Abstract
Purpose
Multiple interventions have been tested in acute respiratory distress syndrome (ARDS). We examined the entire agenda of published randomized controlled trials (RCTs) in ARDS that reported on mortality and of respective meta-analyses.
Methods
We searched PubMed, the Cochrane Library and Web of Knowledge until July 2013. We included RCTs in ARDS published in English. We excluded trials of newborns and children; and those on short-term interventions, ARDS prevention or post-traumatic lung injury. We also reviewed all meta-analyses of RCTs in this field that addressed mortality. Treatment modalities were grouped in five categories: mechanical ventilation strategies and respiratory care, enteral or parenteral therapies, inhaled / intratracheal medications, nutritional support and hemodynamic monitoring.
Results
We identified 159 published RCTs of which 93 had overall mortality reported (n= 20,671 patients) - 44 trials (14,426 patients) reported mortality as a primary outcome. A statistically significant survival benefit was observed in 8 trials (7 interventions) and two trials reported an adverse effect on survival. Among RTCs with >50 deaths in at least 1 treatment arm (n=21), 2 showed a statistically significant mortality benefit of the intervention (lower tidal volumes and prone positioning), 1 showed a statistically significant mortality benefit only in adjusted analyses (cisatracurium) and 1 (high-frequency oscillatory ventilation) showed a significant detrimental effect. Across 29 meta-analyses, the most consistent evidence was seen for low tidal volumes and prone positioning in severe ARDS.
Conclusions
There is limited supportive evidence that specific interventions can decrease mortality in ARDS. While low tidal volumes and prone positioning in severe ARDS seem effective, most sporadic findings of interventions suggesting reduced mortality are not corroborated consistently in large-scale evidence including meta-analyses.
Keywords: Acute respiratory distress syndrome, treatment, survival, mortality
Introduction
The acute respiratory distress syndrome (ARDS) [1] carries high mortality (typically between 27 - 45%) [2, 3]. Patients typically die from the underlying cause of ARDS, sepsis and/or multiorgan failure [4-6]. Currently there are no specific therapies for ARDS that are widely and unequivocally recommended, except for mechanical ventilation (MV) with low tidal volumes [7]. However, there are numerous trials on ARDS and some of them have occasionally reported significant benefits. By examining single trials in isolation it is difficult to judge which results reflect genuine benefits of the tested interventions and which might be due to diverse biases [8]. Furthermore, several trials in which the intervention showed a potential beneficial effect were stopped early, which can inflate estimates of treatment effects [9]. To understand which treatments can reduce mortality in ARDS, one should examine the entire agenda of published trials for this condition, instead of focusing on one intervention at a time [10].
Here, we aimed to review all the agenda of published RCTs on ARDS using an umbrella review of the evidence. In an umbrella review, the data from clinical trials on diverse interventions for a particular disease are juxtaposed, facilitating a bird’s eye view analysis of the strengths, weaknesses and biases of this literature [10, 11]. Here, we analyzed the results of RCTs of treatments for ARDS that reported on mortality outcomes. We also systematically overviewed the results of all the respective meta-analyses in this field reporting mortality outcomes. We aimed to map whether any interventions have robust evidence that they can curtail mortality for this syndrome.
Methods
Eligibility criteria for randomized controlled trials
We considered all published RCTs involving therapies for the treatment of ARDS. Trials have been performed over many decades and definitions of ARDS have evolved over time. We tried to be all-encompassing therefore we considered all definitions of ARDS [1, 12]. RCTs in patients with ARDS published in English were retained if they compared an intervention against placebo or another intervention, regardless of whether there were also common “backbone” interventions (treatments that were provided to all study patients, irrespectively of the treatment arm). We excluded trials performed in newborns and children since causes and management options for ARDS are generally different than those in adults. In addition, we excluded trials that analyzed a subset of patients from a larger study, tested short-term interventions lasting minutes (e.g. different modes of suctioning, single recruitment maneuver), focused on ARDS prevention, or evaluated subjects with post-traumatic or inhalation injury. We also included all meta-analyses of RCTs in ARDS that had mortality as an outcome.
Search strategy
We searched PubMed, Cochrane library and Web of Knowledge with last update on the 7/25/2013. We retrieved articles published in English-language without limits on publication year and perused reference lists of related papers, meta-analysis and review articles for additional pertinent citations. We used the following search algorithm for PubMed search of RCTs: (((((adult respiratory distress syndrome) OR (hypoxemic respiratory failure) OR (acute lung injury)) AND (“random*” OR “controlled trial” OR “randomized controlled trial” OR “placebo” OR “double-blind”)) AND Humans [Mesh] AND English [lang])) NOT infant [MeSH Terms]. Furthermore, we systematically searched PubMed for relevant meta-analyses that included mortality as one of the outcomes. When more than one meta-analysis had tested the same (or overlapping) interventions, we kept all of them, so as to juxtapose their results and see whether they are consistent. However, we did not include the older version of 2 meta-analyses that were published by the very same authors on the same intervention with 2-3 years difference between the old and newer versions.
We employed a similar strategy to search the Web of Knowledge for RCTs excluding Medline (Topic=(((adult respiratory distress syndrome) OR (hypoxemic respiratory failure) OR (acute NEAR/3 lung NEAR/3 injury)) AND (“random*” OR “controlled trial” OR “randomized controlled trial” OR “placebo” OR “double-blind”)) NOT Topic=(infant*) NOT Topic=((rat OR mouse OR mice OR dog OR animal)) Refined by: [excluding] Databases=( MEDLINE ) AND Languages=( ENGLISH ) Timespan=All years.). Furthermore we queried the Cochrane Central Registry of Controlled Trials with ((adult respiratory distress syndrome) OR (hypoxemic respiratory failure) OR (acute lung injury)), Limit to “Trials” and “meta-analysis”.
Data extraction
Two investigators (J.Z. and A.R.T) screened abstracts and articles and identified those that meet inclusion/exclusion criteria. When we identified overlapping reports on the same trial, we analyzed data from the most complete report. We reviewed the full text of all articles selected by the reviewers. Two investigators independently extracted data. Differences were resolved by consensus (all authors). For RCTs, we extracted data regarding the first author’s name, publication year, intervention administered, number of participants per treatment arm, and primary outcome. We recorded mortality data, calculated from 2×2 tables of deaths per arm the respective odds ratios and risk ratios and recorded any reported hazard ratios (adjusted and unadjusted) for time-to-event analyses of mortality. We flagged statistically significant differences in mortality (defined as p<0.05 or 95% CI of a relative risk metric entirely on one side of 1.00).
For meta-analyses we collected the total number of participants and deaths in each arm, follow-up time, risk ratio and odds ratio with 95% CI, model used for analysis (fixed or random) and heterogeneity index I2.
Overall design of the umbrella review
Interventions were grouped in five categories: MV strategies and respiratory care, enteral or parenteral therapies, inhaled / intratracheal medications, nutritional support and hemodynamic monitoring. Specific interventions tested are presented in e-table 1.
Although death is a major ARDS outcome, not all trials reported on mortality. Perhaps some trials did not consider mortality, e.g. if the trials were very grossly underpowered for mortality assessment; or selectively did not report death outcomes. Therefore, we recorded in how many trial reports information on mortality was mentioned at all. We then particularly focused on RCTs that claimed to have overall mortality as a primary outcome or also provided mortality data in the text. We investigated whether any claims were made by the authors that any apparent survival benefits of a particular intervention pertained to the entire or a subset of the study population. Whenever a survival benefit was claimed for a particular subgroup, we determined whether these analyses were defined a priori or post hoc.
In addition, we examined all the available published meta-analyses in ARDS patients on the respective interventions, and compared their results with those of selected trials and against each other, whenever two or more meta-analyses had evaluated the same intervention.
Analyses
We calculated odds and risk ratios with their respective 95% CIs using MedCalc version 12.7 (Ostend, Belgium). When reported, we also presented hazard ratios and 95% CIs, presenting whatever adjustments may have been used by the primary authors.
Mortality outcomes may be assessed at different times of follow-up in the same trial. Whenever treatment effects were provided at different times of follow-up for the same trial, we selected the one that was considered to represent the primary analysis according to the authors; if this was unclear, we selected the longest follow-up data. However, we also recorded in a separate table mortality treatment effect estimates (calculated odds ratios and risk ratios) and their 95% CIs for all time-points provided in the manuscript, so as to assess whether these differed for the same trial (e.g. statistically significant only for some, but not all time points). Methodological aspects of the quality of the trials are shown in the supplemental file. Additional aspects of quality, such as the quality of care and the extent of standardization of the control and active interventions, may be important, but are typically judge to arbitrate based on published information.
Results
Eligible ARDS trials
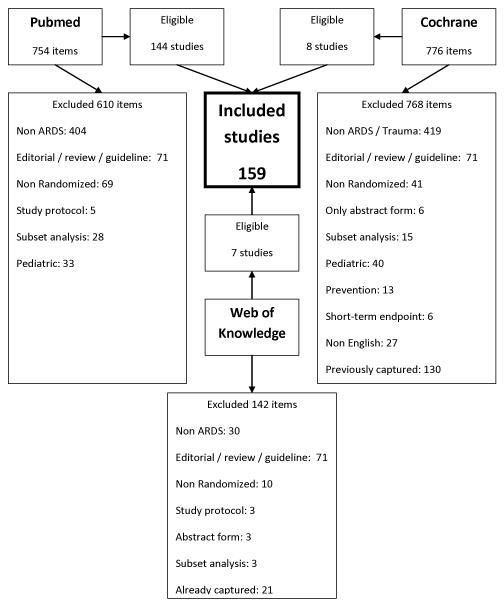
We identified 159 published RCTs that tested a variety of interventions in patients with ARDS (figure 1, e-table 1 and 2). We grouped the interventions tested in 5 groups as shown in table 1 and e-table 1 and 2. Of all selected trials, 93 had overall mortality reported and included 20,671 randomized patients (table 2) [S1-S93]. The other 66 trials with a total of 1,398 randomized patients did not report mortality data and the median (IQR: interquartile range) of patients per study was 18 (12-26) with a range of 5 to 72 subjects. Of the studies without mortality data, the follow-up ranged from 2 hours up to 7 days and 35 (53 %) had crossover design. The outcomes in these studies were changes in the oxygenation, hemodynamics, respiratory mechanics or inflammatory markers (e-tables 3-7).
Figure 1.
Flow chart of published randomized trials in ARDS
Table 1.
Randomized trials in ARDS with death as a reported study outcome.
| Ref. | Randomized comparison |
Number of patients randomi zed (interven tion / control) |
Number of deaths (interven tion / control) |
Terminat ed early |
Mortalit y at |
Calculated OR (95% CI) |
Calculated RR (95% CI) |
Adjusted HR (95% CI) |
Surviv al benefit # |
|---|---|---|---|---|---|---|---|---|---|
| MV strategies and respiratory care | |||||||||
| S1 | Prone vs. supine positioning |
237/229 | 38/75 | No | 28 d* | 0.39 (0.25- 0.61) |
0.49 (0.34- 0.69) |
By SOFA: 0.42 (0.26-0.66) |
+ |
| S2 | Lower tidal volume with extracorporeal CO2 removal vs. protective MV |
40/39 | 7/6 | No | Hospital | 1.17 (0.35- 3.84) |
1.14 (0.42- 3.08) |
NA | |
| S3 | HFOV vs. control ventilation |
275/273 | 129/96 | Yes (futility) |
Hospital * |
1.63 (1.16- 2.3) |
1.33 (1.09- 1.64) |
NA | − |
| S4 | HFOV vs. usual ventilatory care |
398/397 | 166/163 | No | 30 d* | 1.03 (0.77- 1.36) |
1.02 (0.86- 1.20) |
By several variables: 1.03 (0.75-1.40) |
|
| S5 | NIPPV vs. control (high concentration O2 therapy) |
21/19 | 1/5 | Yes (slow recruitm ent) |
Hospital § |
0.14 (0.01- 1.33) |
0.18 (0.02- 1.41) |
NA | |
| S6 | Recruitment maneuver | 55/55 | 16/24 | No | ICU to28 d* |
0.53 (0.24- 1.17) |
0.67 (0.4- 1.11) |
NA | |
| S7 | Airway pressure release ventilation vs. low tidal volume ventilation. |
31/32 | 2/2 | No | 5 d& | 1.03 (0.14- 7.84) |
1.03 (0.14- 6.80) |
NA | . |
| S8 | Referral for ECMO vs. conventional MV |
90/90 | 33/45 | Yes | 6 m | 0.58 (0.32- 1.05) |
0.73 (0.52- 1.03) |
NA | |
| S9 | Prone vs. supine positioning |
168/174 | 52/57 | No | 28 d* | 0.92 (0.58- 1.45) |
0.95 (0.69- 1.29) |
NA | |
| S10 | Decremental PEEP titration following Alveolar recruitment maneuver or a table- based PEEP |
30/27 | 14/15 | No | 60 d§ | 0.70 (0.25- 1.99) |
0.84 (0.5 – 1.40) |
NA | |
| S11 | PEEP guided by esophageal pressure vs. ARDS network recommendations |
30/31 | 8/14 | Yes (effect at interim analysis) |
6 m§ | 0.44 (0.15- 1.29) |
0.59 (0.29- 1.20) |
By APACHE II score was 0.52 (0.22-1.25) |
|
| S12 | Prone vs. supine positioning |
21/19 | 8/10 | Yes (low enrollme nt) |
60 d* | 0.55 (0.16- 1.95) |
0.72 (0.36- 1.45) |
NA | |
| S13 | High vs. moderate PEEP |
385/382 | 107/119 | No | 28 d* | 0.85 (0.62- 1.16) |
0.89 (0.72- 1.11)) |
NA | |
| S14 | Open-lung ventilation vs. low-tidal-volume ventilation |
475/508 | 173/205 | No | Hospital at 28 d* |
0.85 (0.65- 1.1) |
0.90 (0.77- 1.06) |
By several variables: 0.97 (0.84-1.12) |
|
| S15 | High PEEP and low tidal volume vs. low PEEP and higher tidal volume |
50/45 | 16/24 | Yes (mortalit y benefit) |
ICU* | 0.41 (0.18- 0.95) |
0.60 (0.37- 0.98) |
NA | + |
| S16 | Prone vs. supine positioning |
76/60 | 33/35 | Yes (decreas e in enrollme nt) |
ICU* | 0.55 (0.28- 1.09) |
0.74 (0.53- 1.04) |
By several variables: 0.40 (0.17-0.61) |
+ |
| S17 | PLV vs. conventional ventilation |
107/204 | 16/46 | No | 28 d§ | 0.60 (0.32- 1.13) |
0.66 (0.39- 1.11) |
NA | |
| S18 | HFOV vs. conventional ventilation |
24/37 | 8/16 | Yes (slow recruitm ent) |
30 d* | 1.52 (0.52- 4.44) |
1.3 (0.66- 2.55) |
By several variables: 1.15 (0.43-3.1) |
|
| S19 | Prone vs. supine positioning positioning |
413/378 | 134/119 | No | 28 d* | 1.05 (0.78- 1.41) |
1.03 (0.84- 1.26) |
NA | |
| S20 | Higher vs. lower PEEP | 276/273 | 76/68 | Yes (futility) |
Hospital at 60 d* |
1.15 (0.78- 1.68) |
1.11 (0.83- 1.46) |
By several variables: 0.88 (0.6-1.29) |
|
| S21 | APRV or SIMV | 30/28 | 5/5 | Yes (futility) |
28 d& | 0.92 (0.24- 3.59) |
0.93 (0.30- 2.88) |
NA | |
| S22 | HFOV vs. conventional ventilation |
75/73 | 28/38 | No | 30 d* | 0.55 (0.28- 1.08) |
0.72 (0.50- 1.03) |
NA | |
| S23 | PLV vs. conventional MV |
65/25 | 27/9 | No | 28 d§ | 1.26 (0.49- 3.28) |
1.15 (0.64- 2.1) |
NA | |
| S24 | Prone vs. supine positioning |
152/152 | 95/89 | Yes (slow recruitm ent) |
6 m* | 1.18 (0.74- 1.87) |
1.07 (0.89- 1.28) |
NA | |
| S25 | APRV with spontaneous breathing vs. controlled MV |
15/15 | 3/4 | No | NA& | 0.69 (0.12- 3.79) |
0.75 (0.20- 2.79) |
NA | |
| S26 | Prone positioning vs. continuous rotation |
12/14 | 7/9 | No | NA& | 0.78 (0.16- 3.8) |
0.9 (0.49- 1.68) |
NA | |
| S27 | PCV vs. VCV | 37 / 42 | 19 / 33 | No | Hospital * |
0.29 (0.11- 0.77) |
0.65 (0.46- 0.93) |
NA | + |
| S28 | Lower vs. traditional tidal volumes |
432/429 | 134/171 | Yes (lower mortality ) |
Hospital up to 6 m* |
0.68 (0.51- 0.90) |
0.78 (0.65- 0.93) |
NA | + |
| S29 | Computerized decision support for MV vs. not |
100/100 | 36/32 | No | Hospital * |
1.2 (0.67- 2.15) |
1.13 (0.76- 1.66) |
NA | |
| S30 | Reduced vs. traditional tidal volume MV. |
26/26 | 13/12 | Yes (futility) |
Hospital & |
1.17 (0.39- 3.47) |
1.08 (0.62- 1.91) |
NA | |
| S31 | Lung protective MV vs. control |
18/19 | 7/11 | No | 28 d& | 0.46 (0.12- 1.72) |
0.67 (0.34- 1.35) |
NA | |
| S32 | Reduced vs. traditional tidal volume (≥ 10 mL/kg) |
58/58 | 27/22 | Yes (futility) |
60 d* | 1.43 (0.68- 2.99) |
1.23 (0.80- 1.89) |
NA | |
| S33 | Pressure- and volume- limited MV or conventional MV |
60/60 | 30/28 | No | Hospital * |
1.14 (0.56- 2.34) |
1.07 (0.74- 1.55) |
NA | |
| S34 | Protective MV vs. conventional MV |
29/24 | 11/17 | Yes (surviva l benefit) |
28 d* | 0.25 (0.08-0.80) |
0.54 (0.31- 0.91) |
APACHE II score: 0.19 (0.08–0.47) |
+ |
| S35 | MV with “open lung approach” with low distending pressures vs. conventional approach |
15/13 | 5/7 | No | Hospital § |
0.43 (0.09- 1.98) |
0.62 (0.26- 1.48) |
NA | |
| S36 | PCV vs. VCV | 16/11 | 9/7 | No | 25 d& | 0.73 (0.15- 3.55) |
0.88 (0.47- 1.65) |
NA | |
| S37 | Extracorporeal CO2 removal vs. continuous positive pressure MV |
21 / 19 | 14 / 11 | Yes | 30 d* | 1.45 (0.40- 5.26) |
1.15 (0.71- 1.88) |
NA | |
| S38 | HFOV vs. conventional MV |
52/48 | 10/10 | No | Hospital & |
0.9 (0.34- 2.41) |
0.92 (0.42- 2.02) |
NA | |
| S39 | ECMO vs. conventional MV |
42/48 | 38/44 | Yes | 68 d* | 0.86 (0.20- 3.69) |
0.99 (0.97- 1.12) |
NA | |
| Enteral or parenteral therapies | |||||||||
| S40 | IV infusion of GMCSF vs. pl |
64/66 | 11/15 | No | 28 d§ | 0.71 (0.30- 1.68) |
0.76 (0.38- 1.52) |
NA | |
| S41 | Simvastatin PO or placebo |
30/30 | 11/11 | No | 14 d& | 1 (0.35- 2.86) |
1 (0.51- 1.94) |
NA | |
| S42 | Cisatracurium besylate IV vs. pl |
178/162 | 56/66 | No | 90 d* | 0.67 (0.43- 1.04) |
0.77 (0.58- 1.03) |
By several variables: 0.68 (0.48-0.98) |
+ |
| S43 | Ginger extract vs. pl | 16/16 | 3/2 | No | MICU stay to 21 d§ |
1.62 (0.23- 11.26) |
1.5 (0.29- 7.81) |
NA | |
| S44 | Inactivated recombinant factor VIIa IV vs. pl |
144/70 | 36/15 | Yes (higher mortality) |
28 d§ | 1.22 (0.61- 2.42) |
1.17 (0.69- 1.98) |
NA | |
| S45 | Activated protein C IV infusion or placebo |
37/38 | 5/5 | No | 60 d§ | 1.03 (0.27- 3.91) |
1.03 (0.32- 3.26) |
NA | |
| S46 | Oxothiazolidine IV vs. pl |
101/114 | 30/18 | Yes (higher mortality) |
30 d§ | 2.25 (1.16- 4.36) |
1.88 (1.12- 3.16) |
NA | - |
| S47 | Methylprednisone IV vs. pl |
63/28 | 15/12 | No | Hospital § |
0.42 (0.16- 1.07) |
0.56 (0.30- 1.03) |
NA | |
| S48 | Conservative vs. liberal strategy of fluid management |
503/497 | 128/14 1 |
No | Hospital to 60 d* |
0.86 (0.65- 1.14) |
0.9 (0.73- 1.1) |
NA | |
| S49 | Methylprednisolone IV vs. pl |
89/91 | 26/26 | No | Hospital at 60-d* |
1.03 (0.54- 1.97) |
1..02 (0.65- 1.62) |
NA | |
| S50 | Salbutamol IV vs. pl | 19/21 | 11/14 | No | 7 d§ | 0.69 (0.19- 2.49) |
0.87 (0.53- 1.42) |
NA | |
| S51 | Furosemide IV with or without albumin |
20/20 | 7/9 | No | 30 d§ | 0.66 (0.18- 2.35) |
0.78 (0.36- 1.68) |
NA | |
| S52 | Sivelest at sodium IV infusion vs. pl |
12/12 | 3/3 | No | 30 d§ | 1 (0.16- 6.35) |
1 (0.25- 4.00) |
NA | |
| S53 | Sivelestat sodium IV infusion vs. pl |
241/246 | 64/64 | Yes (trend to worsen mortality) |
28 d* | 1.03 (0.69- 1.54) |
1.05 (0.78- 1.41) |
NA | |
| S54 | Cisatracurium IV vs. pl | 28/28 | 10/17 | No | 28 d§ | 0.36 (0.12- 1.06) |
0.59 (0.33- 1.05) |
NA | |
| S55 | Lisofylline IV vs. pl | 116/119 | 37/29 | Yes (futility) |
28 d* | 1.45 (0.82- 2.58) |
1.31 (0.87- 1.98) |
NA | |
| S56 | Liposomal PGE1 IV infusion vs. pl |
70/32 | 21/9 | Yes (futility) |
28 d* | 1.1 (0.43- 2.76) |
1.07 (0.55- 2.06) |
NA | |
| S57 | Ketoconazole (enteral) vs. pl |
117/117 | 41/40 | Yes (futility) |
Hospital at 6 m* |
1.04 (0.61- 1.78) |
1.03 (0.72- 1.46) |
NA | |
| S58 | IV infusion of NAC vs. NAC with rutin vs. pl |
12/12/12 | 5/4/7 | No | 30 d& | 0.36 (0.07- 1.88)& |
0.57 (0.23- 1.45)& |
NA | |
| S59 | Liposomal PGE1 IV infusion vs. pl |
177/171 | 57/50 | No | 28 d§ | 1.14 (0.73- 1.81) |
1.10 (0.8- 1.51) |
NA | |
| S60 | Atrial Natriuretic peptide IV infusion vs. pl |
20/20 | 3/6 | No | NA& | 0.41 (0.09- 1.95) |
0.50 (0.14- 1.73) |
NA | |
| S61 | Prolonged Methylprednisolone (IV/PO) vs. pl |
16/8 | 0/5 | Yes (lower mortality) |
ICU at 32 d* |
0.02 (0.00- 0.44) |
0.05 (0.00- 0.78) |
NA | + |
| S62 | IV infusion of NAC vs. pl |
22/20 | 7/5 | No | ICU* | 1.40 (0.36- 5.41) |
1.27 (0.48- 3.37) |
NA | |
| S63 | IV infusion of NAC vs. procysteine vs. pl |
14/17/15 | 5/6/6 | No | 30 d§ | 0.83 (0.19- 3.75)‡ |
0.89 (0.35- 2.28)‡ |
NA | |
| S64 | IV infusion of Liposomal prostaglandin E1 vs. pl |
17/8 | 1/2 | No | 28 d* | 0.19 (0.01- 2.47) |
0.24 (0.02- 2.23) |
NA | |
| S65 | Human monoclonal antiendotoxin antibody (HA-1A) vs. pl |
30/33 | 15/23 | No | 28 d§ | 0.43 (0.16- 1.22) |
0.72 (0.47- 1.09) |
NA | |
| S66 | NAC IV vs. pl | 32/29 | 7/10 | No | 1 m* | 0.53 (0.17- 1.66) |
0.63 (0.28- 1.45) |
NA | |
| S67 | NAC IV vs. pl | 32/34 | 17/17 | No | 60 d& | 1.13 (0.43- 2.98) |
1.06 (0.67- 1.7) |
NA | |
| S68 | PGE1 IV infusion vs. pl | 72/74 | 42/37 | No | 30 d& | 1.40 (0.73- 2.69) |
1.17 (0.86- 1.57) |
NA | |
| S69 | PGE1 IV infusion vs. pl | 50/50 | 30/24 | Yes (futility) |
30 d* | 1.63 (0.74- 3.59) |
1.25 (0.87- 1.8) |
NA | |
| S70 | IV high dose methylprednisolone vs. pl |
50/49 | 30/31 | Yes (futility) |
45 d* | 0.87 (0.39- 1.96) |
0.95 (0.69- 1.29) |
NA | |
| Inhaled / intratracheal medications | |||||||||
| S71 | Aerosolized β2- adrenergic receptor agonists vs. pl |
152/130 | 35/23 | Yes (futility) |
Hospital to 60 d§ |
1.39 (0.77- 2.51) |
1.30 (0.81- 2.08) |
By baseline covariates: 1.27 (0.68-2.38) |
|
| S72 | Intratracheal recombinant surfactant protein C-based surfactant vs. pl |
419/424 | 95/101 | Yes (futility) |
28 d* | 0.94 (0.68- 1.29) |
0.95 (0.74- 1.22) |
NA | |
| S73 | Intratracheal exogenous natural surfactant vs. usual care |
208/210 | 60/51 | Yes (futility) |
28 d* | 1.26 (0.82- 1.95) |
1.19 (0.86- 1.64) |
NA | |
| S74 | Intratracheal protein C– based recombinant surfactant vs. usual care |
224/224 | 72/81 | No | 28 d§ | 0.84 (0.57- 1.24) |
0.89 (0.68- 1.15) |
NA | |
| S75 | Inhaled NO vs. pl | 192/193 | 44/39 | No | 28 d§ | 1.17 (0.72- 1.91) |
1.13 (0.77- 1.66) |
NA | |
| S76 | Intratracheal recombinant protein C– based surfactant vs. control |
27/13 | 7/5 | No | 28 d§ | 0.56 (0.14- 2.29) |
0.67 (0.26- 1.72) |
NA | |
| S77 | MV with and without inhaled NO |
15/15 | 8/7 | No | 30 d& | 1.30 (0.31- 5.48) |
1.14 (0.56- 2.35) |
NA | |
| S78 | Inhaled NO vs. pl | 93/87 | 41/35 | Yes (slow enrollmen t) |
30 d§ | 1.17 (0.65- 2.12) |
1.10 (0.78- 1.55) |
NA | |
| S79 | Inhaled NO vs. usual care |
15/15 | 9/8 | No | 30 d* | 1.31 (0.31- 5.58) |
1.13 (0.6- 2.11) |
NA | |
| S80 | Inhaled NO vs. usual care |
20/20 | 11/9 | No | Hospital & |
1.49 (0.43- 5.19) |
1.22 (0.65- 2.29) |
NA | |
| S81 | Inhaled NO vs. pl (nitrogen gas) |
120/57 | 35/17 | No | 28 d§ | 0.97 (0.49- 1.93) |
0.98 (0.60- 1.59) |
NA | |
| S82 | Bovine surfactant by endotracheal instillation vs. pl |
43/16 | 10/7 | No | 28 d* | 0.39 (0.12- 1.31) |
0.53 (0.24- 1.16) |
NA | |
| S83 | Aerosolized synthetic surfactant or placebo. |
364/361 | 145/14 3 |
Yes (futility) |
30 d* | 1.01 (0.75- 1.36) |
1.01 (0.84- 1.20) |
NA | |
| S84 | Aerosolized surfactant for 12 vs. 24 hs vs. pl |
17/17/17 | 7/6/8 | No | 30 d* | 0.61 (0.15- 2.43)¶ |
0.75 (0.33- 1.7)¶ |
NA | |
| Nutritional support | |||||||||
| S85 | Trophic vs. full enteral feeding |
508/492 | 118/10 9 |
No | 60 d§ | 1.06 (0.79- 1.43) |
1.05 (0.83- 1.32) |
NA | |
| S86 | Inflammatory modulators vs. control diet |
143/129 | 38/21 | Yes (futility) |
60 d§ | 1.86 (1.02- 3.38) |
1.63 (1.01- 2.63) |
NA | |
| S87 | Inflammatory modulators vs. control diet |
71/61 | 11/11 | No | 28 d§ | 0.83 (0.33- 2.08) |
0.86 (0.40- 1.84) |
NA | |
| S88 | Inflammatory modulators vs. pl |
41/49 | 9/12 | No | 60 d& | 0.87 (0.32- 2.32) |
0.9 (0.42- 1.91) |
NA | |
| S89 | Inflammatory modulators vs. control diet |
46/49 | 20/17 | No | 14 d§ | 1.45 (0.63- 3.31) |
1.25 (0.76- 2.08) |
NA | |
| S90 | Enteral Inflammatory modulators vs. pl |
51/47 | 6/9 | No | 30 d& | 0.56 (0.18- 1.72) |
0.61 (0.24- 1.59) |
NA | |
| Hemodynamic monitoring and others | |||||||||
| S91 | PAOP vs. CVP | 513/488 | 141/12 8 |
No | Hospital at 60 d* |
1.07 (0.81- 1.41) |
1.05 (0.85- 1.29) |
NA | |
| S92 | PAC vs. no PAC | 335/341 | 199/20 8 |
No | 28 d* | 1.17 (0.72- 1.91) |
0.97 (0.86- 1.10) |
NA | |
| S93 | CAVH vs. pl | 9/6 | 4/5 | No | NA& | 0.16 (0.01- 1.98) |
0.53 (0.24- 1.20) |
NA | |
References are provided in the Reference Appendix of the supplemetal file.
Abbreviations: APACHE: Acute Physiology and Chronic Health Evaluation, APVR: airway pressure release ventilation, ARM: alveolar recruitment maneuvers, CI: confidence interval, d: day, ECMO: extracorporeal membrane oxygenation, HFOV: high-frequency oscillatory ventilation, HR: hazard ratio, ICU: intensive care unit, m: month, IV: intravenous, M: mortality, MV: mechanical ventilation, NA: not available, OR: odds ratio, PAC: pulmonary artery catheter, PCV: pressure-controlled ventilation, PEEP: positive end-expiratory pressure, PGE1: prostaglandin E1, pl: placebo, PLV: partial liquid ventilation, PO: by mouth, PPV: positive pressure ventilation, RR: relative risk, SIMV: synchronized intermittent ventilation, SOFA: Sequential Organ Failure Assessment score, VCV: volume-controlled ventilation, vs.:versus.
for the comparison of NAC with rutin versus placebo.
NAC versus placebo.
For the comparison between 24 hs aerosolized surfactant versus placebo.
mortality as the only or as part of primary outcome.
mortality as a secondary outcome. & mortality not as part of a prespecified primary or secondary outcome.
based on statistical significance (crude or adjusted ratios) a positive sign represents a beneficial effect, a negative sign a deleterious effect and an empty box the lack of difference in survival between the study groups.
Table 2.
Analyses at different time points on mortality outcomes
| Author, year, reference |
Randomized comparison |
Mortality at | Calculated OR (95% CI) |
Calculated RR (95% CI) |
Adjusted HR (95% CI) |
Survival benefit # |
|---|---|---|---|---|---|---|
| MV strategies and respiratory care | ||||||
|
Guerin, 2013
[20] |
Prone vs. supine positioning |
28 d* | 0.39 (0.25-0.61) | 0.49 (0.34-0.69) | By SOFA: 0.42 (0.26-0.66) |
+ |
| 90 d | 0.44 (0.29-0.68) | 0.58 (0.43-0.77) | By SOFA: 0.48 (0.32-0.72). |
+ | ||
|
Ferguson et al.
[17] |
HFOV vs. control ventilation |
Hospital* | 1.63 (1.16-2.30) | 1.33 (1.09-1.64) | NA | − |
| 28 d* | 1.69 (1.17-2.46) | 1.41 (1.11-1.81) | NA | − | ||
| Young, 2013 [32] | HFVO vs. usual ventilatory care |
30 d* | 1.03 (0.77-1.36) | 1.02 (0.86-1.20) | By several variables: 1.03 (0.75-1.40) |
|
| Hospital | 1.09 (0.82-1.46) | 1.05 (0.89-1.24) | NA | |||
|
Taccone, 2009
[29] |
Prone vs. supine positioning |
28 d* | 0.92 (0.58-1.45) | 0.95 (0.69-1.29) | NA | |
| 6 m | 0.81 (0.52-1.27) | 0.90 (0.72-1.13) | NA | |||
|
Talmor, 2008
[90] |
PEEP guided by esophageal pressure vs. ARDS network recommendations |
28 d | 0.32 (0.08-1.20) | 0.43 (0.14-1.14) | 0.46 (0.19-1) | |
| 6 m | 0.44 (0.15-1.29) | 0.59 (0.29-1.20) | By APACHE II score was 0.52 (0.22-1.25) |
|||
| Huh, 2009 [91] | PEEP guided by esophageal pressure vs. ARDS network recommendations |
28 d | 1.33 (0.45-3.94) | 1.20 (0.60-2.39) | NA | |
| 6 m | 0.44 (0.15-1.29) | 0.59 (0.29-1.20) | By APACHE II score was 0.52 (0.22-1.25) |
|||
| Meade, 2008[22] | Open-lung ventilation vs. low-tidal-volume ventilation |
Hospital | 0.85 (0.65-1.10) | 0.90 (0.77-1.06) | By several variables: 0.97 (0.84-1.12) |
|
| 28 d | 0.83 (0.63-1.09) | 0.88 (0.73-1.06) | NA | |||
| Villar, 2006 [35] | High PEEP and low tidal volume vs. low PEEP and higher tidal volume |
ICU* | 0.41 (0.18-0.95) | 0.60 (0.37-0.98) | NA | + |
| Hospital | 0.41 (0.18-0.94) | 0.61 (0.38-0.98) | NA | + | ||
|
Mancebo, 2006
[34] |
Prone vs. supine positioning |
ICU* | 0.55 (0.28-1.09) | 0.74 (0.53-1.04) | By several variables: 0.4 (0.17-0.61) |
+ |
| Hospital | 0.62 (0.31-1.24) | 0.81 (0.60-1.10) | NA | |||
|
Guerin, 2004
[19] |
Prone vs. supine positioning |
28 d* | 1.05 (0.78-1.41) | 1.03 (0.84-1.26) | NA | |
| 90 d | 1.05 (0.79-1.39) | 1.03 (0.87-1.21) | NA | |||
|
Varpula, 2004
[92] |
APRV or SIMV | 28 d | 0.92 (0.24-3.59) | 0.93 (0.30-2.88) | NA | |
| 1 y | 0.60 (0.17-2.17) | 0.67 (0.24-1.86) | NA | |||
|
Derdak, 2002
[71] |
HFOV vs. conventional ventilation |
30 d* | 0.55 (0.28-1.08) | 0.72 (0.5-1.03) | NA | |
| 6 m | 0.61 (0.32-1.17) | 0.79 (0.58-1.08) | NA | |||
|
Gattinoni, 2001
[18] |
Prone vs. supine positioning |
ICU | 1.11 (0.71-1.74) | 1.05 (0.84-1.32) | NA | |
| 10 d | 0.80 (0.47-1.37) | 0.84 (0.56-1.27) | NA | |||
| 6 m* | 1.18 (0.74-1.87) | 1.07 (0.89-1.28) | NA | |||
|
Esteban,
2000[37] |
PCV vs. VCV | ICU | 0.42 (0.17-1.10) | 0.70 (0.48-1.04) | NA | |
| Hospital* | 0.29 (0.11-0.77) | 0.65 (0.46-0.93) | NA | + | ||
| Enteral or parenteral therapies | ||||||
|
Steinberg,
2006[93] |
Methylprednisolo ne IV vs. pl |
Hospital | 1.03 (0.54-1.97) | 1..02 (0.65-1.62) | NA | |
| 6 m | 0.98 (0.52-1.84) | 0.99 (0.64-1.52) | NA | |||
| Zeiher, 2004[33] | Sivelestat sodium IV infusion vs. pl |
28 d* | 1.03 (0.69-1.54) | 1.05 (0.78-1.41) | NA | |
| 6 m | 1.48 (1.02-2.15) | 1.29 (1.01-1.64) | NA | − | ||
|
Gainnier, 2004
[76] |
Cisatracurium IV vs. pl |
28 d | 0.36 (0.12-1.06) | 0.59 (0.33-1.05) | NA | |
| 60 d | 0.48 (0.16-1.41) | 0.72 (0.45-1.17) | NA | |||
|
Meduri, 1998
[38] |
Prolonged methylprednisolo ne (IV/PO) vs. pl |
ICU* | 0.02 (0.001-0.44) | 0.05 (0.00-0.78) | NA | + |
| Hospital | 0.09 (0.01-0.67) | 2.00 (0.05-0.81) | NA | + | ||
| Bone, 1989 [94] | PGE1 IV infusion vs. pl |
30 d* | 1.63 (0.74-3.59) | 1.25 (0.87-1.80) | NA | |
| 6 m | 1.29 (0.58-2.88) | 1.1 (0.81-1.51) | NA | |||
| Inhaled / intratracheal medications | ||||||
|
Spragg, 2011
[28] |
Intratracheal recombinant surfactant protein C-based surfactant vs. pl |
28 d* | 0.94 (0.68-1.29) | 0.95 (0.74-1.22) | NA | |
| 90 d | 1.03 (0.78-1.37) | 1.02 (0.85-1.23) | NA | |||
| 6 m | 1.06 (0.80-1.40) | 1.04 (0.87-1.24) | NA | |||
|
Kesecioglu, 2009
[21] |
Intratracheal exogenous natural surfactant vs. usual care |
28 d* | 1.26 (0.82-1.95) | 1.19 (0.86-1.64) | NA | |
| 6 m | 1.47 (0.98-2.21) | 1.24 (0.99-1.56) | NA | |||
| Nutritional support | ||||||
| Singer, 2006 [39] | Inflammatory modulators vs. control diet |
14 d | 1.45 (0.63-3.31) | 1.25 (0.76-2.08) | NA | |
| 28 d | 0.30 (0.13-0.70) | 0.49 (0.29-0.83) | NA | + | ||
| 90 d | 1.02 (0.41-2.55) | 1.01 (0.79-1.28) | NA | |||
| Hemodynamic monitoring and others | ||||||
|
Richard, 2003
[26] |
PAC vs. no PAC | 28 d* | 1.17 (0.72-1.91) | 0.97 (0.86-1.10) | NA | |
| 90 d | 0.93 (0.67-1.30) | 0.98 (0.89-1.08) | NA | |||
Abbreviations: APACHE: Acute Physiology and Chronic Health Evaluation, APVR: airway pressure release ventilation, CI: confidence interval, d: day, HFOV: high-frequency oscillatory ventilation, HR: hazard ratio, ICU: intensive care unit, IV: intravenous, m: month, NA: not available, OR: odds ratio, PAC: pulmonary artery catheter, PCV: pressure-controlled ventilation, PEEP: positive end-expiratory pressure, PGE1: prostaglandin E1, PO: by mouth , RR: relative risk, SIMV: synchronized intermittent ventilation, SOFA: Sequential Organ Failure Assessment score, VCV: volume-controlled ventilation.
primary analysis;
secondary analysis,
based on statistical significance (crude or adjusted ratios) a positive sign represents a beneficial effect, a negative sign a deleterious effect and an empty box the lack of difference in survival between the study groups.
Of the 93 trials [S1-S93] analyzed in more depth (n=20,671 randomized patients with a median (IQR) 99 (49-293) subjects per study (range 15-1001), forty-four included mortality as a primary outcome with 14,426 randomized patients; another 49 trials (n=6,245) reported death as a specified secondary outcome (31 trials, n=5,231) or simply as additional information in the manuscript (18 trials, n=1,014). Mortality was reported during the ICU or hospital stay or during follow-up ranging from 28 days (minimum) to 6 months (maximum) (table 2). A total of 32 studies were prematurely terminated (documentation of beneficial effect considered unlikely (n=18), perceived overwhelming evidence for benefit (n=5), perceived documentation of a detrimental effect (n=3), slow recruitment (n=6)).
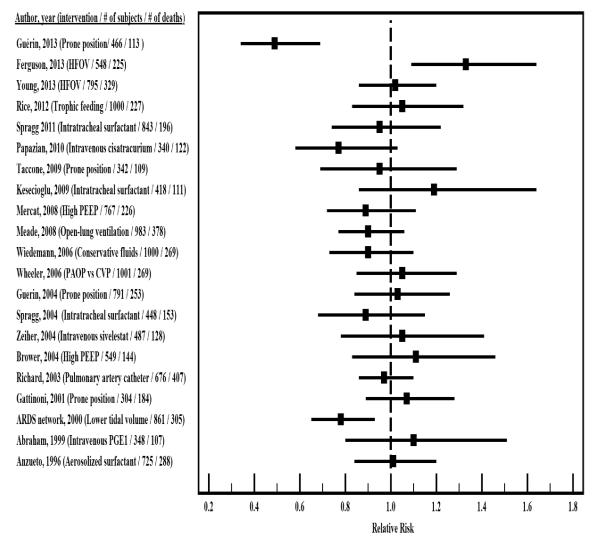
Of the 93 trials, 21 [13-33] had at least 50 deaths in one study arm (Figure 2 and e-table 8) (20 had at least 50 deaths in both study arms).
Figure 2. Calculated unadjusted risk ratios for mortality in randomized trials in ARDS that had more than 50 deaths in at least one arm.
When multiple metrics were provided we focused in the follow-up that defined the primary mortality outcome. In the event that this was not available we considered the time point of the secondary outcome and if none available the longer follow-up time.
Abbreviations: CVP: central venous pressure, HFOV: high-frequency oscillatory ventilation, PAOP: pulmonary artery occlusion pressure, PEEP: positive end-expiratory pressure, PGE1: prostaglandin E1.
Differences in mortality
There was a statistical significant difference in mortality favoring the intervention in 8 studies (prone positioning [2 studies [20, 34]], cisatracurium [24], high PEEP and low tidal volume[35], lower tidal volume [two studies [13, 36]], pressure control ventilation[37] and prolonged methylprednisolone[38]). Only 6 [13, 20, 35-38] of these 8 trials yielded a statistical significant difference using unadjusted metrics; the other two trials showed a statistically significant survival benefit only on adjusted HR (prone positioning[34] and cisatracurium[24]), but the difference was non-significant in unadjusted analyses. Two trials actually reported a statistically significant adverse effect on survival (high-frequency oscillatory ventilation [HFOV] and intravenous oxothiazolidine).
Of the studies that included more than 50 deaths in at least 1 treatment arm (Figure 2 and e-table 8), only three showed a mortality benefit of the intervention (lower tidal volumes [13], prone positioning [20], cisatracurium[24]), and one of them (cisatracurium[24]) did so only in adjusted analyses, as described above. One trial (HFOV[17]) suggested a detrimental effect of the active treatment and 17 showed no statistically significant difference between study arms (Figure 1).
23 trials presented mortality outcome data on 2 or more different time points. In 3 trials one or more analyses had found a non-significant difference, but analysis with different follow-up showed a statistically significant benefit (pressure controlled ventilation [37], primary analysis; inflammatory modulation diet [39], secondary analysis) or a statistically significant harm (sivelestat [33], secondary analysis) regarding survival. In 22/23 trials, the relative risk results for mortality had amply overlapping 95% CIs, while in the case of inflammatory modulation diet [39] the large benefit at 28 days was incongruent with the results at 14 days (primary analysis) and 90 days.
Meta-analyses
Of 147 screened citations (PubMed=91, Cochrane=56), 29 meta-analyses were selected (table 3) [3, 40-67]. These meta-analyses tested a variety of interventions including low tidal volumes (n=3)[47, 50, 59], prone positioning (n=5)[3, 40, 45, 52, 63], higher PEEP (n=6)[46, 48, 55, 57, 59, 60], HFOV (n=1)[61], non-invasive ventilation (n=1)[44], nitric oxide (n=2)[41, 42], exogenous surfactant (n=3)[49, 54, 65], corticosteroids (n=4)[43, 53, 56, 62], cisatracurium (n=1)[64], inflammation modulating diet (n=2)[58, 67], inhaled β2 agonists (n=1)[66] and sivelestat (n=1)[51] - one meta-analysis tested 2 interventions (low tidal volume and higher PEEP)[59]. Interventions that statistically significantly reduced mortality based on the provided summary effects on the overall population included low tidal volume ventilation (in 3/3 meta-analyses) [47, 50, 59], HFOV (in the single meta-analysis performed) [61], high PEEP (in 3 out of 6 meta-analyses) [46, 55, 57], cisatracurium (in the single meta-analysis performed)[64] and inflammation-modulating diet (in the two meta-analysis performed) [58, 67].
Table 3.
Meta-analyses of randomized trials that evaluate mortality in ARDS
| Author, year, reference |
Intervention | n | Participants | Deaths | Time | Risk ratio (95% CI)# |
Fixed (F) or random (R) Effect and heterogeneity |
Interpretation by the authors |
|---|---|---|---|---|---|---|---|---|
| Singh, 2013 [66] | Inhaled β2- agonists vs. pl |
2 | 313/293 | 97/76 | Hospital | 1.22 (0.95- 1.56) |
R (I2=0%) | No survival benefit. |
| 2 | 182/182 | 66/52 | 28 d | 1.04 (0.50- 2.16) |
R (I2=83%) | |||
|
Santa Cruz,
2013 [60] |
High vs. low PEEP without other interventions |
3 | 1136/1163 | 378/429 | Hospital | 0.90 (0.81- 1.01) |
F (I2=0%) | Trend toward mortality benefit. |
| Zhang, 2013 [65] | Exogenous surfactant vs. pl |
8 | 1101/1043 | 368/349 | 28-30 d | 1.00 (0.89- 1.12) |
F (I2=0%) | Intervention was not associated with reduced mortality. No difference among the different types of surfactant. |
|
Alhazzani, 2013
[64] |
Cisatracurium vs. pl |
3 | 223/208 | 70/93 | ICU | 0.70 (0.55- 0.89) |
R (I2=0%) | Cisatracurium reduced 28 days, ICU and hospital mortality |
| 3 | 223/208 | 76/98 | Hospital | 0.72 (0.58- 0.91) |
R (I2=0%) | |||
| 3 | 223/208 | 57/81 | 28 d | 0.66 (0.50- 0.87) |
R (I2=0%) | |||
| Meng, 2012[54] | Exogenous surfactant vs. pl |
9 | 1285/1289 | 396/392 | 28-30 d | OR: 1.02 (0.86-1.20) |
F (I2=0%) | Intervention did not improve survival |
|
Afshari, 2011
[42] |
Inhaled nitric oxide vs. pl |
14 | 660/590 | 265/228 | Variable (1-365 d) |
1.06 (0.93- 1.22) |
F (I2=0%) | No benefit on survival |
| 9 | 578/504 | 208/578 | 28 d | 1.12 (0.95- 1.31) |
F (I2=0%) | |||
| Burns, 2011[47] | Pressure and volume-limited ventilation vs. traditional MV |
10 | 888/861 | 312/366 | Hospital | 0.84 (0.70- 1.00) |
R (I2=43%) | Borderline (p=0.05) statistically significant reduction in mortality. |
|
Dasenbrook,
2011 [48] |
Higher vs. lower PEEP |
4 | 1166/1194 | 311/356 | 28 d | 0.90 (0.79- 1.02) |
F (I2=11%) | No significant difference in 28 d survival. |
|
Abroug, 2011
[40] |
Prone vs. supine positioning |
7 | 862/813 | NA | ICU | 0.91 (0.75- 1.12) |
R (I2=0%) | No significant effect on ICU mortality. Sub-analysis showed a survival benefit in those with more severe forms of ARDS |
| Dee, 2011 [67] | Inflammation- modulating diet vs. control diet |
3 Sa me stu die s |
171/173 | 42/72 | Hospital | 0.58 (0.42- 0.79) |
R (I2=0%) | Intervention improved survival |
| Briel, 2010 [46] | Higher vs. lower PEEP |
3 | 1136/1163 | 324/381 | ICU | 0.87 (0.78- 0.97) |
Log-binomial regression |
No improvement in hospital survival. Survival improved in more severe forms of ARDS. |
| 374/409 | Hospital | 0.94 (0.86- 1.04) |
||||||
| Iwata, 2010 [51] | Sivelestat vs. pl | 4 | 379/379 | NA | 28-30 d | 0.95 (0.72- 1.26) |
R (I2=0%) | No significant survival benefit at 28-30 d but worse survival at |
| 2 | 253/258 | NA | 6 m | 1.27 (1.00- 1.62) |
R (I2=0%) | 6 m | ||
| Sud, 2010 [61] | Prone vs. supine positioning (severe hypoxemia) |
7 | 295/260 | 157/163 | Hospital | 0.84 (0.74- 0.96) |
R (I2=0%) | Prone positioning reduced mortality in patients with severe hypoxemia. Overall, no significant effect. |
| Prone vs. supine positioning (less severe hypoxemia) |
7 | 590/578 | 248/230 | Hospital | 1.07 (0.93- 1.22) |
R (I2=0%) | ||
|
Lamontagne,
2010 [53] |
Corticosteroid therapy vs. pl |
12 | 471/495 | 147/176 | Hospital | 0.84 (0.66- 1.06) |
R (I2=29%) | Low-dose corticosteroid therapy may reduce all-cause mortality |
| Lower corticosteroid dose vs. pl |
9 | 374/396 | 95/128 | Hospital | 0.68 (0.49- 0.96) |
R (I2=30%) | ||
| Sud, 2010 [3] | HFOV vs. conventional MV |
6 | 189/176 | 73/87 | Variable (Hospital or 30 d) |
0.77 (0.61- 0.98) |
R (I2=0%) | Intervention might improve survival |
|
Putensen, 2009
[59] |
Lower vs. higher TV at similar PEEP |
3 | 518/515 | 177/211 | Hospital | OR: 0.75 (0.58-0.96) |
F (I2=18%) | Low TV reduced hospital mortality. Higher PEEP did not improve mortality |
| Higher vs. lower PEEP at low TV |
3 | 1136/1163 | 378/429 | Hospital | OR: 0.86 (0.72-1.02) |
F (I2=0%) | ||
| Lower TV + higher PEEP vs. higher TV and lower PEEP |
2 | 79/69 | 30/42 | Hospital | OR: 0.38 (0.20-0.75) |
F (I2=0%) | ||
| Tang, 2009 [62] | Corticosteroids vs. pl |
4 | 191/150 | 45/53 | Hospital | 0.51 (0.24- 1.09) |
R (I2=51%) | Low-dose steroids was not associated with improved survival |
|
Phoenix, 2009
[57] |
Higher vs. lower PEEP |
6 | 1233/1251 | 415/482 | Early mortality (Hospital and 28 d) |
0.87 (0.79- 0.97) |
R (I2=0%) | PEEP may provide a mortality benefit. |
| Only studies with groups with similar tidal volumes |
3 | 1136/1163 | 378/429 | Hospital | 0.90 (0.81- 1.01) |
R (I2=0%) | ||
|
Kopterides, 2009
[52] |
Prone vs. supine positioning |
4 | 662/609 | 245/230 | ICU | 0.97 (0.77- 1.22) |
R (I2=32%) | No survival differences, however ICU mortality was lower in severely ill patients. |
| Oba, 2009 [55] | High PEEP vs. low PEEP |
5 | 1215/1232 | 408/464 | Hospital | 0.89 (0.80- 0.99) |
F (I2=0%) | Survival benefit in hospital mortality, but statistical and clinical heterogeneity. Effect greater in patients with higher ICU severity scores |
| 3 | 889/914 | 253/296 | 28 d | 0.88 (0.76- 1.01) |
F (I2=0%) | |||
|
Pontes-Arruda
2008 [58] |
Inflammation- modulating diet vs. control diet |
3 | 152/144 | 37/62 | 28 d | OR: 0.40 (0.24-0.68) |
F (I2=0%) | Mortality reduction in those treated. |
| Peter, 2008 [56] | Corticosteroid vs. pl |
5 | 303/268 | 127/141 | Variable (Hospital- 60d)) |
OR: 0.62 (0.23-1.26) |
R (SD=0.53) | No significant survival benefit |
|
Tiruvoipati,
2008 [63] |
Prone vs. supine positioning |
4 | 662/609 | 263/246 | Variable (ICU-6 m) |
OR: 0.98 (0.70-1.30) |
R (I2=18%) | No significant survival benefit |
|
Alsaghir, 2008
[45] |
Prone vs. supine positioning |
3 | 241/225 | 113/113 | ICU | OR: 0.79 (0.45-1.39) |
R (I2=40%) | No difference in mortality. Subgroup analysis suggested a beneficial effect in patients with higher illness severity |
| 3 | 641/590 | 238/223 | 28-30 d | OR: 0.95 (0.71-1.28) |
R (I2=28%) | |||
| 4 | 662/609 | 301/279 | 90 d | OR: 0.99 (0.77-1.27) |
R (I2=10%) | |||
|
Agarwal, 2007
[43] |
Corticosteroids vs. pl (early ARDS) |
3 | 147/153 | 85/105 | Variable (Hospital / 30 d) |
OR: 0.57 (0.25-1.32) |
R (I2=53%) | No benefit in survival |
| Corticosteroids vs. pl (late ARDS) |
3 | 118/117 | 33/41 | Variable (Hospital / 30 d) |
OR: 0.58 (0.22-1.53) |
R (I2=42%) | ||
|
Adhikari, 2007
[41] |
Nitric oxide vs. pl | 9 | 577/509 | 199/162 | Hospital | 1.10 (0.94- 1.30) |
R (I2=0%) | No mortality benefit |
|
Agarwal, 2006
[44] |
Noninvasive ventilation with conventional treatment |
3 | 55/56 | 17/20 | ICU | 0.96 (0.80- 1.12) |
R (I2=0%) | No survival benefit. No difference between intratracheal instillation and aerosolized methods |
|
Davidson,
2006[49] |
Exogenous pulmonary surfactant vs. pl |
6 | 631/639 | 235/255 | 28-30 d | OR: 0.97 (0.73-1.3) |
F (NA) | Intervention did not improve survival |
|
Eichacker, 2002
[50] |
Low vs. control (higher TV and plateau pressure) tidal volumes |
2 | 461/453 | 145/189 | Variable (Hospital- 28 d) |
0.75 (0.63- 0.89)* |
NA | Significant heterogeneity in outcomes that precluded a single summary effect. |
| Low vs. control (lower TV and plateau pressure) tidal volumes |
3 | 144/144 | 70/62 | Variable (Hospital- 60 d) |
1.13 (0.88- 1.45)* |
Abbreviations: d: day, I2: heterogeneity, ICU: intensive care unit, m: month, MV: mechanical ventilation, NA: not available, OR: odds ratio, PEEP: positive end-expiratory pressure.SD: standard deviation among studies, TV: tidal volume.
approximate values obtained from their figure 1.
unless specified the value provided is risk ratio, otherwise odds ratio or risk reduction is reported.
Meta-analyses that have assessed low tidal volume have consistently suggested statistically significant mortality benefits [47, 50, 59] . However, upper 95% CIs are close to 1.00 and one meta-analysis found a benefit only in a subgroup that used a comparator of higher tidal volumes and plateau pressures.
One meta-analysis of HFOV showed a 23% significant reduction in the relative risk [61]; however, the two largest trials (published after this meta-analysis, each of them larger than the meta-analysis in sample size)[17, 32] have found either no benefit (risk ratio 1.02) [32] or a significantly increased risk of death (risk ratio 1.33) [17].
The 6 existing meta-analyses of high PEEP [46, 48, 55, 57, 59, 60] yield similar summary treatment effects with 95% CIs reach close to 1.00. One meta-analysis[46] found a favorable effect of high PEEP in ICU but not hospital mortality, nevertheless the results are consistently non-significant when the different levels of PEEP are compared in patients ventilated with low tidal volumes.
A meta-analysis[64] of intravenous cisatracurium infusion showed a mortality benefit at different time points (ICU, hospital and 28-day); however it included 3 studies of markedly different size performed by the same groups of investigators in France.
In addition, two meta-analysis using the same 3 studies [58, 67] found a reduction in mortality in patients receiving inflammatory modulation diet; however this result applies to data on 28-day mortality and are driven by the study discussed above[39] that had a favorable estimate at 28 days, but showed a trend for increased mortality at 14 days and no benefit at 90 days.
Eight of the 29 meta-analyses made claims for the presence of a survival benefit in subsets of patients with greater background disease severity and/or hypoxemia (n=6)[3, 40, 45, 46, 52, 55], with different doses (n=1)[53] or different settings for the control/background intervention (n=1)[50] (table 3). The most consistent observation seemed to be that prone positioning reduced the hospital mortality in the subgroup of patients with more severe hypoxemia, but not overall. This observation was made by at least 3 of the 5 respective meta-analyses, and it was validated also in a subsequent RCT [68] published after these 5 meta-analyses of prone positioning showing a 51% relative risk reduction (58% in adjusted analysis) in a study population with severe ARDS.
Discussion
Despite 159 RCTs and 29 meta-analyses on ARDS treatment, and sporadic significant findings in single papers, the available evidence seems to consistently support a reduction in overall mortality with low tidal volume ventilation and also with prone positioning among patients with severe ARDS. These two interventions may really be the only ones that can be currently recommended for routine clinical use with rigorous support.
Beyond these two interventions, sporadic claims of mortality benefits seem to be spurious and reflect chance findings or selective analyses, as has been seen also in other fields[69, 70]. This may apply to cisatracurium [24, 64], HFOV[61, 71], high PEEP [35, 46, 55, 57, 60], pressure control ventilation [37], corticosteroids [38, 53, 72], and inflammation-modulating diet [39, 58, 67]. Due to the limited number of patients, often we cannot exclude modest benefits with certainty. However, when large trials have been performed, they have shown no benefit, or even harm, as in the case of HFOV. Conducting additional definitive large trials may be warranted to settle some of the other unclear claims or before universally adopting results of a single large randomized control trial. An alternative approach would be the inclusion of fewer patients who are at higher-risk for the outcome of interest.
Even for the two best documented interventions that apparently decrease mortality in ARDS, the exact range of indications for their application is not fully settled. Mechanical ventilation with low-tidal volume is now a well-established practice in the treatment of ARDS as higher tidal volumes can overstretch the alveoli leading to inflammation and lung injury [73]. It remains unclear, however, whether this intervention provides a survival benefit when compared with relatively higher tidal volumes that limit the airway pressures [50]. Interestingly, this “lung-protective” ventilation modality is likely to be beneficial even among patients without ARDS [74]. As for prone ventilation, it has taken a long time (the first RCT was published in 2001) to decipher how to apply it. Five meta-analyses[3, 40, 45, 52, 63] published between 2008 and 2011 found very similar, non-significant overall effects, but at least 3 of them identified a significant benefit for mortality in patients with more severe ARDS[3, 40, 52]. Then, a recent large study showed a 28-day survival improvement in those patients that received prone positioning [20]. Low tidal volumes and prone positioning may even need to be applied concurrently. According to a meta-analysis published after the end of our search, benefits from prone position have been demonstrated only in trials that use also low tidal volumes [75].
Among other interventions, neuromuscular blockers and high PEEP have interesting tentative signals of benefit. Neuromuscular blockers may improve oxygenation and decrease inflammation [76, 77]. Cisatracurium has shown a 90-day adjusted survival benefit when compared to placebo [24], but not in an unadjusted analysis. Treatment effects that are analysis-dependent are tenuous [78]. A recent meta-analysis [64] also concludes in favor of the short-term infusion of cisatracurium as this treatment may reduce hospital mortality and barotraumas without significant side effects. However, the data come from the same group of investigators and the mortality benefits are driven largely by the trial that has shown significant benefits in adjusted analyses. Further independent corroboration of these results in multi-center trials is needed.
High levels of PEEP have not conclusively shown an improved survival. Meta-analyses have showed high heterogeneity [46, 48, 55, 57, 59, 60]. Perhaps this intervention might be beneficial in patients with severe ARDS, as in the case of prone ventilation, but this hypothesis needs validation in a large trial. Higher levels of PEEP may increase the proportion of aerated lung at end-expiration, preventing lung injury, improving oxygenation and permitting a lower inspiratory fraction of O2, which in turn limits pulmonary oxygen toxicity [79, 80].
The field of ARDS therapeutics has had a large number of RCTs and meta-analyses performed to-date. For some topics, there have been multiple (up to 6) meta-analyses on the same intervention. While some independent validation of meta-analyses is useful, redundancy could be avoided[81]. At this stage, it is unlikely that priority should be given to performing more small trials and more meta-analyses of single interventions. Besides the 155 published RCTs and 29 meta-analyses that we identified, in preliminary searches we identified another 117 unpublished trials in clinicaltrials.gov (37 completed, 21 not yet recruiting, 43 recruiting and 16 terminated) as of July 2013. Considering the possibility of additional trials that are neither published nor registered, the cumulative research agenda of ARDS may currently include over 300 RCTs. However, the large majority of them are small investigations where important outcomes such as mortality are difficult or impossible to investigate meaningfully. Results on mortality are likely to leave substantially uncertainty, even when they seem promising. Mortality benefits claimed on small trials very often represent spurious findings [82, 83]. There are few relatively large trials performed in the field, and the largest trial published to-date has had 1,001 patients. We suggest that modestly large trials (e.g. with 500-1000 patients) should become more common in the field. Such trials have been able to yield conclusive answers for tentative interventions, including both favorable (e.g. prone positioning) and unfavorable conclusions (e.g. HFOV). Nevertheless, of the 117 unpublished registered trials in clinicaltrials.gov, we found only 9 that have an anticipated total sample size exceeding 500 (details in the supplement).
While modestly large trials would require by default multi-center collaborations and sufficient resources, successful precedents such as the PROSEVA trial on prone positioning [20] suggest that such a strategy is worth adopting more commonly. Small trials are likely more susceptible to selective reporting of analyses and outcomes, and results may become even more confusing with emphasis on subgroup analyses and other secondary explorations of the data [8, 84]. Given that ARDS is a common major problem affecting millions of patients annually, recruiting sufficient numbers of patients should be feasible. This applies also to situations where interventions are proposed for testing in specific subsets of patients where there may be biological or prior clinical evidence that they may be more effective.
Another important issue is the lack of standardization in the time-period in which mortality in ARDS studies is reported. RCTs have used time-points that include ICU, hospital, 28-days up to 6-month mortality. This variability makes it difficult to compare the effects of different interventions. In many trials ICU and hospital mortalities were not reported, which are important outcome measures to assess the effect of ICU interventions. Most deaths in ARDS are not directly related to lung disease, but to extrapulmonary organ dysfunction [85], therefore it is challenging to prove than interventions targeting the lung improve overall survival.
Our umbrella review has limitations. Firstly, we are limited by the amount and quality of available information in primary studies [11]. Moreover, it is difficult to generalize the results of these studies given the diverse inclusion/exclusion criteria and severity of disease [3, 20, 24, 86, 87]. Furthermore, there is inequality in the contribution of centers and lack of protocolized general care. Second, the vast majority of the evidence pertained to testing an intervention versus control management. Trials comparing head-to-head effective interventions are not available. Lower tidal volume was incorporated in clinical practice lately and until recently no other interventions have had strong evidence to be used as standard controls. However, what constitutes standard management may change over time. Moreover, as some interventions start showing efficacy, head-to-head comparisons will become more important to perform [10]. One would need to design trials specifically addressing additive or synergistic effects of effective interventions, when these are used concomitantly [88]. Third, the results that we present focus on published information susceptible to reporting biases. Some of the spurious significant signals that we identified might have been reversed if additional unpublished data were available. However, obtaining unpublished data is notoriously difficult. This is one more reason why larger-scale collaboration to perform large multi-center trials are direly needed in the field. Issues of wider data sharing of the conducted trials, ideally at patient-level data, may need to be discussed as well [89]. Fourth, we focused specifically on mortality, while it is possible that some interventions may have beneficial effects on other outcomes, such as the duration of mechanical ventilation, without necessarily affecting mortality. Such interventions may still be useful, but here we focused on the most important outcome that matters in this setting.
Supplementary Material
Acknowledgements
The authors will like to thank the Cleveland Clinic medical librarian Kim Brady for her invaluable help in the PubMed, Cochran and clinicaltrial.gov searches.
Funding sources: Dr ART is supported by CTSA KL2 [Grant # TR000440] (A.R.T.) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- APVR
airway pressure release ventilation
- ARDS
acute respiratory distress syndrome
- ARM
alveolar recruitment maneuvers
- CI
confidence interval
- d
day
- HFOV
high-frequency oscillatory ventilation
- HR
hazard ratio
- ICU
intensive care unit
- m
month
- IV
intravenous
- M
mortality
- MV
mechanical ventilation
- NA
not available
- OR
odds ratio
- PAC
pulmonary artery catheter
- PCV
pressure-controlled ventilation
- PEEP
positive end-expiratory pressure
- PGE1
prostaglandin E1
- PLV
partial liquid ventilation
- PO
by mouth
- PPV
positive pressure ventilation
- RCT
randomized controlled trial
- RR
relative risk
- SIMV
synchronized intermittent ventilation
- SOFA
Sequential Organ Failure Assessment score
- VCV
volume-controlled ventilation
Footnotes
Authors’ contributions: Adriano R. Tonelli MD: Participated in the design of the study, data collection, study selection, analysis and interpretation of the results, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Joe Zein MD: Participated in the design of the study, data collection, study selection, analysis and interpretation of the results, writing of the manuscript and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Jacob Adams DO: Participated in the design of the study, data collection, study selection, analysis and interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
John P.A. Ioannidis, MD, DSc: Participated in the conception and design of the study, study selection, analysis and interpretation of the results, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Conflict of interest: Adriano R. Tonelli MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Joe Zein MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Jacob Adams MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
John P.A. Ioannidis, MD, DSc: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
None of the context of this paper were previously published / presented in any form.
References
- 1.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni L, Rhodes A, Slutsky AS, Vincent JL, Rubenfeld GD, Thompson BT, Ranieri VM. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 2.Villar J, Blanco J, Anon JM, Santos-Bouza A, Blanch L, Ambros A, Gandia F, Carriedo D, Mosteiro F, Basaldua S, Fernandez RL, Kacmarek RM. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 3.Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, Pesenti A, Guerin C, Mancebo J, Curley MA, Fernandez R, Chan MC, Beuret P, Voggenreiter G, Sud M, Tognoni G, Gattinoni L. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med. 2010;36:585–599. doi: 10.1007/s00134-009-1748-1. [DOI] [PubMed] [Google Scholar]
- 4.Bersten AD, Edibam C, Hunt T, Moran J. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med. 2002;165:443–448. doi: 10.1164/ajrccm.165.4.2101124. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery AB, Stager MA, Carrico CJ, Hudson LD. Causes of mortality in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1985;132:485–489. doi: 10.1164/arrd.1985.132.3.485. [DOI] [PubMed] [Google Scholar]
- 6.Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Penuelas O, Abraira V, Raymondos K, Rios F, Nin N, Apezteguia C, Violi DA, Thille AW, Brochard L, Gonzalez M, Villagomez AJ, Hurtado J, Davies AR, Du B, Maggiore SM, Pelosi P, Soto L, Tomicic V, D’Empaire G, Matamis D, Abroug F, Moreno RP, Soares MA, Arabi Y, Sandi F, Jibaja M, Amin P, Koh Y, Kuiper MA, Bulow HH, Zeggwagh AA, Anzueto A. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188:220–230. doi: 10.1164/rccm.201212-2169OC. [DOI] [PubMed] [Google Scholar]
- 7.Girard TD, Bernard GR. Mechanical ventilation in ARDS: a state-of-the-art review. Chest. 2007;131:921–929. doi: 10.1378/chest.06-1515. [DOI] [PubMed] [Google Scholar]
- 8.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, Heels-Ansdell D, Walter SD, Guyatt GH, Flynn DN, Elamin MB, Murad MH, Abu Elnour NO, Lampropulos JF, Sood A, Mullan RJ, Erwin PJ, Bankhead CR, Perera R, Ruiz Culebro C, You JJ, Mulla SM, Kaur J, Nerenberg KA, Schunemann H, Cook DJ, Lutz K, Ribic CM, Vale N, Malaga G, Akl EA, Ferreira-Gonzalez I, Alonso-Coello P, Urrutia G, Kunz R, Bucher HC, Nordmann AJ, Raatz H, da Silva SA, Tuche F, Strahm B, Djulbegovic B, Adhikari NK, Mills EJ, Gwadry-Sridhar F, Kirpalani H, Soares HP, Karanicolas PJ, Burns KE, Vandvik PO, Coto-Yglesias F, Chrispim PP, Ramsay T. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010;303:1180–1187. doi: 10.1001/jama.2010.310. [DOI] [PubMed] [Google Scholar]
- 10.Ioannidis JP, Karassa FB. The need to consider the wider agenda in systematic reviews and meta-analyses: breadth, timing, and depth of the evidence. BMJ. 2010;341:c4875. doi: 10.1136/bmj.c4875. [DOI] [PubMed] [Google Scholar]
- 11.Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ. 2009;181:488–493. doi: 10.1503/cmaj.081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 13.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 14.Abraham E, Baughman R, Fletcher E, Heard S, Lamberti J, Levy H, Nelson L, Rumbak M, Steingrub J, Taylor J, Park YC, Hynds JM, Freitag J. Liposomal prostaglandin E1 (TLC C-53) in acute respiratory distress syndrome: a controlled, randomized, double-blind, multicenter clinical trial. TLC C-53 ARDS Study Group. Crit Care Med. 1999;27:1478–1485. doi: 10.1097/00003246-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Anzueto A, Baughman RP, Guntupalli KK, Weg JG, Wiedemann HP, Raventos AA, Lemaire F, Long W, Zaccardelli DS, Pattishall EN. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. N Engl J Med. 1996;334:1417–1421. doi: 10.1056/NEJM199605303342201. [DOI] [PubMed] [Google Scholar]
- 16.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F, Granton JT, Arabi YM, Arroliga AC, Stewart TE, Slutsky AS, Meade MO. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 18.Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, Malacrida R, Di Giulio P, Fumagalli R, Pelosi P, Brazzi L, Latini R. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 19.Guerin C, Gaillard S, Lemasson S, Ayzac L, Girard R, Beuret P, Palmier B, Le QV, Sirodot M, Rosselli S, Cadiergue V, Sainty JM, Barbe P, Combourieu E, Debatty D, Rouffineau J, Ezingeard E, Millet O, Guelon D, Rodriguez L, Martin O, Renault A, Sibille JP, Kaidomar M. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA. 2004;292:2379–2387. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 20.Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 21.Kesecioglu J, Beale R, Stewart TE, Findlay GP, Rouby JJ, Holzapfel L, Bruins P, Steenken EJ, Jeppesen OK, Lachmann B. Exogenous natural surfactant for treatment of acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2009;180:989–994. doi: 10.1164/rccm.200812-1955OC. [DOI] [PubMed] [Google Scholar]
- 22.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, Austin P, Lapinsky S, Baxter A, Russell J, Skrobik Y, Ronco JJ, Stewart TE. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive endexpiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 23.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, Gervais C, Baudot J, Bouadma L, Brochard L. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 24.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guerin C, Prat G, Morange S, Roch A. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 25.Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, Rock P. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, Boulain T, Lefort Y, Fartoukh M, Baud F, Boyer A, Brochard L, Teboul JL. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2003;290:2713–2720. doi: 10.1001/jama.290.20.2713. [DOI] [PubMed] [Google Scholar]
- 27.Spragg RG, Lewis JF, Walmrath HD, Johannigman J, Bellingan G, Laterre PF, Witte MC, Richards GA, Rippin G, Rathgeb F, Hafner D, Taut FJ, Seeger W. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004;351:884–892. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 28.Spragg RG, Taut FJ, Lewis JF, Schenk P, Ruppert C, Dean N, Krell K, Karabinis A, Gunther A. Recombinant surfactant protein C-based surfactant for patients with severe direct lung injury. Am J Respir Crit Care Med. 2011;183:1055–1061. doi: 10.1164/rccm.201009-1424OC. [DOI] [PubMed] [Google Scholar]
- 29.Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, Caspani L, Raimondi F, Bordone G, Iapichino G, Mancebo J, Guerin C, Ayzac L, Blanch L, Fumagalli R, Tognoni G, Gattinoni L. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302:1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, Connors AF, Jr., Hite RD, Harabin AL. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 31.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr., Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 32.Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, Rowan K, Cuthbertson BH. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368:806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 33.Zeiher BG, Artigas A, Vincent JL, Dmitrienko A, Jackson K, Thompson BT, Bernard G. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med. 2004;32:1695–1702. doi: 10.1097/01.ccm.0000133332.48386.85. [DOI] [PubMed] [Google Scholar]
- 34.Mancebo J, Fernandez R, Blanch L, Rialp G, Gordo F, Ferrer M, Rodriguez F, Garro P, Ricart P, Vallverdu I, Gich I, Castano J, Saura P, Dominguez G, Bonet A, Albert RK. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173:1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 35.Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34:1311–1318. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 36.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 37.Esteban A, Alia I, Gordo F, de Pablo R, Suarez J, Gonzalez G, Blanco J. Prospective randomized trial comparing pressure-controlled ventilation and volume-controlled ventilation in ARDS. For the Spanish Lung Failure Collaborative Group. Chest. 2000;117:1690–1696. doi: 10.1378/chest.117.6.1690. [DOI] [PubMed] [Google Scholar]
- 38.Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, Tolley EA. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 39.Singer P, Theilla M, Fisher H, Gibstein L, Grozovski E, Cohen J. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34:1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [DOI] [PubMed] [Google Scholar]
- 40.Abroug F, Ouanes-Besbes L, Dachraoui F, Ouanes I, Brochard L. An updated study-level meta-analysis of randomised controlled trials on proning in ARDS and acute lung injury. Crit Care. 2011;15:R6. doi: 10.1186/cc9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007;334:779. doi: 10.1136/bmj.39139.716794.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afshari A, Brok J, Moller AM, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome and acute lung injury in adults and children: a systematic review with meta-analysis and trial sequential analysis. Anesth Analg. 2011;112:1411–1421. doi: 10.1213/ANE.0b013e31820bd185. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal R, Nath A, Aggarwal AN, Gupta D. Do glucocorticoids decrease mortality in acute respiratory distress syndrome? A meta-analysis. Respirology. 2007;12:585–590. doi: 10.1111/j.1440-1843.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal R, Reddy C, Aggarwal AN, Gupta D. Is there a role for noninvasive ventilation in acute respiratory distress syndrome? A meta-analysis. Respir Med. 2006;100:2235–2238. doi: 10.1016/j.rmed.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Alsaghir AH, Martin CM. Effect of prone positioning in patients with acute respiratory distress syndrome: a meta-analysis. Crit Care Med. 2008;36:603–609. doi: 10.1097/01.CCM.0000299739.98236.05. [DOI] [PubMed] [Google Scholar]
- 46.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 47.Burns KE, Adhikari NK, Slutsky AS, Guyatt GH, Villar J, Zhang H, Zhou Q, Cook DJ, Stewart TE, Meade MO. Pressure and volume limited ventilation for the ventilatory management of patients with acute lung injury: a systematic review and meta-analysis. PLoS One. 2011;6:e14623. doi: 10.1371/journal.pone.0014623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dasenbrook EC, Needham DM, Brower RG, Fan E. Higher PEEP in patients with acute lung injury: a systematic review and meta-analysis. Respir Care. 2011;56:568–575. doi: 10.4187/respcare.01011. [DOI] [PubMed] [Google Scholar]
- 49.Davidson WJ, Dorscheid D, Spragg R, Schulzer M, Mak E, Ayas NT. Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: results of a meta-analysis. Crit Care. 2006;10:R41. doi: 10.1186/cc4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eichacker PQ, Gerstenberger EP, Banks SM, Cui X, Natanson C. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials testing low tidal volumes. Am J Respir Crit Care Med. 2002;166:1510–1514. doi: 10.1164/rccm.200208-956OC. [DOI] [PubMed] [Google Scholar]
- 51.Iwata K, Doi A, Ohji G, Oka H, Oba Y, Takimoto K, Igarashi W, Gremillion DH, Shimada T. Effect of neutrophil elastase inhibitor (sivelestat sodium) in the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): a systematic review and meta-analysis. Intern Med. 2010;49:2423–2432. doi: 10.2169/internalmedicine.49.4010. [DOI] [PubMed] [Google Scholar]
- 52.Kopterides P, Siempos II, Armaganidis A. Prone positioning in hypoxemic respiratory failure: meta-analysis of randomized controlled trials. J Crit Care. 2009;24:89–100. doi: 10.1016/j.jcrc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Lamontagne F, Briel M, Guyatt GH, Cook DJ, Bhatnagar N, Meade M. Corticosteroid therapy for acute lung injury, acute respiratory distress syndrome, and severe pneumonia: a meta-analysis of randomized controlled trials. J Crit Care. 2010;25:420–435. doi: 10.1016/j.jcrc.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Meng H, Sun Y, Lu J, Fu S, Meng Z, Scott M, Li Q. Exogenous surfactant may improve oxygenation but not mortality in adult patients with acute lung injury/acute respiratory distress syndrome: a meta-analysis of 9 clinical trials. J Cardiothorac Vasc Anesth. 2012;26:849–856. doi: 10.1053/j.jvca.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oba Y, Thameem DM, Zaza T. High levels of PEEP may improve survival in acute respiratory distress syndrome: A meta-analysis. Respir Med. 2009;103:1174–1181. doi: 10.1016/j.rmed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Peter JV, John P, Graham PL, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336:1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phoenix SI, Paravastu S, Columb M, Vincent JL, Nirmalan M. Does a higher positive end expiratory pressure decrease mortality in acute respiratory distress syndrome? A systematic review and meta-analysis. Anesthesiology. 2009;110:1098–1105. doi: 10.1097/ALN.0b013e31819fae06. [DOI] [PubMed] [Google Scholar]
- 58.Pontes-Arruda A, Demichele S, Seth A, Singer P. The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: a meta-analysis of outcome data. JPEN J Parenter Enteral Nutr. 2008;32:596–605. doi: 10.1177/0148607108324203. [DOI] [PubMed] [Google Scholar]
- 59.Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151:566–576. doi: 10.7326/0003-4819-151-8-200910200-00011. [DOI] [PubMed] [Google Scholar]
- 60.Santa Cruz R, Rojas JI, Nervi R, Heredia R, Ciapponi A. High versus low positive endexpiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013;6:CD009098. doi: 10.1002/14651858.CD009098.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sud S, Sud M, Friedrich JO, Meade MO, Ferguson ND, Wunsch H, Adhikari NK. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta-analysis. BMJ. 2010;340:c2327. doi: 10.1136/bmj.c2327. [DOI] [PubMed] [Google Scholar]
- 62.Tang BM, Craig JC, Eslick GD, Seppelt I, McLean AS. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009;37:1594–1603. doi: 10.1097/CCM.0b013e31819fb507. [DOI] [PubMed] [Google Scholar]
- 63.Tiruvoipati R, Bangash M, Manktelow B, Peek GJ. Efficacy of prone ventilation in adult patients with acute respiratory failure: a meta-analysis. J Crit Care. 2008;23:101–110. doi: 10.1016/j.jcrc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Alhazzani W, Alshahrani M, Jaeschke R, Forel JM, Papazian L, Sevransky J, Meade MO. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2013;17:R43. doi: 10.1186/cc12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang LN, Sun JP, Xue XY, Wang JX. Exogenous pulmonary surfactant for acute respiratory distress syndrome in adults: A systematic review and meta-analysis. Exp Ther Med. 2013;5:237–242. doi: 10.3892/etm.2012.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh B, Tiwari AK, Singh K, Mommer SK, Ahmed A, Erwin PJ, Franco PM. Beta-2-Agonist for the Treatment of Acute Lung Injury: A Systematic Review and Meta-Analysis. Respir Care. 2013 doi: 10.4187/respcare.02571. [DOI] [PubMed] [Google Scholar]
- 67.Dee BM, Bruno JJ, Lal LS, Canada TW. Effects of immune-enhancing enteral nutrition on mortality and oxygenation in acute lung injury and acute respiratory distress syndrome: a meta-analysis. Hosp Pharm. 2011;46:33–40. [Google Scholar]
- 68.Lee JM, Bae W, Lee YJ, Cho YJ. The Efficacy and Safety of Prone Positional Ventilation in Acute Respiratory Distress Syndrome: Updated Study-Level Meta-Analysis of 11 Randomized Controlled Trials. Crit Care Med. 2013 doi: 10.1097/CCM.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 69.Contopoulos-Ioannidis DG, Ioannidis JP. Claims for improved survival from systemic corticosteroids in diverse conditions: an umbrella review. Eur J Clin Invest. 2012;42:233–244. doi: 10.1111/j.1365-2362.2011.02584.x. [DOI] [PubMed] [Google Scholar]
- 70.Dwan K, Gamble C, Williamson PR, Kirkham JJ. Systematic review of the empirical evidence of study publication bias and outcome reporting bias - an updated review. PLoS One. 2013;8:e66844. doi: 10.1371/journal.pone.0066844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, Carlin B, Lowson S, Granton J. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:801–808. doi: 10.1164/rccm.2108052. [DOI] [PubMed] [Google Scholar]
- 72.Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson M, Umberger R. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 73.Carney D, DiRocco J, Nieman G. Dynamic alveolar mechanics and ventilator-induced lung injury. Crit Care Med. 2005;33:S122–128. doi: 10.1097/01.ccm.0000155928.95341.bc. [DOI] [PubMed] [Google Scholar]
- 74.Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Pasqualucci Mde O, Damasceno MC, Schultz MJ. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 75.Beitler JR, Shaefi S, Montesi SB, Devlin A, Loring SH, Talmor D, Malhotra A. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med. 2014 doi: 10.1007/s00134-013-3194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, Papazian L. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32:113–119. doi: 10.1097/01.CCM.0000104114.72614.BC. [DOI] [PubMed] [Google Scholar]
- 77.Forel JM, Roch A, Marin V, Michelet P, Demory D, Blache JL, Perrin G, Gainnier M, Bongrand P, Papazian L. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006;34:2749–2757. doi: 10.1097/01.CCM.0000239435.87433.0D. [DOI] [PubMed] [Google Scholar]
- 78.Saquib N, Saquib J, Ioannidis JP. Practices and impact of primary outcome adjustment in randomized controlled trials: meta-epidemiologic study. BMJ. 2013;347:f4313. doi: 10.1136/bmj.f4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Altemeier WA, Sinclair SE. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care. 2007;13:73–78. doi: 10.1097/MCC.0b013e32801162cb. [DOI] [PubMed] [Google Scholar]
- 80.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149:1327–1334. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 81.Siontis KC, Hernandez-Boussard T, Ioannidis JP. Overlapping meta-analyses on the same topic: survey of published studies. BMJ. 2013;347:f4501. doi: 10.1136/bmj.f4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ioannidis JP, Lau J. The impact of high-risk patients on the results of clinical trials. J Clin Epidemiol. 1997;50:1089–1098. doi: 10.1016/s0895-4356(97)00149-2. [DOI] [PubMed] [Google Scholar]
- 83.Pereira TV, Horwitz RI, Ioannidis JP. Empirical evaluation of very large treatment effects of medical interventions. JAMA. 2012;308:1676–1684. doi: 10.1001/jama.2012.13444. [DOI] [PubMed] [Google Scholar]
- 84.Ioannidis JP. Why most discovered true associations are inflated. Epidemiology. 2008;19:640–648. doi: 10.1097/EDE.0b013e31818131e7. [DOI] [PubMed] [Google Scholar]
- 85.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128:525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 86.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 87.Villar J, Perez-Mendez L, Blanco J, Anon JM, Blanch L, Belda J, Santos-Bouza A, Fernandez RL, Kacmarek RM. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting--a prospective, multicenter validation study. Intensive Care Med. 2013;39:583–592. doi: 10.1007/s00134-012-2803-x. [DOI] [PubMed] [Google Scholar]
- 88.Mills EJ, Thorlund K, Ioannidis JP. Calculating additive treatment effects from multiple randomized trials provides useful estimates of combination therapies. J Clin Epidemiol. 2012;65:1282–1288. doi: 10.1016/j.jclinepi.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 89.Doshi P, Goodman SN, Ioannidis JP. Raw data from clinical trials: within reach? Trends Pharmacol Sci. 2013;34:645–647. doi: 10.1016/j.tips.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 90.Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huh JW, Jung H, Choi HS, Hong SB, Lim CM, Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care. 2009;13:R22. doi: 10.1186/cc7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettila VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand. 2004;48:722–731. doi: 10.1111/j.0001-5172.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 93.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 94.Bone RC, Slotman G, Maunder R, Silverman H, Hyers TM, Kerstein MD, Ursprung JJ. Randomized double-blind, multicenter study of prostaglandin E1 in patients with the adult respiratory distress syndrome. Prostaglandin E1 Study Group. Chest. 1989;96:114–119. doi: 10.1378/chest.96.1.114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.