Abstract
Background
Muscle-specific RING finger protein-1 (MuRF1) is an E3 ligase that inhibits cardiac hypertrophy. However, how MuRF1 regulates cardiac hypertrophy and function during pressure overload (PO) remains poorly understood. We investigated the role of endogenous MuRF1 in regulating cardiac hypertrophy in response to PO in vivo.
Methods and Results
Transverse aortic constriction (TAC) for 4 weeks significantly reduced expression of MuRF1 in the mouse heart. After 2 and 4 weeks of TAC, MuRF1 knockout (Murf1−/−) mice exhibited enhanced cardiac hypertrophy and left ventricular (LV) dysfunction compared to non-transgenic (NTg) mice. Histological analyses showed that Murf1−/− mice exhibited more severe fibrosis and apoptosis than NTg mice after TAC. TAC-induced increases in the activity of a nuclear factor of activated T cells (NFAT) luciferase reporter were significantly greater in Murf1−/− than in NTg mice. TAC-induced increases in calcineurin A (CnA) expression were also significantly enhanced in Murf1−/− compared to in NTg mice. Co-immunoprecipitation assays showed that endogenous MuRF1 and CnA interact with one another. Polyubiquitination of CnA was attenuated in Murf1−/− mouse hearts at baseline and in response to TAC, and the protein stability of CnA was enhanced in cardiomyocytes in which MuRF1 was downregulated in vitro. Furthermore, MuRF1 directly ubiquitinated CnA in vitro. Cardiac-specific overexpression of ZAKI-4β, an endogenous inhibitor of CnA, significantly suppressed the enhancement of TAC-induced cardiac hypertrophy and dysfunction, as well as increases in cardiac fibrosis and apoptosis, in Murf1−/− mice.
Conclusions
Endogenous MuRF1 negatively regulates cardiac hypertrophy and dysfunction in response to PO through inhibition of the calcineurin-NFAT pathway.
Keywords: calcineurin, cardiac hypertrophy, E3 ubiquitin ligase, muscle atrophy, ubiquitination
Cardiac hypertrophy is an adaptive response of the heart to hemodynamic overload, as a means of both augmenting systolic function and reducing wall stress. Pathological hypertrophy, which is accompanied by cardiac dysfunction and pathological changes in the myocardium, develops in response to both pressure and volume overload or mutations in sarcomeric genes. The prolonged presence of cardiac hypertrophy is associated with an increased risk of arrhythmia, sudden death and the development of heart failure. Among a plethora of signaling pathways participating in the development of pathological hypertrophy 1, one of the most intensely characterized hypertrophic signaling cascades is the one involving calcineurin, a calcium/calmodulin-dependent serine/threonine phosphatase 2. The primary targets of calcineurin A (CnA) in the heart include the nuclear factor of activated T cells (NFAT) family. CnA dephosphorylates NFATs in the cytoplasm, resulting in their translocation to the nucleus and the subsequent development of cardiac hypertrophy 3. Although the activity of CnA is regulated primarily at the level of protein expression 4 and by endogenous inhibitors of CnA, including MCIP1 5 and CIB1 6, other mechanisms may also exist.
The muscle-specific RING finger (MuRF) family consists of RING finger E3 ubiquitin ligases expressed specifically in cardiac and skeletal muscles 7. MuRF1 is upregulated during skeletal muscle atrophy, and mice lacking MuRF1 showed a reduction in denervation-induced skeletal muscle atrophy 8. MuRF1 is also expressed in the heart and the cardiomyocytes therein. Previous studies have shown that MuRF1 negatively regulates phenylephrine (PE)-induced cardiac hypertrophy in vitro 9 and pressure overload (PO)-induced cardiac hypertrophy in vivo 10. Overexpression of MuRF1 in the heart induces wall thinning and cardiac dysfunction 11, suggesting that MuRF1 may promote heart failure when overexpressed. Recently, mutations of TRIM63, the gene encoding MuRF1, were found in patients with hypertrophic cardiomyopathy. Inducible expression of those TRIM63 mutants in the mouse heart causes cardiac hypertrophy associated with activation of mTOR-S6K and CnA signaling 12. Importantly, however, neither the molecular mechanism by which MuRF1 negatively regulates cardiac hypertrophy in response to PO nor the direct link between the TRIM63 mutation and CnA signaling has been elucidated. Furthermore, whether endogenous MuRF1 plays a protective or detrimental role in cardiac function during PO is not well understood.
In this study, we investigated the role of endogenous MuRF1 in mediating cardiac hypertrophy in response to PO. In particular, we asked whether MuRF1 plays a protective or detrimental role in PO-induced cardiac hypertrophy. In addition, we investigated the downstream signaling mechanism through which MuRF1 regulates cardiac hypertrophy. Surprisingly, contrary to the results obtained from gain-of-function animal models 11, our results obtained from a loss-of-function mouse model suggest that MuRF1 plays a protective role against PO-induced cardiac hypertrophy through downregulation of the CnA-NFAT pathway. The fact that MuRF1 is downregulated during PO suggests that downregulation of the protective mechanism may contribute to the development of pathological hypertrophy.
Methods
An expanded Methods section is available in the online-only Data Supplement.
Animals
MuRF1 knockout (Murf1−/−) mice have been described previously 8. NFAT-luciferase reporter mice and ZAKI-4β transgenic mice were generated using the α-myosin heavy chain (α-MHC) promoter 13. All animal protocols were approved by the review board of the Institutional Animal Care and Use Committee of the New Jersey Medical School.
Isolation of ubiquitinated proteins
Heart homogenate (500 μg) was incubated with Glutathione S Transferase-Tandem Ubiquitin Binding Entity 2 (GST-TUBE2, 20 mg, Life Sensors) at 4°C overnight. After adding glutathione sepharose beads, the samples were incubated at 4°C for 1 hour and then washed with RIPA buffer 5 times. Samples were boiled and subjected to SDS-PAGE analyses.
In vitro ubiquitylation assay
In vitro ubiquitylation was performed as described previously 14. In brief, 2 μM CnA (Non-tagged, Enzo Life Sciences), 100 μM Biotin-ubiquitin, 100 nM E1 enzyme, 5 μM His-UBCH5c (E2), and 1 μM GST-MuRF1 (Boston Biochem) or 1 μM bacterially expressed GST were incubated in 20 μM MOPS (pH 7.2), 100 μM KCl, 5 μM MgCl2, 5 μM ATP, 10 μM DTT, and 1 μM phenylmethylsulfonyl fluoride for 1 hour at 37°C. Samples were analyzed by SDS-PAGE and immunoblotting.
Statistical analysis
All values are expressed as mean ± SEM. Statistical comparisons between groups were conducted by exact permutation test 15 or one-way ANOVA followed by a post hoc Bonferroni-Dunn’s comparison test. A value of p < 0.05 was accepted as significant. Statistical analyses were conducted using GraphPad Prism (GraphPad Software, Inc.).
Results
MuRF1 negatively regulates cardiac hypertrophy
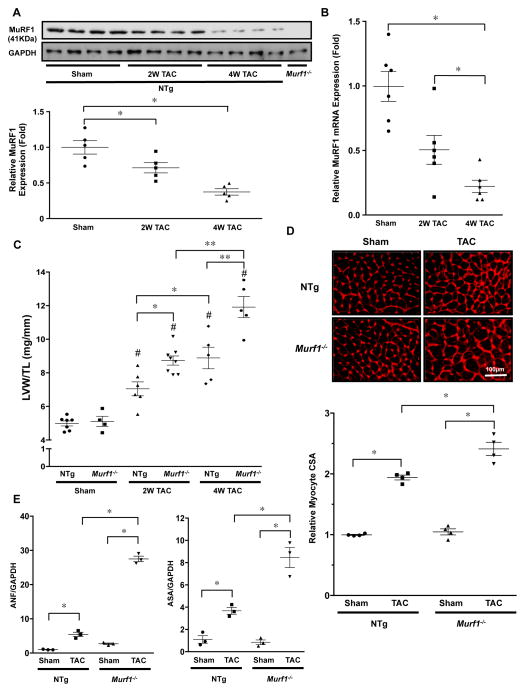
We examined the effect of PO upon protein expression of MuRF1 in the mouse heart. PO applied by TAC for 2 and 4 weeks decreased MuRF1 protein in a time-dependent manner (Figure 1A). PO also decreased mRNA expression of MuRF1 (Figure 1B). We then examined the role of endogenous MuRF1 in regulating PO-induced cardiac hypertrophy. Three-month-old non-transgenic (NTg) and Murf1−/− mice were subjected to TAC for 2 to 4 weeks. Murf1−/− mice exhibited a greater increase in left ventricular (LV) weight/tibia length (TL) than NTg mice (Figure 1C and Online Tables 1 and 2). The TAC-induced increase in cardiomyocyte cross-sectional area was also significantly greater in Murf1−/− than in NTg mice (Figure 1D). Quantitative PCR analysis showed that TAC increased mRNA expression of fetal-type genes, including atrial natriuretic factor (ANF) and α-skeletal actin (ASA), in both Murf1−/− and NTg mice. However, mRNA expression of ANF and ASA after TAC was significantly greater in Murf1−/− than in NTg mice (Figure 1E). These data suggest that a lack of MuRF1 enhances PO-induced cardiac hypertrophy. We examined the role of MuRF1 in regulating hypertrophy induced by PE, an α1-adrenergic receptor agonist, in cultured cardiomyocytes. Adenovirus-mediated upregulation of MuRF1 significantly attenuated PE-induced cardiac hypertrophy (Online Figure IA). On the other hand, MuRF1 had no effect upon IGF-I-induced cardiac hypertrophy (Online Figure IA). These results are consistent with previously reported data 16, indicating that MuRF1 plays an essential role in mediating α1-adrenergic receptor-induced, but not IGF-I-induced cardiac hypertrophy. Treatment with CK59, a CAMKII inhibitor, did not alter the PE-induced downregulation of MuRF1 protein levels in cultured cardiomyocytes, suggesting that the expression level of MuRF1 is not regulated by CAMKII (Online Figure IB).
Figure 1. Gene ablation of MuRF1 enhances pressure overload-induced cardiac hypertrophy.
A, Upper: Expression of MuRF1 in mouse hearts in response to TAC. TAC (2 and 4 weeks) or sham operation was performed on NTg mice and Murf1−/− mice. Protein expression of MuRF1 and GAPDH was evaluated by immunoblotting. Lower: The results of the quantitative analysis of MuRF1 expression are shown. * P<0.05 (N=5). B, mRNA expression of the MuRF1 gene was measured by quantitative RT-PCR. * P<0.05 (N=6). C, LV weight/TL in Murf1−/− and NTg mice 2 or 4 weeks after TAC. # P<0.05 compared to sham-operated mice with the same genotype, * P<0.05 (N=4–8). D, Upper: Wheat germ agglutinin (WGA) staining of cardiac sections 2 weeks after TAC. Lower: Quantification of myocyte cross-sectional area. * P<0.05 (N=4). E, Fetal-type gene mRNA expression 2 weeks after TAC. Left: Atrial natriuretic factor (ANF) expression. Right: α-skeletal actin (ASA) expression. * P<0.05 (N=3).
Lack of MuRF1 exacerbates cardiac dysfunction and histopathological changes in the heart in response to PO
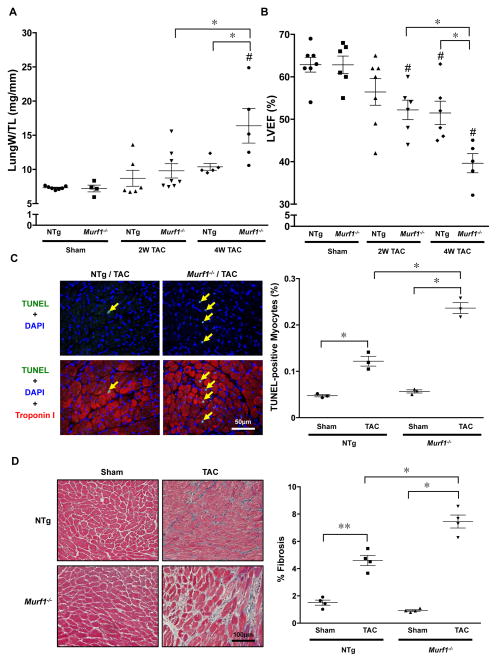
Lung weight/TL, an index of lung congestion, was significantly greater in Murf1−/− mice than in NTg mice 4 weeks after TAC (Figure 2A and Online Tables 1 and 2). Echocardiographic measurements indicated that the ejection fraction (EF) and fractional shortening (FS) were significantly lower in Murf1−/− mice than in NTg mice 2 and 4 weeks after TAC (Figure 2B and Online Tables 3 and 4). The number of TUNEL-positive nuclei was increased in both NTg and Murf1−/− mice 2 weeks after TAC compared to in sham-operated mice. However, the increase in the percentage of TUNEL-positive nuclei was significantly greater in Murf1−/− than in NTg mice (Figure 2C). Similarly, cardiac fibrosis was increased in both NTg and Murf1−/− mice, but the increase in percent fibrosis after 2 weeks of TAC was significantly greater in Murf1−/− than in NTg mice (Figure 2D). These results suggest that more severe cardiac dysfunction was induced after TAC in the absence of MuRF1.
Figure 2. Pressure overload in Murf1−/− mice causes myocardial apoptosis, cardiac fibrosis, and cardiac dysfunction after TAC.
A, Lung weight/TL in Murf1−/− and NTg mice 2 and 4 weeks after TAC. # P<0.05 compared to sham-operated mice with the same genotype, * P<0.05 (N=4–8). B, Statistical analysis of echocardiographically measured LV ejection fraction (LVEF) is shown. # P<0.05 compared to sham-operated mice with the same genotype, * P<0.05 (N=5–7). C, Left: Representative images of TUNEL staining of cardiac sections 2 weeks after TAC. Right: Percent TUNEL-positive myocytes. * P<0.05 (N=3). D, Left: Masson’s Trichrome (MT) staining of cardiac sections 2 weeks after TAC. Right: Percentage of area that is MT-positive. * P<0.05 (N=4).
MuRF1 regulates the CnA-NFAT pathway
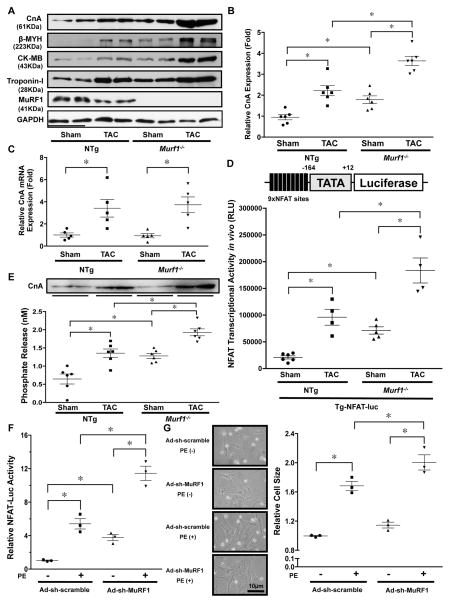
We evaluated how MuRF1 negatively affects cardiac hypertrophy in response to PO. As previously reported, the protein levels of the β-isoform of myosin heavy chain (β-MYH), creatine kinase-MB fraction (CK-MB), and Troponin I, known targets of MuRF1 10, 11, were increased by TAC in NTg mice, and the TAC-induced increase in these proteins was significantly enhanced in Murf1−/− compared to in NTg mice (Figure 3A). Interestingly, the protein level of CnA was also increased by TAC in NTg mice, and the level of CnA after TAC was significantly higher in Murf1−/− than in NTg mice (Figure 3A–B). mRNA expression of CnA was also increased significantly after TAC in both NTg and Murf1−/− hearts, but there was no significant difference between NTg and Murf1−/− hearts (Figure 3C). Based on these results, we hypothesized that MuRF1 promotes ubiquitination-mediated degradation of CnA, thereby negatively regulating cardiac hypertrophy in response to PO. To test this hypothesis, we next addressed whether MuRF1 affects the CnA-NFAT pathway in the heart under PO. To this end, we first evaluated NFAT protein expression by immunoblotting and immunostaining. Neither TAC nor ablation of MuRF1 affected the NFAT protein level (Online Figure IIA). However, TAC significantly increased nuclear staining of NFAT in NTg hearts and the TAC-induced increase in nuclear staining of NFAT was significantly enhanced in Murf1−/− hearts (Online Figure IIB). Similarly, PE-induced upregulation of NFAT in the nucleus was enhanced in the presence of MuRF1 short hairpin RNA (sh-MuRF1) in cultured cardiomyocytes (Online Figure IIC and D). We next crossed transgenic mice harboring an NFAT-luciferase reporter gene (Tg-NFAT-Luc) with Murf1−/− mice (Murf1−/−-Tg-NFAT-Luc). Tg-NFAT-Luc mice showed a significant increase in the NFAT-Luc reporter activity in the heart one week after TAC compared to after sham operation (Figure 3D). The NFAT-Luc reporter activity was significantly enhanced at baseline and in response to TAC in Murf1−/−-Tg-NFAT-Luc mice compared to in Tg-NFAT-Luc mice (Figure 3D). We also evaluated the phosphatase activity of CnA, which was normalized to the amount of total protein in the heart such that changes in activity reflect changes in CnA protein abundance. NTg mice showed a significant increase in CnA activity in the heart two weeks after TAC compared to after sham operation (Figure 3E). The CnA activity was significantly enhanced at baseline and after TAC in Murf1−/− mice compared to in NTg mice (Figure 3E). Knockdown of MuRF1 through transduction of adenovirus harboring sh-MuRF1 (Ad-sh-MuRF1) induced greater NFAT activity (Figure 3F) and a larger cell size (Figure 3G) in cultured cardiomyocytes in response to PE in vitro, suggesting that the effect of MuRF1 downregulation upon CnA activity and hypertrophy is cell-autonomous. Taken together, these results suggest that the lack of MuRF1 enhances CnA-NFAT signaling.
Figure 3. MuRF1 negatively regulates the calcineurin-NFAT pathway.
A, The protein levels of CnA, β-MYH, CK-MB, and Troponin-I at baseline and in response to TAC (4 weeks) in Murf1−/− and NTg mice. B, The results of the quantitative analysis of the CnA protein level shown in Figure 3A. * P<0.05 (N=6). C, mRNA expression of the CnA gene was measured by quantitative RT-PCR. * p<0.05 (N=5). D, Murf1−/− enhances NFAT activity at baseline and in response to TAC. Tg-NFAT-Luc mice were crossed with Murf1−/− or control mice, and each mouse group was subjected to either sham or TAC operation. After one week, NFAT-Luc activity in the heart homogenates was evaluated. * P<0.05 (N=4–6). E, Calcineurin phosphatase activity in the hearts of Murf1−/− or NTg mice subjected to either sham or TAC operation. * P<0.05 (N=6). Upper panel shows an immunoblot of the relative amounts of CnA protein in the homogenates being assessed in these experiments. F, MuRF1 inhibits phenylephrine (PE)-induced cardiac hypertrophy and NFAT activation. Cardiomyocytes were transduced with Ad-sh-MuRF1 and transfected with NFAT-luciferase reporter vector. * P<0.05 (N=3). G, Left: Representative images of neonatal rat cultured cardiomyocytes treated with PE and the indicated adenoviruses. Right: Relative cell size of myocytes treated with the indicated adenoviruses in the presence or absence of PE for 72 hours was examined. * P<0.05 (N=3).
MuRF1 plays an important role in mediating ubiquitination of CnA
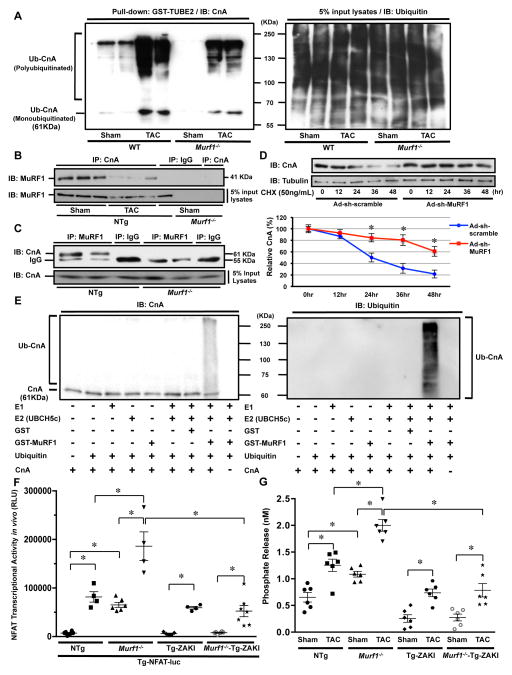
We hypothesized that MuRF1 affects the protein amount of CnA through ubiquitination. To this end, the extent of CnA ubiquitination was evaluated with the GST-Tandem Ubiquitin Binding Entity assay. Ubiquitination of CnA in the presence of PO was significantly attenuated in Murf1−/− mouse hearts (Figure 4A), suggesting that endogenous MuRF1 regulates ubiquitination of CnA in the presence of PO. To examine whether MuRF1 and CnA interact with one another, co-immunoprecipitation assays were performed. Heart homogenates prepared from NTg and Murf1−/− mice were subjected to immunoprecipitation with either an anti-CnA antibody, an anti-MuRF1 antibody or control IgG. MuRF1 was found in the CnA immunoprecipitate prepared from NTg but not Murf1−/− mouse hearts (Figure 4B). MuRF1 was not found in the control IgG immunoprecipitate. Similarly, CnA was found in the MuRF1 immunoprecipitate prepared from NTg but not Murf1−/− mouse hearts (Figure 4C). These results suggest that endogenous MuRF1 and CnA interact with one another. In addition, less MuRF1 was immunoprecipitated with CnA after TAC than after sham operation, suggesting that the interaction between CnA and MuRF1 decreases in response to TAC (Figure 4B). We next evaluated the effect of MuRF1 upon the stability of CnA, using CHX, an inhibitor of protein synthesis. Degradation of CnA was significantly slower when MuRF1 was downregulated with Ad-sh-MuRF1 in cultured cardiomyocytes, suggesting that endogenous MuRF1 is involved in CnA degradation (Figure 4D). To test whether MuRF1 directly ubiquitinates CnA, in vitro ubiquitination assays were performed using recombinant proteins. Glutathione-S-transferase (GST)-MuRF1 and UBCH5c, an E2 ubiquitin-conjugating enzyme, were incubated with biotin-ubiquitin, a ubiquitin-activating enzyme (E1), and CnA, and the reaction mixture was subjected to immunoblotting with anti-ubiquitin and anti-CnA antibodies. Ubiquitination of CnA, as assessed by the detection of high-molecular-weight polyubiquitin chains of ubiquitin and monoubiquitinated CnA, was detected only in a reaction containing E1, UBCH5c, MuRF1, and ubiquitin (Figure 4E). Elimination of any one of the four components in this reaction abolished ubiquitination of CnA. These results suggest that MuRF1 directly polyubiquitinates CnA.
Figure 4. MuRF1 plays a critical role in mediating ubiquitination of CnA.
A, Left: Heart homogenates were incubated with agarose-conjugated GST-TUBE2 to obtain ubiquitinated proteins. The samples were then subjected to immunoblot analyses with anti-CnA antibody. Right: An immunoblot of the input with anti-ubiquitin antibody. The results are representative of three experiments. B, Heart homogenates were subjected to immunoprecipitation with anti-CnA antibody or control IgG. The immunoprecipitates were immunoblotted with anti-MuRF1 antibody. C, Heart homogenates were subjected to immunoprecipitation with anti-MuRF1 antibody or control IgG. The immunoprecipitates were immunoblotted with anti-CnA antibody. D, Cultured cardiomyocytes were transduced with either Ad-sh-MuRF1 or Ad-sh-scramble. Ninety-six hours after transduction, cells were treated with cycloheximide (CHX) and then harvested after the indicated times. Immunoblot analysis was performed using anti-CnA antibody. The amount of CnA was normalized with that of α-tubulin at each time point and expressed as a percentage of the value at time 0 h. * P<0.05 vs. Ad-sh-scramble at that time point (N=3). E, In vitro ubiquitination reactions were performed to test the ubiquitin ligase activity of MuRF1. Ubiquitin conjugates of CnA were detected with anti-CnA (left panel) and anti-ubiquitin antibodies (right panel). The ubiquitination of CnA is represented by the accumulation of slower-migrating species of CnA with concomitant loss of the nonubiquitinated protein. The results are representative of three experiments. F, ZAKI-4β abrogates the enhancement of TAC-induced increases in the CnA activity in Murf1−/− mice. Tg-NFAT-Luc mice were crossed with Tg-ZAKI, Murf1−/−, Murf1−/−-Tg-ZAKI cross or control mice, and each mouse group was subjected to either sham or TAC operation. After one week, NFAT-Luc activity in the heart homogenates was evaluated. * P<0.05 (N=4–7). G, Calcineurin phosphatase activity in the hearts of Tg-ZAKI, Murf1−/−, Murf1−/−-Tg-ZAKI or NTg mice subjected to either sham or TAC operation. * P<0.05 (N=6).
Inhibition of CnA suppresses cardiac hypertrophy and dysfunction induced by MuRF1 gene disruption
To evaluate the involvement of CnA in mediating the enhancement of TAC-induced cardiac hypertrophy in Murf1−/− mice, transgenic mice with cardiac-specific overexpression of ZAKI-4β (Tg-ZAKI) 13, an endogenous inhibitor of CnA, were crossed with Murf1−/− mice (Murf1−/−-Tg-ZAKI). To evaluate whether ZAKI-4β abrogates the enhancement of CnA activity caused by MuRF1 deletion, these mice were further crossed with Tg-NFAT-Luc. One week after TAC, the NFAT-Luc reporter activity was significantly lower in Murf1−/−-Tg-ZAKI mice than in mice with Murf1−/− alone (Figure 4F). We also evaluated the phosphatase activity of CnA in the heart. The CnA activity was significantly lower in Murf1−/−-Tg-ZAKI mice than in mice with Murf1−/− alone (Figure 4G). These results indicate that ZAKI-4β effectively inhibits the enhancement of TAC-induced increases in CnA activity in Murf1−/− mice.
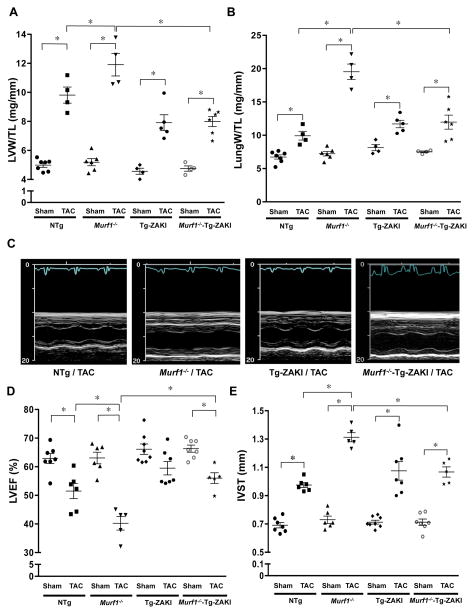
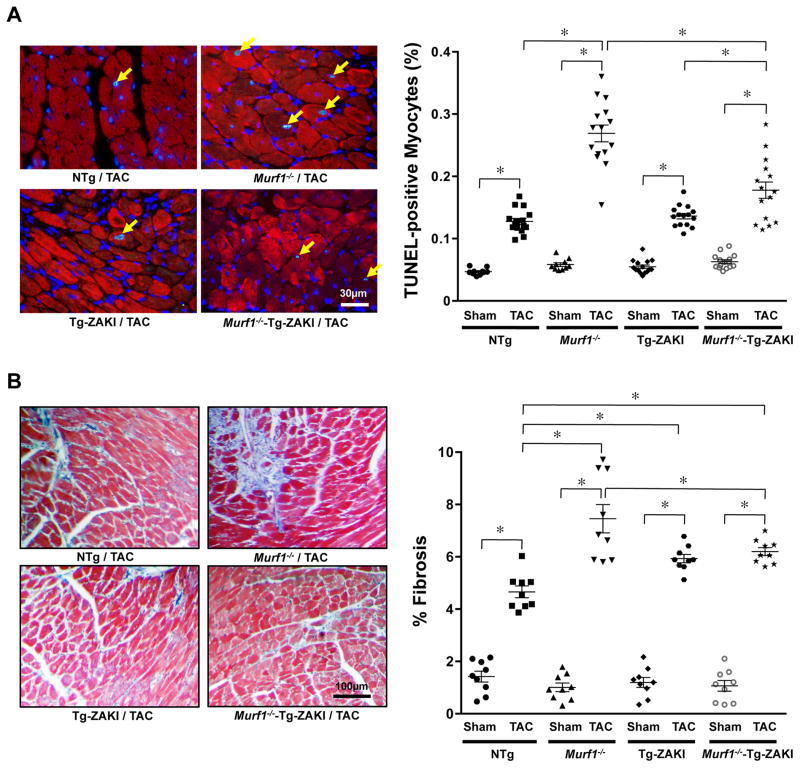
Two weeks after TAC, the enhancement of the increases in LV weight/TL and lung weight/TL in Murf1−/− mice compared to in NTg mice was normalized by ZAKI-4β expression in the Murf1−/− mice (Figure 5A and 5B). Echocardiographic analysis also demonstrated that ZAKI-4β expression in Murf1−/− mice significantly attenuated the reduction in LVEF and the enhanced increase in wall thickness after TAC observed in mice with Murf1−/− alone (Figure 5C–E). Histological analyses indicated that ZAKI-4β expression in Murf1−/− mice significantly attenuated the enhanced increase in TUNEL-positive myocytes and interstitial fibrosis after TAC seen in mice with Murf1−/− alone (Figure 6A and 6B). These results suggest that Murf1−/− enhances cardiac hypertrophy and cardiac dysfunction after TAC through CnA-dependent mechanisms.
Figure 5. Effects of inhibition of CnA on the enhancement of TAC-induced cardiac hypertrophy and cardiac dysfunction in Murf1−/− mice.
Tg-ZAKI, Murf1−/−, Murf1−/−-Tg-ZAKI or NTg mice were subjected to TAC for 4 weeks. A, Postmortem measurements of LV weight/TL. * P<0.05 (N=4–7). B, Postmortem measurements of Lung weight/TL. * P < 0.05 (N=4–7). C, Representative recordings of the M-mode echocardiography are shown. D, Statistical analysis of echocardiographically measured LVEF is shown. * P<0.05 (N=5–8). E, Statistical analysis of echocardiographically measured interventricular septum thickness (IVST) is shown. * P<0.05 (N=5–8).
Figure 6. Effects of inhibition of CnA on the enhancement of TAC-induced fibrosis and apoptosis in Murf1−/− mice.
Tg-ZAKI, Murf1−/−, Murf1−/−-Tg-ZAKI or NTg mice were subjected to TAC for 4 weeks. A, Apoptosis was evaluated with TUNEL staining. The right panel shows the results of the quantitative analysis. * P<0.05 (N=10–15). B, Interstitial fibrosis was evaluated with Masson’s Trichrome staining. The right panel shows the results of the quantitative analysis. * P<0.05 (N=9).
Discussion
Although previous studies have suggested that MuRF1 is a negative regulator of cardiac hypertrophy in response to PO 10, the mechanism by which the E3 ligase negatively regulates cardiac hypertrophy has been unknown. Our results suggest that endogenous MuRF1 is a critical upstream regulator of the CnA-NFAT pathway in the heart and that MuRF1 negatively regulates pathological cardiac hypertrophy and cardiac dysfunction in response to PO through direct downregulation of CnA.
Previous studies have shown that MuRF1 negatively regulates cardiac hypertrophy both in vitro and in vivo 10, 16 and that overexpression of MuRF1 stimulates cardiac dysfunction at baseline and in response to PO 11. However, how endogenous MuRF1 affects cardiac function under stress has not been addressed critically thus far. We confirmed that downregulation of endogenous MuRF1 enhances cardiac hypertrophy caused by PO. Importantly, however, downregulation of endogenous MuRF1 also exacerbated pathological findings in the heart, including cardiac fibrosis and apoptosis, and decreases in LVEF. Thus, our results are in marked contrast with those of the previous study by Willis et al., in which transgenic overexpression of MuRF1 in the heart induced both pathological hypertrophy and cardiac dysfunction at baseline and under PO 11. Although we do not yet know the reason for the discrepancy, it is important to point out that expression of MuRF1 is decreased during PO. Thus, it is possible that the role of endogenous MuRF1 is to inhibit pathological hypertrophy and cardiac dysfunction during PO, and that downregulation of endogenous MuRF1 during PO promotes hypertrophy and cardiac dysfunction. On the other hand, based on the results of Willis et al., excessive upregulation might also promote hypertrophy and dysfunction by some unknown mechanism. We speculate that excessive upregulation of the E3 ligase may affect additional targets that may not be targeted at lower levels of MuRF1.
Although the PO-induced downregulation of MuRF1 was significant after 2 weeks of TAC, downregulation develops gradually. Thus, the immediate early phase of PO-induced hypertrophy may be induced through MuRF1 downregulation-independent mechanisms, such as direct posttranslational modification of class II HDACs 17. On the other hand, PO-induced downregulation of MuRF1 may contribute to the development of later phase cardiac hypertrophy and heart failure.
Transcription of MuRF1 is upregulated during cardiac unloading in the heart 11, and MuRF1 is upregulated in the rat model of chronic heart failure caused by permanent coronary ligation 18. Upregulation of MuRF1 transcription is also observed during denervation-induced atrophy in skeletal muscle 8, where it is induced preferentially in fast fibers rather than slow fibers. On the other hand, MuRF1 is downregulated in the mouse model of PO 19 (and in this study), with estrogen attenuating TAC-induced downregulation of MuRF1 19. MuRF1 is upregulated by NF-κB- 20, PGC-1α- 21 and FoxO-dependent 22 mechanisms. However, the molecular mechanism mediating downregulation of MuRF1 during PO remains to be elucidated.
Previous studies have suggested that the CnA-NFAT pathway plays an important role in mediating pathological hypertrophy 23. Activation of CnA in response to hypertrophic stimuli is mediated by many mechanisms. We show that the TAC-induced increases in the activity of CnA are significantly enhanced in Murf1−/− mice, suggesting that MuRF1 is a negative regulator of CnA in the heart. We believe that the enhancement of CnA is functionally significant because the enhancement of TAC-induced cardiac hypertrophy and LV dysfunction in Murf1−/− mice was significantly attenuated when TAC-induced activation of CnA was inhibited by ZAKI-4β, an endogenous inhibitor of CnA 24.
Several lines of evidence suggest that endogenous MuRF1 directly regulates the protein stability of CnA through ubiquitination. First, downregulation of MuRF1 significantly attenuates degradation of CnA in cardiomyocytes in vitro, indicating that endogenous MuRF1 negatively regulates the stability of CnA in a cell-autonomous manner. Second, endogenous MuRF1 and CnA physically interact with one another. The fact that their interaction decreased during TAC correlates well with the increased protein level of CnA during TAC. Third, the extent of polyubiqutination of CnA after TAC is significantly attenuated in Murf1−/− mice. Finally, MuRF1 is able to directly polyubiquitinate CnA in test tubes, indicating that CnA can be a direct substrate of MuRF1.
The activity of CnA is regulated by many endogenous molecules, including MCIP1 5 and CIB1 6. However, to our knowledge, the functional involvement of MuRF1 in the regulation of CnA has not been demonstrated in the heart. It has previously been shown that MAFbx causes degradation of CnA in cardiomyocytes. However, this was demonstrated using gain-of-function experiments 25. Taking into account our recent observation using loss-of-function experiments that MAFbx mediates, rather than inhibits, cardiac hypertrophy 26, we propose that, under physiological conditions, MuRF1, rather than MAFbx, controls the level of CnA. Importantly, despite modest increases in NFAT activity in Murf1−/− mice subjected to sham operation, these mice do not show obvious cardiac hypertrophy. Thus, it is possible that, although NFAT enhances TAC-induced hypertrophy, modest elevation of NFAT alone may not be sufficient for inducing hypertrophy at baseline.
MuRF1 is localized in the M-line region and colocalized with α-actinin, one of the major components of the Z-disc in heart tissue 7. CnA is tethered to α-actinin at the Z-disc in cardiomyocytes 27. Thus, MuRF1 may regulate CnA signaling in the microdomain near the Z-disc. It has been shown that both MuRF1 and MuRF2 are required for maintenance of type-II muscle fibers, possibly through upregulation of myozenin-1, a negative regulator of calcineurin 28. Although the involvement of myozenin-1 in the regulation of CnA by MuRF1 cannot be excluded, downregulation of MuRF1 was sufficient for upregulation of calcineurin during PO in the heart.
Several other molecules act downstream of MuRF1. MuRF1 regulates PKCε through interaction with RACK1 16 and affects transcription through interaction with serum response factor 10 and glucocorticoid modulatory element binding factor-1 29. MuRF1 also regulates muscle contraction through degradation of myosin heavy chain 30, troponin I 31, and myosin binding protein C 32. In addition, MuRF1 regulates microtubule dynamics 9, myofilament structure 29, and muscle metabolism, including carbohydrate metabolism and branched chain amino acid metabolism 11. Whether these molecules located downstream of MuRF1 are also involved in the progression of pathological hypertrophy and LV dysfunction initiated by downregulation of MuRF1 remains to be determined.
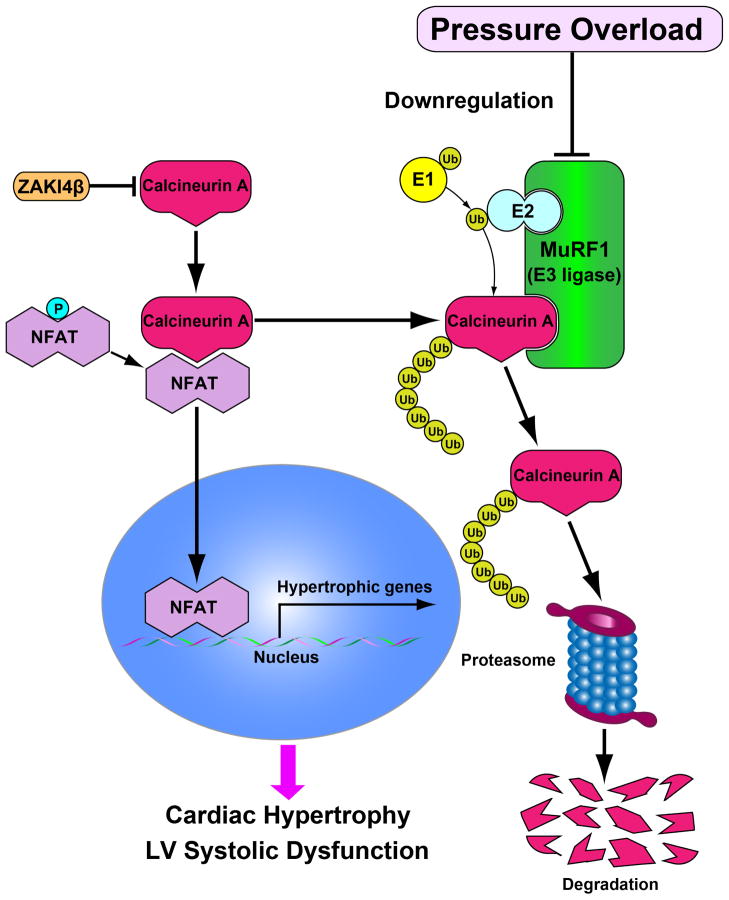
In conclusion, we have identified MuRF1 as an important negative regulator of cardiac hypertrophy (Figure 7). Since endogenous MuRF1 is downregulated during PO, a pharmacological intervention that maintains, but does not increase, the level of MuRF1 may prevent the progression of pathological hypertrophy and heart failure.
Figure 7. Hypothetical model of MuRF1 and CnA interactions in response to pressure overload (PO) in the heart.
Expression of CnA is increased but that of MuRF1 is decreased in response to PO, and MuRF1 negatively regulates pathological hypertrophy in part through proteasomal degradation of CnA.
Supplementary Material
Acknowledgments
We thank Drs. David Glass (Novartis), Jeffery Molkentin (Cincinnati Children’s Hospital) and Jeffrey Robbins (Cincinnati Children’s Hospital) for providing us with Murf1−/− mice, NFAT-Luc mice and the α-MHC promoter vector, respectively.
Sources of Funding
This work was supported by U.S. Public Health Service Grants HL67724, HL91469, HL102738, HL112330, and AG23039 (to JS), a Scientist Development Grant (12SDG12070262) from the American Heart Association (to YM), a Sakakibara Memorial Research Grant from The Japan Research Promotion Society for Cardiovascular Diseases (to YM), a Grant-in-Aid for Young Scientists B (22790697) from the Ministry of Education, Culture, Sports, Science and Technology (to SU), the Japan Heart Foundation/Novartis Grant for Research Award in molecular cellular cardiology, 2010 (to SU), and the Leducq Foundation Transatlantic Network of Excellence (to JS).
Footnotes
Disclosures
None.
References
- 1.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 2.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 4.Oka T, Dai YS, Molkentin JD. Regulation of calcineurin through transcriptional induction of the calcineurin A beta promoter in vitro and in vivo. Mol Cell Biol. 2005;25:6649–6659. doi: 10.1128/MCB.25.15.6649-6659.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothermel BA, McKinsey TA, Vega RB, Nicol RL, Mammen P, Yang J, Antos CL, Shelton JM, Bassel-Duby R, Olson EN, Williams RS. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2001;98:3328–3333. doi: 10.1073/pnas.041614798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heineke J, Auger-Messier M, Correll RN, Xu J, Benard MJ, Yuan W, Drexler H, Parise LV, Molkentin JD. CIB1 is a regulator of pathological cardiac hypertrophy. Nat Med. 2010;16:872–879. doi: 10.1038/nm.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol. 2001;306:717–726. doi: 10.1006/jmbi.2001.4448. [DOI] [PubMed] [Google Scholar]
- 8.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 9.Spencer JA, Eliazer S, Ilaria RL, Jr, Richardson JA, Olson EN. Regulation of microtubule dynamics and myogenic differentiation by MURF, a striated muscle RING-finger protein. J Cell Biol. 2000;150:771–784. doi: 10.1083/jcb.150.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis MS, Ike C, Li L, Wang DZ, Glass DJ, Patterson C. Muscle ring finger 1, but not muscle ring finger 2, regulates cardiac hypertrophy in vivo. Circ Res. 2007;100:456–459. doi: 10.1161/01.RES.0000259559.48597.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willis MS, Schisler JC, Li L, Rodriguez JE, Hilliard EG, Charles PC, Patterson C. Cardiac muscle ring finger-1 increases susceptibility to heart failure in vivo. Circ Res. 2009;105:80–88. doi: 10.1161/CIRCRESAHA.109.194928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SN, Czernuszewicz G, Tan Y, Lombardi R, Jin J, Willerson JT, Marian AJ. Human molecular genetic and functional studies identify TRIM63, encoding Muscle RING Finger Protein 1, as a novel gene for human hypertrophic cardiomyopathy. Circ Res. 2012;111:907–919. doi: 10.1161/CIRCRESAHA.112.270207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelpi RJ, Gao S, Zhai P, Yan L, Hong C, Danridge LM, Ge H, Maejima Y, Donato M, Yokota M, Molkentin JD, Vatner DE, Vatner SF, Sadoshima J. Genetic inhibition of calcineurin induces diastolic dysfunction in mice with chronic pressure overload. Am J Physiol Heart Circ Physiol. 2009;297:H1814–1819. doi: 10.1152/ajpheart.00449.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Hohfeld J, Patterson C. CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J Biol Chem. 2001;276:42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- 15.Christie D. Resampling with Excel. Teaching Statistics. 2004;26:9–14. [Google Scholar]
- 16.Arya R, Kedar V, Hwang JR, McDonough H, Li HH, Taylor J, Patterson C. Muscle ring finger protein-1 inhibits PKC{epsilon} activation and prevents cardiomyocyte hypertrophy. J Cell Biol. 2004;167:1147–1159. doi: 10.1083/jcb.200402033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 18.Adams V, Linke A, Wisloff U, Doring C, Erbs S, Krankel N, Witt CC, Labeit S, Muller-Werdan U, Schuler G, Hambrecht R. Myocardial expression of Murf-1 and MAFbx after induction of chronic heart failure: Effect on myocardial contractility. Cardiovasc Res. 2007;73:120–129. doi: 10.1016/j.cardiores.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Donaldson C, Eder S, Baker C, Aronovitz MJ, Weiss AD, Hall-Porter M, Wang F, Ackerman A, Karas RH, Molkentin JD, Patten RD. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ Res. 2009;104:265–275. doi: 10.1161/CIRCRESAHA.108.190397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li HH, Willis MS, Lockyer P, Miller N, McDonough H, Glass DJ, Patterson C. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J Clin Invest. 2007;117:3211–3223. doi: 10.1172/JCI31757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 24.Kingsbury TJ, Cunningham KW. A conserved family of calcineurin regulators. Genes Dev. 2000;14:1595–1604. [PMC free article] [PubMed] [Google Scholar]
- 25.Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, Patterson C. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest. 2004;114:1058–1071. doi: 10.1172/JCI22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Usui S, Maejima Y, Pain J, Hong C, Cho J, Park JY, Zablocki D, Tian B, Glass DJ, Sadoshima J. Endogenous muscle atrophy F-box mediates pressure overload-induced cardiac hypertrophy through regulation of nuclear factor-kappaB. Circ Res. 2011;109:161–171. doi: 10.1161/CIRCRESAHA.110.238717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frey N, McKinsey TA, Olson EN. Decoding calcium signals involved in cardiac growth and function. Nat Med. 2000;6:1221–1227. doi: 10.1038/81321. [DOI] [PubMed] [Google Scholar]
- 28.Moriscot AS, Baptista IL, Bogomolovas J, Witt C, Hirner S, Granzier H, Labeit S. MuRF1 is a muscle fiber-type II associated factor and together with MuRF2 regulates type-II fiber trophicity and maintenance. J Struct Biol. 2010;170:344–353. doi: 10.1016/j.jsb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McElhinny AS, Kakinuma K, Sorimachi H, Labeit S, Gregorio CC. Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1. J Cell Biol. 2002;157:125–136. doi: 10.1083/jcb.200108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6:376–385. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Kedar V, McDonough H, Arya R, Li HH, Rockman HA, Patterson C. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci U S A. 2004;101:18135–18140. doi: 10.1073/pnas.0404341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mearini G, Gedicke C, Schlossarek S, Witt CC, Kramer E, Cao P, Gomes MD, Lecker SH, Labeit S, Willis MS, Eschenhagen T, Carrier L. Atrogin-1 and MuRF1 regulate cardiac MyBP-C levels via different mechanisms. Cardiovasc Res. 2010;85:357–366. doi: 10.1093/cvr/cvp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.