Abstract
Activation of the angiotensin II type 2 receptors (AT2R) has been considered cardioprotective. However, there are controversial findings regarding the role of overexpressing AT2R in the heart. Using transgenic mice with different levels of AT2R gene overexpression in the heart (1, 4 or 9 copies of the AT2R transgene, Tg1, Tg4 or Tg9), we studied the effect of AT2R overexpression on left ventricular remodeling and dysfunction post-myocardial infarction (MI). Tg1, Tg4, Tg9 and their wild-type (WT) littermates were divided into 1) sham MI, 2) MI + vehicle, and 3) MI + AT2R antagonist. Treatments were started 4 weeks after MI and continued for 8 weeks. AT2R protein and mRNA expression in the heart was significantly increased in transgenic mice and the increase positively correlated with copies of the transgene. AT1R mRNA and protein expression remained unchanged in Tg1 and Tg4 but slightly higher in Tg9 mice. Systolic blood pressure and cardiac phenotypes did not differ among strains under basal conditions. MI caused myocardial hypertrophy, interstitial fibrosis, ventricular dilatation and dysfunction associated with increased protein expression of Nox2 and transforming growth factor (TGF)-β1 expression. These pathological responses were diminished in Tg1 and Tg4 mice. Moreover, the protective effects of AT2R were abolished by AT2R antagonist and also absent in Tg9 mice. We thus conclude that whether overexpression of AT2R is beneficial or detrimental to the heart is largely dependent on expression levels and possibly via regulations of Nox2 and TGF-β1 signaling pathways.
Keywords: angiotensin II type 1 receptor, angiotensin II type 2 receptor, myocardial infarction, transgenic mice
INTRODUCTION
Angiotensin II (Ang II), the principal effector of the renin-angiotensin system (RAS), binds to two distinct receptors, AT1R and AT2R. AT1R is ubiquitous and abundant in adult tissues and known to be responsible for most of the detrimental cardiovascular effects of Ang II, whereas expression of AT2R is high in the fetus, rapidly reduced after birth, and up-regulated in response to pathological stimuli such as hypertension and myocardial ischemia.1,2 AT2R has been suggested to exert cardioprotective properties most likely via kinin/nitric oxide (NO) mediated mechanisms.3–6 Using an animal model of heart failure induced by myocardial infarction (MI), we and others demonstrated that activation of AT2R contributes to the cardioprotective effects of Ang II receptor blocker (ARB).7–9 Studies using in vivo gene-transfer techniques or transgenic mice with AT2R specifically overexpressed in the heart showed that activation/overexpression of AT2R attenuated pressure- or ischemia-induced cardiac remodeling and dysfunction.10–13 Likewise, deletion of AT2R enhanced mortality and aggravated cardiac dysfunction.14 These data support a cardioprotective role of AT2R.
However, controversial findings have been reported. D’Amore et al found that in cultured neonatal cardiomyocytes overexpression of AT2R caused hypertrophy that correlated positively with the level of AT2R expression.15 Yan et al16 and Nakayama et al17 showed that overexpressing AT2R specifically in ventricular cardiomyocytes decreased cardiac contractility and caused dilated cardiomyopathy, and the severity of cardiac dysfunction correlated positively with copy numbers of the AT2R transgene. Because AT2 remains at a very low level in the normal adult heart, we hypothesize that whether activation/overexpression of AT2R is beneficial or detrimental to the heart largely depends on its level of expression. Overexpression at high amounts, associated with increased AT1, could be detrimental via a signaling mechanism similar to AT1. For this, we used transgenic mice with different levels of AT2R overexpression (1, 4 or 9 copies of the AT2R transgene, Tg1, Tg4 or Tg9) specifically in ventricular cardiomyocytes to study 1) whether mice with low copy numbers of AT2R transgene (Tg1 or Tg4) had less severe cardiac hypertrophy and remodeling and better preserved left ventricular (LV) function post-MI and these beneficial effects were diminished by AT2R blockade, and 2) whether AT2R overexpression at high levels (Tg9) worsened LV remodeling and dysfunction post-MI, which involves increased oxidative stress and TGFβ1-mediated fibrotic and inflammatory responses.
MATERIALS AND METHODS
Animals
Male transgenic mice with overexpression of 1, 4 or 9 copies of the AT2R transgene (Tg1, Tg4 or Tg9) were kindly provided by Dr. Xinhua Yan (Tufts University - St. Elizabeth's Medical Center of Boston) and bred in our mutant mouse facility. The AT2R transgene was driven by the myosin light chain promoter and expressed specifically at ventricular cardiomyocytes. Their wild-type (WT) littermates were used as controls. Mice were housed in an air-conditioned room with a 12-hour light/dark cycle and given standard chow with free access to tap water. This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Health System. All studies were conducted in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals.
Induction of myocardial infarction and experimental protocols
When mice were 11–12 weeks of age, MI was created by ligating the left anterior descending coronary artery as described previously.8 Four weeks after surgery, each strain of mice (WT, Tg1, Tg4, and Tg9) was divided into 3 groups: 1) sham MI; 2) MI + vehicle; and 3) MI + AT2-antagonist (AT2-ant; PD123319, 20 mg/kg/day (provided by Pfizer Inc) via osmotic minipump (Alzet, Cupertino, CA). Treatment was started 4 weeks after MI to avoid effects on infarct size (IS) and healing and continued for 8 weeks.
Systolic blood pressure (SBP) and cardiac function were measured in conscious mice as described previously6,18,19.
Histopathological study
Heart weight, myocyte cross-sectional area (MCSA) and interstitial collagen fraction (ICF) were measured as described previously6,18,19.
Real-time PCR of AT1R and AT2R mRNA expression and Western blot for AT2R, endothelial nitric oxide synthase (eNOS), Nox2 and TGF-β1 protein expression
Detailed methods are available in online-only Data Supplements.
Data analysis
All data are expressed as mean ± SE. Student’s two-sample t-test was used to compare differences between strains or between treatments within strains. When multiple comparisons were performed, Hochberg’s step-up procedure was used to adjust p-values. The family-wise type I error rate was set at 0.05.
RESULTS
AT2R and AT1R protein and mRNA expression
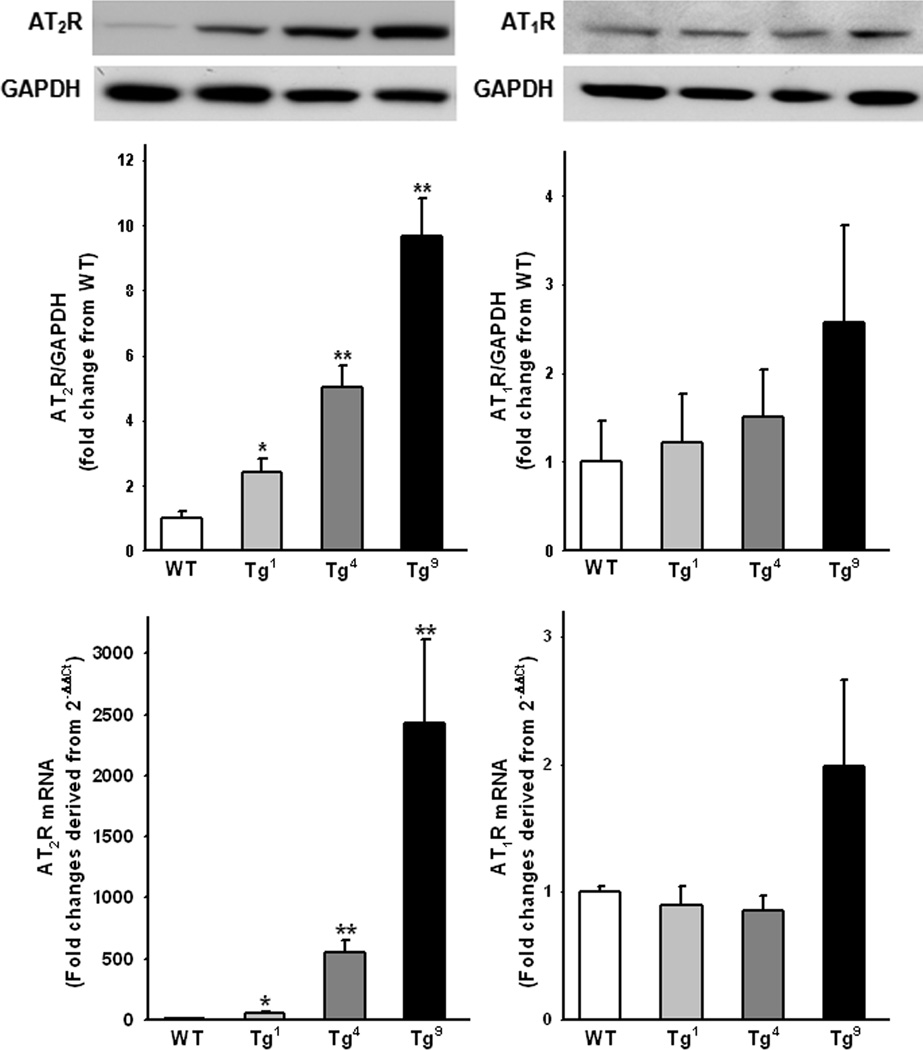
AT2R protein and mRNA expression in the heart was very low in WT mice but significantly increased in Tg1, Tg4 and Tg9 mice. The levels of increase correlated positively with copies of the transgene (Fig 1, left panels). AT1R expression was similar in WT, Tg1 and Tg4 mice but was higher in Tg9 mice, though the increase did not reach statistical significance (Fig. 1, right panels).
Figure 1.
AT2R and AT1R mRNA (top) and protein (bottom) expression in mice with 1, 4 or 9 copies of the AT2R transgene (Tg1, Tg4 or Tg9). Data are presented as fold change relative to wild-type (WT) controls. n = 4 – 6 in each group. *: p < 0.05, **: p < 0.01 Tg1, Tg4 or Tg9 vs WT controls.
Early and late mortality
The early mortality rate (within 24 hours after MI) was similar among the strains. During the first week of MI, 36% of the WT mice, 11% of the Tg1 mice, 23% of the Tg4 mice and 31% of the Tg9 mice died, mostly from cardiac rupture (p < 0.01 WT vs Tg1 and p < 0.05 Tg9 vs Tg1). Thereafter, there was no difference in mortality among groups (see Fig S1. in Online Data Supplement).
SBP and heart rate (HR)
Basal SBP and HR were similar among groups and remained unchanged in sham-operated mice. MI caused a decrease in SBP similarly in all strains, although the change did not reach statistical significance (Table 1). Blockade of AT2R had no effect on SBP in all groups. HR was not affected by MI or treatment with AT2R antagonist.
Table 1.
Effect of cardiac specific overexpression of AT2R on SBP, HR, tissue weight and infarct size post-MI.
| Group | WT | Tg1 | Tg4 | Tg9 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham n = 11 |
MI | Sham n = 9 |
MI | Sham n =12 |
MI | Sham n = 10 |
MI | |||||
| Parameters | Veh n = 14 |
AT2R-ant n = 12 |
Veh n = 12 |
AT2R-ant n = 14 |
Veh n = 8 |
AT2R-ant n = 8 |
Veh n = 11 |
AT2R-ant n = 11 |
||||
| BW (g) | 31.9±1 | 33.0±1 | 31.2±1 | 33.7±2 | 33.9±1 | 31.9±1 | 31.4±1 | 32.7±1 | 31.9±1 | 30.0±2 | 28.2±2 | 28.8±2 |
| SBP (mmHg) | 110±3 | 102±3 | 107±3 | 111±4 | 104±2 | 107±4 | 110±3 | 103±4 | 96±5 | 114±3 | 107±2 | 106±2 |
| HR (beats/min) | 665±13 | 698±12 | 667±12 | 674±12 | 671±17 | 653±14 | 657±18 | 675±13 | 642±30 | 681±11 | 681±9 | 674±9 |
| LVW (mg/10 g) | 30.7±1 | 44.3±2* | 45.4±2 | 28.6±2 | 38.5±2*† | 45.1±2‡ | 28.7±1 | 40.4±2* | 44.2±2 | 32.5±1 | 45.9±2*¶ | 42.8±1 |
| THW (mg/10 g) | 41.0±1 | 61.4±3* | 61.0±3 | 39.6±2 | 51.7±3*† | 62.0±3‡ | 38.9±1 | 56.3±3* | 60.7±3 | 41.8±1 | 59.3±2*¶ | 55.1±2 |
| Lungs (mg/10 g) | 55.0±3 | 61.7±5 | 65.5±6 | 55.5±4 | 55.2±3 | 71.1±5‡ | 57.0±2 | 57.5±6 | 57.1±2 | 54.7±3 | 65.0±4*¶ | 64.1±4 |
| Liver (mg/10 g) | 445±17 | 426±10 | 482±16‡ | 436±20 | 407±8 | 438±9‡ | 421±10 | 391±16 | 488±26‡ | 396±15 | 399±14 | 452±16‡ |
| IS (%) | -- | 33.3±2 | 33.1±4 | -- | 30.2±8 | 34.8±2 | -- | 31.3±2 | 33.9±2 | -- | 30.6±2 | 33.9±5 |
WT: wild-type littermates; Tg1, Tg4 and Tg9: mice with AT2R specifically overexpressed in ventricular cardiomyocytes (1, 4 or 9 copies of the AT2R transgene); MI: myocardial infarction; Veh: vehicle; AT2R-ant: angiotensin II type 2 receptor antagonist, PD123319; BW: body weight; SBP: systolic blood pressure; HR: heart rate; LVW: left ventricular weight corrected by body weight; THW: total heart weight corrected by body weight; lung and liver weight corrected by body weight; IS: infarct size.
: p < 0.05, MI+ vehicle vs sham groups within strain;
: p < 0.05, Tg1, Tg4 or Tg9 vs WT within treatments;
: p < 0.05, AT2R vs vehicle within strain;
: p < 0.05, Tg9 vs Tg1 or Tg4.
Body and tissue weight and infarct size
Body and organ weights were similar among strains with sham MI. MI significantly increased heart weight in all strains but no strain difference was detected. MI per se also increased lung weight in WT and Tg9 strains but not in Tg1 and Tg4 groups. However, this protective effect on lung congestion was reversed by blockade of AT2R in Tg1 mice (Table 1). MI did not affect liver weight; although blockade of AT2R caused significant liver congestion, no strain difference was seen (Table 1). Infarct size was similar in all strains with or without AT2R antagonist (Table 1).
Cardiac function and remodeling
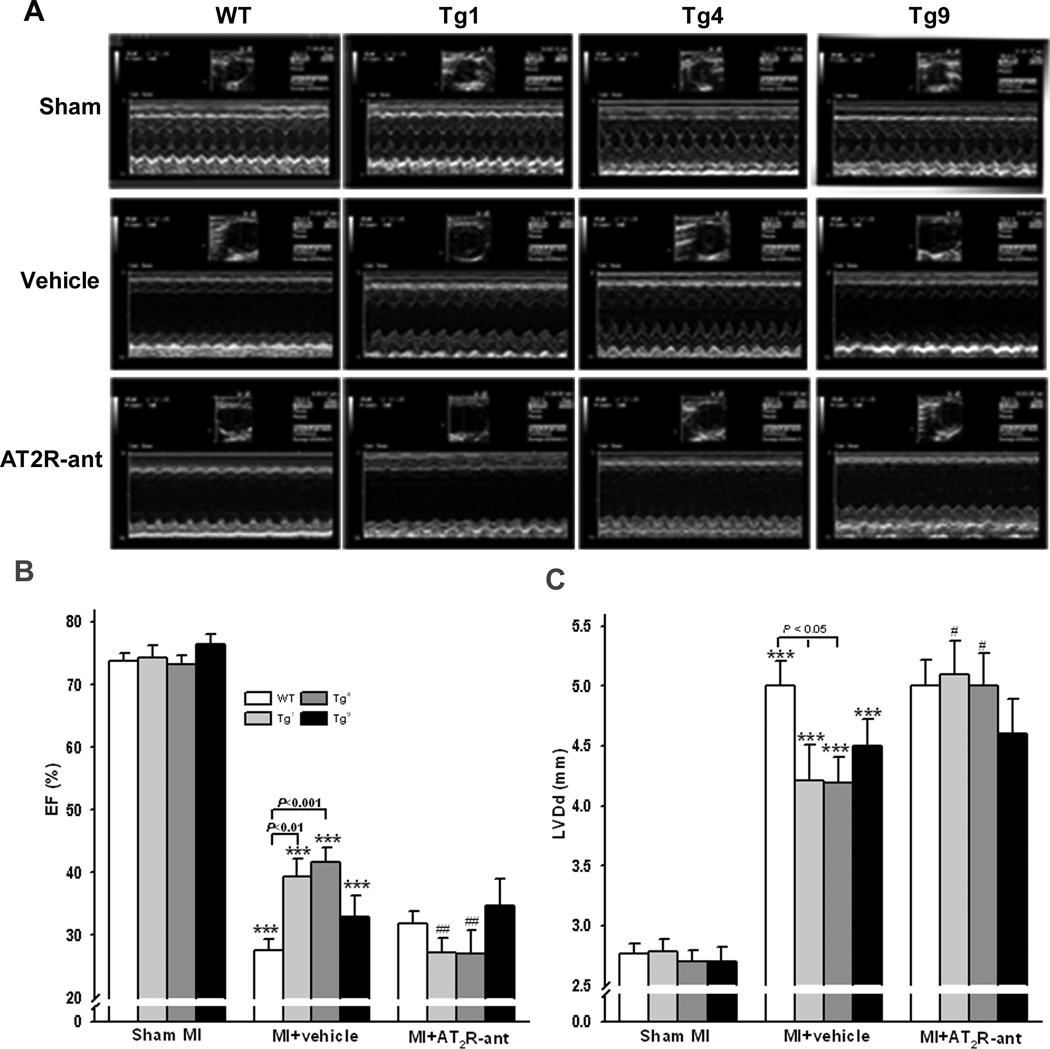
Overexpression of AT2R with different copy numbers had no effect on cardiac morphology and function under basal conditions, since left ventricular diastolic dimension (LVDd) and ejection fraction (EF) were similar among WT, Tg1, Tg4 and Tg9 mice with sham MI (Fig. 2). MI caused LV dilatation and dysfunction in all strains as evidenced by increased LVDd and decreased EF; however, these detrimental cardiac effects were less severe in Tg1 and Tg4 mice compared to WT controls. The cardioprotective effects observed in Tg1 and Tg4 mice were completely blunted by AT2R blockade (Fig. 2).
Figure 2.
Effect of cardiac-specific overexpression of AT2R with or without AT2R antagonist (AT2R-ant, PD123319) on left ventricular (LV) ejection fraction (EF) and diastolic dimension (LVDd) post MI. A: representative echocardiograms; B and C: quantitative analysis of EF and LVDd, respectively. ***: p < 0.001 MI + vehicle vs sham groups within strains; #: p < 0.05, ##: p < 0.01 MI + AT2R-ant vs MI + vehicle within strains.
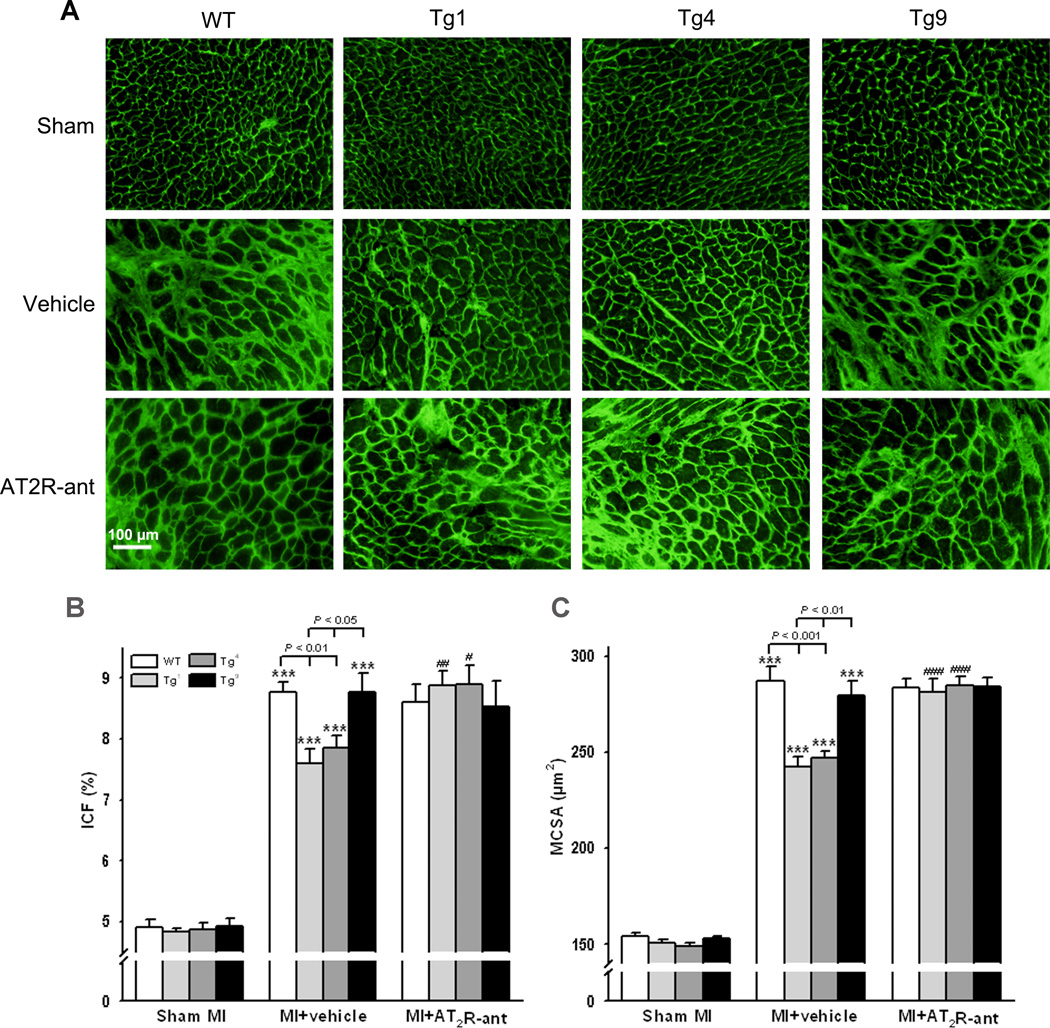
Myocyte hypertrophy and cardiac interstitial fibrosis
MCSA and ICF were similar among WT, Tg1, Tg4, and Tg9 groups receiving sham operations. MI increased MCSA and ICF in all strains; however, the hypertrophic and fibrotic responses were less severe in Tg1 and Tg4 compared to WT controls or Tg9 mice (Fig. 3). Blockade of AT2R reversed the anti-hypertrophic and anti-fibrotic effects observed in Tg1 and Tg4 mice. MCSA and ICF did not differ between WT and Tg9 mice treated with either vehicle or AT2R antagonist (Fig. 3).
Figure 3.
Effect of cardiac-specific overexpression of AT2R with or without AT2R-ant on myocyte cross-sectional area (MCSA) and interstitial collagen fraction (ICF) post- MI. A: representative images of MCSA and interstitial collagen deposition; B and C: quantitative analysis of MCSA and ICF, respectively. ***: p < 0.001 MI + vehicle vs sham groups within strains; #: p < 0.05, ##: p < 0.01, ###: p < 0.001 MI + AT2R-ant vs MI + vehicle within strains.
Protein expression of eNOS, Nox2 and TGF-β1 in the heart
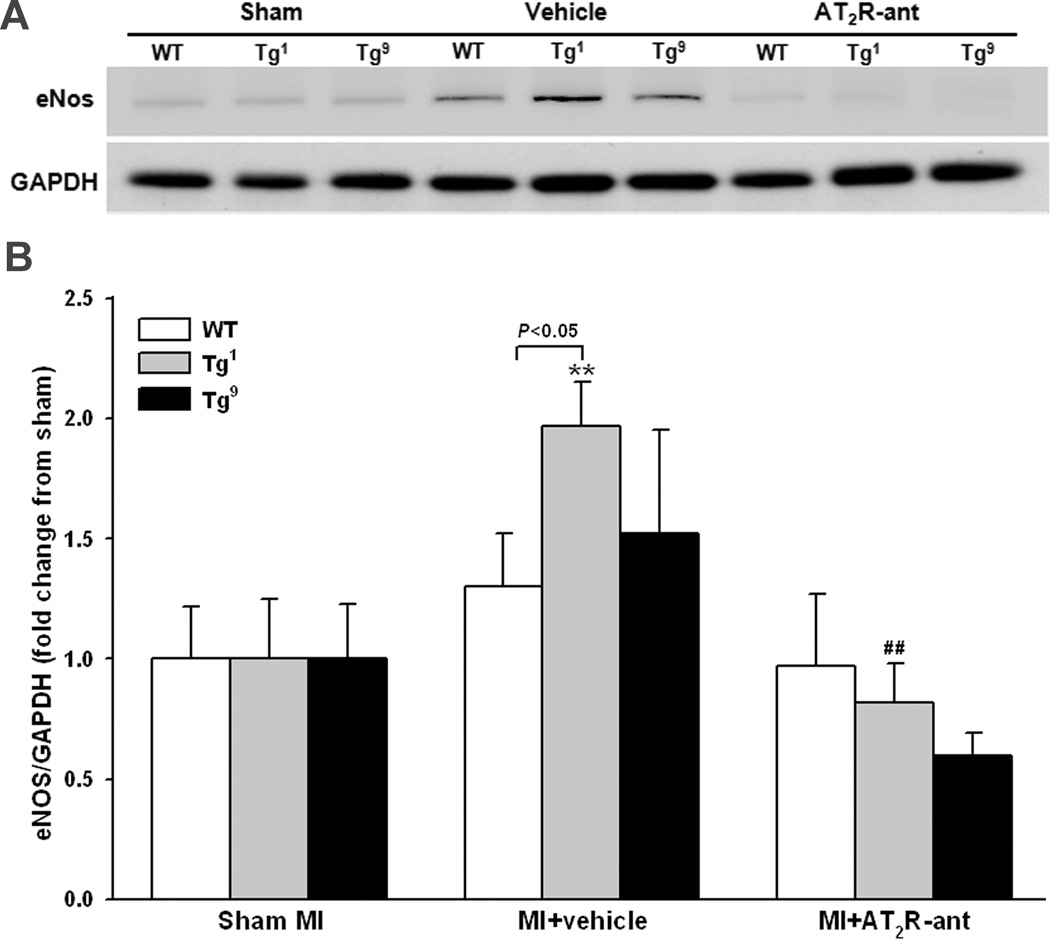
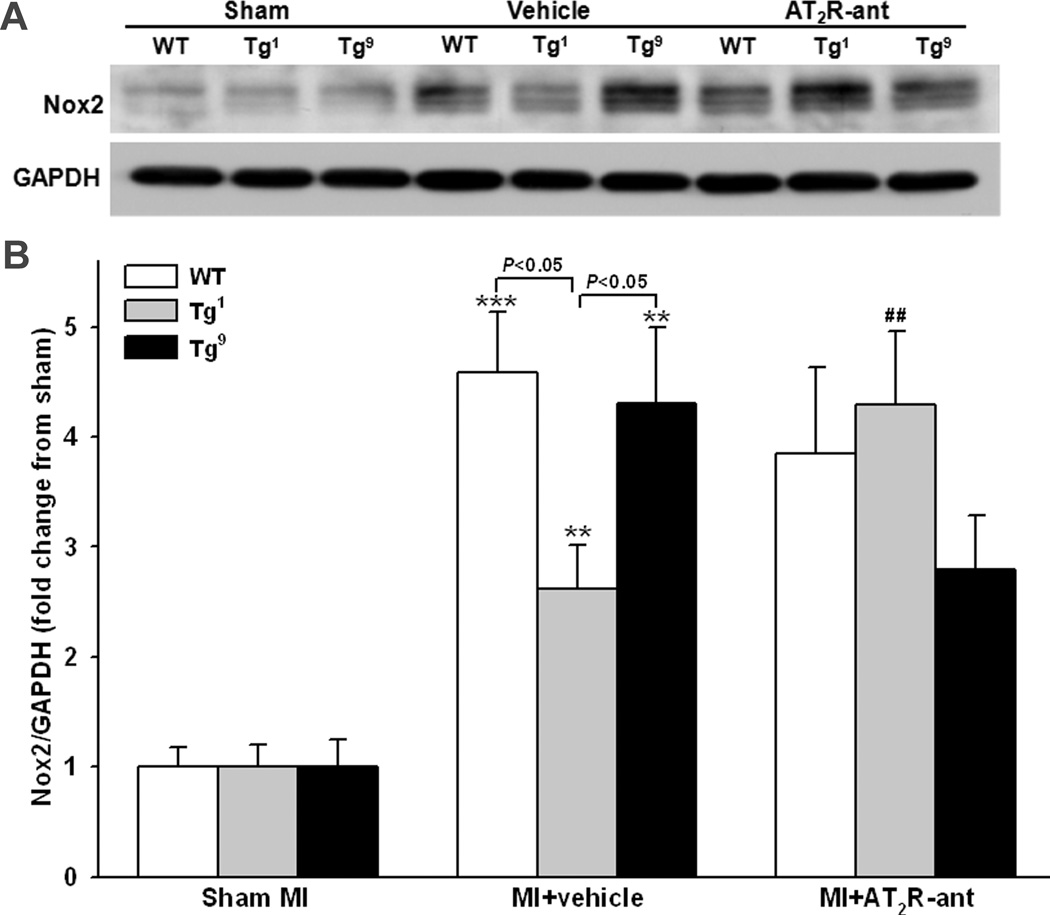
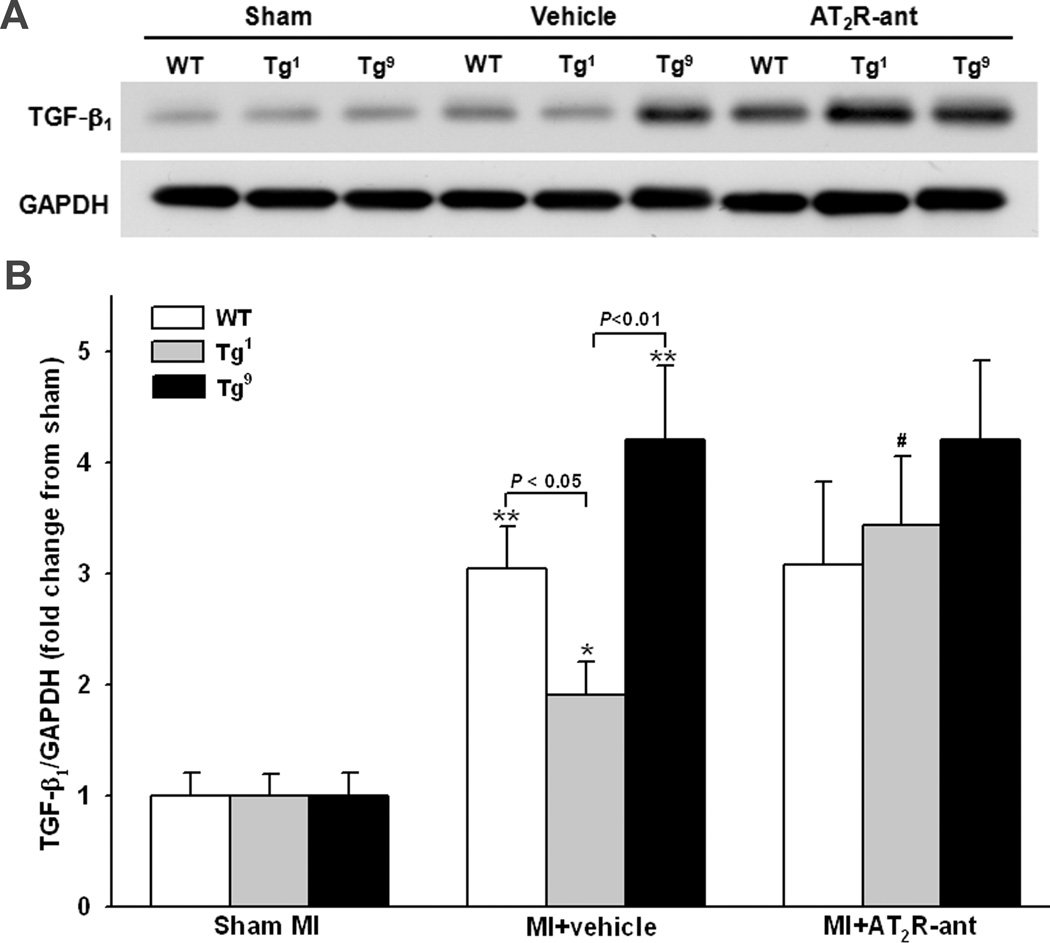
Protein expression of eNOS, Nox2 and TGF-β1 was determined in all strains (since there was no difference between Tg1 and Tg4, only data from Tg1 are presented in the figures). eNOS, Nox2 and TGF-β1 expression did not differ among strains with sham MI. MI increased eNOS expression in Tg1 and Tg4 mice and this effect was completely blocked by AT2R antagonist (Fig. 4). AT2R antagonist also tended to decrease eNOS expression in Tg9 mice compared to vehicle treated mice of same strain, but the difference did not reach statistical significance. Nox2 expression was increased in all groups after MI; however, the increase was less prominent in Tg1 and Tg4 compared to WT and Tg9 groups (Fig. 5). AT2R antagonist reversed the effect observed in Tg1 and Tg4 but had no effect in WT. AT2R antagonist tended to lower Nox2 expression in Tg9 mice but the difference was not statistically significant. TGF-β1 protein expression was also increased in all groups, but the increase was insignificant in Tg1 and Tg4 and this effect was blunted by AT2R antagonist (Fig. 6). Blockade of AT2R did not affect TGF-β1 expression in WT and Tg9 groups.
Figure 4.
Effect of cardiac-specific overexpression of AT2R with or without AT2R-ant on endothelial NOS (eNOS) protein expression post-MI. A: Representative Western blots of eNOS and GAPDH. B: Semi-quantitative analysis of eNOS protein corrected by GAPDH and expressed as fold change relative to sham controls within strain. n = 8 in each group. **: p < 0.01 MI + vehicle vs sham groups within strains; ##: p < 0.01 MI + AT2R-ant vs MI + vehicle within strains.
Figure 5.
Effect of cardiac-specific overexpression of AT2R with or without AT2R-ant on Nox2 protein expression post-MI. A: Representative Western blots of Nox2 and GAPDH. B: Semi-quantitative analysis of Nox2 protein corrected by GAPDH and expressed as fold change relative to sham controls within strain. n = 8 in each group. **: p < 0.01, ***: p < 0.001 MI + vehicle vs sham groups within strains; ##: p < 0.01 MI + AT2R-ant vs MI + vehicle within strains.
Figure 6.
Effect of cardiac-specific overexpression of AT2R with or without AT2R-ant on TGF-β1 protein expression post-MI. A: Representative Western blots of TGF-β1 and GAPDH. B: Semi-quantitative analysis of TGF-β1 protein corrected by GAPDH and expressed as fold change relative to sham controls within strain. n = 8 in each group. *: p < 0.05, **: p < 0.01 MI + vehicle vs sham groups within strains; #: p < 0.05 MI + AT2R-ant vs MI + vehicle within strain.
DISCUSSION
Our present study shows that transgenic mice with cardiac-specific AT2R overexpression had a significant increase in AT2R mRNA and protein expression, which positively correlated with copies of the transgene. Furthermore, overexpression of AT2R at excessive levels as observed in mice with 9 copies of the transgene (Tg9) tended to increase AT1R expression. Under basal conditions, overexpression of AT2R either at high or low levels did not affect blood pressure, cardiac morphology and function. When subjected to MI, mice with a low number of AT2R transgene copies (Tg1 and Tg4) had a lower mortality rate and less severe LV remodeling and dysfunction; these effects were abolished by AT2R antagonist. In mice with higher copies of the transgene (Tg9) and increased AT1R expression, the cardioprotective effect of AT2R vanished. These data suggest that under normal conditions overexpression of AT2R specifically in ventricular cardiomyocytes does not play an important role in the regulation of blood pressure, cardiac morphology or function. However, in response to cardiac injury such as MI, overexpression of cardiac AT2R protects the heart against adverse remodeling and dysfunction, but these protective effects largely rely on the AT2R expression levels. Indeed, low or moderate overexpression of AT2R is beneficial, but excessively overexpressing AT2R could be detrimental to the heart.
Two major Ang II receptors, AT1R and AT2R, have been identified. AT1R is widely expressed in adult organs and mediates the major effects of Ang II. Conversely, AT2R is reportedly expressed at high levels in fetus and its expression decreases rapidly after birth and remains low in the adult heart, but relatively abundant in the vasculature.1 In response to pathological stimuli such as hypertension, cardiac hypertrophy and/or ischemia, AT2R is upregulated in both humans and experimental animals, probably as a compensatory mechanism in response to increased renin-angiotensin activity.1,2 It has been reported that activation of AT2R counteracts AT1-mediated actions, which in most cases is believed to be cardiac beneficial.20 We and others previously demonstrated that activation of AT2R contributes to the cardioprotective effects of ARB, possibly via release of kinins and NO.3,4,6,8,21 Since AT2R is upregulated in cardiovascular disease (CVD)1,2 and activation of AT2R by blocking AT1R by ARB leads to cardioprotection, it is clinically relevant to overexpress AT2R at levels that do not overly exceed the physiological limits and to develop and study new AT2R ligands for the treatment of cardiovascular disease. Indeed, the cardioprotective role of AT2R is further supported by a number of studies involving activation and/or overexpression of AT2 in cardiomyocytes, cardiac tissue and blood vessels.10,12,13 In the present study we found that mice with low or moderate overexpression of AT2R had less severe cardiac hypertrophy, fibrosis and chamber dilatation coupled with better preserved cardiac function compared to WT. MI-induced increase in Nox2 and TGF-β1 protein expression were also attenuated in Tg1 and Tg4 mice. None of these protective effects were seen in Tg9 mice Furthermore, the protective effects observed in Tg1 and Tg4 were abolished by the AT2R antagonist. These data demonstrate that overexpression of AT2R at low or moderate level is cardioprotective and those effects are AT2R specific.
However, conflicting results regarding AT2R overexpression in cardiomyocytes have been reported as well. Yan et al16 and Nakayama et al17 found that ventricle-specific expression of the AT2R receptor (9 and 18 transgene copies, driven by the myosin light chain promoter) caused cardiac hypertrophy and aggravated the development of dilated cardiomyopathy and heart failure. Furthermore, the severity of cardiac dysfunction correlated positively with the copy numbers of the AT2R transgene. Using cultured neonatal myocytes transfected with an adenovirus encoding the AT2R gene, D’Amore et al15 found that increased AT2R did not inhibit Ang II-induced myocyte hypertrophy but rather aggravated the hypertrophic response. In the present study using transgenic mice with different levels of AT2R overexpression in the heart, we found that blood pressure and cardiac morphology and function did not differ among WT and Tg1, Tg4 and Tg9 strains subjected sham operation. Although it is a little surprising that overexpression of AT2R, particularly at an excessive level considered far beyond the physiological levels, did not affect the cardiac phenotypes, we speculate that AT2R is saturated under basal conditions, and thus overexpression of AT2R without a change in the endogenous ligand would not affect the function. Also, in the present study AT2R is specifically overexpressed in the heart and activation of the receptor may initiate mainly local or paracrine effects, which may have little or no effect on systemic hemodynamics.
Although we have no definite answer for these disparate results of AT2R overexpression, based on our findings and others we believe that the levels of AT2R overexpression may play a major role as to whether overexpression of AT2R is beneficial or detrimental to the heart. In studies showing that AT2R overexpression in the heart is beneficial, the levels of AT2R were about 20–35% relative to AT1,3,22 whereas in studies showing that overexpression of AT2R is detrimental or non-cardioprotective, levels of AT2R were often similar to or greater than AT1R.15,16 For example in Yan’s study,16 mice with a high copy number of the AT2R transgene (18 copies) developed dilated cardiomyopathy, and mice with an even higher copy number (34 copies) developed overt heart failure and died prematurely. Unfortunately, AT1R expression was not reported in this study. Qi et al12 recently reported that moderate cardiac-specific AT2R overexpression suppressed AT1R expression. In the present study, we measured both AT2R and AT1R expression and showed that overexpression of AT2R at low or moderate levels did not affect AT1R expression but at a high level it tended to increase AT1R and blunted the cardioprotective effects of AT2R. At the present time we have no explanation as to why overexpression of AT2R increases AT1R expression but this result may warrant further investigation. Nevertheless, our data indicate that levels of AT2 overexpression are important determinants for whether AT2 overexpression in myocytes is beneficial or detrimental.
Several signaling mechanisms have been explored as to the cardioprotective actions of AT2R.23 An increase in NO release via kinins has drawn great attention.3,8 We recently in vitro transfected mouse coronary artery endothelial cells with the AT2R gene and found that overexpression and activation of AT2R increases bradykinin release through prolylcarboxypeptidase (a plasma prekallikrein activator), leading to enhanced NO production.5 In the present study, overexpression of AT2R in mouse heart at low or moderate levels upregulated eNOS expression and attenuated the cardiac remodeling and dysfunction post-MI and these effects were not seen in Tg9 mice. These results suggest that overexpression of AT2R exerts cardioprotective effects post-MI, possibly through activation of eNOS and release of NO.
Anti-oxidative stress could be another potential mechanism responsible for AT2R-mediated cardioprotection. It is well known that reactive oxygen species (ROS) generated by an NADPH oxidase-dependent pathway play a pivotal role in the pathophysiology of heart failure. The gp91phox (Nox2)-containing NADPH oxidase is the major source of ROS in the heart.24 Studies show that vascular overexpression of AT2R blunted Ang II-induced increase in Nox2 mRNA expression and vascular injury,25 whereas deletion or blockade of AT2R augmented oxidative stress, NADPH oxidase activity and renal and vascular injury.26,27 In the present study, we found that MI-induced a significant increase in Nox2 protein expression in the heart but this increase was attenuated in mice with low overexpression of AT2R, which was reversed by AT2R blockade. However, in mice with high overexpression of the AT2R (Tg9) MI-induced increase of Nox2 protein remained high as seen with WT mice. These data may indicate that overexpression of AT2R protects the heart against ischemia-induced injury partly through reducing oxidative stress and this protection is inversely related to the overexpression levels of AT2R.
Increased TGF-β1 expression/signaling contributes to the fibrotic remodeling and tissue damage.28,29 Overexpression/activation of AT2R has been shown to exert anti-fibrotic and anti-growth functions via suppressing TGF-β1. For example, Hashimoto et al30 reported that vascular overexpression of AT2R ameliorated 5/6 nephrectomy-induced upregulation of TGF-β1 and glomerular injury. In renovascular hypertension, activation of AT2R decreased renal TGF-β1 associated with reduction of inflammation.31 Habashi et al32 showed that loss of AT2R accelerates aortic aneurysm formation and rupture in mouse model of Marfan syndrome most likely through TGF-β1-mediated activation of extracellular signal-regulated kinase. In the current study we showed that transgenic mice with 1 or 4 copies of the AT2R gene had less degree of cardiomyocyte hypertrophy, cardiac fibrosis and upregulation of TGF-β1, which were not seen in Tg9 mice. These data indicate that overexpression of AT2R in the heart at low or moderate levels might be responsible for the reduced cardiac hypertrophy, fibrosis and dysfunction post-MI, in part mediated via reduced TGF-β1 signaling.
In summary, the present study demonstrates that AT2R overexpression specifically in the heart does not seem to play an essential role in cardiac hemodynamics, morphology, and function under basal conditions. However, low or moderate levels of overexpression (Tg1, Tg4) of AT2R appear to protect the heart from maladaptive remodeling and dysfunction post-MI. These beneficial effects involve upregulation of eNOS and downregulation of TGF-β1 and oxidative stress signaling pathways, which disappeared when AT2R overexpressed at an excessive level. Thus we conclude that whether overexpression of AT2 is beneficial or detrimental to the heart post-MI is largely dependent on levels of its expression, possible via regulation of eNOS, Nox2 and TGF-β1 signaling pathways.
PERSPECTIVE
AT2R expression is upregulated in patients with cardiovascular disease, probably as a compensatory mechanism. Activation of AT2R also reportedly contributes to the cardioprotective effect of ARB. However, there are conflicting reports regarding the role of AT2R in the heart. Thus, understanding the role of AT2R and defining the mechanism(s) underlying AT2R-mediated cardioprotection could help to develop new therapeutic strategies, such as using an AT2R agonist alone or in combination with ACEi or ARB to treat patients with cardiovascular disease.
Supplementary Material
Novelty and Significance.
What is new
There are conflicting reports regarding the role of AT2R in the heart. We demonstrate for the first time that low or moderate overexpression of AT2R in the heart is beneficial, but high levels may be detrimental.
What is relevant
AT2R expression is upregulated in patients with cardiovascular disease, probably as compensatory mechanisms. Defining the role of AT2R and the mechanism(s) underlying AT2R-mediated cardioprotection could lead to new therapeutic strategies such as specific activation/overexpression of AT2R either alone or combined with ACEi or ARB to treat hypertension, ischemic heart disease and end-organ damage, which has high clinical relevance.
Summary
Low or moderate levels of AT2R overexpression protects the heart from maladaptive remodeling and dysfunction post-MI. An excessive increase in AT2R abolishes such protection. We thus conclude that whether overexpression of AT2R is beneficial or detrimental to the heart is largely dependent on expression levels. The beneficial effects involve upregulation of eNOS and downregulation of TGF-β1 and oxidative stress signaling pathways.
Acknowledgments
We thank Dr. Xinhua Yan from Tufts University - St. Elizabeth's Medical Center of Boston for generously providing us with the AT2R transgenic mice.
Sources of Funding
This work was supported by National Institutes of Health/National Heart, Lung and Blood Institute grants HL-28982 (PPG Project II for X.-P. Yang, and PPG Project I for O.A. Carretero,) and Institutional fund (X.-P. Yang).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Utsunomiya H, Nakamura M, Kakudo K, Inagami T, Tamura M. Angiotensin II AT2 receptor localization in cardiovascular tissues by its antibody developed in AT2 gene-deleted mice. Regul Pept. 2005;126:155–161. doi: 10.1016/j.regpep.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Wharton J, Morgan K, Rutherford RAD, Catravas JD, Chester A, Whitehead BF, De Leval MR, Yacoub MH, Polak JM. Differential distribution of angiotensin AT2 receptors in the normal and failing human heart. J Pharmacol Exp Ther. 1998;284:323–336. [PubMed] [Google Scholar]
- 3.Kurisu S, Ozono R, Oshima T, Kambe M, Ishida T, Sugino H, Matsuura H, Chayama K, Teranishi Y, Iba O, Amano K, Matsubara H. Cardiac angiotensin II type 2 receptor activates the kinin/NO system and inhibits fibrosis. Hypertension. 2003;41:99–107. doi: 10.1161/01.hyp.0000050101.90932.14. [DOI] [PubMed] [Google Scholar]
- 4.Yayama K, Okamoto H. Angiotensin II-induced vasodilation via type 2 receptor: role of bradykinin and nitric oxide. Int Immunopharmacol. 2008;8:312–318. doi: 10.1016/j.intimp.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Zhu L, Carretero OA, Liao TD, Harding P, Li H, Sumners C, Yang XP. Role of prolylcarboxypeptidase in angiotensin II type 2 receptor-mediated bradykinin release in mouse coronary artery endothelial cells. Hypertension. 2010;56:384–390. doi: 10.1161/HYPERTENSIONAHA.110.155051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Carretero OA, Zhu L, Shesely EG, Rhaleb N-E, Dai X, Wang L, Yang JJ, Yang X-P. The protective role of AT2 and B1 receptors in kinin B2 receptor knockout mice with myocardial Infarction. Clin Sci (Lond) 2012;124:87–96. doi: 10.1042/CS20120341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YH, Yang XP, Sharov VG, Nass O, Sabbah HN, Peterson E, Carretero OA. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest. 1997;99:1926–1935. doi: 10.1172/JCI119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Carretero OA, Liu YH, Shesely EG, Yang F, Kapke A, Yang XP. Role of AT2 receptors in the cardioprotective effect of AT1 antagonists in mice. Hypertension. 2002;40:244–250. doi: 10.1161/01.hyp.0000029095.23198.ad. [DOI] [PubMed] [Google Scholar]
- 9.Oishi Y, Ozono R, Yoshizumi M, Akishita M, Horiuchi M, Oshima T. AT2 receptor mediates the cardioprotective effects of AT1 receptor antagonist in post-myocardial infarction remodeling. Life Sci. 2006;80:82–88. doi: 10.1016/j.lfs.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Falcón BL, Stewart JM, Bourassa E, Katovich MJ, Walter G, Speth RC, Sumners C, Raizada MK. Angiotensin II type 2 receptor gene transfer elicits cardioprotective effects in an angiotensin II infusion rat model of hypertension. Physiol Genomics. 2004;19:255–261. doi: 10.1152/physiolgenomics.00170.2004. [DOI] [PubMed] [Google Scholar]
- 11.Metcalfe BL, Huentelman MJ, Parilak LD, Taylor DG, Katovich MJ, Knot HJ, Sumners C, Raizada MK. Prevention of cardiac hypertrophy by angiotensin II type-2 receptor gene transfer. Hypertension. 2004;43:1233–1238. doi: 10.1161/01.HYP.0000127563.14064.FD. [DOI] [PubMed] [Google Scholar]
- 12.Qi Y, Li H, Shenoy V, Li Q, Wang F, Raizada M, Sumners C, Katovich M. Moderate cardiac-selective overexpression of angiotensin type 2 receptor protects cardiac functions from ischemic injury. Exp Physiol. 2011;97:89–101. doi: 10.1113/expphysiol.2011.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Bove CM, French BA, Epstein FH, Berr SS, DiMaria JM, Gibson JJ, Carey RM, Kramer CM. Angiotensin II type 2 receptor overexpression preserves left ventricular function after myocardial infarction. Circulation. 2002;106:106–111. doi: 10.1161/01.cir.0000020014.14176.6d. [DOI] [PubMed] [Google Scholar]
- 14.Adachi Y, Saito Y, Kishimoto I, Harada M, Kuwahara K, Takahashi N, Kawakami R, Nakanishi M, Nakagawa Y, Tanimoto K, Saitoh Y, Yasuno S, Usami S, Iwai M, Horiuchi M, Nakao K. Angiotensin II type 2 receptor deficiency exacerbates heart failure and reduces survival after acute myocardial infarction in mice. Circulation. 2003;107:2406–2408. doi: 10.1161/01.CIR.0000072763.98069.B4. [DOI] [PubMed] [Google Scholar]
- 15.D'Amore A, Black MJ, Thomas WG. The angiotensin II type 2 receptor causes constitutive growth of cardiomyocytes and does not antagonize angiotensin II type 1 receptor-mediated hypertrophy. Hypertension. 2005;46:1347–1354. doi: 10.1161/01.HYP.0000193504.51489.cf. [DOI] [PubMed] [Google Scholar]
- 16.Yan X, Price RL, Nakayama M, Ito K, Schuldt AJ, Manning WJ, Sanbe A, Borg TK, Robbins J, Lorell BH. Ventricular-specific expression of angiotensin II type 2 receptors causes dilated cardiomyopathy and heart failure in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;285:H2179–H2187. doi: 10.1152/ajpheart.00361.2003. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama M, Yan X, Price RL, Borg TK, Ito K, Sanbe A, Robbins J, Lorell BH. Chronic ventricular myocyte-specific overexpression of angiotensin II type 2 receptor results in intrinsic myocyte contractile dysfunction. Am J Physiol Heart Circ Physiol. 2005;288:H317–H327. doi: 10.1152/ajpheart.00957.2003. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Carretero OA, Lin CX, Cavasin MA, Shesely EG, Yang JJ, Reudelhuber TL, Yang XP. Role of cardiac overexpression of ANG II in the regulation of cardiac function and remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H1900–H1907. doi: 10.1152/ajpheart.00379.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang XP, Liu YH, Rhaleb N-E, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol Heart Circ Physiol. 1999;277:H1967–H1974. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]
- 20.Steckelings UM, Rompe F, Kaschina E, Namsolleck P, Grzesiak A, Funke-Kaiser H, Bader M, Unger T. The past, present and future of angiotensin II type 2 receptor stimulation. J Renin Angiotensin Aldosterone Syst. 2010;11:67–73. doi: 10.1177/1470320309347791. [DOI] [PubMed] [Google Scholar]
- 21.Bove CM, Yang Z, Gilson WD, Epstein FH, French BA, Berr SS, Bishop SP, Matsubara H, Carey RM, Kramer CM. Nitric oxide mediates benefits of angiotensin II type 2 receptor overexpression during post-infarct remodeling. Hypertension. 2004;43:680–685. doi: 10.1161/01.HYP.0000115924.94236.91. [DOI] [PubMed] [Google Scholar]
- 22.Masaki H, Kurihara T, Yamaki A, Inomata N, Nozawa Y, Mori Y, Murasawa S, Kizima K, Maruyama K, Horiuchi M, Dzau VJ, Takahashi H, Iwasaka T, Inada M, Matsubara H. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J Clin Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brede M, Roell W, Ritter O, Wiesmann F, Jahns R, Haase A, Fleischmann BK, Hein L. Cardiac hypertrophy is associated with decreased eNOS expression in angiotensin AT2 receptor-deficient mice. Hypertension. 2003;42:1177–1182. doi: 10.1161/01.HYP.0000100445.80029.8E. [DOI] [PubMed] [Google Scholar]
- 24.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 25.Takata H, Yamada H, Kawahito H, Kishida S, Irie D, Kato T, Wakana N, Miyagawa S, Fukui K, Matsubara H. Vascular angiotensin II type 2 receptor attenuates atherosclerosis via a kinin/NO-dependent mechanism. J Renin Angiotensin Aldosterone Syst. 2013 doi: 10.1177/1470320313491794. IN Press. [DOI] [PubMed] [Google Scholar]
- 26.Iwai M, Chen R, Li Z, Shiuchi T, Suzuki J, Ide A, Tsuda M, Okumura M, Min L-J, Mogi M, Horiuchi M. Deletion of angiotensin II type 2 receptor exaggerated atherosclerosis in apolipoprotein E-null mice. Circulation. 2005;112:1636–1643. doi: 10.1161/CIRCULATIONAHA.104.525550. [DOI] [PubMed] [Google Scholar]
- 27.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R117–R124. doi: 10.1152/ajpregu.00476.2002. [DOI] [PubMed] [Google Scholar]
- 28.Lim H, Zhu YZ. Role of transforming growth factor-beta in the progression of heart failure. Cell Mol Life Sci. 2006;63:2584–2596. doi: 10.1007/s00018-006-6085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto N, Maeshima Y, Satoh M, Odawara M, Sugiyama H, Kashihara N, Matsubara H, Yamasaki Y, Makino H. Overexpression of angiotensin type 2 receptor ameliorates glomerular injury in a mouse remnant kidney model. Am J Physiol Renal Physiol. 2004;286:F516–F525. doi: 10.1152/ajprenal.00294.2003. [DOI] [PubMed] [Google Scholar]
- 31.Matavelli LC, Huang J, Siragy HM. Angiotensin AT(2) receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57:308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.