Abstract
Background
The incidence of venous thromboembolism (VTE) among patients undergoing hepatic surgery is poorly defined, leading to varied use of VTE prophylaxis among surgeons. We sought to define the incidence of VTE after liver surgery and identify risk factors associated with VTE.
Methods
Incidence of VTE and associated risk factors within 90 days of hepatic resection between 2006 and 2012 at a major academic center was analyzed. Risk factors for VTE were identified using univariate and multivariate analyses.
Results
A total of 599 patients were included in the study cohort; 30 (5.0 %) had a prior history of VTE. The indications for surgery were malignant (90.8 %) and benign lesions (9.2 %). The majority of patients underwent a minor hepatectomy (<3 Couinaud segments; n =402, 67.1 %) while 195 (32.6 %) patients underwent a major hepatectomy (≥3 Couinaud segments). Three hundred seven (51.3 %) patients were started on VTE chemoprophylaxis preoperatively with 407 (67.8 %) patients receiving VTE chemoprophylaxis within 24 h of surgery. Twenty-eight (4.7 %) patients developed VTE; 20 (3.3 %) had deep venous thrombosis (DVT), 11 (1.8 %) had pulmonary embolism (PE), and three (0.5 %) developed both DVTand PE. Among the VTE patients, 23 (82.1 %) had received VTE chemoprophylaxis. On multivariate analyses, history of VTE (odds ratio [OR] 4.51, 95 % confidence interval [CI] 1.81–17.22, P =0.03), prolonged operative time (OR 1.17 per additional hour, 95 % CI 1.04–1.32, P =0.009), and increased length of stay (LOS) (OR 1.07, 95 % CI 1.02–1.12, P =0.01) were independent risk factors for VTE.
Conclusion
VTE within 90 days of hepatic resection is common, occurring in nearly one in 20 patients. Most VTE events occurred among patients who received current best practice prophylaxis for VTE. More aggressive strategies to identify and reduce the risk of VTE in patients at highest risk of VTE, including those with a history of VTE, extended operative time, and prolonged LOS, are warranted.
Keywords: Venous thromboembolism, Hepatectomy, Prophylaxis, Bleeding, DVT
Introduction
The incidence of deep vein thrombosis (DVT) and pulmonary embolism (PE) has been reported to range from 20-30 % among surgical patients without the administration of chemical and mechanical prophylaxis.1 Chemoprophylaxis with unfractionated or low-molecular weight can reduce the incidence of DVT and PE by about 75 % among general surgery patients.1,2 Because of its efficacy in preventing venous thromboembolism (VTE), the American College of Chest Physicians (ACCP) has issued evidenced-based recommendations for the use of chemoprophylaxis over no prophylaxis in surgical patients of moderate-to-high risk for developing VTE. Indeed, most patients undergoing liver resection would be considered moderate-to-high risk based on the widely used and externally validated Caprini risk scoring system.3 As patients undergoing liver resection are at moderate perioperative bleeding risk, the ACCP has issued recommendations for the prompt institution of chemoprophylaxis once the risk of bleeding is diminished.4 However, due to the paucity of data on the subject, this recommendation is only grade 2C (i.e., weak, based on low-quality evidence).
Practice guidelines regarding prophylaxis for patients undergoing liver resection are, at best, mixed due to a lack of data on VTE incidence as well as VTE-related outcomes after hepatectomy. Surgeons are often weary of the potential for post-operative hemorrhage following hepatectomy. Furthermore, patients undergoing a major hepatectomy are sometimes at risk for post-operative liver insufficiency, leading to the concern that these patients are already “self” or “auto” anti-coagulated. This belief is often supported by the resulting laboratory derangements in measurable liver function, including elevations in the prothombin time/international normalized ratio (PT/INR) and partial thromboplastin time (PTT), as well as occasional thrombocytopenia. In turn, some surgeons delay or completely withhold the administration of VTE prophylaxis, particularly chemoprophylaxis, after liver resection.
Given the paucity of data, lack of clear recommendations, and perceived heterogeneity in practice patterns among liver surgeons, we sought to define the incidence and risk factors associated with the highest likelihood of a VTE event after liver surgery. In addition, our objective was to determine the relative effectiveness of thromboprophylaxis in reducing clinically significant VTE in patients, as well as characterize the incidence of complications possibly attributable to chemoprophylaxis.
Methods
Patient and Data Collection
Patients undergoing planned hepatectomy for benign and malignant disease at Johns Hopkins Hospital were identified between 1990 and 2012. Standard demographic and clinicopathologic data were collected including age, sex, race, American Society of Anesthesiologists (ASA) status, Model for End-Stage Liver Disease (MELD) score, history of previous VTE, and presence or absence of a hypercoagulable state. Laboratory values including total serum bilirubin, albumin, PT/INR, PTT, hematocrit, and serum creatinine were recorded for all patients preoperatively as well as 1, 2, and 3 days after surgery. Operative details, such as type of liver resection, number of concurrent ablations, estimated intraoperative blood loss, operative time, and requirement for transfusion were recorded. Use of chemical and mechanical prophylaxis before, during, and after surgery was recorded. The type, administration and timing of VTE prophylaxis was left to the discretion of the attending surgeon. For patients who did not receive chemoprophylaxis within 24 h post-operatively, the first post-operative day of chemoprophylaxis was recorded, if applicable. Highest-grade morbidity was recorded and classified according to the Clavien–Dindo classification system.5 Perioperative mortality was defined as death in the hospital or within 90 days from the date of surgery.6 DVT and PE within 90 days of surgery were recorded and confirmed radiographically by duplex ultrasonography or chest computerized tomography, respectively, in patients with clinical suspicion. The study was approved by the Johns Hopkins University Institutional Review Board.
Statistical Analysis
Continuous variables were presented as the median with the interquartile range (IQR), where appropriate. Categorical variables were displayed as whole numbers and percentages. Baseline characteristics of the study population were summarized according to administration of prophylaxis. Comparative analyses of continuous variables were performed using Wilcoxon test for parametric and nonparametric data and oneway analysis of variance (ANOVA), as appropriate. Fisher's exact test or Chi-square test was used for comparing categorical variables. The most parsimonious model was created using a stepwise approach that included factors statistically significant on univariate analysis (i.e., P <0.20). For statistical analyses, P values less than 0.05 (two-tailed) were deemed significant. Odds ratios (OR) were presented with 95 % confidence intervals (CI). All analyses were carried out with STATA version 12.0 (StataCorp LP, College Station, TX, USA).
Results
Patient and Surgical Details
The study cohort consisted of 599 patients who underwent a planned hepatectomy. The median patient age was 58 years (interquartile range [IQR], 49–67). Males (49.6 %) and females (50.4 %) were evenly distributed in the cohort. The majority of patients (n =489, 81.6 %) were non-Hispanic white. Most patients (n =441, 74.1 %) were classified as ASA 3 with an ECOG performance status of 0 (n=266, 73.7 %). Thirty patients (5.0 %) were found to have a prior history of VTE (Table 1). The indication for surgery was malignant disease in the overwhelming majority of patients (n =544, 90.8 %), while a subset had benign disease (n =55, 9.2 %). The most common indications for surgery were colorectal liver metastasis (n =243, 47.7 %), non-colorectal liver metastasis (n =136, 26.7 %), primary liver cancer (n =120, 23.6 %), or benign hepatic lesion (n =55, 9.2 %). The majority of patients had not received any previous liver-directed therapy (n =419, 70.0 %); about one-quarter of patients (n =128, 21.4 %) had received preoperative systemic chemotherapy. Of note, patients with malignancy as an indication for surgery were more likely to have had a prior history of VTE (OR 5.24, CI 1.41–19.17, P=0.01).
Table 1.
Clinicopathologic and pre-operative characteristics of patients undergoing planned hepatectomy
| Total (n = 599) | Chemoprophylaxis (n = 454) | No chemoprophylaxis (n = 145) | P value | |
|---|---|---|---|---|
| Age, years (IQR) | 58 (49 to 67) | 58 (50 to 68) | 57 (47 to 64) | 0.05 |
| Male sex | 297 (49.6) | 227 (50) | 75 (51.7) | 0.72 |
| Ethnicity | 0.06 | |||
| Caucasian | 489 (81.6) | 363 (80.0) | 126 (86.9) | |
| Other | 110 (18.4) | 91 (20.1) | 19 (13.1) | |
| ASA Score | 0.60 | |||
| I–II | 154 (25.9) | 113 (25.0) | 41 (28.7) | |
| III–IV | 441 (74.1) | 339 (75.0) | 102 (71.3) | |
| ECOG Performance Status | 0.67 | |||
| 0 | 266 (73.7) | 258 (74.1) | 8 (61.5) | |
| 1 | 90 (24.9) | 85 (24.4) | 5 (38.5) | |
| 2 and 3 | 5 (1.4) | 5 (1.5) | 0 | |
| History of VTE | 30 (5.0) | 29 (6.4) | 1 (0.7) | 0.006 |
| Diagnosis | 0.004 | |||
| Malignant | 544 (90.8) | 421 (92.7) | 123 (84.8)) | |
| Benign lesion | 55 (9.2) | 33 (7.3) | 22 (15.2) | |
| Pre-operative INR (IQR) | 1 (0.9 to 1.0) | 1 (0.9 to 1.0) | 0.9 (0.9 to 1.0) | <0.001 |
| Pre-operative hematocrit (IQR) | 39 (35.8 to 41.2) | 38.8 (35.8 to 41.1) | 39.5 (35.8 to 42.0) | 0.13 |
| Post-operative hematocrit (IQR) | 30.6 (27.1 to 33.5) | 30.4 (26.9 to 33.4) | 31.0 (27.6 to 33.9) | 0.11 |
| Post-operative % hematocrit change (IQR) | –27.1 (–40.8 to –14.5) | –26.4 (–41.7 to –13.9) | –27.2 (–40.0– –14.5) | 0.28 |
| Pre-operative therapy, no. (%) | <0.001 | |||
| None | 419 (70.0) | 316 (69.6) | 103 (71.0) | |
| Surgery | 34 (5.7) | 14(3.1) | 20 (13.8) | |
| Ablation or IAT | 15 (2.5) | 11 (2.4) | 4 (2.8) | |
| Systemic Chemotherapy | 128 (21.4) | 110(24.2) | 18 (12.4) | |
| Portal vein embolization | 3 (0.5 | 3 (0.7) | 0 |
At the time of surgery, the majority of patients underwent a minor hepatectomy (less than three Couinaud segments; n = 402, 67.1 %), 195 (32.6 %) patients underwent a major hepatectomy (three or more Couinaud segments), and two patients (<1 %) underwent ablation only (Table 2). Of those who underwent a minor hepatectomy, 155 (38.6 %) had a non-anatomic hepatic wedge resection with the remaining undergoing a single or bi-segmentectomy (n =247, 61.4 %). Of those who underwent a major hepatectomy, 135 (69.2 %) had a hemi-hepatectomy and 60 (31.8 %) had an extended hemi-hepatectomy. Overall, median operative time was 4.6 h (IQR 3.5–6.2) and median estimated blood loss was 500 ml (IQR 250–900).
Table 2.
Operative and post-operative characteristics of patients undergoing planned hepatectomy
| Total (n = 599) | Chemoprophylaxis (n = 454) | No chemoprophylaxis (n = 145) | P value | |
|---|---|---|---|---|
| Resection type, no. (%) | <0.001 | |||
| Non-anatomic Wedge | 155 (26.0) | 135 (29.9) | 20 (13.8) | |
| <Hemi-hepatectomy | 247 (41.4) | 187 (41.4) | 60 (41.4) | |
| Hemi-hepatectomy | 135 (22.6) | 84 (18.6) | 51 (35.2) | |
| Extended hepatectomy | 60 (10.1) | 46 (10.2) | 14 (9.7) | |
| Operation time, h (IQR) | 4.6 (3.5–6.2) | 4.9 (3.6–6.4) | 4.2 (3.3–5.0) | <0.001 |
| EBL, ml (IQR) | 500 (250–900) | 450 (200–850) | 550 (300–1000) | 0.21 |
| Intra-operative RBC transfusion | 137 (22.9) | 102 (22.9) | 35 (24.3) | 0.80 |
| Post-operative RBC transfusion | 101 (16.9) | 94 (22.7) | 7 (5.0) | <0.001 |
| Overall transfusion | 204 (34.1) | 167 (36.8) | 37 (25.5) | 0.01 |
| Pre-operative VTE Prophylaxis | 307 (51.3) | 307 | – | – |
| Post-operative VTE Prophylaxis within 24 h | 406 (67.8) | 406 | – | – |
| Post-operative deep venous thrombosis within 90 days | 20 (3.3) | 17 (3.7) | 3 (2.1) | 0.33 |
| PE within 90 days | 11 (1.8) | 8 (1.8) | 3 (2.1) | 0.81 |
| LOS, days (IQR) | 5 (4–7) | 5 (4–8) | 5 (4–6) | 0.27 |
| Complication grade ≥3 within 30 days | 39 (6.5) | 35 (7.7) | 4 (2.8) | 0.04 |
| 90-day mortality | 13 (2.2) | 10(2.2) | 3 (2.1) | 0.9 |
VTE Prophylaxis Type and Administration
Nearly all patients (n =592, 98.8 %) received either mechanical and/or chemoprophylaxis during their hospitalization. The majority of patients received chemoprophylaxis (n = 450, 75.1 %), while 142 (23.7 %) patients received only mechanical prophylaxis (i.e., sequential compression devices); seven (1.2 %) patients did not receive any type of VTE prophylaxis. Among the subset of patients receiving chemoprophylaxis, 307 (68.2 %) patients were started on chemoprophylaxis in the preoperative setting prior to surgical incision and 406 (90.2 %) patients received chemoprophylaxis within 24 h post-operatively. The overwhelming majority of patients received subcutaneous heparin either every 8 h (n = 328, 54.8 %) or 12 h (n =111, 18.6 %), while the remaining patients received lovenox (n =15, 3.3 %). Of note, in 2008 a multidisciplinary team at our institution established a specialty-specific, mandatory, computerized clinical decision support module for VTE risk stratification and prevention. This clinical support tool improved best practice VTE prophylaxis from 51.1 % to 97.8 %.
Age, sex, ASA score, pre-operative liver transaminases, bilirubin, serum albumin, INR, and hematocrit all did not differ among patients who received chemoprophylaxis versus those who did not (all P >0.05). Patients undergoing resection for malignant disease were more likely to have received chemoprophylaxis (77.4 %) than those with benign disease (60.0 %) (P =0.02). Of note, patients who were not given chemoprophylaxis were more likely to have undergone a major hepatectomy (no chemoprophylaxis: n =65, 44.9 % vs. chemoprophylaxis: n =130, 28.8 %, P <0.001). EBL (no chemoprophylaxis: 450 ml vs. chemoprophylaxis: 550 ml) and intraoperative transfusion (no chemoprophylaxis: n = 102, 23 % vs. chemoprophylaxis: n =35, 24 %) did not differ between the two groups (all P >0.05). Patients receiving chemoprophylaxis did, however, have a higher incidence of postoperative (n =94, 22.7 %) and overall (n =167, 36.8 %) red blood cell transfusion (both P <0.05). The overall incidence of any complication was higher among patients who did receive chemoprophylaxis (no chemoprophylaxis: n =22, 15.2 % vs. chemoprophylaxis: n =209, 46.0 %, P <0.001). Patients receiving chemoprophylaxis, however, only had a slightly higher incidence of severe complications (grade≥3) (no chemoprophylaxis: n =4, 2.8 % vs. chemoprophylaxis: n =35, 7.7 %, P =0.04). No patient in either group underwent reoperation secondary to bleeding. Both groups had a median length of stay (LOS) of 5 days (P >0.05).
VTE: Incidence, Outcomes and Risk Factors
Out of the entire cohort of 599 patients, 28 (4.7 %) developed VTE; 20 (3.3 %) had DVT, 11 (1.8 %) had PE, and three (0.5 %) developed both DVT and PE. Patient demographics, ASA score, prior history of VTE did not differ among those who developed a post-operative VTE and those who did not (all P >0.05) (Table 3). In addition, VTE incidence did not change over time (1990–2007: 2.9 % vs. 2008–2012: 6.3 %; P =0.15).
Table 3.
Clinicopathologic and operative characteristics of patients with and without VTE within 90 days
| Post-operative VTE (n = 28) | No Post-operative VTE (n = 571) | P value | |
|---|---|---|---|
| Age, years (IQR) | 61.3 (53.7 to 68.2) | 57.7 (49 to 67) | 0.40 |
| Male sex | 16 (57.1) | 286 (50.1) | 0.47 |
| Ethnicity | 0.67 | ||
| Caucasian | 22 (78.6) | 467 (81.8) | |
| Other | 6 (21.4) | 104 (18.2) | |
| ASA Score | 0.18 | ||
| I–II | 4 (14.8) | 150 (26.4) | |
| III–IV | 23 (85.2) | 418 (73.6) | |
| ECOG Performance Status | 0.57 | ||
| 0–1 | 22 (100) | 334 (98.5) | |
| 2–3 | 0 | 5 | |
| History of VTE | 4 (14.3) | 26 (4.6) | 0.02 |
| Diagnosis | 0.78 | ||
| Malignant | 25 (89.3) | 519 (90.9) | |
| Benign lesion | 3 (10.7) | 52 (9.1) | |
| Pre-operative INR (IQR) | 1 (1 to 1) | 1 (0.9 to 1) | 0.91 |
| Pre-operative hematocrit (IQR) | 36.6 (34.8 to 39.8) | 39 (36 to 41.4) | 0.03 |
| Post-operative hematocrit (IQR) | 29.6 (25.8 to 33.3) | 30.7 (27.2 to 33.5) | 0.46 |
| Post-operative hematocrit % change (IQR) | –25.2 (–43.6 to –2.9) | –27.1 (–40.8 to –14.8) | 0.001 |
| Pre-operative therapy | 9 (32.1) | 171 (30.0) | <0.001 |
| Resection type | 0.11 | ||
| <Hemi-hepatectomy | 15 (53.6) | 387 (68.0) | |
| ≥Hemi-hepatectomy | 13 (46.4) | 182 (32.0) | |
| Operation time, h (IQR) | 6.75 (4.2 to 9.5) | 4.55 (3.5 to 6.0) | |
| EBL, ml (IQR) | 625 (400 to 1,000) | 475 (250 to 850) | 0.27 |
| Intra-operative RBC transfusion | 11 (39.3) | 126 (27.1) | 0.03 |
| Post-operative RBC transfusion | 7 (25.0) | 94 (16.5) | 0.24 |
| Overall transfusion | 15 (53.6) | 189 (33.1) | 0.03 |
| LOS, days (IQR) | 11.5 (6.5 to 21) | 5 (4 to 7) | 0.005 |
| VTE prophylaxis | 23 (82.1) | 431 (75.5) | 0.42 |
| Pre-operative VTE Prophylaxis | 17 (60.7) | 290 (50.8) | 0.31 |
| Post-operative VTE Prophylaxis within 24 h | 20 (70.4) | 386 (67.6) | 0.67 |
| Complication grades 3–5 within 30 days | 9 (32.1) | 30 (5.3) | <0.001 |
| 90-day mortality | 2 (7.1) | 11 (1.9) | 0.06 |
Among patients who had data on INR available (n =495), the incidence of VTE was higher among patients who had a post-operative peak INR ≥1.5 (peak INR ≥1.5, 14.3 % vs. INR <1.5, 3.6 %; P <0.001). In contrast, among those who had data on platelet count available (n =577), the incidence of VTE was the same among patients who had a platelet count less than (7.3 %) or greater than (4.5 %) 100,000 (P =0.32). Patients on preoperative DVT prophylaxis with coumadin were uncommon (n =3); none of these patients had a preoperative hypercoaggulable state or history of previous DVT documented and none of these patients had VTE. The VTE incidence was also similar among patients undergoing surgery for a malignant (4.6 %) versus a benign (5.5 %) indication (P =0.77). In particular, among patients with an underlying malignancy, the incidence of VTE was no different among patients with hepatocellular carcinoma (HCC) (2.2 %) versus non-HCC cancers (4.8 %) (P =0.41). Of note, the incidence of VTE was higher among the 107 patients who had a liver resection with a concurrent simultaneous colon resection (simultaneous liver with concurrent colorectal procedure, 11.2 % vs. staged liver-only, 3.9 %; P =0.008).
Chemoprophylaxis was not associated with a difference in the incidence of DVT (no chemoprophylaxis: n =3, 2.1 % vs. chemoprophylaxis: n =17, 3.7 %) or PE (no chemoprophylaxis: n =3, 2.1 % vs. chemoprophylaxis: n =8, 1.8 %) (both P >0.05). Specifically, among patients who developed a VTE, 23 (82.1 %) had received VTE chemoprophylaxis within 24 h of surgery.
Patients who experienced a VTE had a higher incidence of any post-operative complication (no VTE n =212, 37.1 % vs. VTE n =19, 67.9 %), as well as severe complications (no VTE, 5.3 % vs. VTE, 32.1 %) (both P <0.05). Patients who experienced a VTE also had a longer LOS (no VTE, 5 days vs. VTE, 11.5 days; P =0.005). In addition, VTE was associated with a trend toward increased 90-day mortality (no VTE, 1.9 % vs. VTE, 7.1 %; P =0.06).
On univariate analyses, several factors were associated with the risk of post-operative VTE (Table 4). Specifically, patient-level factors included prior history of VTE (OR 3.49, 95 % CI 1.13–10.81, P =0.03), as well as an initial lower pre-operative hematocrit (OR 0.92 for each additional percent increase, 95 % CI 0.85–0.99, P =0.03) (Table 4). Operative factors associated with VTE risk were extent of hepatic resection (extended hemi-hepatectomy: OR 3.97, 95 % CI 1.60–9.82, P =0.003), operative time (OR 1.22 per additional hour, 95 % CI 1.09–1.37, P <0.001), overall RBC transfusion (OR 2.29, 95 % CI 1.04–5.00, P =0.04), and reoperation (OR 9.33, 95 % CI 3.07–28.39, P <0.001). LOS (OR 1.01, 95 % CI 1.00–1.02, P =0.06) and post-operative complication (OR 8.54, 95 % CI 3.56–20.50, P <0.001) were also associated with VTE risk. On multivariate analyses, after adjusting for competing risk factors, factors that remained independently associated with VTE risk included history of VTE (OR 4.51, 95 % CI 1.81–17.22, P =0.03), operative time (OR 1.17 per additional hour, 95 % CI 1.04–1.32, P =0.009), and LOS (OR 1.07, 95 % CI 1.02–1.12, P =0.01) (Figs. 1 and 2).
Table 4.
Clinical factors associated with development of VTE within 90 days after hepatic surgery
| Univariate analysis |
P value | Adjusted analysis |
P value | |||
|---|---|---|---|---|---|---|
| Variables | OR | 95 % CI | OR | 95 % CI | ||
| Age | 1.01 | 0.98–1.04 | 0.40 | – | – | – |
| Race | 0.82 | 0.32–2.06 | 0.67 | – | – | – |
| Male sex | 0.75 | 0.35–1.62 | 0.47 | – | – | – |
| ASA score (1, 2 vs. 3, 4) | 2.06 | 0.70–6.06 | 0.19 | 1.61 | 0.45–5.73 | 0.46 |
| History of VTE | 3.49 | 1.13–10.81 | 0.03 | 4.51 | 1.18–17.22 | 0.03 |
| Elevated AST | 1.46 | 0.67–3.19 | 0.34 | – | – | – |
| Elevated ALT | 1.26 | 0.54–2.92 | 0.59 | – | – | – |
| Elevated ALP | 1.31 | 0.60–2.90 | 0.50 | – | – | – |
| Hematocrit | 0.92 | 0.85–0.99 | 0.03 | 0.94 | 0.86–1.04 | 0.24 |
| Type of hepatic surgery | ||||||
| Hemi-hepatectomy | 0.99 | 0.35–2.78 | 1.00 | – | – | – |
| Extended hepatectomy | 3.97 | 1.60–9.82 | 0.003 | 2.08 | 0.65–6.62 | 0.22 |
| Operative time | 1.22 | 1.09–1.37 | 0.001 | 1.17 | 1.04–1.32 | 0.009 |
| LOS | 1.01 | 1.00–1.02 | 0.06 | 1.07 | 1.02–1.12 | 0.01 |
| Re-operation | 9.33 | 3.07–28.39 | <0.001 | 3.30 | 0.95–11.46 | 0.06 |
| Transfusion | 2.33 | 1.09–5.00 | 0.03 | 0.69 | 0.22–2.15 | 0.52 |
| Complication (1–2 vs. 3–5) | 8.54 | 3.56–20.50 | <0.001 | 3.30 | 0.95–11.46 | 0.06 |
| VTE prophylaxis | 1.49 | 0.56–4.00 | 0.43 | – | – | – |
Fig. 1.
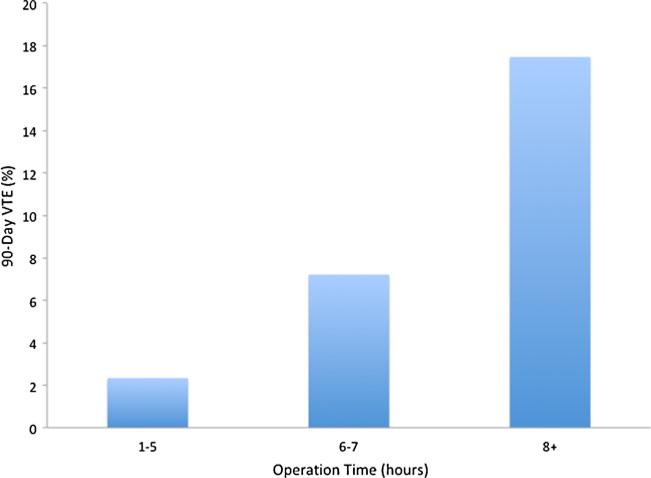
Proportion of patients with VTE within 90 days of operation based on operative time
Fig. 2.
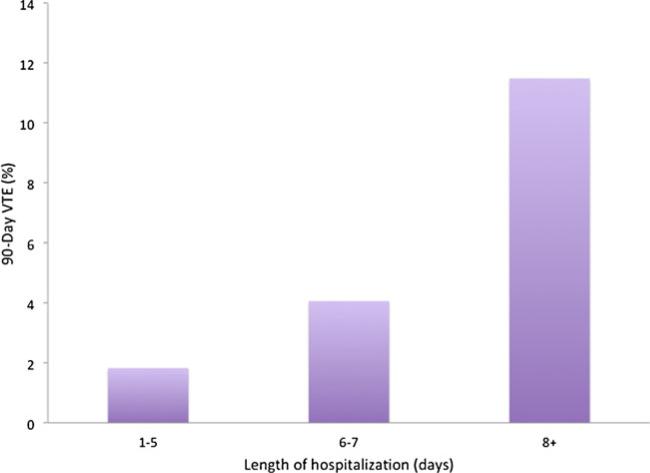
Proportion of patients with VTE within 90 days of operation based on length of hospitalization
Discussion
VTE prophylaxis is an important surgical issue. The incidence of VTE, including DVT and PE, can be substantial among general surgery patients – especially those who do not receive appropriate prophylaxis around the time of surgery.1 In fact, Ho et al.7 reported an incidence of 3.0 % for DVT and 1.8 % for PE among patients undergoing colon resections. The issue of VTE prophylaxis may be even more important among patients undergoing hepatic resection. De Martino et al.8 reported that patients undergoing hepatectomy had the highest risk of VTE among any abdominal surgical procedure; in addition, the risk of VTE was more than twice that of patients undergoing non-abdominal procedures such as breast surgery. Mechanical and chemical prophylaxes have been shown to reduce VTE events in surgical patients.1,2,7 As such, the ACCP has issued a grade IB (strong, based on moderate-quality evidence) or 2C (weak, based on low quality evidence) recommendation depending on the patient's perceived risk for bleeding.4 The risk of post-operative bleeding following hepatectomy has been reported to range from <1 % to 5 %.9–12 Due to the potential for bleeding, as well as the occasional perturbations in the synthesis of coagulation factors following major hepatectomy, some surgeons avoid routine VTE prophylaxis. Despite the controversy around the routine use of VTE prophylaxis, data on hepatic resection, risk of VTE, and the impact of VTE prophylaxis on peri-operative outcomes are scarce. Turley et al. reported data from a large cohort derived from the National Surgical Quality Improvement Program (NSQIP); however, this study was significantly limited by the fact that the authors had no information on the actual use of VTE prophylaxis in the study cohort.13 While Reddy et al. examined VTE after hepatectomy using institutional data, the study involved multiple centers with different utilization patterns of VTE prophylaxis.14 It is interesting to note that heterogeneity with regard to VTE prophylaxis similarly existed at our own single center. Because of this variability, in 2008 a multidisciplinary team at our institution established a specialty-specific, mandatory, computerized clinical decision support module for VTE risk stratification and prevention. Interestingly, while this clinical support tool improved best practice VTE prophylaxis it did not lead to changes in VTE incidence over time. The current study is also important because we examined a large cohort of patient (n = 599) treated at a single center. More importantly, we elucidated the overall incidence of VTE following hepatic resection, identified the risk factors associated with the highest risk of VTE, and the impact of VTE chemoprophylaxis on perioperative outcomes.
Interestingly, data from even a single center demonstrate heterogeneity with regard to the utilization of VTE prophylaxis. Specifically, while the majority of patients received VTE chemoprophylaxis (75.8 %), a full one-quarter (24.2 %) of patients did not receive chemoprophylaxis around the time of hepatic resection. In the current study, we identified a number of factors that were associated with receipt of perioperative VTE chemoprophylaxis. While several factors such as age and sex did not impact the likelihood of a patient receiving chemoprophylaxis, surgical indication did influence a provider's likelihood of using chemoprophylaxis. Specifically, patients with a malignant indication for surgical resection were more likely to receive peri-operative chemoprophylaxis compared with patients who had benign disease (Table 1). In addition, patients who underwent a major hepatectomy were 1.5 times less likely to receive chemoprophylaxis. While the reason for this may be multifactorial, the lower utilization of chemoprophylaxis after major hepatectomy is probably related to the concern over liver insufficiency and the belief that even mild liver insufficiency may induce an “auto-anticoagulation”, decreasing the risk of thrombotic events. Despite having alterations in platelets, PT/INR, and PTT, patients with liver insufficiency actually often have significant increased risk for venous thrombosis.15 In fact, patients with transient liver insufficiency may actually have progressive development of hypercoagulability based on post-operative thromboelastogram analysis.16 Barton et al.15 demonstrated a relative hypercoagulable state after liver resection using thromboelastogram analysis despite an increase in PT and INR. Similarly, medical patients with liver insufficiency and alterations in laboratory coagulation values have been shown to have a higher incidence of thrombotic complications than the general hospital population, leading to the routine use of chemoprophylaxis in these patients.17–21 In the current study, we noted that nearly one in seven patients who underwent an extended hepatectomy developed a VTE (Fig. 3). Use of prophylaxis in chronic liver disease has been shown to decrease VTE without an increase in bleeding complications.22 As such, VTE prophylaxis should strongly be considered, even among patients undergoing major hepatic resection.
Fig. 3.
Proportion of patients with VTE based on type of liver resection
We found that patients undergoing hepatectomy were at significant risk of VTE within 90 days of surgery. Specifically, the incidence of VTE in the current study was 4.7 %, which was consistent with other published data.23–25 Importantly, we identified several factors that were significantly associated with the risk of VTE. Factors most strongly associated with VTE risk included prior history of VTE, prolonged operative time, and an extended LOS (Table 4). The effect of LOS was most pronounced among patients whose hospitalization was greater than 8 days (Fig. 2).
Prolonged operative time and long hospital stays likely contribute to increased venous stasis due to decreased mobility. Given the overall incidence of VTE of 5 %, according to the Caprini VTE risk calculation, the majority of patients undergoing liver resection would be considered moderate (~3 %) to high risk (~6 %) and should therefore receive routine chemoprophylaxis.3,4
The main reason why some surgeons avoid routine use of VTE chemoprophylaxis around the time of liver surgery is the concern over perioperative bleeding. The risk of nonfatal postoperative major bleeding secondary to chemoprophylaxis is a matter of debate, with several studies reporting conflicting results.1,26–29 Mita et al.30 reported a higher rate of postoperative bleeding after gastric cancer surgery among patients receiving chemoprophylaxis (8.1 %) compared with no chemoprophylaxis (0.7 %). In contrast, Reddy et al.14 reported that VTE chemoprophylaxis did not increase the rate of red blood cell transfusion after major hepatectomy. In the current study, while we noted a slight increase in the incidence of post-operative and overall transfusion among patients receiving chemoprophylaxis, no patient required reoperation secondary to bleeding. Taken together, the data strongly suggest that VTE chemoprophylaxis does not increase the risk of major bleeding following liver resection.
The current study had several limitations that should be considered when interpreting the data. While the current study included a relatively large number of patients (n ≈600), the number of VTE events in the cohort was relatively small (n = 28). As such, the finding that receipt of VTE chemoprophylaxis was not associated with VTE risk needs to be interpreted with caution. Given the small number of events and the fact that “only” 145 patients did not receive chemoprophylaxis, any analyses examining the impact of prophylaxis on VTE were likely underpowered. In contrast, other studies have noted that chemoprophylaxis was indeed associated with a lower incidence of VTE after hepatectomy.14
In conclusion, VTE within 90 days of hepatic resection is common, occurring in nearly one in 20 patients. Most VTE events occurred among patients who received current best practice prophylaxis for VTE. More aggressive strategies to identify and reduce the risk of VTE in patients at highest risk of VTE, including those who have an extended operative time and LOS, are warranted.
Contributor Information
Aslam Ejaz, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Gaya Spolverato, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Yuhree Kim, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Donald L. Lucas, Department of Surgery, Walter Reed National Military Medical Center, Bethesda, MD, USA
Brandyn Lau, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Matthew Weiss, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Fabian M. Johnston, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Marian Kheng, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Kenzo Hirose, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Christopher L. Wolfgang, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Elliott Haut, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Timothy M. Pawlik, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA Department of Surgery, Johns Hopkins Hospital, 600 N. Wolfe Street, Blalock 688, Baltimore, MD 21287, USA.
References
- 1.Collins R, Scrimgeour A, Yusuf S, Peto R. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318(18):1162–73. doi: 10.1056/NEJM198805053181805. [DOI] [PubMed] [Google Scholar]
- 2.Mismetti P, Laporte S, Darmon JY, et al. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88(7):913–30. doi: 10.1046/j.0007-1323.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- 3.Caprini JA, Arcelus JI, Hasty JH, et al. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17(Suppl 3):304–12. [PubMed] [Google Scholar]
- 4.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S–77S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayo SC, Shore AD, Nathan H, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB (Oxford) 2011;13(7):473–82. doi: 10.1111/j.1477-2574.2011.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho YH, Seow-Choen F, Leong A, et al. Randomized, controlled trial of low molecular weight heparin vs. no deep vein thrombosis prophylaxis for major colon and rectal surgery in Asian patients. Dis Colon Rectum. 1999;42(2):196–202. doi: 10.1007/BF02237127. discussion 202–3. [DOI] [PubMed] [Google Scholar]
- 8.De Martino RR, Goodney PP, Spangler EL, et al. Variation in thromboembolic complications among patients undergoing commonly performed cancer operations. J Vasc Surg. 2012;55(4):1035–1040. e4. doi: 10.1016/j.jvs.2011.10.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyder O, Pulitano C, Firoozmand A, et al. A risk model to predict 90-day mortality among patients undergoing hepatic resection. J Am Coll Surg. 2013;216(6):1049–56. doi: 10.1016/j.jamcollsurg.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato M, Tateishi R, Yasunaga H, et al. Mortality and morbidity of hepatectomy, radiofrequency ablation, and embolization for hepato-cellular carcinoma: a national survey of 54,145 patients. J Gastroenterol. 2012;47(10):1125–33. doi: 10.1007/s00535-012-0569-0. [DOI] [PubMed] [Google Scholar]
- 11.Bhayani NH, Hyder O, Frederick W, et al. Effect of metabolic syndrome on perioperative outcomes after liver surgery: a National Surgical Quality Improvement Program (NSQIP) analysis. Surgery. 2012;152(2):218–26. doi: 10.1016/j.surg.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236(4):397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turley RS, Reddy SK, Shortell CK, et al. Venous thromboembolism after hepatic resection: analysis of 5,706 patients. J Gastrointest Surg. 2012;16(9):1705–14. doi: 10.1007/s11605-012-1939-x. [DOI] [PubMed] [Google Scholar]
- 14.Reddy SK, Turley RS, Barbas AS, et al. Post-operative pharmacologic thromboprophylaxis after major hepatectomy: does peripheral venous thromboembolism prevention outweigh bleeding risks? J Gastrointest Surg. 2011;15(9):1602–10. doi: 10.1007/s11605-011-1591-x. [DOI] [PubMed] [Google Scholar]
- 15.Barton JS, Riha GM, Differding JA, et al. Coagulopathy after a liver resection: is it over diagnosed and over treated? HPB (Oxford) 2013 doi: 10.1111/hpb.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerutti E, Stratta C, Romagnoli R, et al. Thromboelastogram monitoring in the perioperative period of hepatectomy for adult living liver donation. Liver Transpl. 2004;10(2):289–94. doi: 10.1002/lt.20078. [DOI] [PubMed] [Google Scholar]
- 17.Senzolo M, Sartori MT, Lisman T. Should we give thromboprophylaxis to patients with liver cirrhosis and coagulopathy? HPB (Oxford) 2009;11(6):459–64. doi: 10.1111/j.1477-2574.2009.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salami A, Qureshi W, Kuriakose P, et al. Frequency and predictors of venous thromboembolism in orthotopic liver transplant recipients: a single-center retrospective review. Transplant Proc. 2013;45(1):315–9. doi: 10.1016/j.transproceed.2012.06.060. [DOI] [PubMed] [Google Scholar]
- 19.Aldawood A, Arabi Y, Aljumah A, et al. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J. 2011;9(1):1. doi: 10.1186/1477-9560-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesmana CR, Inggriani S, Cahyadinata L, Lesmana LA. Deep vein thrombosis in patients with advanced liver cirrhosis: a rare condition? Hepatol Int. 2010;4(1):433–8. doi: 10.1007/s12072-010-9166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabbagh O, Oza A, Prakash S, et al. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137(5):1145–9. doi: 10.1378/chest.09-2177. [DOI] [PubMed] [Google Scholar]
- 22.Barclay SM, Jeffres MN, Nguyen K, Nguyen T. Evaluation of pharmacologic prophylaxis for venous thromboembolism in patients with chronic liver disease. Pharmacotherapy. 2013;33(4):375–82. doi: 10.1002/phar.1218. [DOI] [PubMed] [Google Scholar]
- 23.Tzeng CW, Katz MH, Fleming JB, et al. Risk of venous thromboembolism outweighs post-hepatectomy bleeding complications: analysis of 5651 National Surgical Quality Improvement Program patients. HPB (Oxford) 2012;14(8):506–13. doi: 10.1111/j.1477-2574.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merkow RP, Bilimoria KY, McCarter MD, et al. Post-discharge venous thromboembolism after cancer surgery: extending the case for extended prophylaxis. Ann Surg. 2011;254(1):131–7. doi: 10.1097/SLA.0b013e31821b98da. [DOI] [PubMed] [Google Scholar]
- 25.Morris-Stiff G, White A, Gomez D, et al. Thrombotic complications following liver resection for colorectal metastases are preventable. HPB (Oxford) 2008;10(5):311–4. doi: 10.1080/13651820802074431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hacker RI, Ritter G, Nelson C, et al. Subcutaneous heparin does not increase postoperative complications in neurosurgical patients: An institutional experience. J Crit Care. 2012;27(3):250–4. doi: 10.1016/j.jcrc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Cohen AT, Wagner MB, Mohamed MS. Risk factors for bleeding in major abdominal surgery using heparin thromboprophylaxis. Am J Surg. 1997;174(1):1–5. doi: 10.1016/S0002-9610(97)00050-0. [DOI] [PubMed] [Google Scholar]
- 28.McLeod RS, Geerts WH, Sniderman KW, et al. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the canadian colorectal DVT prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233(3):438–44. doi: 10.1097/00000658-200103000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De A, Roy P, Garg VK, Pandey NK. Low-molecular-weight heparin and unfractionated heparin in prophylaxis against deep vein thrombosis in critically ill patients undergoing major surgery. Blood Coagul Fibrinolysis. 2010;21(1):57–61. doi: 10.1097/MBC.0b013e3283333505. [DOI] [PubMed] [Google Scholar]
- 30.Mita K, Ito H, Murabayashi R, et al. Postoperative bleeding complications after gastric cancer surgery in patients receiving anticoagulation and/or antiplatelet agents. Ann Surg Oncol. 2012;19(12):3745–52. doi: 10.1245/s10434-012-2500-6. [DOI] [PubMed] [Google Scholar]