Abstract
Study Objectives:
Despite several polysomnographic normative studies and multiple surveys of sleep disorders in the general population, few data have been collected on healthy sleepers. We aimed to survey the characteristics of healthy sleep.
Methods:
We prospectively investigated the sleep history of 100 subjects of a representative population sample who reported undisturbed sleep and in whom relevant sleep disorders were ruled out by a two-step screening procedure. Approximately four subjects had to be contacted for identifying 1 eligible subject who participated.
Results:
The median reported time in bed was from 23:00 (21:30–02:00) to 07:00 (05:30–11:00). The total sleep duration was 7.3 h (5–10 h), varying from 7.5 h in the age group ≤ 30 years to 7 h in subjects aged 40–60 years and to 8 h in subjects > 60 years (p = 0.002). The median sleep efficiency was high (93.3%, range: 55.6% to 100%). Fifty-one subjects reported occasional snoring. Forty-five subjects reported sporadic non-bothersome sleep-related movement disorders (25 sleep-related leg cramps, 22 lifetime bruxism, 5 restless legs syndrome), and 36 had a history of sporadic non-bothersome parasomnias (27 nightmares, 12 sleepwalking, 1 sleep paralysis).
Conclusion:
In this population of healthy sleepers, snoring is the most common finding. Moreover, non-bothersome forms of recognizable sleep-related movement disorders and parasomnias are surprisingly common. These findings may suggest that diagnostic criteria of sleep disorders should not only be based on the presence of symptoms but also account for a minimum frequency or discomfort.
Citation:
Frauscher B, Mitterling T, Bode A, Ehrmann L, Gabelia D, Biermayr M, Walters AS, Poewe W, Högl B. A prospective questionnaire study in 100 healthy sleepers: non-bothersome forms of recognizable sleep disorders are still present. J Clin Sleep Med 2014;10(6):623-629.
Keywords: physiological sleep, questionnaire, gender, elderly, parasomnia, sleep-related movement disorder, healthy sleeper
Sleep disorders are common in the general population with prevalences of up to 28% in industrialized countries.1–3 In a study investigating the prevalence and type of sleep disorders across different countries, one in four subjects irrespective of the individual country complained of poor sleep.4 In addition, the authors found that despite the high prevalence of sleep disorders, awareness of disturbed sleep and its negative impact on health was low in the general population.4 Increasing trends of sleep complaints over the last decades have been reported by some authors.5 In a large survey of 155,877 participants of the general population, Grandner et al. demonstrated that in contrast to the literature, advancing age was not associated with increased self-reported sleep disturbance, whereas poor health status and depressed mood were.6 The authors speculated that the often-reported increase in sleep problems with age might be a nonlinear phenomenon, mediated by factors other than physiologic aging.6
Despite several polysomnographic studies on sleep7 and multiple large surveys of sleep disorders, especially of insomnia, in the general population,8 survey data on healthy sleepers are largely lacking. Further knowledge on sleep in healthy sleepers, however, would be of interest considering the significant negative impact of poor sleep on quality of life and physical health.9,10 In this light, we aimed to survey the sleep characteristics of a sample of healthy sleepers between 18 and 80 years of age with a special emphasis on parasomnias and sleep-related movement disorders.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Despite several polysomnographic studies on sleep and multiple large surveys of sleep disorders in the general population, survey data on healthy sleepers are largely lacking. Further knowledge on sleep in healthy sleepers, however, would be of interest considering the significant negative impact of poor sleep on quality of life and physical health.
Study Impact: In this population of healthy sleepers, occasional snoring, non-bothersome forms of recognizable sleep-related movement disorders, and parasomnias are still surprisingly common. These findings may suggest that diagnostic criteria of sleep disorders should not only be based on the presence of symptoms but also account for a minimum frequency or discomfort.
METHODS
To be considered a healthy sleeper, subjects had to report undisturbed sleep, and had to pass both step 1 and step 2 of a two-step screening procedure. Potential study subjects were recruited randomly from an existing population sample representative for the population of Tyrol/Austria by a market research organization (Karmasin Institute).
Two-Step Screening Procedure
Step 1 consisted of a structured telephone interview of an average duration of 30 minutes (min) performed by trained interviewers of Karmasin Institute. Approximately 4 subjects had to be interviewed for every one willing or eligible subject that passed on to step 2.
The interview was terminated when an exclusion criterion was met, and further data were not collected on these excluded subjects. The initial screening consisted of questions designed to rule out any neurological or psychiatric disease, medical problems (such as pulmonary, renal, cardiac and liver disease, diabetes mellitus), body mass index (BMI) > 30 kg/m2, pregnancy, regular alcohol consumption ≥ 3 glasses of wine or 3 bottles of beer per day, use of pain medication > 3 times per week, or use of central nervous system active medication including hypnotics during the year prior to study participation. Sleep disorders were also queried. Patients who were previously diagnosed with a sleep disorder (irrespective of its severity), presence of shiftwork, presence of non-restorative sleep or problems initiating or maintaining sleep > 2 times per week, reported cessation of breathing during sleep noticed by the subject or the bed partner, presence of daytime sleepiness and unintended falling asleep during the day, as well as restless legs syndrome (RLS) > twice a week and RLS symptoms considered bothersome by the patients, when specifically asked for complaints, were ruled out. Circadian rhythm disorders, movement disorders during sleep (except RLS) and parasomnias were not assessed in step 1 and therefore presented no exclusion criteria. All subjects who passed inclusion and exclusion criteria of step 1 were allowed to go on to step 2.
Step 2 comprised a personal face-to-face semi-structured expert interview which was performed at the sleep clinic of the Department of Neurology at Innsbruck Medical University to exclude the possibility that some participants gave unintentional incorrect answers in step 1. The duration of this interview was on average one hour. During this interview, all subjects' weight was checked, and subjects with a BMI > 30 kg/m2 were excluded. Also participants with neurological, psychiatric, or medical disorders were excluded according to the criteria as in step 1. For ruling out depression, we applied the Hospital Anxiety and Depression Scale.11 A score > 10 was used as an exclusion criterion. We also performed a structured patient sleep interview assessing all sleep diagnoses according to the criteria of the International Classification of Sleep Disorders, 2nd edition (ICSD-2) main categories and major subcategories.12 Sleep-related exclusion criteria corresponded to the exclusion criteria of step 1. In addition, participants were also excluded in step 2 if they had an Epworth Sleepiness Scale score ≥ 10, which is indicative for pathological daytime sleepiness,13 or any circadian rhythm disorder (delayed sleep phase type, advanced sleep phase type, irregular sleep-wake type, free running type). Movement disorders during sleep were used as further exclusions only if the participants considered these symptoms as subjectively bothersome when specifically asked about them using a yes/no question. These movement disorders included sleep-related leg cramps, sleep-related bruxism, or sleep-related rhythmic movement disorder; and past or current history of parasomnias such as confusional arousals, sleepwalking, and sleep terrors, REM sleep behavior disorder (RBD), recurrent isolated sleep paralysis, nightmares, or sleep-related hallucinations. Subjects with a previous history of a non-current sleep disorder were also allowed to be included in the present study. Subjects were rated as eligible if they passed both screening steps.
Sleep Questionnaire and Validated Scales
After passing both screening steps, all participants completed a sleep questionnaire between August and November 2011 applied by physicians trained in sleep medicine under the supervision of board certified neurologists specialized in sleep medicine. Apart from general questions concerning sleep times, circadian chronotype, sleep quality, and additional sleep features (sleep-related movement disorders, parasomnias, isolated findings), the sleep questionnaire contained several validated scales. Sleep times were rated with 15-min accuracy. Sleep quality was assessed by the Pittsburgh Sleep Quality Index,14 which is composed of 7 subscores aiming at subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of hypnotics, and daytime sleepiness. The maximum total score is 21 points. In case of sporadic RLS, defined as presence of RLS symptoms < 2 times per week, RLS severity was assessed by the International RLS Severity Rating Scale.15 This scale comprises 10 items with a maximum score of 40 points. Mild RLS is defined as a score of 1–10 points, moderate RLS with a score of 11–20 points, severe RLS with 21–30 points, and very severe RLS with 31–40 points. Screening for RBD was performed with the Innsbruck RBD inventory.16 The Innsbruck RBD inventory is a 5-item screening questionnaire for RBD. A cutoff of 0.25 has been shown to be suggestive of RBD.16 Sporadic presence of a non-bothersome parasomnia or movement disorder was defined with a frequency < 2 times per week. Of note, the Pittsburgh Sleep Quality Index, the International RLS Severity Rating Scale, and the Innsbruck RBD inventory were not used to further exclude subjects.
This study was approved by the local ethical committee of Innsbruck Medical University. All participants granted written informed consent prior to investigation.
Statistics
Statistics were calculated with SPSS 19.0 for Windows. All data were tested for normal distribution using the Shapiro Wilks test. Participants were grouped into subjects 18–30 years, 31–40 years, 41–50 years, 51–60 years, and subjects 61–80 years of age. Since data were not normally distributed, nonparametric statistics (Mann-Whitney U-test in case of 2 groups, Kruskal-Wallis test in case of more than 2 groups) were applied. For categorical variables, χ2 tests were performed. A p-value below 0.05 was considered to indicate statistical significance.
RESULTS
Demographics of the Study Sample
One hundred ten subjects were recruited by Karmasin Institute after undergoing a positive screening according to inclusion and exclusion criteria (approximately 400 subjects had to be screened to identify 110 eligible subjects who were willing to go on to step 2). Of these 110 potential study candidates, 10 subjects had to be excluded during the second step of the screening because of BMI > 30 kg/m2 (n = 4), drop out (n = 2), hepatitis C (n = 1), major depression (n = 1), spastic cerebral palsy (n = 1), and sick sinus syndrome (n = 1). A total of 100 healthy sleepers (60 women, 40 men) participated in this study. The median age of study participants was 43 (19–77) years. For demographic data see Table 1.
Table 1.
Demographic characteristics of the study sample

Sleep Timing on Working Days
The median time in bed on working days was from 23:00 (21:30–02:00) to 07:00 (05:30–11:00). The median sleep latency was estimated to be 10 min (range: 0–60 min). The estimated median nocturnal wake time was 30 min (range: 0–240 min). The median sleep duration was 7.3 hours (h, 5–10 h). Information on sleep times over the different age groups is provided in Table 2: Subjects ≤ 30 years had later bedtimes and rise times than subjects in the older age groups (bedtime: p = 0.003, rise time: p = 0.001). Of note, total sleep duration was 7.5 h in the age group ≤ 30 years, decreasing to 7 h in subjects aged 40–60 years, and again increasing to 8 h in subjects older than 60 years (p = 0.002). Twenty of the 22 subjects > 60 years of age were retired. Retired subjects slept a median of 8.0 h per night (5.5–10 h), compared to 7.0 h per night (5–9 h) in non-retired subjects (p = 0.042). Figure 1 illustrates different sleep durations of the total sample. The median sleep efficiency was high at 93.3% (55.6 to 100%). Women showed earlier bedtimes than men (p = 0.005). Rise time, sleep latency, nocturnal wake time, total sleep duration, and sleep efficiency did not differ between women and men (all p-values > 0.05). See Table 3 for further information. Twenty subjects (12 men, 8 women) with a mean age of 43.3 ± 16.6 years reported a planned afternoon nap for a median duration of 30 min (12–120) 2.9 times per week (1–7).
Table 2.
Sleep parameters of the healthy sleepers across different age groups
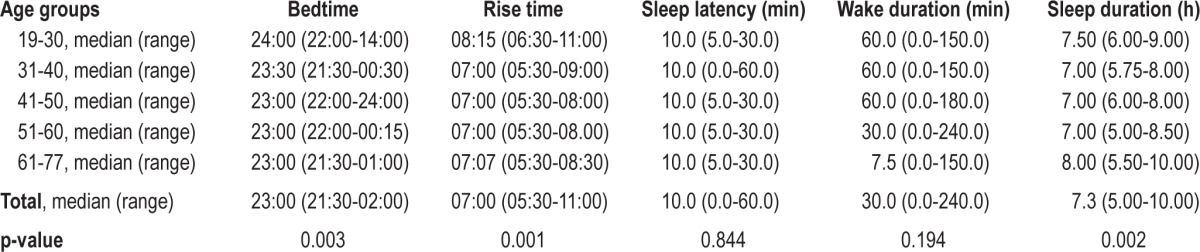
Figure 1. Distribution of sleep duration of the total study sample.
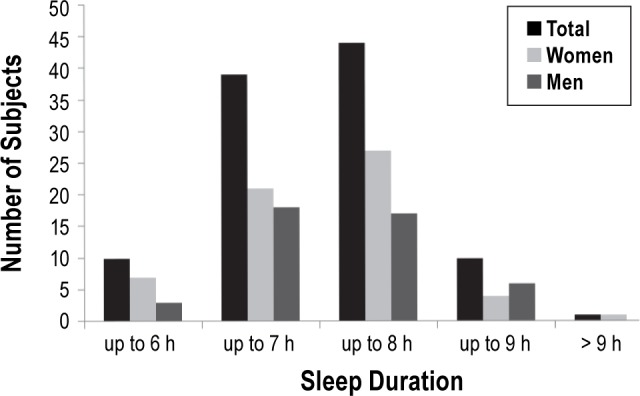
Table 3.
Influence of gender on sleep parameters
Self-Rated Chronotype and Sleep Quality
Thirty-three subjects (33%) rated themselves according to best physical performance as morning types, 37 (37%) as evening types, and 26 (26%) as indifferent types. Of note, subjects classified as evening types were more common in the age group ≤ 30 years (n = 12/23) than the age group > 60 years (5/22). Morning types were more common in the age group > 60 years (4/22) compared to the age group ≤ 30 years (11/23, p = 0.078). The median score on the Pittsburgh Sleep Quality Index was 3 (range: 0–7). No significant age group or gender difference was present (age group: p = 0.740 gender: p = 0.938). Fourteen percent of subjects (12 women, 2 men) scored > 5 points on the Pittsburgh Sleep Quality Index; none scored > 7 points.
Sporadic Non-Bothersome Sleep-Related Movement Disorders, Sporadic Non-Bothersome Parasomnias, and Clinically Isolated Syndromes (Table 4)
Table 4.
Sporadic non-bothersome parasomnias, sporadic non-bothersome sleep-related movement disorders, and isolated symptoms in healthy sleepers across different age groups
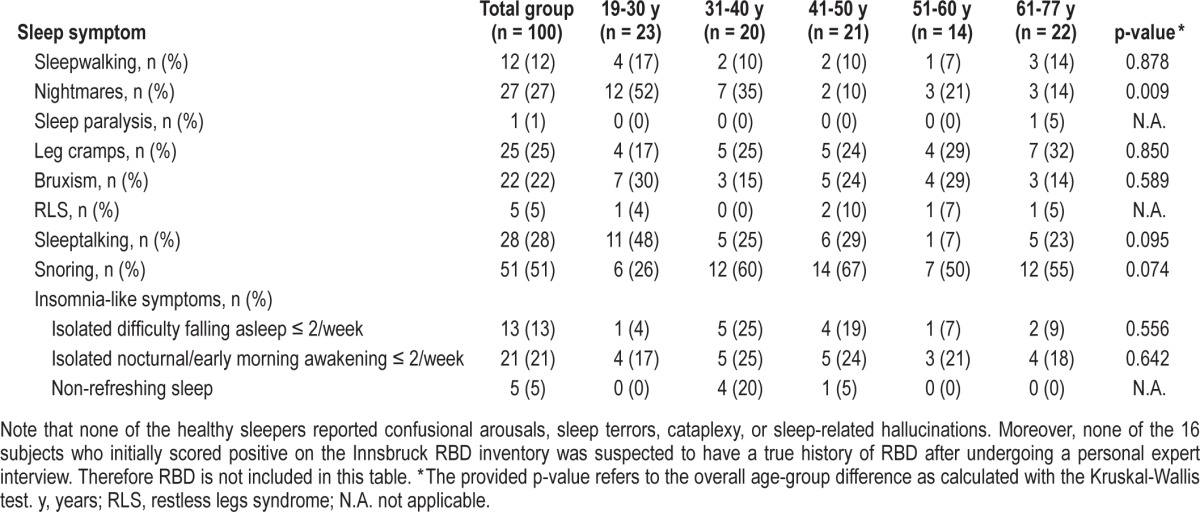
Forty-five participants (45%) reported sporadic non-bothersome sleep-related movement disorders (25 sleep-related leg cramps, 22 bruxism, 5 RLS). Thirty-six subjects (36%) had a current or past history of sporadic and non-bothersome parasomnias (27 occasional nightmares, 12 sleepwalking, 1 sleep paralysis). According to the International RLS Severity Rating Scale, one of the 5 subjects was classified as mild RLS, and 4 had no RLS symptoms during the week prior to investigation. Sixteen subjects scored positive on the Innsbruck RBD inventory, but personal expert interview suggests that none of them had a “true” RBD history. Reasons for false-positive screening results were a history of sporadic non-bothersome NREM parasomnias, potential non-bothersome periodic leg movements in sleep, and non-bothersome occasional nightmares. For further specifications and distribution with respect to age, see Table 4. Fifty-one percent of the total sample were reported to snore at least occasionally; 28% reported sleeptalking. Isolated insomnia-like symptoms are reported in Table 4: 21% reported nocturnal/early morning awakening ≤ 2/week; 13% reported difficulties with falling asleep ≤ 2/week, and 5% reported non-refreshing sleep.
Past Experience with Hypnotics
Fourteen participants (14%, 10 women, 4 men) reported on situational past use of hypnotics (4 did not remember the type of hypnotic, 3 benzodiazepines, 3 antidepressants, 3 herbals, 1 antihistaminergic agent). In all cases, the use was transient. The difference in gender was not significant (p = 0.395).
DISCUSSION
To the best of our knowledge, this is the first expert-interview based sleep survey performed in healthy sleepers recruited from a population-based sample of the general population. The main findings were a large inter-individual range of sleep timing, less than expected changes across the lifespan, a high proportion of subjects reporting at least occasional snoring, and an unexpectedly high proportion of sporadic and non-bothersome sleep comorbidity even in this cohort of healthy sleepers. As we aimed to investigate healthy subjects with no apparent sleep disorder who perceived their sleep as good according to their impression, we decided a priori not to use exclusion criteria of simple snoring, presence of non-restorative sleep, problems with initiating or maintaining sleep less than twice per week, and the sporadic lifetime presence of any non-bothersome movement disorders or parasomnias.
Inter-Individual Variability of Sleep Timing
There were marked inter-individual differences in sleep timing including bedtimes, sleep latency, total sleep duration, and duration of nocturnal wake episodes. This finding well supports the notion that there is not a single normal sleep pattern but that sleep rather presents an individual distinct trait. Given that at least a portion of the healthy sleepers in this study had sleep patterns comparable to patients suffering from insomnia, we would emphasize the importance of not only assessing quantitative but also qualitative parameters such as sleep quality and daytime performance to correctly distinguish normal from pathological sleep. Apart from these inter-individual differences in sleep timing, up to 21% of the participants reported isolated insomnia-like symptoms occurring 1–2 days per week. The interesting question of whether these subjects will develop clinical insomnia in the future awaits longitudinal observation and cannot be answered by the present study.
A median bedtime on working days of 23:00 corresponds well to that documented in other central European countries and is earlier than the usual bedtime in southern European countries.4 The median self-perceived sleep duration was 7.3 h ranging from 5 to 10 h. This corresponds well to a recent telephone interview-based questionnaire study in the Austrian general population, which demonstrated that approximately half of the subjects reported less than seven h sleep per night.17
Less than Expected Variability of Sleep Timing across the Lifespan
In this survey, the distribution of morning and evening types over the different age groups was well in line with that reported in the literature.18,19 As expected, a higher proportion of participants rating themselves as evening types was present in young adults ≤ 30 years of age than participants older than 60 years, whereas, in contrast, morning types were more common in the group of participants aged 60 years and older than those ≤ 30 years. In line with this self-perception were the findings of bedtimes over different age groups. They were later in the younger participants than the older subjects.
Participants older than 60 years had a total sleep time of 8 h, which was higher than the median sleep duration of 7.3 h of the total group. At first glance, this finding seems to be in contrast to the literature, since numerous studies investigating objective sleep parameters have demonstrated age-related changes with decreasing total sleep times over the lifespan.20–24 A very thorough and meticulous meta-analysis of Ohayon et al., who investigated 65 polysomnographic studies representing 3,577 subjects aged 5 to 102 years, demonstrated that total sleep time generally markedly decreased with age with a loss of approximately 10 minutes of sleep per decade.7 However, our data are in line with a recent large survey demonstrating that poor general health and depressed mood, and not advanced age were associated with self-reported disturbed sleep.6 One possible explanation for the longer sleep duration in subjects over 60 years in the current study could be retirement, allowing for more sleep. In Austria, the average retirement age in 2011 was 59 years for women and 63 years for men.25 Indeed, the vast majority of subjects > 60 years of age were retired, and retired subjects reported longer sleep durations than non-retired subjects. Moreover, current data are subjective, based on participants' histories, while the aforementioned studies were based on objective polysomnographic studies.7,20–24
Present or Past Occurrence of Sporadic or Non-Bothersome Sleep Comorbidity (Sleep-Related Movement Disorders, Parasomnias, Isolated Findings)
Taking into account that all participating subjects were healthy sleepers and that clinically relevant sleep-related movement disorders and parasomnias were exclusion criteria for the present study, it was striking and unexpected that there was a high number of non-bothersome sleep-related movement disorders or parasomnias in the present study. Forty-five percent of subjects reported sporadic non-bothersome sleep-related movement disorders, and 36% had a present or past occurrence of sporadic non-bothersome parasomnias. This result underlines the vast spectrum of various phenomena during sleep ranging from only mild and rare manifestations, which are not at all bothersome, to severe and frequent manifestations which severely influence sleep and daytime functioning.26 In line with our observation of a broad clinical spectrum of parasomnias is a very recent study on the prevalence and comorbidity of nocturnal wandering performed in the US adult general population.27 Ohayon et al. found a lifetime prevalence of nocturnal wandering of up to 30%, which was mostly due to nocturnal wandering in childhood and adolescence, a prevalence of up to 4% in the year prior to investigation, and a prevalence of up to 1% for more than 1 episode per month, whereas episodes occurring on a weekly basis were rare at approximately 0.3%.27 Hence it is important to not only ask for the presence of motor phenomena during sleep but also for their severity, frequency, and impact on sleep and quality of life to correctly differentiate incidental findings from clinically relevant disorders. This is of utmost importance in considering therapeutic issues. Parasomnias and sleep-related movement disorders should only be treated if patients consider them bothersome. On the other hand, one cannot rule out the possibility that subjects who have currently non-bothersome or sporadic sleep disorders will eventually develop a relevant sleep disturbance over time. In addition, approximately half of our healthy sleepers reported snoring at least occasionally with no further history of suggestive sleep apnea, as this had been an exclusion criterion. Whether further polysomnographic work-up for sleep apnea is recommended should be decided on an individual basis.
Screening Questionnaires vs. Expert Interview
Questionnaires for sleep disturbances and sleep quality are useful instruments for screening purposes and for providing rough estimations but do not take the place of a face-to-face expert interview. All patients in the present were rated as healthy sleepers based on sleep history and according to inclusion and exclusion criteria. When looking at the scores of the Pittsburgh Sleep Quality Index, however, 14% of these healthy sleepers scored more than 5 points which is generally used as the cutoff for poor sleep.14 Nevertheless, neither subjective sleep perception nor expert evaluation confirmed disturbed sleep in any of these subjects. However, not a single subject scored higher than 7 points on this 21-point scale. Moreover, 16% of participants scored as positive on the Innsbruck RBD inventory for screening of RBD,16 whereas eliciting a thorough history for RBD by expert interview did not suggest potential RBD in any of these subjects. This strongly suggests that the Innsbruck RBD inventory16 as well as other RBD inventories28 are useful for screening purposes but cannot be recommended as a substitute for expert-based interviews.
Experience with Hypnotics
Experience with situation-related hypnotic use was reported by 14% of healthy sleepers. Given the ready availability and convenience of hypnosedatives, it may simply reflect habits of a society that uses hypnotics easily as shown by various different authors in industrialized countries.2–4,29
Potential Limitations
The sample size of the present study is small compared to surveys performed in the general population. However, in contrast to population-based surveys, the present study aimed to investigate the variable expression of normal sleep in a carefully selected sample of healthy normal sleepers. For our sample of 100 healthy sleepers, approximately 4 randomly selected subjects of a representative population-based sample had to be investigated in order to identify one eligible study candidate who participated in the study. In addition, gender was not equally distributed over the different age groups. A similar experience was reported by Fietze and Diefenbach, who found that via advertisement only one of five subjects qualified for a control group for sleep studies.30 As all data are based on personal interviews of the study participants, we cannot rule out that an independent bed partner interview might have led to a slight change in the frequency or findings of this study. In the present study, all interviews were done personally by physicians experienced in sleep medicine. Another benefit was that all participants were recruited from an existing population sample representative for the Austrian population. This mode of recruitment was chosen to avoid a healthy volunteer bias.
CONCLUSION
In this paper we used a strict definition of a healthy sleeper. Approximately 400 subjects of a representative population sample had to be contacted to identify 100 eligible subjects who participated in our study. In this population of healthy sleepers, snoring was the most common finding. In addition, non-bothersome forms of recognizable sleep disorders were still surprisingly common. These findings may suggest that diagnostic criteria of sleep disorders should not only be based on the presence of symptoms but also account for a minimum frequency or discomfort of symptoms.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by the intramural funding program of the Medical University Innsbruck for young scientists, Project 2010012005. The work was performed at the Department of Neurology, Innsbruck Medical University. Dr. Frauscher has participated in speaking engagements for UCB and has received research funding from the Austrian Science Fund (KLI236) and the National Bank of Austria (15127). Dr. Walters has served as a consult to UCB Pharma on Restless Legs Syndrome and has also received research grant funding from UCB Pharma and Mundipharma. Werner Poewe has received consultancy and lecture fees from AbbVie, Astra Zenca, Teva, Lundbeck, Novartis, GSK, Boehringer-Ingelheim, UCB, Orion Pharma, Merck Serono and Merz Pharmaceuticals in relation to clinical drug development programs for PD. Birgit Högl: paid speaking engagements, advisory boards or consulting for: UCB, Pfizer, Sanofi, GSK, BI, Mundipharma, Respironics. Travel support from Habel Medizintechnik and Vivisol. Grant to institution by UCB. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Ishigooka J, Suzuki M, Isawa S, Muraoka H, Murasaki M, Okawa M. Epidemiological study on sleep habits and insomnia of new outpatients visiting general hospitals in Japan. Psychiatry Clin Neurosci. 1999;53:515–22. doi: 10.1046/j.1440-1819.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM, Smirne S. Prevalence and consequences of insomnia disorders in the general population of Italy. Sleep Med. 2002;3:115–20. doi: 10.1016/s1389-9457(01)00158-7. [DOI] [PubMed] [Google Scholar]
- 3.Zeitlhofer J, Seidel S, Klösch G, et al. Sleep habits and sleep complaints in Austria: current self-reported data on sleep behaviour, sleep disturbances and their treatment. Acta Neurol Scand. 2010;122:398–403. doi: 10.1111/j.1600-0404.2010.01325.x. [DOI] [PubMed] [Google Scholar]
- 4.Soldatos CR, Allaert FA, Ohta T, Dikeos DG. How do individuals sleep around the world? Results from a single-day survey in ten countries. Sleep Med. 2005;6:5–13. doi: 10.1016/j.sleep.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Santos-Silva R, Bittencourt LR, Pires ML, et al. Increasing trends of sleep complaints in the city of Sao Paulo, Brazil. Sleep Med. 2010;11:520–4. doi: 10.1016/j.sleep.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Grandner MA, Martin JL, Patel NP, et al. Age and sleep disturbances among American men and women: data from the U.S. Behavioral Risk Factor Surveillance System. Sleep. 2012;35:395–406. doi: 10.5665/sleep.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 8.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 9.Hajak G SINE Study Group. Study of Insomnia in Europe. Epidemiology of severe insomnia and its consequences in Germany. Eur Arch Psychiatry Clin Neurosci. 2001;251:49–56. doi: 10.1007/s004060170052. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–61. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann-Lingen C, Buss U, Snaith RP. Bern: Verlag Hans Huber; 1995. HADS-D Hospital Anxiety and Depression Scale-Deutsche Version. [Google Scholar]
- 12.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders, 2nd ed.: diagnostic and coding manual. [Google Scholar]
- 13.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 14.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 15.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 16.Frauscher B, Ehrmann L, Zamarian L, et al. Validation of the Innsbruck REM sleep behavior disorder inventory. Mov Disord. 2012;27:1673–8. doi: 10.1002/mds.25223. [DOI] [PubMed] [Google Scholar]
- 17.Falkenstetter T, Frauscher B, Anderer P, et al. Erhöhte Tagesschläfrigkeit in Österreich. Somnologie. 2010;14:15–22. [Google Scholar]
- 18.Reyner LA, Horne JA, Reyner A. Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults. Sleep. 1995;18:127–34. [PubMed] [Google Scholar]
- 19.Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–38. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- 21.Williams RL, Karacan I, Hursch CJ. New York: Wiley; 1974. Electroencephalography (EEG) of human sleep: clinical applications. [Google Scholar]
- 22.Bixler EO, Kales A, Jacoby JA, Soldatos CR, Vela-Bueno A. Nocturnal sleep and wakefulness: effects of age and sex in normal sleepers. Int J Neurosci. 1984;23:33–42. doi: 10.3109/00207458408985343. [DOI] [PubMed] [Google Scholar]
- 23.Hirshkowitz M, Moore CA, Hamilton CR, 3rd, Rando KC, Karacan I. Polysomnography of adults and elderly: sleep architecture, respiration, and leg movement. J Clin Neurophysiol. 1992;9:56–62. [PubMed] [Google Scholar]
- 24.Danker-Hopfe H, Schäfer M, Dorn H, et al. Percentile Reference Charts for Selected Sleep Parameters for 20- to 80-Year-Old Healthy Subjects from the SIESTA Database. Somnologie. 2005;9:3–14. [Google Scholar]
- 25. Online from Statistik Austria: http://www.statistik.at/web_de/statistiken/soziales/sozialleistungen_auf_bundesebene/pensionen_und_renten/index.html.
- 26.Frauscher B, Gschliesser V, Brandauer E, et al. The severity range of restless legs syndrome (RLS) and augmentation in a prospective patient cohort: association with ferritin levels. Sleep Med. 2009;10:611–5. doi: 10.1016/j.sleep.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Ohayon MM, Mahowald MW, Dauvilliers Y, Krystal AD, Léger D. Prevalence and comorbidity of nocturnal wandering in the U.S. adult general population. Neurology. 2012;78:1583–9. doi: 10.1212/WNL.0b013e3182563be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stiasny-Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov Disord. 2007;22:2386–93. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- 29.Calem M, Bisla J, Begum A, et al. Increased prevalence of insomnia and changes in hypnotics use in England over 15 years: analysis of the 1993, 2000, and 2007 National Psychiatric Morbidity Surveys. Sleep. 2012;35:377–84. doi: 10.5665/sleep.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fietze I, Diefenbach K. Healthy sleepers are rare: problems and success rates in establishing a control group for sleep studies. Neuropsychopharmacology. 2003;28:558–61. doi: 10.1038/sj.npp.1300082. [DOI] [PubMed] [Google Scholar]


