Abstract
Nicotine dependence is a chronic and difficult to treat disorder. While environmental stimuli associated with smoking precipitate craving and relapse, it is unknown whether smoking cues are cognitively processed differently than neutral stimuli. To evaluate working memory differences between smoking-related and neutral stimuli, we conducted a delay-match-to-sample (DMS) task concurrently with functional magnetic resonance imaging (fMRI) in nicotine dependent participants. The DMS task evaluates brain activation during the encoding, maintenance, and retrieval phases of working memory. Smoking images induced significantly more subjective craving, and greater midline cortical activation during encoding in comparison to neutral stimuli that were similar in content yet lacked a smoking component. The insula, which is involved in maintaining nicotine dependence, was active during the successful retrieval of previously viewed smoking vs. neutral images. In contrast, neutral images required more prefrontal cortex-mediated active maintenance during the maintenance period. These findings indicate that distinct brain regions are involved in the different phases of working memory for smoking-related vs. neutral images. Importantly the results implicate the insula in the retrieval of smoking-related stimuli, which is relevant given the insula’s emerging role in addiction.
Keywords: insula, memory, nicotine dependence
Introduction
Nicotine dependence is a major cause of preventable mortality leading to approximately 440,00 annual deaths in the United States (CDC, 2008). To address this health crisis, it is critical to study factors maintaining nicotine addiction such as reactivity to smoking-related cues, which can trigger craving and relapse (Caggiula et al., 2002). Smoking cue-reactivity has been extensively studied by passively exposing nicotine dependent individuals to smoking cues while evaluating brain reactivity using functional magnetic resonance imaging (fMRI; Engleman et al., 2012; Franklin et al., 2007; Janes et al., 2010). Less clear are the cognitive processes underlying smoking cue-reactivity, such as the memory for smoking cues. Specifically, we focused on identifying differences in brain activity during working memory for smoking and neutral stimuli. Using the standard working memory delay-match-to-sample (DMS) task (LoPresti et al., 2008; Schon et al., 2004, 2008), nicotine dependent participants were shown smoking-related and neutral images concurrently with fMRI. The DMS task permits the assessment of brain activation during the encoding, maintenance, and retrieval phases of working memory for smoking-related images and neutral images that serve as a control condition.
The insula is one brain region we hypothesize to play a greater role in the recognition of smoking vs. neutral stimuli due to this region’s role in nicotine dependence, memory, and awareness of visceral states. The insula has been implicated in maintaining nicotine dependence (Naqvi et al., 2007), as well as associations between visceral drug effects and drug-related stimuli (Conteras et al., 2012; Forget and Le Foll, 2010; Janes et al., 2010). Specifically, insula inactivation disrupts drug cue/drug effect associations leading to a reduction in conditioned place preference (Conteras et al., 2012) and a reduction in cue-induced nicotine self-administration (Forget and Le Foll, 2010). More broadly, the insula has been implicated in various forms of memory (Xie et al., 2012, Levens and Phelps et al., 2010 Ross and Slotnick, 2008, Miranda and Bermudez-Rattoni, 2007; Bermudez-Rattoni et al., 2005; Bermudez-Rattoni and McGaugh, 1991) and the integration of affective states with cognition (Wager and Feldman Barrett, 2005). The link between the insula and nicotine dependence, drug/cue associations, and memory in general, suggests that the insula may play a key role in smoking-related cognitive processes such as memory.
The insula was initially suggested to be part of the sensory system due to its involvement in visceral and somatosensory functions (Penfield and Faulk, 1955; Mesulam and Mufson 1982). Since then, the insula’s participation in specific visceral processes was expanded to include interoception, which Craig (2008, pg. 273) defines as “the ongoing status of all tissues and organs of the body, including skin, muscle, and visera”. Thus, the insula plays a more global role in all bodily feelings ranging from sensations linked to cardiovascular function (Critchley et al., 2004) to more complex sensations related to emotion (Craig, 2008, 2002) and the desire to smoke (Naqvi et al., 2007). The insula’s link with interoception in general suggests that this region may facilitate memory for stimuli associated with a range of visceral states including stimuli related to the visceral effect of nicotine. While this research focuses on the insula, the current study also allows for the identification of additional brain regions that may preferentially play a role in the memory for smoking stimuli.
Methods
Participants
Eighteen nicotine-dependent smokers (8 men/10 women, 2 Left-handed) between the ages of 18–33 completed all study measures at the McLean Imaging Center of McLean Hospital. Inclusion criteria included: reported smoking ≥ 10 cigarettes/day over the past 6 months and were moderately to heavily nicotine dependent as measured by the Fagerstrom test for nicotine dependence (FTND; Fagerström, 1978). Participants were assessed by the Structured Clinical Interview for DSM (SCID) and met criteria for current nicotine dependence. Potential participants were excluded if they had a lifetime diagnosis of the following conditions: organic mental disorder, bipolar or unipolar depression, or schizophrenia spectrum disorder. Participants were excluded for current substance use disorder other than nicotine dependence. Smokers also were excluded for pregnancy, current psychotropic drug use, recent drug use or excessive alcohol use. Participants were recruited using online advertisements and fliers posted in the Boston metropolitan area. All participants provided both verbal and written informed consent prior to participating in the study and the institutional review board at McLean Hospital approved this study.
Participant Assessments
Lifetime tobacco use was assessed by pack-years (packs of cigarettes smoked per day x years as a smoker), while smoking was measured by expired carbon monoxide (CO; Micro Smokerlyzer II, Bedfont Scientific Instruments) immediately prior to scanning.
Functional Neuroimaging
Scans were acquired on a Siemens Trio 3 Tesla scanner (Erlangen, Germany) with a 32-channel head coil. Multiplanar rapidly acquired dual echo gradient-echo structural images used the following parameters (TR = 2.1 s, TE 3.3 ms, slices = 128, matrix = 256 × 256, flip angle = 7°, resolution = 1.0 mm × 1.0 mm × 1.33 mm) and gradient echo echo-planar images were acquired using the following parameters (TR = 2 s, TE = 30 ms, matrix = 64 × 64, flip angle = 75°, 37 3.5 mm slices with a 0.35mm gap). To standardize the time since a cigarette was last smoked, all participants smoked one of their own cigarettes immediately following the informed consent. MRI scanning began approximately 1.5 h after smoking.
Delay Match to Sample Task
The delay-match-to-sample task is a standard working memory task, which followed a design similar to our previous work (LoPresti et al., 2008; Schon et al., 2004, 2008). During the DMS task, participants were shown a sample image (2 s), followed by a 10 s delay, and then a test image (2 s). Consistent with our prior work (LoPresti et al., 2008; Schon et al., 2004, 2008), the duration of the delay period was kept fixed as different delay lengths result in different activation patterns (Elliot and Dolan, 1999). Using a fiber optic response button box, participants were asked to determine whether the test image matched or did not match the sample image. Responses were recorded during the test image presentation. Each trial was separated by a jittered inter-trial interval (ITI) that ranged from 6 – 14 s, at 2 s intervals with an average of 10 s (Fig. 1). During fMRI scanning, participants performed the DMS task with 6 runs comprised of 16 trials each. During each run half of the trials were the match condition and the other half were the non-match condition. Match and non-match trials were equally divided into smoking or neutral image types such that in each run there were 4 smoking match, 4 smoking non-match, 4 neutral match, and 4 neutral non-match trials with a total of 24 of each trial type presented across the 6 runs. Prior to scanning, participants viewed the directions for the task and performed a practice version of the task during which only neutral images were used; these neutral images were not subsequently displayed during the in-scanner task. Smoking and neutral images were comprised of images from various sources including the International Smoking Image Series (Gilbert and Rabinovich, 1999) and those used in our prior work (Janes et al., 2010) and by Kober et al., 2010. To control for visual characteristics all images were converted to gray scale. The smoking images represented people smoking, hands holding cigarettes, and items such as cigarettes. The neutral images, which lacked the smoking element, were matched for content and included images of people, hands holding objects such as paintbrushes, and items such as pens. Except in the case of match trials, where the sample and target images were identical, each image shown during the DMS task was novel. Seventy-two unique smoking and seventy-two unique neutral images were shown during the DMS task. The DMS task was presented using E-prime 2.0 software (Psychology Software Tools) and data on behavioral performance (accuracy and reaction time) were collected.
Fig 1.
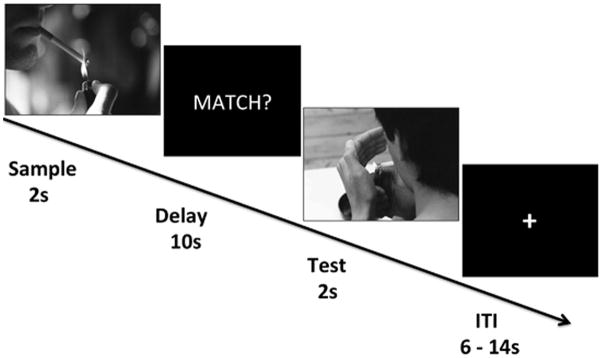
Delay match to sample task. During fMRI, participants were instructed to remember an image over a 10 second delay. Participants were then asked to determine whether the image shown during the Test period matched or did not match the image shown during the sample period. Each block of image presentations was separated by a variable-length ITI. As the image types could either be smoking or neutral there were four conditions; 1) smoking match, 2) smoking non-match (as depicted in the figure), 3) neutral match, 4) neutral non-match.
fMRI preprocessing
Analysis of fMRI data was conducted with FSL 4.1.9 (FMRIB Software Library; Analysis Group, FMRIB; Oxford, UK www.fmrib.ox.ac.uk/fsl). Within FSL, preprocessing was implemented using FEAT 5.98 (FMRI Expert Analysis Tool) using the following steps: motion correction using MCFLIRT, slice time correction using Fourier-space time series phase-shifting, spatial smoothing (6mm full with half max), non-brain removal using the brain extraction tool (BET) and high pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50 s). Image registration was first carried out using FMRIB’s Linear Image Registration Tool (FLIRT) and further refined using FMRIB’s Nonlinear Image Registration Tool (FNIRT). Images were warped to Montreal Neurological Institute atlas (MNI) space and resampled to 2 mm isotropic voxels.
fMRI data analysis
Included in the general linear model at the first level of analysis were ten regressors, each convolved with a gamma hemodynamic response function, corresponding to sample, delay, ITI, test match, and test non-match periods for the smoking and neutral conditions. Only the test periods were divided into match or non-match conditions, as participants were unable to predict whether a trial was a match or non-match condition prior to the test phase. Sample, delay, and ITI regressors were constructed using boxcar functions of length equal to the duration of the event. Test regressors were modeled as boxcars of length equal to the participant’s reaction time for that specific trial. Confound regressors were also included to remove incorrect trials (omission and commission), to model motion effects using 6 motion regressors, and to remove data spikes.
Contrasts were created between the smoking and neutral image conditions at the sample, delay, test match and test non-match periods. Memory encoding activity was equated with the sample period while maintenance and retrieval activity were equated with the delay period and test period respectively. First level results were combined (across runs) using a second level fixed effects analysis. Results averaged over each run for each subject where analyzed at the third level (group) using a mixed model. At the group level, multiple comparisons were corrected to p < 0.05 using a cluster-based threshold across the entire brain Z = 2.3 (Worsley et al, 2001).
Subjective Image Ratings
Following scanning, participants were again shown the smoking and neutral images and were asked to rate them on three measures. Each image was evaluated on a 5-point scale for affective valence, arousal, and craving. Subjective ratings for the average of all smoking vs. neutral images were compared using repeated measures ANOVA followed by post-hoc paired t-tests.
Results
Participant Demographics
Participants were 25.06 ± 4.75 (mean ± standard deviation) years old with 15.22 ± 2.08 years of education. Participants had moderate to high nicotine dependence as measured by the FTND (6.27 ± 1.02) and 6.38 ± 4.69 pack-years of tobacco cigarette use. Prior to scanning participants had expired CO levels at 25.67 ± 13.11 ppm, confirming recent cigarette use.
Delay match to sample behavioral performance
On average across all trial types, subjects performed at or near ceiling (0.95 ± 0.07 accuracy, Table 1). Repeated-measures ANOVA on mean accuracy showed no main effect of image type, but did reveal a significant effect of test condition (F = 4.8, p < 0.05), and an interaction of image type and test condition (F = 9.6, p < 0.01). Post hoc analysis revealed that smokers were significantly less accurate when performing the smoking image non-match condition relative to the neutral image non-match condition (t (17) = 3.3, p < 0.01) and relative to the smoking test match condition (t (17) = 2.3, p < 0.05). However, while subjects performed significantly worse on the smoking non-match condition, their average accuracy was still high for this trial type (0.92 ± 0.01). There was no influence of image type (smoking/neutral) or test condition (match/non-match) on reaction time.
Table 1.
Delay-match-to-sample behavioral performance. Each trial type is listed followed by the mean ± standard deviation for accuracy and reaction time in ms. Smokers performed significantly worse on the non-match smoking relative to the non-match neutral and match smoking trials.
| Trial Type | Accuracy | Reaction Time (ms) |
|---|---|---|
| Match Neutral | 0.95 +/− 0.06 | 880.7 +/− 149.8 |
| Match Smoking | 0.95 +/− 0.08 | 867.1 +/− 164.9 |
| Non-Match Neutral | 0.97 +/− 0.07 | 885.5 +/− 165.6 |
| Non-Match Smoking | 0.92 +/− 0.01 | 891.7 +/− 194.4 |
Subjective image ratings
There was a significant main effect of image type (smoking/neutral, F = 7.2, p < 0.01) and rating type (affect/arousal/craving, F = 12.7, p < 0.01) and an interaction between image and rating (F = 21.1, p < 0.001). Post hoc t-tests revealed that participants reported significantly greater levels of cigarette craving when viewing smoking vs. neutral images (t (17) = 7.1, p < 0.0001). Subjective ratings for affect and arousal did not differ between smoking and neutral images.
Functional Magnetic Resonance Imaging Results
DMS Encoding Period
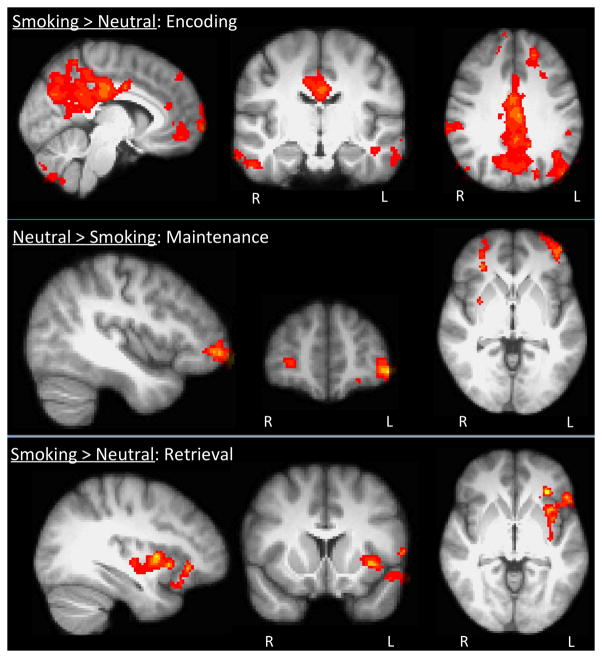
During the sample period, significant activation was clearly evident for the smoking > neutral contrast in cortical midline structures including the medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and precuneus. Activation was also found bilaterally in the frontal pole, extrastriate cortex and temporal gyrus, the right IFG and the cerebellum. (Fig. 2., Table 2, pcorrected ≤ 0.05). No significant activation was revealed for the neutral > smoking contrast during encoding.
Fig 2.
Top Panel Smoking > Neutral Encoding: Greater fMRI reactivity in midline cortical structures during the encoding phase of smoking vs. neutral memory. Middle Panel Neutral > Smoking Maintenance: Smokers have greater fMRI reactivity in the VLPFC and DLPFC during the maintenance neutral vs. smoking memory. Bottom Panel Smoking > Neutral Retrieval: Greater fMRI activity in the left insula and IFG during the test (retrieval) period.
Table 2.
Brain reactivity to smoking vs. neutral images during delayed match to sample encoding, maintenance, and test periods. Brain area and Brodmann area refer to the location of each cluster of contiguous voxels. MNI coordinates (X, Y, Z) refer to the region of maximum cluster activation for each cluster. Z-max refers to the maximum Z- statistic in each cluster (pcluster corrected < 0.05). Voxels refers to the total number of voxels within the cluster.
| Brain Area | Brodmann Area | X | Y | Z | Z-Max | Voxels | |
|---|---|---|---|---|---|---|---|
| Smoking > Neutral | |||||||
| Encoding: | left parahippocampal gyrus, bilateral posterior cingulate cortex, bilateral precuneus, left superior, middle, inferior temporal gyrus, left fusiform gyrus | 7, 19, 21, 22, 23, 29, 30, 37 | −48 | −48 | −4 | 5.78 | 12750 |
| bilateral cerebellar crus I and II, right inferior temporal gyrus, right fusiform | 19, 37 | 24 | −82 | −52 | 4.2 | 4887 | |
| bilateral superior and middle frontal gyrus | 9, 10 | −6 | 70 | −2 | 4.29 | 2593 | |
| right Inferior frontal gyrus, right superior temporal gyrus | 22, 47 | 52 | 24 | −8 | 4.25 | 1236 | |
| right inferior parietal lobule | 40 | 68 | −42 | 20 | 4.39 | 1039 | |
| left superior frontal gyrus | 8 | −18 | 38 | 30 | 4.12 | 997 | |
| medial frontal gyrus, anterior cingulate cortex | 10, 24, 32 | −4 | 50 | 0 | 3.16 | 652 | |
| right inferior and middle temporal gyrus | 20, 21 | 54 | −18 | −30 | 4.15 | 537 | |
| Retrieval Match: | left insula, left claustrum, left inferior frontal gyrus | 47 | −32 | 30 | −6 | 4.05 | 1134 |
| Neutral > Smoking | |||||||
| Maintenance: | right ventrolateral prefrontal cortex, right dorsolateral prefrontal cortex, right putamen | 9, 46, 10, 47 | 38 | 30 | 26 | 3.43 | 1072 |
| left ventrolateral prefrontal cortex | 46 | −46 | 54 | −4 | 3.51 | 588 | |
DMS Maintenance Period
Memory maintenance was assessed during the delay period, which revealed that there was significant activation for the neutral > smoking contrast in bilateral ventrolateral (VLPFC) and right dorsolateral prefrontal cortex (DLPFC) and right putamen (Fig. 2., Table 2, pcorrected ≤ 0.05). No significant activation was apparent for the smoking > neutral contrast during maintenance.
DMS Test Period
During the test period significant activation was found during the smoking > neutral match contrast in the left insula extending into the inferior frontal gyrus (IFG, Fig. 2., Table 2, pcorrected ≤ 0.05). No activation occurred during retrieval for the neutral > smoking match contrast Additionally, no significant differences in activation were found when comparing the smoking > neutral non-match condition.
Discussion
Supporting the insula’s role in the memory for smoking-related stimuli, the insula was significantly more activated during the recognition of smoking vs. neutral images. Our findings also indicate that drug-related and neutral memory processes differ as distinct patterns of brain activity were observed during encoding, maintenance, and retrieval for smoking vs. neutral stimuli. The smoking vs. neutral contrast revealed that greater brain activity was present during encoding and retrieval whereas the maintenance phase was the only period where the neutral vs. smoking contrast resulted in heightened brain activity.
Insula activity during recognition of addiction-related images
During the target match period for smoking-related versus neutral images, the left insula extending into the adjacent IFG was more active. Specifically, both mid and anterior regions of the insula were more active for the recognition of smoking images. Mid-regions of the insula receive interoceptive information about the physical condition of the body, which is then passed to more anterior sub-regions (Craig, 2008) to be used in higher-order processes such as affect and cognition (Craig, 2008, Wager and Feldman Barrett, 2005). We suggest the insula plays a greater role in memory for smoking-related vs. neutral images due to the interoceptive states induced during the retrieval of smoking cues. In the current study, smokers reported experiencing significantly more craving when viewing smoking vs. neutral images. According to the somatic marker theory of addiction, cue-induced craving is experienced due to the recall of drug-related interoceptive states, which are partly mediated by the insula (Verdejo-Garcia and Bechara, 2009; Naqvi and Bechara, 2009).
One possibility is that smoking cue-induced interoception leads to relatively more insula involvement in smoking image recognition. The insula may aid interoceptive memory as this brain region integrates internal states with external cognitive task demands (Farb et al., 2012; Gu et al., 2013). Specifically, interoceptive states evoked by smoking-related images may provide internal information that guides retrieval when a cue is externally presented a second time as in the case of the target match condition. In fact, individuals who are more aware of their internal states have an easier time remembering previously viewed affective images. These same individuals do not show memory facilitation for neutral stimuli (Pollatos and Schandry, 2008), suggesting that memory recognition is facilitated by the internal states associated with specific stimuli.
During recognition of smoking vs. neutral images, the left but not right insula was significantly active. While the bilateral insula has been implicated in nicotine dependence (Naqvi et al., 2007; Janes et al., 2010), the left insula is beginning to emerge as playing a more prominent role in nicotine dependence. Not only does the left insula of smokers have larger gray matter density in comparison to non-smoker controls (Zhang et al., 2011), but also an fMRI meta-analysis indicated that smoking cues result in consistent left insula activity (Engleman et al., 2012). In relation to memory, the left insula is expressly involved in matching a target image with a previously viewed image, but does not activate to simple image repetition where recall is not required (Jiang et al., 2000). Finally, a meta-analysis showed left lateralized insula activity during working memory (Wager and Smith et al., 2003). Taken together these studies implicate the left insula in both smoking cue-reactivity and recognition memory when sample and target images match. The current findings expand the insula’s role to include memory retrieval of smoking-associated stimuli, which is inline with preclinical work implicating the insula in memory for drug-cue associations (Contreras et al., 2012).
During the test phase of the non-match condition, no brain activity differences were found between smoking and neutral cues, indicating that similar strategies may have been used to correctly identify novel images regardless of cue type. Insula activity during the smoking vs. neutral match, but not non-match, condition is consistent with the finding that awareness of somatic states leads to better recognition of previously viewed affective images, but not neutral memory or correct rejections (Pollatos and Schandry, 2008).
Neutral memory relies on active maintenance
During the neutral vs. smoking delay contrast more activity was found in the DLPFC and VLPFC, which play a primary role in actively maintaining the memory of stimuli across a delay (Levy and Goldman-Rakic, 2000; Schon et al., 2008; Smith and Jonides, 1999; Courtney et al., 1997; Owen et al., 2005; Wager and Smith, 2003; Curtis and D’Esposito, 2003). The putamen also was active during this contrast, which is consistent with other reports of putamen activation during memory maintenance (Cairo et al., 2004). While the insula facilitates memory retrieval for stimuli that induce craving, successful recall of neutral memory may depend more on PFC-mediated active maintenance over the delay period, suggesting that different brain-related processes are used for addiction-related vs. neutral working memory. Alternatively, craving induced by smoking stimuli may have made it more difficult for participants to maintain neutral memory as craving may tax working memory (Hoffman et al., 2012). Thus, due to cigarette craving induced by exposure to smoking stimuli, participants may have required additional PFC resources to maintain neutral memory during the delay period.
Addiction-related images encoded as self-referential
Enhanced brain activity, primarily in cortical midline brain regions, was found during encoding of smoking vs. neutral images. These cortical midline brain areas show strong overlap other smoking cue-reactivity work (Engelman et al., 2012) and with patterns of brain activity reported during self-referential processing (Heatherton et al., 2006, Macrae et al., 2004, Moran et al., 2006, Northoff et al., 2006), which facilitates memory for stimuli particularly relevant to the individual (Rogers et al., 1977; Fossati et al., 2004; Gutchess et al., 2007; Turk et al., 2008). While the network of cortical midline structures is collectively linked in self-referential processing, the mPFC specifically is implicated in the facilitation of self-referential memory (Macrae et al., 2004). Medial PFC activation during encoding predicts self-related memory (Macrae et al., 2004). Critically, mPFC damage abolishes the ability of self-reference to enhance memory (Philippi et al., 2012). In relation to the current findings, activation of cortical midline structures, and the mPFC in particular, suggests that nicotine dependent individuals encode smoking images as self-referential. While plausible, this interpretation requires independent confirmation.
Unfortunately, it is not possible to directly determine the mPFC’s contribution to successful recognition of smoking cues using behavioral performance on the DMS task because participants performed at or close to ceiling levels of accuracy when recalling both stimuli types. While the current DMS task was designed to maximize the number of correct trials to investigate brain activity differences between stimuli, future research involving more difficult variations of the DMS task could test whether brain regions associated with self-reference contribute to a memory advantage for smoking cues. However, we suggest that smoking cues may be more effectively encoded relative to neutral cues given that greater DLPFC and VLPFC activation was needed to maintain neutral vs. smoking memory. Future research involving larger sample sizes also may be beneficial to determine whether additional variables such as sex and smoking history impact memory for smoking stimuli.
Conclusions
The present results offer strong evidence that different brain areas are engaged during working memory for smoking-related images. When smoking images are presented to smokers, they are encoded as self-relevant, require less active maintenance across a delay, and may rely on insula mediated internal states for their successful recognition. Our previous work showed that insula reactivity to smoking stimuli was highest in relapse vulnerable smokers (Janes et al., 2010). Combined with the current finding we suggest that the insula’s involvement in retrieval of addiction-associated stimuli may facilitate relapse vulnerability, which is in line with the somatic marker theory of addiction (Verdejo-Garcia and Bechara, 2009; Naqvi and Bechara, 2009).
Acknowledgments
This work was supported by the National Institute on Drug Abuse Grant K01DA029645.
Footnotes
Author Contribution
AJ, RR, CS, and SL were responsible for the study concept and design. AJ and SF collected all data. SF performed all data entry. AJ performed all data analysis guided by RR, LN, and BF. AJ drafted the manuscript. All authors critically reviewed the content of this manuscript for publication.
References
- Bermudez-Rattoni F, McGaugh JL. Insular cortex and amygdala lesions differentially affect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Research. 1991;549:165–170. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Okuda S, Roozendaal, McGaugh JL. Insular cortex is involved in consolidation of object recognition memory. Learn Mem. 2005;12:447–449. doi: 10.1101/lm.97605. [DOI] [PubMed] [Google Scholar]
- Cairo TA, Liddle PF, Woodward TS, Ngan ETC. The influence of working memory load on phase specific patterns of cortical activity. Brain Res Cog Brain Res. 2004;21:377–387. doi: 10.1016/j.cogbrainres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses – United States, 2000 – 2004. Morbidity and Mortality Weekly Report. 2008;57:1226–28. [PubMed] [Google Scholar]
- Contreras M, Billeke P, Vicencio S, Madrid C, Perdomo G, Gonzalez M, Torrealba F. A role for the insular cortex in long-term memory for context-evoked drug craving in rats. Neuropsychopharmacology. 2012;37:2101–2108. doi: 10.1038/npp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception and emotion: a neuroanatomical perspective. In: Lewis ML, Haviland-Jones JM, Barrett LF, editors. Hadbook of Emotion. 3. New York (NY): The Guilford Press: 2008. pp. 272–288. [Google Scholar]
- Critchley HD, Wiens S, Roshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cog Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ. Differential neural response during performance of matching and nonmatching to sample tasks at two delay intervals. J Neurosci. 1999;19:5066–5073. doi: 10.1523/JNEUROSCI.19-12-05066.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman JM, Versace F, Fobinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Anderson AK. Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb Cortex. 2013;23:114– 126. doi: 10.1093/cercor/bhr385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psych. 2010;68:265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H. Distributed self in episodic memory; neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22:1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: A perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. International smoking image series (with neutral counterparts), version 1.2. Carbondale, IL: Integrative Neuroscience Laboratory, Department of Psychology, Southern Illinois University; 1999. [Google Scholar]
- Gu X, Liu X, Van Dam NT, Hof PR, Fan J. Cognition-emotion integration in the anterior insular cortex. Cereb Cortex. 2013;23:20–7. doi: 10.1093/cercor/bhr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Yoon C, Schacter DL. Ageing and the self-reference effect in memory. Memory. 2007;15:822–837. doi: 10.1080/09658210701701394. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. SCAN. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends Cogn Sci. 2012;16:174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick BB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psych. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman Complementary neural mechanisms for tracking items in human working memory. Science. 2000;287:643–646. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatl pathway underlies cognitive regulation of craving. Proc NatlAcad Sci USA. 2010;107:14811–14846. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens SM, Phelps EA. Insula and orbital frontal cortex activity underlying emotion interference resolution in working memory. J Cog Neurosci. 2010;22:2790–2803. doi: 10.1162/jocn.2010.21428. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Experimental Brain Research. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- LoPresti M, Schon K, Tricarico MD, Swisher JD, Celone KA, Stern CE. Working memory for social cues recruits orbitofrontal cortex and amygdala: a functional magnetic resonance imaging study of delayed matching to sample for emotional expressions. J Neurosci. 2008;28:3718–3728. doi: 10.1523/JNEUROSCI.0464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelly WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1– 22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Miranda MI, Bermudez-Rattoni F. Cholinergic activity in the insular cortex is necessary for acquisition and consolidation of contextual memory. Neurobiol Learn Mem. 2007;87:343–351. doi: 10.1016/j.nlm.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, Kelley WM. Modulation of cortical midline structures by implicit and explicit self-relevance evaluation. Social neurosci. 2006;4:197–211. doi: 10.1080/17470910802250519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain-A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Faulk ME. The insula: Further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Duff MC, Denburg NL, Tranel D, Rudrauf D. Medial PFC damage abolishes the self-reference effect. Journal of Cogn Neurosci. 2012;24:475–481. doi: 10.1162/jocn_a_00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Schandry R. Emotional processing and emotional memory are modulated by interoceptive awareness. Cognition and Emotion. 2008;22:272–287. [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. J Pers Soc Psychol. 1977;35:677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Ross RS, Slotnick SD. The hippocampus is preferentially associated with memory for spatial context. J Cog Neurosci. 2008;20:432–46. doi: 10.1162/jocn.2008.20035. [DOI] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, LoPresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Tinaz S, Somers DC, Stern CE. Delayed match to object or place: an event-related fMRI study of short-term stimulus maintenance and the role of stimulus pre-exposure. Neuroimage. 2008;39:857–872. doi: 10.1016/j.neuroimage.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Turk DJ, Cunningham SJ, Macrae CN. Self-memory biases in explicit and incidental encoding of trait adjectives. Conscious Cog. 2008;17:1040–1045. doi: 10.1016/j.concog.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56:48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Feldman-Barrett L. From affect to control: Functional specialization of the insula motivation and regulation. 2004 Published online at PsychExtra: http://www.columbia.edu/cu/psychology/tor/
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. CABIN. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. Ch 14. 2001. [Google Scholar]
- Xie C, Bai F, Yu H, Shi Y, Yuan Y, Chen G, Li W, Chen G, Zhang Z, Li S. Abnormal insula functional network is associated with episodic memory decline in amnestic mild cognitive impairment. Neuroimage. 2012;15:320–7. doi: 10.1016/j.neuroimage.2012.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54:42–48. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]