Abstract
G protein–coupled receptors (GPCRs) are versatile molecular machines that regulate the majority of physiological responses to chemically diverse hormones and neurotransmitters. Recent breakthroughs in structural studies have advanced our understanding of GPCR signaling, particularly the selectivity of ligand recognition and receptor activation of G proteins.
Membrane proteins have a critical role in cell-cell communication. Among them, the GPCRs evolved to sense a large number of extracellular signals such as photons, ions, monoamines, nucleosides, lipids, peptides and proteins1. GPCRs transmit these various chemical signals across the plasma membrane by activating G proteins and arrestins. The era of GPCR structural biology started in the 1990s with a series of studies using two-dimensional crystallography to determine the three-dimensional structure of rhodopsin (reviewed in ref. 2), a receptor for photons, showing for the first time the arrangement of the seven transmembrane segments within the membrane plane. This was followed in 2000 with the first high-resolution three-dimensional crystal structure of rhodopsin3. The first crystal structure of a GPCR activated by a diffusible ligand, the β2-adrenergic receptor (β2-AR)4, took another seven years, due in part to the low natural abundance and inherent instability of these membrane proteins. These structures provided high-resolution insights into GPCR function, particularly into how antagonists bind within the seven–transmembrane-segment hydrophobic core of the receptor and stabilize the inactive state. They also provided a structural context for a large body of published work that used mutagenesis and biophysical approaches to characterize receptor structure and function. Since then, a continuous flow of GPCR structures, predominantly in antagonist-bound conformations, has been published. The recent successes in GPCR crystallography are a consequence of several technological developments that helped overcome the three primary impediments to crystallogenesis: biochemical instability of purified GPCR protein in detergents, structural flexibility and the paucity of polar surface for forming crystal lattice contacts. These developments included protein engineering to decrease protein heterogeneity, enhance stability, reduce flexibility and increase the polar surface available for crystal lattice contacts; the development of lipid mesophase crystallography, a critical approach for membrane proteins that are unstable in short-chain detergents and thus are not amenable to the vapor diffusion strategy; the development of conformationally selective antibodies to increase polar surface available for crystal lattice contacts and stabilize specific receptor conformations; and the availability of small, intense X-ray sources (minibeams)5 required for collection of diffraction data from small, radiation-sensitive crystals. The latest breakthrough in the field of GPCR structural biology was the first structure of a GPCR–G protein complex, specifically the β2-AR coupled to the Gs protein, the stimulatory G protein for adenylyl cyclase6. Acquiring this long-awaited crystal structure was made possible not only by using many of the technologies cited above but also by trapping a highly stable state in an otherwise unstable signaling complex. Obtaining a stable complex involved the use of an enzyme to hydrolyze GDP and stabilize an empty G protein bound to its receptor, the use of an antibody from llamas that stabilizes the G protein’s active form, the addition of a highly crystallizable protein at the N terminus of the receptor (T4 lysozyme) and the development of a new detergent that stabilized the purified complex. Analysis of the growing body of structural data highlights the process by which chemically divergent stimuli are recognized as well as the mechanisms by which these various ligands lead to activation of cytosolic signaling proteins. Yet there is still much to be learned about these versatile signaling molecules before we will fully understand the molecular basis of receptor activation, G protein coupling specificity and arrestin signaling and the functional consequences of receptor oligomerization.
Sensing chemical diversity
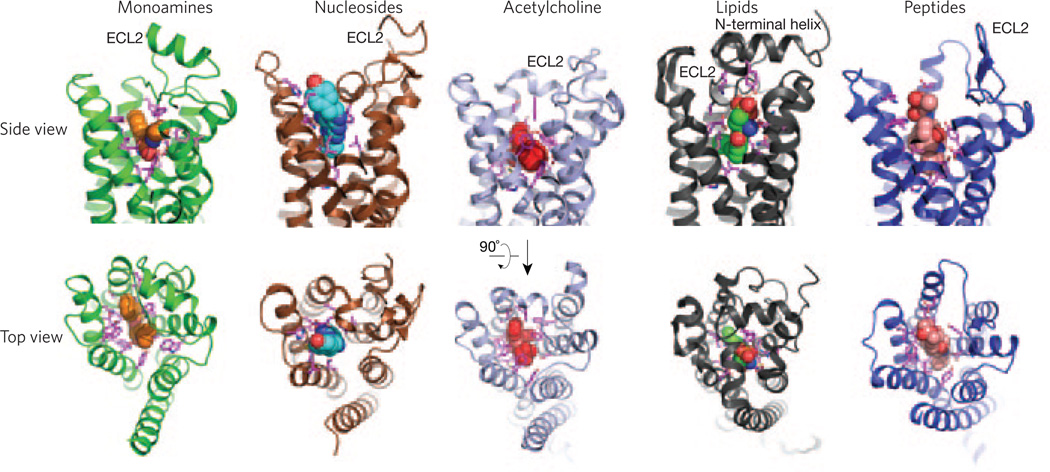
All GPCRs present a canonical fold of seven transmembrane segments embedded in the plasma membrane and connected by three loops facing the extracellular environment. These loops and the extracellular part of the seven–transmembrane-segment bundle constitute the ligand-binding domain for the class A GPCRs and are responsible for their ligand recognition versatility (Fig. 1). In addition to modulation via the binding site for hormones and neurotransmitters, commonly referred to as the orthosteric binding site, the function of some GPCRs can be modulated by ligands that bind outside of the orthosteric site. These ligands are known as allosteric ligands. The muscarinic receptors have become a model system for regulation by small allosteric modulators. Structural studies captured the details of the GPCR binding pocket architecture responsible for recognizing chemically different diffusible orthosteric ligands such as monoamines (β2-AR), nucleosides (A2A receptor), acetylcholine (M3 receptor), lipids (S1P1 receptor) and peptides (μ-opioid receptor (OR))7–10. All of these structures share a similar seven–transmembrane-segment architecture, with the orthosteric ligands used in the crystallization of each of these receptors bound in a pocket that can penetrate as deep as ~15 Å into the hydrophobic core of the protein. The size, shape and amino acid composition of these binding pockets explain the ability of GPCRs to discriminate between diverse chemical signals (Fig. 1).
Figure 1.
Diversity of ligand binding domains in class A GPCRs. View from the side (top) and from the extracellular surface (bottom) of receptors for chemically diverse ligands. Shown from left to right are the β2-adrenergic receptor bound to carazolol (orange spheres; Protein Data Bank (PDB) code 2RH1), the A2A adenosine receptor bound to ZM24138 (blue spheres; PDB code 3EML), the M2 muscarinic receptor bound to QNB (red spheres; PDB code 3UON), the sphingosine 1-phosphate receptor bound to ML056 (green spheres; PDB code 3V2Y) and the μ-opioid receptor bound to β-FNA (salmon spheres; PDB code 4DKL). A great diversity in extracellular domains, and most notably in ECL2, can be observed. Side chains of residues within 4 Å of the ligands forming the binding pocket are shown in pink.
Many hormones and neurotransmitters can activate more than one GPCR. For example, there are 9 adrenergic receptors that respond to adrenaline and noradrenaline, 5 muscarinic receptor subtypes and 13 serotonin receptor subtypes. When comparing members within a subfamily, there is high sequence conservation within the seven–transmembrane-segment core and more diversity within the cytoplasmic loops and C terminus. Each of these subtypes within a subfamily of GPCRs carries out a specific physiological function. For this reason, drug discovery efforts typically target one subtype rather than the entire subfamily. This has proven difficult for many subfamilies of GPCRs, particularly the muscarinic receptors. The first insights into the challenges of developing subtype-selective drugs come from the β-adrenergic, muscarinic and opioid receptor families, for which the structures of more than one family member have been determined.
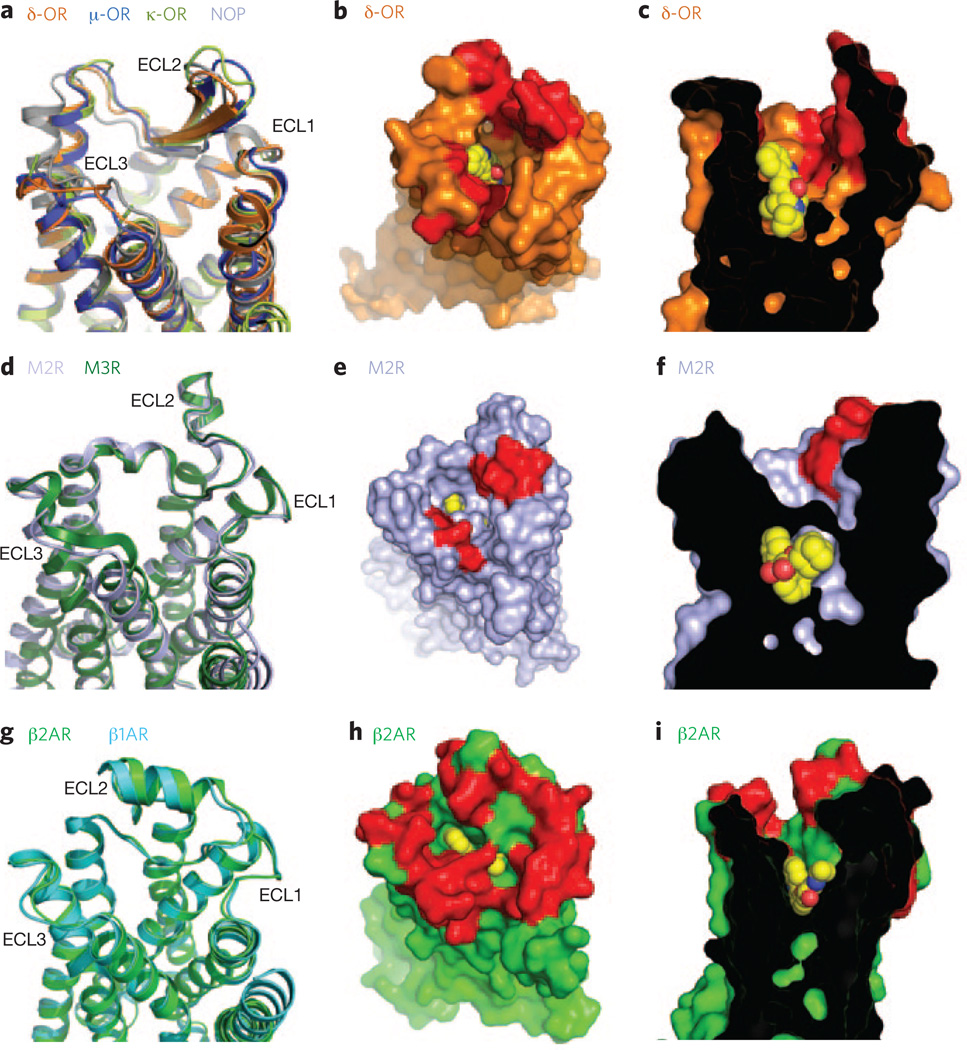
For the opioid receptors, the structures of all four subtypes have recently been determined (μ-OR, δ-OR, K-OR and nociceptin/orphanin FQ peptide receptor)10–13. Of interest, even with low conservation in their extracellular loop (ECL) sequences, the opioid receptors share a similar structural fold in their three ECLs, including a β-hairpin loop in the ECL2 (Fig. 2a). A surface view highlighting the amino acids implicated in opioid receptor–selective ligand binding clearly suggests the presence of a selectivity filter in the outer part of the binding pockets (Fig. 2b,c). For example, naltrindole selectively binds δ-OR, and this can be attributed to a single amino acid difference.
Figure 2.
Comparison of binding pockets within receptor subtypes. (a) Extracellular view of the opioid receptors (δ-OR (PDB code 4EJ4), δ-OR (PDB code 4DKL), K-OR (PDB code 4DJH) and nociceptim/ orphanin FQ peptide (NOP) (PDB code 4EA3)) showing structural conservation of the extracellular loops (ECLs 1–3). (b,c) Top (b) and side (c) views of the δ-OR bound to naltrindole (yellow spheres), showing the selectivity determinants in red. (d) Extracellular view of the M2 and M3 muscarinic receptors (M2R (PDB code 3UON) and M3R (PDB code 4DAJ)) showing a similar fold in ECLs 1, 2 and 3. (e,f) Top (e) and side (f) views of the M2R bound to QNB (yellow spheres), with the residues that form the allosteric binding pocket shown in red. (g) Extracellular view of the β1-AR and β2-AR (PDB codes 2Y04 and 2RH1, respectively) showing the structural conservation of extracellular loops (ECLs 1–3). (h,i) Top (h) and side (i) views of the β2-AR bound to carazolol (yellow spheres), with the residues that differ between β1-AR and β2-AR shown in red.
Interestingly, this selectivity filter in the opioid receptor family is structurally analogous to the allosteric binding region found in the structure of muscarinic M2 and M3 receptors14,15. Though the orthosteric binding site is strictly conserved between these two muscarinic receptor subtypes, the allosteric pocket is poorly conserved and forms an extracellular vestibule acting as a potential selective filter (Fig. 2d–f). Molecular dynamic simulations suggest that this allosteric site may contribute some selectivity to orthosteric ligand binding14. Transient interactions between orthosteric ligands and amino acids in the extracellular vestibule may preferentially influence association or dissociation kinetics, even though these amino acids do not form part of the orthosteric pocket. A better understanding of the nature of interactions between ligands and the extracellular vestibule may provide a new opportunity for designing more subtype-specific ligands.
The same concept of a selectivity filter could be applied to the binding selectivity of β-ARs. The orthosteric binding pockets of β1-AR and β2-AR are nearly identical, differing by only one amino acid. However, as in the muscarinic receptors, the amino acid composition of the extracellular vestibules leading to the orthosteric binding pockets is more diverse. This diversity not only contributes to greater subtype selectivity of larger ligands that extend into the vestibule but also may contribute as a selectivity filter, as small ligands go into and out of the orthosteric pocket (Fig. 2g–i). As with the muscarinic receptors, unbiased molecular dynamics simulations suggest that binding of adrenergic ligands follows a multistep binding process16, the first binding interaction in the extracellular vestibule being the most energetically demanding16 and thus the most likely to contribute to binding selectivity.
Turning the receptor on
GPCRs can be viewed as molecular engines idling at the cell surface and oscillating between the inactive and active states, with the inactive state being the most stable and the most highly represented. Agonist binding stabilizes conformational states that bind and activate G proteins and other signaling molecules such as arrestins. Comparison of antagonist and agonist binding pockets is now possible for several GPCRs (reviewed in ref. 17). In addition, differences in the binding pockets of agonist-bound GPCR structures in various states of activation, as reflected in conformational changes in the cytoplasmic domains, have been observed for the β2-AR. These range from the agonist-bound and inactive state to the agonist-bound and fully active state in complex with a G protein6,18. The availability of multistate structural data facilitates the application of computational approaches to approximate active-state binding pockets from inactive-state crystal structures, enabling structure-based docking studies to identify new agonists. As the antagonist molecules dominate the docking against inactive structure, it is expected that agonists will dominate docking studies performed against the active state.
There is a growing body of evidence that GPCRs can adopt multiple conformations that include more than one active state. In fact, the chemical structure of ligands can be differentially decoded by receptors to select which specific signaling pathway to activate. For example, some synthetic agonists can stimulate G protein signaling but not β-arrestin–mediated signaling. The reverse situation has also been observed, in which a ligand can block G protein signaling and stimulate arrestin signaling. Recent studies demonstrate that this phenomenon is made possible by the structural plasticity of GPCRs, whose active conformation for the G protein pathway is different from that for the β-arrestin signaling pathway. This has been demonstrated for the β2-AR and the vasopressin type 2 receptor using biochemical and biophysical approaches19–21. A biased ligand is thus able to stabilize the active state for primarily one of several possible signaling pathways. Movement of transmembrane segment 7 and helix 8 was found to be important for the β-arrestin active state, whereas conformational changes in transmembrane segment 6 are associated with G protein activation19–21.
Current challenges and future directions
GPCRs represent the largest target class of all US Food and Drug Administration–approved drugs. Since the 1990s, advances in the structural and biochemical characterization of GPCRs have led to a better understanding of these signaling molecules. However, the application of structural approaches to developing safer, more effective drugs for these important clinical targets is just beginning. The current limitations for structure-based drug discovery are discussed below.
GPCR stability
For soluble protein targets such as kinases and proteases, new crystal structures can be obtained in a matter of months. Though the time required to obtain a new GPCR structure has been reduced substantially during the past several years, it is still highly dependent on the properties of the specific target and the availability of high-affinity ligands to stabilize the protein during expression and crystallogenesis. Furthermore, the size and stability of GPCR crystals, together with the limitations of in meso crystallization methods, makes exchanging ligands within a formed crystal impractical or impossible. Therefore, obtaining structures of low-affinity lead compounds requires starting from purified protein, and the low affinity of a lead compound may be insufficient to stabilize the receptor during crystallogenesis.
Conformational diversity
As noted above, GPCRs can activate more than one G protein or other signaling proteins such as arrestins in a ligand-dependent manner. Evidence suggests that the use of in silico screening will identify ligands of the same efficacy class as the ligand used to obtain the crystal structure. Therefore, we need more structures describing different active states of GPCRs. To fully understand the structural basis of receptor–G protein coupling specificity, we need structures of GPCRs in complex with Gi, Gq and other G proteins. GPCRs are also tightly regulated by GPCR kinases (GRKs) that phosphorylate the C terminus or intracellular loops of receptors. This process also is ligand dependent, and the pattern of phosphorylation may be GRK or ligand specific and thereby influence both desensitization and arrestin signaling. Obtaining GPCR–GRK and GPCR–arrestin complex structures will help us understand the intricate behavior of ligand-biased signaling through arrestins22.
New targets
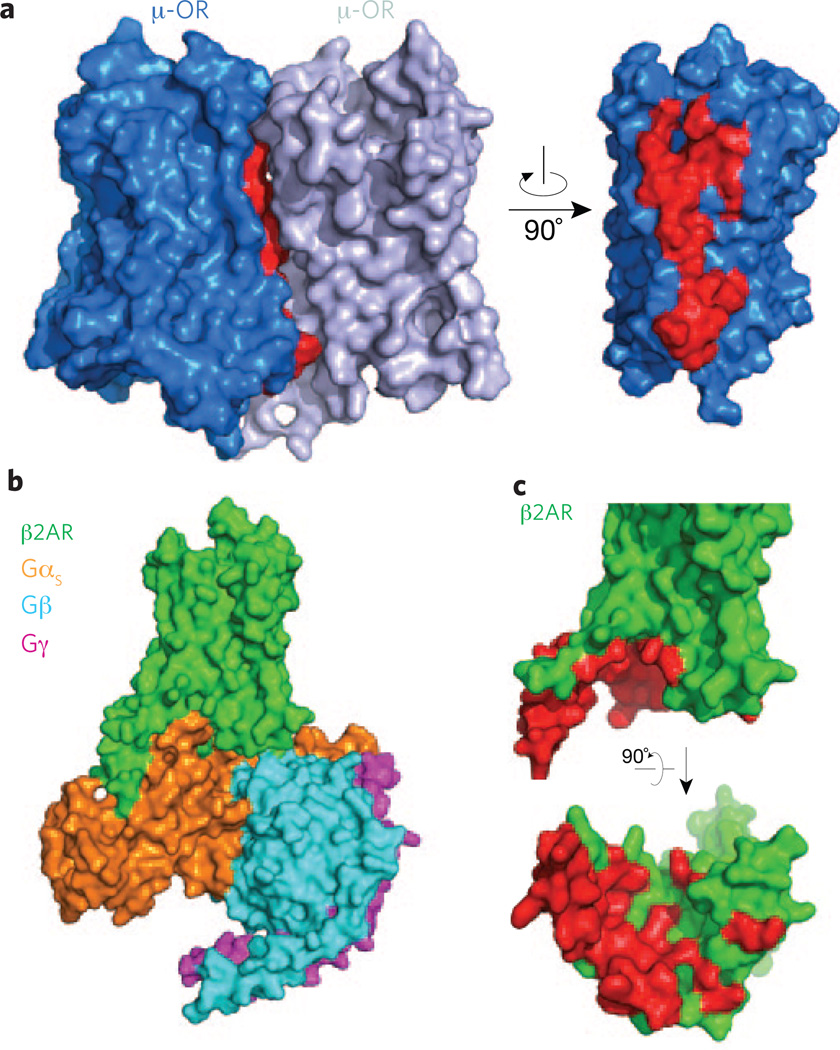
In spite of the challenges that remain, recent GPCR structures have identified new opportunities for drug discovery. These potential targets include the dimeric interfaces, such as those found in the μ-OR and CXCR4 receptor structures10,23 (Fig. 3a). Developing compounds based on these structures that could alter receptor dimers or oligomers will undoubtedly help those in the field to determine the potential role of oligomerization in receptor function, an issue that has been unresolved for more than 15 years. In addition, we now have access to the structure of a G protein–binding pocket (Fig. 3b,c), another potential target for developing small diffusible ligands that are able to penetrate within the cells to modulate GPCR function. This was shown to be feasible with the success of small molecules targeting the β- and γ-subunits of G proteins and modulating GPCR function24.
Figure 3.
Potential domains to target for regulating receptor function. (a) Dimer interface observed in the δOR crystal structure (PDB code 4DKL) involving transmembrane segments 5 and 6 of each receptor molecule. Residues at the interface are shown in red. (b) Overall view from within the membrane plane of the β2-AR signaling complex (PDB code 3SN6) comprising the β2-AR and the Gs heterotrimer with the αs, β and γ subunits. (c) Side (top) and bottom (bottom) views of β2-AR, with the residues forming the binding pocket of the Gs protein highlighted in red.
Understanding GPCR dynamics
As noted above, it is now generally accepted that GPCRs can adopt multiple conformations. Evidence from both functional and biophysical studies on several well-characterized GPCR models demonstrates that structurally distinct ligands can stabilize functionally distinct conformations25. However, the mechanisms by which ligands induce or stabilize specific conformations and the timescales over which transitions between different conformations occur are poorly understood. These parameters will most likely differ in different cellular contexts because of differences in associated signaling proteins and the composition of the lipid bilayer. A better understanding of the dynamic character of GPCRs may contribute to the development of more selective and effective drugs.
Conclusions
The structural biology of GPCRs, though far from common, has become more tractable. Crystal structures obtained thus far have contributed to our understanding of ligand binding specificity and the process of G protein activation. However, we still know relatively little about how these versatile signaling proteins work at a molecular level. Future efforts to determine the structures of complexes between GPCRs and other signaling proteins and to characterize the role of protein dynamics in receptor function will further our understanding of their complex signaling behavior. The challenge for the field will be to integrate these structural insights and structural approaches into the drug discovery process to facilitate more efficient and economical development of safer and more effective drugs.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Bockaert J, Pin JP. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schertler GF. Eye (Lond.) 1998;12:504–510. doi: 10.1038/eye.1998.138. [DOI] [PubMed] [Google Scholar]
- 3.Palczewski K, et al. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen SG, et al. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 5.Kobilka B, Schertler GF. Trends Pharmacol. Sci. 2008;29:79–83. doi: 10.1016/j.tips.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen SG, et al. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbaum DM, et al. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 8.Jaakola VP, et al. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanson MA, et al. Science. 2012;335:851–855. doi: 10.1126/science.1215904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manglik A, et al. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granier S, et al. Nature. 2012;485:400–404. doi: 10.1038/nature11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, et al. Nature. 2012;485:327–332. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson AA, et al. Nature. 2012;485:395–399. doi: 10.1038/nature11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruse AC, et al. Nature. 2012;482:552–556. doi: 10.1038/nature10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haga K, et al. Nature. 2012;482:547–551. doi: 10.1038/nature10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dror RO, et al. Proc. Natl. Acad. Sci. USA. 2011;108:18684–18689. doi: 10.1073/pnas.1110499108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebon G, Warne T, Tate CG. Curr. Opin. Struct. Biol. 2012 Apr 3; doi: 10.1016/j.sbi.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum DM, et al. Nature. 2011;469:236–240. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahsai AW, et al. Nat. Chem. Biol. 2011;7:692–700. doi: 10.1038/nchembio.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu JJ, Horst R, Katritch V, Stevens RC, Wuthrich K. Science. 2012;335:1106–1110. doi: 10.1126/science.1215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahmeh R, et al. Proc. Natl. Acad. Sci. USA. 2012;109:6733–6738. doi: 10.1073/pnas.1201093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajagopal S, Rajagopal K, Lefkowitz RJ. Nat. Rev. Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu B, et al. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonacci TM, et al. Science. 2006;312:443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- 25.Kobilka BK, Deupi X. Trends Pharmacol. Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]