Abstract
Background
Fibroblast growth factor-23 (FGF-23) is a phosphate regulatory hormone that directly stimulates left ventricular hypertrophy in experimental models. The role of FGF-23 in cardiovascular disease development in the general population is unclear. We tested associations of FGF-23 with major subclinical and clinical cardiovascular disease outcomes in a large prospective cohort.
Methods and Results
We evaluated 6,547 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) who were initially free of cardiovascular disease. We measured serum FGF-23 using the Kainos immunoassay. The MESA measured left ventricular (LV) mass by magnetic resonance imaging, coronary calcium (CAC) by computed tomography, and carotid intima-medial thickness (IMT) by ultrasound. The MESA adjudicated incident heart failure, coronary heart disease, and stoke by medical record review. After adjustment, the highest FGF-23 quartile was associated with an estimated 2.4 gram greater LV mass (95% CI 0.4, 4.5 greater) and a 26% greater odds of higher CAC scores (95% CI 9% to 46% greater) compared to the lowest quartile. Over 7.5 years follow-up, each 20-pg/mL higher FGF-23 concentration was associated with a 19% greater risk of heart failure (95% CI 3% to 37% greater) and a 14% greater risk of coronary heart disease (95% CI 1% to 28% greater). FGF-23 was not associated with carotid IMT or stroke.
Conclusions
Higher serum FGF-23 concentrations are associated with subclinical cardiac disease and with new heart failure and coronary disease events, but not with carotid IMT or stroke. FGF-23 may be a novel cardiovascular risk factor in the general population.
Keywords: Fibroblast growth factor-23, FGF-23, left ventricular mass, left ventricular hypertrophy, coronary artery calcium, carotid intima-media thickness, heart failure, coronary heart disease, stroke, cardiovascular disease
Fibroblast growth factor-23 (FGF-23) is a major phosphate regulatory hormone that is produced in bone and acts on the kidneys to enhance urinary phosphate excretion and inhibit synthesis of calcitriol, the biologically active form of vitamin D.1, 2 Serum FGF-23 concentrations are elevated in rare phosphate wasting disorders, such as hypophosphatemic rickets and tumor induced osteomalacia. FGF-23 concentrations also rise substantially in the setting of chronic kidney disease (CKD) presumably to defend against phosphate overload.3,4
The phosphaturic effects of FGF-23 on the kidneys coincide with potentially adverse effects on the myocardium. In isolated cardiac myocytes, FGF-23 mimics the activity of FGF-2, a general fibroblast growth factor, by activating hypertrophic gene programs, promoting cardiomyocyte growth, and stimulating the release of natriuretic peptides.5 Administration of recombinant FGF-23 substantially increases left ventricular mass in animal models.5 In humans who have established CKD, higher serum FGF-23 concentrations are associated with left ventricular hypertrophy and heart failure events.5, 6 Moreover, human vascular tissue expresses FGF receptors and klotho, the target for FGF-23 binding.7 Higher FGF-23 concentrations are also associated with atherosclerosis burden, ischemic cardiovascular events and all-cause mortality in cohorts of established CKD patients8, individuals with stable coronary disease,9 and general older adults.6, 10
Previous human studies of FGF-23 have assessed ethnically homogenous populations, focused on individuals who have pre-existing cardiovascular and kidney diseases, and utilized differing methods to measure cardiovascular outcomes. To evaluate FGF-23 as a potential novel cardiovascular risk factor in the general population we measured serum FGF-23 concentrations in a prospective, multi-ethnic cohort of 6,547 individuals who were initially free of clinical cardiovascular disease. We delineated associations of FGF-23 with left ventricular mass, assessed by cardiac magnetic resonance imaging, coronary artery calcification, and carotid intima-media thickness. We then determined prospective associations of FGF-23 with incident heart failure, coronary heart disease, and stroke events during long-term follow-up.
Methods
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study of cardiovascular disease among 6,814 community-living individuals.11 Between 2000–2002, the MESA recruited participants aged 45–84 years from study sites in Baltimore, MD; Chicago, IL; St. Paul, MN; Forsyth County, NC; New York, NY; and Los Angeles CA. By design, the MESA recruited a final population that was 38% White, 28% African-American, 22% Hispanic, and 12% Asian. The MESA excluded participants who had any previous self-reported diagnosis of cardiovascular disease, defined by myocardial infarction, angina, stroke, transient ischemic attack, heart failure, atrial fibrillation, nitroglycerin use, angioplasty, coronary artery bypass grafting, valve replacement, pacemaker or defibrillator, or any surgery on the heart or arteries. All participants gave informed consent and Institutional Review Board approval was obtained for each site.
We evaluated 6,552 participants (96.2%) who had adequate stored serum for FGF-23 measurements. We excluded 2 subjects who had evidence of severe chronic kidney disease (CKD; estimated glomerular filtration rate [GFR] <15 ml/min/1.73m2) and 3 subjects who had FGF-23 values that were extremely out of range and were considered by the laboratory to represent laboratory error.
Measurement of Serum FGF-23 Concentrations
MESA study personnel collected blood and urine samples in the morning after an overnight fast. The University of Vermont Laboratory for Clinical Biochemistry (LCBR) stored samples using established methods12 and shipped first use samples on dry ice to the University of Washington. We measured serum FGF-23 concentrations using the Kainos immunoassay,13 which detects the full-length, biologically intact FGF-23 molecule via mid-molecule and distal epitopes. We used standardized high and low value FGF-23 controls within each run to monitor quality control. The coefficient of variation for singlicate high and low control samples across 81 plates were 6.7% and 12.4%, respectively.
Measurements of Subclinical Disease
MESA personnel performed cardiac magnetic resonance imaging (MRI) using scanners with 1.5-Tesla magnets. Central readers blinded to other study data read cardiac MRI images using commercial software (MASS 4.2; Leiden, the Netherlands). Left ventricular mass was calculated as the difference between the epicardial and endocardial areas, multiplied by slice thickness and the section gap, and then multiplied by the specific gravity of myocardium. Among 75 subjects who underwent repeat MRI measurements, the intraclass correlation coefficient for left ventricular mass was 0.98.14 We also assessed left ventricular mass indexed to height, weight, and sex using equations that were developed in previous MESA analyses.15 The MESA defined left ventricular hypertrophy (LVH) by a left ventricular mass index >85.3 g/m2 for women or >107.8 g/m2 for men, which correspond to sex-specific 95th percentile scores in subjects without diabetes or hypertension.16
Study personnel at each site measured coronary artery calcium (CAC) using ECG-gated electron beam computed tomography (CT) or multi-detector row helical CT. Personnel scanned participants twice in succession over phantoms of known physical calcium concentration. Central readers blinded to other study data averaged results from the two scans and calculated coronary calcium scores using the Agatston method.17 Interobserver and intraobserver agreement for CAC were 0.90 and 0.93, respectively.18
Trained examiners performed carotid ultrasound imaging centered on a 10 mm segment of the common carotid arteries about 1cm below the common carotid bulb. Common carotid IMT was measured centrally by trained readers and defined as the mean of the maximum IMT of the near and far walls of the right and left sides. In replicate readings, the between-reader correlation coefficient was 0.84–0.86.19 Carotid artery plaques were defined as any versus none in the common or internal artery.
Ascertainment of Cardiovascular Events
MESA personnel screened participants for incident events through telephone contacts and scheduled follow-up exams.11 Potential events prompted collection of hospitalization records, outpatient reports, and/or death certificates. Two study physicians blinded to other study data independently reviewed the medical records. For the purposes of this study we considered incident heart failure events as probable or definite cases of heart failure. The MESA Events Committee defined probable heart failure by a physician diagnosis of heart failure plus medical treatment for heart failure. The Events Committee defined definite heart failure by the above criteria plus either echocardiographic or chest X-ray evidence of heart failure.15 The committee defined coronary heart disease as myocardial infarction, definite angina, probable angina if followed by coronary artery bypass grafting or percutaneous coronary intervention, resuscitated cardiac arrest, or coronary heart disease death. The committee defined ischemic stroke as a focal neurological deficit lasting more than 24 hours or stroke symptoms lasting <24 hours with clinically relevant lesion on brain imaging.
Other Study Variables
MESA personnel ascertained medical and personal histories using standardized questionnaires, and assessed medication use via the inventory method. Study personnel calculated dietary micronutrient intake using a 127-item Block-style food frequency questionnaire and Nutrition Data System for Research (NDSR) software. The LCBR measured serum creatinine using a modified Jaffe reaction that was indirectly calibrated to Cleveland Clinic laboratory standards, serum cystatin C and C-reactive protein concentrations using a BNII nephelometer, and urine albumin and creatinine from spot morning collections using nephelometry and the rate Jaffe reaction, respectively. We measured serum and urine phosphate from previously frozen samples using timed-rate colorimetry on a Beckman-Coulter DxC chemistry analyzer.
MESA investigators defined diabetes by the use of a diabetes medication or a fasting blood glucose level ≥126 mg/dl. MESA personnel calculated metabolic equivalent-minutes/day of moderate or vigorous physical activity from self-reported frequency, duration, and intensity of individual activities. We estimated glomerular filtration rate (GFR) using serum creatinine and cystatin C concentrations from the 2012 CKD-EPI equation that incorporates both of these markers.20 We defined CKD by the presence of either eGFRCKD-EPI <60ml/min/1.73m2 or a urine albumin to creatinine ratio ≥30 mg/g. Cardiac biomarkers (NT-proBNP and troponin T) were previously measured as part of a MESA ancillary study in a subset 5,438 participants.
Statistical Analysis
We tabulated baseline characteristics by FGF-23 quartiles and used Kendall’s tau statistic to report correlations. We used linear regression to estimate cross-sectional associations of FGF-23 with continuous cardiac MRI variables and carotid IMT. We examined spline and polynomial models to investigate potential non-linear associations of FGF-23 with LV mass; however, the linear model provided the best statistical fit to the data. We used a proportional odds model to estimate associations of FGF-23 with previously established categories of coronary calcification that predict cardiovascular events: 0, 1–100, 101–300, and >300 Agatston units.21 Coefficients from this polytomous model are interpreted as odds ratios for having a higher CAC score per unit difference in exposure (FGF-23 quartile). We used the Kaplan-Meier method to construct cumulative incidence plots and used proportional hazards models to estimate adjusted hazard ratios (HRs) for time to each binary event. In race-stratified proportional hazard analyses, there was no evidence of non-proportional hazards. We defined time at risk as elapsed time from the baseline exam until the first occurrence of each event or the data were censored due to death, dropout, or end of follow-up.
We adjusted for potential confounders using two nested models that were specified prior to the analyses. A first model stratified by race, and adjusted for age, sex, study site, height, and weight. A second model added adjustment for diabetes (yes versus no), systolic blood pressure (continuous), anti-hypertension medications (yes versus no), highest education level (high school or less, high school to some college, college degree or more), current smoking (current versus former or non-smoker), C-reactive protein, eGFRCKD-EPI, and ln (urine albumin to creatinine ratio). In sensitivity analyses we added adjustment for serum, urine, and dietary phosphate, and for cardiovascular medication use (beta-blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and lipid lowering agents). For analyses of urine phosphate we included adjustment for ln(urine creatinine), measured on the same spot urine sample.
Due to non-response, approximately 5% of subjects were missing covariate data on education, smoking, urine albumin, creatinine, and cystatin C. These values were multiply imputed using chained equations on the basis of observed baseline covariates, including the exposure and outcome variables. Multiple analyses (n=5) over the imputations were combined using Rubin’s rules to account for variability in the imputation procedure22. Missing covariate data accounted for <1% of variation in coefficient estimates. We used the Wald test to assess interactions.
All p-values were 2-sided. Analyses were conducted using the open-source package R version 2.15 (R Foundation, Vienna, Austria) and STATA 12.1 (College Station, TX).
Results
Description of Serum Fibroblast Growth Factor-23 Concentrations
The median and mean (standard deviation) serum FGF-23 concentration was 37.7 and 40.0 (15.2) pg/mL, respectively; 99.4% of FGF-23 values were <100 pg/mL. Mean serum FGF-23 concentrations were quantitatively similar, though statistically different by race/ethnicity: 41.7 pg/mL in Caucasians, 39.3 pg/mL in African Americans, 39.8 pg/mL in Chinese Americans, and 38.1 pg/mL in Hispanics (ANOVA p<0.001). Mean FGF-23 concentrations were on average 1.0 pg/mL higher in men compared to women (p=0.01), and this gender difference was numerically similar across race/ethnicity group. Serum FGF-23 concentrations were most robustly associated with estimated GFR (Table 1; rank correlation [τ] = −0.18), and less strongly related to serum phosphate (τ = +0.06), urinary phosphate (τ = +0.02), and dietary phosphate intake (τ = +0.03).
Table 1.
Baseline characteristics by fibroblast growth factor-23 quartile
| Serum fibroblast growth factor-23 concentration (pg/mL) | ||||
|---|---|---|---|---|
| < 30.5 | 30.5 – 37.7 | 37.7 – 46.4 | 46.4 – 223 | |
| Number of subjects | 1,636 | 1,639 | 1,634 | 1,637 |
| Age (years) | 60.8 (10.2) | 61.8 (10.0) | 62.3 (10.3) | 63.6 (10.3) |
| Male | 678 (41.4) | 786 (48.0) | 784 (48.0) | 812 (49.6) |
| Race | ||||
| White | 525 (32.1) | 621 (37.9) | 656 (40.1) | 741 (45.3) |
| Chinese | 191 (11.7) | 214 (13.1) | 194 (11.9) | 195 (11.9) |
| African American | 485 (29.6) | 448 (27.3) | 429 (26.3) | 415 (25.4) |
| Hispanic | 435 (26.6) | 356 (21.7) | 355 (21.7) | 286 (17.5) |
| Diabetes | 203 (12.4) | 202 (12.3) | 175 (10.7) | 231 (14.1) |
| Current smoking | 274 (16.8) | 218 (13.3) | 199 (12.2) | 152 (9.3) |
| Alcohol drinks per week | ||||
| None | 414 (41.1) | 386 (39.3) | 409 (41.9) | 409 (40.3) |
| 1–7 | 439 (43.6) | 433 (44.1) | 421 (43.1) | 454 (44.7) |
| More than 7 | 154 (15.3) | 162 (16.5) | 147 (15.0) | 152 (15.0) |
| Physical activity1* | 16.4 (27.0) | 16.4 (27.0) | 16.4 (24.5) | 14.9 (24.0) |
| Education | ||||
| High school or less | 630 (38.7) | 598 (36.6) | 594 (36.5) | 524 (32.1) |
| Some college | 472 (29.0) | 430 (26.3) | 458 (28.1) | 503 (30.8) |
| College degree or more | 527 (32.4) | 607 (37.1) | 575 (35.3) | 606 (37.1) |
| Body mass index (kg/m2) | 27.8 (5.4) | 27.9 (5.2) | 28.5 (5.4) | 29 (5.6) |
| Systolic blood pressure (mmHg) | 125.5 (22.0) | 125.2 (20.9) | 126.4 (21.1) | 128.6 (21.8) |
| Any hypertension medication | 510 (31.2) | 537 (32.8) | 581 (35.6) | 773 (47.3) |
| ACE or ARB | 244 (14.9) | 254 (15.5) | 266 (16.3) | 394 (24.1) |
| Beta blocker | 128 (7.8) | 144 (8.8) | 139 (8.5) | 207 (12.7) |
| Lipid-lowering medication | 223 (13.6) | 247 (15.1) | 261 (16.0) | 328 (20.1) |
| Estimated GFR (ml/min/1.73m2)* | 90.0 (20.2) | 90 (22.1) | 81.5 (23.3) | 73.7 (27.2) |
| Estimated GFR ≤ 60 ml/min/1.73m2 | 160 (9.9) | 202 (12.4) | 234 (14.4) | 421 (25.9) |
| Urine albumin to creatinine ratio (mg/g)* | 6.7 (6.7) | 6.7 (6.8) | 6.7 (7.6) | 8.2 (10.5) |
| Serum C-reactive protein (mg/L)* | 2.0 (3.7) | 1.8 (3.0) | 1.8 (3.2) | 2.0 (3.6) |
| Serum 25-hydroxyvitamin D (ng/mL) | 23.8 (11.3) | 25.4 (11.0) | 25.5 (11.0) | 27.4 (12.9) |
| Serum calcium (mg/dL) | 9.6 (0.4) | 9.6 (0.4) | 9.7 (0.4) | 9.7 (0.4) |
| Serum phosphate (mg/dL) | 3.6 (0.5) | 3.6 (0.5) | 3.7 (0.5) | 3.7 (0.6) |
| Urine phosphate (mg/dL) | 49.4 (33.1) | 49.8 (33.8) | 51.8 (37.7) | 53.2 (38.3) |
| Dietary phosphate (mg/day) | 1,016.8 (585.8) | 1,003.5 (555.4) | 1,038 (577.2) | 1,072.3 (618.9) |
| NT-proBNP (pg/mL) | 95.9 ±177.7 | 89.0 ±123.5 | 101.2 ±185.4 | 113.7 ±258.7 |
| Troponin T (pg/mL) | 0.009 ±0.002 | 0.009 ±0.002 | 0.009 ±0.005 | 0.010 ±0.007 |
Values in the table expressed as mean (standard deviation),
geometric mean (interquartile range), or number (percent).
Physical activity expressed as hours per week of moderate or vigorous physical activity.
Associations with Subclinical Cardiovascular Disease
Higher serum FGF-23 concentrations were associated with progressively greater left ventricular mass and a greater prevalence of LVH (Table 2). Associations of FGF-23 with left ventricular mass were found to be approximately linear and persisted after adjustment for established LVH risk factors. When analyzed continuously, each 20-pg/mL higher serum FGF-23 concentration was associated with an estimated 1.2-gram greater left ventricular mass (95% CI 0.2, 2.2 grams greater) after full adjustment for covariates in model 2. Higher serum FGF-23 concentrations were similarly associated with left ventricular mass directly indexed to height, weight, and sex, and with the left ventricular mass-to-volume-ratio, but not with end diastolic volume, stroke volume, or ejection fraction (Supplemental Table 1).
Table 2.
Associations of FGF-23 with subclinical cardiovascular disease
| FGF-23 (pg/mL) | Left ventricular mass (N=4,832) | Mean differences in left ventricular mass (g) | ||
|---|---|---|---|---|
| LVH (%) | Mean (g; SD) | Model 1 | Model 2 | |
| < 30.5 | 10.5 | 139.5 (37.8) | 0 (reference) | 0 (reference) |
| 30.5 – 37.7 | 8.9 | 144.6 (38.6) | 0.38 (−1.65, 2.40) | 1.02 (−0.90, 2.93) |
| 37.7 – 46.4 | 9.5 | 146.7 (39.7) | 0.87 (−1.21, 2.95) | 1.46 (−0.49, 3.42) |
| 46.4 – 223 | 11.7 | 149.9 (41.1) | 2.74 (0.54, 4.94) | 2.44 (0.37, 4.51) |
| p-for-trend | 0.014 | 0.020 | ||
| FGF-23 (pg/mL) | Coronary artery calcium (N=6,547) | Odds ratios for higher CAC score | ||
|---|---|---|---|---|
| Prevalence (%) | Median score1 | Model 1 | Model 2 | |
| < 30.5 | 43.1 | 74.8 (223.4) | 1.0 (reference) | 1.0 (reference) |
| 30.5 – 37.7 | 48.2 | 77.6 (271.9) | 1.09 (0.95, 1.25) | 1.12 (0.97, 1.29) |
| 37.7 – 46.4 | 49.9 | 88.1 (283.7) | 1.08 (0.94, 1.24) | 1.09 (0.94, 1.26) |
| 46.4 – 223 | 57.3 | 106.3 (334.8) | 1.32 (1.15, 1.52) | 1.26 (1.09, 1.46) |
| p-for-trend | <0.001 | 0.005 | ||
| FGF-23 (pg/mL) | Carotid IMT (N=6,470) | Mean differences in carotid IMT (μm) | ||
|---|---|---|---|---|
| Any plaque (%)1 | Mean (μm; SD) | Model 1 | Model 2 | |
| < 30.5 | 37.6 | 852.1 (181.1) | 0 (reference) | 0 (reference) |
| 30.5 – 37.7 | 40.3 | 860.5 (181.7) | −4.06 (−15.21, 7.09) | 0.21 (−10.69, 11.1) |
| 37.7 – 46.4 | 40.9 | 866.0 (197.7) | −4.28 (−15.68, 7.12) | −1.79 (−13.04, 9.46) |
| 46.4 – 223 | 46.2 | 896.7 (210.6) | 13.09 (0.89, 25.28) | 11.77 (−0.34, 23.88) |
| p-for-trend | 0.045 | 0.090 | ||
LVH = left ventricular hypertrophy. IMT = intima-media thickness.
Median Agatston score among participants who had a non-zero coronary calcium score.
Model 1: Stratified by race and adjusted for age, sex, study site, height and weight.
Model 2: Adds diabetes, systolic blood pressure, any hypertension medication, current smoking, log C-reactive protein concentration, and education level (high school or less, high school to some college, college degree or more), estimated glomerular filtration rate (creatinine/cystatin C) and log(urine albumin to creatinine ratio).
Average of maximal on right and left (calculated variable in MESA)
The overall prevalence of coronary calcium was 49.6% and the median coronary calcium score among subjects who had a positive score was 86.5 Agatston units. In unadjusted analyses, higher serum FGF-23 concentrations were associated with a greater prevalence and a greater extent of coronary calcification (Table 2). After full adjustment, higher FGF-23 concentrations were associated with significantly greater coronary calcium scores. Carotid IMT values tended to be greatest in the highest FGF-23 quartile; however, associations of FGF-23 with carotid IMT were not statistically significant. Associations of FGF-23 concentrations with coronary calcium and IMT measures were generally linear (Supplemental Figure 1).
Associations with Incident Cardiovascular Events
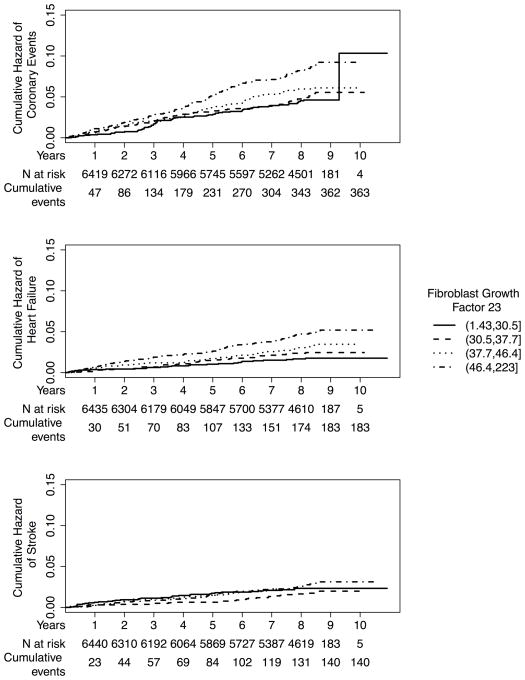
Median (intraquartile range) follow-up times for incident heart failure events (N=183), incident coronary heart disease events (N=363), and incident stroke events (N=140) were 8.5 years (7.7, 8.6 years), 8.5 years (7.6, 8.6 years), and 8.5 years (7.7, 8.6 years), respectively. Serum FGF-23 concentrations were most strongly associated with incident heart failure (Table 3; Figure 1). Crude heart failure incidence rates were more than two-fold greater, comparing the highest to the lowest quartile of FGF-23. After full adjustment, each 20-pg/mL greater FGF-23 concentration was associated with an estimated 19% greater risk of heart failure (HR 1.19; 95% CI 1.03, 1.37) and a 14% greater risk of incident coronary heart disease (HR 1.14; 95% CI 1.01, 1.28). FGF-23 was not associated with stroke (HR 1.04; 95% CI 0.86, 1.27). Associations of FGF-23 with heart failure and coronary disease events were approximately linear (Supplemental Figure 2).
Table 3.
Associations of FGF-23 with incident cardiovascular events
| FGF-23 (pg/mL) | Heart Failure (N=183) | Adjusted hazard ratio (95% confidence interval) | |
|---|---|---|---|
| Rate per 1,000 person-years (events) | Model 1 | Model 2 | |
| < 30.5 | 2.1 (26) | reference | reference |
| 30.5 – 37.7 | 2.9 (36) | 1.25 (0.75, 2.06) | 1.33 (0.78, 2.25) |
| 37.7 – 46.4 | 3.9 (49) | 1.56 (0.96, 2.52) | 1.55 (0.93, 2.58) |
| 46.4 – 223 | 5.8 (72) | 2.02 (1.27, 3.22) | 1.72 (1.06, 2.80) |
| P-for-trend | 0.001 | 0.022 | |
| FGF-23 (pg/mL) | Coronary Heart Disease (N=363) | Adjusted hazard ratio (95% confidence interval) | |
|---|---|---|---|
| Rate per 1,000 person-years (events) | Model 1 | Model 2 | |
| < 30.5 | 5.5 (68) | reference | reference |
| 30.5 – 37.7 | 6.3 (78) | 1.03 (0.74, 1.44) | 1.09 (0.78, 1.52) |
| 37.7 – 46.4 | 7.2 (89) | 1.14 (0.83, 1.58) | 1.19 (0.85, 1.65) |
| 46.4 – 223 | 10.4 (128) | 1.51 (1.11, 2.05) | 1.39 (1.00, 1.92) |
| P-for-trend | 0.005 | 0.038 | |
| FGF-23 (pg/mL) | Stroke (N=140 events) | Adjusted hazard ratio (95% confidence interval) | |
|---|---|---|---|
| Rate per 1,000 person-years (events) | Model 1 | Model 2 | |
| < 30.5 | 2.8 (35) | reference | reference |
| 30.5 – 37.7 | 2.1 (27) | 0.72 (0.43, 1.2) | 0.82 (0.49, 1.39) |
| 37.7 – 46.4 | 2.8 (35) | 0.89 (0.56, 1.42) | 1.04 (0.64, 1.69) |
| 46.4 – 223 | 3.4 (43) | 0.98 (0.62, 1.54) | 1.00 (0.61, 1.65) |
| P-for-trend | 0.821 | 0.804 | |
Model 1: Stratified by race and adjusted for age, sex, study site, height and weight.
Model 2: Adds diabetes, systolic blood pressure, any hypertension medication, current smoking, log C-reactive protein concentration, and education level (high school or less, high school to some college, college degree or more), estimated glomerular filtration rate (creatinine/cystatin C) and log(urine albumin to creatinine ratio).
Figure 1. Cumulative incidences of cardiovascular events by serum FGF-23 concentration.
Y-axis depicts the unadjusted cumulative incidences of heart failure, coronary heart disease, and stroke, respectively; X-axis depicts follow-up time in the study with interval censoring. Data are presented by quartiles of serum FGF-23 concentration with the number at risk and cumulative number of events by year described below the X-axis.
Sensitivity Analyses
Associations of FGF-23 with heart failure and coronary disease events were minimally altered by further adjustment for serum and urine phosphate concentrations, dietary phosphate intake, and serum 25-hydroxyvitamin D concentrations (Table 4). Moreover, associations of FGF-23 with cardiovascular events were unaltered by adjustment for cardiovascular medications or for serum concentrations of low density lipoprotein, high density lipoprotein, and triglycerides plus statin use. The associations of FGF-23 with coronary heart disease events were qualitatively similar after excluding angina cases from this outcome. Among the 182 heart failure events, 30 were preceded by a coronary heart disease event. Associations of FGF-23 with heart failure were unchanged when analyses were censored for coronary heart disease.
Table 4.
Sensitivity analyses for incident heart failure and coronary heart disease events
| Heart Failure | Coronary Heart Disease | |
|---|---|---|
| Adjusted hazard ratio (95% confidence interval) | Adjusted hazard ratio (95% confidence interval) | |
| Association per 20 pg/mL greater FGF-23 | 1.19 (1.03, 1.37) | 1.13 (1.01, 1.26) |
| Additional adjustments | ||
| Add adjustment for serum phosphate | 1.17 (1.01, 1.35) | 1.12 (1.00, 1.25) |
| Add adjustment for urine phosphate | 1.19 (1.03, 1.37) | 1.14 (1.02, 1.27) |
| Add adjustment for dietary phosphate | 1.18 (1.01, 1.37) | 1.12 (0.99, 1.25) |
| Add adjustment for serum 25-hydroxyvitamin D | 1.21 (1.04, 1.41) | 1.15 (1.02, 1.29) |
| Modified definitions of events | ||
| Exclude angina from coronary heart disease events | 1.12 (0.96, 1.30) | |
| Censor heart failure analyses for coronary heart disease | 1.20 (1.03, 1.40) | |
Model 2: stratified by race, and adjusted for age, sex, study site, height, weight, diabetes, systolic blood pressure, any hypertension medication, current smoking, log C-reactive protein concentration, and education level (high school or less, high school to some college, college degree or more), estimated glomerular filtration rate (creatinine/cystatin C) and log(urine albumin to creatinine ratio).
Subgroup Analyses
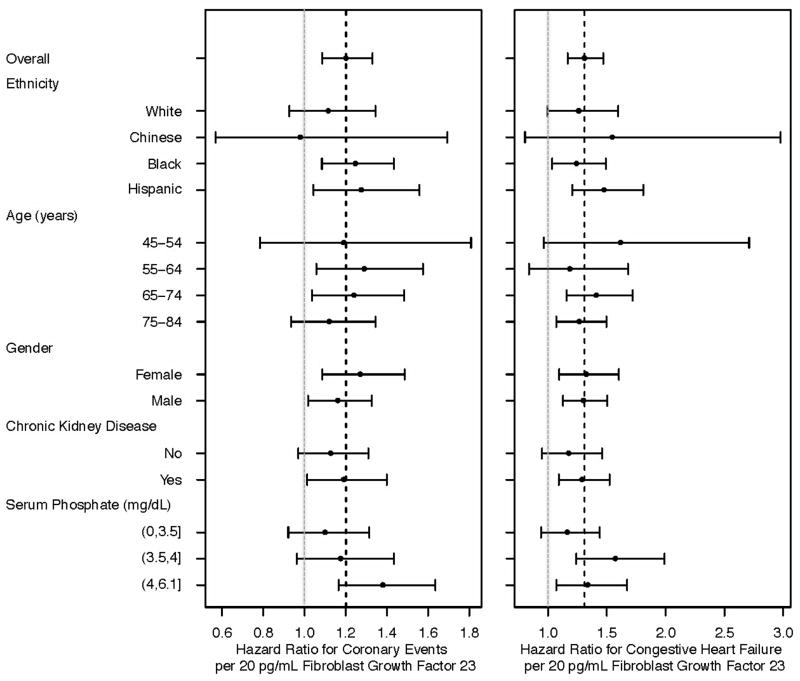
The size of associations of FGF-23 with heart failure and coronary heart disease events did not differ materially or statistically by race/ethnicity (Figure 2; p-for-interaction >0.4). Similarly, associations of FGF-23 with cardiovascular events were numerically and statistically similar by age, sex, serum phosphate concentration, and CKD status. The association of FGF-23 with heart failure and coronary heart disease events was also similar across quartiles of the urinary phosphate to creatinine ratio (p-for-interaction >0.5).
Figure 2. Associations of serum FGF-23 concentration with incident heart failure and coronary heart disease events by subgroup.
Forest plot depicting associations of 20-pg/mL greater serum FGF-23 concentrations with incident heart failure and incident coronary heart disease events by subgroup. The X-axis represents the relative risk (closed circles) and 95% confidence interval (horizontal lines) for each association. The vertical hashed lines represent hazards ratio for the full cohort and the light grey vertical lines represent a hazard ratio of 1.0.
Discussion
In a community-based, multi-ethnic cohort that was initially free of clinical cardiovascular disease, we found higher serum FGF-23 concentrations to be associated with modest differences in subclinical cardiovascular disease: left ventricular mass and coronary artery calcification, and with clinical cardiovascular events: heart failure and coronary heart disease. We did not observe associations of FGF-23 with carotid IMT or incident stroke. Although power to detect interactions by race/ethnicity may be limited, associations of FGF-23 with cardiovascular events were quantitatively similar in Caucasian, African American, Chinese, and Hispanic participants.
Experimental evidence supports a possible causal role for FGF-23 in cardiovascular disease development. In isolated rat ventricular myocytes, FGF-23 promotes dose-dependent expansion of myocardial surface area and stimulates the expression of atrial and brain natriuretic peptides (BNP).5 Injection of recombinant FGF-23 into adult mice significantly increases heart weight and left ventricular wall thickness. FGF-23 also inhibits 1,25-dihydroxyvitamin D, the potent biologic form of vitamin D, via effects on CYP27B1 and CYP24A1 enzymes.23 Experimental disruption of the vitamin D receptor directly modulates the contractile properties of cardiomyocytes, stimulates the renin-angiotensin system, and promotes cardiac hypertrophy.24, 25 Klotho, a key cofactor for FGF-23 binding, is linked with an advanced aging phenotype in animal models, and some klotho gene polymorphisms are associated with cardiovascular diseases in humans.26–28
Most human studies of FGF-23 have focused on established CKD patients, who tend to have markedly elevated serum FGF-23 concentrations and prevalent cardiovascular diseases. In the Chronic Renal Insufficiency Cohort study, the largest prospective study of CKD to date, higher serum FGF-23 concentrations are associated with prevalent LVH, new-onset LVH during follow-up, and all-cause mortality.5, 29 Among 1,099 late-stage CKD patients from the Homocysteinemia in Kidney and End Stage Renal Disease Trial, higher serum FGF-23 concentrations are associated with a composite outcome of myocardial infarction, amputation, or stroke.8 However, FGF-23 was not associated with stroke in a sub-analysis of individual events.
Emerging data from non-CKD populations also demonstrate associations of FGF-23 with LVH and cardiovascular events. A study of 795 general older Swedish adults reported associations of higher serum FGF-23 concentrations with a greater prevalence of LVH, assessed by echocardiography.30 A related study using whole-body magnetic resonance angiography in 306 older adults found higher FGF-23 concentrations to be associated with greater total atherosclerosis burden.10 A cohort study of older U.S. adults (mean age 78 years) found associations of higher serum FGF-23 concentrations with a composite outcome of myocardial infarction, stroke, or cardiovascular death, and separately with heart failure.31 FGF-23 is also associated with all-cause mortality among stable outpatients who have established coronary artery disease.9 In contrast, a nested case-control study of male participants in the Health Professionals Follow-up Study found no association of FGF-23 with a composite outcome of non-fatal myocardial infarction plus fatal coronary heart disease.32
Our study builds on previous work in several ways. First, we assessed associations of FGF-23 with concurrent measurements of subclinical cardiovascular disease among individuals who were free of clinically apparent disease, strengthening inference for a possible role of FGF-23 in disease development. Second, we evaluated separate associations for adjudicated heart failure, coronary disease, and stroke events, which may occur through divergent biological pathways. Third, we adjusted for a set of established cardiovascular risk factors that were measured using uniform procedures, including estimates of kidney function by serum cystatin C, serum creatinine, and urine albumin excretion. Fourth, we demonstrated numerically similar associations with cardiovascular events across four major race/ethnicity categories in a large, multi-ethnic study population that specifically oversampled African American, Chinese, and Hispanic individuals. Stratified analyses by race/ethnicity are important given known race-specific differences in mineral metabolism and cardiovascular disease development.
Our study has some limitations. We cannot prove causal relationships of FGF-23 with cardiovascular disease outcomes because observed associations may be confounded by other factors that were not measured in this study. FGF-23 represents an individual biomarker within complex pathways of bone and phosphate metabolism. Other factors within these pathways may be linked with both FGF-23 and cardiovascular disease development. Measurements of FGF-23 and subclinical cardiovascular disease were contemporaneous, precluding demonstration of temporal relationships. Heart failure cases in MESA were defined based on the presence of symptomatic disease; however cases were not subcategorized as to systolic versus diastolic dysfunction, because information necessary for this distinction was not uniformly available.
In summary, we observed associations of higher serum FGF-23 concentrations with subclinical and clinical cardiovascular disease outcomes in a large, multi-ethic, community-based cohort study. Our findings suggest that FGF-23 may be a novel risk factor for the development of heart failure and coronary heart disease in the general population. Important next steps include confirmation of these findings in other general, healthy populations, clarification of clinically relevant FGF-23 concentrations in terms of cardiovascular risk, and further exploration of putative mechanisms that may link FGF-23 with cardiovascular disease.
Supplementary Material
Acknowledgments
The authors wish to thank the investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
This research was supported by grant R01-HL-096875. MESA is supported by contracts N01-HC-95159 – N01-HC-95166, N01-HC-95169, R01-HL-071739 and R01-HL-072403 from the NHLBI.
Footnotes
Disclosures
Dr. Kestenbaum reports receiving consulting fees and grant support from Amgen Inc.
References
- 1.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of fgf23 demonstrates an essential physiological role of fgf23 in phosphate and vitamin d metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine MA, Kumar R, Schiavi SC. Fgf-23 inhibits renal tubular phosphate transport and is a phex substrate. Biochem Biophys Res Commun. 2001;284:977–981. doi: 10.1006/bbrc.2001.5084. [DOI] [PubMed] [Google Scholar]
- 3.White KE, Jonsson KB, Carn G, Hampson G, Spector TD, Mannstadt M, Lorenz-Depiereux B, Miyauchi A, Yang IM, Ljunggren O, Meitinger T, Strom TM, Juppner H, Econs MJ. The autosomal dominant hypophosphatemic rickets (adhr) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab. 2001;86:497–500. doi: 10.1210/jcem.86.2.7408. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 5.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. Fgf23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: Chs (cardiovascular health study) J Am Coll Cardiol. 2012;60:200–207. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donate-Correa J, Mora-Fernandez C, Martinez-Sanz R, Muros-de-Fuentes M, Perez H, Meneses-Perez B, Cazana-Perez V, Navarro-Gonzalez JF. Expression of fgf23/klotho system in human vascular tissue. International journal of cardiology. 2013;165:179–183. doi: 10.1016/j.ijcard.2011.08.850. [DOI] [PubMed] [Google Scholar]
- 8.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M. Fgf-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH. The associations of fibroblast growth factor 23 and uncarboxylated matrix gla protein with mortality in coronary artery disease: The heart and soul study. Ann Intern Med. 2010;152:640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirza MA, Hansen T, Johansson L, Ahlstrom H, Larsson A, Lind L, Larsson TE. Relationship between circulating fgf23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009;24:3125–3131. doi: 10.1093/ndt/gfp205. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Lewis MR, Callas PW, Jenny NS, Tracy RP. Longitudinal stability of coagulation, fibrinolysis, and inflammation factors in stored plasma samples. Thromb Haemost. 2001;86:1495–1500. [PubMed] [Google Scholar]
- 13.Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D, Kassem M, Rackoff P, Zimering M, Dalkin A, Drobny E, Colussi G, Shaker JL, Hoogendoorn EH, Hui SL, Econs MJ. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2006;91:2055–2061. doi: 10.1210/jc.2005-2105. [DOI] [PubMed] [Google Scholar]
- 14.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: Normal values by age, sex, and ethnicity. Ajr. 2006;186:S357–365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 15.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: The mesa (multi-ethnic study of atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edvardsen T, Rosen BD, Pan L, Jerosch-Herold M, Lai S, Hundley WG, Sinha S, Kronmal RA, Bluemke DA, Lima JA. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging--the multi-ethnic study of atherosclerosis (mesa) Am Heart J. 2006;151:109–114. doi: 10.1016/j.ahj.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 18.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac ct in population-based studies: Standardized protocol of multi-ethnic study of atherosclerosis (mesa) and coronary artery risk development in young adults (cardia) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 19.Polak JF, Pencina MJ, O’Leary DH, D’Agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in multi-ethnic study of atherosclerosis. Stroke. 2011;42:3017–3021. doi: 10.1161/STROKEAHA.111.625186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin c. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 22.Rubin DB. Multiple imputation for nonresponse in surveys. Wiley; New York: 1987. [Google Scholar]
- 23.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. Fgf-23 is a potent regulator of vitamin d metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 24.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-dihydroxyvitamin d(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. 1,25(oh)2-vitamin d3 actions on cell proliferation, size, gene expression, and receptor localization, in the hl-1 cardiac myocyte. J Steroid Biochem Mol Biol. 2007;103:533–537. doi: 10.1016/j.jsbmb.2006.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jo SH, Kim SG, Choi YJ, Joo NR, Cho GY, Choi SR, Kim EJ, Kim HS, Kim HJ, Rhim CY. Klotho gene polymorphism is associated with coronary artery stenosis but not with coronary calcification in a korean population. International heart journal. 2009;50:23–32. doi: 10.1536/ihj.50.23. [DOI] [PubMed] [Google Scholar]
- 27.Majumdar V, Nagaraja D, Christopher R. Association of the functional kl-vs variant of klotho gene with early-onset ischemic stroke. Biochem Biophys Res Commun. 2010;403:412–416. doi: 10.1016/j.bbrc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 28.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 29.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. Jama. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact fgf23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study) J Am Coll Cardiol. 2012;60:200–7. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor EN, Rimm EB, Stampfer MJ, Curhan GC. Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. Am Heart J. 2011;161:956–962. doi: 10.1016/j.ahj.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.