Abstract
Objective
Lymphatic vessels collect extravasated fluid and proteins from tissues to blood circulation as well as play an essential role in lipid metabolism by taking up intestinal chylomicrons. Previous studies have shown that impairment of lymphatic vessel function causes lymphedema and fat accumulation, but clear connections between arterial pathologies and lymphatic vessels have not been described.
Approach and Results
Two transgenic mouse strains with lymphatic insufficiency (sVEGFR3 and Chy) were crossed with atherosclerotic mice (LDLR−/−/ApoB100/100) to study the effects of insufficient lymphatic vessel transport on lipoprotein metabolism and atherosclerosis. Both sVEGFR3 × LDLR−/−/ApoB100/100 mice and Chy × LDLR−/−/ApoB100/100 mice had higher plasma cholesterol levels compared to LDLR−/−/ApoB100/100 control mice during both normal chow diet (16.3 mmol/l and 13.7 mmol/l vs. 8.2 mmol/l, respectively) and Western-type high fat diet (e.g. after 2 weeks of fat diet 45.9 mmol/l and 42.6 mmol/l vs. 30.2 mmol/l, respectively). Cholesterol and triglyceride levels in very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL) fractions were increased. Atherosclerotic lesions in young and intermediate cohorts of sVEGFR3 × LDLR−/−/ApoB100/100 mice progressed faster than in control mice (e.g. intermediate cohort mice at 6 weeks 18.3% vs. 7.7% of the whole aorta, respectively). In addition, lesions in sVEGFR3 × LDLR−/−/ApoB100/100 mice and Chy × LDLR−/−/ApoB100/100 mice had much less lymphatic vessels than lesions in control mice (0.33% and 1.07% vs. 7.45% of podoplanin positive vessels, respectively).
Conclusions
We show a novel finding linking impaired lymphatic vessels to lipoprotein metabolism, increased plasma cholesterol levels and enhanced atherogenesis.
Keywords: Atherosclerosis, Lymphatic capillary, Lipoprotein
INTRODUCTION
Cardiovascular complications caused by atherosclerosis, such as coronary artery disease and stroke, are major health problems in the Western world. Several factors contribute to atherogenesis but its main causes are elevated levels of low-density lipoprotein (LDL) and low levels of high-density lipoprotein (HDL) resulting from genetic factors and excessive consumption of dietary fat. The primary characteristics of atherosclerotic lesions are intimal accumulation of cholesterol-rich macrophages and proliferation of smooth muscle cells.1-3 In addition, some advanced lesions stimulate proliferation of vasa vasorum that arise from the adventitia and invade into the vessel wall. The increased vascularity around atheromas is hypothesized to provide oxygen and nutrients, and conduits for recruitment of inflammatory cells to the expanding vessel wall. However, they can also lead to hemorrhages that make lesions more prone to rupture.4-6
Lymphatic vessels are responsible for collecting and transporting protein-rich extravasated fluid from tissues to blood circulation and are required for chylomicron absorption from the intestinal microvillus. In addition, they participate in inflammatory reactions by transporting antigens and inflammatory cells to lymph nodes and sites of inflammation.7,8 Vascular endothelial growth factor receptor 3 (VEGFR3) is a receptor for VEGF-C and VEGF-D, and a key mediator of lymphangiogenesis and maintenance of lymphatic endothelium.9,10 VEGFR3 is required for normal vascular development11, but its postnatal expression is mainly restricted to lymphatic vessels.12
We have here studied effects of high-fat feeding on serum lipids and atheroma formation in mouse models of lymphatic dysfunction. Soluble VEGFR3 (sVEGFR3) mice have impaired lymphatic vessels in the skin and some more distant organs because they secrete, under the control of the basal keratinocyte K14-promoter, a chimeric fusion protein which consists of the ligand binding portion of VEGFR3 extracellular domain and the fragment crystallizable (Fc) domain of immunoglobulin (Ig) γ-chain.13 Chy mice have impaired lymphangiogenesis due to an inactivating point mutation in the VEGFR3 gene.14 Crossing the mice with atherogenic mice deficient of low-density lipoprotein receptor and apolipoprotein B48 (LDLR−/−/ApoB100/100)15 showed that sVEGFR3 × LDLR−/−/ApoB100/100 mice and Chy × LDLR−/−/ApoB100/100 mice have increased cholesterol levels leading to accelerated atherogenesis especially in young and intermediate mouse cohorts. Our results suggest that lymphatic vessels have an important role in maintaining proper lipoprotein metabolism and vascular homeostasis.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Effects of Western diet on blood lipid levels in mice with dysfunctional lymphatic vessels
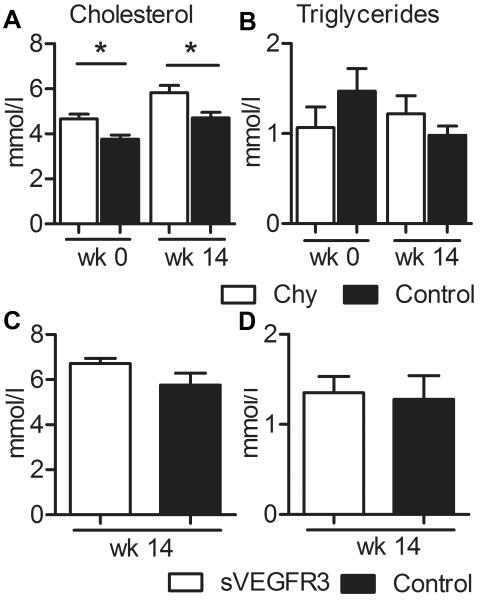
Weight gain and food consumption was similar between the Chy mice and their wild type littermates over the 14-week feeding period on a Western diet (data not shown). Dual-energy X-ray absorptiometry (DEXA) analysis demonstrated a modest but significant reduction of fat mass in Chy mice compared with wild type littermates (11.6 ± 1.1 g vs. 15.1 ± 0.8 g, p = 0.03). Serum cholesterol levels were significantly higher in Chy mice, both on chow and Western diet (Fig. 1A). However, no significant differences in the triglyceride levels were observed between the mice of the two genotypes (Fig. 1B). The sVEGFR3 mice and their wild type littermates had similar body weight and food consumption after 14 wk on Western diet. No significant changes in total cholesterol or triglycerides (Fig 1C and D) levels were observed between the genotypes although a small trend towards higher cholesterol values was apparent in the sVEGFR3 animals.
Figure 1.
Effects of Western diet on Chy and sVEGFR3 mice. Total cholesterol (A) and triglycerides (B) were measured in male Chy mice and their wild type littermates on chow diet and after 14 wk on Western diet and in male sVEGFR3 mice and their littermates after 14 wk on Western diet (C and D, respectively). Conversion factors from mmol/l to mg/dl for cholesterol and triglycerides are 38.67 and 88.57, respectively. Data is presented as mean ± SEM. * p < 0.05.
Characterization of sVEGFR3 × LDLR−/−/ApoB100/100 mice and Chy × LDLR−/−/ApoB100/100 mice
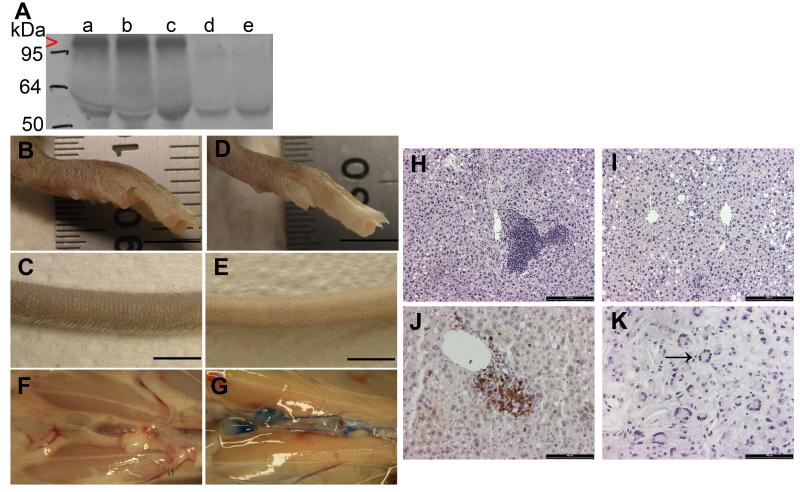
sVEGFR3 × LDLR−/−/ApoB100/100 mice were viable and fertile and there was no difference in weight gain compared to controls. sVEGFR3 protein was present in blood plasma in detectable amounts (Fig. 2A). Chy × LDLR−/−/ApoB100/100 mice had a normal lifespan and weight, but remarkably smaller litter sizes (3.8 pups / litter) and a lower frequency of the mutant allele (17 % of born pups). In addition, approximately 10 % of the Chy × LDLR−/−/ApoB100/100 pups developed severe ascites in the abdomen and needed to be sacrificed at weaning.
Figure 2.
Characterization of the sVEGFR3 × LDLR−/−/ApoB100/100 mouse model. Expression of sVEGFR3 in plasma was confirmed by western blotting (A). sVEGFR3 was expressed in sVEGFR3 × LDLR−/−/ApoB100/100 mice (lanes a, b and c, red arrowhead), but not in LDLR−/−/ApoB100/100 mice (lanes d and e). Antibody cross-reacted with mouse IgG heavy chain (55 kDa). Lymphatic drainage was affected in sVEGFR3 × LDLR−/−/ApoB100/100 mice causing swelling in the feet (B) and tails (C) compared to LDLR−/−/ApoB100/100 mice (D and E, respectively). Lymphatic insufficiency was confirmed with Evans Blue dye injections into footpads. No visible dye was detected in the abdominal collecting lymphatic vessels in sVEGFR3 × LDLR−/−/ApoB100/100 mice 30 min after dye injection (F), whereas collecting lymphatic vessels in LDLR−/−/ApoB100/100 mice were clearly stained (G). On Western diet, sVEGFR3 × LDLR−/−/ApoB100/100 mice had accumulation of inflammatory cells around hepatic central veins in the liver (H) whereas livers in LDLR−/−/ApoB100/100 mice were normal (I). Most inflammatory cells were CD3 positive T-lymphocytes (J). Compromised lymphatic function caused also swelling in the jaw area of sVEGFR3 × LDLR−/−/ApoB100/100 mice characterized by cholesterol crystal-like structures and multinuclear inflammatory giant cells (K, black arrow). Bar 5 mm (B-E), 200 μm (H and I) and 100 μm (J and K).
Lymphatic vessels were absent in the skin of sVEGFR3 × LDLR−/−/ApoB100/100 mice and Chy × LDLR−/−/ApoB100/100 mice. In particular, sVEGFR3 × LDLR−/−/ApoB100/100 mice had swollen feet and tails (Fig. 2B and C) compared to LDLR−/−/ApoB100/100 mice (Fig. 2D and E), which indicated accumulation of fluid and fat in the tissues. Lymphatic function was investigated after Evans Blue dye injections into footpads. In sVEGFR3 × LDLR−/−/ApoB100/100 mice, transport of Evans Blue from feet to collecting lymphatic vessels was inhibited (Fig. 2F) and no blue dye was visible in the thoracic duct 30 min after the injection. Transport of Evans Blue dye was normal in LDLR−/−/ApoB100/100 mice (Fig. 2G). The old cohort of sVEGFR3 × LDLR−/−/ApoB100/100 mice on Western diet had accumulation of inflammatory cells in the livers (Fig. 2H), such cells were not present in the livers of Chy × LDLR−/−/ApoB100/100 mice or LDLR−/−/ApoB100/100 control mice (Fig. 2I). These inflammatory cells were mostly CD3 positive T-cells that accumulated in large quantities around the central veins (Fig. 2J). In addition, some sVEGFR3 × LDLR−/−/ApoB100/100 mice had swelling in their jaws, with histologic evidence of fat tissue and inflammatory giant cells in their neck area (Fig. 2K).
Analysis of metabolic factors in sVEGFR3 × LDLR−/−/ApoB100/100 mice and Chy × LDLR−/−/ApoB100/100 mice
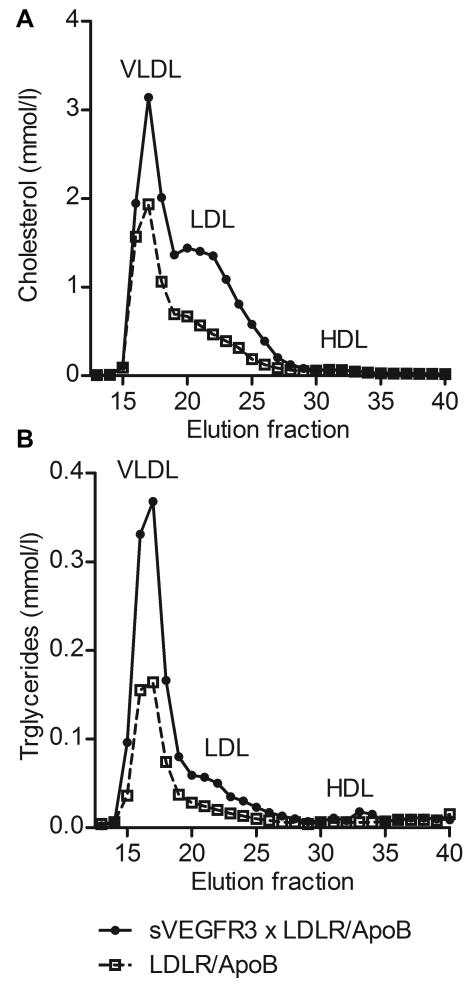
Cholesterol and triglyceride levels in blood plasma were measured to determine the effect of impaired lymphatic function on blood lipids. Cholesterol levels (Table 1) were significantly elevated in sVEGFR3 × LDLR−/−/ApoB100/100 mice (16.3 ± 1.2 mmol/l) and Chy × LDLR−/−/ApoB100/100 mice (13.7 ± 0.8 mmol/l) on chow diet compared to LDLR−/−/ApoB100/100 control mice (8.2 ± 0.4 mmol/l). Significantly higher cholesterol levels were maintained in sVEGFR3 × LDLR−/−/ApoB100/100 mice and in Chy × LDLR−/−/ApoB100/100 mice after starting the Western diet (Table 1). Triglyceride levels were also significantly higher in sVEGFR3 × LDLR−/−/ApoB100/100 mice before Western diet compared to controls (Table 1). To assess the distribution of cholesterol and triglycerides in different lipoprotein fractions, lipoprotein profiles were determined by fast protein liquid chromatography (FPLC) analysis of plasma samples from sVEGFR3 × LDLR−/−/ApoB100/100 mice and LDLR−/−/ApoB100/100 mice after 6 weeks on Western diet. sVEGFR3 × LDLR−/−/ApoB100/100 mice had higher levels of cholesterol (Fig. 3A) and triglycerides (Fig. 3B) in VLDL and LDL fractions when compared to control mice. Both groups had low levels of cholesterol and triglycerides in HDL fraction.
Table 1.
Cholesterol and triglyceride levels of sVEGFR3 × LDLR−/−/ApoB100/100 mice, Chy × LDLR−/−/ApoB100/100 mice and LDLR−/−/ApoB100/100 control mice on chow (wk 0) and Western diet for 2, 4, 6 and 12 weeks.
| Cholesterol (mmol/l) |
|||||
|---|---|---|---|---|---|
| wk 0 | wk 2 | wk 4 | wk 6 | wk 12 | |
|
|
|||||
| sVEGFR3 × LDLR/ApoB |
16.3±1.2(19)**** | 45.9±3.0(20)**** | 58.8±5.2(11)**** | 50.8±4.4(16)* | 52.7±8.7(7) |
| Chy × LDLR/ApoB |
13.7±0.8(5)# | 42.6±0.7(5)# | 46.3±8.1(4) | 53.5±10.5(3) | 53.5±3.1(2) |
| LDLR/ApoB | 8.2±0.4(21) | 30.2±1.2(21) | 31.8±4.8(14) | 36.9±3.0(20) | 46.0±3.0(9) |
| Triglycerides (mmol/l) |
|||||
| wk 0 | wk 2 | wk 4 | wk 6 | wk 12 | |
|
|
|||||
| sVEGFR3 × LDLR/ApoB |
1.9 ± 0.1(20)*** | 2.6 ± 0.3(21) | 4.3 ± 0.8(12) | 3.8 ± 0.8(16) | 4.2 ± 1.9 (7) |
| Chy × LDLR/ApoB |
1.6 ± 0.1(5) | 3.4 ± 0.5(5) | 2.3 ± 0.7(4) | 4.3 ± 1.9(3) | 3.1 ± 0.6(2) |
| LDLR/ApoB | 1.4 ± 0.1(21) | 2.9 ± 0.3(21) | 2.5 ± 0.4(10) | 3.3 ± 0.4(19) | 2.5 ± 0.4(9) |
Values are mean ± SEM. No. of animals is marked in parenthesis. Values from sVEGFR3 × LDLR−/−/ApoB100/100 (*) mice and Chy × LDLR−/−/ApoB100/100 (#) mice are compared to values from LDLR−/−/ApoB100/100 control mice at the same time point. Values are combined from all age cohorts and both genders. Conversion factors from mmol/l to mg/dl for cholesterol and triglycerides are 38.67 and 88.57, respectively.
p < 0.0001,
p < 0.001,
p < 0.01,
p < 0.05 and
< 0,05
Figure 3.
Analysis of cholesterol and triglyceride levels in plasma lipoproteins. Distribution of cholesterol (A) and triglycerides (B) in different lipoprotein fractions in young sVEGFR3 × LDLR−/−/ApoB100/100 mice fed Western diet for 6 wk show clear increases in VLDL and LDL fractions in sVEGFR3 × LDLR−/−/ApoB100/100 mice compared to LDLR−/−/ApoB100/100 mice. Both genders have been used in the analysis.
To further analyze lipid metabolism in sVEGFR3 × LDLR−/−/ApoB100/100 mice, intestinal triglyceride absorption was measured with radioactive Triolein either with or without the lipoprotein lipase (LPL) inhibitor Triton WR1339. After lipid bolus, radioactivity in plasma did not differ between sVEGFR3 × LDLR−/−/ApoB100/100 mice and LDLR−/−/ApoB100/100 control mice (Supplement IA). To test if the lipoprotein uptake in the liver is altered, LDL turnover was analyzed. sVEGFR3 × LDLR−/−/ApoB100/100 mice tended to have a slower clearance rate of 125I-LDL than controls (75.1 % vs. 64.5% of 125I-LDL remaining in the blood 12 h after the injection, respectively). However, the difference was not statistically significant. The half-life of 125I-LDL was around 24 h in both groups (Supplement IB). To identify differences in HDL functions, in vivo reverse cholesterol transfer (RCT) rates were compared between these 2 strains after injection of macrophages labeled with 3H-cholesterol in the peritoneum. In vivo RCT was similar in both groups (Supplement IC). In addition, the effect of impaired lymphatic vessels on glucose metabolism was analyzed with glucose tolerance tests. Both sVEGFR3 × LDLR−/−/ApoB100/100 mice and Chy × LDLR−/−/ApoB100/100 mice had similar fasting glucose levels compared to controls and plasma glucose levels returned to basal levels 60 min after the glucose injection in all groups (Supplement ID).
Progression of atherosclerosis in sVEGFR3 × LDLR−/−/ApoB100/100 mice and Chy × LDLR−/−/ApoB100/100 mice
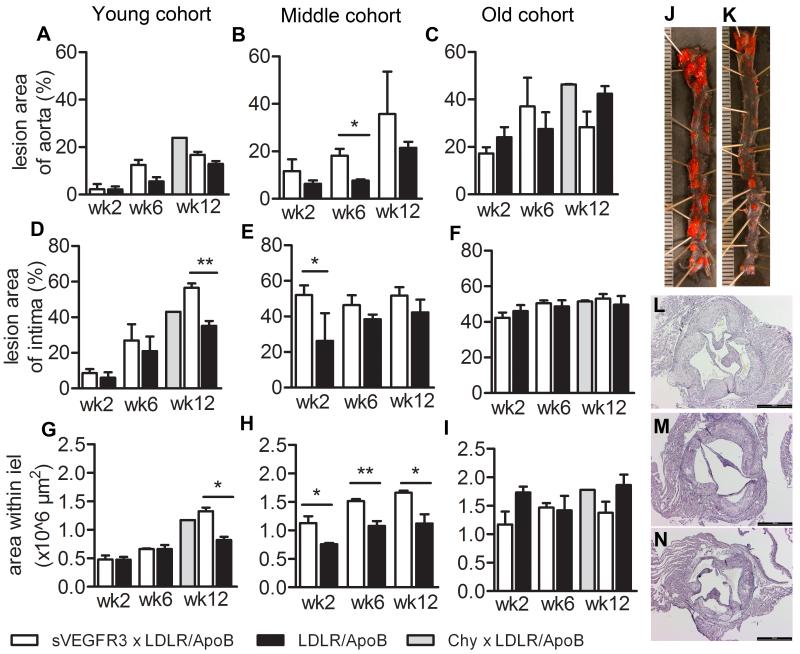
Atherosclerotic lesions were measured from young, intermediate and old mouse cohorts fed Western diet for 3 – 12 weeks as the percent area of lesions in the entire aorta and by the mean cross-sectional area of intima at the aortic root. sVEGFR3 × LDLR−/−/ApoB100/100 mice had increased areas of atherosclerotic lesions in en face opened aortas compared to controls and reached statistical significance in the intermediate cohort on Western diet for 6 weeks (Fig. 4A-C). Intimal lesion areas in the aortic roots were also generally larger in sVEGFR3 × LDLR−/−/ApoB100/100 mice and reached statistical significance compared to controls in the young and intermediate cohorts on Western diet for 12 and 2 weeks, respectively (Fig. 4D-F). Atherosclerotic lesion size was also increased in Chy × LDLR−/−/ApoB100/100 mice. Lesion development was compensated with outward remodeling in sVEGFR3 × LDLR−/−/ApoB100/100 mice since the area within the internal elastic lamina (IEL) was increased at the latest time point in young cohort and at all time points in intermediate cohort of sVEGFR3 × LDLR−/−/ApoB100/100 mice compared to controls (Fig. 4G-I). In the old cohort, area within internal elastic lamina was similar between groups. Differences in the lesion sizes can be easily visualized in the en face opened aortas (Fig 4J and K) and Hematoxylin-Eosin stained cross-sections (Fig. 4L-N, respectively).
Figure 4.
Evaluation of atherosclerotic lesion areas in sVEGFR3 × LDLR−/−/ApoB100/100 mice, Chy × LDLR−/−/ApoB100/100 mice and LDLR−/−/ApoB100/100 mice. Data is presented as the total en face lesion area of the total aortic area (A, B and C), as the total intimal lesion area of the aortic root (D, E and F) and as area within internal elastic lamina (G, H and I). Lesion areas were measured on 2, 6 and 12 weeks after starting the Western diet in the young, intermediate and old cohorts (3-4, 7-8, and 11-12 months of age, respectively). Both genders were used in the analysis. Representative pictures of En face aortas in intermediate cohort of sVEGFR3 × LDLR−/−/ApoB100/100 mice (J) and LDLR−/−/ApoB100/100 mice (K) after 4 weeks of Western diet and Hematoxylin-Eosin stainings showing atherosclerotic lesions in young cohorts sVEGFR3 × LDLR−/−/ApoB100/100 mice (G), Chy × LDLR−/−/ApoB100/100 mice (H) and LDLR−/−/ApoB100/100 mice (I) after 12 weeks of Western diet. Data is presented as mean ± SEM. * p < 0.05, ** p < 0.01. Unit 500 μm (J and K) and bar 500 μm (L-N).
The impact of aging and Western diet on atherosclerotic lesion composition was examined on modified Movat’s stains of aortic cross-sections of intermediate cohort sVEGFR3 × LDLR−/−/ApoB100/100 mice and LDLR−/−/ApoB100/100 control mice after 2, 6 and 12 weeks of Western diet. Lesions from sVEGFR3 × LDLR−/−/ApoB100/100 mice contained cholesterol crystals as early as 2 weeks on Western diet, whereas lesions developed more slowly in control LDLR−/−/ApoB100/100 mice and consisted mainly of foam cells up to 6 weeks on Western diet (Fig.5A-D). After 12 weeks of Western diet, many lesions in LDLR−/−/ApoB100/100 mice contained atheromatous cores, whereas lesions in sVEGFR3 × LDLR−/−/ApoB100/100 mice still had some cellularity (Fig. 5E and F). Results were also confirmed with immunohistochemical staining for macrophages (Fig. 5G and H). Intermediate cohorts of sVEGFR3 × LDLR−/−/ApoB100/100 mice and LDLR−/−/ApoB100/100 appeared to have similar quantities of lipids in the lesions (Fig. 5 I and J). Lesions of young cohort Chy × LDLR−/−/ApoB100/100 mice after 12 wk on Western diet showed cholesterol crystals and some foam cells (Fig. 5K and L).
Figure 5.
Evaluation of lesion composition in sVEGFR3 × LDLR−/−/ApoB100/100 mice, Chy × LDLR−/−/ApoB100/100 mice and LDLR−/−/ApoB100/100 mice. Representative pictures of modified Movat’s pentachrome stainings of atherosclerotic lesions in aortic roots after 2, 6 and 12 weeks of Western diet. Intermediate cohort sVEGFR3 × LDLR−/−/ApoB100/100 mice show increased amounts of cholesterol crystals (arrow) and collagen compared to LDLR−/−/ApoB100/100 control mice (A-F). mMQ stainings for macrophages in intermediate cohort sVEGFR3 × LDLR−/−/ApoB100/100 mice (G) and LDLR−/−/ApoB100/100 mice (H) after 6 weeks of fat diet show accumulation of macrophages and expanding core in a lesion in sVEGFR3 × LDLR−/−/ApoB100/100 mice. No difference in lipid deposition was detected between strains in Oil-red-O for intermediate after 4 weeks of Western diet (I and J). Movat’s staining (K) and mMQ (L) staining for lesions in young cohort Chy × LDLR−/−/ApoB100/100 mice after 12 weeks of Western diet show accumulation of cholesterol crystals and some macrophages. Bar 200 μm.
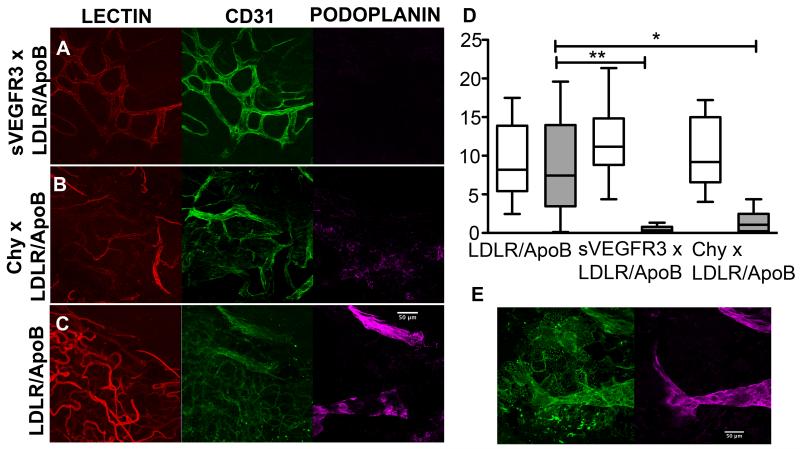
Plaque neovascularization and lymphatic vessels associated with atherosclerotic lesions in the descending aorta were visualized by confocal microscopy after intravenous injection of GSL-1 (glucan synthase-like-1) lectin in vivo, followed by in situ staining with antibodies reactive for podoplanin, a marker on lymphatics. CD31 was expressed on both blood vessels and lymphatics, but only blood perfused blood vessels stained positive for GSL-1. Lymphatic vessels stained positive for podoplanin and CD31 but excluded hematogenously delivered GSL-I lectin. (Figure 6). Atherosclerotic regions in the descending aortas from all genotype groups had similar densities of plaque-associated neovascularization (sVEGFR3 × LDLR−/−/ApoB100/100 median 11.2% area of GSL-I lectin+ vessels per high power field, range 4.36-21.34; Chy × , LDLR−/−/ApoB100/100 median 9.18%, range 4.03-17.2 and LDLR−/−/ApoB100/100 controls median 8.20,% range 2.45-17.5 (Kruskal Wallis P = 0.5491, Dunn’s post test comparison between groups P > 0.05) (Fig. 6D). Despite their similar or greater extents of atherosclerosis, lymphatic vessels were less abundant in the adventitia around atheromas of sVEGFR3 × LDLR−/−/ApoB100/100 mice (median 0.33% area podoplanin+vessels / hpf, range 0.09 - 1.33, Kruskal Wallis P = 0.0014, Dunn’s post test comparison, P < 0.05) and Chy × LDLR−/−/ApoB100/100 mice (median 1.07%, range 0.04-4.36, P < 0.05) compared to controls (median 7.45%, range 0.12 - 19.59) (Fig. 6D).
Figure 6.
Confocal images of neovascularization and lymphatic vessels associated with atherosclerotic lesions in descending aortas of old cohort sVEGFR3 × LDLR−/−/ApoB100/100 mice (A), Chy × LDLR−/−/ApoB100/100 mice (B) and LDLR−/−/ApoB100/100 mice (C) on Western diet. See Methods for in vivo and whole mount in situ staining. Images shown are Z-stack projections of vessels in depth of 24 μm from the adventitia into the media. Intravascularly labeled GSL-I lectin vessels (red) colocalized with most but not all CD31-reactive vessels (green). Podoplanin-positive vessels (magenta) had a larger caliber and expressed CD31 but did not contain GSL-I lectin. The densities of vasa vasorum in plaques (D) (open bars) from all groups were similar (sVEGFR3 × LDLR−/−/ApoB100/100 median 11.2 % area of GSLI/hpf range 4.36-21.34, Chy × , LDLR−/−/ApoB100/100 median 9.18 %, range 4.03-17.2 and LDLR−/−/ApoB100/100 controls median 8.20%, range 2.45-17. (Kruskal Wallis P = 0.5491, Dunn’s post test comparison between groups P > 0.05)). Lymphatic vessels (grey bars) were nearly absent or irregularly formed in sVEGFR3 × LDLR−/−/ApoB100/100 mice (median 0.33% area podoplanin+ vessels / hpf, range 0.09-1.33) and Chy × LDLR−/−/ApoB100/100 mice (median 1.07%, range 0.04-4.36) compared to LDLR−/−/ApoB100/100 controls (median 7.45%, range 0.12-19.59) (Kruskal Wallis P = 0.0014, Dunn’s post test comparisons P < 0.05 for sVEGFR3 ** and Chy* relative to controls). Both genders were used in the analysis. Single slice images of lymphatic vessels located at 8 μm depth in the adventitia (E) show spatial localization of podoplanin staining (magneta) with some CD31+ (green) vessels.
DISCUSSION
This study showed that two different mouse strains with impaired lymphatic vessel function develop increased cholesterol levels and atherosclerosis when bred into an atherosclerotic background. Indeed, lymphatic vessels are not only required for lipid absorption from the intestine, but may have systemic effects on circulating levels of lipoproteins, inflammatory reactions in peripheral tissues and atherosclerosis.1,5
In normal physiological situations, chylomicrons are taken up from intestinal enterocytes into lacteals, small lymphatic vessels in the mesentery, and transported to blood circulation via the thoracic duct.16 In addition, lymphatic vessels are involved in endogenous lipoprotein metabolism as extravasated lipoproteins are taken back to blood through lymphatic vessels.17 Peripheral lymph contains all classes of lipoproteins, but concentrations of LDL and VLDL are significantly lower than in plasma, suggesting that these lipoproteins are cleared before returning to blood.18-20 If the function of lymphatic vessels is impaired either in experimental animals13,14,21 or in humans with hereditary conditions or surgical procedures22,23, tissues develop lymphedema and increased adipose tissue. Emerging evidence now indicates that lymphatic vessels have roles in lipid transport, reverse cholesterol transport (RCT) and in modulating adipose tissue, but knowledge of their functions in hypercholesterolemia and atherosclerosis is limited24. Both sVEGFR3 × LDLR−/−/ApoB100/100 mice13 and Chy × LDLR−/−/ApoB100/100 mice14, which have impaired lymphatic function especially in their skin and some developmental lymphatic defects in other parts of the body, developed higher plasma cholesterol on a western-type high-fat diet compared to controls in the non-atherosclerotic background and atherosclerosis-prone LDLR−/−/ApoB100/100 background. It should be noted that the lipoprotein profiles of the mice used in this study and in other studies by our group25 differ from originally published lipoprotein profiles of LDLR−/−/ApoB100/100 mice.15 Mice used in this study have clearly a hypercholesterolemic phenotype and higher levels of cholesterol especially in VLDL-sized particles and lower levels of cholesterol in LDL-sized particles than in the original publication.15 This difference could be due to the natural transgenic drift of the mouse strains, use of the different background strain or differences in the lipoprotein analysis methods. However, these differences do not explain the main findings of this study since adequate controls have been used throughout the experiments.
Lipid absorption, LDL turnover and in vivo RCT after intraperitoneal injection of labeled macrophages were not different between sVEGFR3 × LDLR−/−/ApoB100/100 mice and LDLR−/−/ApoB100/100 control mice. Thus, sVEGFR3 × LDLR−/−/ApoB100/100 mice appeared to have no changes in lipoprotein absorption from the intestine or uptake by the liver when compared to LDLR−/−/ApoB100/100 control mice. Other studies have shown that RCT is reduced when cholesterol-labeled macrophages were injected in the skin of Chy mice or into distal extremities or peripheral tissues where lymphatics were interrupted.24 It is possible the RCT rates from intraperitoneal macrophages into plasma were not significantly altered in sVEGFR × LDLR−/−/ApoB100/100 mice compared to controls because peritoneal lymphatics were not sufficiently impaired by sVEGFR3. In addition, since hyperlipidemia itself has shown to alter lymphatic vessels in hypercholesterolemic ApoE−/− mice,26,27 dyslipidemic conditions may have affected lymphatic function also in control mice in this study and obviated RCT differences. On the other hand, VEGF-A and VEGF-B can alter genes involved in lipoprotein metabolism and lipid uptake.25,28 Such effects have not been reported for VEGF-C, but if present, then sVEGFR3 might have altered cholesterol and lipoprotein metabolism directly. Therefore, future studies, such as analyzing the lipoprotein production from the liver and evaluating the expression of genes related to lipid metabolism, are needed to clarify the mechanisms how the deficient lymphatic vessels affect hypercholesterolemia and atherosclerosis.29
Since lymphatic vessels actively participate in immune cell trafficking and adaptive immunity, attenuated function of lymphatic vessels might alter inflammatory reactions and have secondary effects on atherosclerosis and lipid metabolism. Older sVEGFR3 × LDLR−/−/ApoB100/100 mice developed massive infiltrations of inflammatory cells in their livers and giant cells in their neck area, whereas livers in old Chy × LDLR−/−/ApoB100/100 mice resembled livers of control mice. Accumulation of fat in the liver may further activate pro-inflammatory cytokines (e.g. tumor necrosis factor (TNF)-α) and reactive oxygen species that augment vascular inflammation.30 Recent studies have shown that while Chy mice have normal immune cell transport from the skin to lymph nodes31, K14-VEGFR3-Ig mice have impaired dendritic cell trafficking and B-cell function in the skin, altered systemic T-cell ratios, and one-year old K14-VEGFR3-Ig mice have many signs of autoimmunity32. Depletion of regulatory T-cells alone can increase plasma cholesterol levels and induce an atherogenic lipoprotein profile33. These results indicate that abnormal lymphatic function could have systemic effects on inflammatory cell homing and lipoprotein metabolism that promote atherosclerosis.1-3
Although systemic changes in hypercholesterolemia or immune functions may account for the increased atherosclerosis in mice with deficient lymphatic vessels, impaired lymphatic functions in aortic plaques could have exerted a local effect on plaque growth. An important step in atherosclerotic progression is angiogenesis and growth of vasa vasorum.34 Intravital microscopy studies of atherosclerotic mice have demonstrated that plaque-associated vessels function as more efficient conduits for inflammatory cell recruitment compared to the arterial endothelium overlying atheromas.35 Angiogenesis is driven by several factors (e.g. VEGF) produced as a response to hypoxia and inflammatory stimuli.36 In addition to arterial and venous microvessels, lymphatic vessels develop in the adventitia of large arteries.37-39 Macrophages in human atherosclerotic lesions express lymphangiogenic factors VEGF-C and VEGF–D and their receptor VEGFR340,41 and lymphatic growth occurs in the human arteries with calcified and fibrous atherosclerotic plaques.38,42 In this study, the density of plaque-associated neovascularization imaged en face from the adventitial surface by confocal microscopy remained at similar levels in all mouse strains, but lymphatic vessels were less abundant in vascularized atheromas of either sVEGFR3 × LDLR−/−/ApoB100/100 mice or Chy × LDLR−/−/ApoB100/100 mice compared to control LDLR−/−/ApoB100/100 mice.
The study by Martel24 observed absorptive lymphatics in transverse sections of the aortic sinus and descending aorta and noted the lymphatics weaved in and out of adjacent adipose tissue and were not absent in Chy mice. The Chy mice however were not evaluated in the atherosclerotic background. Other differences should be noted compared to this study. First, this study quantified only plaque-associated lymphatics that adhered to the descending aorta, resided within the plaque borders and did not invade periaortic fat. Lymphatics and plaque neovascularization in the aortic sinus may vary compared to other aortic regions. Second, plaque-associated lymphatics and neovascularization were both examined and were observed in close proximity. Since normal aortas of mice rarely contain CD31+ blood vessels or vasa vasorum in the adventitia, the close proximity of plaque lymphatics and neovascularization to plaques imaged in this study might have been more specific to lymphatics acquired during atherosclerosis. Indeed, absorptive lymphatics residing near the aorta and in periaortic fat may handle interstitial fluid flow through the aortic wall, but this effect likely diminishes as a function of distance from the aorta and is altered by serosal compartments, branch vessels and anatomic variations throughout the aorta to designate a strict boundary for lymphatics that regulate interstitial fluid flow across and around the aorta. In agreement with others, plaque lymphatics were confined to the adventitia layer, but lymphatic vessels did not invade as deeply towards the media compared to plaque neovascularization, which sometimes extends into the intima at sites of attenuated media in more advanced lesions.43 Similar to lymphatics residing in the peripheral capsule of tumors, the high tissue pressures of the artery wall may compress and impair the development of functional lymphatics in plaques.24 Finally, this study identified lymphatics associated with advanced atherosclerosis in aortas, while the experimental design of aorta RCT studies utilized aorta transplant models that disrupted periaortic lymphatics and measured clearance of cholesterol when new lymphatics were allowed to form or were blocked with a VEGFR3 inhibitor.24 The regrowth of aorta lymphatics in the surgical model may be altered by transient hypoxia in the transplanted tissue, acute wounding responses in the surgical site, different blood flow patterns and changes in interstitial fluid flow in the transplanted arch segment, which could all differ from mechanisms that regulate local lymphatic growth around atherosclerotic plaques. It is also recognized that antagonists of angiogenesis and lymphangiogenesis may have different responses on physiologic and pathologic vessel growth in different contexts.
This study raises important questions for investigations of the functions of adventitial lymphatics in atherosclerotic and normal blood vessels. In addition to RCT, adventitial plaque lymphatics might provide a route for transporting unretained cholesterol and lipoproteins out of the vessel wall, since lymphatic vessels are thought to be the main carriers of lipoproteins from interstitium to blood circulation.24,27 Alternatively, lymphangiogenesis found at sites of inflammation38,44,45 may provide routes for the entry or egress of immune cells and clearance of pathogens.46 Lymphatic channels in adventitial aortic tertiary lymphoid organs (ATLOs) contain T and B cell aggregates, T regulatory cells and nearby high endothelial venules. Together the lymphatic conduits and immune cell aggregates in the vessel wall likely have complex effects on vascular immune responses that are involved during both progression and regression phases of atherosclerosis.47
In conclusion, we show a novel association between impaired lymphatic vessels, lipoprotein metabolism and increased atherogenesis. These results support the emerging view that lymphatic vessels play an important role in regulating lipoprotein metabolism and vascular biology, and may provide new options for the treatment of lipid-related diseases.
Supplementary Material
SIGNIFICANCE.
Increasing evidence suggests that lymphatic vessels play an important role in the lipoprotein metabolism. Here we show that deficiency of lymphatic vessels affects cholesterol and triglyceride levels and accelerates the development of atherosclerosis in mice. Further understanding of lymphatic functions in lipoprotein metabolism may reveal new avenues for the treatment of vascular diseases.
ACKNOWLEDGEMENTS
We acknowledge Professor M. Jauhiainen for lipoprotein profile analysis and J. Malinen and S. Laidinen for excellent technical assistance.
SOURCES OF FUNDING This study was supported by grants from Academy of Finland, Academy of Finland Collaborative Research Consortium on Genome-Scale Cancer Biology (decision number 262976), Center of Excellence in Cardiovascular Diseases and Type 2 Diabetes, European Research Council (FUTUREGENES, Grant Agreement Number 250050 and TX-FACTORS, Grant Agreement Number 268804), Leducq Transatlantic Network of Excellence (Lymph Vessels in Obesity and Cardiovascular Disease) and Finnish Foundation for Cardiovascular disease and Kuopio University Hospital (EVO grant). Confocal imaging experiments were supported by grant R21 HL108177 and performed with assistance of Radu Maldovan in the University of Colorado Anschutz Medical Campus Advance Light Microscopy Core, supported in part by NIH/NCRR Colorado CTSI Grant UL1 RR025780.
Non-standard Abbreviations and Acronyms
- ApoB
apolipoprotein B
- GSL-1
glucan synthase-like-1
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- LDLR
low density lipoprotein receptor
- sVEGFR3
soluble vascular endothelial growth factor 3
- RCT
reverse cholesterol transport
- VEGF
vascular endothelial growth factor
- VLDL
very low density lipoprotein
Footnotes
DISCLOSURES None
REFERENCES
- 1.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 2.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 4.Moulton KS. Angiogenesis in atherosclerosis: gathering evidence beyond speculation. Curr Opin Lipidol. 2006;17:548–555. doi: 10.1097/01.mol.0000245261.71129.f0. [DOI] [PubMed] [Google Scholar]
- 5.Yla-Herttuala S, Bentzon JF, Daemen M, et al. Stabilisation of atherosclerotic plaques. Position paper of the European Society of Cardiology (ESC) Working Group on atherosclerosis and vascular biology. Thromb Haemost. 2011;106:1–19. doi: 10.1160/TH10-12-0784. [DOI] [PubMed] [Google Scholar]
- 6.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 7.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 8.Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 9.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:1751. [PMC free article] [PubMed] [Google Scholar]
- 11.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 12.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 14.Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI, Yla-Herttuala S, Finegold DN, Ferrell RE, Alitalo K. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veniant MM, Zlot CH, Walzem RL, Pierotti V, Driscoll R, Dichek D, Herz J, Young SG. Lipoprotein clearance mechanisms in LDL receptor-deficient “Apo-B48-only” and “Apo-B100-only” mice. J Clin Invest. 1998;102:1559–1568. doi: 10.1172/JCI4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon JB. Mechanisms of chylomicron uptake into lacteals. Ann N Y Acad Sci. 2010;1207(Suppl 1):E52–7. doi: 10.1111/j.1749-6632.2010.05716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon JB. Lymphatic lipid transport: sewer or subway? Trends Endocrinol Metab. 2010;21:480–487. doi: 10.1016/j.tem.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanjee MN, Cooke CJ, Olszewski WL, Miller NE. Lipid and apolipoprotein concentrations in prenodal leg lymph of fasted humans. Associations with plasma concentrations in normal subjects, lipoprotein lipase deficiency, and LCAT deficiency. J Lipid Res. 2000;41:1317–1327. [PubMed] [Google Scholar]
- 19.Sloop CH, Dory L, Roheim PS. Interstitial fluid lipoproteins. J Lipid Res. 1987;28:225–237. [PubMed] [Google Scholar]
- 20.Reichl D. Extravascular circulation of lipoproteins: their role in reverse transport of cholesterol. Atherosclerosis. 1994;105:117–129. doi: 10.1016/0021-9150(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 21.Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, Hurley KE, Riedel ER, Van Zee KJ. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26:5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanton AW, Modi S, Mellor RH, Levick JR, Mortimer PS. Recent advances in breast cancer-related lymphedema of the arm: lymphatic pump failure and predisposing factors. Lymphat Res Biol. 2009;7:29–45. doi: 10.1089/lrb.2008.1026. [DOI] [PubMed] [Google Scholar]
- 24.Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, Kreisel D, Swartz MA, Sorci-Thomas MG, Randolph GJ. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest. 2013;123:1571–1579. doi: 10.1172/JCI63685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinonen SE, Kivela AM, Huusko J, Dijkstra MH, Gurzeler E, Makinen PI, Leppanen P, Olkkonen VM, Eriksson U, Jauhiainen M, Yla-Herttuala S. The effects of VEGF-A on atherosclerosis, lipoprotein profile, and lipoprotein lipase in hyperlipidaemic mouse models. Cardiovasc Res. 2013;99:716–723. doi: 10.1093/cvr/cvt148. [DOI] [PubMed] [Google Scholar]
- 26.Lim HY, Rutkowski JM, Helft J, Reddy ST, Swartz MA, Randolph GJ, Angeli V. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol. 2009;175:1328–1337. doi: 10.2353/ajpath.2009.080963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, Angeli V. Lymphatic Vessels Are Essential for the Removal of Cholesterol from Peripheral Tissues by SR-BI-Mediated Transport of HDL. Cell Metab. 2013;17:671–684. doi: 10.1016/j.cmet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Hagberg CE, Falkevall A, Wang X, et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 29.Horra A, Salazar J, Ferre R, Vallve JC, Guardiola M, Rosales R, Masana L, Ribalta J. Prox-1 and FOXC2 gene expression in adipose tissue: A potential contributory role of the lymphatic system to familial combined hyperlipidaemia. Atherosclerosis. 2009;206:343–345. doi: 10.1016/j.atherosclerosis.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 30.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 31.Platt AM, Rutkowski JM, Martel C, Kuan EL, Ivanov S, Swartz MA, Randolph GJ. Normal dendritic cell mobilization to lymph nodes under conditions of severe lymphatic hypoplasia. J Immunol. 2013;190:4608–4620. doi: 10.4049/jimmunol.1202600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, Randolph GJ, Swartz MA. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J Immunol. 2012;189:2181–2190. doi: 10.4049/jimmunol.1103545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klingenberg R, Gerdes N, Badeau RM, et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barger AC, Beeuwkes R, 3rd, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson EE. Intravital microscopy on atherosclerosis in apolipoprotein e-deficient mice establishes microvessels as major entry pathways for leukocytes to advanced lesions. Circulation. 2011;124:2129–2138. doi: 10.1161/CIRCULATIONAHA.111.030627. [DOI] [PubMed] [Google Scholar]
- 36.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 37.Eliska O, Eliskova M, Miller AJ. The absence of lymphatics in normal and atherosclerotic coronary arteries in man: a morphologic study. Lymphology. 2006;39:76–83. [PubMed] [Google Scholar]
- 38.Kholova I, Dragneva G, Cermakova P, Laidinen S, Kaskenpaa N, Hazes T, Cermakova E, Steiner I, Yla-Herttuala S. Lymphatic vasculature is increased in heart valves, ischaemic and inflamed hearts and in cholesterol-rich and calcified atherosclerotic lesions. Eur J Clin Invest. 2011;41:487–497. doi: 10.1111/j.1365-2362.2010.02431.x. [DOI] [PubMed] [Google Scholar]
- 39.Sacchi G, Weber E, Comparini L. Histological framework of lymphatic vasa vasorum of major arteries: an experimental study. Lymphology. 1990;23:135–139. [PubMed] [Google Scholar]
- 40.Rutanen J, Leppanen P, Tuomisto TT, Rissanen TT, Hiltunen MO, Vajanto I, Niemi M, Hakkinen T, Karkola K, Stacker SA, Achen MG, Alitalo K, Yla-Herttuala S. Vascular endothelial growth factor-D expression in human atherosclerotic lesions. Cardiovasc Res. 2003;59:971–979. doi: 10.1016/s0008-6363(03)00518-2. [DOI] [PubMed] [Google Scholar]
- 41.Schmeisser A, Christoph M, Augstein A, Marquetant R, Kasper M, Braun-Dullaeus RC, Strasser RH. Apoptosis of human macrophages by Flt-4 signaling: implications for atherosclerotic plaque pathology. Cardiovasc Res. 2006;71:774–784. doi: 10.1016/j.cardiores.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Nakano T, Nakashima Y, Yonemitsu Y, Sumiyoshi S, Chen YX, Akishima Y, Ishii T, Iida M, Sueishi K. Angiogenesis and lymphangiogenesis and expression of lymphangiogenic factors in the atherosclerotic intima of human coronary arteries. Hum Pathol. 2005;36:330–340. doi: 10.1016/j.humpath.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Moulton KS, Olsen BR, Sonn S, Fukai N, Zurakowski D, Zeng X. Loss of collagen XVIII enhances neovascularization and vascular permeability in atherosclerosis. Circulation. 2004;110:1330–1336. doi: 10.1161/01.CIR.0000140720.79015.3C. [DOI] [PubMed] [Google Scholar]
- 44.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baluk P, Tammela T, Ator E, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones D, Min W. An overview of lymphatic vessels and their emerging role in cardiovascular disease. J Cardiovasc Dis Res. 2011;2:141–152. doi: 10.4103/0975-3583.85260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grabner R, Lotzer K, Dopping S, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206:233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.