was the case in the present study. The EEG and MEG signals differ in their biophysical properties including greater cancellation of the MEG signal compared to EEG, much greater leadfield for referential EEG than that of MEG planar gradiometers, and different sensitivities to source orientation (Ahlfors et al., 2010a; Ahlfors et al., 2010b; Irimia et al., 2012). Consequently, EEG and MEG are likely to reveal different aspects of the underlying generators when those generators are highly distributed as is the case with language processing. Furthermore, the uncertainty of source estimates is influenced by a variety of factors and different MEG inverse models provide very different estimates of the M400 even when applied on the same data set (Halgren et al., 2002). Nevertheless, the current results are consistent with previous evidence indicating that language is subserved by distributed temporo-prefrontal generators. Intracranial EEG studies have reported left-dominant N400 generators in the anterior temporal and ventrolateral prefrontal areas, suggesting that the N400 is not a singular entity, but a summation of volume-conducted activity of distributed simultaneously active generators (Halgren et al., 1994a; Halgren et al., 1994b; Nobre and McCarthy, 1995). The distributed nature of the N400-generating activity has been confirmed by aMEG studies showing that its magnetic counterpart (M400) is estimated to lie in the left-dominant temporo-prefrontal cortices (Dhond et al., 2001; Halgren et al., 2002; Helenius et al., 1998; Marinkovic et al., 2003; Marinkovic et al., 2011). Such estimates are consistent with extensive fMRI evidence showing that left-lateralized temporal and prefrontal areas are activated by language tasks (Binder et al., 2009; Liakakis et al., 2011; Price, 2010; Visser et al., 2010). Therefore, localization uncertainty notwithstanding, the present study has provided further insight into regional differentiation as a function of meaning and orthographic regularity and processing stages. Furthermore, the current aMEG estimates confirm previous ERP evidence on alcohol effects (Marinkovic et al., 2004b) and clarify that they seem to be generated in the left avPFC, resulting from alcohol-induced greater difficulty of semantic processing.
3.4. Conclusions and limitations
In summary, our results are consistent with models proposing that different types of letter strings are processed by a highly overlapping network (Tagamets et al., 2000). Parallel engagement of the temporo-fronto-cingulate network seems to indicate that semantic retrieval in the context of an executive task is subserved by a distributed system (Binder et al., 2009; Noppeney et al., 2004). The visual word processing is left-lateralized, with the exception of nonprounceable letter strings that uniquely engage the right temporal cortex. Alcohol intoxication attenuates the early visual activity and it increases prefrontal activity as a function of the difficulty of lexicosemantic access and retrieval.
One of the study’s limitations concerns the efficacy of placebo beverage administration. Participants were only told that the beverage may or may not contain alcohol. However, the post-experimental questionnaire indicates that they were able to deduce the type of beverage they were given. All of the participants have had experience with drinking mixed alcoholic beverages and they all drink regularly in social situations. Given their familiarity with the timeline and subjective effects of alcohol metabolism, they were able to discern the beverage content from a variety of sensations during the course of the entire session. Previous studies using balanced placebo design indicate that ERPs and autonomic physiological measures are influenced by the pharmacological effects of alcohol rather than expectancy (Marinkovic et al., 2000; Marinkovic et al., 2001; Marinkovic et al., 2004b). Nevertheless, the effects of intoxication should be considered within the larger context of the social setting and are likely a combination of both influences. Another limitation is an imprecise measurement of the BrAC. Because no electronic devices can be used inside magnetically shielded MEG chamber, the breathalyzer readings could not be obtained in a continuous manner and the peak BrAC must be inferred from pre- and post-experimental measurements but is not known.
4. Experimental procedure
4.1. Participants
All sessions of the experiment were completed by twenty-two participants (12 men, mean ± SD age = 24.9 ± 4.5 years) who were all healthy, right-handed, non-smoking native English speakers. Prior to the study, all prospective participants were carefully screened on a number of criteria and were excluded if they were left-handed, if they were under 21 years of age, if they were not native speakers of English, if they were on any medication, if they had a history of seizures or heady injury leading to loss of consciousness longer than 2 min, if they had a neurological or psychiatric disorder or other health problems, if they had been in treatment or arrested for drug or alcohol related offenses, if they smoked tobacco regularly or smoked within the previous month, if they had any implanted ferromagnetic objects, or if they suspected they were pregnant. Furthermore, they were excluded if they reported using illegal substances regularly or if they had used them within the previous 3 months.
The enrolled participants reported no alcohol- or drug-related problems, no previous head injury and no medication use at the time of the study. They described themselves as light social drinkers and reported drinking alcohol 2.0 ± 1.1 times per week and in low-to-moderate amounts (2.8 ± 0.9 drinks per occasion) (adapted Alcohol Use Questionnaire Cahalan et al., 1969). Men and women did not differ in the quantity or frequency of drinking. Participants reported no family history of alcohol or drug abuse (i.e. first or second degree relatives) and no alcoholism-related symptoms (Short Michigan Alcoholism Screening Test, SMAST Selzer et al., 1975). Before participation, all subjects gave written, informed consent approved by the relevant protection committee.
4.2. Double-duty lexical decision task
Lexical decision task relies on detecting real words mixed in among nonword letter strings and is a common probe of visual word recognition (Meyer and Schvaneveldt, 1971). Given the vulnerability of the executive system to alcohol intoxication, we additionally manipulated response conflict in a double-duty paradigm. This modified lexical decision task required subjects to respond to each real word (RW) with their right index finger unless the word denoted animals (AW), in which case they had to respond with their left index finger. They were asked to disregard all nonwords which were of two types: pseudowords were pronounceable nonwords (PN) that were orthographically and phonologically legal letter strings with no meaning, such as "stigor"; nonpronounceable nonwords (NN) were consonant strings such as "bxrtc". Participants were instructed to respond as quickly as possible, without losing accuracy. Two stimulus lists were created and used in a counterbalanced manner across subjects and beverage sessions. Stimuli were presented in a random order as white letters on a black background for 300 ms with Presentation software and were followed by a fixation string (xxxx) with a total trial duration of 2.5 sec. Short breaks were given every ~4 min. There were 110 stimuli in each condition. All stimuli were matched for the word length, RW: 6.3 ± 1.6 letters; AW: 6.2 ± 1.7, PN: 6.2 ± 1.7; NN: 6.0 ± 1.3. The AW and RW conditions were also matched for the number of syllables at 2.0 ± 0.7, and 2.1 ± 0.8 respectively. Finally, all meaningful words had low frequency of occurrence and were matched across the two conditions, AW: 5.5 ± 8.9; RW: 4.3 ± 2.4 per million (Francis and Kucera, 1982), although this database did not contain all animal words. Perceptual novelty (larger font) and repetition of RW and PW were additionally manipulated. However, because the results of these manipulations did not interact with the effects of beverage, they will not be discussed further here. Behavioral results are shown in Fig. 2.
4.3. Experimental Design and Procedure
Each subject completed two MEG recording sessions and underwent a structural MRI scan in addition to the introductory session. During the initial visit to the lab, participants provided detailed information about their current and past drinking habits, medical history, and filled out questionnaires. No beverage was administered at this time, but the subjects participated in a brief MEG recording and were familiarized with the experimental procedure with the purpose of reducing situation-induced arousal (Maltzman and Marinkovic, 1996).
Subsequently, the participants took part in both alcohol and placebo sessions in the within-subject design. The two experimental sessions were administered 16 ± 13 days apart on average in a counterbalanced manner so that half of the subjects received alcohol in the first and placebo in the second session. Both sessions followed the identical protocol with the exception of the administered beverage. Upon arrival to the laboratory, subjects were asked about their compliance with a requirement to abstain from food for 3 hours and alcohol for at least 48 hours prior to each session and practiced the task. Women were given a urine tests prior to each session to confirm that they were not pregnant. Subjects rated their momentary moods and feelings with the Biphasic Alcohol Effects Scale (BAES, Martin et al., 1993) prior to drinking (at baseline), immediately before and after the task, on the ascending and descending limbs of the breath alcohol concentration curve (BrAC) respectively. During the alcohol session, a beverage containing 0.60 g/kg of ethanol for men and 0.55 g/kg for women (Breslin et al., 1997) was administered as a cocktail of 20% v/v of vodka (Grey Goose, Bacardi Limited) mixed with orange juice. The placebo beverage contained the same volume of orange juice (Marinkovic et al., 2012a; Marinkovic et al., 2012b; Marinkovic et al., 2013). Breath alcohol concentration (BrAC) was measured with a breathalyzer (Draeger, Inc.) on multiple occasions during the session except when the subjects were inside the recording chamber. Since no electronic devices can be used inside the magnetically shielded MEG chamber, direct BrAC measurements could not be obtained during the actual task performance. The ascending BrAC measured before the task (22 ± 0.07 min after drinking), was 0.044% ± 0.012. The task was administered 38 ± 9 minutes after drinking and it encompassed the BrAC peak. By the time that it was measured again at 86 ± 11 min, the BrAC descended to 0.044% ± 0.011.
Upon task completion, the participants were asked to rate the contents of the beverage, the perceived task difficulty, and how intoxicated they felt on a 1 – 5 Likert scale. They also estimated the number of alcoholic drinks they imbibed in 0.5 drink increments. At the end of each session subjects were provided transportation to their homes. High-resolution structural MRI scans were obtained from all participants in a separate session. Further details can be found in the companion paper (Marinkovic et al., 2012b).
4.4. Data acquisition and analysis
4.4.1. MRI
Structural MRI images were acquired with a 3T Siemens Trio whole-body scanner (Siemens, Erlangen). For each subject, two high-resolution 3D MP-RAGE T1-weighted sequences were obtained with the following parameters: TR = 2.53 sec, TE = 3.25 ms, flip angle = 7 degrees, FOV = 256, 128 sagittal slices, 1.33 mm thickness, in-plane resolution 1×1 mm. Each subject's cortical surface was reconstructed from these images with FreeSurfer https://surfer.nmr.mgh.harvard.edu/ (Dale et al., 1999; Fischl et al., 1999a) and served to constrain inverse estimates, defining the solution space with 5124 free-rotating dipoles spaced ~7mm apart (Dale et al., 2000). The inner skull surface was derived from the segmented MRI data and used for a boundary element model of the volume conductor in the forward calculations (Hamalainen and Sarvas, 1989; Oostendorp and van Oosterom, 1991). For purposes of group averaging, the reconstructed individual surfaces were morphed into an average representation based on the sulcal-gyral pattern alignment (Fischl et al., 1999b).
4.4.2. MEG
High-density MEG signals were recorded from 204 channels comprising 102 pairs of planar gradiometers, with a whole-head Vectorview instrument (Elekta-Neuromag) in a magnetically and electrically shielded room. The signal was minimally filtered (0.5 to 100 Hz) and recorded continuously at a sampling rate of 601Hz. A precise co-registration with the structural MRI images was achieved by digitizing the nasion and preauricular points, the position of magnetic coils attached to the skull, and a large array of random points with a 3Space Isotrak II system. Data analysis relied on custom-made MATLAB (Mathworks, Natick, MA) routines and made use of publically available packages including EEGLAB (Delorme and Makeig, 2004) and FieldTrip (Oostenveld et al., 2011). Epochs extending −300 ms to 800 ms relative to stimulus onset were bandpass filtered 0.5 and 40 Hz, downsampled to 300 Hz, and baseline corrected using the prestimulus period as the baseline. A combination of independent component analysis (Delorme and Makeig, 2004) and automatic threshold rejection was used to remove epochs contaminated with eye-blinks and other artifacts. In order to eliminate potential statistical bias, the tallies of artifact-free correct trials were equalized across beverage and task conditions by removing superfluous trials at random. There were 88 ± 13 trials on average in each task condition.
Anatomically-constrained linear minimum-norm estimation procedure was used to obtain noise-normalized source estimates (Dale and Sereno, 1993; Dale et al., 2000; Hamalainen and Ilmoniemi, 1994). Noise covariance was calculated from the prestimulus periods across data epochs and used for inverse calculation, resulting in "brain movies", or dynamic statistical parametric maps (dSPM) of cortical current dipoles (Dale et al., 2000; Dhond et al., 2003; Marinkovic et al., 2003). The source estimates are expressed as the square root of an F-statistic reflecting the likelihood that a particular patch of cortex is more active than baseline at each timepoint. Group average dSPMs of the overall activity pattern are shown in Fig. 3, and the placebo vs. alcohol dSPM differences in Fig. 5. Region-of-interest (ROI) analysis was conducted in order to examine the temporal dynamics of the activity estimated at each cortical location and to ascertain the statistical reliability of the observed effects of the factors of task conditions and beverage (Fig. 4). Unbiased ROIs were selected based on the overall group average across all subjects, task and beverage conditions and were applied to all subjects by an automatic spherical morphing procedure (Fischl et al., 1999b) in a manner blind to each subject's activation pattern. The ROIs encompassed the left-lateralized fronto-temporal network associated with language, as well as the relevant subset of the comparable regions on the right (Fig. 4). ROIs included the lateral temporal cortex (LTC), anterior inferior prefrontal cortex (avPFC) bordering on insula, and motor cortex centered on hand area (Mot) bilaterally. The left lateral occipital (Occ) and the anterior cingulate cortex (ACC) were additionally included. Repeated measures ANOVAs (SPSS, 2001; Woodward et al., 1990) were carried out with the factors of condition (RW, AW, PN, NN) and beverage (alcohol, placebo) for both imaging and behavioral data. The factor of gender was also included, but since it exerted no significant effects, the reported results are pooled across the gender factor. The ANOVAs were performed for each ROI on the estimated noise-normalized dipole strength values averaged over time points in the following time windows reflecting successive processing stages peaking in different areas: early visual processing in the occipital cortex (100–180 ms), early top-down prelexical effects prefrontally (150–250 ms), lexical access and phonological recoding (230–280 ms) in the left temporal cortex, incongruence monitoring and lexicosemantic access (350–450 ms), lexicosemantic retrieval (450–550 ms) in the left frontal and bilateral temporal cortices, motor preparation (600–700 ms), Figs 3,4,5. Effects of letter string type are explored for the placebo condition in 2.2.1., whereas the effects of alcohol intoxication are described in 2.2.2. Bonferroni correction was used for all pairwise condition comparisons and only the corrected p-values are reported for those contrasts. The statistics pertaining to main effects and interactions are reported with their original values.
4.4.3. Scalp ERPs
EEG signal referenced to the nose was measured at Fz and Cz sites simultaneously with the MEG signal. The electrooculogram (EOG) was recorded with electrodes placed just above the nasion and at the outer canthus of the left eye. Electrode impedance was kept well below 5 kOhms. Good quality complete EEG data sets were acquired from twenty participants and analyzed with the signal-space analysis described above. Grand average ERPs recorded at Cz are shown in Fig. 6. Repeated measures ANOVA with the factors of condition and beverage was carried out on the peak latency and peak amplitudes of the negative deflections within the N400 (300–500 ms) time window and the positive deflections within the P600 (450–700 ms) latency range.
Anatomically-constrained MEG is used to examine neurofunctional correlates of reading
Healthy adults consume alcohol and placebo in a within-subject crossover design
Compared to real, pseudowords elicit weaker M400 in the left temporo-prefrontal area
Consonant strings uniquely engage the right hemisphere during the M400
Alcohol attenuates early visual activity and increases difficulty of semantic retrieval
Acknowledgments
This research was supported by funds from the National Institutes of Health (K01-AA13402 and R01-AA016624 (to KM), K01-MH079146 (to DJH), RR031599), and MIND Institute. The data were collected at Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, USA. We thank Jonathan Dan, Jason Sherfey, and Sanja Kovacevic for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson SK, Stein RM, Swartzwelder HS. Impairment of semantic and figural memory by acute ethanol: age- dependent effects. Alcohol Clin Exp Res. 1998;22:1437–1442. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Ahlfors SP, Han J, Belliveau JW, Hamalainen MS. Sensitivity of MEG and EEG to source orientation. Brain Topogr. 2010a;23:227–232. doi: 10.1007/s10548-010-0154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlfors SP, Han J, Lin FH, Witzel T, Belliveau JW, Hamalainen MS, Halgren E. Cancellation of EEG and MEG signals generated by extended and distributed sources. Hum Brain Mapp. 2010b;31:140–149. doi: 10.1002/hbm.20851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Porjesz B, Blangero J, Goate A, Edenberg HJ, Chorlian DB, Kuperman S, O'Connor SJ, Rohrbaugh J, Bauer LO, Foroud T, Rice JP, Reich T, Begleiter H. Genetics of event-related brain potentials in response to a semantic priming paradigm in families with a history of alcoholism. Am J Hum Genet. 2001;68:128–135. doi: 10.1086/316936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Ben-Yishai R, Bar-Or RL, Sompolinsky H. Theory of orientation tuning in visual cortex. Proc Natl Acad Sci U S A. 1995;92:3844–3848. doi: 10.1073/pnas.92.9.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, McCarthy G, Wood CC. Event-related potentials, lexical decision and semantic priming. Electroencephalogr Clin Neurophysiol. 1985;60:343–355. doi: 10.1016/0013-4694(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Bentin S, Mouchetant-Rostaing Y, Giard MH, Echallier JF, Pernier J. ERP manifestations of processing printed words at different psycholinguistic levels: Time course and scalp distribution. Journal of Cognitive Neuroscience. 1999;11:235–260. doi: 10.1162/089892999563373. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. Neuroimage. 2005;27:677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin FC, Kapur BM, Sobell MB, Cappell H. Gender and alcohol dosing: a procedure for producing comparable breath alcohol curves for men and women. Alcohol Clin Exp Res. 1997;21:928–930. doi: 10.1111/j.1530-0277.1997.tb03860.x. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. Monograph #6. New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. American drinking practices: A national study of drinking behavior and attitudes. [Google Scholar]
- Calhoun VD, Altschul D, McGinty V, Shih R, Scott D, Sears E, Pearlson GD. Alcohol intoxication effects on visual perception: An fMRI study. Hum Brain Mapp. 2004;21:298–299. doi: 10.1002/hbm.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, Houston RJ, Smith ND, Bauer LO, Taylor RE. N400 as an index of semantic expectancies: differential effects of alcohol and cocaine dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:936–943. doi: 10.1016/j.pnpbp.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Chan AM, Baker JM, Eskandar E, Schomer D, Ulbert I, Marinkovic K, Cash SS, Halgren E. First-pass selectivity for semantic categories in human anteroventral temporal lobe. J Neurosci. 2011;31:18119–18129. doi: 10.1523/JNEUROSCI.3122-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Xia J, Li G, Zhou Y. The effects of acute alcohol exposure on the response properties of neurons in visual cortex area 17 of cats. Toxicol Appl Pharmacol. 2010;243:348–358. doi: 10.1016/j.taap.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Clark D, Wagner AD. Assembling and encoding word representations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia. 2003;41:304–317. doi: 10.1016/s0028-3932(02)00163-x. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J. Medial frontal cortex and response conflict: evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res. 2008;1238:127–142. doi: 10.1016/j.brainres.2008.07.114. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: a dual route cascaded model of visual word recognition and reading aloud. Psychol Rev. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Cuffin BN. Effects of head shape on EEG's and MEG's. IEEE Trans Biomed Eng. 1990;37:44–52. doi: 10.1109/10.43614. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Datta R, DeYoe EA. I know where you are secretly attending! The topography of human visual attention revealed with fMRI. Vision Res. 2009;49:1037–1044. doi: 10.1016/j.visres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon D, Dynowska A, Ritter W, Grose-Fifer J. Repetition and semantic priming of nonwords: implications for theories of N400 and word recognition. Psychophysiology. 2004;41:60–74. doi: 10.1111/1469-8986.00120. [DOI] [PubMed] [Google Scholar]
- Dehghani N, Cash SS, Chen CC, Hagler DJ, Jr, Huang M, Dale AM, Halgren E. Divergent cortical generators of MEG and EEG during human sleep spindles suggested by distributed source modeling. PLoS One. 2010a;5:e11454. doi: 10.1371/journal.pone.0011454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani N, Cash SS, Rossetti AO, Chen CC, Halgren E. Magnetoencephalography demonstrates multiple asynchronous generators during human sleep spindles. J Neurophysiol. 2010b;104:179–188. doi: 10.1152/jn.00198.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dhond RP, Buckner RL, Dale AM, Marinkovic K, Halgren E. Spatiotemporal maps of brain activity underlying word generation and their modification during repetition priming. J Neurosci. 2001;21:3564–3571. doi: 10.1523/JNEUROSCI.21-10-03564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhond RP, Marinkovic K, Dale AM, Witzel T, Halgren E. Spatiotemporal maps of past-tense verb inflection. Neuroimage. 2003;19:91–100. doi: 10.1016/s1053-8119(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Eldar E, Cohen JD, Niv Y. The effects of neural gain on attention and learning. Nat Neurosci. 2013;16:1146–1153. doi: 10.1038/nn.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federmeier KD, Laszlo S. Time for Meaning: Electrophysiology Provides Insights into the Dynamics of Representation and Processing in Semantic Memory. Psychology of Learning and Motivation: Advances in Research and Theory. 2009;Vol 51:51, 1–44. [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC. Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcohol Clin Exp Res. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn P. Acute effects of alcohol on cognition and impulsive-disinhibited behavior. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's neuroscience and behavioral research portfolio. Vol 34. Bethesda MD: US Department of health and human services; 2000. pp. 337–356. [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency analysis of English usage: Lexicon and grammar. Vol. Boston: Houghton Mifflin; 1982. [Google Scholar]
- Glosser G, Friedman RB, Kohn SE, Sands L, Grugan P. Cognitive mechanisms for processing nonwords: evidence from Alzheimer's disease. Brain Lang. 1998;63:32–49. doi: 10.1006/brln.1997.1924. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Grainger J, Holcomb PJ. Watching the Word Go by: On the Time-course of Component Processes in Visual Word Recognition. Lang Linguist Compass. 2009;3:128–156. doi: 10.1111/j.1749-818X.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P, van Berkum J. Beyond the sentence given. Philos Trans R Soc Lond B Biol Sci. 2007;362:801–811. doi: 10.1098/rstb.2007.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E. Insights from evoked potentials into the neuropsychological mechanisms of reading. In: Scheibel AB, Wechsler AF, editors. Neurobiology of higher cognitive function. Vol. New York: Guilford; 1990. pp. 103–150. [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K. Spatio-temporal stages in face and word processing. I. Depth-recorded potentials in the human occipital, temporal and parietal lobes [corrected] [published erratum appears in J Physiol Paris 1994;88(2):following 151] J Physiol Paris. 1994a;88:1–50. doi: 10.1016/0928-4257(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Chauvel P. Spatio-temporal stages in face and word processing. 2. Depth-recorded potentials in the human frontal and Rolandic cortices [published erratum appears in J Physiol Paris 1994;88(2):following 151] J Physiol Paris. 1994b;88:51–80. doi: 10.1016/0928-4257(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, Van Petten C, Marinkovic K, Lewine JD, Dale AM. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. Neuroimage. 2002;17:1101–1116. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- Halgren E, Wang C, Schomer DL, Knake S, Marinkovic K, Wu J, Ulbert I. Processing stages underlying word recognition in the anteroventral temporal lobe. Neuroimage. 2006;30:1401–1413. doi: 10.1016/j.neuroimage.2005.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography - theory, instrumentation, and applications to noninvasive studies of the working human brain. Reviews of Modern Physics. 1993;65:413–497. [Google Scholar]
- Hamalainen MS, Sarvas J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng. 1989;36:165–171. doi: 10.1109/10.16463. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput. 1994;32:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Harm MW, Seidenberg MS. Computing the meanings of words in reading: cooperative division of labor between visual and phonological processes. Psychol Rev. 2004;111:662–720. doi: 10.1037/0033-295X.111.3.662. [DOI] [PubMed] [Google Scholar]
- Hart J, Jr, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990;27:226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Helenius P, Salmelin R, Service E, Connolly JF. Distinct time courses of word and context comprehension in the left temporal cortex. Brain. 1998;121:1133–1142. doi: 10.1093/brain/121.6.1133. [DOI] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Hetzler BE, Wiesman JM, Dobbs CM, Oaklay KE. Acute effects of alcohol on photic evoked potentials of rats: lateral geniculate nucleus and reticular formation. Pharmacol Biochem Behav. 1983;18(Suppl 1):483–487. doi: 10.1016/0091-3057(83)90222-8. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ. Semantic priming and stimulus degradation: implications for the role of the N400 in language processing. Psychophysiology. 1993;30:47–61. doi: 10.1111/j.1469-8986.1993.tb03204.x. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Grainger J, O'Rourke T. An electrophysiological study of the effects of orthographic neighborhood size on printed word perception. J Cogn Neurosci. 2002;14:938–950. doi: 10.1162/089892902760191153. [DOI] [PubMed] [Google Scholar]
- Irimia A, Van Horn JD, Halgren E. Source cancellation profiles of electroencephalography and magnetoencephalography. Neuroimage. 2012;59:2464–2474. doi: 10.1016/j.neuroimage.2011.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Stadler W, Pollhuber D, Sauseng P, Rohm D. Episodic retrieval is reflected by a process specific increase in human electroencephalographic theta activity. Neurosci Lett. 2001;302:49–52. doi: 10.1016/s0304-3940(01)01656-1. [DOI] [PubMed] [Google Scholar]
- Kovacevic S, Azma S, Irimia A, Sherfey J, Halgren E, Marinkovic K. Theta oscillations are sensitive to both early and late conflict processing stages: effects of alcohol intoxication. PLoS One. 2012;7:e43957. doi: 10.1371/journal.pone.0043957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends Cogn Sci. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annu Rev Psychol. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo S, Federmeier KD. A Beautiful Day in the Neighborhood: An Event-Related Potential Study of Lexical Relationships and Prediction in Context. J Mem Lang. 2009;61:326–338. doi: 10.1016/j.jml.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo S, Stites M, Federmeier KD. Won't get fooled again: An event-related potential study of task and repetition effects on the semantic processing of items without semantics. Lang Cogn Process. 2012;27:257–274. doi: 10.1080/01690965.2011.606667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin JM, Ross MH, Mendelson JH, Kaufman MJ, Lange N, Maas LC, Mello NK, Cohen BM, Renshaw PF. Reduction in BOLD fMRI response to primary visual stimulation following alcohol ingestion. Psychiatry Res. 1998;82:135–146. doi: 10.1016/s0925-4927(98)00022-5. [DOI] [PubMed] [Google Scholar]
- Liakakis G, Nickel J, Seitz RJ. Diversity of the inferior frontal gyrus--a meta-analysis of neuroimaging studies. Behav Brain Res. 2011;225:341–347. doi: 10.1016/j.bbr.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Liu AK, Dale AM, Belliveau JW. Monte Carlo simulation studies of EEG and MEG localization accuracy. Hum Brain Mapp. 2002;16:47–62. doi: 10.1002/hbm.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltzman I, Marinkovic K. Alcohol, alcoholism, and the autonomic nervous system: A critical account. In: Begleiter H, Kissin B, editors. The Pharmacology of Alcohol and Alcohol Dependence. Vol. New York: Oxford University Press; 1996. pp. 248–306. [Google Scholar]
- Marinkovic K, Halgren E, Klopp J, Maltzman I. Alcohol effects on movement-related potentials: a measure of impulsivity? J Stud Alcohol. 2000;61:24–31. doi: 10.15288/jsa.2000.61.24. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Halgren E, Maltzman I. Arousal-related P3a to novel auditory stimuli is abolished by moderately low alcohol dose. Alcohol and Alcoholism. 2001;36:529–539. doi: 10.1093/alcalc/36.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron. 2003;38:487–497. doi: 10.1016/s0896-6273(03)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K. Spatiotemporal dynamics of word processing in the human cortex. The Neuroscientist. 2004;10:142–152. doi: 10.1177/1073858403261018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Cox B, Reid K, Halgren E. Head position in the MEG helmet affects the sensitivity to anterior sources. Neurology and Clinical Neurophysiology. 2004a;2004:30. [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Halgren E, Maltzman I. Effects of alcohol on verbal processing: An ERP study. Alcohol Clin Exp Res. 2004b;28:415–423. doi: 10.1097/01.alc.0000117828.88597.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Baldwin S, Courtney MG, Witzel T, Dale AM, Halgren E. Right hemisphere has the last laugh: neural dynamics of joke appreciation. Cogn Affect Behav Neurosci. 2011;11:113–130. doi: 10.3758/s13415-010-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, Artsy E. Acute alcohol intoxication impairs top-down regulation of Stroop incongruity as revealed by blood oxygen level-dependent functional magnetic resonance imaging. Hum Brain Mapp. 2012a;33:319–333. doi: 10.1002/hbm.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rosen BQ, Cox B, Kovacevic S. Event-related theta power during lexical-semantic retrieval and decision conflict is modulated by alcohol intoxication: Anatomically-constrained MEG. Frontiers in Psychology. 2012b;3 doi: 10.3389/fpsyg.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, Artsy E, Lee AK. Effects of acute alcohol intoxication on saccadic conflict and error processing. Psychopharmacology (Berl) 2013;230:487–497. doi: 10.1007/s00213-013-3173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Loeches M, Hinojosa JA, Gomez-Jarabo G, Rubia FJ. An early electrophysiological sign of semantic processing in basal extrastriate areas. Psychophysiology. 2001;38:114–124. [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Rabbitt PM, James GH, Kerr SA. Comparing the effects of alcohol and intelligence on text recall and recognition. Br J Psychol. 1990;81:299–313. doi: 10.1111/j.2044-8295.1990.tb02363.x. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. J Neurosci. 2005;25:8303–8310. doi: 10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DE, Schvaneveldt RW. Facilitation in recognizing pairs of words: evidence of a dependence between retrieval operations. J Exp Psychol. 1971;90:227–234. doi: 10.1037/h0031564. [DOI] [PubMed] [Google Scholar]
- Moriguchi S, Zhao X, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on excitatory and inhibitory synaptic transmission in rat cortical neurons. Alcohol Clin Exp Res. 2007;31:89–99. doi: 10.1111/j.1530-0277.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int. 1995;26:305–336. doi: 10.1016/0197-0186(94)00139-l. discussion 337–42. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Tivis R, Ceballos N, Varner JL, Rohrbaugh J. Neurophysiological efficiency in male and female alcoholics. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:919–927. doi: 10.1016/s0278-5846(02)00206-3. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related field potentials in the anterior-medial temporal lobe: II. Effects of word type and semantic priming. J Neurosci. 1995;15:1090–1098. doi: 10.1523/JNEUROSCI.15-02-01090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Phillips J, Price C. The neural areas that control the retrieval and selection of semantics. Neuropsychologia. 2004;42:1269–1280. doi: 10.1016/j.neuropsychologia.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Olivas ND, Quintanar-Zilinskas V, Nenadic Z, Xu X. Laminar circuit organization and response modulation in mouse visual cortex. Front Neural Circuits. 2012;6:70. doi: 10.3389/fncir.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostendorp T, van Oosterom A. The potential distribution generated by surface electrodes in inhomogeneous volume conductors of arbitrary shape. IEEE Trans Biomed Eng. 1991;38:409–417. doi: 10.1109/10.81559. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Schendan HE. Asymmetries of brain function in alcoholism: Relationship to aging. In: Obler L, Connor LT, editors. Neurobehavior of language and cognition: Studies of normal aging and brain damage. Vol. New York: Kluwer Academic Publishers; 2000. [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout L, Holcomb P. Event-related potentials and language comprehension. In: Rugg MD, Coles MGH, editors. Electrophysiology of mind: Event-related brain potentials and cognition. Vol. Oxford: Oxford University Press; 1995. [Google Scholar]
- Parsons OA. Intellectual impairment in alcoholics: persistent issues. Acta Med Scand Suppl. 1987;717:33–46. doi: 10.1111/j.0954-6820.1987.tb13040.x. [DOI] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorusso M, Pesenti S, Gallagher A, Perani D, Price C, Frith CD, Frith U. A cultural effect on brain function. Nat Neurosci. 2000;3:91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- Pearson P, Timney B. Effects of moderate blood alcohol concentrations on spatial and temporal contrast sensitivity. J Stud Alcohol. 1998;59:163–173. doi: 10.15288/jsa.1998.59.163. [DOI] [PubMed] [Google Scholar]
- Philiastides MG, Ratcliff R, Sajda P. Neural representation of task difficulty and decision making during perceptual categorization: a timing diagram. J Neurosci. 2006;26:8965–8975. doi: 10.1523/JNEUROSCI.1655-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Vecchi L, Zani A. From orthography to phonetics: ERP measures of grapheme-to-phoneme conversion mechanisms in reading. J Cogn Neurosci. 2004;16:301–317. doi: 10.1162/089892904322984580. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Zotto MD, Zani A. Greek language processing in naive and skilled readers: Functional properties of the VWFA investigated with ERPs. Cogn Neuropsychol. 2006;23:355–375. doi: 10.1080/02643290442000536. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Roopesh BN, Rangaswamy M, Kamarajan C, Chorlian DB, Stimus A, Bauer LO, Rohrbaugh J, O'Connor SJ, Kuperman S, Schuckit M, Porjesz B. Priming deficiency in male subjects at risk for alcoholism: the N4 during a lexical decision task. Alcohol Clin Exp Res. 2009;33:2027–2036. doi: 10.1111/j.1530-0277.2009.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopesh BN, Rangaswamy M, Kamarajan C, Chorlian DB, Pandey AK, Porjesz B. Reduced resource optimization in male alcoholics: N400 in a lexical decision paradigm. Alcohol Clin Exp Res. 2010;34:1905–1914. doi: 10.1111/j.1530-0277.2010.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Nagy ME. Lexical contribution to nonword-repetition effects: evidence from event-related potentials. Mem Cognit. 1987;15:473–481. doi: 10.3758/bf03198381. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Price CJ. Explaining left lateralization for words in the ventral occipitotemporal cortex. J Neurosci. 2011;31:14745–14753. doi: 10.1523/JNEUROSCI.2238-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychol Rev. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, Van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) Journal of Studies on Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Smith ME, Halgren E. Event-related potentials during lexical decision: effects of repetition, word frequency, pronounceability, and concreteness. Electroencephalogr Clin Neurophysiol Suppl. 1987;40:417–421. [PubMed] [Google Scholar]
- Sprague TC, Serences JT. Attention modulates spatial priority maps in the human occipital, parietal and frontal cortices. Nat Neurosci. 2013;16:1879–1887. doi: 10.1038/nn.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS. SPSS for Windows. Vol. Chicago, Il: SPSS Inc.; 2001. [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Tagamets MA, Novick JM, Chalmers ML, Friedman RB. A parametric approach to orthographic processing in the brain: an fMRI study. J Cogn Neurosci. 2000;12:281–297. doi: 10.1162/089892900562101. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Eichhorn GR, Grabowski T, Ponto LL, Hichwa RD. Neural correlates of naming animals from their characteristic sounds. Neuropsychologia. 2003;41:847–854. doi: 10.1016/s0028-3932(02)00223-3. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Luka BJ. Neural localization of semantic context effects in electromagnetic and hemodynamic studies. Brain Lang. 2006;97:279–293. doi: 10.1016/j.bandl.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Vartiainen J, Liljestrom M, Koskinen M, Renvall H, Salmelin R. Functional magnetic resonance imaging blood oxygenation level-dependent signal and magnetoencephalography evoked responses yield different neural functionality in reading. J Neurosci. 2011;31:1048–1058. doi: 10.1523/JNEUROSCI.3113-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Jobard G, Mazoyer B, Tzourio-Mazoyer N. Word and non-word reading: what role for the Visual Word Form Area? Neuroimage. 2005;27:694–705. doi: 10.1016/j.neuroimage.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph MA. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J Cogn Neurosci. 2010;22:1083–1094. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci. 2005;25:604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Franceschi D, Fowler JS, Thanos PK, Scherbaum N, Pappas N, Wong CT, Hitzemann RJ, Felder CA. Regional brain metabolism during alcohol intoxication. Alcohol Clin Exp Res. 2000;24:822–829. [PubMed] [Google Scholar]
- Weschke S, Niedeggen M. Differential effects of moderate alcohol consumption on motion and contrast processing. Psychophysiology. 2012;49:833–841. doi: 10.1111/j.1469-8986.2012.01356.x. [DOI] [PubMed] [Google Scholar]
- Woodward JA, Bonett DG, Brecht ML. Introduction to linear models and experimental design. Vol. San Diego: Harcourt Brace Jovanovich; 1990. [Google Scholar]
- Wydell TN, Vuorinen T, Helenius P, Salmelin R. Neural correlates of letter-string length and lexicality during reading in a regular orthography. J Cogn Neurosci. 2003;15:1052–1062. doi: 10.1162/089892903770007434. [DOI] [PubMed] [Google Scholar]
- Ziegler JC, Besson M, Jacobs AM, Nazir TA, Carr TH. Word, pseudoword, and nonword processing: A multitask comparison using event-related brain potentials. Journal of Cognitive Neuroscience. 1997;9:758–775. doi: 10.1162/jocn.1997.9.6.758. [DOI] [PubMed] [Google Scholar]