Abstract
To determine whether outcome after allogeneic hematopoietic cell transplantation (HCT) could be estimated by using peripheral white blood cell count (WBC) as a metric that integrates several aspects of HCT recovery, we conducted a retrospective study of 1109 adult patients who underwent first allogeneic HCT from 2003 through 2009. WBC at 1, 2, and 3 months after HCT was categorized as low (<2), normal (2-10), and high (>10×109 cells/L). Overall survival (OS) and progression-free survival (PFS) were lower for patients with low or high WBC at 1, 2, or 3 months after HCT (p<0.0001). We developed a predictive 3-group risk model based on the pattern of WBC recovery early after HCT. Five-year OS was 47%, 30%, and 15% (p<0.0001) and 5-year PFS was 39%, 22%, and 14% for patients in the 3 different risk groups (p<0.0001). The pattern of WBC recovery early after HCT provides prognostic information for relapse, non-relapse mortality, progression-free survival, and overall survival. A scoring system based on the trajectory of the WBC in the first 3 months after HCT can effectively stratify patients into 3 groups with different PFS and OS. If validated, this system could be useful in the clinical management of patients after HCT, and to stratify patients enrolled on HCT clinical trials.
Keywords: White blood cell count, Allogeneic transplantation, Prognostic risk group
INTRODUCTION
The success of allogeneic hematopoietic stem cell transplantation (HCT) depends, in large part, on the robust recovery of the lymphoid and myeloid hematopoietic system. While the complexity of immunologic reconstitution is still being unraveled, studies have suggested that early hematopoietic recovery after HCT can be useful prognostic markers for HCT outcome. Early recovery of absolute lymphocyte count (ALC) [1-8], lymphocyte subsets [9-10], absolute monocyte counts [7-8], number of circulating NK cells [11] or invariant natural killer T (iNKT)/T cell ratios [12] have been reported to be associated with survival, non-relapse mortality, relapse, and acute GVHD. Recently, pre-transplantation ALC level was also shown to be associated with relapse and survival [13]. In contrast to leukocyte subsets, the total circulating white blood cell count (WBC) integrates both myeloid and lymphoid elements, and could also be a marker of immunohematopoietic function. Perturbations of the WBC early after HCT could reflect poor stem cell number and function, host microenvironment defect, inflammation, infection, and medication effect. We hypothesized that WBC recovery as a whole, and the patterns of WBC changes early after HCT might provide a simple, yet independent prognostic marker for clinical outcomes after HCT. To test this hypothesis, we conducted a large retrospective analysis of patients undergoing HCT for hematologic malignancies at our institution.
METHODS
Patients
The study cohort was comprised of 1109 consecutive adult patients who underwent first peripheral blood or bone marrow allogeneic HCT with myeloablative or reduced intensity conditioning at Dana Farber Cancer Institute/Brigham and Women’s Hospital between 2003 and 2009. Patients receiving umbilical cord blood transplantation, haplo-identical transplant, or transplantation for benign hematologic conditions were excluded. Patients who died or relapsed within one month of HCT were also excluded since WBC at 1 month could not be assessed. All patients provided consent for use of protected health data for research on a protocol approved by the Institutional Review Board of the Dana-Farber/Harvard Cancer Center. Demographic, clinical and laboratory data, as well as HCT outcomes were retrieved from our comprehensive institutional transplantation database.
Attainment of WBC data after HCT
The WBC at or nearest (usually within 1week) the set time points of the study were retrieved from complete blood counts (CBC) drawn as part of routine clinical care after transplantation.
Transplantation
Patients were transplanted on a variety of investigational protocols and treatment plans. Myeloablative conditioning (MAC) regimens consisted mostly of cyclophosphamide (3600 mg/m2 or 120 mg/kg) plus total body irradiation (1400 cGy in 7 fractions), or intravenous busulfan (12.8 mg/kg) plus cyclophosphamide (3600 mg/m2). Reduced intensity conditioning (RIC) regimens consisted primarily of fludarabine (120 mg/m2) plus intravenous low-dose busulfan (3.2-6.4 mg/kg). Patients received bone marrow or filgrastim mobilized peripheral blood stem cells from HLA-matched or mismatched, related or unrelated donors. Graft-versus-host disease (GVHD) prophylaxis consisted primarily of a calcineurin inhibitor (cyclosporine or tacrolimus) combined with methotrexate, with or without sirolimus (Table 1). Based on the clinical protocols, filgrastim (G-CSF) was usually started on day+12 after MAC transplantation, or on day +1 after RIC to hasten neutrophil engraftment, and discontinued when the absolute neutrophil counts is over 1000/μL for 2 consecutive days. Supportive care for all patients followed institutional standards.
Table 1.
Baseline Characteristics
| All (N=1109) | MAC (N=528) | RIC (N=581) | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
|
| ||||||
| Age, median (range) | 51 (18, 74) | 44 (18, 61) | 57 (18, 74) | |||
| Patient Sex | ||||||
| Male | 658 | 59.3 | 289 | 54.7 | 369 | 63.5 |
| Female | 451 | 40.7 | 239 | 45.3 | 212 | 36.5 |
| Donor Sex | ||||||
| Male | 639 | 57.6 | 299 | 56.6 | 340 | 58.5 |
| Female | 470 | 42.4 | 229 | 43.4 | 241 | 41.5 |
| Patinet-Donor Sex | ||||||
| FF | 205 | 18.5 | 111 | 21 | 94 | 16.2 |
| FM | 246 | 22.2 | 128 | 24.2 | 118 | 20.3 |
| MF | 265 | 23.9 | 118 | 22.3 | 147 | 25.3 |
| MM | 393 | 35.4 | 171 | 32.4 | 222 | 38.2 |
| ECOG PS | ||||||
| 0 | 374 | 33.7 | 200 | 37.9 | 174 | 29.9 |
| 1 | 480 | 43.3 | 214 | 40.5 | 266 | 45.8 |
| 2-3 | 99 | 8.9 | 34 | 6.4 | 65 | 11.1 |
| unknown | 156 | 14.1 | 80 | 15.2 | 76 | 13.1 |
| Disease | ||||||
| AML | 404 | 36.4 | 232 | 43.9 | 172 | 29.6 |
| ALL | 100 | 9 | 85 | 16.1 | 15 | 2.6 |
| CLL | 102 | 9.2 | 19 | 3.6 | 83 | 14.3 |
| CML | 78 | 7 | 61 | 11.6 | 17 | 2.9 |
| Hodgkin lymphoma | 50 | 4.5 | 8 | 1.5 | 42 | 7.2 |
| Multiple Myeloma | 35 | 3.2 | 1 | 0.2 | 34 | 5.9 |
| MDS | 130 | 11.7 | 50 | 9.5 | 80 | 13.8 |
| Myeloproliperative Neoplasms | 25 | 2.4 | 7 | 1.3 | 19 | 3.3 |
| Non-Hodgkin lymphoma | 180 | 16.2 | 62 | 11.7 | 118 | 20.3 |
| Other Acute Leukemia | 4 | 0.4 | 3 | 0.6 | 1 | 0.2 |
| HLA Type | ||||||
| Matched/Unrelated | 565 | 50.9 | 245 | 46.4 | 320 | 55.1 |
| Matched/Related | 450 | 40.6 | 235 | 44.5 | 215 | 37 |
| Mismatched/Unrelated | 86 | 7.8 | 42 | 8 | 44 | 7.6 |
| Mismatched/Related | 8 | 0.7 | 6 | 1.1 | 2 | 0.3 |
| Graft Source | ||||||
| Bone Marrow | 70 | 6.4 | 54 | 10.3 | 16 | 2.8 |
| PBSC | 1039 | 93.7 | 474 | 89.8 | 565 | 97.2 |
| Disease Risk Indexa | ||||||
| 0: Low | 177 | 16 | 57 | 10.8 | 120 | 20.7 |
| 1: Intermediate | 591 | 53.3 | 278 | 52.7 | 313 | 53.9 |
| 2: High | 304 | 27.4 | 171 | 32.4 | 133 | 22.9 |
| 3: Very high | 37 | 3.3 | 22 | 4.2 | 15 | 2.6 |
| Patient or Donor CMV seropositivity | ||||||
| No | 421 | 38 | 216 | 40.9 | 205 | 35.3 |
| Yes | 688 | 62 | 312 | 59.1 | 376 | 64.7 |
| aGVHD prophylaxis | ||||||
| CI+Sirolimus | 697 | 62.8 | 288 | 54.5 | 409 | 70.4 |
| CI+Other | 376 | 33.9 | 229 | 43.4 | 147 | 25.3 |
| Sirolimus+MMF | 15 | 1.4 | 15 | 2.6 | ||
| Ex vivo TCD/Other | 21 | 1.9 | 11 | 2.1 | 10 | 1.7 |
| Year of HCT, median (range) | 2005 (2003, 2009) |
2005 (2003, 2009) |
2005 (2003, 2009) |
|||
| CD34 cells/kg (×106), medain (range) | 7.8 (0.3, 47.7) | 7.7 (0.3, 32.7) | 7.9 (0.8, 47.7) | |||
| no. of missing CD34 | 5 | 0.5 | 3 | 0.6 | 2 | 0.3 |
| Day 30 Chimerismb, median (range) | 90 (0, 100) | |||||
| no. of missing chimerism | 9 | 1.5 | ||||
| Years of follow-up for survivors median (range) |
5.1 (1.1, 9.6) | 5.9 (2.0, 9.2) | 5.0 (1.1, 9.6) | |||
Armand et al, Blood, 2012;
patients with reduced intensity conditioning only. CI: Calcineurin Inhibitor; TCD: T cell depletion.
Chimerism Analysis
In patients undergoing RIC transplantation, day 30 total donor chimerism was assessed from bone marrow aspirates and/or blood approximately 30 days after HCT. Genotyping was determined by short tandem repeat typing using the ABI Profiler Plus Kit (Applied Biosystems Inc.) and ABI 310 Genetic Analyzer. “Informative” alleles specific to donor or recipient were used for chimerism determination.
Endpoints and Statistical Analysis
The primary goal of the study was to assess the relationship of WBC recovery at 1, 2, and 3 months after HCT to overall survival (OS) and progression-free survival (PFS). To rule out the direct influence of relapse on the WBC, a landmark analysis was performed at 1,2,3 months post HCT excluding relapse or death prior to each time point. In fact, throughout the analysis, all WBC values that were measured after disease relapse within 3 months of HCT were censored at each time point. OS and PFS were previously defined [14]. Log-rank test was used for comparisons of Kaplan-Meier curves. Cumulative incidences for non-relapse death and relapse with or without death were estimated reflecting time to relapse and time to non-relapse death respectively as competing risks. Gray test [15] was used for comparison of cumulative incidence curves. Multivariable proportional hazards models stratified by conditioning intensity were constructed to examine the effect of WBC after adjusting for other potential prognostic factors that are detailed in Table 1 and occurrence of grade II-IV acute GVHD and grade 3 or higher infection within 100 days of HCT. The occurrence of grade II-IV and grade ≥3 infection were included as time dependent variables in multivariable analysis. The proportional hazards assumption for each variable was tested and interaction terms were examined. The linearity assumption for continuous variables was examined using restricted cubic spline estimates of the relationship between the continuous variable and log relative hazard [16] and the cutoff points of these variables were based on the change of the log relative hazards. In particular, WBC was categorized as 0-<2×109 cells/L, 2-<4×109 cells/L, and >10×109 cells/L and age is dichotomized as ≥40 vs. <40 for MAC and ≥60 vs. <60 in RIC patients. CD34 cells/kg was categorized as ≤4×106, 4-15 ×106, and >15 ×106 cells/kg. Risk factors for low or high WBC were examined using multivariable multinomial logistic regression analysis (i.e., low vs. normal, high vs. normal). All p-values are two-sided. A significance level of 0.025 was employed to take multiple comparisons (low vs. normal, high vs. normal WBC) into account. However, when exploring potential risk factors for low or high WBC, a significance level of 0.05 was employed. All calculations were done using SAS 9.3 (SAS Institute Inc, Cary, NC), and Rversion 2.13.2 (the CRAN project).
RESULTS
Patient Characteristics
The baseline characteristics of the 1109 patients are shown in Table 1. The median age was 51 years (range 18-74). The median follow up time among surviving patients was 5.1 years (range 1.1-9.6). Fifty-two percent of patients received reduced intensity conditioning (RIC) and 48% received myeloablative conditioning (MAC). Seventy-seven percent of patients were ECOG performance status 0 or 1 at baseline; 41% of patients underwent transplantation from HLA-matched related donors, while 51% were transplanted from HLA-matched unrelated donors, and 8.5% received HLA-mismatched transplantation; 94% of patients received stem cells from G-CSF mobilized peripheral blood. The distribution of disease risk index (DRI), which was created based on disease and disease status at transplantation [14], was 16% for low, 53% intermediate, 27% for high and 3.3% for very high risk.
White Blood Cell Counts After HCT and Threshold Value
The distribution of WBC (excluding values after disease relapse) at 1, 2 and 3 months after HCT is shown in Figure 1-(A). The median WBC at these 3 time points was between 4.4 and 4.8×109 cells/L, and 36% - 42% of patients had WBC below 4×109 cells/L during this period. This suggests that many patients experience a prolonged period of leukopenia after HCT. Since the lower limit of WBC (4×109 cells/L) may not be clinically the most relevant threshold in this patient population, we determined empirically the optimal thresholds by using restricted cubic spline estimates of the relationship between WBC and log relative hazard of overall survival (14). The spline smoothing curve suggests that there is a U-shaped relationship between the level of WBC and hazard of overall survival. To confirm this U-shaped relationship, we plotted hazard ratios of overall survival by intervals of WBC counts (Figure 1-(B)). The hazard ratio of WBC 0-<2×109 cells/L relative to the reference group of WBC 4-<6×109 cells/L was 1.51 (p=0.01), 1.6(p=0.004), and 1.5 (p=0.04), respectively, at 1,2,3 months after HCT. Similarly, the hazard ratio of WBC ≥10×109 cells/L relative to the reference group was 1.76 (p<0.0001), 1.74(p=0.002), and 1.62 (p=0.016), respectively, at 1,2,3 months after HCT. It is noted that the hazard ratio of WBC 2-4×109 cells/L to the reference group is close to 1 at each time point (0.91, 1.03, 0.98 at 1,2,3 months after HCT, respectively). Based on this information, we categorized WBC as “low” (0-<2×109 cells/L), “normal” (2-10×109 cells/L), or “high” (>10×109 cells/L). Using this definition, 6-7% of patients had low WBC and 5-10% of patients had high WBC at 1,2, or 3 months post HCT. Overall, 27% of patients had either low or high WBC at some point during the first 3 months of HCT.
Figure 1.
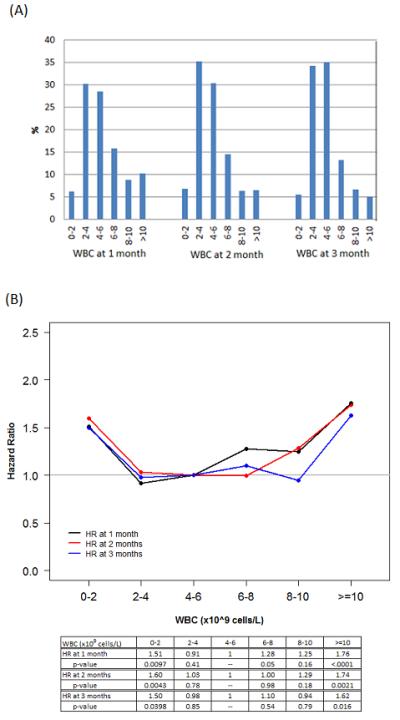
(A) Distribution of WBC at 1,2,3 months after HCT. Landmark analysis was performed excluding relapse or death prior to each time point. (B) Hazard ratios of WBC at 1,2,3 months after HCT. The reference group is WBC 4.6 × 109 cells/L. The gray horizontal line represents hazard ratio of 1.
Impact of WBC on Survival Outcomes
Regardless of the time point (1, 2 or 3 months after HCT), a low or a high WBC was associated with inferior OS and PFS. Five-year OS was 33%, 45%, 33% for patients with low, normal, or high WBC at 1 month after HCT, respectively (p<0.0001), 35%, 50%, 37% at 2 months after HCT, respectively (p<0.0001), and 41%, 53%, 48% at 3 months after HCT, respectively (p≤0.0025). Five-year PFS was 25%, 38%, 30% for patients with low, normal, or high WBC at 1 month after HCT, respectively (p=0.0001), 27%, 42%, 33% at 2 months after HCT, respectively (p<0.0001), and 37%, 45%, 42% at 3 months after HCT, respectively (p=0.03 for L vs. N, 0.037 for H vs. N) (Figure 2, Table S1). This was true even when RIC and MAC patients were analyzed separately except for MAC at 3 months after HCT. For MAC patients, low or high WBC at 3 months after HCT was not statistically different compared to patients with normal WBC (Table S1).
Figure 2.
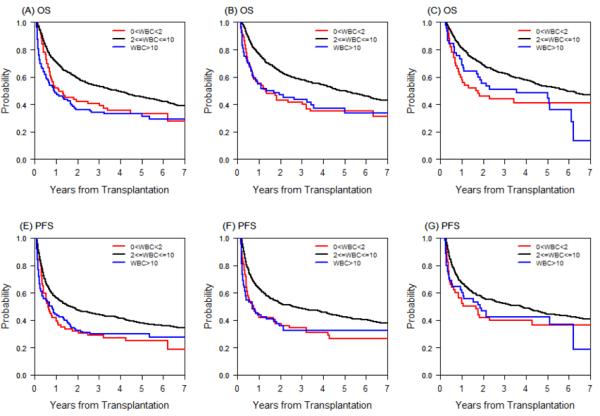
(A) Overall survival (OS) by WBC category at 1 month after HCT. (B) OS by WBC category at 2 months after HCT. (C) OS by WBC category at 3 months after HCT. (D) Progression-free survival (PFS) by WBC category at 1 month after HCT. (E) PFS by WBC category at 2 months after HCT. (F) PFS by WBC category at 3 months after HCT.
Pattern of WBC recovery
Since some patients with either low or high WBC at 1 or 2 months after HCT recovered at the subsequent time point(s), we investigated the pattern of WBC recovery and its impact on survival outcome. We noted that many patients developed newly low or high WBC at 2 or 3 months after HCT, and this was an equally high risk of poor outcome as it was for patients whose WBC was low or high at 1 month as well. When each type of WBC trend (i.e., normal to low/high or vice versa) was examined using Cox models stratified by conditioning intensity, we found that patients with high WBC at 1 month who subsequently recovered their WBC to normal at 2 and 3 months after HCT had similar OS compared to patients with sustained normal WBC throughout the 3 month period (n=46, HR=1.05, p=0.84). Also, patients with normal WBC at 1 month who had one time transient low or high WBC during the 3 month period had similar OS compared to patients with sustained normal WBC throughout the 3 month period (n=84, HR=1.1, p=0.54). Thus we combined these groups as a referent group (WBC risk group 0). The remaining patients with first low WBC at 1 or 2 months (WBC risk group 1) or patients with first high WBC at 1 or 2 months after HCT (WBC risk group 2) had poor OS compared to the referent group (Table 2). Patients who died or relapsed after 1 month were scored based on their 1 month WBC value. Likewise, patients who died or relapsed after 2 months of HCT were scored based on their 1 and 2 months WBC values. In addition, patients with missing WBC at 2 months (n=4) and at 3 months after HCT (n=24) were categorized based on two available WBC values. To investigate whether these patients were a random subset of the entire cohort, the OS was compared between these 29 patients and 1080 patients and the OS was similar between these two cohorts (5-year OS 43% vs. 41%, p=0.86).
Table 2.
WBC risk score assignment
| WBC risk score | WBC 1m | WBC 2m | WBC 3m | ||
|---|---|---|---|---|---|
| 0 | Normal or High | & | Normal | & | Normal |
| Normal | & | Normal | |||
| Normal | & | Normal | |||
|
| |||||
| 1 | Low | ||||
| Normal | & | Low | |||
|
| |||||
| 2 | High | ||||
| Normal | & | High | |||
Blank means ‘any’ WBC category except that (Normal, Low, and Normal) or (High, Normal, and Normal) at 1,2,3 months after HCT, respectively, were categorized as WBC risk group 0. 4 patients with missing WBC at 2 months and 24 patients with missing WBC at 3 months after HCT were categorized based on 2 available WBC values. Scores for patients who died or relapsed after 1 month were based on their 1 month WBC values; scores for patients who died or relapsed after 2 months of HCT were based on their 1 and 2 months WBC values.
Based on this classification, the 5-year OS was 47%, 30%, and 15% for WBC risk group 0,1, and 2, respectively (p<0.0001) and the 5-year PFS was 39%, 22%, and 14% for WBC risk group 0,1, and 2, respectively (p<0.0001) (Table 3, Figures 3A and 3B). This result was consistent when other prognostic factors were adjusted for in multivariable Cox models stratified by conditioning intensity (HR 1.64, 3.06 for OS for WBC risk group 1 and 2 respectively, p≤0.0002; HR 1.49, 2.88 for PFS for WBC risk group 1 and 2 respectively, p≤0.0017) (Table 4). Other factors that were significantly associated with OS in the multivariable analysis were age (HR 1.27 for age>=40 in MAC and age>=60 in RIC, p=0.005), ECOG performance status (HR 1.31 for 1 vs 0, p=0.007; HR 1.58 for 2-3 vs 0, p=0.002), disease risk index (HR 1.73 for Intermediate vs. Low, p<0.0001, HR 2.68 for High/very high vs. Low, p<0.0001), occurrence of grade II-IV acute GVHD (HR 1.5, p<0.0001), and any grade ≥3 infection within day 100 (HR 1.89, p<0.0001). Other factors that were significantly associated with PFS in the multivariable analysis were ECOG performance status (HR 1.29 for 1 vs. 0, p=0.007; HR 1.42 for 2-3 vs. 0, p=0.01), disease risk index (HR 1.93 for Intermediate vs. Low, p<0.0001, HR 2.98 for High/very high vs. Low, p<0.0001), year of transplantation (HR 0.95, p=0.03), CD34 cell dose (HR 1.31 for ≤4×106 vs 4-15×106 cells/kg, p=0.03) and occurrence of grade ≥3 infection within day 100 (HR 1.71, p<0.0001). Detailed results of the multivariable analysis are provided in Table 4. In multivariable analysis, the occurrence of grade II-IV acute GVHD and day 100 grade ≥3 infection status were included as time dependent variables.
Table 3.
Survival outcomes by WBC risk group
| WBC risk groupa |
Univariable Analysis |
||||
|---|---|---|---|---|---|
| N | 1-yr (%), (95% CI) | 5-yr (%), (95% CI) | p-valueb | ||
|
| |||||
| OS | 0 | 937 | 73 (70,76) | 47 (44, 51) | ref |
| 1 | 91 | 46 (36, 56) | 30 (21, 40) | <0.0001 | |
| 2 | 81 | 31 (21, 41) | 15 (8, 24) | <0.0001 | |
|
| |||||
| PFS | 0 | 937 | 58 (55, 61) | 39 (36, 43) | ref |
| 1 | 91 | 35 (26, 45) | 22 (14, 31) | <0.0001 | |
| 2 | 81 | 25 (16, 34) | 14 (7, 22) | <0.0001 | |
|
| |||||
| NRM | 0 | 937 | 9 (7, 11) | 19 (17, 22) | ref |
| 1 | 91 | 24 (16, 33) | 28 (19, 37) | 0.008 | |
| 2 | 81 | 32 (22, 42) | 37 (27, 48) | 0.0005 | |
|
| |||||
| Relapse | 0 | 937 | 32 (29, 35) | 41 (38, 44) | ref |
| 1 | 91 | 41 (30, 51) | 51 (39, 61) | 0.29 | |
| 2 | 81 | 43 (32, 54) | 49 (38, 60) | 0.0005 | |
0: referent group; 1: low WBC during the first 3 months of HCT; 2: high WBC during the first 3 months of HCT, 3: normal WBC initially but disease progression or relapse during the first 3 months of HCT.
stratified by conditioning intensity
Figure 3.
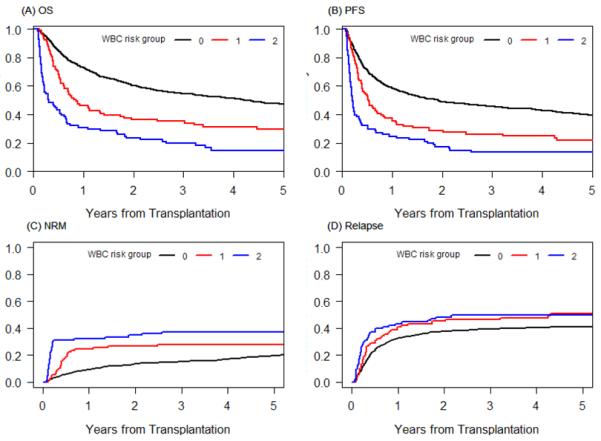
(A) Overall survival, (B) progression-free survival, (C) cumulative incidence of NRM and (D) relapse by WBC risk group. 0: reference group, 1: low WBC during the first 3 months of HCT; 2: high WBC during the first 3 months of HCT.
Table 4.
Multivariable Cox modelsa
| OS |
PFS |
NRM |
Relapse |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |||||
|
| ||||||||||||||||
| Ageb | 1.27 | 1.08 | 1.49 | 0.005 | 1.11 | 0.95 | 1.30 | 0.19 | 1.34 | 1.02 | 1.76 | 0.036 | 0.99 | 0.82 | 1.20 | 0.93 |
| Patient-Donor Sex | ||||||||||||||||
| M<-F vs. Other | 1.08 | 0.90 | 1.30 | 0.39 | 1.03 | 0.86 | 1.23 | 0.76 | 1.10 | 0.82 | 1.49 | 0.53 | 1.00 | 0.81 | 1.25 | 0.98 |
| HLA type | ||||||||||||||||
| MUD vs MRD | 1.06 | 0.90 | 1.27 | 0.48 | 0.98 | 0.83 | 1.15 | 0.78 | 1.65 | 1.22 | 2.22 | 0.001 | 0.77 | 0.63 | 0.93 | 0.008 |
| Mismatched vs MRD | 1.26 | 0.94 | 1.68 | 0.12 | 1.08 | 0.82 | 1.43 | 0.59 | 1.95 | 1.25 | 3.03 | 0.003 | 0.80 | 0.55 | 1.15 | 0.23 |
| Patient or Donor CMV | ||||||||||||||||
| seropositivity: Y vs. N | 1.06 | 0.90 | 1.25 | 0.47 | 1.02 | 0.87 | 1.19 | 0.83 | 1.14 | 0.87 | 1.51 | 0.34 | 0.97 | 0.80 | 1.17 | 0.73 |
| Disease Relapse Index | ||||||||||||||||
| Intermediate vs. Low | 1.73 | 1.32 | 2.26 | <.0001 | 1.93 | 1.50 | 2.49 | <.0001 | 1.12 | 0.76 | 1.66 | 0.56 | 2.50 | 1.78 | 3.52 | <.0001 |
| High/Very high vs. Low | 2.68 | 2.02 | 3.56 | <.0001 | 2.98 | 2.28 | 3.89 | <.0001 | 1.22 | 0.79 | 1.88 | 0.37 | 4.61 | 3.24 | 6.56 | <.0001 |
| Year of Transplantation | 0.97 | 0.93 | 1.02 | 0.28 | 0.95 | 0.91 | 1.00 | 0.03 | 0.98 | 0.91 | 1.07 | 0.69 | 0.94 | 0.89 | 1.00 | 0.04 |
| CD34 cells/kg (×106)c | ||||||||||||||||
| <=4 vs <4-15 | 1.28 | 0.99 | 1.66 | 0.06 | 1.31 | 1.03 | 1.66 | 0.03 | 1.37 | 0.90 | 2.09 | 0.15 | 1.21 | 0.91 | 1.63 | 0.19 |
| >15 vs <4-15 | 1.20 | 0.94 | 1.53 | 0.15 | 1.17 | 0.93 | 1.49 | 0.19 | 0.83 | 0.53 | 1.32 | 0.43 | 1.33 | 1.01 | 1.76 | 0.045 |
| ECOG performance Status | ||||||||||||||||
| 1 vs. 0 | 1.31 | 1.07 | 1.58 | 0.007 | 1.29 | 1.07 | 1.54 | 0.007 | 1.21 | 0.89 | 1.66 | 0.23 | 1.32 | 1.05 | 1.65 | 0.017 |
| 2-3 vs. 0 | 1.58 | 1.18 | 2.11 | 0.002 | 1.42 | 1.08 | 1.88 | 0.01 | 1.89 | 1.19 | 3.00 | 0.007 | 1.26 | 0.89 | 1.79 | 0.20 |
| missing vs. 0 | 1.29 | 0.99 | 1.67 | 0.06 | 1.23 | 0.96 | 1.58 | 0.10 | 1.08 | 0.69 | 1.67 | 0.75 | 1.31 | 0.96 | 1.78 | 0.09 |
| Graft Source | ||||||||||||||||
| BM vs. PBSC | 0.77 | 0.51 | 1.15 | 0.19 | 0.81 | 0.56 | 1.19 | 0.29 | 0.58 | 0.31 | 1.07 | 0.08 | 1.02 | 0.63 | 1.66 | 0.94 |
| Grade 2-4 aGVHD | 1.50 | 1.26 | 1.78 | <.0001 | 1.19 | 1.00 | 1.42 | 0.05 | 2.28 | 1.75 | 2.98 | <.0001 | 0.74 | 0.57 | 0.96 | 0.02 |
| Gr. ≥3 Infection within D100 | 1.89 | 1.57 | 2.27 | <.0001 | 1.71 | 1.40 | 2.08 | <.0001 | 3.39 | 2.45 | 4.70 | <.0001 | 1.14 | 0.88 | 1.48 | 0.33 |
| WBC risk group | ||||||||||||||||
| 1 vs. 0 | 1.64 | 1.26 | 2.13 | 0.0002 | 1.49 | 1.16 | 1.92 | 0.0017 | 2.04 | 1.34 | 3.11 | 0.0009 | 1.27 | 0.93 | 1.74 | 0.13 |
| 2 vs. 0 | 3.06 | 2.36 | 3.97 | <.0001 | 2.88 | 2.23 | 3.73 | <.0001 | 3.23 | 2.15 | 4.85 | <.0001 | 2.86 | 2.03 | 4.03 | <.0001 |
stratified by conditioning intensity.
dichotomized as 1 if age>=40 in MAC or >=60 in RIC; 0 otherwise.
5 missing CD34 are combined with CD34<=4 because of a similar hazard ratio.
d: time dependent variable. MUD: matched unrelated donor; MRD: matched related donor.
To further evaluate the impact of WBC on cumulative incidences of relapse and NRM, competing risks data analysis was performed. The 5-year cumulative incidence of NRM was 19%, 28%, 37% for WBC risk group 0, 1, and 2, respectively (p=0.008, p=0.0005, respectively), and the 5-year cumulative incidence of relapse was 41%, 51%, and 49% for WBC risk group 0,1, and 2 respectively (p=0.29, p=0.0005, respectively) (Table 3, Figures 3C and 3D). As in the survival outcome, this result was consistent when other prognostic factors were adjusted for in multivariable regression models stratified by conditioning intensity (HR 2.04, p=0.0009; HR 3.23, p<0.0001 for NRM for WBC risk group 1 and 2, respectively; HR 1.27, p=0.13; HR 2.86, p<0.0001 for relapse for WBC risk group 1 and 2, respectively) (Table 4). Other factors that were significantly associated with NRM in the multivariable analysis were age (p=0.036), matched unrelated donor (p=0.001), mismatched donor (p=0.003), ECOG performance status ≥2 (p=0.007), occurrence of grade II-IV acute GVHD (p<0.0001), and any grade ≥3 infection within day 100 (p<0.0001) (Table 4). Other factors that were significant for relapse in the multivariable analysis were matched unrelated donor (p=0.008), disease risk index (p<0.0001), CD34 cell dose (p=0.045), ECOG performance status (p=0.017), year of transplantation (p=0.04), and occurrence of grade II-IV acute GVHD (p=0.02) (Table 4).
Landmark Analysis
To confirm that the WBC risk group was not just a predictor of imminent relapse or NRM, but have prognostic significance for survival outcomes further into the future, we performed a landmark analysis restricted to patients who were alive and relapse-free beyond day 100 of HCT. The 5-year OS was 55%, 40%, 38% for WBC risk group 0,1, and 2, respectively (p≤0.0002) and 5-year PFS was 47%, 29%, 34% for WBC risk group 0,1, and 2, respectively (p≤0.0005) (Figure S1).
Factors affecting abnormal WBC
Because the impact of WBC recovery early after HCT on clinical outcome was statistically significant, we sought to identify factors that affect WBC during the first 3 months after HCT. In multivariable logistic regression analysis, patients receiving MAC were more likely to develop high WBC (OR=2.47 compared to RIC, p<0.0001); sirolimus use as GVHD prophylaxis (OR=0.62, p=0.009) and low CD34 cells infused (OR=0.50 for < 4×106 vs 4-15×106 cells/kg, p=0.04) were less likely to develop high WBC. For low WBC, patients receiving peripheral blood stem cells were less likely to develop low WBC (OR=0.40, p=0.04). For RIC patients, failure to achieving >=90% day 30 chimerism level was also associated with low WBC (OR=1.71, p=0.039).
We also investigated association of low or high WBC and infection that occurred within 100 days of HCT and found that low or high WBC was associated with viral and fungal infection, but not with bacterial infection (data not shown). Grade 3 or higher viral or fungal infection rate was 19%, 9%, 17% for patients with low, normal, high WBC, respectively (p=0.0002).
DISCUSSION
Recovery of the total WBC after HCT is affected by engraftment kinetics, infection, drug toxicity, persistence or relapse of hematologic malignancy, and marrow microenvironment defects. While much attention has been paid to post transplant metrics such as absolute lymphocyte count, and lymphoid chimerism, the impact of WBC as a whole early after HCT on survival has not been evaluated. We hypothesized that the WBC, as a ubiquitous and standard laboratory metric measured across the first few months after transplantation, can be a useful predictor of HCT outcomes.
Our analysis showed that leukocytosis in the first 3 months after HCT (WBC risk score 2) is a significant predictor of poor OS and PFS. In the case of high WBC, this may reflect the development of transplant-related complications such as infections, or presence of GVHD since WBC de-margination is expected from the high dose corticosteroids used as primary GVHD treatment. In the case of low WBC, this may reflect, marrow dysfunction associated with underlying malignancy, or defective marrow microenvironment. Leukopenia may also result from direct myelosuppression by infectious agents (especially viruses) or drugs. Finally, it is also possible that persistent low or high WBC early after HCT reflect impaired immunologic recovery, which predispose to infection or relapse.
We also proposed that the lower boundary for normal WBC for HCT prognostic purposes should be 2×109 cells/L, as opposed to the standard reference threshold of 4×109 cells/L. Indeed, our results suggest that patients with mild leukopenia (WBC 2-4 ×109 cells/L) after HCT have similar outcome as those with WBC 4-10 ×109 cells/L (HR=1.04 for OS, p=0.8).
We acknowledge that the single institution and retrospective design are inherent flaws of our study, although these limitations are mitigated to some degree by the large size of the cohort, the consistency of the results for RIC and MAC HCT, and the completeness of data at the different time points. As such, our results should be interpreted with some caution until it is validated in a large independent cohort. We also acknowledge that fluctuations of the WBC are expected in the course of routine clinical care, and thus the designation of prognostic value on single set point values in time needs to be interpreted cautiously, although this issue is not limited to this study only and is inherent to many studies with biologic measurements. It is reassuring, however, that our conclusions were also supported in analyses looking at the WBC trajectory over time, which is less likely to be affected by these fluctuations.
In summary, our finding suggest that the WBC at 1,2, and 3 months after HCT may have significant prognostic implications: patients with persistent leukopenia or leukocytosis in the first 3 months after HCT have severely compromised survival. Based on the WBC trajectory in the first 3 months after HCT, we have developed a WBC risk scoring system that effectively stratified 3 groups of patients with different PFS and OS. If validated, this system could potentially be a useful and readily available tool to guide prognostication and stratification of patients enrolled on HCT clinical trials, and alert clinicians toward earlier restaging of disease, closer monitoring of infection, donor chimerism, and potential pre-emptive interventions in patients with high risk scores.
Supplementary Material
Figure S1. Landmark analysis for overall survival (A) and progress-free survival (B) by WBC riskgroup. All death and relapse prior to day 100 of HCT were excluded.
Acknowledgement of Research Funding
This work was supported in part by NCI PO1 CA18029.
Financial disclosure: This research was funded in part by P01 CA142106 (to J.H.A.).
We acknowledge Kimberly Philips and the data management staff for their tireless work in maintaining the DFCI BMT database, without which this study would not be possible.
This study was presented in abstract at the annual meeting of the American Society of Hematology, Atlanta, Georgia, 2011.
Footnotes
Conflict of Interest Disclosures
The authors have no relevant conflicts of interest to disclose.
AUTHORSHIP CONTRIBUTIONS
H. T. K. designed and performed the research, analyzed the data, and wrote the paper.
D. F. collected data and reviewed the paper.
P. A. collected data, reviewed and wrote the paper.
E. A. collected data and reviewed the paper.
G. K. collected data and reviewed the paper.
C. C. collected data and reviewed the paper.
J. K. collected data and reviewed the paper.
E. P. A. collected data and reviewed the paper.
J. H. A. collected data and reviewed the paper.
R. J. S. collected data and reviewed the paper.
J. R. collected data and reviewed the paper.
V. T. H. collected data, oversaw the data collection team from the database, and wrote on the paper.
REFERENCES
- 1.Pavletic ZS, Joshi SS, Pirruccello SJ, et al. Lymphocyte reconstitution after allogeneic blood stem cell transplantation for hematologic malignancies. Bone Marrow Transplant. 1998;21(1):33–41. doi: 10.1038/sj.bmt.1701037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar S, Chen MG, Gastineau DA, et al. Lymphocyte recovery after allogeneic bone marrow transplantation predicts risk of relapse in acute lymphoblastic leukemia. Leukemia. 2003;17(9):1865–70. doi: 10.1038/sj.leu.2403055. [DOI] [PubMed] [Google Scholar]
- 3.Savani BN, Mielke S, Rezvani K, et al. Absolute lymphocyte count on day 30 is a surrogate for robust hematopoietic recovery and strongly predicts outcome after T cell-depleted allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(10):1216–23. doi: 10.1016/j.bbmt.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishaqi MK, Afzal S, Dupuis A, Doyle J, Gassas A. Early lymphocyte recovery post-allogeneic hematopoietic stem cell transplantation is associated with significant graft-versus-leukemia effect without increase in graft-versus-host disease in pediatric acute lymphoblastic leukemia. Bone Marrow Transplant. 2008;41:245–252. doi: 10.1038/sj.bmt.1705891. [DOI] [PubMed] [Google Scholar]
- 5.Le Blanc K, Barrett AJ, Schaffer M, et al. Lymphocyte recovery is a major determinant of outcome after matched unrelated myeloablative transplantation for myelogenous malignancies. Biol Blood Marrow Transplant. 2009;15(9):1108–15. doi: 10.1016/j.bbmt.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke MJ, Vogel RI, Janardan SK, et al. Early lymphocyte recovery and outcomes after umbilical cord blood transplantation (UCBT) for hematologic malignancies. Biol Blood Marrow Transplant. 2011;17(6):831–40. doi: 10.1016/j.bbmt.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thoma MD, Huneke TJ, DeCook LJ, et al. Peripheral blood lymphocyte and monocyte recovery and survival in acute leukemia postmyeloablative allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2012 Apr;18(4):600–7. doi: 10.1016/j.bbmt.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Decook LJ, Thoma M, Huneke T, et al. Impact of lymphocyte and monocyte recovery on the outcomes of allogeneic hematopoietic SCT with fludarabine and melphalan conditioning. Bone Marrow Transplant. 2012 Oct 29; doi: 10.1038/bmt.2012.211. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Sohn SK, Won DI, et al. Rapid helper T-cell recovery above 200 × 10 6/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37(12):1119–28. doi: 10.1038/sj.bmt.1705381. [DOI] [PubMed] [Google Scholar]
- 10.Fedele R, Martino M, Garreffa C, et al. The impact of early CD4+ lymphocyte recovery on the outcome of patients who undergo allogeneic bone marrow or peripheral blood stem cell transplantation. Blood Transfus. 2012 Apr;10(2):174–80. doi: 10.2450/2012.0034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buhlmann L, Buser AS, Cantoni N, et al. Lymphocyte subset recovery and outcome after T-cell replete allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46(10):1357–62. doi: 10.1038/bmt.2010.306. [DOI] [PubMed] [Google Scholar]
- 12.Rubio MT, Moreira-Teixeira L, Bachy E, et al. Early aftertransplantation donor-derived invariant natural killer T-cell recovery predicts the occurrence of acute graft-versus-host disease and overall survival. Blood. 2012 Sep 6;120(10):2144–54. doi: 10.1182/blood-2012-01-404673. [DOI] [PubMed] [Google Scholar]
- 13.Storb R, Gyurkocza B, Storer BE, et al. Allogeneic Hematopoietic Cell Transplantation following Minimal Intensity Conditioning: Predicting Acute Graft-versus-Host Disease and Graft-versus-Tumor Effects. Biol Blood Marrow Transplant. 2013 May;19(5):792–8. doi: 10.1016/j.bbmt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120(4):905–13. doi: 10.1182/blood-2012-03-418202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray R. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;(16):1140–54. [Google Scholar]
- 16.Harrell F. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer-Verlag; New York: 2001. Springer Series in Statistics. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Landmark analysis for overall survival (A) and progress-free survival (B) by WBC riskgroup. All death and relapse prior to day 100 of HCT were excluded.


