Abstract
Objectives
Smaller hippocampal volumes are observed in depression but it remains unclear how antidepressant response and persistent depression relate to changes in hippocampal volume. We examined the longitudinal relationship between hippocampal atrophy and course of late-life depression.
Setting
Academic medical center.
Participants
Depressed and never-depressed cognitively intact subjects age 60 or older.
Measurements
Depression severity was measured every three months with the Montgomery-Asberg Depression Rating Scale (MADRS). Participants also completed cranial 1.5T MRI every two years. We compared two-year change in hippocampal volume based on remission status, then in expanded analyses examined how hippocampal volumes predicted MADRS score.
Results
In analyses of 92 depressed and 70 never-depressed subjects, over two years the cohort whose depression never remitted exhibited greater hippocampal atrophy than the never-depressed cohort. In expanded analyses of a broader sample of 152 depressed elders, depression severity was significantly predicted by a hippocampus by time interaction where smaller hippocampus volumes over time were associated with greater depression severity.
Conclusions
Hippocampal atrophy is associated with greater and persistent depression severity. Neuropathological studies are needed to determine if this atrophy is related to the toxic effects of persistent depression or related to underlying Alzheimer’s disease.
Keywords: Geriatrics, depression, hippocampus, MRI, neuroimaging, longitudinal
INTRODUCTION
Substantial evidence implicates the hippocampus in the pathophysiology of major depressive disorder (MDD). Such work began with the recognition that hippocampal volumes are smaller in patients with MDD,1 particularly in those individuals with a longer duration of depression.2,3 These findings support a neurotoxicity hypothesis 4,5 proposing that stress-related increases in glucocorticoids and decreases in neurotrophic factors adversely affect hippocampal neurons and result in hippocampal volume loss. However, other evidence supports that smaller hippocampi may create a predisposition or vulnerability to MDD.6 Genetic or early environmental influences may adversely affect hippocampal structure and function, in turn increasing vulnerability to stress-related psychiatric disorders.7 Thus there is potentially a reciprocal relationship between depressive episodes and hippocampal structure.8,9
This picture is even more complicated in older adults with MDD, or late-life depression (LLD). As in younger and midlife adults, compared to nondepressed cohorts, LLD is associated with smaller hippocampal volumes 10–12 and greater reductions in hippocampal volume over time.13,14 Smaller hippocampal volumes in LLD are also associated with greater depression severity,15 while incident depressive episodes and greater depression severity are associated with a greater longitudinal reduction in hippocampal volume over 6 to 7 years.16 Factors contributing to smaller hippocampal volumes in LLD include the same factors contributing to smaller hippocampi in younger adults. However, depression is considered a risk factor for or early symptom of Alzheimer’s disease (AD),17–19 itself characterized by progressive hippocampal atrophy.20,21
A number of studies also demonstrate that hippocampal volume is associated with poor response to antidepressant treatment. Studies in both general adult MDD populations and LLD specifically demonstrate that smaller baseline hippocampal volumes are associated with poorer acute response to antidepressants.22–24 Similarly, smaller hippocampal volumes at baseline can predict poorer long-term antidepressant treatment outcomes of up to three years.25–28 The longitudinal relationship between change in hippocampal volume and antidepressant outcomes is less well studied. This is a crucial issue as studies examining animal models demonstrate that antidepressants may alleviate the effects of stress on the hippocampus while also improving neurogenesis.9,29–31 Such antidepressant effects on the hippocampus could be seen using MRI in human populations, as demonstrated in studies investigating post-traumatic stress disorder (PTSD).32,33 However, results from studies in MDD are mixed, with reports that antidepressant treatment either increases 34,35 or has no effect on hippocampal volumes.36 Similarly, a study by Frodl and colleagues demonstrated that individuals who took antidepressants consistently over three years showed increases in hippocampal volumes.26 However, they did not find significant differences in hippocampal volumes in the broader sample of depressed and nondepressed subjects.
Although incident depression and greater depression severity in older adults is associated with greater hippocampal atrophy,16 the relationship between change in hippocampal volume and treatment course has not been well addressed. The purpose of this study is to examine the longitudinal relationship between the persistence of depression and change in hippocampal volume. We hypothesized that, over the study period, greater hippocampal atrophy would be associated with poorer antidepressant treatment outcomes and higher levels of depression severity. As antidepressant effects on hippocampal neurogenesis decline with age 37 and as hippocampal atrophy occurs both in normal aging and with neurodegenerative processes,38 we did not expect to observe any statistically significant increases in hippocampal volumes over the study period.
METHODS AND MATERIALS
Study Participants
Participants entered this longitudinal study through several mechanisms at Duke University Medical Center. Starting in 1994, participants began enrolling in the National Institute of Mental Health (NIMH)-sponsored Mental Health Clinical Research Center for the study of Depression in Later Life and its longitudinal sister study. In 2001, these programs transitioned to the Conte Center for the Neuroscience of Depression in the Elderly and the companion Neurocognitive Outcomes of Depression in the Elderly (NCODE) longitudinal study.
Eligible depressed subjects were aged 60 years or older and met diagnostic criteria for MDD, single episode or recurrent episodes. Diagnosis was based on the NIMH Diagnostic Interview Schedule (DIS) 39 and confirmed by clinical interview. Exclusion criteria included other major psychiatric illnesses, including bipolar disorder and lifetime alcohol or substance abuse or dependence. Individuals with primary neurologic illnesses that could affect structural brain MRI scans were excluded, including dementia, Parkinson disease, multiple sclerosis, and seizure disorders. Contraindications for magnetic resonance imaging (MRI) were also an exclusion criterion. Although individuals meeting diagnostic criteria for anxiety disorders were excluded, participants with comorbid anxiety symptoms were included, as long as major depressive disorder was judged by the study psychiatrist to be the primary diagnosis.
Nondepressed comparison subjects were recruited through the Center for Aging Subject Registry at Duke University, which includes community-dwelling elders in central North Carolina. Eligible comparison subjects were age 60 years or older, had a nonfocal neurologic examination, no self-report of neurologic disease or depressive disorder, and no evidence of depression based on the DIS.39
The study was approved by the Duke University Medical Center Institutional Review Board. All study participants provided written informed consent prior to enrollment. We have previously published longitudinal results from this cohort examining the relationship between change in hippocampal volume, stress,40 and subsequent cognitive decline.14,41 These prior studies did not examine the relationship between hippocampal volume and course of depression severity with treatment.
Clinical evaluation and treatment
At baseline, a study geriatric psychiatrist interviewed each depressed subject and completed standardized assessments, including the Montgomery-Asberg Depression Rating Scale (MADRS).42 Nondepressed participants were not evaluated with the MADRS. All participants completed the Mini-Mental State Examination (MMSE) 43 at baseline, and individuals who scored below 25 were excluded from the study. Clinical assessments of depressed participants with repeat MADRS scoring was performed every three months and when clinically indicated. All study psychiatrists are trained on completion of the MADRS with high interrater reliability (κ > 0.9).
Depressed subjects were treated according to the Duke Somatic Treatment Algorithm for Geriatric Depression.44 This algorithm engages a stepwise approach that allows broad use of commercially available antidepressant modalities. Although the majority of depressed subjects were prescribed sertraline on study entry, the antidepressant regimen differed across the sample based on depression severity, past treatments, medication tolerability, and response. Switching antidepressant medications and augmentation strategies were allowed as necessary for subjects who did not respond to initial treatment. Participants were evaluated at least every three months and were seen more frequently as necessary as clinically indicated. Although not routinely recommended to all subjects, psychotherapy and electroconvulsive therapy were also treatment options. However, no participants in the current study received electroconvulsive therapy.
MRI Acquisition and Analysis
After screening for contraindications, cranial MRI was performed using a 1.5 Tesla, whole-body MRI system (Signa, GE Medical Systems, Milwaukee, WI) using the standard head (volumetric) radiofrequency coil. Alignment was confirmed by a rapid sagittal localizer scan and then two dual-echo, fast spin-echo acquisitions were obtained: the first in the axial plane for morphometry of cerebral structures and the second in a coronal oblique plane for morphometry of the hippocampus. Our MRI acquisition protocol has been previously described.10,45 MRI was repeated on the same scanner approximately every two years. However, the coronal acquisition was added after study initiation, so not all participants had hippocampal volume measures at study entry.
Image processing occurred at the Duke Neuropsychiatric Imaging Research Laboratory (NIRL). Tissue segmentation and measurement of total cerebral volume was performed using previously described methods.45 This segmentation process utilized the different image contrasts to identify differing tissues (white matter, gray matter, CSF) and identified and allowed for quantification of white matter hyperintensity (WMH) volume. Total cerebral volume included total white and gray matter, WMH and CSF volumes in both hemispheres.45
The hippocampus was delineated using previously described methods.10 Beginning with the most posterior coronal slice and moving anteriorly, analysts measured the hippocampus on each side where the pulvinar nucleus of the thalamus obscured the crura fornicis. Both the fimbria and the thin strip of gray matter along the medial border of the hippocampus were cut at their narrowest points. Tracing continued around the hippocampal body to the starting point. The amygdala-hippocampal transition zone appeared as a diffuse area of gray matter between the anterior portion of the hippocampus and the posterior portion of the amygdala, and was also transected at its narrowest point. The anterior border of the hippocampus was defined as the slice on which the inferolateral ventricle appeared horizontally without any body of gray matter visible below it.
All analysts received extensive training. Reliability was established by repeated measurements separated by at least a week on multiple MRIs before raters were approved to process study data. Intraclass correlation coefficients were: left hippocampus=0.8; right hippocampus=0.7; left WMH = 0.988; right WMH = 0.994; and total cerebral volume=0.997.
Analytic Plan
We planned two sets of analyses involving different samples. First, we tested for differences in change in hippocampal volume over two years based on diagnosis and antidepressant response. We limited these analyses to those participants who had hippocampal measures at both study entry and at the 2-year assessment. Second, we examined the longitudinal relationship over time between depression severity, measured by MADRS, and hippocampal volume. These analyses were limited to depressed subjects as the nondepressed cohort did not have MADRS data. However, we did not limit the analyses only to subjects with baseline and 2-year hippocampal data. We also included subjects who had baseline-only measures and longitudinal hippocampal measures, but no baseline hippocampus measure. Missing baseline measures were due to subject enrollment prior to initiation of the coronal MRI acquisition needed for hippocampus measures or subject inability to complete MRI. All analyses were conducted using SAS version 9.2 (Cary, NC).
For the two-year analyses, we divided the sample into four cohorts. This included a) never depressed comparison subjects, b) depressed subjects who achieved and maintained remission (defined as achieving a MADRS ≤ 5 for two consecutive assessments), c) depressed subjects who remitted but later relapsed (subsequent MADRS > 10), and d) depressed subjects who never remitted. We limited these analyses to subjects who had hippocampal data at baseline study entry. For univariate analyses of demographic and neuroimaging data, we used ANOVA for continuous variables and chi square tests for categorical analyses.
This set of analyses used mixed models (PROC MIXED). Our dependent variable was change in hippocampus volume, included as a repeated measure to account for right or left hemisphere. For our primary models, covariates included diagnostic cohort, total cerebral volume, hemisphere, age, and time between scans. For secondary models, we planned to incorporate demographic variables that differed significantly among the diagnostic cohorts. We additionally included WMH volume as a covariate, as change in WMH volume is associated with depression outcomes.46 If change in hippocampus volume was significantly associated with diagnostic cohort, we anticipated using least square means analyses to test for pairwise comparisons of the adjusted means. For these analyses, we conducted uncontrolled t-tests.
The second set of analyses examining the relationship between depression severity and hippocampal volumes used mixed models (PROC MIXED) to predict MADRS scores over the study period. Covariates included hippocampus volume, cerebral volume, hemisphere, baseline MADRS, WMH volume, sex, age, and time. Hippocampus volume was a repeated measure, both in hemisphere and in time. In these models we were specifically interested in an interaction between time and hippocampus volume, examining how change in hippocampus volume over time predicted MADRS scores over the study period.
RESULTS
Diagnostic status and two-year change in hippocampal volume
Initial analyses tested for differences in two-year change in hippocampal volume between diagnostic cohorts. These analyses included data on 162 elderly individuals, consisting of 47 depressed individuals who achieved and maintained remission, 18 who remitted but then relapsed, 27 who never achieved remission, and 70 never-depressed comparison subjects. Most demographic variables did not differ between the cohorts (Table 1). Exceptions included education, wherein the never-depressed cohort was significantly more educated than all depressed cohorts, and sex representation, where the never-depressed cohort had a higher representation of women.
Table 1.
Demographic differences by diagnostic cohort
| Variable | Remitted (N=47) | Relapsed (N=18) | Nonremitted (N=27) | Never depressed (N=70) | Test value | p value |
|---|---|---|---|---|---|---|
| Age | 69.3 (6.5) | 70.0 (6.1) | 72.1 (7.3) | 69.7 (6.2) | F 3,158 = 1.19 | 0.3154 |
| Sex | 59.6 (28) | 66.7 (12) | 55.6 (15) | 81.4 (57) | χ2 = 9.36 | 0.0249 |
| Race | 83.0 (39) | 88.9 (16) | 85.2 (23) | 88.6 (62) | Fisher’s exact | 0.8550 |
| Education | 14.5 (2.2) | 13.9 (3.2) | 14.6 (2.1) | 15.6 (1.6) | F 3,158 = 4.50 | 0.0047 |
| MADRS, baseline | 26.5 (7.4) | 25.0 (7.7) | 26.0 (5.4) | - | F 2,89 = 0.29 | 0.7477 |
| MADRS, 2y | 3.2 (3.3) | 13.8 (12.0) | 18.1 (8.4) | - | F 2,89 = 36.44 | < 0.0001 |
| MMSE, baseline | 28.7 (1.2) | 28.6 (2.0) | 28.4 (1.7) | 28.7 (1.7) | F 3,158 = 0.23 | 0.8771 |
| MMSE, 2y | 28.7 (2.0) | 28.2 (2.9) | 28.3 (2.0) | 28.7 (1.3) | F 3,142 = 0.76 | 0.5200 |
| WMH volume, baseline | 6.9 (9.3) | 3.7 (2.2) | 6.2 (7.4) | 4.9 (5.4) | F 3,158 = 1.28 | 0.2844 |
| WMH volume, 2y | 7.7 (10.5) | 5.1 (3.5) | 8.7 (10.9) | 6.1 (7.9) | F 3,158 = 0.93 | 0.4287 |
Demographic data for the cohort of subjects included in two-year analyses. Data for continuous variables presented as mean (SD) and analyzed with ANOVA with degrees of freedom presented with the F values in the table. Categorical variables are presented as % (N) and analyzed through chi-square tests with 3 degrees of freedom. Analyses included all subjects except the 2-year MMSE, where missing data resulted in a sample of 146 individuals. Age and education presented in years, sex as % female, and race as % white. MADRS = Montgomery-Asberg Depression Rating Scale; MMSE = Mini-Mental State Exam; WMH volume = white matter hyperintensity volume, in milliliters.
In univariate comparisons, unadjusted hippocampal volume measures at both baseline and year 2 did not significantly differ between the cohorts (Table 2). To determine if year 2 measures differed significantly from baseline measures, we conducted two-tailed t-tests comparing these measures within each cohort. There were no significant differences between baseline and year 2 hippocampal measures for any cohort (data not shown) except in the nonremitting cohort, where year 2 measures were significantly smaller than baseline measures for the total hippocampus volume (t = 2.17, 26df, p = 0.0385) and left hemisphere volume (t = 2.43, 26df, p = 0.0218) but not right hippocampus (t = 1.65, 26df, p = 0.1107).
Table 2.
Unadjusted baseline and Year 2 neuroimaging measures by diagnostic cohort
| Variable | Remitted (N=47) | Relapsed (N=18) | Nonremitted (N=27) | Never depressed (N=70) | Test value | p value |
|---|---|---|---|---|---|---|
| Hippocampus, left | ||||||
| • Baseline | 2.97 (0.38) | 2.92 (0.29) | 3.09 (0.54) | 2.95 (0.43) | F 3,158 = 0.97 | 0.4083 |
| • Year 2 | 2.95 (0.50) | 2.95 (0.40) | 2.89 (0.50) | 3.02 (0.47) | F 3,158 = 0.60 | 0.6147 |
| Hippocampus, right | ||||||
| • Baseline | 3.16 (0.40) | 3.04 (0.30) | 3.14 (0.54) | 3.08 (0.42) | F 3,158 = 0.51 | 0.6738 |
| • Year 2 | 3.09 (0.48) | 3.08 (0.41) | 2.98 (0.61) | 3.13 (0.45) | F 3,158 = 0.61 | 0.6065 |
| Cerebral volume | 1159.6 (130.5) | 1133.7 (116.0) | 1191.0 (144.0) | 1132.6 (112.7) | F 3,158 = 1.64 | 0.1820 |
Data presented in milliliters, mean (SD). Analyses used ANOVA with degrees of freedom presented with the F values in the table
We then used models to test for cohort differences in change in hippocampus volume (Table 3). The a priori parsimonious model (Model 1) included baseline cerebral volume, age, hemisphere (left or right hippocampus), and time between scans as covariates. A secondary model (Model 2) incorporated WMH volume but also sex and education as covariates as these variables differed among cohorts in univariate analyses (Table 1). In Model 1, change in hippocampal volume was significantly predicted by cohort assignment. Through pairwise comparisons of adjusted means, this finding was due to a significant difference between the nonremitted subjects and the never-depressed subjects (uncorrected t-test, t = 2.81, 163df, p = 0.0055). Although we did not plan on controlling for multiple comparisons, this difference remains statistically significant after a Bonferroni correction (6 comparisons, resulting in an adjusted significance level of 0.0083). No other cohort comparisons demonstrated statistically significant differences. However, in Model 2, hippocampal volume change did not significantly differ by cohort after controlling for sex, education, and WMH volume. None of the added variables significantly predicted change in hippocampus volume.
Table 3.
Models examining cohort differences in hippocampal volume change over 2 years
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Variable | F value | p value | F value | p value |
| • Cohort | F 3,163 = 2.79 | 0.0385 | F 3,164 = 2.23 | 0.0866 |
| • Age | F 1,163 = 0.19 | 0.6606 | F 1,164 = 0.02 | 0.8862 |
| • Hemisphere | F 1,163 = 0.45 | 0.5045 | F 1,164 = 0.45 | 0.5045 |
| • Cerebral volume | F 1,163 = 0.10 | 0.7551 | F 1,164 = 0.64 | 0.4248 |
| • Time | F 1,163 = 0.00 | 0.9738 | F 1,164 = 0.01 | 0.9427 |
| • Sex | - | - | F 1,164 = 1.05 | 0.3077 |
| • Education | - | - | F 1,164 = 0.50 | 0.4817 |
| • WMH volume | F 1,164 = 0.46 | 0.5003 | ||
These mixed models predict two-year change in hippocampus volume. Parsimonious Model 1 was developed according to our a priori plan. Model 2 includes sex, education, and WMH developed as those demographic variables differed between diagnostic cohorts or had previously been associated with hippocampal volumes. Time refers to time between scans; hemisphere refers to right or left hippocampus. WMH = white matter hyperintensity volume.
Longitudinal Depression severity and hippocampus volume change
Subsequent analyses examined if longitudinal hippocampus volume measures predicted depression severity. For these analyses, we included an additional 60 depressed subjects with hippocampal data. These individuals either had a baseline-only MRI or had longitudinal hippocampal data but with baseline scans prior to initiation of the coronal acquisition needed for hippocampal measurement. Including the 92 depressed subjects examined in our 2-year analyses described above, this resulted in an expanded cohort of 152 depressed adults. Subjects had a mean age at study entry of 69.7y (SD = 6.9y, range 60–88y). The cohort was 63% (N=96) women and 85% (N=129) Caucasian, with the majority of the other subjects being African-American. Other demographic characteristics of this expanded cohort were comparable to those displayed in Table 1. Participants were in the study from 0 days (baseline-only assessments) to 3,123 days, with a mean duration of participation of 942 days (SD=902 days).
We examined mixed models predicting MADRS score over the course of study participation. Covariates included baseline MADRS, age, education, sex, and time. Hippocampus volume, WMH volume, and cerebral volume were included as repeated measures, using hemisphere as a variable to discriminate between the left and right hippocampus. As we hypothesized we would see a relationship between MADRS score and change in hippocampus over time, we examined an interaction term between time and hippocampus volume (Table 4). Examination of this interaction term showed that individuals with smaller hippocampal volumes over time demonstrated increasing or non-decreasing MADRS trajectories. Notably, there was no direct effect of WMH volumes on MADRS scores. We also examined an interaction term between WMH volume and time, but as this did not reach a threshold of statistical significance, it was removed from the model and is not reported.
Table 4.
Models predicting longitudinal relationship between hippocampus volume and MADRS score
| Variable | F value | p value |
|---|---|---|
| Hippocampus Volume | F 1,5603 = 18.03 | < 0.0001 |
| Cerebral volume | F 1,5603 = 0.99 | 0.3206 |
| WMH volume | F 1,5603 = 0.05 | 0.8183 |
| Baseline MADRS | F 1,5603 = 0.03 | 0.8664 |
| Sex | F 1,5603 = 0.76 | 0.6362 |
| Age | F 1,5603 = 0.76 | 0.3823 |
| Education | F 1,5603 = 0.02 | 0.8865 |
| Time | F 1,5603 = 7.42 | 0.0065 |
| Hemisphere | F 1,5603 = 0.29 | 0.5876 |
| Hippocampus * Time interaction | F 1,5603 = 24.68 | < 0.0001 |
This repeated measure mixed model analysis includes 152 depressed elders who had hippocampal volume measures at any time during their study participation. This analysis includes the 92 depressed participants included in the previous analyses detailed in Tables 1–3. Time refers to time in the study; hemisphere refers to right or left hippocampus. WMH = white matter hyperintensity volume. MADRS = Montgomery-Asberg Depression Rating Scale.
DISCUSSION
Although smaller hippocampal volumes have previously been associated with LLD and poorer antidepressant response in LLD, to our knowledge this is the first report to associate progressive hippocampal atrophy with persistence of depressive symptoms in LLD. Our primary finding is that in a cohort with LLD, persistent depression severity is associated with hippocampal atrophy.
Importantly, our two analytic models resulted in similar conclusions. Compared with the never-depressed cohort, in parsimonious models the nonremitting cohort had greater atrophy of the hippocampus bilaterally (Table 3). Similarly, smaller hippocampal volumes over time were associated with increasing or non-decreasing depression severity (Table 4). The consistency of our findings is important as in the full model (Table 3), cohort differences in hippocampal volume change were not statistically significant after controlling for sex, education, and WMH volume. Although this difference between models necessitates caution when interpreting the results, we feel confident in relying on the parsimonious model as none of the additional covariates added to the full model were significantly associated with change in hippocampal volume. Our finding is largely concordant with past work associating smaller hippocampal volumes with poorer long-term course of depression.25–28 Moreover, our findings are concordant with population studies finding that depression in older adults is associated with brain atrophy, including volumetric differences in the hippocampus.12,16
Although not the focus of this report, WMH volumes were not significantly associated with the dependent variables in either model. The lack of a significant association between change in WMH volume and hippocampal volume change (Table 3) is concordant with past reports that did not find significant associations between these measures in other elderly populations with cognitive impairment.47,48 Although we have previously reported a relationship between change in WMH volume and course of antidepressant response in LLD,46 in this study we did not find a significant relationship between WMH volumes and longitudinal depression severity. Given differences in the analytic approach used across these studies, this requires more study. It is possible that WMHs may influence response to antidepressants, but are less associated with fluctuations in mood over the course of treatment. As we have previously proposed, it is also possible that the effects of WMH on depression and the antidepressant response depend on hyperintensity location.49
In contrast to past reports in MDD and PTSD,32–34 we did not observe statistically significant increases in hippocampal volume in any cohort. One explanation for these discrepant findings may that the antidepressant effect on hippocampal neurogenesis declines with age,37 thus limiting our ability to observe a positive effect of antidepressants on hippocampus structure. However, as some other studies in MDD have also not found an effect of antidepressant treatment on hippocampal volume,36 there may not be a measurable effect to observe. When considering this issue, it is important to recognize that we examined an elderly cohort over two years. It is possible that the effect of aging on hippocampus structure may counterbalance the acute effects of antidepressants on neurogenesis and hippocampal morphology, particularly if neurogenesis is reduced with aging.
Our study has clinical implications. If replicated, it is possible that a reduction in hippocampal volume is an important biomarker associated with antidepressant nonresponse in LLD. Moreover, hippocampal atrophy is followed by cognitive decline and conversion to AD in both nondepressed 21,50,51 and depressed elders.13,14 Thus we are observing a phenomenon wherein a failure to remit with antidepressant treatment is associated with greater hippocampal atrophy. Such atrophy is then associated with subsequent cognitive impairment. This is an important subgroup requiring further study that may benefit from targeted interventions.
The study’s limitations include issues related to antidepressant treatment. Although participants met diagnostic criteria for MDD at entry, many depressed participants were taking antidepressants at enrollment. However, duration of depressive symptoms and duration of antidepressant use prior to enrollment was not available. Moreover, antidepressant treatment varied in the population over the course of the study, reflecting real-world concerns of working to provide participants with the best chance possible of achieving remission. Although addressed clinically, antidepressant treatment compliance was not quantified, and so it is possible that individuals who were less compliant had a poorer treatment response and also greater hippocampal atrophy. These issues complicate interpretation of study data, but do not diminish the importance of our primary finding and the variability in antidepressant use reflects clinical practice for refractory patients.
Other limitations relate to the imaging methods. These data were acquired on 1.5T MRI, resulting in lower resolution of structures that likely contributed to the lower ICCs (0.7 – 0.8). Finally, this study was focused solely on the hippocampus. Atrophy or changes in the prefrontal cortex, cingulate gyrus, or entorhinal cortex could also affect treatment outcomes.52 We could not examine that issue as we did not have volumetric data for discrete gray matter regions. Additionally, we do not have discrete data on the presence or severity of vascular risk factors that are associated with hippocampal volumes, such as hypertension, diabetes, or smoking.53 Finally, our initial models are limited by a relatively small sample size in some cohorts. However, we address this concern by combining all depressed subjects in our additional models examining depression severity.
In conclusion, hippocampal atrophy is associated with greater depression severity in older adults over two years. Such hippocampal atrophy may potentially serve as a biomarker predicting both reduced likelihood of response to antidepressants but also risk of cognitive decline. Future research should investigate this relationship further by also including probes of Alzheimer pathology, such as amyloid imaging. Depressed elders are an important population to study further to determine what treatments may improve both affective and cognitive symptoms.
Figure 1.
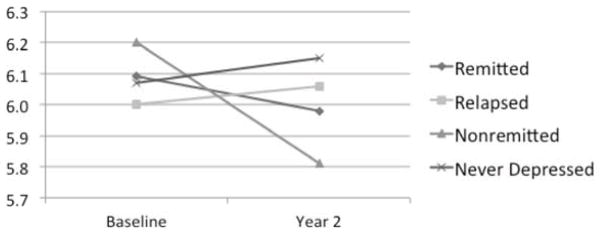
Two-year change in proportional hippocampus volumes
Total hippocampal volumes at baseline and year two. Presented are adjusted means for each time point, controlling for age and baseline cerebral volume. After controlling for these covariates in addition to time and hemisphere, hippocampal volume change differed significantly between cohorts (Table 3). This was primarily related to differences between the nonremitted and never-depressed cohorts. Other cohort comparisons did not demonstrate statistically significant differences.
Acknowledgments
This project was supported by the National Institute of Mental Health (NIMH) grants R01 MH077745, R01 MH054846, K24 MH070027, and P50 MH60451
Footnotes
The authors report no financial conflicts of interest.
Preliminary data from this study were presentation at the 2013 Annual Meeting of the Society of Biological Psychiatry in San Francisco, California, USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arnone D, McIntosh AM, Ebmeier KP, et al. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 3.McKinnon MC, Yucel K, Nazarov A, et al. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatr Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci USA. 2001;98:12320–12322. doi: 10.1073/pnas.231475998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amico F, Meisenzahl E, Koutsouleris N, et al. Structural MRI correlates for vulnerability and resilience to major depressive disorder. J Psychiatry Neurosci. 2011;36:15–22. doi: 10.1503/jpn.090186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Science’s STKE : signal transduction knowledge environment. 2004;2004:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- 8.Sheline YI. Depression and the hippocampus: cause or effect? Biol Psychiatry. 2011;70:308–309. doi: 10.1016/j.biopsych.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 10.Steffens DC, Byrum CE, McQuoid DR, et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 11.Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry. 2013;21:184–195. doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Geerlings MI, Sigurdsson S, Eiriksdottir G, et al. Associations of current and remitted major depressive disorder with brain atrophy: the AGES-Reykjavik Study. Psychol Med. 2013;43:317–328. doi: 10.1017/S0033291712001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien JT, Lloyd AJ, McKeith IG, et al. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 14.Steffens DC, McQuoid DR, Payne ME, et al. Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2011;19:4–12. doi: 10.1097/JGP.0b013e3181d6c245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezzati A, Zimmerman ME, Katz MJ, et al. Hippocampus. 2013. Hippocampal correlates of depression in healthy elderly adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.den Heijer T, Tiemeier H, Luijendijk HJ, et al. A study of the bidirectional association between hippocampal volume on magnetic resonance imaging and depression in the elderly. Biol Psychiatry. 2011;70:191–197. doi: 10.1016/j.biopsych.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Steffens DC, Plassman BL, Helms MJ, et al. A twin study of late-onset depression and apolipoprotein E epsilon 4 as risk factors for Alzheimer’s disease. Biol Psychiatry. 1997;41:851–856. doi: 10.1016/S0006-3223(96)00247-8. [DOI] [PubMed] [Google Scholar]
- 18.Devanand DP, Sano M, Tang M-X, et al. Depressed mood and incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 19.Heun R, Kockler M, Ptok U. Depression in Alzheimer’s disease: is there a temporal relationship between the onset of depression and the onset of dementia? Eur Psychiatry. 2002;17:254–258. doi: 10.1016/s0924-9338(02)00678-8. [DOI] [PubMed] [Google Scholar]
- 20.Jack CR, Jr, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desikan RS, Fischl B, Cabral HJ, et al. MRI measures of temporoparietal regions show differential rates of atrophy during prodromal AD. Neurology. 2008;71:819–825. doi: 10.1212/01.wnl.0000320055.57329.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh M-H, McQuoid DR, Levy RM, et al. Hippocampal volume and antidepressant response in geriatric depression. Int J Geriatr Psychiatry. 2002;17:519–525. doi: 10.1002/gps.611. [DOI] [PubMed] [Google Scholar]
- 23.Vakili K, Pillay SS, Lafer B, et al. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- 24.MacQueen GM, Yucel K, Taylor VH, et al. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry. 2008;64:880–883. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Frodl T, Meisenzahl EM, Zetzsche T, et al. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry. 2004;65:492–499. doi: 10.4088/jcp.v65n0407. [DOI] [PubMed] [Google Scholar]
- 26.Frodl T, Jager M, Smajstrlova I, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatr Neurosci. 2008;33:423–430. [PMC free article] [PubMed] [Google Scholar]
- 27.Kronmuller KT, Pantel J, Kohler S, et al. Hippocampal volume and 2-year outcome in depression. Br J Psychiatry. 2008;192:472–473. doi: 10.1192/bjp.bp.107.040378. [DOI] [PubMed] [Google Scholar]
- 28.Hoogenboom WS, Perlis RH, Smoller JW, et al. Feasibility of studying brain morphology in major depressive disorder with structural magnetic resonance imaging and clinical data from the electronic medical record: A pilot study. Psychiatry Res. 2012 doi: 10.1016/j.pscychresns.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malberg JE, Eisch AJ, Nestler EJ, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 31.Manji HK, Moore GJ, Rajkowska G, et al. Neuroplasticity and cellular resilience in mood disorders. Mol Psychiatry. 2000;5:578–593. doi: 10.1038/sj.mp.4000811. [DOI] [PubMed] [Google Scholar]
- 32.Vermetten E, Vythilingam M, Southwick S, et al. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bremner JD, Vermetten E. Neuroanatomical changes associated with pharmacotherapy in posttraumatic stress disorder. Ann N Y Acad Sci. 2004;1032:154–157. doi: 10.1196/annals.1314.012. [DOI] [PubMed] [Google Scholar]
- 34.Arnone D, McKie S, Elliott R, et al. State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.150. [DOI] [PubMed] [Google Scholar]
- 35.Schermuly I, Wolf D, Lieb K, et al. State dependent posterior hippocampal volume increases in patients with major depressive disorder. J Affect Disord. 2011;135:405–409. doi: 10.1016/j.jad.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 36.Vythilingam M, Vermetten E, Anderson GM, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Couillard-Despres S, Wuertinger C, Kandasamy M, et al. Ageing abolishes the effects of fluoxetine on neurogenesis. Mol Psychiatry. 2009;14:856–864. doi: 10.1038/mp.2008.147. [DOI] [PubMed] [Google Scholar]
- 38.Jack CR, Jr, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robins LN, Helzer JE, Croughan J, et al. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 40.Zannas AS, McQuoid DR, Payne ME, et al. Negative life stress and longitudinal hippocampal volume changes in older adults with and without depression. J Psychiatr Res. 2013 doi: 10.1016/j.jpsychires.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawyer K, Corsentino E, Sachs-Ericsson N, et al. Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non-depressed controls. Aging Ment Health. 2012;16:753–762. doi: 10.1080/13607863.2012.678478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 43.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 44.Steffens DC, McQuoid DR, Krishnan KRR. The Duke Somatic Treatment Algorithm for Geriatric Depression (STAGED) approach. Psychopharmacol Bull. 2002;36:58–68. [PubMed] [Google Scholar]
- 45.Payne ME, Fetzer DL, MacFall JR, et al. Development of a semi-automated method for quantification of MRI gray and white matter lesions in geriatric subjects. Psychiatry Res. 2002;115:63–77. doi: 10.1016/s0925-4927(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 46.Taylor WD, Steffens DC, MacFall JR, et al. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003;60:1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- 47.Du AT, Schuff N, Laakso MP, et al. Effects of subcortical ischemic vascular dementia and AD on entorhinal cortex and hippocampus. Neurology. 2002;58:1635–1641. doi: 10.1212/wnl.58.11.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Pol LA, van der Flier WM, Korf ES, et al. Baseline predictors of rates of hippocampal atrophy in mild cognitive impairment. Neurology. 2007;69:1491–1497. doi: 10.1212/01.wnl.0000277458.26846.96. [DOI] [PubMed] [Google Scholar]
- 49.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.20. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kramer JH, Mungas D, Reed BR, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mungas D, Harvey D, Reed BR, et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerritsen L, Comijs HC, van der Graaf Y, et al. Depression, Hypothalamic Pituitary Adrenal Axis, and Hippocampal and Entorhinal Cortex Volumes-The SMART Medea Study. Biol Psychiatry. 2011;70:373–380. doi: 10.1016/j.biopsych.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 53.Qiu C, Zhang Y, Bronge L, et al. Medial temporal lobe is vulnerable to vascular risk factors in men: a population-based study. Eur J Neurol. 2012;19:876–883. doi: 10.1111/j.1468-1331.2011.03645.x. [DOI] [PubMed] [Google Scholar]


