Abstract
In many social species group, members share acoustically similar calls. Functional hypotheses have been proposed for call sharing, but previous studies have been limited by an inability to distinguish among these hypotheses. We examined the function of vocal sharing in female budgerigars with a two-part experimental design that allowed us to distinguish between two functional hypotheses. The social association hypothesis proposes that shared calls help animals mediate affiliative and aggressive interactions, while the password hypothesis proposes that shared calls allow animals to distinguish group identity and exclude nonmembers. We also tested the labeling hypothesis, a mechanistic explanation which proposes that shared calls are used to address specific individuals within the sender–receiver relationship. We tested the social association hypothesis by creating four–member flocks of unfamiliar female budgerigars (Melopsittacus undulatus) and then monitoring the birds’ calls, social behaviors, and stress levels via fecal glucocorticoid metabolites. We tested the password hypothesis by moving immigrants into established social groups. To test the labeling hypothesis, we conducted additional recording sessions in which individuals were paired with different group members. The social association hypothesis was supported by the development of multiple shared call types in each cage and a correlation between the number of shared call types and the number of aggressive interactions between pairs of birds. We also found support for calls serving as a labeling mechanism using discriminant function analysis with a permutation procedure. Our results did not support the password hypothesis, as there was no difference in stress or directed behaviors between immigrant and control birds.
Keywords: Budgerigar, Parrot, Communication, Label, Melopsittacus undulatus, Vocal label, Vocal signature
Introduction
Group living can provide members with a number of benefits including improved foraging and hunting efficiency (Valone 1989; Uetz and Hieber 1997), protection from predation or parasitism (Mooring and Hart 1992; Hass and Valenzuela 2002; Ebensperger and Blumstein 2006), increased mating opportunities (Baglione et al. 2002; Mitani and Watts 2002), and the opportunity to form coalitions or other useful social relationships (O'Brien 1991; Mitani and Watts 2002). Group living can also entail many costs; one such cost may be the necessity of recognizing many individuals to operate within a dominance hierarchy, form coalitions, or engage in other social interactions (Brown and Farabaugh 1997; Höjesjö et al. 1998; Tibbetts 2002; Goymann and Wingfield 2004; D'Ettorre and Heinze 2005; Cortopassi and Bradbury 2006). In some species, this recognition is based on individually distinctive vocal signatures that may develop either innately or through vocal production learning (Mundinger 1970; Cheney and Seyfarth 1980; Caldwell et al. 1990; Rendall et al. 1996; Janik 2000; Janik and Slater 2000; McComb et al. 2000; Tyack 2000b; Nousek et al. 2006; Berg et al. 2012). In other species, shared vocalizations develop within groups through social learning of signals; such sharing has been observed in a number of taxa including cetaceans (Smolker and Pepper 1999; Janik 2000; Miller et al. 2004; Watwood et al. 2004), parrots (Farabaugh and Dooling 1996; Wanker et al. 2005; Scarl and Bradbury 2009; Salinas–Melgoza and Wright 2012), songbirds (Mammen and Nowicki 1981; Farabaugh et al. 1988; Brown and Farabaugh 1991; Sewall 2009), hummingbirds (Stiles 1979; Yang et al. 2007), and bats (Boughman 1997). The sharing of vocal signals can occur at different levels, ranging from the small scale dyadic sharing between two individuals (Wanker et al. 2005; Moravec et al. 2006; Balsby and Scarl 2008) to large-scale geographic dialects (Baker and Cunningham 1985; Groth 1993; Myasato and Baker 1999; Wright et al. 2008; Kershenbaum et al. 2012). In addition, sharing can occur via imitation, in which one individual imitates another's call, or convergence, which we define as two or more birds developing new or intermediate vocalizations with aspects of each other's calls.
A number of hypotheses have been proposed to explain vocal sharing within social groups. The password hypothesis proposes that groups are exclusive and shared calls act as passwords that allow group members to distinguish between strangers and residents and expel strangers (Mammen and Nowicki 1981; Feekes 1982; Tyack 2008; Young 2011). Immigrants must imitate the group call in order to integrate fully and gain access to resources and would experience increased aggression until the process is complete. The group cohesion hypothesis also proposes that group specific calls allow animals to differentiate group identity, but rather than excluding individuals, these calls function to coordinate the activities of group members. This hypothesis proposes that by facilitating group recognition, shared calls help animals avoid joining foreign groups and increase group cohesion during activities such as foraging and predator avoidance (Janik and Slater 1998; Price 2003; Vehrencamp et al. 2003; Tyack 2008; Candiotti et al. 2012).
Shared call types may also help regulate social interactions and associations within a group. The affiliative hypothesis proposes that the process of forming shared calls helps new members integrate into a group and strengthens social bonds, particularly during the formation of new groups (Mammen and Nowicki 1981; Vehrencamp et al. 2003; Tyack 2008; King et al. 2013). Shared calls may also be used to mediate agonistic or competitive interactions. For example, in songbirds, territorial males may match or avoid matching the songs sung by competitors in order to escalate or de-escalate the level of threat of attack (Baptista 1985; Slater 1989; Bradbury and Vehrencamp 1998; Vehrencamp 2001). Although less well-studied, it is possible that nonterritorial species could use shared calls to mediate aggressive interactions. The social association hypothesis subsumes both these ideas to propose that shared calls could be used to mediate both aggressive and affiliative interactions that can occur among group members.
All of the previous hypotheses are similar in that they attempt to provide a functional explanation for the sharing of calls by group members. In contrast, the labeling hypothesis provides a mechanistic explanation for how shared calls allow animals to recognize and distinguish among group members and address one another (Janik and Slater 2000; Tyack 2000a; Price 2003; Wanker et al. 2005; Balsby and Scarl 2008; Tyack 2008). Animals could potentially label themselves (i.e., a “signature”), the individual(s) they are directing their calls to (i.e., a “name”), or their relationship with the labeled individual.
One species that may have multiple functions for call sharing is the budgerigar, Melopsittacus undulatus, a small, nomadic, highly social parrot in which group composition may change regularly (Wyndham 1980). The most common call used by budgerigars is the contact call, a short, frequency-modulated vocalization that develops through social learning (Brittan-Powell et al. 1997). Budgerigars placed in groups typically develop contact calls that are shared among group members over a period of several weeks (Farabaugh and Dooling 1996; Brittan-Powell et al. 1997; Brown and Farabaugh 1997; Bartlett and Slater 1999; Hile et al. 2000; Hile and Striedter 2000; Fujiwara et al. 2011; Young 2011). Call sharing in this species appears to exhibit different patterns depending on how groups are formed. In male–female pairs, males typically imitate female calls, and this process appears to contribute to pair bond formation and maintenance (Hile et al. 2000; Hile et al. 2005; Moravec et al. 2006; Moravec and Striedter 2010). Imitation also occurs in all-male groups when immigrants are introduced to a new flock (Bartlett and Slater 1999; Young 2011). In contrast, some degree of mutual convergence occurs among group members when all members are new (Farabaugh et al. 1994; Hile and Striedter 2000; Young 2011). A recent study by Young (2011) tested the password hypothesis by moving male budgerigars into new groups and monitoring patterns of call learning, social behavior and stress levels [via fecal glucocorticoid metabolites (FGMs)]. Contrary to the predictions of the password hypothesis, immigrant birds did not experience heightened aggression or lower affiliation relative to control birds that were not moved into a new social group. Elevated stress levels were associated with social disruption, but not with the lack of a shared group call. Furthermore, rather than exhibiting a single shared call that could function as a password of group membership, males had repertoires containing multiple contact call types that had formed through alternative patterns of call sharing, with different calls shared by different sets of birds within the group (Young 2011). To date, no studies have tested alternative hypotheses for the function of call sharing in groups of budgerigars.
The goal of this study was to test multiple hypotheses for call sharing in female budgerigars. With the exception of work by Hile and Striedter (2000), little research has focused on sharing in female budgerigars, which may exhibit different patterns of call sharing and/or have different functions for call sharing than males. In addition, we examined how two different extremes of group formation affected the development of shared calls. In the first phase of our experiment, the Novel Group Formation Phase, all birds were unfamiliar to each other before being placed in groups of four individuals and allowed to develop shared call types. In the second phase of the experiment, the Immigrant Transfer Phase, one bird (immigrant) was transferred from its original group to a new social group, while individuals in the other cages were unchanged (controls). Our use of a two-part experimental design and captive flocks allowed us to test whether call sharing helps mediate interactions among group members (social association hypothesis) or whether it acts to exclude individuals from a group (password hypothesis). In addition, we also tested the mechanistic labeling hypothesis.
To test our hypotheses, we recorded the birds’ behavior toward one another, their vocalizations in groups and pairs, and tested their stress by measuring FGMs from feces. FGM is an indicator of the concentrations of circulating corticosterone, the primary stress hormone in birds. Corticosterone levels indicate general stress (Dehnhard et al. 2003; Goymann 2005; Touma and Palme 2005), breeding status (Ebensperger et al. 2011), or social standing (Saltzman et al. 1994; Creel 2001; Goymann and Wingfield 2004). We predicted that FGM levels in budgerigars would reflect the degree to which an individual shared calls with others, or engaged in either aggressive or affiliative interactions with group members.
The social association hypothesis predicts multiple shared call types developing in each cage with different patterns of sharing among individuals, a positive relationship between the number of social interactions between pairs and the number of call types they share, and an association between the FGM levels of the birds and the number of calls they share overall. The password hypothesis predicts a single shared call type within each group, imitation of this call type by immigrants entering a new flock, higher levels of aggressive behavior and/or lower levels of affiliative behavior toward immigrants before imitation occurs, and increased FGM levels in immigrants after transfer but prior to imitation. To test the mechanistic labeling hypothesis, we also conducted additional recording sessions of pairs of birds to test the prediction that individuals would use different call variants depending on whom they were recorded with.
Methods
Animal housing and care
These methods generally follow Young (2011) with modifications as follows. In July and August of 2010, we acquired 52 adult female budgerigars from a local breeder and several pet stores. Budgerigars were given water, a commercial seed diet (Brooks Brand Seeds, Lawton, OK), and a vitamin supplement (Lafeber, Cornell, IL) in their water ad libitum and were housed on a 12/12 light/dark cycle. Budgerigars were habituated to the experimental conditions by placing them in sound chambers for 30-min intervals (2–4 sessions per bird) and observing their behaviors for 20-min sessions (4–11 sessions per bird). We then chose 40 of the budgerigars that vocalized most extensively during habituation trials for inclusion in the experiment. These 40 budgerigars were divided into ten cages of four individuals that were unfamiliar to each other. The birds were housed in three different rooms (four cages per room, one room containing two cages with non-experimental birds) and were visually isolated from one another with plywood barriers covered in acoustic foam to reduce sound propagation and reverberation. Birds in separate cages could still hear one another, but prior experiments indicated that auditory contact without social interaction does not promote call sharing (Farabaugh et al. 1994; Hile and Striedter 2000; Young 2011). One cage was later excluded from the analyses because one of the birds was male; the remaining birds were confirmed to be female via genetic sexing from blood following protocols in Pease et al. (2012).
Experimental outline
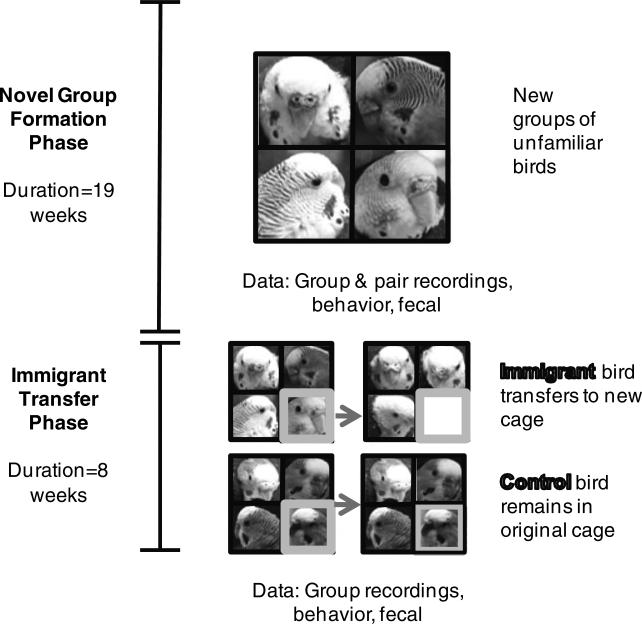
Our experiment consisted of two phases, the 19-week Novel Group Formation Phase, which tested the social association and labeling hypotheses, and the 8-week Immigrant Transfer Phase, which tested the password hypothesis (Fig. 1). During the Novel Group Formation Phase, groups of four unfamiliar birds were placed together. During the Immigrant Transfer Phase, there were two experimental conditions, immigrant cages in which the focal bird (the immigrant) was moved to a different cage, and control cages in which the focal bird was not moved.
Fig. 1.
A depiction of the timeline and two phases of the general experiment. The Novel Group Formation Phase tested the social association hypothesis. Here each group consisted of four unfamiliar individuals. The Immigrant Transfer Phase had two conditions: experimental cages in which focal birds (immigrants) were moved between cages, and control cages in which focal birds (controls) remained in their original cage
Social association hypothesis
During the Novel Group Formation Phase of the experiment, all birds were placed into groups of four unfamiliar individuals. Birds remained in these groups until group-specific calls had developed for all cages and been present for several weeks (total duration=19 weeks). During those weeks, vocalizations, behavioral interactions, and FGM levels were monitored (details below).
Vocalizations
We placed birds in acoustic isolation chambers twice a week for 30-min sessions and recorded vocalizations using Audio-Technica Pro 37 microphones (frequency responses=30–15,000 Hz). All recordings were processed with a Saffier Pro digitizer and directly saved to a Dell DHMPC computer using the sound program Syrinx 2.6 (Programmed by Burt 2006), with a sampling rate of 22,050. Syrinx was programmed to automatically partition calls into individual sounds files. The isolation chambers were 52 quart coolers (Igloo, Shelton, CT) lined with acoustic foam and had transparent plexiglass doors, allowing individuals to see one another during recording sessions. The interior dimensions of the cooler were 23×25.5×48 cm. We played recordings of unfamiliar budgerigar vocalizations at low levels to promote vocalizing (Hile and Striedter 2000).
To assess whether convergence had occurred within groups, two researchers experienced with budgerigar calls (CD and BC) visually examined and sorted spectrograms of up to 100 contact calls per bird per recording session. We created spectrograms in Raven 1.3 and 1.4 (Cornell Lab of Ornithology) with a window size of 256 samples, a filter bandwidth of 124 Hz with a Hanning (Hann) window, a discrete Fourier transform (DFT) size of 256 samples, grid spacing of 86.1, and 50 % overlap. Contact calls were scanned for different types and placed into lexicons (a catalog) of types for each 2-week period starting with the first week. The call type that was produced most frequently by each individual was considered that individual's “dominant” contact call (Farabaugh et al. 1994; Bartlett and Slater 1999), which we are referring to as the “bird's own dominant call” (BODC) following Young (2011). Calls given by two or more birds were identified as shared types, and the call type that was given the most frequently by a minimum of three birds in the group was identified as the group dominant call (GDC). We determined vocal convergence based on the presence of a GDC following Young (2011) rather than examining the individual dominant call types as in some previous research (Farabaugh et al. 1994; Bartlett and Slater 1999). Some individuals consistently failed to call in the acoustic chambers; if 50 % or more of the individuals in a given cage gave fewer than three calls per week for 3 or more weeks, we removed the cage from analysis. Thus, only eight cages were included in acoustic analyses.
We counted the number of call types as well as the number of shared calls for each individual. We considered a call to be shared when the duration of the call and the shape of the dominant harmonic band were categorized into the same call category by both researchers. In addition, we examined how call sharing occurred between immigrants and residents during the Immigrant Transfer Phase (details described below). The goal was to determine whether call sharing was occurring through imitation of the residents’ calls by the immigrants, or through convergence between immigrants and residents.
Although visual sorting of call types by human observers has been shown to be highly accurate (Janik 1999; Nowicki and Nelson 2010), we also verified our sorting with a test of observer agreement using two cages from the Novel Group Formation Phase (cages 1 and 8) and two cages from the Immigrant Transfer Phase (cage 2, a transfer cage, and cage 5, a control cage). To test observer agreement, three observers, one trained in budgerigar call discrimination and two trained in spectrogram analysis but not budgerigar calls, independently sorted up to 80 calls for each cage. A maximum of 20 calls from each bird were included in the analysis. During the Novel Group Formation Phase, we included up to ten calls per bird from weeks 15–16 and ten from weeks 18–19. For the Immigrant Transfer Phase, we included up to ten calls from weeks 1–2 and ten calls from weeks 3–4 for each bird. The observers were naive to the week the call was recorded and the individual that gave the call, and they were presented in random order. Pairwise measures of agreement (Kappa statistic, IBM SPSS 20) were calculated between each observer.
For final verification of our visual sorting, we verified the sorting process with a DFA using calls from cage 1. We examined weeks 17 and 19, in which the number of calls given per bird ranged from 55 to 159 and the entire cage had seven call types. We chose up to ten examples of each call type per bird when available, and made 13 automated feature measurements including the duration and the mean and variation of the pitch (pitch estimates are essentially measures of the fundamental frequency), frequency, frequency modulation, amplitude modulation, goodness of pitch (a measure of how periodic a sound is), and Wiener entropy (a measure of the width and uniformity, e.g., noisiness of the power spectrum) using Sound Analysis Pro 1.2 (Tchernichovski et al. 2000). We then processed the data in a discriminant function analysis (DFA) in JMP 8.0 (SAS Institute, Cary, NC). We examined the percent classification accuracy and compared the accuracy to the a priori probability, which is the probability that a call is classified to the correct category by chance. A priori probabilities were calculated using the number of calls given for each type of call per bird. We also calculated Wilks’ Lambda values, in which low values indicates a high difference in group means. We report results from a cross-validated set of data (Lengagne 2001; Kirschel et al. 2011). We are aware that this analysis pseudo-replicates the data since subjects contributed multiple calls per call type. However, all call types except one occurred in a single subject, and hence, a pDFA (see below) was not possible. Practically this means we could not test discriminability of call types while controlling for caller identity. However, since the sole aim of this test was whether the human rater categorization can be confirmed by an analysis of the call parameters, we believe this approach is appropriate.
Behavior
We used continuous behavioral sampling methods to gather data in 20-min sessions twice a week for each cage on Palm Pilots (m500) encoded with software designed by Jim Ha (University of Washington). Behavior recordings took place between 8 a.m. and noon when birds were most active. Recordings were conducted by two observers (C.D. and B.C.) who trained together until their behavioral observations were consistent. We recorded directed affiliative behaviors (allopreening, courting, allofeeding, beak-touches, and warbling) and directed aggressive behaviors (threatening, displacement, and pecking) as events following Young (2011). Several affiliative behaviors including allopreening, courting, and warbling were also recorded for duration because they could continue for several seconds.
Fecal hormone analysis
FGM offers the advantages of being collected noninvasively and providing an integrated measure of circulating levels of corticosterone over a longer period of time than blood samples (Möstl et al. 2005). To determine FGM concentrations, we initially collected fecal samples that comprised the entire fecal and urate mass after birds had been isolated for 30 min in sound chambers. Samples were placed in 0.2 mL PCR tubes and stored at either –20 or –80 °C. We consolidated samples for each individual within each week into 12×75 mm borosilicate glass tubes. We then subsampled among individuals (three birds from each cage were randomly selected) and weeks for the radioimmunoassay (RIA); the assay was performed for weeks 1–4, 7, 10, 13, 16, and 19. Determination of FGM in consolidated fecal samples was performed by double-antibody RIA in the Animal and Range Sciences Endocrinology Laboratory at New Mexico State University. The assay utilized a nonspecific corticosterone antiserum and 125I-corticosterone obtained from MP Biomedical (Orangeburg, NY) that was validated for use with budgerigar feces (Young and Hallford 2012). In the current experiment, increasing amounts of feces (10, 20, and 40 mg) yielded a displacement curve that was parallel to the standard curve. The extraction efficiency was 87.9±0.83 %. When 25, 32, or 62 pg/mg of corticosterone was added to 20 mg samples, 89 %, 88 %, and 94 % of the corticosterone was recovered. The sensitivity at 95 % displacement was 4 pg/mg. The inter-assay coefficient of variation for five assays was 7.7 %, and the average intra-assay variation was 6 %.
Interactions among vocal sharing, behavior, and FGM
To assess whether call sharing reflects underlying social relationships, we calculated the number of shared calls between each pair of birds during weeks 10–19 (when both shared pair calls and many GDCs had developed) as well as the number of behavioral interactions among each pair of birds. We then ran a principal components analysis (PCA) on counts of each affiliative and aggressive behavior as well as the sum of the duration of the state affiliative behaviors. We conducted a quartimin rotation on the factors (considered optimal for oblique rotations). We then ran two generalized linear mixed models (one for each of the two factors derived; Baayen 2008) with Poisson error structure and log link function testing whether the number of shared call types per dyad (response variable) was influenced by the factor scores describing the relationship of the individuals of a dyad (predictor variable with fixed effect). To control for potential differences between groups with regard to the overall amount of call type sharing, we included the group ID as a random effect into the model, and to control for the possibility that the number of shared calls is a simple function of the call type repertoire sizes of the two birds of a dyad, we included the log transformed sum of their repertoire sizes as an offset term into the model. Since the data were nonindependent in the sense that each individual was involved in several dyads, we established the significance of the model using a permutation test (Adams and Anthony 1996; Manly 1997). For this, we permuted the matrix with the factor scores per dyad, whereby we permuted rows and columns simultaneously as in a Mantel test (Sokal and Rohlf 1995) and restricted the permutation to happen only within groups. Since for two subjects (TUG and PRL) we had incomplete data, we excluded them from the data. We conducted 1,000 permutations into which we included the original data as one permutation and determined the P value associated with the effect of the factor score as the proportions of permutations revealing an absolute estimate of its effect at least as large as that revealed from the original data. The permutation was conducted using a procedure for R (R Core Team 2013) and the GLMMs were conducted using the function lmer of the R-package lme4 (Bates et al. 2013). Prior to the analysis, we square-root transformed factor 1 (after subtracting the minimum) to achieve a more symmetrical distribution. To compare whether the number of calls shared with cagemates reflects underlying levels of stress, we calculated average FGM measurements for each bird for weeks 10–19 and ran a regression comparing their average FGM concentration to the number of shared calls held by each individual.
Password hypothesis
We tested the password hypothesis with the Immigrant Transfer Phase of the experiment, in which one individual from half of the cages was transferred to a new cage (the immigrant) while the individuals in the other half of the cages remained in their same cages (controls). The duration of this phase of the experiment was 8 weeks, and we again monitored vocalizations, behavioral interactions, and FGM levels.
Vocalizations
General methods are the same as described for the Novel Group Formation Phase. One important change was that we created lexicons on a weekly basis to follow more closely the development of shared calls. We only analyzed the vocalizations of birds from seven of the ten cages due to limited calling in three cages. We determined whether the transfer and residents had developed shared calls by visually sorting spectrograms of their calls. After an immigrant bird produced the GDC, we considered that week and all remaining weeks to be the post-sharing period. We combined the post-sharing weeks because some subjects vocalized infrequently during some weeks, and the sample size was inadequate to conduct a week-by-week analysis for all cages. We also examined lexicons to verify that the immigrant birds were not producing calls of their new group prior to transfer. We assessed the extent of call imitation by measuring call features using SAP 1.2 as described above and then calculated the Mahalanobis distances, which was the distance between the focal birds’ version of the contact call and the multivariate mean of each call type of the resident birds (the centroid; Silva and Stam 1996; Young 2011). Up to ten examples of each call type per bird were included in the analysis. Due to one of the immigrants rapidly developing a shared call during week one of the Immigrant Transfer Phase, we could not compare the focal bird's centroid to the resident's centroid between weeks one and later weeks. Instead, we compared weeks 18–19 (the baseline period) to post-transfer weeks. Since immigrant birds were still in their original cage during the baseline period, we generated Mahalanobis distances between the immigrant bird's GDC in their original cage and the centroid of the call type that they converged to later. Thus, we assessed call sharing at two time periods; the baseline (weeks 18–19 from the Novel Group Formation Phase), the post-transfer period (for control birds) and the post-call-sharing period (for immigrants). Five control birds and three immigrant birds (the ones that vocalized regularly) were included in this analysis. We analyzed the data with a repeated-measures analysis of variance (ANOVA) with Treatment (Control vs. Transfer) and Time Period as main effects and a Treatment×Time Period interaction.
Behavior and fecal hormone analysis
Methods are the same as described for the Novel Group Formation Phase for collection of behavior and FGM data. For the FGM analyses, we subsampled individuals (choosing the focal bird and two randomly selected residents in each cage) and also subsampled weeks (analyzing transfer weeks 1–4, 6, and 8).
Interactions between vocal sharing, behavior, and FGM
We analyzed FGM and behavioral data with repeated-measures ANOVAs with main effects of Treatment, Time Period, and a Treatment×Time interaction. Since all immigrant birds converged onto shared calls at different rates, we compared both FGM and behavior values from the first 2 weeks of the post-transfer period (when most birds had not converged) and the last 2 weeks (seven and eight), when all immigrant birds had converged. Since we did not have FGM values for week 7, only week 8 was included in the analysis. For the FGM data we used a percent change from the baseline values (FGM value from week 19 pre-transfer) rather than the raw values, because individuals can differ in overall patterns of FGM levels and we were interested primarily on the relative change from baseline (Brownie 1992). For the behavior analyses, we ran a PCA on counts of each affiliative and aggressive behavior as well as the sum of the duration of the state affiliative behaviors, using a quartimin rotation, as was also done for the social affiliation hypothesis. In addition, behaviors directed toward the focal bird and by the focal bird were combined (e.g., all beak-touches given by or to bird A were included in a single beak-touch category). All five control and four immigrant birds were included in these analyses. Although birds in some cages vocalized too infrequently to be included in vocalization analyses, they interacted at normal levels and were thus included in the behavioral analyses.
Labeling hypothesis
During the Novel Group Formation Phase, we conducted pair recording sessions, in which each cage member was recorded three times a week, once with every other member of her cage. During those sessions, we recorded four birds, two from one cage and two from another cage. Birds from the same cage could see and hear one another during the session through the clear plexiglass doors of the acoustic chambers. Birds from the other cage were visually isolated by a plywood barrier covered in acoustic foam to attenuate sound.
We created separate lexicons from the pair recording sessions for each pair of birds for weeks 18–19. We measured call features using SAP 1.2 as described above. To test whether calls differed according to the identity of the caller or the recipient we used DFAs in combination with a permutation procedure (pDFA; Mundry and Sommer 2007). For predictor categories, we followed Wanker et al. (2005) with some modifications:
Signature: Calls were classified according to the identity of the sender, regardless of who the receiver was (e.g., bird A calling with bird B, bird A calling with bird C, and bird A calling with bird D were assigned to group 1)
Name: Calls were classified according to the identity of the receiving individual, regardless of who the sender was (e.g., bird B calling with bird A, bird C calling with bird A, and bird D calling with bird Awere assigned to group 1)
Sender-directed: Calls were classified based on the identity of the senders and the receivers that the calls were directed toward (e.g., bird A calling to bird B was assigned to group 1, bird B calling to bird Awas assigned to group 2, bird A calling to bird C was assigned to group 3, etc.)
A pDFA approach allows for testing the discriminability between, subjects by their calls while controlling for nonindependence of the calls, e.g., due to senders calling repeatedly in the presence of the same recipients. More, specifically, to test for discriminability between senders (Signature), we assumed calls given by the same sender in the presence of the same recipient to be nonindependent and, hence, used such blocks of calls identified by the same sender and recipient as the units of permutation (i.e., the identity of the sender was permuted between such blocks of calls). Furthermore, to control for nonindependence of calls recorded from the same group (i.e., cage), we restricted the permutation such that sender identities were only permuted within cages. We used the percentage of correctly cross-classified calls as the test statistic and conducted a total of 1,000 permutations (into which we included the result for the original data as one permutation). To balance the contribution of senders as well as of recipients per sender in this analysis, we derived discriminant functions based on data sets comprising equal numbers of calls per combination of sender and recipient (randomly selected) and also the same number of recipients (randomly selected) per sender before deriving the discriminant functions (numbers of calls per combination of sender and recipient and number of recipients per sender were determined according to the respective minimum available). To rule out undue influence of any particular random selection when determining the percentage of correctly cross-classified calls for the original (unpermuted data), we created 100 such random selections and averaged the result. In order to have sufficient samples sizes (with regard to the number of calls per sender and per combination of recipient and sender) and a reasonable sample (with regard to the number of recipients per sender and senders per cage), we restricted the data set to combinations of recipients and senders for which we had at least three calls, senders for which we had calls given toward at least two recipients, and cages out of which we had at least two senders (in that sequence). This led to the final data comprising a total of 1,614 calls from 20 subjects out of seven cages [number of calls per caller and cage: Cage 1: CEL (141), FIE (98), JEW (29); Cage 3: LAD (24), SWA (109), TAN (150); Cage 5: DAN (113), PET (60), POW (101); Cage 7: LIL (13), MOS (108), PAN (23), SLA (45); Cage 8: PIC (70), ZUC (87); Cage 9: AZU (107), JOY (57); and Cage 10: LIM (98), OLI (72), SEA (109)]. Of these, we used six calls per sender to derive the discriminant functions and the remainder for cross-classification. Having only six calls per subject available for deriving the discriminant functions meant that we had to reduce the number of call parameters to ideally five (Tabachnick and Fidell 2001). We achieved this by running standard multiple regressions with the call parameters as the predictors (and a random variable as the response) and iteratively excluded the call parameter with the largest variance inflation factor (VIF; Quinn and Keough 2002; Field 2005). This eventually left the call parameters syllable duration, Wiener entropy, mean frequency, variance in Wiener entropy, and variance in mean frequency in the data set. Prior to running the multiple regressions and the pDFA, we square-root transformed variance in pitch and variance in goodness of pitch (each after subtracting the respective minimum) and log-transformed variance in mean frequency to achieve more symmetrical distributions.
For the analysis of discriminability between calls of different senders with regard to the recipient (Name), we proceeded correspondingly. Here, the final data set comprised 1,604 calls given by 25 subjects out of seven cages [Cage 1: CEL (64), FIE (57), JEW (81), TUR (78); Cage 3: LAD (100), SWA (55), TAN (43), TUG (85); Cage 5: DAN (53), PET (65), POW (96), SLE (60); Cage7: LIL (57), MOS (25), PAN (49), SLA (58); Cage 8: PIC (97), TUQ (56); Cage 9: AZU (53), BRI (26), PRL (46); and Cage 10: CAR (59), LIM (53), OLI (119), SEA (69)]. As before, the units of permutation were blocks of calls given from a particular sender in the presence of a particular recipient, and again the permutation was restricted to within cages. However, this time, blocks of calls were randomized between recipients rather than between senders.
While the recipient model addressed the question of whether calls of different senders are discriminable across them with regard to the identity of the recipient, the next model addressed the question of whether calls can be discriminated between recipients within senders (Sender-directed). For this model, we selected those senders for which we had calls uttered in the presence of at least two receivers whereby per dyad (combination of sender and receiver) at least six calls were required. The final data consisted of 1,633 calls given by 18 senders in the presence of 31 recipients out of eight cages [number calls and recipients per caller and cage: Cage 1: CEL (141, 3), FIE (98, 3), JEW (29, 3); Cage 2: CLO (85, 3); Cage 3: SWA (109, 3), TAN (150, 3); Cage 5: DAN (113, 3), PET (57, 2), POW (101, 3); Cage 7: MOS (108, 3), SLA (45, 3); Cage 8: PIC (70, 3), ZUC (87, 2); Cage 9: AZU (107, 3), JOY (57, 2); and Cage 10: LIM (98, 2), OLI (69, 2), SEA (109, 3)]. For this analysis, we randomized individual calls within callers.
The pDFAs were conducted using a function written in R (R Core Team 2013) by R. Mundry, available by request. The function is based on the function lda of the R package MASS (Venables and Ripley 2002).
Results
Call classification and accuracy of visual sorting
There was high agreement among the three sorters who visually classified calls for cages 1 and 8 from the Novel Group Formation Phase, and cages 2 and 5 from the Immigrant Transfer Phase (all P values<0.0001). For cage 1, the average agreement (Cohen's Kappa, K) was 0.81 (Avg T =11.8), and the average agreement increased to 0.90 when six rare calls were removed from the sample. For cage 8, the average agreement (K) was 0.79 (Avg T =10.33), and the average agreement increased to 0.88 when six rare calls were removed from the sample. For the Immigrant Transfer Phase, the average agreement for cage 2 (K) was 0.77 (Avg T =11.6), and the agreement increased to 0.88 when seven rare calls were removed from the sample. The average agreement for cage 5 was 0.76 (Avg T=9.3)
The DFA was also very accurate, and the overall discrimination accuracy for the call types given by members of cage 1 during weeks 18–19 was 100 %, which was considerably higher than the mean a priori probability of 14 % (N =72, Wilks’ lambda=0.000, P <0.0001). The cross-validation procedure was slightly less accurate with a classification accuracy of 95.8 %. Thus, we considered the visual sorting of calls to be in agreement with quantitative measures of the calls. The two most critical predictor variables were duration and mean frequency. The discrimination to call type was also higher than the discrimination to individual bird, which was 88.9 % using the cross-validation procedure (N =72, Wilks’ lambda=0.035, P <0.0001).
Social association hypothesis
Call sharing and repertoire size
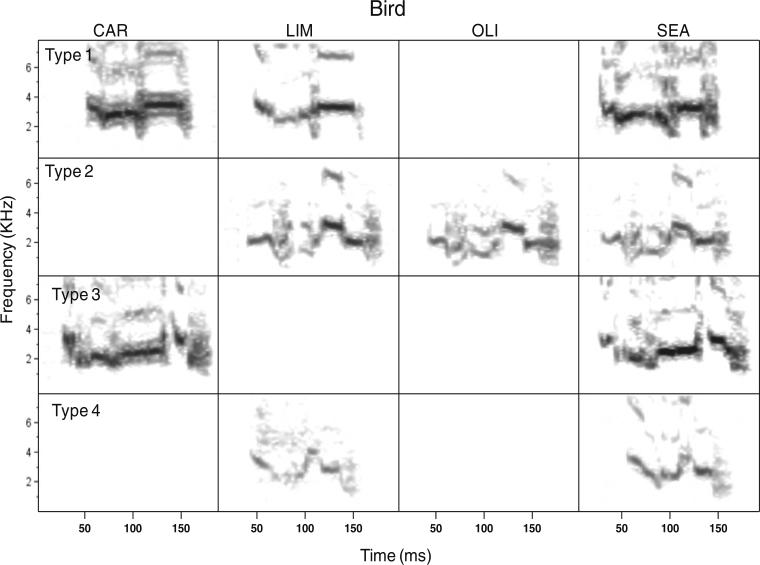
Some shared calls emerged among pairs of birds during the first week of the Novel Group Formation Phase. The earliest GDCs (group calls shared among at least three birds) did not emerge until week 9. During weeks 18–19, of the eight cages in which at least three birds called regularly, seven of those cages exhibited a call type shared by three or four birds. The number of call types shared by at least two birds per cage ranged from 1 to 4, and the median, 25 %, and 75 % quartiles were 2, 2, and 3.75, respectively. Individual repertoire size for weeks 18–19 was also fairly small, with a range of 1–9 calls, and a median, 25 %, and 75 % quartile value of 2, 2, and 3, respectively. A typical lexicon for a cage is shown in Fig. 2. Details on the development of shared calls during the Immigrant Transfer Phase are described in the password hypothesis section below.
Fig. 2.
A lexicon from cage 10 showing all call types shared among cage members. Unshared call types are not depicted
Call sharing, behavior, and FGM
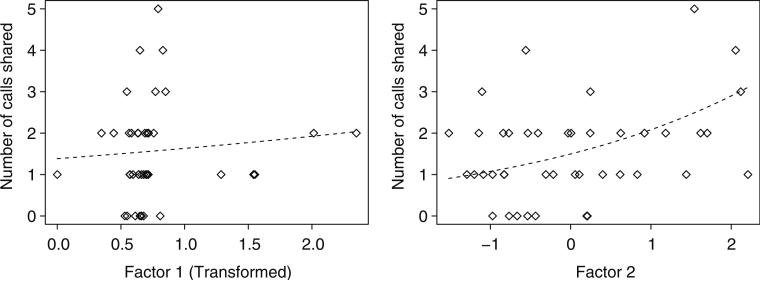
The first two rotated factors for the behavior PCA accounted for 46 % of the total variance, with factor one accounting for affiliative behaviors and factor two accounting for aggressive behaviors (Table 1). Variables with the largest loading values for factor one (>±0.35 and identified by JMP) included the number of beak touches and warbles and the summed duration of the affiliative state behaviors. Two aggressive variables including threats and pecks had the largest loading values for factor two as well as courtship, which was an affiliative behavior. There was a significant positive impact of factor two on the number of shared calls, but no such effect of factor one (permutation test; factor one: estimate=0.16, P =0.444; factor two: estimate=0.33, P =0.006; Fig. 3). A regression of FGM on number of calls an individual shared was not significant (R2=0.003, F1,24=0.08, P =0.77).
Table 1.
Loading values for the principal component analysis for weeks 10–19
| Behavior | Rotated factor 1 | Rotated factor 2 |
|---|---|---|
| Beak touch | 0.56 | –0.19 |
| Warble | 0.52 | 0.00 |
| Allofeed | –0.01 | 0.14 |
| Court | 0.23 | 0.69 |
| Threat | 0.06 | 0.48 |
| Peck | –0.07 | 0.39 |
| Displace | –0.15 | 0.28 |
| Duration of Warble and Court | 0.99 | 0.09 |
The values shown are for a quartimin rotation
Fig. 3.
Number of calls shared between pairs of birds plotted against rotated factors one and two from the PCA. Included in the analysis are weeks 10–19 after the formation of novel groups in the Social Association Phase. Variables with high loading values (all positive) for factor one were affiliative behaviors including the number of beak touches and warbles and the summed duration of the affiliative state behaviors. Variables with high loading values (all positive) for factor two were primarily aggressive behaviors (threats, pecks, and displacements) with the addition of courtship. The model was significant for factor two (aggression) only
Password hypothesis
Call sharing
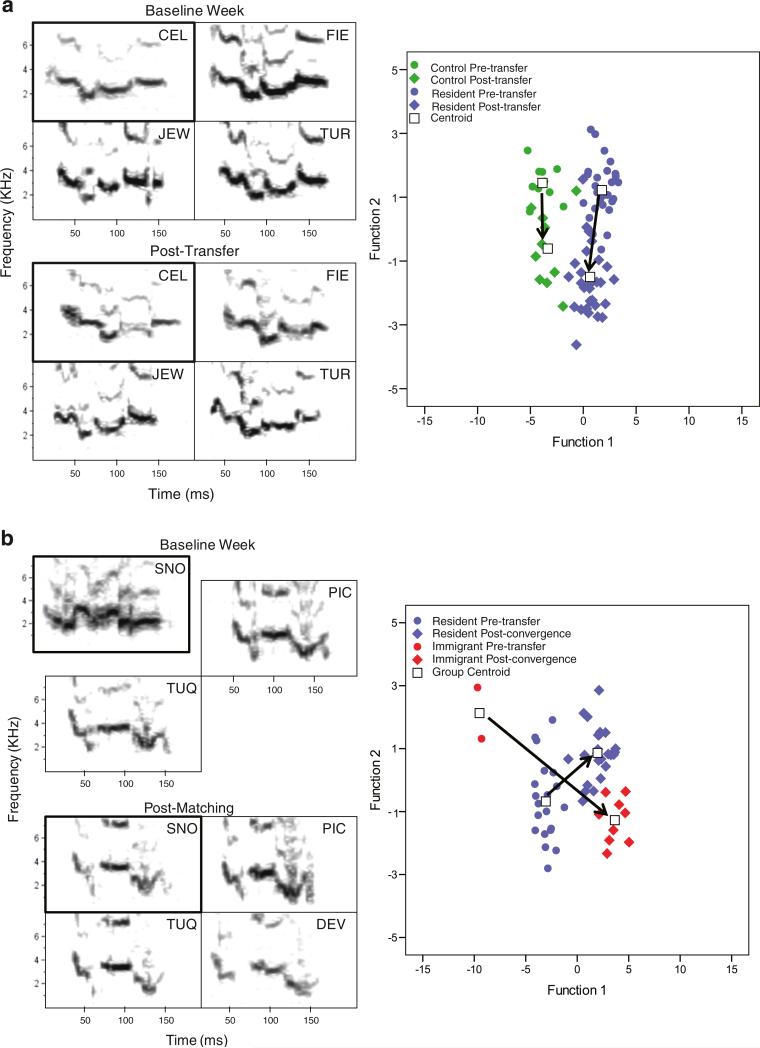
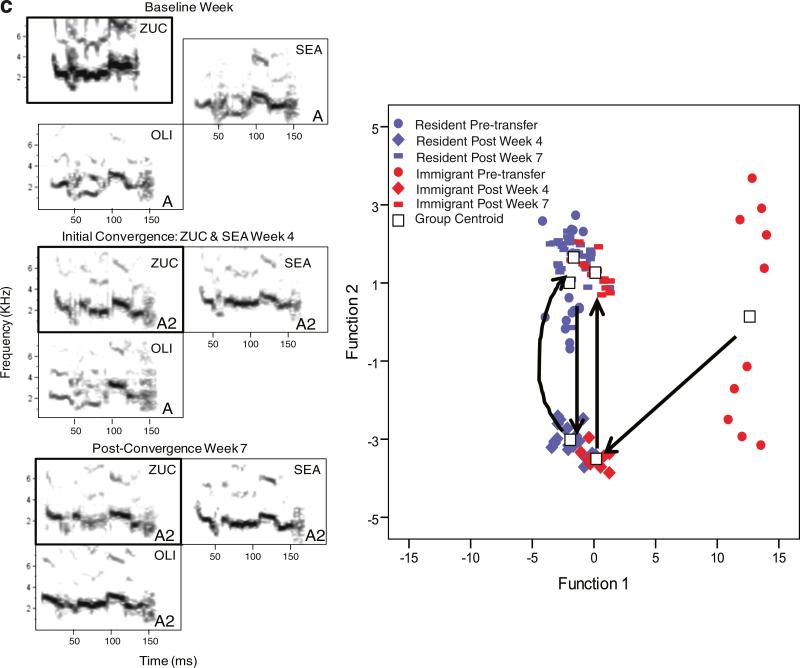
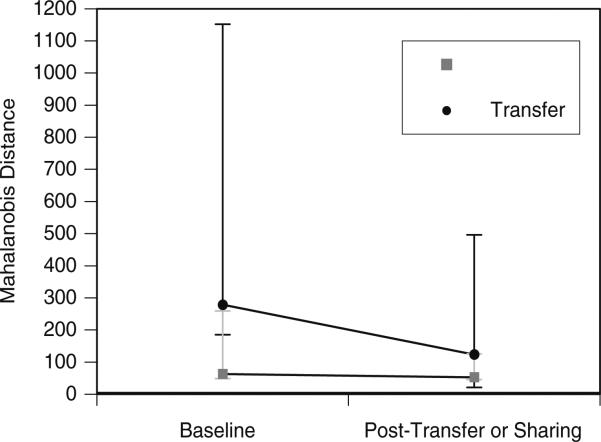
Call sharing after transfer happened at different rates and in different forms depending on the cage (Fig. 4). Of the three immigrant birds that vocalized (LIM, SNO, and ZUC), SNO matched the GDC during transfer week 1 (Fig. 4b). LIM matched one of the resident's calls during transfer week 3 but did not match the groups GDC until week 6. In the remaining transfer cage, the immigrant and resident birds converged on a new group call type. In week 4, ZUC and one resident began giving a call that appeared to be a hybrid of one of the ZUC's baseline call types and the GDC. By week 7, the GDC had shifted to that new variant (Fig. 4c). It appeared that the calls of both immigrant and control birds became more similar to the calls of resident birds after transfer (the Mahalanobis distances decreased) and that there was greater change between the calls of immigrants and residents as opposed to control birds and residents, but none of the ANOVA effects were significant (Table 2, Fig. 4a–c and 5).
Fig. 4.
Vocal imitation of contact calls with the baseline week and transfer weeks in control and immigrant birds. Boxes around the focal birds’ calls are in bold. The boxes of the immigrant birds are offset during the baseline week to indicate that they had not yet been moved to their new group. The birds’ initials are in the top right corners of the boxes and call types (if necessary) are in the bottom right corners. If a resident bird did not give the GDC type, they were excluded from the figure and the analysis. Canonical plots are of a DFA of budgerigar contact call acoustic measurements from the same cage. Each point represents a call. Arrows between the group centroids (denotes mean value for a particular subset of calls) indicates the direction of movement for the call structure. Included are (a) an example of a control cage (cage 1), (b) an example of a transfer cage (cage 8) in which the immigrant bird matched the resident birds call type, and (c) an example of a transfer cage (cage 10) in which there was mutual convergence between transfer and control birds. For cage 10, initial convergence happened during week 4 when ZUC and SEA began giving a call type (type A2) that appeared to be intermediate between ZUC's baseline call and the group call (type A). At week 7, the group call shifted to that new call type (type A2). To illustrate the change in calls for cage 10, two post-transfer time periods are shown; week 4 and weeks 7–8
Table 2.
Results of repeated-measures ANOVAs comparing control and immigrant birds in the Immigrant Transfer Phase for (1) behavior (PCA-rotated factors one and two), (2) FGM, and (3) The level of call convergence using Mahalanobis distances
| Variable | Control X ±SE | Transfer X±SE | Subject (Trt) |
Treatment |
Time period |
Trt×Time period |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| F statistic | P value | F statistic | P value | F statistic | P value | F statistic | P value | |||
| Behavior PCA factor one | –0.10±0.31 | 0.13±0.34 | 0.747,7 | 0.65 | 0.251,7 | 0.63 | 0.661,7 | 0.44 | 0.081,7 | 0.78 |
| Behavior PCA factor two | –0.19±0.29 | 0.24±0.33 | 1.457,7 | 0.32 | 0.971,7 | 0.36 | 6.491,7 | 0.04 | 0.071,7 | 0.79 |
| FGM | 94.5±11.2 | 100.7±12.5 | 2.97,7 | 0.08 | 0.131.7 | 0.72 | 0.191,7 | 0.67 | 0.121,7 | 0.73 |
| Call convergence | 95.5±89.5 | 285.9±103.4 | 1.035,5 | 0.48 | 1.931,5 | 0.22 | 4.351,5 | 0.09 | 2.781,5 | 0.16 |
For FGM, we used a percentage of change from the baseline values (FGM value from week 19 pre-transfer) rather than the raw values. The time period for behavior and FGM included two time periods: weeks 1–2 and weeks 7–8. For vocal convergence, we divided week into two time periods: baseline and post-convergence
Fig. 5.
Mahalanobis distances from the focal birds to the centroid of acoustic variation of the resident birds during the baseline week and post-sharing weeks. The median, 25th and 75th percent quartiles are shown
Behavior and FGM
For behavior, the first two rotated factors accounted for 60.4 % of the total variance, with factor one accounting for affiliative behaviors and factor two accounting for aggressive behaviors (Table 3). Variables with the largest loading values (>±0.35 and identified by JMP) for factor one included beak-touches, allofeeding, courtship, and the summed duration of the affiliative state behaviors. All aggressive variables had large positive loading values for factor two with the number of beak touches having a negative loading value. There were no significant differences in the rotated PCA components depending on the treatment (control or immigrant) or the treatment by time period interaction (P >0.05; Table 2). There was one difference between time periods in that factor two, which accounted for aggressive behaviors, was lower between all birds during weeks 7–8 than weeks 1–2 (mean± SE for weeks 1 and 2=0.49±0.26, weeks 7 and 8=–0.44±0.26). There was no difference in the FGM levels depending on the Treatment (control or immigrant), Time Period or the Treatment×Time Period interaction (P >0.05; Table 2).
Table 3.
Loading values for the principal component analysis for behavior for post-transfer weeks
| Behavior | Rotated factor 1 | Rotated factor 2 |
|---|---|---|
| Beak touch | 0.44 | –0.51 |
| Warble | 0.36 | 0.16 |
| Allofeed | 0.58 | –0.16 |
| Court | 0.79 | 0.33 |
| Threat | 0.04 | 0.79 |
| Peck | 0.16 | 0.83 |
| Displace | –0.04 | 0.56 |
| Duration ofWarble and Court | 0.94 | –0.25 |
The values shown are for a quartimin rotation
Labeling hypothesis
Predictor categories one and three were significant (Signature and Sender-directed), while category two, Name, was not. Senders were quite clearly discriminable by their calls and thus exhibited individual signatures (pDFA: percentage of correctly cross-classified calls=41.5, expected percentage of correctly cross-classified calls (derived as the average of the permuted data)=25.8, P =0.001). When repeating the analysis separately for each group used in the overall test, we found significant (P <0.05) discriminability in five out of seven cages, a trend in one cage (P =0.072), and only one cage (number 8) where discriminability between subjects did not reach significance (P =0.166).
When testing for discriminability between calls with regard to the receiver (Name), we found no obvious discriminability when considering recipients regardless of which bird uttered the call (percent correctly cross-classified calls: 22.6, expected percentage of correctly cross-classified calls=24.8, P =0.752). This finding was replicated when we repeated the analysis per cage, where we found no significant discriminability in any of the cages (N =7 cages, smallest P =0.16, average P =0.62, no error level correction applied).
Within callers we found a clear discriminability between calls given to different recipients (Sender-directed; percentage of correctly cross-classified calls: 31.5, expected percentage of correctly cross-classified calls=20.5, P =0.001). This finding was largely replicated when we repeated the analysis separately per cage where we found significant (P <0.05) discriminability in five out of eight cages, a trend for discriminability in two cages (P =0.063 and P =0.073, respectively), and no obvious discriminability in only one cage (cage 2, P =0.289).
Discussion
We tested two alternative functional hypotheses for call sharing in groups of female budgerigars, the social association hypothesis, and the password hypothesis, with a two-part experimental design that allowed us to explicitly test and make predictions for each hypothesis. We found support for the social association hypothesis, as well as support for a potential mechanism that may facilitate social interactions, that budgerigar contact calls act as labels. In contrast, we found little support for the password hypothesis.
The social association hypothesis received support from vocal and behavioral data, but not from stress data. Birds developed multiple shared call types and we found a positive relationship between the number of calls shared by any two birds and a PCA factor that consisted primarily of aggressive behavior. In addition, during the Immigrant Transfer Phase of the experiment, we observed convergence between the immigrant's calls and the focal birds’ calls in one cage, as predicted by the social association hypothesis. Contrary to our predictions, we did not observe any association between FGM levels and the number of calls shared. Although variables such as dominance status can affect stress, it is has not previously been tested whether strength of social interactions or call sharing would have such an effect in animals (Sapolsky 1987; Saltzman et al. 1994; Creel 2001; Goymann and Wingfield 2004). In the context of our experimental groups, we see no relationship between call sharing and stress as measured by FGM in budgerigars.
We also found support for a labeling mechanism that could allow shared calls to facilitate social interactions. We tested the discriminatory accuracy of three predictive categories: Signature, Name, and Sender-directed. As a predictive variable, Category II (Name) was not significant, but two other categories, I (Signature) and III (Sender-directed), were effective at grouping the call types. Previous work showed that budgerigars are capable of discriminating between contact calls of different individuals and types and that they use acoustic structure within the 2–4 kHz range for call discrimination (Dooling 1986; Brittan-Powell et al. 1997). Our work suggests that particular calls can be used to identify not only who is giving the call but also to whom the call is being directed. Similar results were found in spectacled parrotlets, where the sender-directed grouping variable was also found to be the most accurate predictor of call type (Wanker et al. 2005). An important future direction will be to investigate how receivers make use of these identifying characteristics in calls to identify both senders and intended receivers.
Individual signatures in animals are not uncommon and have been found in such diverse species as bottle-nosed dolphins Tursiops truncatus, eagle owls Bubo bubo, jungle crows Corvus macrorhynchos, fur seals Arctocephalus tropicalus, and torrent frogs Odorrona tormota (Sayigh et al. 1990; Lougheed and Handford 1992; Charrier et al. 2001; Sayigh et al. 2007; Feng et al. 2009; Janik 2009; Kondo et al. 2010). Less common though is the ability to alter calls depending on the receiver. Such alterations suggest not only that budgerigars label other birds individually but also that they have mental representations for individuals in their group and tailor their vocalizations accordingly (Striedter et al. 2003; Tyack 2003; Wanker et al. 2005). We expect that call sharing or matching in other species may also function as labels, but currently evidence only supports this in dolphins and orange-fronted parakeets (Tyack 1997; Janik 2000; Tyack 2003; Balsby et al. 2012; King et al. 2013). The generally small repertoires of the female birds that we observed (mean=2.5 call types) makes it unlikely for each bird to have a unique label for every other bird, so variation within types may also be important for identifying the sender and the receiver. This pattern of calling is different than what is observed in humans, in which each person has a given name that is used as a common label across senders. However, in human languages, a person may be identified with different labels by different people depending on the relationship or status (e.g., Mom, Doctor, Coach, or Professor). Budgerigars may also qualify their relationships by using distinct signals, although any individual bird may not necessarily use the same signal as another bird.
Budgerigars are a highly social, flock-dwelling species, and it is probable that the ability to label oneself as well as a recipient may help mediate social interactions and establish the sender–receiver relationship within the context of large flocks. The positive relationship between the number of shared call types and the number of aggressive interactions supports the idea that different call types or distinct variants of calls may be important during such interactions. Although matching of song types by territorial male songbirds has been shown to indicate aggression(Searcy and Beecher 2009), these are, to our knowledge, the first data in any species that indicates a role for shared calls in mediating aggressive behavior beyond that territorial context. Previous research has found more evidence for an association between affiliation and call sharing. For example, male bottle-nosed dolphins converge onto shared signature whistles after forming an alliance (Smolker and Pepper 1999), and in an experimental setting, dolphins match the whistles of close social associates in an affiliative manner (King et al. 2013). Male chimpanzees, Pan troglodytes, that associate closely and chorus with one another also converge on some call features (Mitani and Brandt 1994), and female Campbell's monkeys Cercopithecus campbelli that associate during play converge on acoustic features in their calls (Lemasson et al. 2003). Interestingly, we found almost no pattern between affiliative behavior and call sharing in female budgerigars. The exception was courtship, which was included as an important variable in PCA factor two in addition to the aggressive behaviors. Although courtship behavior is more commonly observed between males and females, it may also be used as a means to maintain positive relationships between some females. For example, our observations of a mixed sex flock of budgerigars indicated that most pairs consisted of a male and female, but we observed one exception in which the pair consisted of two females who, despite having their eggs fertilized by males in the flock, shared a nest box and participated in affiliative behaviors with one another (Dahlin and Cordier, personal observation). So while females habitually exhibit lower levels of affiliative behavior toward one another than males do (Lahaye et al. 2012), they may still be an important component of some relationships. Aggressive interactions, in general, are approximately ten times higher than affiliative interactions in captive females indicating that such interactions are an important form of social engagement among females (Dahlin, personal observation). It would be interesting to determine whether males, who exhibit more sociosexual behavior than females, would exhibit stronger positive relationships between call sharing and other affiliative behavior.
Shared calls could operate in several ways. Matching of signature whistles in dolphins has been suggested as a means to seek the attention of a conspecific (Janik and Slater 1998; Tyack 2003). It has also been hypothesized that converged, shared whistles among groups of allied male dolphins could act as an identifier of the alliance, rather than a single individual (Smolker and Pepper 1999). Orange-fronted conures can rapidly match and then diverge from the calls of conspecifics, and this process may involve some form of negotiation between individuals regarding joining or leaving fission–fusion flocks (Adams et al. 2009; Balsby and Bradbury 2009). Shared calls in budgerigars may also serve as a means to attract the attention of conspecifics. Since shared calls are more associated with aggression in females, they could also play a role in the escalation or de-escalation of conflict.
Unlike the social association hypothesis, the password hypothesis received little support in our study, as found in a previous study on male budgerigars (Young 2011). During the Immigrant Transfer Phase, the predictions for the password hypothesis were partially supported by development of shared calls between immigrant birds and their cagemates. Contrary to predictions, however, this similarity was not always achieved through imitation of established resident calls by the immigrants but, in one cage, occurred through convergence between the immigrant and residents onto a new call type. Although we could observe the imitation and convergence processes through visual inspection of spectrograms and canonical plots of the calls, we did not find any statistical differences in the Mahalanobis distances between control birds and immigrants. Significant differences may have been obscured by a considerable amount of variation in the initial Mahalanobis distances for the immigrant birds during the baseline period as well as a small sample size. We also observed that calls continued to change even among groups of long-term residents (e.g., the control cages).
The password hypothesis also received little support from behavior or stress data. Our data indicated that there was less aggressive behavior between all birds during weeks 7 and 8 as opposed to the first 2 weeks. Contrary to the predictions of the password hypothesis, however, there was no difference between control and immigrant birds. Thus, although aggressive behavior may be more frequent in birds that are less familiar with one another, those repercussions do not stem from the birds’ failure to vocalize a password call. There was also no evidence that stress levels, as measured by FGM concentrations, were affected by whether or not an immigrant had developed shared calls.
Other hypotheses have been proposed for call sharing including the mate attraction and pair-bonding (Hile et al. 2000; Moravec et al. 2006; Keenan and Benkman 2008; Sewall 2009; Moravec and Striedter 2010) and group cohesion (Brown 1985; Bradbury et al. 2001; Yurk et al. 2002) hypotheses. The mate attraction and pair-bonding hypothesis is specific to mated pairs and suggests that the process of convergence or imitation can have two effects on pair bond formation: (1) it may help reinforce social bonds, and (2) it may enhance individual recognition of one's mate (Hile et al. 2000; Moravec et al. 2006; Keenan and Benkman 2008; Sewall 2009; Moravec and Striedter 2010). The mate attraction and pair-bonding hypothesis thus is not relevant for single-sex groups. The group cohesion hypothesis remains a valid hypothesis for testing in single-sex groups, but would be more appropriate to test in a situation where fission–fusion population dynamics could be more realistically simulated.
Comparisons to previous work in budgerigars
Call sharing has been studied in all-female, all-male, and male–female pairs of budgerigars (Farabaugh and Dooling 1996; Brittan-Powell et al. 1997; Brown and Farabaugh 1997; Bartlett and Slater 1999; Hile et al. 2000; Hile and Striedter 2000; Young 2011). Although both sexes can imitate the calls of other individuals, females do so more slowly, and when in male–female pairs, the males imitate the females’ calls (Hile et al. 2000; Hile and Striedter 2000). Support for a sexual selection and/or pair-bonding function has been supported in male–female studies (Hile et al. 2000; Hile et al. 2005; Moravec et al. 2006; Moravec and Striedter 2010), but since call sharing also occurs between same-sex individuals, it is likely that imitation serves additional functions beyond promotion of the pair bond. Our work supports the hypothesis that call matching may serve different function(s) depending on the social relationships between the individuals.
Some of the call convergence patterns and behavior of budgerigars in our experiment differ from previous work. Previous research has found that females converge to a group call within 4–7 weeks (Hile and Striedter 2000). In contrast, formation of group calls appeared to take longer in our females. Convergence between some pairs of birds occurred quickly, however, with some pairs developing shared calls in the first week. Females in our study also appeared to interact more; they regularly engaged in agonistic behavior and occasionally in affiliative behavior in contrast to females in a previous study (Hile and Striedter 2000), in which females were not observed to engage in any interactions during observation periods. Our extensive sampling of behavior may have provided a more complete picture of their behavioral repertoire. Call-sharing patterns of our immigrant birds in the Immigrant Transfer Phase also contrasted somewhat from previous work, which indicated that transferred birds imitated calls of their new cagemates, while the other cage members did not change their calls (Bartlett and Slater 1999). In contrast, we saw in one case convergence between the immigrant and residents rather than imitation of residents by the immigrant, which supports observations of convergence seen in a parallel study of male budgerigars (Young 2011).
Conclusion
A diversity of taxa including cetaceans, songbirds, parrots, and bats exhibit shared vocalizations. The manner in which calls are shared, how sharing occurs and the function of sharing is as variable as the taxa in which it is found (Mammen and Nowicki 1981; Farabaugh et al. 1988; Brown and Farabaugh 1991; Farabaugh and Dooling 1996; Boughman 1997; Brittan-Powell et al. 1997; Wanker et al. 1998; Smolker and Pepper 1999; Janik 2000; Miller et al. 2004; Wanker et al. 2005; Yang et al. 2007; Scarl and Bradbury 2009; Sewall 2009). Many species with shared calls or sequences live in social groups, and these shared calls appear to mediate social interactions to some degree. Our study is one of the first to test multiple hypotheses for the function of call sharing. Our results support the social association hypothesis and suggest that labeling may be a mechanism that promotes associations within groups. In contrast, we find little support for the password hypothesis. The ability to address vocalizations to specific individuals by changing their structure may be important in large groups where social interactions are frequent.
Acknowledgments
We thank Dr. Dennis Hallford and Chelsea Felker for conducting the FGM RIA. We gratefully acknowledge the assistance of Jim Ha who wrote the Palm Pilot program. We thank Anna Berglund and Josh Criswell for assistance with recording the budgerigars and fecal processing. We thank Alfredo Montoya and the staff of the New Mexico State University Animal Care Facility for maintaining our population of budgerigars and providing laboratory space. We also thank Shannon Pease and Daniel Acosta for assistance with genetic sexing. Additional thanks go to Chelsea Blake and Amy Dundorf for assistance with randomized call sorting. We also thank two anonymous reviewers and Dr. Henrik Brumm for insightful comments that improved the quality of the manuscript. Funding was provided to T.W. by NICHD grant SC1HD068128 and a New Mexico State University Arts and Sciences mini-grant.
Footnotes
Ethical Standards Our experiments comply with the current laws of the USA and were approved by the New Mexico State University IACUC (protocol number 2010–001).
Contributor Information
Christine R. Dahlin, Department of Biology, University of Pittsburgh at Johnstown, Johnstown, PA 15904, USA
Anna M. Young, Department of Biology and Earth Science, Otterbein University, Westerville, OH 43081, USA
Breanne Cordier, Department of Biology, MSC 3AF, New Mexico State University, Las Cruces, NM 88003-0001, USA.
Roger Mundry, Max Planck Institute for Evolutionary Anthropology, Deutscher Platz 6, 04103 Leipzig, Germany.
Timothy F. Wright, Department of Biology, MSC 3AF, New Mexico State University, Las Cruces, NM 88003-0001, USA
References
- Adams DC, Anthony CD. Using randomisation techniques to analyse behavioural data. Anim Behav. 1996;51:733–738. [Google Scholar]
- Adams DM, Balsby TJS, Bradbury JW. The function of double chees in orange-fronted conures (Aratinga canicularis; Psittacidae). Behaviour. 2009;146:171–188. [Google Scholar]
- Baayen RH. Analyzing linguistic data. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- Baglione V, Marcos J, Canestrari D, Ekman J. Direct fitness benefits of group living in a complex cooperative society of carrion crows, Corvus corone corone. Anim Behav. 2002;64:887–893. [Google Scholar]
- Baker MC, Cunningham MA. The biology of bird-song dialects. Behav Brain Sci. 1985;8:85–100. [Google Scholar]
- Balsby TJS, Bradbury JW. Vocal matching by orange-fronted conures (Aratinga canicularis). Behav Process. 2009;82:133–139. doi: 10.1016/j.beproc.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Balsby TJS, Momberg JV, Dabelsteen T. Vocal imitation in parrots allows addressing of specific individuals in a dynamic communication network. PloS ONE. 2012;7:e49747. doi: 10.1371/journal.pone.0049747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsby TJS, Scarl JC. Sex-specific responses to vocal convergence and divergence of contact calls in orange-fronted conures (Aratinga canicularis). Proc R Soc Lond B. 2008;275:2147–2154. doi: 10.1098/rspb.2008.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista LF. The functional significance of song-sharing in the white-crowned sparrow. Can J Zool. 1985;63:1741–1752. [Google Scholar]
- Bartlett P, Slater PJB. The effect of new recruits on the flock specific call of budgerigars (Melopsittacus undulatus). Ethol Ecol Evol. 1999;11:139–147. [Google Scholar]
- Bates D, Maechler M, Bolker B. Ime4: linear mixed-effects models using S4 classes. R package version 0.999999-2. 2013 http://cran.r-project.org/web/packages/lme4/index.html.
- Berg KS, Delgado S, Cortopassi KA, Beissinger SR, Bradbury JW. Vertical transmission of learned signatures in a wild parrot. Proc R Soc Lond B. 2012;279:585–591. doi: 10.1098/rspb.2011.0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughman J. Greater spear-nosed bats give group-distinctive calls. Behav Ecol Sociobiol. 1997;40:61–70. [Google Scholar]
- Bradbury JW, Cortopassi KA, Clemmons JR. Geographical variation in the contact calls of orange-fronted parakeets. Auk. 2001;118:958–972. [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of animal communication. Sinauer Associates; Sunderland: 1998. [Google Scholar]
- Brittan-Powell EF, Dooling RJ, Farabaugh SM. Vocal development in budgerigars (Melopsittacus undulatus): contact calls. J Comp Psychol. 1997;111:226–241. doi: 10.1037/0735-7036.111.3.226. [DOI] [PubMed] [Google Scholar]
- Brown ED. The role of song and vocal imitation among common crows (Corvus brachyrhynchos). Z Tierpsychol. 1985;68:115–136. [Google Scholar]
- Brown ED, Farabaugh SM. Song sharing in a group-living song-bird, the Australian Magpie Gymnorhina tibicen: Part III. Sex specificity and individual specificity of vocal parts in communal chorus and duet songs. Behaviour. 1991;118:244–274. [Google Scholar]
- Brown ED, Farabaugh SM. What birds with complex social relationships can tell us about vocal learning: vocal sharing in avian groups. In: Snowdon CT, Farabaugh SM, editors. Social influence on vocal development. Cambridge University Press; Cambridge: 1997. [Google Scholar]
- Brownie AC. In: The metabolism of adrenal cortical steroids. James VHT, editor. The adrenal gland; Raven, NY: 1992. [Google Scholar]
- Caldwell MC, Caldwell DK, Tyack PL. Review of the signature-whistle hypothesis for the Atlantic bottlenose dolphins. In: Leatherwood S, Reeves RR, editors. The bottlenose dolphins. Academic; New York: 1990. pp. 191–234. [Google Scholar]
- Candiotti A, Zuberbuhler K, Lemasson A. Convergence and divergence in Diana monkey vocalizations. Biol Lett. 2012;8:382–385. doi: 10.1098/rsbl.2011.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier I, Mathevon N, Jouventin P. Mother's voice recognition by seal pups. Nature. 2001;412:873. doi: 10.1038/35091136. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. Vocal recognition in free-ranging vervet monkeys. Anim Behav. 1980;28:362–367. [Google Scholar]
- Cortopassi KA, Bradbury JW. Contact call diversity in wild orange-fronted parakeet pairs, Aratinga canicularis. Anim Behav. 2006;71:1141–1154. [Google Scholar]
- Creel S. Social dominance and stress hormones. Nature. 2001;379:212. [Google Scholar]
- D'Ettorre P, Heinze J. Individual recognition in ant queens. Curr Biol. 2005;15:2170–2174. doi: 10.1016/j.cub.2005.10.067. [DOI] [PubMed] [Google Scholar]
- Dehnhard M, Schreer A, Krone O, Jewgenow K, Krause M, Grossman R. Measurement of plasma corticosterone and fecal glucocorticoid metabolites in the chicken (Gallus domesticus), the great cormorant (Phalacrocorax carbo), and the goshawk (Accipiter gentilis). Gen Comp Endocrinol. 2003;131:345–352. doi: 10.1016/s0016-6480(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Dooling RJ. Perception of vocal signal by budgerigars (Melopsittacus undulatus). Exp Biol. 1986;45:195–218. [PubMed] [Google Scholar]
- Ebensperger LA, Blumstein DT. Sociality in New World hystricognath rodents is linked to predators and burrow digging. Behav Ecol. 2006;12:227–236. [Google Scholar]
- Ebensperger LA, Ramirez-Estrada J, Leon C, Castro RA, Tolhuysen LO, Sobrero R, Quirici V, Burger JB, Soto-Gamboa M, Hayes LD. Sociality, glucorticoids and direct fitness in the communally rearing rodent, Octodon degus. Horm Behav. 2011;60:346–352. doi: 10.1016/j.yhbeh.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Farabaugh SM, Brown ED, Veltman CJ. Song sharing in a group-living songbird, the Australian Magpie Gymnorhina tibicen: Part II. Vocal sharing between territorial neighbors, within and between geographic regions, and between sexes. Behaviour. 1988;104:105–125. [Google Scholar]
- Farabaugh SM, Dooling RJ. Acoustic communication in parrots: laboratory and field studies of Budgerigars, Melopsittacus undulatus. In: Kroodsma DE, Miller EH, editors. Ecology and evolution of acoustic communication in birds. Cornell University Press; Ithaca: 1996. pp. 97–117. [Google Scholar]
- Farabaugh SM, Linzenbold A, Dooling RJ. Vocal plasticity in budgerigars (Melopsitticus undulatus): evidence for social factors in the learning of contact calls. J Comp Psychol. 1994;108:81–92. doi: 10.1037/0735-7036.108.1.81. [DOI] [PubMed] [Google Scholar]
- Feekes F. Song mimesis within colonies in Cacicus c. cela (Icteridae, Aves). A colonial password? Z Tierpsychol. 1982;58:119–152. [Google Scholar]
- Feng AS, Riede T, Arch VS, Yu Z, Zu Z, Yu X, Shen J. Diversity of the vocal signals of concave-eared torrent frogs (Odorrana tormota): evidence for individual signatures. Ethology. 2009;115:1015–1028. [Google Scholar]
- Field A. Discovering statistics using SPSS. Sage Publications; London: 2005. [Google Scholar]
- Fujiwara HE, Kanesada A, Okamoto Y, Satoh R, Watanabe A, Miyamoto T. Long-term maintenance and eventual extinction of preference for a mate's call in the female budgerigar. Anim Behav. 2011;82:971–979. [Google Scholar]
- Goymann W. Non-invasive monitoring of hormones in bird droppings: physiological validation, sampling, extraction, sex differences and the influence of diet on hormone metabolite levels. Ann NY Acad Sci. 2005;1046:35–53. doi: 10.1196/annals.1343.005. [DOI] [PubMed] [Google Scholar]
- Goymann W, Wingfield JC. Allostatic load, social status and stress hormones: the costs of social status matter. Anim Behav. 2004;67:591–602. [Google Scholar]
- Groth JG. Call matching and positive assortative mating in red crossbills. Auk. 1993;110:398–401. [Google Scholar]
- Hass CC, Valenzuela D. Anti-predator benefits of group living in white-nosed coatis (Nasua narica). Behav Ecol Sociobiol. 2002;51:570–578. [Google Scholar]
- Hile AG, Burley NT, Coopersmith CB, Foster VS, Striedter GF. Effects of male vocal learning on female behavior in the budgerigar, Melopsittacus undulatus. Ethology. 2005;111:901–923. [Google Scholar]
- Hile AG, Plummer TK, Striedter GF. Male vocal imitation produces call convergence during pair bonding in budgerigars, Melopsittacus undulatus. Anim Behav. 2000;59:1209–1218. doi: 10.1006/anbe.1999.1438. [DOI] [PubMed] [Google Scholar]
- Hile AG, Striedter GF. Call convergence within groups of female budgerigars (Melopsittacus undulatus). Ethology. 2000;106:1105–1114. doi: 10.1006/anbe.1999.1438. [DOI] [PubMed] [Google Scholar]
- Höjesjö J, Johnsson JI, Petersson E, Järvi T. The importance of being familiar: individual recognition and social behavior in sea trout (Salmo trutta). Behav Ecol. 1998;9:445–451. [Google Scholar]
- Janik VM. Pitfalls in the categorization of behaviour: a comparison of dolphin whistle classification methods. Anim Behav. 1999;57:133–143. doi: 10.1006/anbe.1998.0923. [DOI] [PubMed] [Google Scholar]
- Janik VM. Whistle matching in wild bottlenose dolphins (Tursiops truncatus). Science. 2000;289:1355–1357. doi: 10.1126/science.289.5483.1355. [DOI] [PubMed] [Google Scholar]
- Janik VM. Acoustic communication in delphinids. Adv Stud Behav. 2009;40:123–157. [Google Scholar]
- Janik VM, Slater PJB. Context-specific use suggests that bottlenose dolphin signature whistles are cohesion calls. Anim Behav. 1998;56:829–838. doi: 10.1006/anbe.1998.0881. [DOI] [PubMed] [Google Scholar]
- Janik VM, Slater PJB. The different roles of social learning in vocal communication. Anim Behav. 2000;60:1–11. doi: 10.1006/anbe.2000.1410. [DOI] [PubMed] [Google Scholar]
- Keenan PC, Benkman CW. Call imitation and call modification in red crossbills. Condor. 2008;110:93–101. [Google Scholar]
- Kershenbaum A, Llany A, Blaustein L, Geffen G. Syntactic structure and geographical dialects in the songs of male rock hyraxes. Proc R Soc Lond B. 2012;279:2974–2981. doi: 10.1098/rspb.2012.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SL, Sayigh LS, Wells RS, Fellner W, Janik VM. Vocal copying of individually distinctive signature whistles in bottlenose dolphins. Proc R Soc Lond B. 2013;280 doi: 10.1098/rspb.2013.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschel ANG, Cody ML, Harlow ZT, Promponas VJ, Tayloer EE, Tayloer CE. Territorial dynamics of Mexican Ant-thrushes Formicarius moniliger revealed by individual recognition of their songs. Ibis. 2011;153:255–268. [Google Scholar]
- Kondo N, Izawa EI, Watanabe S. Perceptual mechanism for vocal individual recognition in jungle crows (Corvus macrorhynchos): contact call signature and discrimination. Behaviour. 2010;147:1051–1072. [Google Scholar]
- Lahaye SEP, Eens M, Darras VM, Pinxten R. Testosterone stimulates the expression of male-typical socio-sexual and song behaviors in female budgerigars (Melopsittacus undulatus): an experimental study. Gen Comp Endocrinol. 2012;178:82–88. doi: 10.1016/j.ygcen.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Lemasson A, Gautier J, Hausberger M. Vocal similarities and social bonds in Campbell's money (Cercopithecus Campbelli). Ethology. 2003;326:1185–1193. doi: 10.1016/j.crvi.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Lengagne T. Temporal stability in the individual features in the calls of eagle owls (Bubo bubo). Behaviour. 2001;138:1407–1419. [Google Scholar]
- Lougheed SC, Handford P. Vocal dialects and the structure of geographic variation in the morphological and allozymic characters in the rufous-collared sparrow, Zonotrichia capensis. Evolution. 1992;46:1443–1456. doi: 10.1111/j.1558-5646.1992.tb01135.x. [DOI] [PubMed] [Google Scholar]
- Mammen DL, Nowicki S. Individual differences and within-flock convergence in chickadee calls. Behav Ecol Sociobiol. 1981;9:179–186. [Google Scholar]
- Manly BFJ. Randomization, bootstrap and Monte Carlo methods in biology. Chapman & Hall; New York: 1997. [Google Scholar]
- McComb K, Moss C, Sayialel S, Baker L. Unusually extensive networks of vocal recognition in African elephants. Anim Behav. 2000;59:1103–1109. doi: 10.1006/anbe.2000.1406. [DOI] [PubMed] [Google Scholar]
- Miller PJO, Shapiro AD, Tyack PL, Solow AR. Call-type matching in vocal exchanges of free-ranging killer whales, Orcinus orca. Anim Behav. 2004;67:1099–1107. [Google Scholar]
- Mitani JC, Brandt KL. Social factors influence the acoustic variability in the long-distance calls of male chimpanzees. Ethology. 1994;96:233–252. [Google Scholar]
- Mitani JC, Watts DPaL, JS . Ecological and social correlates of chimpanzee party size and composition. In: Boesch C, Lohmann G, Marchant LL, editors. Behavioural diversity in chimpanzees and bonobos. Cambridge Univeristy Press; Cambridge, UK: 2002. pp. 102–111. [Google Scholar]
- Mooring MS, Hart BL. Animal grouping for protection from parasites: selfish herd and encounter-dilution effects. Behaviour. 1992;123:173–193. [Google Scholar]
- Moravec ML, Striedter GF. “Virtual parrots” confirm mating preferences of female budgerigars. Ethology. 2010;116:961–971. [Google Scholar]
- Moravec ML, Striedter GF, Burley NT. Assortative pairing based on contact call similarity in budgerigars, Melopsittacus undulatus. Ethology. 2006;112:1108–1116. [Google Scholar]
- Möstl E, Rettenbacher S, Palme R. Measurement of corticosterone metabolites in birds’ droppings: an analytical approach. Ann NY Acad Sci. 2005;1046:17–34. doi: 10.1196/annals.1343.004. [DOI] [PubMed] [Google Scholar]
- Mundinger PC. Vocal imitation and individual recognition of finxh calls. Science. 1970;168:480–482. doi: 10.1126/science.168.3930.480. [DOI] [PubMed] [Google Scholar]
- Mundry R, Sommer C. Discriminant function analysis with nonindependent data: consequences and an alternative. Anim Behav. 2007;74:965–976. [Google Scholar]
- Myasato LE, Baker MC. Black-capped chickadee call dialects along a continuous habitat corridor. Anim Behav. 1999;6:1311–1318. doi: 10.1006/anbe.1999.1109. [DOI] [PubMed] [Google Scholar]
- Nousek AE, Slater PJB, Wang C, Miller PJO. The influence of social affiliation on individual vocal signatures of northern resident killer whales (Orcinus orca). Biol Lett. 2006;22:481–484. doi: 10.1098/rsbl.2006.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki S, Nelson DA. Defining natural categories in acoustic signals: comparison of three methods applied to ‘chick-a-dee’ call notes. Ethology. 2010;86:89–101. [Google Scholar]
- O'Brien TG. Female–male social interactions in wedge-capped capuchin monkeys: benefits and costs of group living. Anim Behav. 1991;41:555–567. [Google Scholar]
- Pease SM, Salina-Melgoza A, Escalante P, Renton K, Wright TF. Offspring sex ratios in the lilac-crowned parrot (Amazona finschi). Wilson J Ornithol. 2012;124:393–396. [Google Scholar]
- Price JJ. Communication with shared call repertoires in the cooperatively breeding stripe-backed wren. J Field Ornithol. 2003;74:166–171. [Google Scholar]
- Quinn GP, Keough MJ. Experimental designs and data analysis for biologists. Cambridge University Press; Cambridge: 2002. [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. http://www.R-project.org. [Google Scholar]
- Rendall D, Rodman PS, Emond RE. Vocal recognition of individuals and kin in free-ranging rhesus monkeys. Anim Behav. 1996;51:1007–1015. [Google Scholar]
- Salinas-Melgoza A, Wright TF. Vocal learning and limited dispersal as dual mechanisms for dialect maintenance in a parrot. PLoS ONE. 2012;7:e48667. doi: 10.1371/journal.pone.0048667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W, Schulz-Darken NJ, Scheffler G, Wegner FH, Abbott DH. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiol Behav. 1994;56:801–810. doi: 10.1016/0031-9384(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, social status, and reproductive physiology in free-living baboons. Prentice-Hall; Englewood Cliffs: 1987. [Google Scholar]
- Sayigh LS, Esch HC, Janik VM. Facts about signature whistles of bottlenose dolphins (Tursiops truncatus). Anim Behav. 2007;74:1631–1642. [Google Scholar]
- Sayigh LS, Tyack PL, Wells RS, Scott MD. Signature whistles of free-ranging bottlenosed dolphins, Tursiops truncatus: stability and mother-offspring comparisons. Behav Ecol Sociobiol. 1990;26:247–260. [Google Scholar]
- Scarl JC, Bradbury JW. Rapid vocal convergence in an Australian cockatoo, the galah Eolophus roseicapillus. Anim Behav. 2009;77:1019–1026. [Google Scholar]
- Searcy WA, Beecher MD. Song as an aggressive signal in song-birds. Anim Behav. 2009;78:1281–1292. [Google Scholar]
- Sewall KS. Limited adult vocal learning maintains call dialects but permits pair-distinctive calls in red crossbills. Anim Behav. 2009;77:1303–1311. [Google Scholar]
- Silva APD, Stam A. Discriminant Analysis. In: Grimm LG, Yarnold PR, editors. Reading and understanding multivariate statistics. American Psychological Association; Washington: 1996. pp. 277–318. [Google Scholar]
- Slater JB. Bird song learning: causes and consequences. Ethol Ecol Evol. 1989;1:19–46. [Google Scholar]
- Smolker R, Pepper JW. Whistle convergence among allied male bottlenose dolphins (Delphinidae, Tursiops sp.). Ethology. 1999;105:595–617. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry—the principles and practice of statistics in biological research. Freeman; New York: 1995. [Google Scholar]
- Stiles GF. Ecology and evolution of lek mating ehavior in the long-tailed hermit hummingbird. Ornithol Monogr. 1979;27:1–74. [Google Scholar]
- Striedter GF, Freibott L, J Höjesjö AG, Burley NT. For whom the male calls: an effect of audience on contact call rate and repertoire in budgerigars, Melopsittacus undulatus. Anim Behav. 2003;65:875–882. [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. Allyn & Bacon; Boston: 2001. [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho CE, Bijan P, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- Tibbetts EA. Visual signals of individual identity in the wasp Polistes fuscatus. Proc R Soc Lond B. 2002;269:1423–1428. doi: 10.1098/rspb.2002.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma C, Palme R. Measuring fecal glucorticoid metabolites in mammals and birds: the importance of validation. Ann NYAcad Sci. 2005;1046:54–74. doi: 10.1196/annals.1343.006. [DOI] [PubMed] [Google Scholar]
- Tyack P. Convergence of calls as animals form social bonds, active compensation for noisy communication channels, and the evolution of vocal learning in mammals. J Comp Psychol. 2008;122:319–331. doi: 10.1037/a0013087. [DOI] [PubMed] [Google Scholar]
- Tyack PL. Development and social functions of signature whistles in bottlenose dolphins Tursiops truncatus. Bioacoustics. 1997;8:21–46. [Google Scholar]
- Tyack PL. Dolphins whistle a signature tune. Anim Behav. 2000a;289:1310–1311. doi: 10.1126/science.289.5483.1310. [DOI] [PubMed] [Google Scholar]
- Tyack PL. Functional aspects of cetacean communication. In: Mann J, Connor RC, Tyack PL, Whitehead H, editors. Field studies of dolphins and whales. University of Chicago Press; Chicago: 2000b. pp. 270–307. [Google Scholar]
- Tyack PL. Dolphins communicate about individual-specific social relationships. In: De Waal FBM, Tyack PL, editors. Animal social complexity, intelligence and individualized societies. Harvard University Press; Cambridge: 2003. [Google Scholar]
- Uetz GW, Hieber CS. Colonial web-building spiders: balancing the costs and benefits of group-living. In: Choe JC, Crespo BJ, editors. The evolution of social behavior in insects and arachnids. Cambridge University Press; Cambridge: 1997. [Google Scholar]
- Valone TJ. Group foraging, public information, and patch estimation. Oikos. 1989;56:357–363. [Google Scholar]
- Vehrencamp SL. Is song-type matching a conventional signal of aggressive intentions? Proc R Soc Lond B. 2001;268:1637–1642. doi: 10.1098/rspb.2001.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehrencamp SL, Ritter AF, Keever M, Bradbury JW. Responses to playback of local vs distant contact calls in the orange-fronted conure, Aratinga canicularis. Ethology. 2003;109:37–54. [Google Scholar]
- Venables WN, Ripley BD. Modern Applied Statistics with S. Springer; New York: 2002. [Google Scholar]
- Wanker R, Apcin J, Jennerjahn B, Waibel B. Discrimination of different social companions in spectacled parrotlets (Forpus conspicillatus): evidence for individual vocal recognition. Behav Ecol Sociobiol. 1998;43:197–202. [Google Scholar]
- Wanker R, Sugama Y, Prinage S. Vocal labelling of family members in spectacled parrotlets, Forpus conspicillatus. Anim Behav. 2005;70:111–118. [Google Scholar]
- Watwood SL, Tyack PL, Wells RS. Whistle sharing in paired male bottlenose dolphins, Tursiops truncatus. Behav Ecol Sociobiol. 2004;55:531–543. [Google Scholar]
- Wright TF, Dahlin CR, Salinas-Melgoza A. Stability and change in vocal dialects of the yellow-naped amazon. Anim Behav. 2008;76:1017–1027. doi: 10.1016/j.anbehav.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndham E. Diurnal cycle, behavior and social organization of the budgerigar, Melopsittacus undulatus. Emu. 1980;80:25–33. [Google Scholar]
- Yang X, Lei F, Wang G, Jesse A. Syllable sharing and inter-individual syllable variation in Anna's hummingbird Calypte anna songs, in San Francisco, California. Folia Zool. 2007;56:307–318. [Google Scholar]
- Young A. Dissertation. New Mexico State University; Las Cruces: 2011. Vocal learning in budgerigars, Melopsittacus undulatus: process and function. [Google Scholar]
- Young A, Hallford D. Validation of a fecal glucocorticoid metabolite assay to assess stress in the budgerigar (Melopsittacus undulatus). Zoo Biol. 2012;10:112–116. doi: 10.1002/zoo.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurk H, Barrett-Lennard L, Ford JKB, Matkins CO. Cultural transmission within maternal lineages: vocal clans in resident killer whales in southern Alaska. Anim Behav. 2002;63:1103–1119. [Google Scholar]