Abstract
The Seveso Women’s Health Study (SWHS) is a historical cohort study of the female population residing near Seveso, Italy, on July 10, 1976, when a chemical explosion resulted in the highest known residential exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Individual TCDD concentration was measured in serum collected near the time of the explosion, and in 1996, we collected adequate blood for TCDD and total dioxin toxic equivalent (TEQ) measurement.
Polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls were measured in 1996 serum for a sample (n=225, 23%) of the SWHS cohort and WHO 2005 TEQs were calculated. We examined characteristics that predict 1996 TCDD concentrations and estimated TCDD elimination half-life over the 20 year period since the explosion.
Median lipid-adjusted TCDD and total TEQ concentrations in 1996 serum were 7.3 and 26.2 ppt, respectively. Initial 1976 TCDD and age at explosion were the strongest predictors of 1996 TCDD. The TCDD elimination half-life was 7.1 years for women older than 10 years in 1976, but was shorter in those who were younger.
Twenty years after the explosion, TCDD concentrations in this SWHS sample, the majority of who were children in 1976, remain elevated relative to background. These data add to the limited data available on TCDD elimination half-life in children.
Keywords: dioxin, half-life, TCDD, TEQ, PCDD, PCDF
INTRODUCTION
Polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and biphenyls (PCBs) constitute a group of polyhalogenated aromatic hydrocarbons produced by chemical reactions and combustion processes (1, 2). These environmental contaminants are highly lipophilic, resist metabolism, and bioaccumulate; the general population of most countries has some level of background exposure (1–3). The most toxic congener, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), is a known human carcinogen and has been shown to disrupt multiple endocrine pathways in animals (4–10).
On July 10, 1976, as a result of a chemical explosion, residents of Seveso, Italy experienced among the highest human exposure concentrations to TCDD ever recorded (11). Up to 30 kilograms of TCDD were deposited over the surrounding 18-km2 area, which was divided into exposure zones (A, B, R, non-ABR) based on surface soil TCDD measurements (12), but no individual-level TCDD exposure data were initially available. As evidence of the significant level of TCDD exposure, 193 cases of chloracne were reported among residents of the area, mostly among children (13). In the late 1980s, a method for measuring TCDD in serum became available (14). A limited analysis of pooled 1976 serum samples revealed residents were highly exposed to TCDD, but not to other PCDDs and PCDFs (15).
In 1996, we initiated the Seveso Women’s Health Study (SWHS), a historical cohort study of the female population residing around Seveso at the time of the explosion in 1976 (16). The SWHS cohort includes 981 women who were newborn to 40 years of age in 1976 and resided in the most exposed zones, A or B. Individual TCDD concentration was measured in serum collected shortly after the explosion, but the volume was inadequate to measure individual-level total dioxin toxic equivalents (TEQ). The median lipid-adjusted serum TCDD concentration for the SWHS cohort was 56 ppt, but there was a wide range of TCDD concentrations (2.5–56,000 ppt) and the youngest women were the highest exposed (17). Zone of residence and younger age at explosion were the strongest predictors of 1976 TCDD concentrations (17). In 1996, we collected adequate blood for total TEQ measurement.
Here we present TCDD and total TEQ concentrations in serum collected 20 years after the explosion for a sample of the SWHS cohort. We examine characteristics that predict TCDD and total TEQ concentrations measured in serum collected in 1996 and estimate the TCDD elimination half-life over the 20 year period. Although other studies have estimated TCDD elimination half-life, most have included occupationally-exposed adult males with TCDD measures several years after last exposure (18, 19). The SWHS represents a unique population-based female cohort with individual-level TCDD measurements near the explosion, or time of highest exposure. To our knowledge, there is no other population with serial measures of TCDD collected from the time of highest/initial exposure that has been as well characterized. For this sample, the majority were children at the time of highest exposure (<18 years in 1976) and about 40% were < 10 years, groups under-represented with respect to half-life studies.
METHODS
Study Population
Details of the SWHS study design are presented elsewhere (16). Briefly, eligible women were newborn to age 40 years in 1976, resided in the most highly contaminated areas at time of explosion, and had adequate stored sera collected soon after the explosion. Enrollment took place from March 1996 to July 1998 and 981 women (80% of eligible) participated. Between April 2008 and December 2009, we conducted a follow-up study of the SWHS cohort. For the present analysis, we included 225 women who reported a live birth after January 1, 1994 (either at enrollment in 1996–98 or follow-up in 2008–09), had a 1976 serum TCDD concentration, and had archived serum collected in 1996. Compared to the full cohort, this study sample was younger at explosion.
Procedure
The study was approved by the Institutional Review Boards of the participating institutions. Details of the study procedure for the initial study (1996–1998) and the follow-up study (2008–2009) are presented elsewhere (16, 20). Briefly, participation included signed informed consent, fasting blood draw, anthropometric measurements (including height (cm) and weight (kg)), a personal interview, and a review of medical records. The initial study also included a gynecologic examination and ultrasound, while the follow-up study included blood pressure measurements, a memory test, and for a subset, a bone density examination.
For both the initial and follow-up study, interviews were conducted in private by trained nurse-interviewers who were unaware of the zone of residence and serum TCDD concentrations of the participants. During the interviews, a detailed reproductive and medical history was recorded and information on demographic and lifestyle factors was collected.
Laboratory Analyses
All laboratory analyses were performed at the Centers for Disease Control and Prevention. Individual TCDD concentration was previously measured in archived sera collected in 1976 or 1977 by high-resolution gas chromatography/high-resolution mass spectrometry methods (14). Total lipid content of each specimen was estimated using a “summation” method (21) and analytical results for TCDD were reported on a lipid-adjusted basis as pg/g lipid or parts per trillion (ppt).
A 15-mL archived serum sample collected during the initial SWHS study (1996–1998) was analyzed for 7 PCDDs, 10 PCDFs, 4 coplanar PCBs (c-PCBs; PCB 77, 81, 126, 169), and 8 mono-ortho PCBs (m-PCBs; PCB 105, 114, 118, 123, 156, 157, 167, 189) by high-resolution gas chromatography/isotope-dilution high-resolution mass spectrometry methods (22, 23). Total lipid content was estimated (21) and analytical results were reported on a lipid-adjusted basis as pg/g lipid or ppt for PCDDs, PCDFs, and c-PCBs, and ng/g lipid or parts per billion (ppb) for m-PCBs. Quantifiable results less than the respective method detection limits were reported when observed. For individual analytes below the limit of detection (LOD), a value equal to one-half the LOD was assigned (24).
TEQs were calculated separately for PCDDs (PCDD -TEQ), PCDFs (PCDF -TEQ), c-PCBs (c -PCB-TEQ), m-PCBs (m -PCB-TEQ), and total TEQ based on the 2005 WHO-Toxicity Equivalency Factor (TEF) system (25). Congeners with < 50% of samples above LOD were excluded from TEQ calculations. As a sensitivity analysis, TEQ concentrations were calculated including congeners with <50% above the LOD and the results did not change.
Statistical Analyses
Statistical analyses were performed using STATA 11.0 (26). The distributions of individual 1976 and 1996 TCDD and 1996 total TEQ data were initially examined graphically and with standard descriptive statistics. Because the distributions were right skewed and approximately log-normal, serum TCDD and total TEQ were log10-transformed. Analysis of variance was used to examine the relation of 1976 TCDD, 1996 TCDD, and 1996 total TEQ concentrations with covariates. We used multiple linear regression to determine predictors of 1996 TCDD and total TEQ concentrations and TCDD elimination half-life including exposure-related covariates such as initial 1976 TCDD and age at explosion, and elimination-related covariates assessed at the time of serum collection in 1996, such as age, body mass index (BMI), parity, lactation, and smoking history. For all regression models, standard errors were estimated using the robust Huber-White sandwich estimator.
We calculated the TCDD elimination half-life for each woman based on her TCDD concentrations measured in 1976 and 1996 serum, assuming a first-order kinetic model and the exact number of days between the two fasting blood draws. For 15 (6.7%) women with 1996 TCDD concentrations below the LOD, half-life was calculated by conservatively assuming the 1996 TCDD concentration was equal to the LOD, since assuming a value of LOD/2 would reduce the individual half-life estimate. In sensitivity analyses, we reassigned the 1996 TCDD concentration for these 15 women a value of LOD/2 and the overall results did not change. We graphically present the relation of TCDD elimination half-life to age at explosion, after controlling for initial 1976 TCDD concentration, by means of a robust nonparametric lowess curve. To adjust for initial 1976 TCDD concentration, the lowess curve is plotted against the residuals of the regression of 1996 TCDD concentrations with 1976 TCDD concentrations.
RESULTS
The distribution of serum TCDD and TEQ concentrations for the SWHS sample (n=225) by select characteristics at explosion and follow-up are presented in Table 1. In 1976, the median serum TCDD concentration was 105 ppt and ranged from 12.7 to 56,000 ppt (see Eskenazi et al. (17) for the descriptive statistics for the entire cohort). Twenty years later in 1996, the median (range) serum TCDD and total TEQ concentrations were 7.3 (0.4 – 175) and 26.2 (9.2 – 216.8) ppt, respectively. On the date of the explosion in 1976, the average (range) age of the 225 women was 10.2 (0–27) years and 61% were premenarche. At follow-up in 1996, women averaged 30.6 years, over half were parous (59%), 46% had breastfed, and average BMI was 22.2 kg/m2. As reported for the full cohort (17), in this subsample, 1976 TCDD concentrations were highest among women who resided in Zone A, reported a chloracne diagnosis or animal mortality on their property, or were youngest (<10 years) or premenarche at the time of the explosion. Twenty years later, TCDD and total TEQ concentrations were still highest among Zone A residents, and those reporting a chloracne diagnosis or animal mortality. In contrast, both 1996 TCDD and total TEQ concentrations were lowest among women who were youngest at explosion (<10 years).
Table 1.
Distribution of serum TCDD (1976 and 1996–1998) and TEQ (1996–1998) concentrations by characteristics at explosion and follow-up for the sample of SWHS cohort (n=225), Seveso, Italy.
| 1976 TCDD
|
1996 TCDD
|
1996 TEQ
|
||
|---|---|---|---|---|
| Characteristic | n (%) | Med (IQR)a | Med (IQR)a | Med (IQR)a |
| Total | 225 (100) | 105.0 (47–263) | 7.3 (4–14) | 26.2 (20–36) |
| Characteristics at Explosion (1976) | ||||
| Zone of residence*,†,‡ | ||||
| B | 176 (78) | 75.2 (44–166) | 6.7 (4–11) | 23.9 (19–31) |
| A | 49 (22) | 455.0 (174–1060) | 22.3 (8–37) | 42.4 (25–59) |
| Age (years)*,‡ | ||||
| 0–5 | 50 (22) | 245.5 (130–460) | 6.4 (4–13) | 22.6 (18–35) |
| 6–10 | 63 (28) | 144.0 (56–276) | 7.0 (5–14) | 24.0 (19–34) |
| 11–13 | 60 (27) | 69.9 (42–115) | 7.6 (5–13) | 26.5 (23–35) |
| > 13 | 52 (23) | 61.6 (32–167) | 7.9 (4–20) | 29.1 (22–45) |
| Menarche status*,‡ | ||||
| Premenarche | 138 (61) | 163.0 (68–339) | 7.3 (4–13) | 24.2 (19–34) |
| Postmenarche | 87 (39) | 59.4 (31–117) | 7.3 (5–14) | 28.4 (21–42) |
| Chloracne diagnosedb,*,†,‡ | ||||
| No | 204 (91) | 86.0 (47–213) | 7.0 (4–12) | 24.9 (20–34) |
| Yes – Self or Family | 19 (9) | 1240.0 (144–5710) | 36.5 (9–63) | 50.2 (31–79) |
| Animal mortality on propertyb,*,†,‡ | ||||
| None | 182 (81) | 85.0 (45–212) | 6.9 (4–12) | 25.1 (20–33) |
| Any | 34 (15) | 269.5 (87–553) | 14.7 (7–24) | 35.5 (24–50) |
| Don’t Know | 9 (4) | 54.3 (48–196) | 9.6 (6–12) | 24.3 (22–36) |
| Characteristics at Follow-Up (1996–1998) | ||||
| Age (years)* | ||||
| 20–25 | 45 (20) | 247.0 (130–464) | 5.7 (4–13) | 22.1 (17–31) |
| 26–30 | 60 (27) | 164.5 (66–304) | 7.5 (5–15) | 24.9 (20–36) |
| 31–33 | 61 (27) | 73.8 (42–120) | 7.6 (6–13) | 26.4 (23–34) |
| > 33 | 59 (26) | 60.4 (31–135) | 7.1 (4–16) | 28.0 (20–45) |
| Post-explosion parity* | ||||
| 0 | 92 (41) | 207.5 (82–403) | 7.6 (4–16) | 26.3 (19–36) |
| 1 | 77 (34) | 70.0 (45–179) | 6.8 (5–13) | 24.4 (20–36) |
| ≥ 2 | 56 (25) | 63.3 (32–120) | 7.3 (4–13) | 27.5 (21–37) |
| Post-explosion lactation (months)* | ||||
| None | 122 (54) | 158.5 (62–389) | 7.6 (4–15) | 26.9 (21–36) |
| ≤ 6 | 64 (29) | 68.3 (42–177) | 6.8 (5–14) | 26.1 (21–40) |
| > 6 | 39 (17) | 62.1 (40–112) | 6.9 (4–11) | 24.2 (19–28) |
| Body mass index (kg/m2)† | ||||
| ≤ 18.5 | 20 (9) | 143.0 (61–340) | 5.8 (3–9) | 22.3 (17–27) |
| 18.6–24.9 | 166 (74) | 102.0 (47–251) | 7.4 (5–14) | 26.4 (21–36) |
| 25.0–29.9 | 28 (12) | 68.0 (46–259) | 7.9 (5–15) | 29.9 (20–40) |
| ≥ 30.0 | 11 (5) | 107.0 (28–126) | 7.6 (5–26) | 29.7 (20–62) |
| Cigarette smoking | ||||
| Never | 137 (61) | 112.0 (50–238) | 7.3 (5–13) | 25.7 (21–36) |
| Former | 37 (16) | 72.6 (42–212) | 5.2 (4–9) | 22.4 (16–31) |
| Current | 51 (23) | 130.0 (47–389) | 9.4 (6–17) | 28.4 (22–39) |
Med = median; IQR = interquartile range.
Numbers do not add to 100% due to missing data.
p < 0.05 for log10[1976-TCDD]
p < 0.05 for log10[1996-TCDD]
p < 0.05 for log10[1996-TEQ]
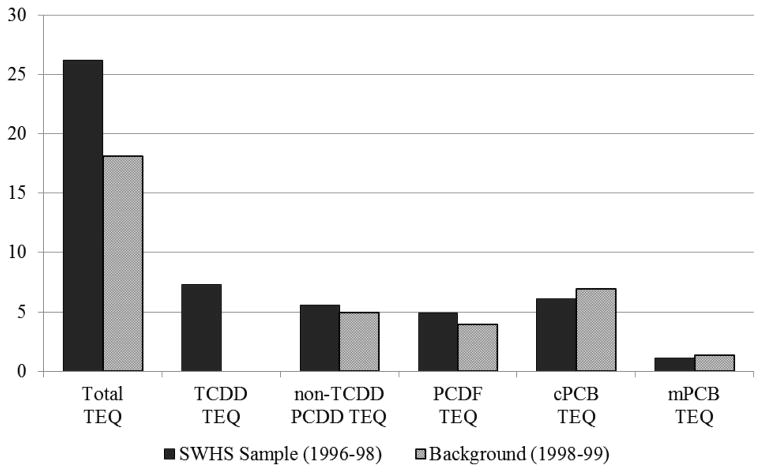
Serum concentrations and corresponding WHO 2005 TEQs for PCDDs, PCDFs, and PCBs for the 225 women from bloods drawn in 1996–98 are summarized in Table 2. The congeners with the largest individual contribution to total TEQ were TCDD (32.7%), followed by PCB 126 (19.3%), 2,3,4,7,8-PeCDF (15.0%), 1,2,3,7,8-PeCDD (10.5%), 1,2,3,6,7,8-HxCDD (5.5%), and PCB 169 (5.2%). The predominant congeners in terms of concentration were OCDD and 1,2,3,4,6,7,8-HpCDD among dioxins, 2,3,4,7,8-PeCDF among furans, and PCB 126 and PCB169 among PCBs. Overall the largest contribution to the total TEQ was made by PCDDs (51.7%), followed by c-PCBs (24.55%), PCDFs (19.2%), and m-PCBs (4.5%). Figure 1 illustrates the relative TEQ concentrations of TCDD, non-TCDD PCDDs, PCDFs, and dioxin-like PCBs (c-PCBs and m-PCBs) compared to similar-aged females with background exposure (27). As illustrated in Figure 1, the elevated total TEQ observed in the SWHS sample can be explained almost entirely by elevated TCDD.
Table 2.
Summary of serum PCDDs/PCDFs/c-PCBs/m-PCBs concentrations and 2005 WHO TEQs for a sample of the SWHS cohort (n=225), Seveso, Italy, 1996–98.
| Analyte | WHO 2005 TEF | Average Detection Limit | % Above Detection Limit | Concentrationa Median (range) | WHO-TEQ05 (pg TEQ/g lipid) Median (IQR) |
|---|---|---|---|---|---|
| PCDDs (pg/g lipid) | |||||
| 2378-TCDD | 1 | 1.0 | 93 | 7.3 (0.4–175.0) | 7.3 (4.4–13.7) |
| 12378-PeCDD | 1 | 1.0 | 63 | 3.6 (0.3–15.4) | 3.6 (0.6–4.8) |
| 123478-HxCDD | 0.1 | 1.5 | 56 | 1.6 (0.4–6.5) | 0.2 (0.1–0.2) |
| 123678-HxCDD | 0.1 | 1.4 | 99 | 13.8 (0.6–50.6) | 1.4 (1.1–1.7) |
| 123789-HxCDD | 0.1 | 1.5 | 80 | 3.0 (0.5–12.7) | 0.3 (0.2–0.4) |
| 1234678-HpCDD | 0.01 | 1.7 | 100 | 20.8 (2.6–97.1) | 0.2 (0.2–0.3) |
| OCDD | 0.0003 | 33.9 | 100 | 288.0 (74.7–1790) | 0.1 (0.1–0.1) |
| PCDFs (pg/g lipid) | |||||
| 2378-TCDF | 0.1 | 5.0 | 0 | 2.6 (0.4–7.7) | --- (--) |
| 12378-PeCDF | 0.03 | 1.0 | 2 | 0.5 (0.3–4.1) | --- (--) |
| 23478-PeCDF | 0.3 | 1.0 | 100 | 12.5 (1.3–41.3) | 3.8 (3.0–4.7) |
| 123478-HxCDF | 0.1 | 1.0 | 94 | 4.1 (0.3–10.0) | 0.4 (0.3–0.5) |
| 123678-HxCDF | 0.1 | 1.0 | 94 | 4.9 (0.3–15.0) | 0.5 (0.4–0.6) |
| 123789-HxCDF | 0.1 | 1.0 | 0 | 0.5 (0.3–3.8) | --- (--) |
| 234678-HxCDF | 0.1 | 1.0 | 62 | 1.7 (0.3–6.0) | 0.2 (0.1–0.2) |
| 1234678-HpCDF | 0.01 | 1.1 | 97 | 5.4 (0.5–24.4) | 0.1 (0.0–0.1) |
| 1234789-HpCDF | 0.01 | 1.1 | 0 | 0.5 (0.3–4.8) | --- (--) |
| OCDF | 0.0003 | 1.7 | 5 | 0.8 (0.5–12.5) | --- (--) |
| c-PCBs (pg/g lipid) | |||||
| PCB 77 | 0.0001 | 2.3 | 100 | 42.0 (9.4–609.0) | 0.0 (0.0–0.0) |
| PCB 81 | 0.0003 | 2.4 | 96 | 6.8 (1.8–44.9) | 0.0 (0.0–0.0) |
| PCB 126 | 0.1 | 2.1 | 100 | 46.4 (13.6–151.0) | 4.6 (3.6–6.3) |
| PCB 169 | 0.03 | 2.0 | 100 | 42.1 (13.8–160.0) | 1.3 (1.0–1.7) |
| m-PCBs (ng/g lipid) | |||||
| PCB 105 | 0.00003 | 1.0 | 100 | 2.8 (0.4–11.9) | 0.08 (0.07–0.11) |
| PCB 114 | 0.00003 | 1.0 | 26 | 0.7 (0.3–3.9) | --- (--) |
| PCB 118 | 0.00003 | 1.0 | 100 | 17.8 (8.2–86.7) | 0.53 (0.44–0.71) |
| PCB 123 | 0.00003 | 1.0 | 1 | 0.5 (0.1–1.9) | --- (--) |
| PCB 156 | 0.00003 | 1.0 | 100 | 8.3 (2.4–32.0) | 0.25 (0.19–0.33) |
| PCB 157 | 0.00003 | 1.0 | 95 | 2.1 (0.6–8.9) | 0.06 (0.05–0.08) |
| PCB 167 | 0.00003 | 1.0 | 96 | 4.0 (0.5–17.1) | 0.12 (0.09–0.16) |
| PCB 189 | 0.00003 | 1.0 | 67 | 1.2 (0.4–9.8) | 0.04 (0.03–0.05) |
| PCDD TEQ | 13.2 (9.4–20.7) | ||||
| PCDF TEQ | 4.9 (4.0–6.1) | ||||
| c-PCB TEQ | 6.1 (4.9–7.7) | ||||
| m-PCB TEQ | 1.1 (0.9–1.4) | ||||
| Total TEQ | 26.2 (20.3–36.0) | ||||
Samples below LOD were assigned a value of ½ LOD.
Figure 1.
Median serum TEQ concentrations (ppt) of PCDDs, PCDFs, and dioxin-like PCBs in 1996 serum for the SWHS sample compared with concentrations in 1998 serum for Italian women with background exposure, Seveso, Italy, 1996–1998.
The multivariate prediction models for TCDD and total TEQ concentrations in 1996 serum and TCDD elimination half-life are presented in Table 3. Age at explosion and log10[1976-TCDD] were the strongest predictors of log10[1996-TCDD], together explaining 46.0% of the total variance. Adding smoking, BMI, days from explosion to initial blood sample, and time between 1976 and 1996 blood samples improved the prediction model only slightly, with the final model explaining 47.9% of the total variance (see Table 3a). The strongest predictors of log10[1996-TEQ] were also age at explosion and log10[1976-TCDD], but explained less of the total variance (36.9%). Adding other variables, i.e., duration of lactation, smoking history and oral contraceptive use (see Table 3b), improved the prediction model, with 45.0% of the total variance explained by the final model. Likewise, the strongest predictors of TCDD elimination half-life were log10[1976-TCDD] and age at explosion, which together explained 52.4% of the total variance. When BMI and pack-years of smoking were added to the multivariate model (see Table 3c), 54.0% of the total variance was explained.
Table 3.
Predictors of TCDD and total TEQ concentrations in 1996 serum for a sample of the SWHS cohort (n=225), Seveso, Italy, 1996–1998.
| a. Dependent variable: log10[1996-TCDD]
| |||
|---|---|---|---|
| Independent variable | Adjusteda β (95% CI) | p-value | Partial r2(%) |
| log10[1976-TCDD] | 0.546 (0.462, 0.630) | < 0.001 | 46.2 |
| Age at explosion (years) | 0.027 (0.018, 0.035) | < 0.001 | 14.0 |
| Smoking (pack-years) | −0.017 (−0.033, 0.000) | 0.051 | 2.7 |
| BMI in 1996 (kg/m2) | 0.009 (−0.001, 0.019) | 0.069 | 1.1 |
| b. Dependent variable: log10[1996-TEQ]
| |||
|---|---|---|---|
| Independent variable | Adjusteda β (95% CI) | p-value | Partial r2(%) |
| log10[1976-TCDD] | 0.210 (0.163, 0.256) | < 0.001 | 36.0 |
| Age at explosion (years) | 0.020 (0.015, 0.024) | < 0.001 | 27.4 |
| Duration of lactation (months) | −0.006 (−0.011, −0.002) | 0.005 | 4.9 |
| Smoking (pack-years) | −0.010 (−0.016, −0.004) | 0.001 | 4.6 |
| Oral contraceptive use (years) | 0.007 (0.001, 0.012) | 0.023 | 2.7 |
| c. Dependent variable: log10[TCDD half-life]
| |||
|---|---|---|---|
| Independent variable | Adjusteda β (95% CI) | p-value | Partial r2(%) |
| log10[1976-TCDD] | −0.170 (−0.197, −0.144) | < 0.001 | 40.6 |
| Age at explosion (years) | 0.010 (0.007, 0.013) | < 0.001 | 15.7 |
| BMI (kg/m2) | 0.003 (−0.000, 0.007) | 0.064 | 1.1 |
| Smoking (pack-years) | −0.006 (−0.011, −0.001) | 0.024 | 2.6 |
Adjusted for time (days) between explosion and 1976 blood collection and between 1976 and 1996 blood collections and variables in the list (total adjusted R2 = 0.479)
Adjusted for time (days) between explosion and 1976 blood collection and between 1976 and 1996 blood collections and variables in the list (total adjusted R2 = 0.450)
Adjusted for time (days) between explosion and 1976 blood collection and between 1976 and 1996 blood collections and variables in the list (total adjusted R2 = 0.540)
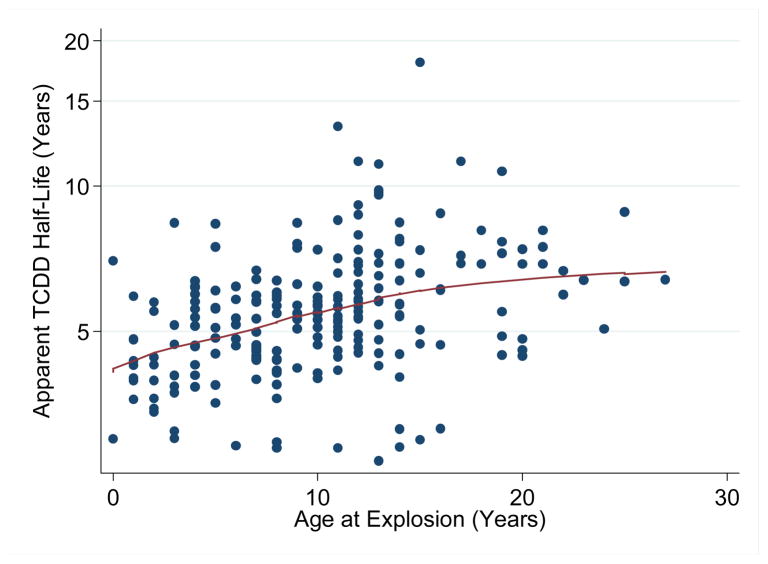
Overall, the TCDD elimination half-life for the SWHS sample averaged 5.9 (±2.7) years and ranged from 1.9 to 22 years. The TCDD elimination half-life varied significantly with age at explosion, after adjusting for initial 1976 serum concentrations. For women who were older than 10 years in 1976, TCDD elimination half-life averaged 7.1 (±3.0) years, but it was significantly shorter in younger age groups (<5 years = 4.3 (±1.7) years; 6–10 years = 5.2 (±1.7) years) (p-value<0.001). Figure 2 illustrates a lowess plot of the relationship between log10[1976-TCDD]-adjusted TCDD elimination half-life and age at explosion.
Figure 2.
Lowess plot of the relatonship between log10[1976-TCDD]-adjusted TCDD elimination half-life and age at explosion for a sample of the SWHS cohort (n=225), Seveso, Italy, 1996–1998.
DISCUSSION
We measured individual TCDD and total TEQ concentrations in serum collected approximately twenty years after the 1976 explosion for a sample of the Seveso Women’s Health Study cohort. TCDD concentrations measured in 1996 serum were significantly reduced compared to the concentrations previously measured in 1976 serum, but remain elevated relative to background (27). Total TEQ concentrations in 1996 serum were also elevated, and a large proportion of the serum TEQ concentrations was due to TCDD. Initial 1976 serum TCDD concentration and age at explosion were significant predictors of 1996 serum TCDD concentrations. The TCDD elimination half-life for those who were older than 10 years of age at the time of the explosion was 7.1 years, but was shorter in those who were younger.
Compared to the general population, serum TCDD concentrations in SWHS remain significantly elevated twenty years after the explosion. TCDD and total TEQ concentrations measured in 1996 serum were significantly higher than concentrations reported for 78 similar-aged female Italian residents with background exposure in 1998–1999 serum (median TCDD = 1.6 pg/g lipid and median TEQ05 = 18.1 pg/g lipid) (27). Worldwide general population concentrations of TCDD and total TEQ05 for the period 1989–2010 are also lower, estimated to be about 1.3 pg/g lipid and 12.5 pg/g lipid, respectively (3). Given that concentrations have been declining rapidly during the 21-year period, these likely underestimate historical concentrations and overestimate current concentrations (3). Concentrations of other dioxin-like compounds measured in 1996 serum including PCDDs, PCDFs, and dioxin-like PCBs, however, were not elevated above background (3, 27), confirming that the increase in total TEQ in SWHS was mainly due to TCDD.
The 1996 serum TCDD concentrations in SWHS are consistent with those reported in a study of Seveso residents from Zones A, B, and non-ABR about 17 years after the explosion (28). PCDDs, PCDFs, and c-PCBs were measured in plasma collected between December 1992 and March 1994 for a sample of residents (n=110). TCDD was detected in 79 percent of samples. Among females (n=56), TCDD concentrations ranged from 45.3 to 80.7 ppt for Zone A (n=2), from 1.3 to 62.6 ppt for Zone B (n=26) and 1.8 to 18.1 ppt for Zone non-ABR (n=28), compared to SWHS, where concentrations ranged from 0.6 to 175 ppt for Zone A and 0.4 to 43 ppt for Zone B. Likewise, in both studies, no other congeners were significantly elevated above background.
In the U.S. general population, the percent contribution to the total TEQ for women age 20–59 years using the 2005 TEFs was 62% PCDDs, 22% for c-PCBs, 14% for PCDFs and 4% for m-PCBs (29). A similar pattern has been reported in other populations with background exposure (3). We also found a similar pattern with the largest contribution to the total TEQ made by PCDDs, followed by c -PCBs and PCDFs, then m-PCBs. However, unlike other populations with background exposure where TCDD contributes only a very small percentage to total TEQ, we found that TCDD contributed 33% to total TEQ. Except for TCDD, the other individual congeners comprising the highest percentage of the total TEQ were PCB 126, 2,3,4,7,8-PeCDF, and 1,2,3,7,8-PeCDD, similar to those reported in the general population (3, 29, 30).
We found the strongest predictor of 1996 serum TCDD was initial 1976 serum TCDD concentration. In addition, age, BMI, and smoking were significant predictors of 1996 TCDD. In populations with background exposure, the most important determinant of TCDD and total TEQ concentrations is age, reflecting accumulation in the body not balanced by corresponding elimination (3, 29, 30). Landi et al. (28) also reported age, BMI, and smoking were significant predictors of plasma TCDD in Zone B. We also found the strongest predictors of 1996 serum total TEQ were initial 1976 serum TCDD concentration and age. This was expected, given the strong contribution of TCDD to TEQ.
The half-life, defined as the change in concentration in the body over time, is the net result of elimination from the body, changes in body composition, and intake from the environment (31). Over the 20 years since the explosion, we found the TCDD elimination half-life for Seveso women who were more than 10 years of age at explosion was 7.1 years, but was shorter in those who were younger. Our results are consistent with others (19, 32–34) and with the suggested reference half-life in adults of 7.2 years (31), and suggest TCDD elimination varies with age and concentration, especially at the very high initial doses seen in Seveso. We also confirm the significant inter-individual variability of TCDD elimination efficiency found in other studies (31, 34). Our findings add to the very limited data available on TCDD elimination half-life in children. In the only other study of children, serial TCDD measurements spanning up to 16 years after the explosion were available for a select group of 45 Seveso children (0–17 years in 1976) (33). TCDD elimination half-life was calculated for each age at subsequent sampling by comparing the subsequent TCDD measure to the child’s initial peak TCDD measurement, assuming a first-order kinetic model. When age at subsequent sampling was less than 18 years, a strong association between half-life and age was found, and children had significantly shorter half-lives than adults. The authors noted several limitations to this study; the number of samples per child was highly variable (ranging from 2 to 10), the time span between sequential TCDD measures was not uniform, female participants with a chloracne diagnosis were over-represented, and children younger than 7 years were under-represented. Our results in this larger sample both confirm and add to the findings of Kerger et al. (33) by suggesting that among children, TCDD half-life is shorter in those who are even younger. This is likely due to dilution resulting from child growth and increased body mass, as well as hepatic elimination at the highest exposure doses (32).
Our findings that half-life is influenced by age and initial TCDD concentration may have important implications for studies that estimate initial TCDD exposure by back-extrapolating from many years later, as this methodology may lead to an “underestimate” of highest TCDD exposure. Age-related variation in TCDD elimination may be particularly important for back-extrapolation in study populations that include children and adolescents such as the Yusho or Yucheng accident cohorts. The SWHS cohort is unique in that initial highest TCDD dose is available for all.
This study has a few limitations. First, compared to the full SWHS cohort, the sample was younger at explosion and had higher 1976 serum TCDD concentrations. Thus, we cannot necessarily generalize to the full SWHS cohort. However, these data shed light on the TCDD half-life particularly for those who were youngest at the time of the explosion. Second, we are reporting total TEQ concentrations in 1996 and we know from the present research of 1996 total TEQ that non-TCDD TEQ was not elevated relative to background. Over the last 40 years, background TEQ concentrations have been substantially reduced. Although we still do not know what total TEQ in 1976 was on an individual-basis, we do know, based on pooled data for females 0 to 40 years from zone non-ABR (17) and corrected for 2005 TEF (see Supplementary Table 1), that background total TEQ05 in 1976 was about 80 ppt.
In summary, twenty years after the explosion serum TCDD concentrations remain significantly elevated in this Seveso Women’s Health Study sample, the majority of who were children in 1976. No other dioxin-like compounds were elevated above background. We found that the TCDD elimination half-life varied with age, and was shorter in those who were younger at the time of the explosion. These findings add to the limited data available on TCDD elimination half-life in children.
Supplementary Material
Acknowledgments
We gratefully acknowledge Aliza Parigi for coordinating data collection at Hospital of Desio and Larry L. Needham for the significant contributions he made to the Seveso Women’s Health Study. This study was supported by Grant Numbers R01 ES07171 and F06 TW02075-01 from the National Institutes of Health, R82471 from the U.S. Environmental Protection Agency, 2P30-ESO01896-17 from the National Institute of Environmental Health Sciences, and #2896 from Regione Lombardia and Fondazione Lombardia Ambiente, Milan, Italy.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Zook D, Rappe C. Environmental sources, distribution, and fate. In: Schecter A, editor. Dioxins and Health. Plenum Press; New York: 1994. pp. 79–113. [Google Scholar]
- 2.Wikoff D, Fitzgerald L, Birnbaum L. Persistent organic pollutants: an overview. In: Schecter A, editor. Dioxins and Health. 3. John Wiley & Sons, Inc; Hoboken, New Jersey: 2012. pp. 1–36. [Google Scholar]
- 3.Consonni D, Sindaco R, Bertazzi P. Blood levels of dioxins, furans, dioxin-like PCBs, and TEQs in general populations: a review, 1989–2010. Environ Int. 2012;44:151–62. doi: 10.1016/j.envint.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 4.IARC. Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. IARC Monogr Eval Carcinog Risks Hum. 1997;69:33–342. [PMC free article] [PubMed] [Google Scholar]
- 5.Baan R, Grosse Y, Straif K, Secretan B, El Ghissassi F, Bouvard V, et al. A review of human carcinogens--Part F: chemical agents and related occupations. Lancet Oncol. 2009;10:1143–4. doi: 10.1016/s1470-2045(09)70358-4. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaum L. Developmental effects of dioxins and related endocrine disrupting chemicals. Toxicol Lett. 1995;82–83:743–50. doi: 10.1016/0378-4274(95)03592-3. [DOI] [PubMed] [Google Scholar]
- 7.Birnbaum LS. The mechanism of dioxin toxicity: relationship to risk assessment. Environ Health Perspect. 1994;102 (Suppl 9):157–67. doi: 10.1289/ehp.94102s9157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birnbaum LS, Tuomisto J. Non-carcinogenic effects of TCDD in animals. Food Addit Contam. 2000;17:275–88. doi: 10.1080/026520300283351. [DOI] [PubMed] [Google Scholar]
- 9.White SS, Birnbaum LS. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology. J Environ Science Health Environ Carcinog Ecotoxicol Rev. 2009;27:197–211. doi: 10.1080/10590500903310047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King-Heiden TC, Mehta V, Xiong KM, Lanham KA, Antkiewicz DS, Ganser A, et al. Reproductive and developmental toxicity of dioxin in fish. Mol Cell Endocrinol. 2012;354:121–38. doi: 10.1016/j.mce.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mocarelli P, Pocchiari F, Nelson N. Preliminary report: 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure to humans--Seveso, Italy. MMWR Morb Mortal Wkly Rep. 1988;37:733–6. [PubMed] [Google Scholar]
- 12.di Domenico A, Silano V, Viviano G, Zapponi G. Accidental release of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) at Seveso, Italy. II. TCDD distribution in the soil surface layer. Ecotoxicol Environ Saf. 1980;4:298–320. doi: 10.1016/0147-6513(80)90032-9. [DOI] [PubMed] [Google Scholar]
- 13.Assennato G, Cervino D, Emmett EA, Longo G, Merlo F. Follow-up of subjects who developed chloracne following TCDD exposure at Seveso. Am J Ind Med. 1989;16:119–25. doi: 10.1002/ajim.4700160203. [DOI] [PubMed] [Google Scholar]
- 14.Patterson DG, Jr, Hampton L, Lapeza CR, Jr, Belser WT, Green V, Alexander L, et al. High-resolution gas chromatographic/high-resolution mass spectrometric analysis of human serum on a whole-weight and lipid basis for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Anal Chem. 1987;59:2000–5. doi: 10.1021/ac00142a023. [DOI] [PubMed] [Google Scholar]
- 15.Mocarelli P, Patterson DJ, Marocchi A, Needham L. Pilot study (Phase II) for determining polychlorinated dibenzo-p-dioxin (PCDD) and polychlorinated dibenzofuran (PCDF) levels in serum of Seveso, Italy residents collected at the time of exposure: Future plans. Chemosphere. 1990;20:967–74. [Google Scholar]
- 16.Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, et al. Seveso Women’s Health Study: a study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere. 2000;40:1247–53. doi: 10.1016/s0045-6535(99)00376-8. [DOI] [PubMed] [Google Scholar]
- 17.Eskenazi B, Mocarelli P, Warner M, Needham L, Patterson DG, Jr, Samuels S, et al. Relationship of serum TCDD concentrations and age at exposure of female residents of Seveso, Italy. Environ Health Perspect. 2004;112:22–7. doi: 10.1289/ehp.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flesch-Janys D, Becher H, Gurn P, Jung D, Konietzko J, Manz A, et al. Elimination of polychlorinated dibenzo-p-dioxins and dibenzofurans in occupationally exposed persons. J Toxicol Environ Health. 1996;47:363–78. doi: 10.1080/009841096161708. [DOI] [PubMed] [Google Scholar]
- 19.Michalek J, Pirkle JL, Needham L, Patterson D, Caudill SP, Tripathi RC, et al. Pharmacokinetics of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Seveso adults and veterans of operation Ranch Hand. J Expo Anal Environ Epidemiol. 2002;12:44–53. doi: 10.1038/sj.jea.7500201. [DOI] [PubMed] [Google Scholar]
- 20.Warner M, Mocarelli P, Samuels S, Needham L, Brambilla P, Eskenazi B. Dioxin exposure and cancer risk in the Seveso Women’s Health Study. Environ Health Perspect. 2011;119:1700–5. doi: 10.1289/ehp.1103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akins JR, Waldrep K, Bernert JT., Jr The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clin Chim Acta. 1989;184:219–26. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- 22.Patterson DG, Turner WE. Method 28: Measurement of PCDDs, PCDFs, and Coplanar PCBs in Serum by HRGC/ID-HRMS. Atanta, GA: National Center for Enviromental Health, CDC; 2005. [Google Scholar]
- 23.Patterson DG, Turner WE. Method 28: Measurement of PCBs and Persistent Pesticides in Serum by HRGC/ID-HRMS. Atlanta, GA: National Center for Enviromental Health, CDC; 2005. [Google Scholar]
- 24.Hornung RW, Reed LD. Estimation of average concentration in the presence of non-detectable values. Appl Occup Environ Hyg. 1990;5:48–51. [Google Scholar]
- 25.Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–41. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stata Corp. Stata Statistical Software Release 11.0. College Station, TX: Stata Press; 2009. [Google Scholar]
- 27.Warner M, Eskenazi B, Patterson DG, Clark G, Turner WE, Bonsignore L, et al. Dioxin-Like TEQ of women from the Seveso, Italy area by ID-HRGC/HRMS and CALUX. J Expo Anal Environ Epidemiol. 2005;15:310–8. doi: 10.1038/sj.jea.7500407. [DOI] [PubMed] [Google Scholar]
- 28.Landi MT, Consonni D, Patterson DG, Jr, Needham LL, Lucier G, Brambilla P, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin plasma levels in Seveso 20 years after the accident. Environ Health Perspect. 1998;106:273–7. doi: 10.1289/ehp.98106273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson D, Turner W, Caudill SP, Needham L. Total TEQ reference range (PCDDs, PCDFs, cPCBs, mono-PCBs) for the US population 2001–2002. Chemosphere. 2008;73:S261–S77. doi: 10.1016/j.chemosphere.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 30.Patterson D, Wong L, Turner W, Caudill SP, DiPietro E, McClure P, et al. Levels in the U.S. population of those persistent organic pollutants (2003–2004) included in the Stockholm convention or in other long-range transboundary air pollution agreements. Environ Sci Technol. 2009;43:1211–8. doi: 10.1021/es801966w. [DOI] [PubMed] [Google Scholar]
- 31.Milbrath M, Wenger Y, Chang C, Emond C, Garabrant D, Gillespie B, et al. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast feeding. Environ Health Perspect. 2009;117:417–25. doi: 10.1289/ehp.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emond C, Michalek JE, Birnbaum LS, DeVito MJ. Comparison of the use of a physiologically based pharmacokinetic model and a classical pharmacokinetic model for dioxin exposure assessments. Environ Health Perspect. 2005;113:1666–8. doi: 10.1289/ehp.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerger BD, Leung HW, Scott P, Paustenbach DJ, Needham LL, Patterson DG, Jr, et al. Age- and concentration-dependent elimination half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Seveso children. Environ Health Perspect. 2006;114:1596–602. doi: 10.1289/ehp.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aylward LL, Brunet RC, Carrier G, Hays SM, Cushing CA, Needham LL, et al. Concentration-dependent TCDD elimination kinetics in humans: toxicokinetic modeling for moderately to highly exposed adults from Seveso, Italy, and Vienna, Austria, and impact on dose estimates for the NIOSH cohort. J Expo Anal Environ Epidemiol. 2005;15:51–65. doi: 10.1038/sj.jea.7500370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.