Abstract
Improvements in screening and adjuvant therapy for breast cancer are associated with decreased recurrence, which may have the effect of increasing the proportion of patients presenting with first-line de novo versus recurrent metastatic breast cancer (MBC). Here, we describe and compare patients with de novo versus recurrent human epidermal growth factor 2 (HER2)-positive MBC. registHER was a prospective observational cohort study (late 2003–early 2006) of 1,023 patients with HER2-positive MBC. Baseline characteristics, treatment patterns, and clinical outcomes were examined in patients with newly diagnosed de novo (n = 327) compared with recurrent HER2-positive MBC after prior treatment for early-stage disease (n = 674). Patients with de novo HER2-positive MBC were less likely to have lung metastases, more likely to have lymph node, bone, and/or liver metastases and >4 sites of metastases and more likely to receive combined or concurrent chemotherapy and hormonal therapy with or without trastuzumab than those with recurrent HER2-positive MBC. Median follow-up was 29 months. Median progression-free survival was 12.1 versus 9.3 months [hazard ratio = 0.716 (95 % confidence interval (CI) 0.617–0.831)], and overall survival was 41.7 versus 32.8 months [hazard ratio = 0.766 (95 % CI 0.633–0.928)] for patients with de novo versus recurrent HER2-positive MBC, respectively. Patients with recurrent HER2-positive MBC had similar outcomes regardless of whether they received prior adjuvant therapy, excluding hormonal therapy. Despite presenting with more advanced-stage disease and higher tumor burdens, patients with de novo HER2-positive MBC have more favorable clinical outcomes than those with recurrent HER2-positive MBC. These differences may be due to effects of prior drug exposure and could have implications for designing and interpreting clinical trials.
Keywords: Metastatic breast cancer, Observational study, De novo MBC, Recurrent MBC, registHER, HER2-positive
Introduction
Data from the last 20 years have shown a decrease in mortality from breast cancer in the United States of more than 30 % from its peak, at least in part because of better screening and treatments [1, 2]. Breast cancer remains the most common type of cancer diagnosed in women and is expected to account for ~29 % of new US cancer cases in 2013 [1]. Earlier identification of the disease and improvements in local and adjuvant therapy [2–7] have resulted in decreasing rates of relapse in patients with early-stage breast cancer. This effectively increases the proportion of patients who initially present with de novo first-line metastatic breast cancer (MBC) in the overall MBC patient population. This overall shift could have implications on treatment choices and the design and interpretation of results from clinical trials in the MBC setting.
Human epidermal growth factor receptor 2 (HER2), overexpressed in approximately 15–20 % of breast cancer cases, is a predictive and prognostic marker of treatment outcomes [8–13]. Prior to the introduction of HER2-targeted therapy, the prognosis for patients with HER2-positive MBC was markedly worse compared with that for patients with HER2-negative MBC [10, 11]. Since the approval of trastuzumab for MBC by the US Food and Drug Administration in 1998, the repertoire of HER2-targeted treatments for HER2-positive MBC has expanded to include lapatinib [14], pertuzumab [15], and trastuzumab emtansine [16], in 2007, 2012, and 2013, respectively. In addition, trastuzumab use in the adjuvant setting [3–5] was approved in 2006, and pertuzumab was approved in the neoadjuvant setting in 2013. As treatments for HER2-positive breast cancer continue to improve, it is important to understand the evolution of the natural history of patients with de novo or recurrent HER2-positive MBC in a real-world setting.
The registHER observational cohort study, which enrolled patients with newly diagnosed HER2-positive MBC between late 2003 and early 2006, offers a unique opportunity to describe and compare patient demographics, clinical characteristics, prior and first-line MBC treatment patterns, and clinical outcomes in patients presenting with de novo or recurrent HER2-positive MBC prior to the approval of adjuvant trastuzumab for the treatment of early-stage breast cancer. By investigating the natural history of de novo and recurrent HER2-positive MBC, this analysis may help us to understand and interpret results from current and future clinical studies in this patient population.
Methods
Study design and patients
registHER was a multicenter, prospective, US-based observational cohort study of 1,023 patients with HER2-positive MBC who were recruited from community and academic settings between December 2003 and February 2006. Study design and recruitment details are described in depth elsewhere [17]. The objectives were to examine the natural history, treatment patterns, and outcomes of patients with HER2-positive MBC in the clinical practice setting. Patients were enrolled within 6 months of diagnosis of HER2-positive MBC and observed until death, disenrollment, or the study end date of June 2009. Patients received treatment based on the standard practice of their physician and without scheduled study-specified evaluations. Prior or planned treatment with any specific HER2-directed therapy was not a requirement for enrollment. All enrolled patients provided informed consent.
Patient information was collected at enrollment and every 3 months thereafter. The evaluation schedule and tumor response were reported by the treating physicians according to their standard practice and judgment.
Statistical analysis
This analysis incorporates data from the June 15, 2009, database lock. Only patients reported to have received MBC treatment during the study were included in this analysis. Patients with de novo HER2-positive MBC were defined as those with ≤90 days between the initial diagnosis of early-stage breast cancer and the diagnosis of MBC (i.e., they likely had occult metastatic disease at the time of initial diagnosis). Patients with recurrent HER2-positive MBC were defined as those with >90 days between the early-stage breast cancer and MBC diagnoses. A 90-day cutoff was used to differentiate de novo and recurrent HER2-positive MBC, because early breast cancer staging information was missing from the records of 215 patients.
Descriptive analyses of demographic and clinical characteristics at MBC diagnosis, tumor staging, biomarkers and treatment history (including prior early-stage breast cancer treatment for patients with recurrent HER2-positive MBC), first-line MBC treatment patterns, and tumor response were performed. Hormone receptor (HR) status was determined according to individual institutional criteria. The term “HR-positive” was defined as estrogen receptor [ER]-positive and/or progesterone receptor [PR]-positive; “HR-negative” was defined as ER-negative and PR-negative. First-line MBC treatment patterns were based on treatment received before first disease progression but after MBC diagnosis; treatment may have been given sequentially or concurrently. Median progression-free survival (PFS), overall survival (OS), and their corresponding 95 % confidence intervals (CIs) were estimated by the Kaplan–Meier method. “PFS” was defined as the time from MBC diagnosis to first progression reported by physicians’ standard practice or death from any cause, whichever occurred first. PFS data for patients without disease progression or death as of the database lock date were censored at the time of the last tumor response evaluation. “OS” was defined as the time from MBC diagnosis to the date of death from any cause. OS data for patients without an event as of the database lock date were censored at the time of the last follow-up. Estimates of best first-line response rates were calculated. Univariate and multivariate Cox regression analyses were used to generate unadjusted and adjusted hazard ratios and their corresponding 95 % CIs. Two multivariate Cox regression analyses for PFS and OS adjusted for age at enrollment, race, Eastern Cooperative Oncology Group performance status (ECOG PS), HR status, visceral/nonvisceral sites, and number of metastatic sites at diagnosis. These covariates were chosen for the multivariate Cox regression analysis on the basis of the covariates’ clinical significance rather than statistical significance.
After using disease-free interval to stratify patients with de novo or recurrent HER2-positive MBC, it became apparent that 24 patients with de novo HER2-positive MBC had initially been staged as early breast cancer and treated with adjuvant or neoadjuvant therapy immediately prior to their metastatic diagnosis. To ensure that the inclusion of these 24 patients did not impact the overall outcomes reported for the other patients with de novo HER2-positive MBC, a sensitivity analysis was performed.
Results
Baseline characteristics and treatment history
Of the 1,023 patients enrolled in registHER, 22 patients were not reported to have received MBC treatment during the study and were excluded from this analysis (N = 1,001). Baseline demographics and clinical characteristics for the registHER cohort were examined for the 327 (33 %) patients presenting with de novo and 673 (67 %) with recurrent HER2-positive MBC (Table 1). A higher proportion of patients with de novo versus recurrent HER2-positive MBC were <50 years old, non-white, had an ECOG PS of 0 or 1, had HR-positive disease, and had ≥4 metastatic disease sites. Patients with recurrent HER2-positive MBC were more likely to be ≥65 years old, have a single metastatic disease site, have node-positive disease, and have HR-negative disease. Patients with de novo HER2-positive MBC were more likely to have metastatic disease in the lymph nodes, bone, or liver, while patients with recurrent HER2-positive MBC were more likely to have metastatic sites in the lung and central nervous system (CNS).
Table 1.
Baseline patient demographics and clinical characteristics
| Characteristic, n (%) | De novo patients (n = 327) |
Recurrent patients (n = 674) |
|---|---|---|
| Age at study enrollment | ||
| <50 years | 133 (40.7) | 246 (36.5) |
| 50–64 years | 132 (40.4) | 276 (40.9) |
| ≥65 years | 62 (19.0) | 152 (22.6) |
| Race | ||
| White | 251 (76.8) | 542 (80.4) |
| Black | 49 (15.0) | 77 (11.4) |
| Other | 27 (8.3) | 55 (8.2) |
| ECOG PS | ||
| 0–1 | 171 (52.3) | 284 (42.1) |
| ≥2 | 24 (7.3) | 36 (5.3) |
| Missing | 132 (40.4) | 354 (52.5) |
| Node status at initial diagnosis | ||
| Positive | 137 (41.9) | 378 (56.1) |
| Negative | 14 (4.3) | 196 (29.1) |
| Unknown | 176 (53.8) | 100 (14.8) |
| HR status at initial diagnosis | ||
| Positive | 180 (55.0) | 349 (51.8) |
| Negative | 136 (41.6) | 297 (44.1) |
| Unknown/missing | 11 (3.4) | 28 (4.2) |
| Number of metastatic disease sites | ||
| 1 | 114 (34.9) | 346 (51.3) |
| 2 | 99 (30.3) | 174 (25.8) |
| 3 | 58 (17.7) | 98 (14.5) |
| ≥4 | 56 (17.1) | 56 (8.3) |
| Site of metastatic disease | ||
| Bone | 181 (55.4) | 269 (39.9) |
| Liver | 154 (47.1) | 246 (36.5) |
| Lung | 88 (26.9) | 229 (34.0) |
| Lymph nodes | 111 (33.9) | 167 (24.8) |
| Breast | 93 (28.4) | 38 (5.6) |
| Mediastinum | 25 (7.6) | 54 (8.0) |
| Central nervous system | 17 (5.2) | 55 (8.2) |
| Pleural effusion | 19 (5.8) | 36 (5.3) |
| Other abdominal | 17 (5.2) | 29 (4.3) |
| Pelvis | 3 (0.9) | 13 (1.9) |
| Ascites | 2 (0.6) | 3 (0.4) |
| Other | 13 (4.0) | 19 (2.8) |
ECOG PS Eastern Cooperative Oncology Group performance status, HR hormone receptor
Of the 587 patients with recurrent HER2-positive MBC who received therapy in the adjuvant or neoadjuvant settings (Table 2), only 55 (9.4 %) received chemotherapy with trastuzumab: 40 (72.7 %) patients were treated with doxorubicin and cyclophosphamide (AC) with trastuzumab and of these, 37 (67.3 %) received AC plus a taxane (ACT) with trastuzumab. Eleven (20.0 %) patients received adjuvant hormonal therapy with chemotherapy. In total, 532 patients with recurrent HER2-positive MBC received non-trastuzumab-based adjuvant or neoadjuvant regimens. Of these 532 patients, 470 received chemotherapy: 396 (74.4 %) received AC, and of these, 248 (46.6 %) were treated with ACT. In addition, of the 532 patients, 60 (11.3 %) received adjuvant hormonal therapy only and 164 (30.8 %) received hormonal therapy with chemotherapy.
Table 2.
Prior adjuvant or neoadjuvant therapies for patients with recurrent HER2-positive MBC
| Treatment, n (%) | Recurrent patients (n = 587) |
|---|---|
| Trastuzumab-based regimens | n = 55 |
| With chemotherapy only | 44 (80.0) |
| With hormonal therapy only | 0 |
| With chemotherapy and hormonal therapy | 11 (20.0) |
| Trastuzumab alone | 0 |
| Non–trastuzumab-based regimens | n = 532 |
| Chemotherapy only | 306 (57.5) |
| Hormonal therapy only | 60 (11.3) |
| Chemotherapy and hormonal therapy | 164 (30.8) |
| Trastuzumab with specific chemotherapy regimens | n = 55 |
| AC | 40 (72.7) |
| ACT | 37 (67.3) |
| Docetaxel | 34 (61.8) |
| Paclitaxel | 19 (34.5) |
| Specific chemotherapy regimens without trastuzumab | n = 532 |
| AC | 396 (74.4) |
| ACT | 248 (46.6) |
| Docetaxel | 146 (27.4) |
| Paclitaxel | 148 (27.8) |
AC doxorubicin and cyclophosphamide, ACT AC and a taxane, HER2 human epidermal growth factor receptor 2
First-line MBC treatment patterns
Treatment patterns for first-line metastatic disease were compared between patients presenting with de novo and those with recurrent HER2-positive MBC (Table 3). Although the proportions of patients who received only trastuzumab plus chemotherapy were similar between the two groups (59 % each), more patients with de novo vs recurrent HER2-positive MBC were treated with both chemotherapy and hormonal therapy (either concurrently or sequentially) with and without trastuzumab (22.9 vs 13.8 % and 2.4 vs 0.9 %, respectively). The proportion of patients who received sequential versus concurrent hormonal therapy with trastuzumab-based regimens was similar between the two groups. Fewer patients with de novo versus recurrent HER2-positive MBC were treated with trastuzumab alone in the first-line MBC setting (2.4 vs 9.2 %). The most commonly used trastuzumab-based combination chemotherapy for both groups was a taxane and a platinum agent doublet. The next most common trastuzumab-based regimens were trastuzumab with vinorelbine for patients with recurrent HER2-positive MBC and trastuzumab with docetaxel for patients with de novo HER2-positive MBC.
Table 3.
First-line treatment for MBC
| Treatment, n (%) | De novo patients (n = 327) | Recurrent patients (n = 674) |
|---|---|---|
| Trastuzumab-based regimens | n = 288 | n = 592 |
| With chemotherapy only | 193 (59.0) | 398 (59.1) |
| With hormonal therapy only | 12 (3.7) | 39 (5.8) |
| With chemotherapy and hormonal therapy | 75 (22.9) | 93 (13.8) |
| Sequential hormonal therapy | 50 (66.7) | 60 (64.5) |
| Concurrent hormonal therapy | 25 (33.3) | 33 (35.5) |
| Trastuzumab alone | 8 (2.4) | 62 (9.2) |
| Non-trastuzumab-based regimens | n = 39 | n = 82 |
| Chemotherapy only | 18 (5.5) | 35 (5.2) |
| Hormonal therapy only | 13 (4.0) | 41 (6.1) |
| Chemotherapy and hormonal therapy | 8 (2.4) | 6 (0.9) |
| Trastuzumab and specific chemotherapy combinations | n = 268 | n = 491 |
| Taxane/platinum | 70 (26.1) | 107 (21.8) |
| Docetaxel | 23 (8.6) | 39 (7.9) |
| Anthracycline/cyclophosphamide/taxane | 21 (7.8) | 3 (0.6) |
| Paclitaxel | 18 (6.7) | 38 (7.7) |
| Vinorelbine | 13 (4.9) | 75 (15.3) |
First-line therapy is the regimen received between the date of the first treatment following the diagnosis of metastasis and the date of first disease progression
Clinical outcomes
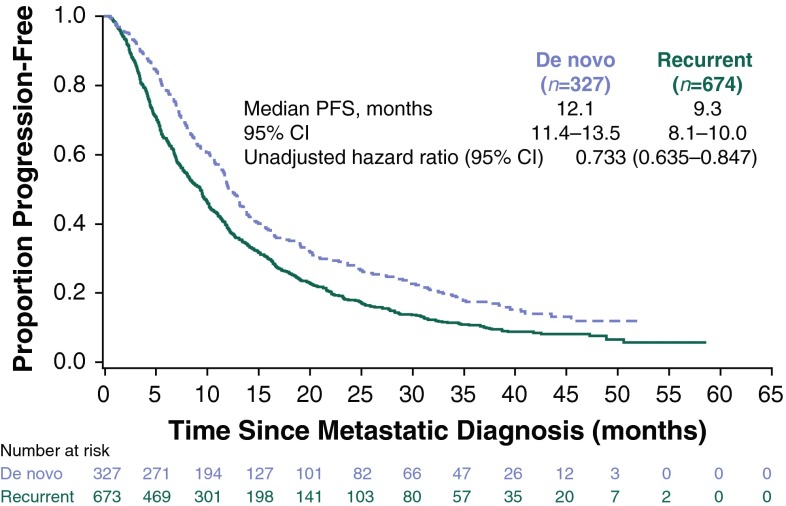
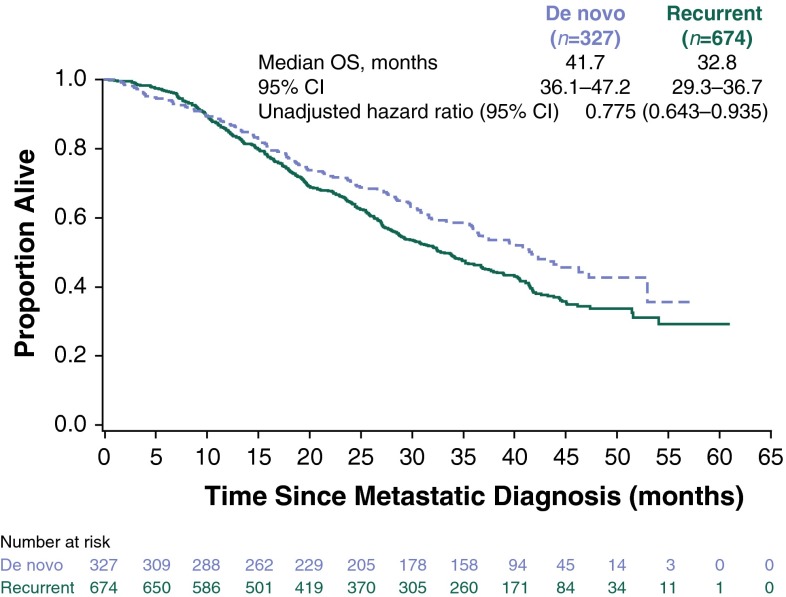
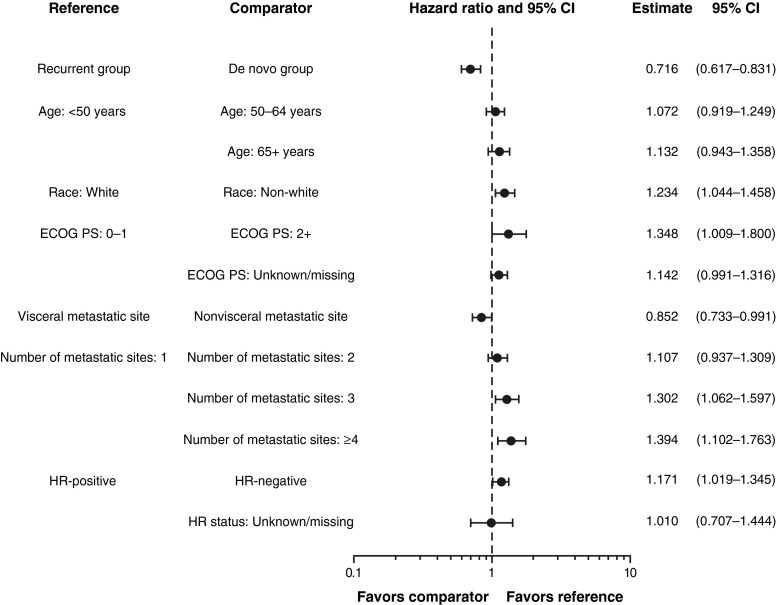
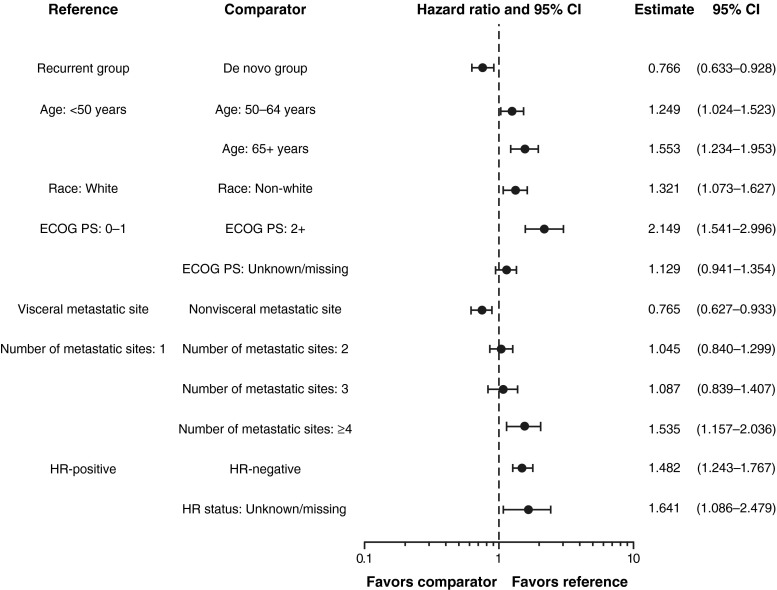
Median follow-up was 29 months (range 0.5–60.9). Median PFS was longer for patients with de novo (12.1 months; 95 % CI 11.4–13.5) versus recurrent (9.3 months; 95 % CI 8.1–10.0) HER2-positive MBC (Fig. 1). The unadjusted hazard ratio was 0.733 (95 % CI 0.635–0.847), favoring patients with de novo HER2-positive MBC. Median OS was 41.7 months (95 % CI 36.1–47.2) versus 32.8 months (95 % CI 29.3–36.7) for patients with de novo versus recurrent HER2-positive MBC, respectively (Fig. 2). The unadjusted HR was 0.775 (95 % CI 0.643–0.935), also favoring patients with de novo HER2-positive MBC. The adjusted PFS hazard ratio was 0.716 (95 % CI 0.617–0.831) (Fig. 3). The adjusted OS hazard ratio was 0.766 (95 % CI 0.633–0.928) (Fig. 4). A higher percentage of patients with de novo versus recurrent HER2-positive MBC had a complete response or a partial response/stable disease as their best first-line response (82.9 vs 72.7 %; Table 4).
Fig. 1.
Progression-free survival following metastatic diagnosis. CI confidence interval, PFS progression-free survival
Fig. 2.
Overall survival following metastatic diagnosis. CI confidence interval, OS overall survival
Fig. 3.
Multivariate Cox regression analyses for progression-free survival with adjusted hazard ratios. CI confidence interval, ECOG PS Eastern Cooperative Oncology Group performance status, HR hormone receptor
Fig. 4.
Multivariate Cox regression analyses for overall survival with adjusted hazard ratios. CI confidence interval, ECOG PS Eastern Cooperative Oncology Group performance status, HR hormone receptor
Table 4.
Best response to first-line treatment for MBC
| Best response, n (%) | De novo patients (n = 327) | Recurrent patients (n = 674) |
|---|---|---|
| Complete response | 70 (21.4) | 105 (15.6) |
| Partial response/stable disease | 201 (61.5) | 385 (57.1) |
| Progressive disease | 48 (14.7) | 162 (24.0) |
| No assessment | 8 (2.4) | 22 (3.3) |
Best observed response confirmed by clinical symptoms, physical examination, or radiology
Median PFS [9.3 (95 % CI 8.0–10.0) vs 9.7 months (95 % CI 6.7–11.1)] and OS [32.8 (95 % CI 28.7–36.8) vs 33.8 months (95 % CI 27.7–41.7)] were similar for patients with recurrent HER2-positive MBC who received prior adjuvant therapy (n = 541) versus those who did not (n = 133). When analyzed according to HR status, patients in both the de novo and recurrent groups with HR-positive disease generally had longer median PFS and OS than patients with HR-negative disease (Table 5). For patients with HR-positive disease, those presenting with de novo MBC had longer median PFS [14.4 (95 % CI 11.8–19.0) vs 9.9 months (95 % CI 8.8–11.4)] and OS [52.9 (95 % CI 43.8–not available [NA]) versus 37.7 months (95 % CI 33.7–41.7)] than patients with recurrent MBC. For patients in the recurrent group with HR-positive disease who had received adjuvant therapy, median OS was longer for those who had received adjuvant hormonal therapy [40.3 months (95 % CI 32.8–43.8)] versus those who had not [34.6 months (95 % CI 25.6–41.5)]. Median OS was the shortest for patients in the recurrent group with HR-negative disease who had received adjuvant therapy [26.6 months (95 % CI 23.9–33.9)].
Table 5.
PFS and OS in patients with recurrent HER2-positive MBC by HR status and prior adjuvant therapy
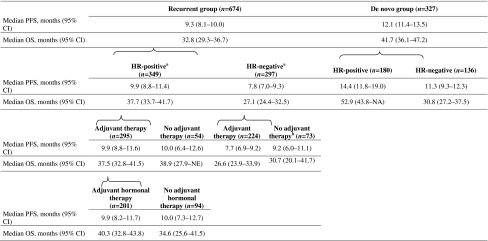
|
CI confidence interval; HR hormone receptor; NE not estimable; OS overall survival; PFS progression-free survival
aThere were 28 patients with missing or unknown HR status
bIncludes patients who did not receive or report the receipt of adjuvant therapy
In patients with de novo HER2-positive MBC, the site of first disease progression was most frequently bone/breast and less frequently visceral sites compared with patients with recurrent HER2-positive MBC (Table 6). Time to first CNS progressive disease was 11.8 months (95 % CI 9.7–13.5) for patients with de novo HER2-positive MBC versus 7.7 months (95 % CI 6.9–10.5) for patients with recurrent HER2-positive MBC. When adjusted for baseline demographics or clinical characteristics, the multivariate Cox regression analysis demonstrated that the hazard of progression and death in patients with de novo HER2-positive MBC were lower than that for recurrent HER2-positive MBC by 28 and 23 %, respectively (Figs. 2, 4). In addition, the following characteristics were also associated with more favorable clinical outcomes: younger age, white race, an ECOG PS of 0 or 1, HR-positive disease, nonvisceral disease, and fewer metastatic disease sites.
Table 6.
Sites of first disease progression following first-line therapy for HER2-positive MBC
| Site at progressive disease, n (%) | De novo patients (n = 327) | Recurrent patients (n = 674) |
|---|---|---|
| Bone/breast only | 103 (31.5) | 174 (25.8) |
| Visceral | 73 (22.3) | 226 (33.5) |
| Any central nervous system | 57 (17.4) | 105 (15.6) |
| Node/local | 16 (4.9) | 61 (9.1) |
| Other sites | 0 (0) | 1 (0.1) |
Site of first disease progression after metastatic diagnosis HER2 human epidermal growth factor receptor 2
Sensitivity analysis
There were no noticeable differences between the baseline and clinical characteristics of patients with de novo HER2-positive MBC in the overall analysis compared with those in the sensitivity analysis that excluded the 24 patients with de novo MBC later found to be treated with adjuvant or neoadjuvant therapy (data not shown). The sensitivity analysis showed that these patients had slightly longer median PFS and OS compared with patients in the overall analysis: median PFS was 12.5 months (95 % CI 11.4–13.8), and median OS was 42.0 months (95 % CI 36.9–NA). For best first-line response, 63 (20.8 %) had complete response, 186 (61.4 %) had partial response/stable disease, 46 (15.2 %) had progressive disease, and 8 (2.6 %) had no assessment. While the proportion of patients with CNS metastases at first disease progression in the sensitivity analysis remained similar to that in the overall analysis (17.5 %), the median time to CNS metastases was higher at 13.0 months (95 % CI 9.3–14.5).
Discussion
The registHER observational cohort study provides a unique opportunity to examine the natural disease history, first-line MBC treatment patterns, and clinical outcomes for patients presenting with de novo or recurrent HER2-positive MBC in a real-world population. Despite presenting with more advanced-stage disease with a higher tumor burden at first diagnosis, patients with de novo HER2-positive MBC had a 28 and 23 % lower hazard of progression and death, respectively, compared with patients with recurrent HER2-positive MBC.
One possible explanation for poorer outcomes for patients with recurrent HER2-positive MBC may be that their metastatic disease is more resistant and/or refractory owing to the selection of more resistant or aggressive clones of recurrent cells as a consequence of prior adjuvant chemotherapy treatment. Effectively addressing treatment resistance resulting from the selective effect of therapy on clonal evolution in a heterogeneous tumor is one of the great challenges of oncology medicine [18]. Intrinsic insensitivity may also be an obstacle to the effective treatment of HER2-positive disease [19, 20]. Additional predictive markers for response to HER2-directed therapies are being studied, and these may help to identify patients with refractory disease in the future [21].
In registHER, patients presenting with de novo HER2-positive MBC had longer PFS and OS in the first-line setting than patients with recurrent MBC regardless of whether they had received prior adjuvant treatment (PFS: 12.1 vs 9.3 months and OS: 41.7 vs 32.8 months, respectively; see Table 5). This trend is nuanced, however, when OS is examined by HR status. For HR-negative disease, the median OS for patients with de novo MBC was similar to that of patients with recurrent MBC who did not receive adjuvant therapy (30.8 vs 30.7 months); both were longer than patients who did receive adjuvant therapy (26.6 months), suggesting selection of more aggressive disease with therapy. Interestingly, when comparing patients with HR-positive disease in the de novo group with those in the recurrent group who had not received adjuvant therapy, median OS was much longer for patients in the de novo group (52.9 vs 38.9 months). Median OS was also longer for patients in the recurrent group with HR-positive disease who had received adjuvant hormonal therapy (n = 201, 68.1 %) (who may have been expected to have developed resistance) versus those who had not (n = 94, 31.9 %) (40.3 vs 34.6 months). These results raise the question of whether the difference in outcomes between patients with de novo or recurrent HER2-positive MBC is a result of treatment patterns or tumor characteristics. In accordance with this, the results of a recent retrospective analysis (N = 331) of women with de novo stage IV or recurrent HER2-positive MBC also underscore the impact of treatment patterns on clinical outcomes, even if possible selection bias due to disease burden is taken into account [22]. Although no differences in PFS or OS were observed between patients with de novo or recurrent MBC in that analysis, median OS was significantly longer for patients with de novo MBC who received surgery [60 months (95 % CI 41–79); n = 46] compared with those who did not receive surgery [26 months (95 % CI 20–32); n = 31] and those with recurrent MBC [40 months (95 % CI 37–43); n = 254] [22].
Due to the timing of the registHER study, only 55 of the 674 patients with recurrent HER2-positive MBC received adjuvant or neoadjuvant trastuzumab. The addition of adjuvant trastuzumab to chemotherapy decreases the risk of recurrence in patients with HER2-positive early-stage breast cancer compared with chemotherapy alone [3–5, 23] and, as a result, is now well established as the standard of care in the adjuvant setting, though ~15 % of patients will still develop metastases [20]. As fewer patients with early-stage breast cancer relapse, it is likely that the ratio of patients presenting with de novo HER2-positive MBC to those with recurrent HER-positive MBC will increase. However, while relevant clinical trials will have enrolled patients with both de novo and recurrent HER2-positive MBC, few published data are available providing this information. In an analysis of patients with breast cancer diagnosed between 1992 and 2007, Dawood et al. [24] found that, of the patients with non-missing HER2 status data (n = 563), 23 % of patients had de novo stage IV HER2-positive disease and 77 % had relapsed HER2-positive disease, based on staging information. In the registHER population, enrolled between 2003 and 2006, the proportion of patients with de novo versus recurrent HER2-positive MBC was 33 vs 67 %, respectively. In the randomized phase 2, TDM4450g study in patients treated with trastuzumab emtansine or trastuzumab plus docetaxel who were enrolled between 2008 and 2009, ~30 % of patients with non-missing staging data had de novo stage IV HER2-positive MBC at initial diagnosis [25]. In that study, however, the number of patients with de novo HER2-positive MBC may have been skewed because patients with an interval <6 months from neoadjuvant or adjuvant chemotherapy or ≤21 days from prior trastuzumab therapy were excluded from enrollment. Also, as an international study, adjuvant therapy practice patterns may be different from the US-based cohort.
To further characterize the changing face of HER2-positive MBC, especially in light of the standard use of trastuzumab for early-stage disease and the subsequent approvals of additional HER2-targeted therapies (such as trastuzumab emtansine in the metastatic setting and pertuzumab in the metastatic and neoadjuvant settings), the Systematic Therapies for HER2-Positive Metastatic Breast Cancer Study (SystHERs; clinicaltrials.gov, NCT01615068) was planned. SystHERs is a US-based multicenter, prospective observational cohort that began enrolling patients with HER2-positive MBC in 2012. The data generated will reflect not only standard trastuzumab use in the adjuvant setting but also the evolving standard of dual HER2-targeted therapy in the metastatic and neoadjuvant settings. Clinical outcomes and characteristics inherent to patients with de novo HER2-positive MBC remain of interest and may have to be taken into account during the study design or data analysis of future HER2-positive clinical trials.
The limitations of this analysis include the use of an arbitrary 90-day cutoff to define patients with de novo or recurrent HER2-positive MBC in order to account for patients with occult de novo HER2-positive MBC. The early-stage breast cancer and MBC dates used to calculate the cutoff interval were collected retrospectively at study enrollment. If the groups had been defined based on staging information instead, the proportions of patients with de novo vs recurrent HER2-positive MBC may have been different. However, data such as those presented here could ultimately provide a more evidence-based method to set these types of criteria. In addition, this analysis was not preplanned in the protocol and thus lacks a predefined hypothesis. As mentioned, the cutoff used led to the inclusion of a small number of patients with occult de novo HER2-positive MBC who were treated with adjuvant therapy before their MBC diagnosis; however, as demonstrated by the sensitivity analysis, the inclusion of these patients did not affect the overall outcomes.
As the relative proportion of patients with de novo HER2-positive MBC compared with recurrent HER2-positive MBC is likely to increase, differences between the groups may become more pronounced. These findings from registHER, together with ongoing and upcoming studies—such as SystHERs—and advances in predictive markers for metastatic treatments, may help in understanding the contributions of tumor characteristics or treatment patterns to differences in de novo and recurrent HER2-positive MBC. The outcomes of these studies will help guide the effective management of patients with HER2-positive MBC in the future.
Acknowledgments
The authors would like to thank Bokai Xia and Jonathan Squire for their statistical programming expertise. Support for third-party writing assistance for this manuscript was provided by Genentech, Inc.
Conflict of interest
PAK, MUY, and AMB have had a consultant/advisory role with Roche/Genentech. HSR and PAK have received research funding for their institutions from Genentech. BY and CQ are full-time employees of Genentech. BY has stock ownership in Roche. MM, DT, and DAY have no disclosures.
Ethical standards
This manuscript complies with the current laws of the country in which the research was performed.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ, Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD, Herceptin Adjuvant (HERA) Trial Study Team Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 4.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu M-C, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay M, Riva A, Crown J, Breast Cancer International Research Group Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 10.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 11.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehrenbacher L, Habel L, Capra A, Anthony A, Li X, Quesenberry C, Fulton R (2009) Incidence and demographic and tumor characteristics of HER2-positive invasive breast cancer in a large, unselected population, 2000–2006. Cancer Res 69 (suppl 3):abstract 3058
- 13.Pathmanathan N, Provan PJ, Mahajan H, Hall G, Byth K, Bilous AM, Balleine RL. Characteristics of HER2-positive breast cancer diagnosed following the introduction of universal HER2 testing. Breast. 2012;21:724–729. doi: 10.1016/j.breast.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D (2007) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743. Erratum in: N Engl J Med 356:1487 [DOI] [PubMed]
- 15.Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM, CLEOPATRA Study Group Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K, EMILIA Study Group Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, Tudor IC, Wang LI, Brammer MG, Shing M, Yood MU, Yardley DA. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834–4843. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 18.Aparicio S, Caldas C. The implications of clonal genome evolution for cancer medicine. N Engl J Med. 2013;368:842–851. doi: 10.1056/NEJMra1204892. [DOI] [PubMed] [Google Scholar]
- 19.Hurvitz SA, Hu Y, O’Brien N, Finn RS. Current approaches and future directions in the treatment of HER2-positive breast cancer. Cancer Treat Rev. 2013;39:219–229. doi: 10.1016/j.ctrv.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa RB, Kurra G, Greenberg L, Geyer CE. Efficacy and cardiac safety of adjuvant trastuzumab-based chemotherapy regimens for HER2-positive early breast cancer. Ann Oncol. 2010;21:2153–2160. doi: 10.1093/annonc/mdq096. [DOI] [PubMed] [Google Scholar]
- 21.Esteva FJ, Yu D, Hung MC, Hortobagyi GN. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010;7:98–107. doi: 10.1038/nrclinonc.2009.216. [DOI] [PubMed] [Google Scholar]
- 22. Rossi V, Nolè F, Redana S, Adamoli L, Martinello R, Aurilio G, Verri E, Sapino A, Viale G, Aglietta M, Montemurro F (2014) Clinical outcome in women with HER2-positive de novo or recurring stage IV breast cancer receiving trastuzumab-based therapy. Breast 23:44–49 [DOI] [PubMed]
- 23.Yin W, Jiang Y, Shen Z, Shao Z, Lu J. Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: a meta-analysis of published randomized controlled trials. PLoS One. 2011;6:e21030. doi: 10.1371/journal.pone.0021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21:2169–2174. doi: 10.1093/annonc/mdq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, Guardino E, Song C, Tong B, Ng V, Chu YW, Perez EA. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–1163. doi: 10.1200/JCO.2012.44.9694. [DOI] [PubMed] [Google Scholar]