Abstract
We investigated whether stem cells remember past physical signals and whether these can be exploited to dose cells mechanically. We found that the activation of the Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding domain (TAZ) as well as the pre-osteogenic transcription factor RUNX2 in human mesenchymal stem cells (hMSCs) cultured on soft poly(ethylene glycol) (PEG) hydrogels (Young’s modulus E ~ 2 kPa) depended on prior culture time on stiff tissue culture polystyrene (TCPS; E ~ 3 GPa). Additionally, mechanical dosing of hMSCs cultured on initially stiff (E ~ 10 kPa) and then soft (E ~ 2 kPa) phototunable PEG hydrogels resulted in either reversible - or above a threshold mechanical dose, irreversible - activation of YAP/TAZ and RUNX2. We also found that increased mechanical dosing on supraphysiologically stiff TCPS biases hMSCs toward osteogenic differentiation. We conclude that stem cells possess mechanical memory - with YAP/TAZ acting as an intracellular mechanical rheostat - that stores information from past physical environments and influences the cells’ fate.
A growing body of evidence supports the notion that stem cells respond to mechanical signals presented by the local extracellular matrix (ECM). Recent reports have begun to clarify and quantify the specific effects of modulus, adhesive ligand density and presentation, as well as nanotopography on cell fate.1-9 These studies are founded on the principle of cellular mechanotransduction: the hypothesis that cells sense and integrate mechanical cues from the ECM, which ultimately direct gene expression and cell fate decisions.10 Seminal work in this field demonstrated that culture geometry or modulus alone affects cell proliferation, angiogenic sprouting, and stem cell differentiation. 8,11-13 More recently, Trappmann et al. and Khetan et al. expanded this paradigm by highlighting the importance of ECM structure and cell-ECM binding interactions in determining stem cell fate.6,7
A profound experiment by Gilbert et al. revealed that muscle stem cell engraftment in vivo is dependent upon the elasticity of the substrate used during in vitro culture.14 This implies that cells remember past mechanical environments and that this memory, or mechanical dosing, may influence long-term fate, even after translocation into the body. In another set of studies, Dupont et al. reported a critical link between the extracellular mechanical environment and intracellular signaling, namely that the transcription factors Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding domain (TAZ) translate physical information into protein expression by localizing to the nucleus and regulating mRNA expression.15,16 Clearly, the culture context matters, yet many questions remain. For example, we began to wonder about the effects, intended or unintended, of standard methods of culturing and expanding stem cells on tissue culture plasticware, the implications of this environment on stem cell plasticity, and whether or not stem cell fate is influenced by culture history (i.e., the sum of all the physical environments with which it has interacted).
Mechanical memory and dosing
To test whether or not stem cells possess such a mechanical memory, we assayed human mesenchymal stem cell (hMSC) behavior during culture on supraphysiologically stiff tissue culture polystyrene (TCPS; E ~ 3 GPa) in growth media (Fig. 1a,b). Experimental conditions are labeled according to the mechanical dose the cells experienced, such that DT1 corresponds to a mechanical dose on TCPS for 1 day (Fig. 1a). hMSCs expressed basal levels of the pre-osteogenic transcription factor, RUNX2, when cultured on TCPS for 1 day (DT1; Fig. 1b). However, RUNX2 gene expression increased with the duration of culture on TCPS (1 to 7 days; DT1 to DT7; Fig. 1b). These data suggested to us that the extent of exposure to a culture environment, or mechanical dose, alone can bias hMSC behavior.17 That is, hMSCs store information from physical environments and this mechanical history influences future fate.
Figure 1. Mechanical dosing and memory of hMSCs.
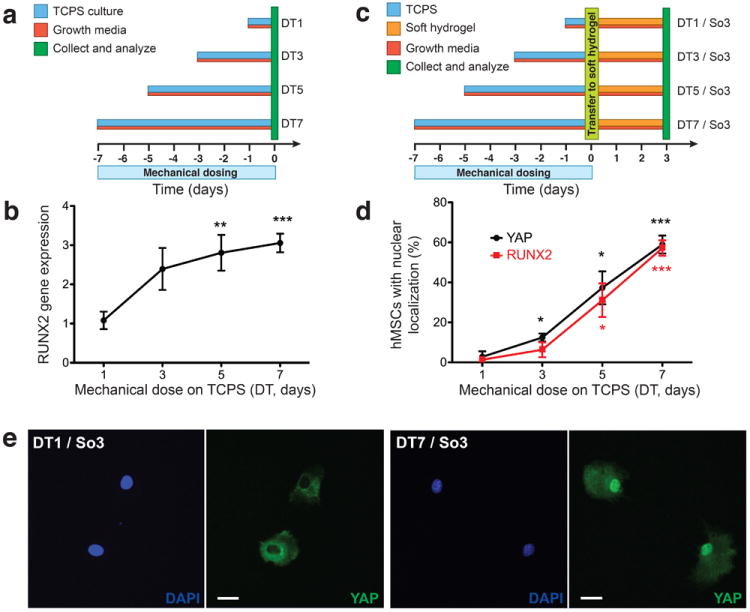
a, hMSCS were cultured on TCPS (blue) in growth media (red) for 1 to 7 days prior to collection and analysis (green, day 0). b, RUNX2 gene expression in hMSCs with mechanical dosing on TCPS as quantified by qRT-PCR. c, hMSCs were cultured on TCPS (blue) in growth media (red) for 1 to 7 days prior to trypsinization and transfer (light green, day 0) to soft hydrogels (orange). hMSCs were cultured subsequently on soft hydrogels in growth media for 3 days prior to collection and analysis (green, day 3). d, YAP and RUNX2 nuclear localization in hMSCs after 3 days on soft hydrogels with previous mechanical dosing on TCPS (DT1 / So3 to DT7 / So3). e, YAP localization in hMSCs on soft hydrogels with 1 day of mechanical dosing on TCPS (DT1 / So3) and 7 days of mechanical dosing on TCPS (DT7 / So3). DAPI, blue; YAP, green. Scale bars, 20 μm. n.s., not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared to DT1 or DT1 / So3.
Based on these data, we hypothesized that an intracellular mechanical sensor - analogous to a rheostat that influences the threshold probability of cellular activation in response to mechanical signals - may exist, which enables cells to retain mechanical information from previous culture conditions. Recent observations revealed that YAP/TAZ were activated (located in the nucleus) in hMSCs on substrata with stiff moduli (E ~ 40 kPa) and de-activated (located in the cytoplasm) on substrata with soft moduli (E ~ 1 kPa); further, this work showed that YAP/TAZ activation correlated with osteogenic differentiation.15,16 Thus, we investigated the role of YAP location, or activation, during mechanical dosing and in the mechanical memory of hMSCs. We designed a set of experiments in which hMSCs were cultured on TCPS (mechanical dosing) prior to trypsinization and re-location to a soft, de-activating hydrogel (E ~ 2 kPa; Fig. 1c). In this manner, we were able to test whether past physical environments (TCPS), which should activate YAP and RUNX2, can override the current mechanical signal (soft hydrogel), which should de-activate YAP and RUNX2. Experimental conditions are labeled according to the mechanical dose and culture time on soft hydrogels, such that DT7 / So3 corresponds to a mechanical dose on TCPS for 7 days prior to transfer (/) to a soft hydrogel for 3 days (Fig. 1c).
YAP/TAZ store mechanical information
In control experiments, YAP was located in the nucleus (activated) of hMSCs when cultured strictly on TCPS and in the cytoplasm (de-activated) of hMSCS when cultured continuously, for up to 13 days, on soft hydrogels (Supplementary Fig. S1). However, in transfer experiments from activating to de-activating substrata, there appeared to be a threshold dose after which YAP remained nuclear even after transfer to a soft hydrogel. Specifically, with 1 day of mechanical dosing on TCPS, YAP de-activated after 3 days on the soft hydrogel (DT1 / So3; Fig. 1d). Whereas, YAP persisted in the activated state in hMSCs after 3 days on the soft hydrogel with 7 days of mechanical dosing on TCPS (DT7 / So3; Fig. 1d). Further, increased mechanical dosing on TCPS (1 to 7 days) corresponded with an increase in the percent of hMSCs with activated (nuclear localized) YAP and RUNX2 (DT1 / So3 to DT7 / So3; Fig. 1d). Correspondingly, immunocytochemistry of YAP in hMSCs (Fig. 1e) revealed distinct intracellular localization; YAP re-located to the cytoplasm for DT1 / So3 and remained in the nuclei for DT7 / So3. Additional staining confirmed that TAZ localized with YAP (cytoplasmic on soft substrates and nuclear on stiff substrates); YAP/TAZ together form a functional mechanosensitive entity (Supplementary Fig. S7).15,16 These findings demonstrate that extended culture on supraphysiologically stiff substrata (TCPS) leads to persistent activation of YAP in hMSCs even after transfer to de-activating, soft hydrogels. The correlation between activated YAP and nuclear RUNX2 on soft hydrogels, which normally suppress RUNX2 nuclear co-localization, after mechanical dosing suggests a potential role of YAP/TAZ as intracellular mechanical rheostats or transducers.
To support the functional role of YAP/TAZ in mechanical memory and dosing, we measured RUNX2 expression levels as a function of mechanical dose in the presence of siRNAs that knockdown YAP and TAZ expression (Supplementary Fig. S11). RUNX2 expression was similar to the original mechanical dosing experiment (Fig. 1b) when treated with non-targeting siRNA. However, treatment with siRNA for YAP/TAZ ablated YAP signaling on TCPS and suppressed RUNX2 expression on TCPS to similar levels observed for hMSCs cultured on soft hydrogels (Supplementary Fig. S11). Therefore, without YAP/TAZ signaling, cells were unable to translate mechanical signals into the expression of pre-osteogenic transcription factors. Additionally, we treated hMSCs with leptomycin B (LMB), a nuclear export inhibitor, to drive YAP activation even on soft hydrogels (Supplementary Fig. S12). With LMB treatment, hMSCs expressed high levels of RUNX2 on soft, de-activating substrates by day 3. This indicates that forced activation of YAP/TAZ can drive the activation of pre-osteogenic transcription factors even on de-activating soft gels.
Mechanical dosing on dynamic substrates
These initial experiments indicated that stem cells possess a mechanical memory and that mechanical dosing may irreversibly influence cells, in part, through YAP/TAZ activation. However, the initial experimental design required that cells be treated with trypsin and passaged from stiff, activating substrata (TCPS) to compliant, de-activating substrata (soft hydrogel), which introduces several inherent complications that may confound interpretation of the results. Therefore, we developed a material that can be modulated from an activating to a de-activating substrate in situ and in the presence of cells. To accomplish this, we synthesized dynamically tunable, photodegradable hydrogels based on a poly(ethylene glycol) di-photodegradable acrylate (PEGdiPDA) crosslinker (Fig. 2a).18-20 PEGdiPDA photodegradable hydrogels enable the user to tune material properties exogenously with precise control21-23 and have been exploited to investigate the cellular response to dynamic alterations in substrate mechanics.24-28
Figure 2. Influence of phototunable substrate modulus on transcription factor activation.

a, Photodegradable hydrogels were fabricated from the free radical polymerization of a photodegrable crosslinker, PEGdiPDA, with a monoacrylated PEG, PEGA. Polymerization results in a stiff hydrogel (~ 10 kPa) that activates YAP in hMSCs (green nucleus, blue cytoplasm). Light exposure (λ = 365 nm; I0 = 10 mW/cm2) for 360s in the presence of cells softens the substrate to a soft hydrogel (~ 2 kPa), upon which YAP de-activates in hMSCs (blue nucleus, green cytoplasm). b, Light exposure can be used to fabricate culture substrata with a range of moduli by exposing the samples to defined doses of light. In this work, hydrogels with average moduli of ~ 10, 6, 4, and 2 kPa were generated. c, Comparison of Young’s moduli of TCPS, stiff hydrogel, and soft hydrogel. d, YAP activation in hMSCs (nuclear localization) increased with increasing modulus. e, Similarly, RUNX2 activation in hMSCs (nuclear localization) increased with increasing modulus. f, YAP and RUNX2 were both excluded from the nucleus (de-activated) in hMSCs on soft hydrogels (2 kPa). g, YAP and RUNX2 were both localized to the nucleus (activated) in hMSCs on stiff hydrogels (10 kPa). DAPI, blue; YAP, green; RUNX2, red. Scale bars, 20 μm. n.s., not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
In this study, we fabricated a photodegradable hydrogel with an initial Young’s modulus (E) of ~10 kPa, which can be softened in situ at any time during culture (Supplementary Fig. S4). The unique use of light enables precise control over the hydrogel modulus, as the photoreaction ceases when the light is turned off and the extent of reaction is governed by the dose of light (Fig. 2b).18,21 Exploiting this property, initially stiff hydrogels were formed into a set of discrete hydrogels with defined moduli (E ~2, 4, 6, and 10 kPa; Fig. 2b). For reference, TCPS possesses a modulus that is several orders of magnitude higher than both extremes of the hydrogel conditions (stiff and soft; Fig. 2c). hMSCs cultured on the various hydrogel surfaces displayed a dose-dependent activation of YAP; increased modulus correlated with increased YAP activation (Fig. 2d). Correspondingly, nuclear co-localization of RUNX2 increased monotonically with increasing modulus (Fig. 2e). This positive correlation between the extent of YAP activation and substrata moduli further corroborates the hypothesis that YAP acts as an intracellular mechanical rheostat.
The localization of the hMSC transcription factors (YAP and RUNX2) diverged on the extremes of the modulus range that we tested (E ~ 2 to 10 kPa). YAP and RUNX2 were both excluded from the nucleus on 2 kPa hydrogels (Fig. 2e), whereas YAP and RUNX2 were primarily translocated to the nucleus on 10 kPa hydrogels (Fig. 2f). YAP was located in the nucleus of 89 ± 4% of the hMSCs cultured on the stiff, activating hydrogel; 6 ± 4% of the hMSCs cultured on the soft, de-activating hydrogel demonstrated nuclear localization of YAP. That is, the stiff (E ~ 10 kPa) photodegradable hydrogel formulation comprises an activating culture substrate and in situ softening with light to the soft (E ~ 2 kPa) hydrogel creates a microenvironment that is associated with de-activation of YAP in hMSCs. Therefore, the photodegradable gel system provides a unique experimental platform to further study mechanical memory and dosing by investigating the dynamic response of stem cells when the underlying substrate is switched from an activating modulus (E ~ 10 kPa) to a de-activating modulus (E ~ 2 kPa) at selected time points.
(Ir)reversible effects of mechanical dosing
To investigate the phenomena of mechanical dosing and mechanical memory on our phototunable substrates, we mechanically dosed hMSCs on the stiff hydrogel (E ~ 10 kPa) for varying extents of time prior to softening the hydrogel in the presence of cells to the soft hydrogel (E ~ 2 kPa). We monitored the localization of YAP and RUNX2 transcription factors for up to 10 days after softening to quantify the temporal response and potential reversible and irreversible effects of the mechanical dose. YAP and RUNX2 re-location to the cytoplasm would indicate de-activation upon softening while persistent nuclear localization of YAP and RUNX2 would indicate that cells ‘remember’ the stiff gel environment. Experimental conditions are labeled according to the mechanical dose on stiff hydrogels and culture time on soft hydrogels prior to analysis, such that DSt7-So1 corresponds to a mechanical dose on a stiff hydrogel for 7 days prior to in situ softening (-) to a soft hydrogel for 1 day (Fig. 3a). hMSCs were seeded at a low density (1000 cells/cm2) to isolate the role of cell-matrix interactions as opposed to cell-cell interactions; and the cells were treated with mitomycin C to inhibit proliferation and limit differences in cell density between the substrates.
Figure 3. Reverisble and irreversible effects of mechanical dosing on phototunable hydrogels.

a, hMSCs were cultured on stiff hydrogels (orange crosshatch) in growth media (red) for 1 to 10 days prior to in situ softening (purple, day 0) the underlying culture substrata to soft hydrogels (orange). hMSCS were cultured subsequently on the softened hydrogels for 1 to 10 days in growth media prior to collection and analysis (green). b, YAP and RUNX2 response to in situ softening after 1 day of mechanical dosing on stiff hydrogels. Stiff control is the average hMSC expression of YAP or RUNX2 over 3, 5, 7, and 10 days on stiff hydrogels and demonstrates full activation. Soft control is the average hMSC expression of YAP or RUNX2 over 3, 5, 7, and 10 days of soft hydrogels and demonstrates basal levels of activation (Supplementary Fig. S2). After softening, YAP and RUNX2 demonstrated transient activation (DSt1-So1) but de-activated to basal levels by day 3 (DSt1-So3). De-activation persisted to day 5 (DSt1-So5). With 1 day of mechanical dosing on stiff hydrogels, hMSCs demonstrated a transient and fully reversible activation of YAP and RUNX2. c, YAP and RUNX2 response to in situ softening after 7 days of mechanical dosing on stiff hydrogels. Upon softening, YAP remained above basal levels out to 5 days after softening (DSt7-So5). On the other hand, RUNX2 activation relaxed to basal levels by day 5 after softening (DSt7-So5). With 7 days of mechanical dosing on stiff hydrogels, hMSCs demonstrated a partially reversible activation of YAP and RUNX2. d, YAP and RUNX2 response to in situ softening after 10 days of mechanical dosing on stiff hydrogels. 10 days after softening, YAP and RUNX2 persisted at active levels significantly above basal levels for soft hydrogels (DSt10-So1 to DSt10-So10). Thus, 10 days of mechanical dosing on stiff hydrogels induced an irreversible activation of YAP and RUNX2. n.s., not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Initially, hMSCs were cultured on activating hydrogels for 1 day prior to softening to the de-activating modulus (Fig. 3b). YAP and RUNX2 were activated and remained in the nucleus transiently after softening (DSt1-So1), but after 3 days on the soft hydrogel, the transcription factors re-located to the cytoplasm with basal nuclear expression (DSt1-So3). De-activation of the transcription factors persisted 5 days after softening (Dst1-So5), which indicated that the initial activation of hMSCs was fully reversible at this mechanical dose (Fig. 3b). To increase the mechanical dose, hMSCs were then cultured on activating hydrogels for 7 days prior to softening (Fig. 3c). Here, a more gradual and partial de-activation of the transcription factors was observed (DSt7-So1 to DSt7-So5), namely 5 days after softening (DSt7-So5) the activation of YAP remained above basal levels on the de-activated hydrogel, though RUNX2 approached basal levels (Fig. 3c). These data suggest that, with this dose, hMSCs retain some information from the initial activating substrate, but that the activation is still partially reversible (Fig. 3c). Finally, hMSCs were cultured on stiff hydrogels for 10 days prior to softening (DSt10-So1 to DSt10-So10; Fig. 3d). With this mechanical dose, YAP and RUNX2 remained activated in the nucleus even 10 days after softening (Supplementary Fig. S6). Experimental replicates with an additional hMSC donor confirmed that these phenomena are general and not specific to a single source of hMSCs (Supplementary Fig. S15). These data imply that even a relatively compliant, activating hydrogel can mechanically dose stem cells in a similar fashion to TCPS (Fig. 1; Fig. 2c). On both TCPS and stiff hydrogels, a sufficient mechanical dose activates YAP constitutively irrespective of the current mechanical context, indicating that this transcription factor is involved in translating the memory of past physical signals (i.e., mechanical history or mechanical dosing of the cells).
These reversible and irreversible effects of mechanical dosing on hMSCs highlight a unique mechanism by which stem cells may integrate and store signals from the ECM. These data also call attention to the manner in which cells are cultured ex vivo (e.g., passage number and substrate modulus).17 Experiments on cell differentiation, cell function, or cell transplantation, in particular those employing multipotent stem cells, may be confounded by unintended mechanical dosing.14 Additionally, stem cells are differentiated, in standard protocols, by delivering specific doses of chemical factors during culture.29 The present findings suggest that an analogous strategy may exist to bias differentiation through the mechanical dosing of stem cells during culture.
Mechanical dosing influences stem cell fate
To test for a functional role of mechanical dosing in controlling cell differentiation, hMSCs were treated for varying lengths of time on TCPS, thereby controlling the mechanical dose, prior to culture on soft hydrogels for 7 days in an adipogenic/osteogenic mixed media (Fig. 4a). In these experiments, we hypothesized that mechanical dosing would bias cells toward the osteogenic lineage based on the results that increased mechanical dosing increased RUNX2 expression. Cells were cultured in a mixed, bipotential adipogenic/osteogenic medium for these experiments to remove any confounding effect of chemical dosing and to explore how mechanical dosing primes cells in a bipotential landscape. As a control, hMSCs were cultured strictly on soft hydrogels for 7 days in the mixed media (So7). In the So7 samples, cells expressed the adipogenic markers peroxisome proliferator-activated receptor gamma (PPARγ; Fig. 4b,d) and Oil Red O (Fig. 4f) as well as osteogenic markers alkaline phosphatase (ALP; Fig. 4c,e) and osteocalcin (OCN; Fig. 4d). Thus, without prior mechanical dosing, hMSCs remain plastic and are able to differentiate toward adipogenesic and osteogenic lineages. As the mechanical dose on TCPS increased from 1 day to 10 days prior to transfer to soft hydrogels (DT1 / So7 to DT10 / So7), differentiation became increasingly biased toward osteogenesis. PPARγ gene expression decreased as a function of mechanical dose (Fig. 4b,d); whereas, ALP and OCN expression increased as a function of mechanical dose (Fig. 4c,e,g). Additionally, Oil Red O staining was only observed in the So7 and DT1 / So7 samples (Fig. 4f). Collectively, these data reveal that mechanical dosing history, prior to culturing hMSCs in a condition that maintains plasticity (soft hydrogel with mixed media), can override the current signal and bias differentiation toward the osteogenic lineage.
Figure 4. Influence of mechanical dosing on differentiation of hMSCs.
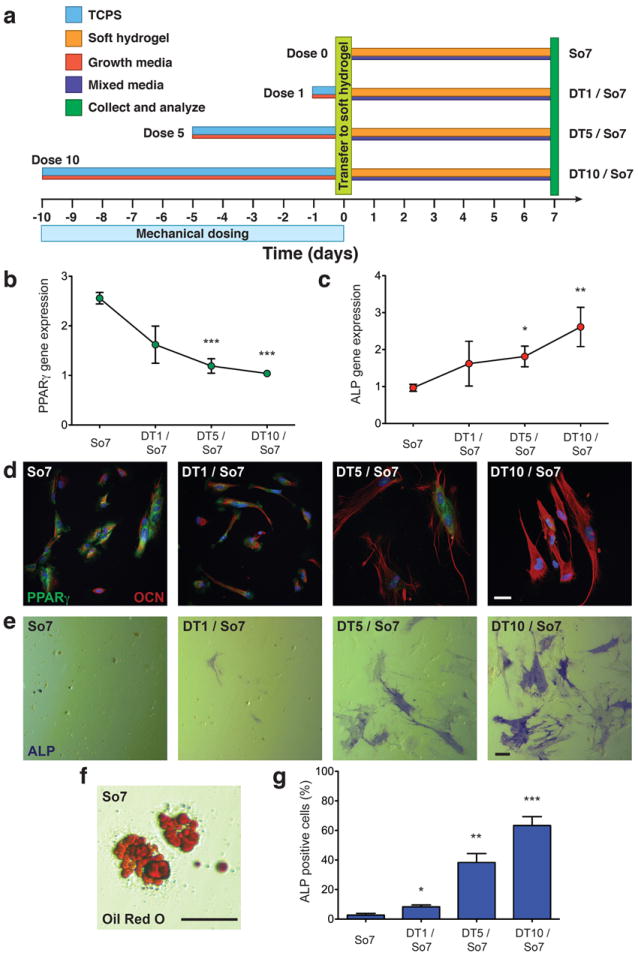
a, hMSCs were cultured on TCPS (blue) in growth media (red) for 1 to 10 days prior to trypsinization and transfer (light green, day 0) to soft hydrogels (orange). hMSCs were cultured subsequently on soft hydrogels for 7 days in mixed media (dark purple) prior to collection and analysis (green, day 7). Control samples were cultured on soft hydrogel in mixed media without mechanical dosing on TCPS (So7). b, PPARγ gene expression in hMSCs with mechanical dosing on TCPS prior to culture on soft hydrogels as quantified by qRT-PCR. c, ALP gene expression in hMSCs with mechanical dosing on TCPS prior to culture on soft hydrogels as quantified by qRT-PCR. d, Immunocytochemistry of PPARγ (green) and osteocalcin (OCN; red) in hMSCs after 7 days on soft hydrogels with various mechanical dose on TCPS. e, Staining for alkaline phosphatase (ALP) in hMSCs with mechanical dosing on TCPS prior to culture on soft hydrogels. f, Representative image of staining for Oil Red O in hMSCs on soft hydrogels. g, Quantification of the percentage of ALP positive cells as a function of mechanical dose on TCPS prior to culture on soft hydrogels. Scale bars, 20 μm. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared to So7.
Outlook
We conclude that hMSCs retain mechanical information from past physical environments and that this mechanical dosing influences future cell fate decisions. Further, we demonstrate that the YAP transcription factor may act as an intracellular mechanical rheostat mediating the effects of mechanical dosing on stem cell plasticity by persistent presence in the nucleus. Mechanical dosing for brief periods leads to reversible activation of YAP; however, a threshold dose occurs that leads to constitutive activation of YAP even after the mechanical dose is removed. Additionally, we show that mechanical dosing on TCPS biases hMSC differentiation toward osteogenesis during culture on soft hydrogels, which initially favor adipogenesis. These findings, that cells respond to mechanical dosing and possess a mechanical memory, deepen our basic understanding of cellular mechanotransduction. Further, they may have significant implications from both the fundamental view of stem cell plasticity during development, disease, and aging, as well as from the practical perspective of how culture and expansion outside of the body affects stem cell function and differentiation. In fact, one can envision potential synergies between mechanical dosing and chemical dosing in manipulating stem cell differentiation.
In total, this manuscript suggests a temporal role in cellular mechanotransduction that involves the history of a cell’s microenvironment. YAP shuttles between the cytoplasm and nucleus, as a mediator of extracellular microenvironmental signals.16 We show that continual exposure to culture environments that activate YAP transiently can lead to constitutive activation. Persistent activation influences the multipotency of hMSCs and appears to act like a promoter toward osteogenesis. This points to a unique mechanism in cellular mechanotransduction, namely one that cells use to retain information from the ECM and at the same time raises several questions as to how short term exposure to microenvironmental cues may lead to reversible and irreversible (long-term) effects on stem cells. Are the irreversible changes induced by constitutive YAP activation mediated genetically, epigenetically, or structurally?30 There is strong evidence that YAP/TAZ mechanotransduction depends directly on the organization of the actin cytoskeleton.15,16 In preliminary experiments in this setting, the effects of mechanical dosing persisted even after disruption of the actin cytoskeleton with latrunculin A (Supplementary Fig. S16), suggesting that there may be secondary players in mechanical memory and dosing. Do the irreversible effects caused by mechanical dosing employ similar signaling pathways as chemical dosing? Can one erase the mechanical memory by excluding YAP from the nucleus during culture or for therapeutic purposes? An improved understanding of the mechanotransduction machinery involved in mechanical dosing and memory may lead to the development of culture additives/conditions that better maintain stem cell multipotency. What are the individual and additive effects of modulus, integrin expression, surface chemistry, protein conformation, and topography on mechanical memory and dosing? The literature is converging on the paradigm that cellular mechanotransduction is not governed by modulus alone, but through a complex interplay of all of these factors.4-7 What additional information will be revealed by studying mechanical memory and dosing on substrates with defined and independently varied topography, surface chemistry, protein conformation, and modulus? Investigating such questions and concepts will not only help evolve more relevant culture systems, especially for stem cells, but improve our collective understanding of how extracellular cues, both soluble and insoluble, are integrated and stored during the life of a cell.
Methods
For complete methods, see Supplementary Information.
Synthesis of hydrogel components
Polyethylene glycol di-photodegradable acrylate (PEGdiPDA) was synthesized and characterized as previously described.18,19 The adhesive peptide OOGRGDSG (diethylene glycol-diethylene glycol-glycine-arginine-glycine-aspartic acid-serine-glycine) was synthesized (Protein Technologies Tribute peptide synthesizer) through Fmoc solid-phase methodology and HATU activation.26 Acrylic acid was coupled on resin to the N-terminal amine with HATU to synthesize Acryl-OOGRGDSG.
Fabrication of photodegradable hydrogels for cell seeding
The preparation of PEGdiPDA, photodegradable hydrogels was adapted from previously described protocols. 20,25 Briefly, PEGdiPDA was co-polymerized with poly(ethylene glycol) monoacrylate (PEGA; Mn ~ 400 Da; Monomer-Polymer and Dajac Laboratories, Inc.) and Acryl-OOGRGDSG in PBS via redox-iniated free radical polymerization. Gel solutions were prepared with 2.5 wt% PEGdiPDA, 10 wt% PEGA, 5 mM Acryl-OOGRGDSG, 0.2 M ammonium persulfate (APS), and 0.1 M tetramethylethylenediamine (TEMED). Gels were formed on acrylated cover glass with diameter of 18 or 22 mm and a thickness of 100 μm. Gels were rinsed in PBS prior to cell seeding. Soft hydrogels (~ 2 kPa) were prepared by irradiating the initial photodegradable hydrogels (~ 10 kPa) with UV light (λ = 365nm; I0 = 10 mW/cm2) for 360s.
hMSC isolation and culture
Human mesenchymal stem cells (hMSCs) were isolated from human bone marrow (Lonza) based on their preferential adhesion to tissue culture polystyrene (TCPS) plates.31 Freshly isolated hMSCs were frozen down in 95% fetal bovine serum (FBS; Invitrogen) and 5% DMSO and marked as P1 hMSCs. P1 hMSCs were used and cultured in growth media, except as noted. Media was changed every 2 to 3 days and hMSCs were treated with mitomycin (10 μg/ml; Sigma) for 2h, to inhibit proliferation, 24 h after seeding. Samples that were used in RT-PCR for RUNX2 expression analysis were not treated with mitomycin. For hMSC differentiation studies, a bipotential adipogenic/osteogenic inductive medium (‘mixed media’) was made by combining adipogenic and osteogenic inductive media 1:1.
Gene expression analysis
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to quantify the mRNA expression levels of ALP, RUNX2, and PPARγ relative to GAPDH. RNA was extracted from the culture samples using TRI Reagent (Sigma) following manufacturer’s instructions. The quantity and purity of extracted RNA was measured via spectrophotometry (ND-1000; NanoDrop). cDNA was synthesized from total RNA using the iScript Synthesis kit (Bio-Rad). Relative mRNA expression levels were measured via qRT-PCR, normalized to GAPDH, using SYBR Green reagents (Bio-Rad) on an iCycler (Bio-Rad).
Immunocytochemistry
hMSCs cultured on TCPS or photodegradable hydrogels were fixed, permeabilized with 0.1% TritonX-100 (Sigma), and blocked with 5 wt% BSA (Sigma). Samples were incubated with primary antibodies for YAP, RUNX2, OCN, and/or PPARg overnight at 4°C. Subsequently, samples were incubated with secondary antibodies (1:1000; Invitrogen) and DAPI (1 μg/ml; Sigma) for 1h at room temperature. Samples were imaged using laser scanning confocal microscopy (LSM 710 NLO; Carl Zeiss AG). DAPI was used to quantify cell number. The percentages of hMSCs with nuclear YAP or RUNX2 were obtained by manually counting cells with nuclear co-localized YAP or RUNX2 and then dividing by the total number of cells and multiplying by 100.
Mechanical dosing on TCPS
For the initial mechanical dosing on TCPS, hMSCs were seeded at 1000 cells/cm2 on TCPS (6-well or 60 cm2 plates) in growth media. Samples (n ≥ 3) were harvested 1, 3, 5, and 7 days after seeding for qRT-PCR to analyze for RUNX2 gene expression. In an additional experiment, hMSCs were seeded at 1000 cells/cm2 on TCPS in growth media prior to treatment with 0.05% trypsin-EDTA (Gibco) and transfer to soft hydrogels at 1000 cells/ cm2 in growth media 1, 3, 5, and 7 days after initial seeding. After 3 days on the soft hydrogels, samples were harvested (n ≥ 3) for immunocytochemistry. To test the effect of mechanical dosing on TCPS on hMSC differentiation, hMSCs were seeded at 2000 cells/cm2 on TCPS in growth media. The cells were treated with trypsin and transferred to soft hydrogels at 3000 cells/ cm2 1, 5, and 10 days after initial seeding. The media was replaced with mixed media 48h after hMSCs were transferred to the soft hydrogels. A control sample was included in which hMSCs were seeded directly on soft hydrogels; 48h after seeding, control samples were exposed to mitomycin (5 μg/ml) for 1h, followed by mixed media. Samples were harvested (n ≥ 3) after 7 days in mixed media for qRT-PCR and immunocytochemistry.
Mechanical dosing on photodegrable hydrogels
hMSCs were seeded on stiff hydrogels at 1000 cells/cm2 in growth media. Hydrogels were softened in situ 1, 7, and 10 days after seeding with UV light (λ = 365nm; I0 = 10 mW/cm2) for 360s in growth media without phenol red. hMSCs were cultured on the soft hydrogels in growth media and harvested 1, 3, and 5 days after in situ softening for immunostaining. For samples that were softened 10 days after seeding, samples were harvested 1, 5, and 10 days after in situ softening.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (DMR 1006711; K.S.A.), the National Institutes of Health (1R21 AR057904, R01 DE016523), the Howard Hughes Medical Institute (K.S.A.), the Teets Family Endowed Doctoral Fellowship (M.W.T.), and the Molecular Biophysics Training Grant from the National Institutes of Health (T32 GM-065103; M.W.T.). We would like to thank R. Tjian and I. Grubisic for helpful discussions on the work as well as E. A. Appel and T. A. Tauer for advice on figure preparation.
Footnotes
Author contributions
M.W.T., C.Y., and K.S.A. conceived the ideas and designed the experiments. C.Y., L.B., and M.W.T. conducted the experiments and analyzed the data. M.W.T., C.Y., L.B., and K.S.A. interpreted the data and wrote the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and rhoa regulate stem cell lineage commitment. Developmental Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 3.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMurray RJ, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011;10:637–644. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- 5.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and longterm cellular responses to dynamic mechanics. Nature Communications. 2012;3 doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 6.Trappmann B, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 7.Khetan S, et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12:458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guilak F, et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watt FM, Jordan PW, Oneill CH. Cell-chape controls terminal differentiation of human epidermal-keratinocytes. Proc Natl Acad Sci U S A. 1988;85:5576–5580. doi: 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingber DE. Fibronectin controls capillary endothelial-cell growth by modulating cell-shape. Proc Natl Acad Sci U S A. 1990;87:3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert PM, et al. Substrate elasticity regulates skeletal muscle stem cell selfrenewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont S, et al. Role of yap/taz in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 16.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by yap and taz. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 17.Maloney JM, et al. Mesenchymal stem cell mechanics from the attached to the suspended state. Biophys J. 2010;99:2479–2487. doi: 10.1016/j.bpj.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloxin AM, Tibbitt MW, Anseth KS. Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nature Protocols. 2010;5:1867–1887. doi: 10.1038/nprot.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kloxin AM, Tibbitt MW, Kasko AM, Fairbairn JF, Anseth KS. Tunable hydrogels for external manipulation of cellular microenvironments through controlled photodegradation. Adv Mater. 2010;22:61–66. doi: 10.1002/adma.200900917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tibbitt MW, Kloxin AM, Anseth KS. Modeling controlled photodegradation in optically thick hydrogels. J Polym Sci Pol Chem. 2013;51:1899–1911. doi: 10.1002/pola.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tibbitt MW, Kloxin AM, Sawicki LA, Anseth KS. Mechanical properties and degradation of chain and step-polymerized photodegradable hydrogels. Macromolecules. 2013;46:2785–2792. doi: 10.1021/ma302522x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong DY, Griffin DR, Reed J, Kasko AM. Photodegradable hydrogels to generate positive and negative features over multiple length scales. Macromolecules. 2010;43:2824–2831. [Google Scholar]
- 24.Kloxin AM, Benton JA, Anseth KS. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials. 2010;31:1–8. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tibbitt MW, Kloxin AM, Dyamenahalli KU, Anseth KS. Controlled two-photon photodegradation of peg hydrogels to study and manipulate subcellular interactions on soft materials. Soft Matter. 2010;6:5100–5108. doi: 10.1039/C0SM00174K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Haeger SM, Kloxin AM, Leinwand LA, Anseth KS. Redirecting valvular myofibroblasts into dormant fibroblasts through lightmediated reduction in substrate modulus. Plos One. 2012;7:e39969. doi: 10.1371/journal.pone.0039969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frey MT, Wang YL. A photo-modulatable material for probing cellular responses to substrate rigidity. Soft Matter. 2009;5:1918–1924. doi: 10.1039/b818104g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Tibbitt MW, Langer SJ, Leinwand LA, Anseth KS. Hydrogels preserve native phenotypes of valvular fibroblasts through an elasticity-regulated pi3k/akt pathway. Proc Natl Acad Sci U S A. 2013;110:19336–19341. doi: 10.1073/pnas.1306369110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 30.Shin JW, et al. Mechanobiology of bone marrow stem cells: From myosin-ii forces to compliance of matrix and nucleus in cell forms and fates. Differentiation. 2013;86:77–86. doi: 10.1016/j.diff.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariner PD, Johannesen E, Anseth KS. Manipulation of mirna activity accelerates osteogenic differentiation of hmscs in engineered 3d scaffolds. J Tissue Eng Regen Med. 2012;6:314–324. doi: 10.1002/term.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


