Summary
Intragenic transcripts initiate within the coding region of a gene, thereby producing shorter mRNAs and proteins. Although intragenic transcripts are widely expressed [1], their role in the functional regulation of genes remains largely unknown. In budding yeast, DNA replication stress activates the S-phase checkpoint that stabilizes replication forks and arrests cells in S-phase with a short spindle [2-4]. When yeast cells were treated with hydroxyurea (HU) to block DNA synthesis and induce replication stress, we found that Ase1, a conserved spindle midzone protein [5], appeared as two short protein isoforms in addition to the full-length protein. We further demonstrated that the short isoforms result from intragenic transcription of ASE1, which depends on the S-phase checkpoint. Blocking generation of the short isoforms leads to a destabilized S-phase spindle, characterized by increased spindle dynamics and frequent spindle collapse. Because the short Ase1 isoforms localize at the spindle in HU-treated cells and overexpression of the short Ase1 isoforms impairs the spindle midzone localization of full-length Ase1, it is likely that the presence of short Ase1 isoforms stabilizes the spindle by antagonizing full-length Ase1. Together, our results reveal intragenic transcription as a unique mechanism to down-regulate gene functions in response to DNA replication stress.
Keywords: Intragenic transcription, S-phase checkpoint, Ase1, Rad53, Spindle
Results and Discussion
The development of new technologies such as next generation RNA sequencing has allowed the identification of a large number of intergenic, intragenic, antisense and non-coding RNAs [1, 6-9]. Previous studies have described intragenic transcript isoforms where transcription initiates within the open reading frame, leading to translation from downstream start codons to generate truncated proteins [10-13]. However, we know little about the regulation and functional relevance of intragenic transcription. Yeast cells treated with DNA synthesis inhibitor HU arrest in S-phase with a short spindle structure [2-4], which has been shown to facilitate kinetochore-microtubule interaction [14], but cells defective in the S-phase checkpoint show elongated spindles when treated with HU. Ase1 is a spindle midzone protein that stabilizes the anaphase spindle by bundling anti-parallel microtubules [5, 15, 16]. Interestingly, overexpression of Ase1 leads to premature spindle elongation in HU-treated cells [5]. However, it is unclear how Ase1 protein function is regulated in response to DNA replication stress.
DNA replication stress induces expression of Ase1 protein fragments
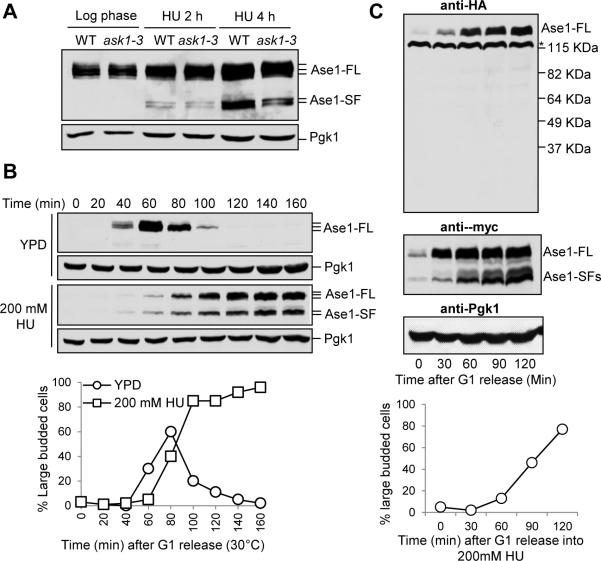
Because we found that ask1-3 kinetochore mutants show an elongated spindle after HU treatment [14], we examined if altered Ase1 protein expression is the cause. However, no obvious difference in Ase1 protein levels was detected between wild-type (WT) and ask1-3 cells with ASE1-13myc after HU treatment. Surprisingly, two short protein isoforms of Ase1 appeared in both WT and ask1-3 cells only after HU treatment (Figure 1A). The short Ase1 isoforms were also induced in synchronized cells during HU treatment, but appeared only as very faint bands during unperturbed S-phase (Figure 1B). Expression of the Ase1 short isoforms is likely specific to HU treatment because we were unable to detect them in cdc13-1 mutant cells incubated at 34°C or in cells treated with MMS (Figure S1A and B), conditions that activate the DNA damage checkpoint [17, 18]. Therefore, HU treatment induces the expression of Ase1 short protein isoforms.
Figure 1. HU-induced expression of Ase1 short protein fragments.
(A) The expression of Ase1 in HU-treated WT and ask1-3 mutant cells. ASE1-13myc and ask1-3 ASE1-13myc cells were grown to log phase at 30°C and released to YPD containing 200 mM HU. Cells were collected at the indicated time points and protein samples were prepared for western blotting. Full length Ase1 migrates around 140 kDa and the short isoforms migrate around 105 kDa. Pgk1 protein migrates at 45 kDa and levels are shown as a loading control. (B) Expression of Ase1 isoforms in synchronized cells treated with HU. G1-arrested ASE1-13myc cells were released into YPD or YPD containing 200 mM HU. α-factor was restored in untreated cells to block the second round of cell cycle. Cells were collected at the indicated time points to detect the expression of Ase1 protein using western blotting. Budding index was used to indicate cell cycle stage and Pgk1 protein levels are shown as a loading control. (C) Ase1 short isoforms are not protein cleavage products. G1-arrested ase1Δ cells containing 3HA-ASE1-13myc plasmid were released into fresh selective media containing 200 mM HU and protein samples were prepared at the indicated time points. Western blotting with anti-HA and anti-myc antibodies was used to determine the expression of full length (Ase1-FL) and short isoforms (Ase1-SF) of Ase1 protein. Budding index was used to indicate cell cycle stage and Pgk1 protein levels are shown as a loading control. Asterisk indicates a non-specific band. Sell also Figure S1.
We first hypothesized that full-length Ase1 protein was being cleaved to generate the short fragments after HU treatment, but we failed to detect any Ase1 N-terminal fragments in HU-treated cells (Figure 1C). Additionally, no N-terminal protein fragments were detected in pre9Δ mutants, which show reduced protein degradation [19] (Figure S1C). These results suggest that the Ase1 short protein isoforms are not cleavage products.
Intragenic transcription of ASE1 produces a shorter mRNA isoform
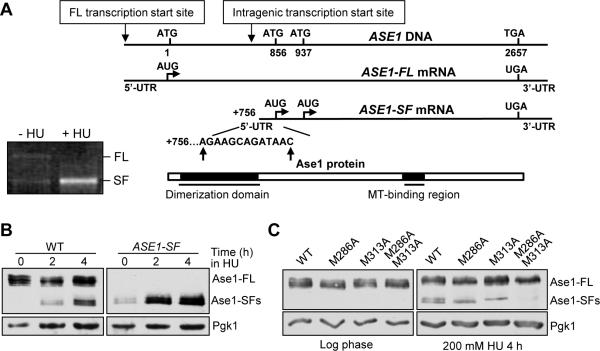
To determine if shorter ASE1 mRNAs are present during HU treatment, we performed 5’ Rapid Amplification of cDNA Ends (5’-RACE), which is used to identify the sequence of the 5’-end of specific mRNAs. HU-treated cells produced much more shorter ASE1 PCR product, while longer ASE1 PCR products were only present in untreated cells (Figure 2A). After sequencing, we found that the short mRNA initiates between nucleotide +756 and +766 within the ASE1 coding region (Figure 2A). To test the possibility that the full length ASE1 mRNA is being processed to generate the short RNA, we constructed a plasmid lacking the endogenous ASE1 promoter and the first 313 nucleotides of the coding region (ASE1-SF). ase1Δ cells with this plasmid also express short Ase1 isoforms after HU treatment (Figure 2B). Therefore, the expression of full-length ASE1 mRNA is not required for the production of short Ase1 protein isoforms, and ASE1 gene likely undergoes intragenic transcription during replicative stress. We noticed an increase in Ase1 short isoforms in ase1Δ cells containing ASE1-SF plasmid after HU treatment (Figure 2B), raising the possibility that the full-length ASE1 mRNA may suppress the expression of the short mRNA. However, constitutive expression of ASE1 did not inhibit the production of Ase1 short isoforms from the ASE1-SF plasmid, arguing against this possibility (Figure S2A-C).
Figure 2. The short Ase1 protein isoforms are a consequence of intragenic transcription.
(A) Transcription of ASE1 short mRNA isoform begins at bp +756 to +766. Gel image for ASE1 gene specific PCR products after 5-RACE. Schematic of results from 5-RACE after cloning and sequencing of PCR bands (10 colonies each). (B) Full-length ASE1 mRNA is not required for expression of Ase1-SFs. ase1Δ cells containing WT ASE1 or ASE1-SF plasmid were grown to log phase at 30°C and released into selective media containing 200 mM HU. Cells were collected at the indicated time points and protein samples were prepared for western blotting to determine the expression of Ase1-FL and Ase1-SFs. (C) Translation of Ase1-SFs starts at M286 and M313. ase1Δ cells containing ASE1-13myc, ase1M286A-13myc, ase1M313A-13myc or ase1M286A-M313A-13myc plasmid were grown to log phase in selective media and released into 200 mM HU for 4 hrs. Cells were collected and protein samples were prepared for western blotting with anti-myc antibody. Pgk1 protein levels are shown as a loading control. See also Figure S2.
Next, we identified the start codons responsible for the short Ase1 protein isoforms. Since sequence analysis indicates that methionine residues 286 and 313 are ideal candidates, one or both of these two AUG codons were mutated to GCC (Ala) to generate plasmids with myc-tagged ase1M286A, ase1M313A and ase1M286A/M313A (ase1-AA). Mutation of each start codon abolished the expression of one corresponding Ase1 short isoform after HU treatment, while no isoforms were detected after both start codons were mutated (Figure 2C). Thus, methionine residues 286 and 313 are the translational start sites for the short Ase1 protein isoforms.
The expression of Ase1 short isoforms is S-phase checkpoint dependent
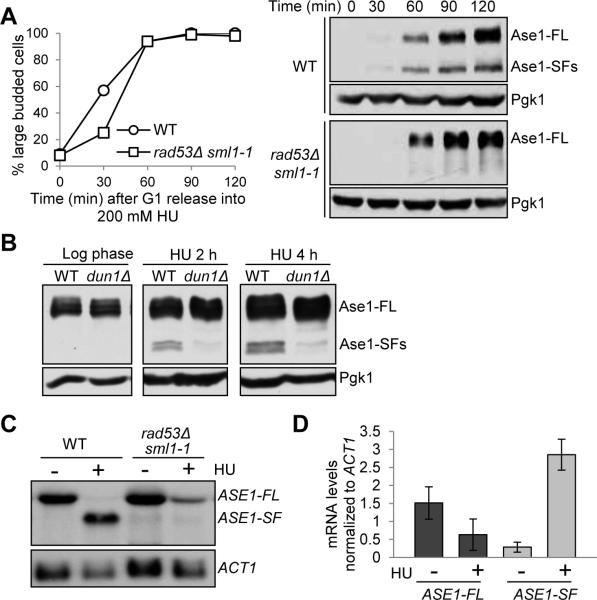
Since the Ase1 short isoforms are only induced after HU treatment that activates the S-phase checkpoint, we asked if this induction depends on Rad53, an essential checkpoint kinase. For this purpose, we examined Ase1 protein expression in rad53Δ sml1-1 checkpoint mutants, wherein the sml1-1 mutation suppresses the lethality of rad53Δ deletion by increasing dNTP production [20]. Interestingly, the Ase1 short isoforms did not appear after HU treatment in rad53Δ mutant cells but were clearly induced in WT cells (Figure 3A). The expression of Ase1 short isoforms also depends on Dun1, another checkpoint kinase downstream of Rad53 [21] (Figure 3B). Together, these results indicate that the induction of Ase1 short protein isoforms requires an intact S-phase checkpoint.
Figure 3. The expression of the Ase1 short isoforms depends on the S-phase checkpoint.
(A) Rad53 is required for Ase1 short protein isoform expression. G1-arrested ASE1-13myc and rad53Δ sml1-1 ASE1-13myc cells were released into YPD containing 200 mM HU. Cells were collected at the indicated time points and protein samples were prepared for western blotting. Budding index was used to indicate cell cycle stage. (B) The expression of Ase1 isoforms in dun1Δ mutants. ASE1-13myc and dun1Δ ASE1-13myc cells were grown to log phase at 30°C and released into YPD containing 200 mM HU. Cells were collected at the indicated time points and protein samples were prepared for western blotting to determine the expression of Ase1 protein. Pgk1 protein levels are shown as a loading control. (C) Intragenic transcription of ASE1 depends on Rad53. G1-arrested WT and rad53Δ sml1-1 cells were released to fresh YPD at 30°C. Twenty min later 200 mM HU was added to half of the cell culture. Cells were harvested 40 min later, washed with 1xPBS and flash frozen with liquid nitrogen. mRNA was prepared and examined by northern blotting with a probe corresponding to nucleotides 2109 to 2411 of the ASE1 gene. ACT1 probe was used for loading control. (D) Quantification of the ASE1 mRNAs in WT cells treated with or without HU from three experiments normalized to ACT1 mRNA using ImageJ software: ASE1-FL –HU (1.51 ± 0.45), ASE1-FL + HU (0.63 ± 0.43), ASE1-SF – HU (0.26 ± 0.14), ASE1-SF + HU (2.86 ± 0.43). See also Figure S3.
We further performed northern blotting to examine the isoforms of ASE1 mRNA using a probe specific to the 3’-region of the ASE1 gene. In untreated cells, only the full-length ASE1 mRNA transcript was detected. In contrast, a shorter ASE1 transcript isoform was detected in cells treated with HU along with an obvious decrease in full-length mRNA (Figure 3C and 3D), confirming the intragenic transcription of the ASE1 gene. In spite of the high levels of short ASE1 mRNA in HU-treated cells, the short protein isoforms are relatively less abundant compared to full-length Ase1 protein (Figure 3A). This inconsistency could be due to a difference in protein stability. Indeed when cells were treated with HU as well as the protein synthesis inhibitor cycloheximide, full-length Ase1 remained stable while the short isoform disappeared (Figure S3A), indicating that the short isoform is less stable than the full-length protein.
We also examined the levels of ASE1 mRNAs in rad53Δ sml1-1 cells after HU treatment using northern blotting. Compared with WT cells, rad53Δ mutants exhibited a reduced level of ASE1 intragenic transcript and increased full-length ASE1 mRNA (Figure 3C and S3B). Similar results were obtained with a rad53-21 mutant. Rad53 has been shown to regulate replication origin firing [22], and ARS1501 is located 229 bp upstream of the ASE1 start codon. In a strain wherein the origin is replaced with TRP1 (TRP1-ASE1), HU treatment also induced the expression of Ase1 short protein isoforms (Figure S3C), indicating that Rad53-dependent intragenic transcription of ASE1 is not a consequence of altered transcription at the replication origin after HU treatment. Together, these data support the conclusion that intragenic transcription of ASE1 is induced during DNA replication stress, which depends on the S-phase checkpoint.
Abolishment of the expression of Ase1 short isoforms causes HU sensitivity and altered spindle dynamics
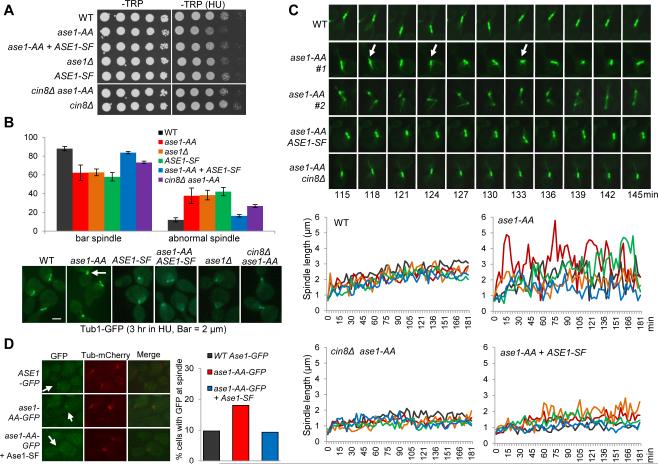
One important question is why cells produce Ase1 short isoforms in response to HU treatment. We first examined if ase1-AA mutants that cannot produce the short isoforms are sensitive to HU. Indeed, ase1-AA cells grew slowly on plates containing 100 mM HU compared to the control cells (Figure 4A). Because ase1Δ mutants also show slightly slower growth on HU plates, it is possible that the mutated Ase1-AA protein loses its function. The ase1Δ mutant shows synthetic growth defects with other mutants such as cin8Δ, kar3Δ and nip100Δ [23, 24]. Our observation that the introduction of ase1-AA plasmid suppressed the growth defects of ase1Δ nip100Δ double mutants as well as the normal spindle structure in untreated ase1-AA mutant cells indicates that Ase1-AA protein maintains most of its function (Figure S4A). Therefore, blocking the generation of short Ase1 protein isoforms in ase1-AA mutants causes some defective response to replicative stress.
Figure 4. Expression of the Ase1 short isoforms stabilizes the S-phase spindle.
(A) ase1-AA mutants are sensitive to HU. Saturated cell cultures with indicated genotypes were 10-fold serial diluted and spotted onto –TRP and –TRP + 100 mM HU plates and scanned after incubation at 30°C for 2 days. (B) ase1-AA cells show abnormal spindles after HU treatment. Cells with indicated genotypes were arrested in G1 and released to fresh selective media containing 200 mM HU for 3 hrs at 30°C. The spindle morphology (Tub1-GFP) in representative cells is shown in the bottom panel. The white arrow indicates a collapsed spindle. The percentage of cells with a normal bar-shaped spindle and an abnormal collapsed or dot-like spindle from three biological replicates is shown in the top panel (n > 100). Normal: WT 88% ± 2.3, ase1-AA 62.3% ± 8.2, ase1Δ 62.6% ± 3.6, ASE1-SF 57.9% ± 4.4, ase1-AA + ASE1-SF 83.7% ± 1.3, cin8Δ ase1-AA 73.2% ± 1.5. Abnormal: WT 12% ± 2.3, ase1-AA 37.7% ± 8.2, ase1Δ 38.4% ± 5.1, ASE1-SF 42.1% ± 4.4, ase1-AA + ASE1-SF 16.3% ± 1.3, cin8Δ ase1-A 26.8% ± 1.5. (C) Spindles are more dynamic and experience more collapse in ase1-AA mutants during HU treatment. Thirty min following G1 release, the cells with indicated genotypes were spotted onto the surface of a slide with an agarose medium pad containing 200 mM HU and subjected to live-cell microscopy. Five representative cells for each genotype were selected and spindle length was measured using Andor iQ2 software. Time zero was set to one image prior to the first separation of spindle poles and spindle length was plotted over time (bottom panel). Live-cell images of the spindle morphology in representative cells are shown in the top panel. Arrows indicate spindle collapse. See also Figure S4. (D) Ase1-SF reduces Ase1-FL spindle localization. G1-arrested cells with indicated genotypes were released to liquid media containing 200 mM HU for 1 hr, and then mounted to a slide as described in (C). The cells were subjected to live-cell microscopy for 30 min after G1 release for 2 hrs. The percentage of cells showing GFP localization to the spindle in several time points during the 30 min window is shown (n >100). See also Figure S4.
Because Ase1 is a microtubule-associated protein [5], the generation of the short isoforms may affect spindle morphology in HU-treated cells. Therefore, we examined the spindle structure in ase1Δ TUB1-GFP cells containing either ASE1 or ase1-AA plasmid after G1 release into HU for 3 hrs. Interestingly, we noticed a 3-fold increase in the number of cells with abnormal curved or dot-like spindles in ase1-AA mutants compared to control cells (the arrow in Figure 4B). We further compared spindle dynamics in those cells using live-cell microscopy. The spindle in cells with WT ASE1 stayed relatively stable during HU arrest (Figure 4C). In contrast, the spindle in most of the ase1-AA cells was much more dynamic with larger variation in length. In many time points, we observed spindle collapse as evidenced by the short distance between the two spindle ends (Figure 4C, arrows). This collapse is unlikely an artifact of spindle orientation since two closely localized spindle poles were observed in the same Z stack. Introduction of ASE1-SF into ase1-AA mutant cells suppressed the abnormal spindle morphology as well as the increased spindle dynamics (Figure 4B and C). Introduction of a second copy of ase1-AA showed similar spindle morphology as ase1-AA mutant cells (data not shown), indicating that the phenotype is attributed to the absence of Ase1 short isoforms and the rescue is not simply due to higher Ase1 protein levels. Additionally, ase1Δ and ASE1-SF cells showed less dynamic spindles, although they were shorter overall (Figure S4B). Therefore, when the expression of the short Ase1 isoforms is blocked, the presence of full length Ase1 alone results in spindle destabilization during HU treatment.
The balance of pushing and pulling forces between microtubule motors regulates spindle length [25]. Ase1 recruits kinesin-5 Cin8 onto the spindle where it slides antiparallel microtubules apart [26]. Interestingly, cin8Δ suppressed both the HU sensitivity and the abnormal spindle structure in ase1-AA cells treated with HU (Figure 4A and B). cin8Δ also suppressed the increased spindle dynamics in ase1-AA (Figure 4C). These results suggest that the highly dynamic spindle morphology in ase1-AA mutant cells depends on the microtubule motor Cin8, at least partially. Consistently, increased Cin8 levels in HU treated cells can induce spindle elongation [27]. Thus, it is likely that Ase1-dependent Cin8 recruitment in HU-treated ase1-AA cells promotes the sliding of antiparallel microtubules, resulting in destabilized spindles.
The Ase1 short protein isoforms contain the microtubule-binding domain but lack the dimerization domain (Figure 2A). These isoforms likely bind microtubules but are unable to dimerize and crosslink antiparallel microtubules, thereby playing a dominant negative role by antagonizing full-length Ase1 protein. To test this idea, we first examined the growth of cin8Δ, kar3Δ and nip100Δ mutants overexpressing the Ase1 short isoform from a galactose inducible promoter, PGAL-ASE1-SF (Figure S4C). Previous studies show that these mutants exhibit synthetic growth defects with ase1Δ [23, 24]. Interestingly, overexpression of ASE1-SF also caused sick growth in these mutants, supporting the dominant negative role of the short isoform (Figure S4D). We also examined the localization of endogenous full-length Ase1 in cells overexpressing ASE1-SF. Similar to previous studies [5, 28], we observed the restricted localization of Ase1 at the spindle midzone in control cells (Figure S4E). In 23 out of 25 cells overexpressing ASE1-SF, however, the localization of full-length Ase1 was dispersed and towards one end of the spindle as it elongated. Moreover, a defect in spindle elongation and spindle breakage was observed frequently in these cells (Figure S4E), similar to ase1Δ mutants [5]. These data support the dominant negative role of the short Ase1 protein isoforms. Importantly, Ase1-AA-GFP shows increased localization to the spindle during HU treatment compared to WT Ase1-GFP and this increase was suppressed by introduction of the ASE1-SF plasmid, indicating that expression of Ase1 short isoforms reduces the spindle association of full length Ase1 (Figure 4D). Therefore, we speculate that the expression of short isoforms of Ase1 stabilizes the spindle during HU treatment by antagonizing full-length Ase1. We noticed that only a small portion of cells showed spindle localization of Ase1-GFP, which may be explained by dynamic Ase1-microtubule interaction. In ase1-AA cells, the association of Ase1 to the anti-parallel microtubules might be stable, but the sliding likely causes spindle collapse and the dissociation of Ase1 from the spindle.
Conclusions
Recent genome-wide isoform profiling in budding yeast identified transcription boundaries for millions of RNA molecules, revealing the presence of multiple transcript isoforms for most genes in the genome [1]. Here we provide the first evidence for S-phase checkpoint-dependent intragenic transcription in response to DNA replication stress. We found that the ASE1 gene is subjected to intragenic transcription upon HU treatment, generating two short Ase1 protein isoforms. Moreover, intragenic transcription of ASE1 depends on the S-phase checkpoint kinase Rad53. Importantly, our results suggest that the expression of the short Ase1 isoforms stabilizes the short spindle structure in HU-arrested cells likely by antagonizing full-length Ase1. Together, our data uncover a novel checkpoint-dependent mechanism to maintain a stable spindle structure during replication stress by inducing intragenic transcription of the ASE1 gene. Additional genes may also undergo intragenic transcription during the cell cycle and differentiation or when cells encounter environmental challenges. Therefore, intragenic transcription is likely a unique mechanism to regulate gene function.
Supplementary Material
Highlights.
Replicative stress induces intragenic transcription of the ASE1 gene.
The intragenic transcription of ASE1 depends on the S-phase checkpoint.
The short Ase1 protein isoforms promote spindle stability during replicative stress.
Acknowledgements
There is no any financial interest that might be construed to influence the results of interpretation of this manuscript. We thank Drs. Gunjan and Kato for yeast strains and reagents. We thank Ruth Didier and the FSU Biology Core Facility for technical support. We thank Drs. Kaplan, Yu, Megraw and Gunjan for reading through the manuscript. This work was supported by R15GM097326 and RO1GM102115 from NIH/NIGMS to Y.W.
Abbreviations
- YPD
Yeast Peptone Dextrose
- HU
Hydroxyurea
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pelechano V, Wei W, Steinmetz LM. Extensive transcriptional heterogeneity revealed by isoform profiling. Nature. 2013;497:127–131. doi: 10.1038/nature12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachant J, Jessen SR, Kavanaugh SE, Fielding CS. The yeast S phase checkpoint enables replicating chromosomes to bi-orient and restrain spindle extension during S phase distress. J Cell Biol. 2005;168:999–1012. doi: 10.1083/jcb.200412076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcasabas AA, Osborn AJ, Bachant J, Hu F, Werler PJ, Bousset K, Furuya K, Diffley JF, Carr AM, Elledge SJ. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat Cell Biol. 2001;3:958–965. doi: 10.1038/ncb1101-958. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Bachant J, Alcasabas AA, Wang Y, Qin J, Elledge SJ. The mitotic spindle is required for loading of the DASH complex onto the kinetochore. Genes Dev. 2002;16:183–197. doi: 10.1101/gad.959402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuyler SC, Liu JY, Pellman D. The molecular function of Ase1p: evidence for a MAP-dependent midzone-specific spindle matrix. Microtubule-associated proteins. J Cell Biol. 2003;160:517–528. doi: 10.1083/jcb.200210021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berretta J, Morillon A. Pervasive transcription constitutes a new level of eukaryotic genome regulation. EMBO Rep. 2009;10:973–982. doi: 10.1038/embor.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z, Wei W, Gagneur J, Perocchi F, Clauder-Munster S, Camblong J, Guffanti E, Stutz F, Huber W, Steinmetz LM. Bidirectional promoters generate pervasive transcription in yeast. Nature. 2009;457:1033–1037. doi: 10.1038/nature07728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan X, Ruan Y. Genome wide full-length transcript analysis using 5′ and 3′ paired-end-tag next generation sequencing (RNA-PET). Methods Mol Biol. 2012;809:535–562. doi: 10.1007/978-1-61779-376-9_35. [DOI] [PubMed] [Google Scholar]
- 9.Waern K, Snyder M. Extensive transcript diversity and novel upstream open reading frame regulation in yeast. G3 (Bethesda) 2013;3:343–352. doi: 10.1534/g3.112.003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu M, Tzagoloff A. Mitochondrial and cytoplasmic fumarases in Saccharomyces cerevisiae are encoded by a single nuclear gene FUM1. J Biol Chem. 1987;262:12275–12282. [PubMed] [Google Scholar]
- 11.Chiu MI, Mason TL, Fink GR. HTS1 encodes both the cytoplasmic and mitochondrial histidyl-tRNA synthetase of Saccharomyces cerevisiae: mutations alter the specificity of compartmentation. Genetics. 1992;132:987–1001. doi: 10.1093/genetics/132.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benanti JA, Matyskiela ME, Morgan DO, Toczyski DP. Functionally distinct isoforms of Cik1 are differentially regulated by APC/C-mediated proteolysis. Mol Cell. 2009;33:581–590. doi: 10.1016/j.molcel.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson M, Botstein D. Two differentially regulated mRNAs with different 5′ ends encode secreted with intracellular forms of yeast invertase. Cell. 1982;28:145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Liang F, Jin F, Wang Y. The coordination of centromere replication, spindle formation, and kinetochore-microtubule interaction in budding yeast. PLoS Genet. 2008;4:e1000262. doi: 10.1371/journal.pgen.1000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellman D, Bagget M, Tu YH, Fink GR, Tu H. Two microtubule-associated proteins required for anaphase spindle movement in Saccharomyces cerevisiae. J Cell Biol. 1995;130:1373–1385. doi: 10.1083/jcb.130.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotwaliwale CV, Frei SB, Stern BM, Biggins S. A pathway containing the Ipl1/aurora protein kinase and the spindle midzone protein Ase1 regulates yeast spindle assembly. Dev Cell. 2007;13:433–445. doi: 10.1016/j.devcel.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garvik B, Carson M, Hartwell L. Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol Cell Biol. 1995;15:6128–6138. doi: 10.1128/mcb.15.11.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tercero JA, Diffley JF. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature. 2001;412:553–557. doi: 10.1038/35087607. [DOI] [PubMed] [Google Scholar]
- 19.Velichutina I, Connerly PL, Arendt CS, Li X, Hochstrasser M. Plasticity in eucaryotic 20S proteasome ring assembly revealed by a subunit deletion in yeast. EMBO J. 2004;23:500–510. doi: 10.1038/sj.emboj.7600059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao X, Muller EG, Rothstein R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell. 1998;2:329–340. doi: 10.1016/s1097-2765(00)80277-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Z, Elledge SJ. DUN1 encodes a protein kinase that controls the DNA damage response in yeast. Cell. 1993;75:1119–1127. doi: 10.1016/0092-8674(93)90321-g. [DOI] [PubMed] [Google Scholar]
- 22.Santocanale C, Diffley JF. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature. 1998;395:615–618. doi: 10.1038/27001. [DOI] [PubMed] [Google Scholar]
- 23.Tong AH, Lesage G, Bader GD, Ding H, Xu H, Xin X, Young J, Berriz GF, Brost RL, Chang M, et al. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–813. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- 24.Pan X, Ye P, Yuan DS, Wang X, Bader JS, Boeke JD. A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell. 2006;124:1069–1081. doi: 10.1016/j.cell.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 25.Saunders W, Lengyel V, Hoyt MA. Mitotic spindle function in Saccharomyces cerevisiae requires a balance between different types of kinesin-related motors. Mol Biol Cell. 1997;8:1025–1033. doi: 10.1091/mbc.8.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khmelinskii A, Roostalu J, Roque H, Antony C, Schiebel E. Phosphorylation-dependent protein interactions at the spindle midzone mediate cell cycle regulation of spindle elongation. Dev Cell. 2009;17:244–256. doi: 10.1016/j.devcel.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan V, Nirantar S, Crasta K, Cheng AY, Surana U. DNA replication checkpoint prevents precocious chromosome segregation by regulating spindle behavior. Mol Cell. 2004;16:687–700. doi: 10.1016/j.molcel.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Khmelinskii A, Schiebel E. Assembling the spindle midzone in the right place at the right time. Cell Cycle. 2008;7:283–286. doi: 10.4161/cc.7.3.5349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.