Abstract
Study Objectives:
Alternative therapies for childhood obstructive sleep apnea syndrome (OSAS) are needed as OSAS may persist despite adenotonsillectomy, and continuous positive airway pressure (CPAP) adherence is low. Nasal expiratory positive airway pressure (NEPAP) devices have not been studied in children. We hypothesized that NEPAP would result in polysomnographic improvement. Further, we aimed to determine NEPAP adherence, effects on sleepiness, behavior, and quality of life.
Methods:
A randomized, double-blind, placebo-controlled, crossover pilot study was performed. CPAP candidates, 8-16 years old, underwent NEPAP and placebo polysomnograms. Subjects with ≥ 50% reduction in the apnea hypopnea index (AHI) from placebo to NEPAP night or AHI < 5/h on NEPAP night wore NEPAP at home for 30 days. Adherence was assessed by daily phone calls/emails and collecting used devices.
Results:
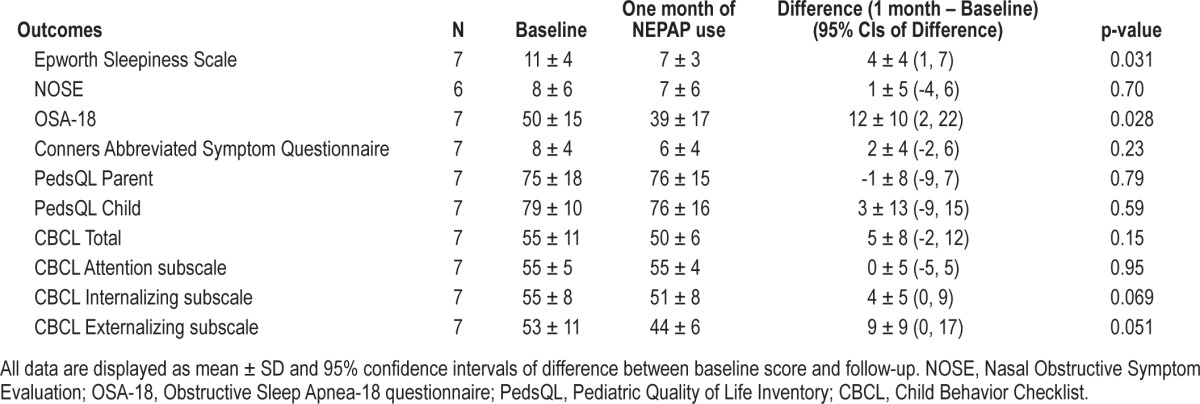
Fourteen subjects (age 13.4 ± 1.9 years, BMI z-scores 2.2 ± 1 [mean ± SD]) were studied. There was significant improvement in the obstructive apnea index with NEPAP vs. placebo: 0.6 (0-21.1)/h vs. 4.2 (0-41.9)/h (median [range], p = 0.010) and trends for improvement in other polysomnographic parameters. However, responses were variable, with 3 subjects not improving and 2 worsening. Older children and those with less hypercapnia had a better response. Eight subjects were sent home with devices; one was lost to follow-up, and adherence in the remainder was 83% of nights; these subjects had a significant improvement in sleepiness and quality of life.
Conclusions:
NEPAP devices are a potential alternative therapy for OSAS in a small subset of children. Due to variability in individual responses, efficacy of NEPAP should be evaluated with polysomnography.
Clinical Trial Registration:
www.clinicaltrials.gov, identifier: NCT01768065
Citation:
Kureshi SA, Gallagher PR, McDonough JM, Cornaglia MA, Maggs J, Samuel J, Traylor J, Marcus CL. Pilot study of nasal expiratory positive airway pressure devices for the treatment of childhood obstructive sleep apnea syndrome. J Clin Sleep Med 2014;10(6):663-669.
Keywords: obstructive sleep apnea syndrome, pediatrics, nasal expiratory positive airway pressure
The childhood obstructive sleep apnea syndrome (OSAS) is common. Prevalence rates range from 1% to 4%.1,2 Although adenotonsillectomy is the usual first step for treatment, a recent study found that up to 20% of otherwise-healthy children may have residual OSAS following surgery.3 Furthermore, children with additional medical conditions, such as neurologic disease or craniofacial anomalies, may need further treatment. Continuous positive airway pressure (CPAP) is the usual second line treatment, but adherence rates in children are low.4–6 Thus, alternative therapies for children with OSAS are desperately needed.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Alternative therapies for children with obstructive sleep apnea syndrome (OSAS) are needed since OSAS may persist after adenotonsillectomy, and suboptimal adherence occurs with continuous positive airway pressure (CPAP). We conducted a pilot study to gain preliminary data to determine the efficacy, tolerability, and adherence of nasal expiratory positive airway pressure (NEPAP) devices in children.
Study Impact: This pilot study is the first pediatric study to our knowledge that utilized NEPAP devices for the treatment of OSAS. NEPAP devices may be a potential alternative therapy for OSAS for a small subset of children with persistent OSAS following surgery or those who are not able to tolerate CPAP.
The nasal expiratory positive airway pressure (NEPAP) device has been used to treat OSAS in adults.7–10 The device acts as a one-way valve. During inspiration the valve opens, with negligible resistance to flow.11 During expiration, the valve closes and airflow is routed through small air channels, which increases resistance.11 The NEPAP devices have been reported to have an expiratory resistance of 80 cm H2O*sec/L at a flow rate of 100 mL/sec.11 The increased resistance has been theorized to help maintain the upper airway pressure during the critical endexpiratory period when the upper airway has been postulated to be most constricted in the breaths preceding an apneic event.11 In contrast to CPAP, which provides positive pressure during both inspiration and expiration, the NEPAP device creates pressure during expiration only. We hypothesized that adherence to NEPAP would be higher than adherence to CPAP as reported in the pediatric literature,5 as NEPAP was less cumbersome and laborious than CPAP.
As NEPAP has never been studied in children, we conducted a small pilot study to gain preliminary data to determine the efficacy and tolerability of NEPAP devices in children. We hypothesized that NEPAP would result in polysomnographic (PSG) improvement of OSAS. Further, we aimed to determine adherence with NEPAP. We also hypothesized that, after using NEPAP for one month, there would be improvements in sleepiness, behavior, and quality of life.
METHODS
This small pilot study was a prospective, randomized, double blind, placebo-controlled, crossover clinical trial. The institutional review board of Children's Hospital of Philadelphia approved the study design for human subjects (IRB# 12-008691). Informed consent was obtained from a parent/guardian, and assent was obtained from children older than 7 years of age. The trial was posted on www.clinicaltrials.gov (NCT01768065).
Pediatric patients diagnosed with moderate-to-severe OSAS who were candidates for CPAP treatment, unable to tolerate CPAP, or used CPAP but desired an alternate treatment, were recruited from the Sleep Center. We first tried the NEPAP devices for size in a few children and found that the devices did not fit into the nares of most children < 8 years of age. Inclusion criteria were therefore ages 8-16 years, baseline clinical OSAS with an AHI ≥ 5/h, and the ability to tolerate NEPAP during an initial instruction session. Subjects were given instructions on habituation techniques during a coaching session with a clinician (JM) who coached them on respiratory techniques. Children with severe developmental delay where aspiration was considered a risk, severe pulmonary or heart disease, and children whose families who did not speak English well enough to complete the psychometric measurements, were excluded.
The study design involved randomization of each subject to perform 2 PSG nights with alternating devices: NEPAP and placebo, performed in random order within approximately 1 month of each other. The NEPAP devices utilized for the study were the same devices currently marketed for use in adults (Provent, Ventus Medical, Belmont, CA). Placebo devices appeared similar to the NEPAP treatment device but had no internal valves. Subjects who were previously using CPAP therapy were instructed to discontinue CPAP for 72 h as a washout period preceding the study PSG nights. Subjects who had ≥ 50% reduction in AHI from the placebo to NEPAP night, or those who had an AHI < 5/h on the NEPAP night, were considered responders and wore NEPAP at home for 30 days. Similar to the intended use for adults, the subjects wore the devices nightly and disposed the devices after one use. Adherence was assessed by daily phone calls/emails and collecting used devices. Questionnaires regarding behavioral measurements and quality of life were obtained at baseline and after 30 days of home use.
PSGs were performed using standard pediatric techniques. PSGs included electroencephalogram, electromyogram (submental and tibial), electrooculogram (right/left), electrocardiogram, oxygen saturation and oximeter pulse waveform, transcutaneous CO2, thoracic and abdominal motion (respiratory inductance plethysmography), and digital video and audio. Oronasal air flow (thermistor), nasal pressure, and end-tidal CO2 (ETCO2) were measured using proprietary cannulas that clipped on to the valves. Data were recorded on a Rembrandt system (Embla, San Carlos, CA).
PSGs were scored in blinded fashion according to the American Academy of Sleep Medicine pediatric guidelines.12 The home phase of the study consisted of an open label extension to use the NEPAP devices for 30 days. Three methods were used to assess adherence. Families were requested to: (1) keep a daily log of NEPAP device use, (2) phone, text, or email daily with a report of the previous night's NEPAP device use and any adverse effects, and (3) keep all discarded devices in a provided sealed container. After using the devices for 1 month, the subjects returned for their final visit and the adherence log and container of used devices were collected.
Subjects and their parents completed questionnaires prior to the initial PSG and after using NEPAP devices at home for 30 days. Sleepiness was evaluated using the Epworth Sleepiness Scale Modified for Children.13 Symptoms of the attention deficit hyperactivity disorder was evaluated with the Conners Abbreviated Symptom Questionnaire.14 Behavior problems were measured with the Child Behavior Checklist.15,16 OSAS symptoms and quality of life were evaluated with the Obstructive Sleep Apnea-18 questionnaire.17 General health-related quality of life was evaluated by the Pediatric Quality of Life Inventory.18 Nasal symptoms for side effects were calculated using the Nasal Obstruction Symptom Evaluation.19 Study data were collected and managed using Research Electronic Data Capture (REDCap).20
In Vitro Testing
The bi-directional pressure versus flow characteristics of the NEPAP devices were independently measured in vitro using a precision pneumotachometer (Hans Rudolph, Inc., Shawnee, KS) and pressure transducer (Validyne, Northridge, CA). Pressure versus flow curves were characterized by pumping air from a calibration syringe bi-directionally through matching left and right nares devices mounted on a plate. Flow was delivered and pressure measured on the nares-side of the devices. A custom computer program sampled pneumotachometer flow and driving pressure at 100 samples/sec over the range of −0.6 to 0.6 L/sec, which is typical of resting tidal peak flows in subjects younger than 18 years.
Statistical Analysis
The distributions of study variables were examined for normality using histograms and one-sample Kolmogorov-Smirnov tests of normal distribution, and appropriate parametric or nonparametric tests were subsequently applied. Demographic characteristics measured on a continuous scale were compared between responders versus non-responders using 2-group t-tests or Mann-Whitney tests as appropriate. Fisher exact tests were used to examine the association between responder status and categorical demographic and baseline variables. For the crossover PSG data, a series of 2-group t-tests (or Mann-Whitney tests) were used to examine effects of carryover, period, and treatment, following Jones and Kenward.21 The first test analyzed carry-over effects by applying the test to the subject averages (or totals). The second test for determining the effect of period applied the test to the period differences. Finally, provided that there were no carry-over or period effects, the third test analyzed the treatment effects by applying the test to the crossover differences. A series of SAS Mixed Effects Models was also used to re-examine and confirm effects of carry-over, period, and treatment, on the various PSG parameters. Paired t-tests or Wilcoxon matched pairs signed ranks tests were used to examine differences between initial visit and repeat visit for the questionnaires regarding behavioral and quality of life outcomes.
RESULTS
Study Group
Details of enrollment are shown in Figure 1. Sixteen subjects were approached; one subject declined enrollment in the study due to time commitments. The remaining 15 subjects that trialed the NEPAP devices in their nares all tolerated the devices during the introductory session and enrolled in the study (Table 1). Most subjects had obesity as the etiology of their OSAS, except for one subject with Down syndrome and one subject who had neurofibromatosis with an upper airway plexiform neurofibroma. There were 6 subjects who used CPAP therapy prior to study enrollment. Prior to entering the study, 4 subjects used nasal steroids (2 NEPAP responders and 2 non-responders), and 2 subjects (both NEPAP responders) used montelukast. There were no changes in the use of these drugs during the study period.
Figure 1. Consort diagram of study participants.
Table 1.
Subject demographics
Polysomnography
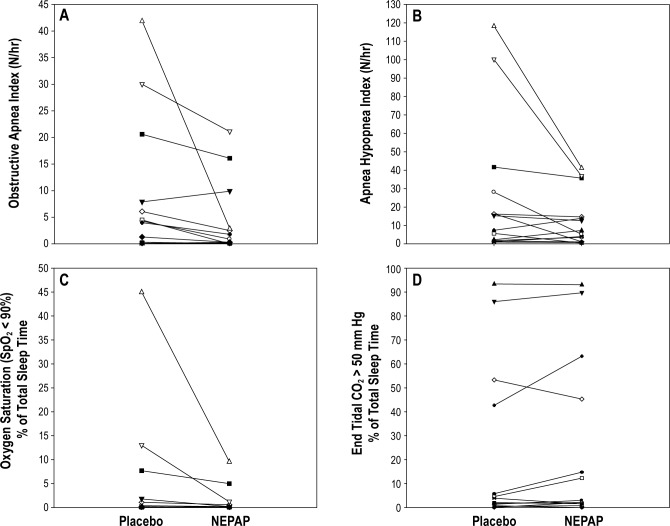
One subject withdrew from the study after the first (placebo) PSG as she did not like sleeping without her CPAP. All subjects tolerated the NEPAP well overnight. For the group as a whole, there was a significant improvement in the obstructive apnea index (Table 2). However, in this small study, there were trends in reduction of AHI, arousal index, and the percent of total sleep time with oxygen saturation < 90% (Table 2, Figure 2), but these were not statistically significant. There were no significant changes in ETCO2 levels or sleep architecture. BMI did not change significantly between the 2 PSG nights (p = 0.77). There were no statistically significant effects of carry-over, period, or treatment for any of the PSG parameters.
Table 2.
Polysomnography outcomes by treatment night
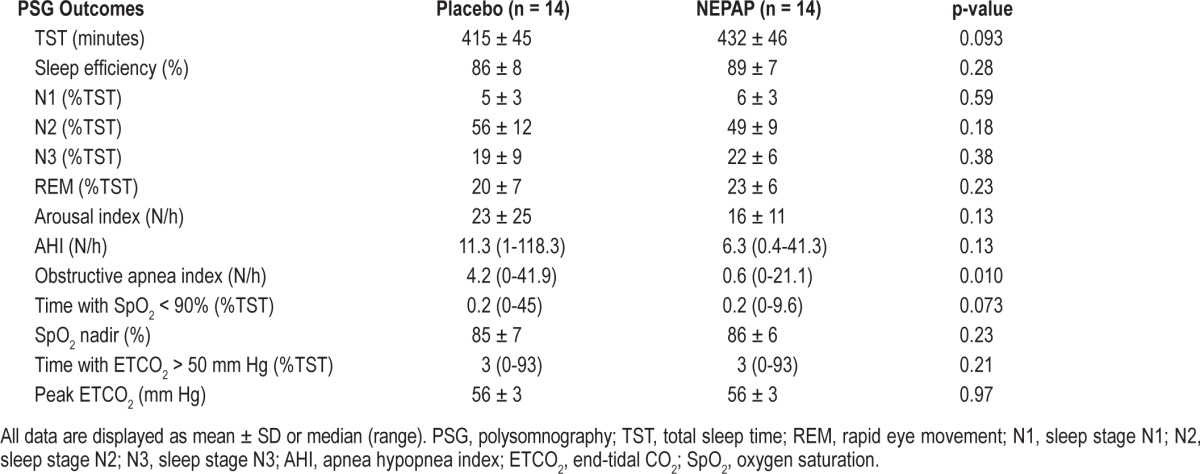
Figure 2. Changes in polysomnographic (PSG) parameters between placebo and nasal expiratory positive airway pressure (NEPAP) devices for the individual subjects (n = 14).
(A) Obstructive apnea index; (B) obstructive apnea hypopnea index; (C) percent of total sleep time with oxygen saturation (SpO2) < 90%; and (D) percent of total sleep time with end-tidal CO2 > 50 mm Hg. There was a significant decrease in the obstructive apnea index from placebo to NEPAP PSG night (p = 0.010). TST, total sleep time.
There was a variable individual response to NEPAP (Figure 2). Two subjects with very severe OSAS had a pronounced response (AHI declined from 118 to 41/h and 100 to 37/h, respectively; Figure 2), although their AHI on NEPAP remained in the clinically severe range. Four subjects had a decrease in AHI to a normal pediatric level of < 1.5/h during the NEPAP PSG night. Two subjects had a paradoxical response to NEPAP with an increase in AHI with the treatment devices compared to placebo, from 2 to 7/h and 7 to 13/h. In addition, although all subjects had a clinical AHI > 5/h prior to study entry, 4 subjects had an AHI < 5/h on both the placebo and NEPAP nights. When those 4 subjects were excluded from analysis, a similar trend was reached for decreases in the AHI (40.1 ± 44.3 vs. 16.9 ± 15.4/h, p = 0.21) and the obstructive apnea index (14.5 ± 14.8 vs. 5.6 ± 7.5/h, p = 0.173) on NEPAP compared to placebo, but these did not reach statistical significance. When analyzing for predictors of NEPAP PSG response versus non-response, the NEPAP responders were older than non-responders (Table 1). During the placebo PSG night, NEPAP responders also had less hypercapnia compared to nonresponders, as shown by a percentage of total sleep time with ETCO2 > 50 mm Hg of 2.1 (0-14.8) vs. 63.2 (1.6-93.1) mm Hg (p = 0.007) compared to non-responders. Otherwise, there were no significant predictors of response to NEPAP based on gender, BMI z-score, or baseline clinical AHI.
Home Phase
Nine subjects were eligible for the home phase of the study. One of these responders did not agree to be in the home phase, and a further subject entered the home phase but was lost to follow-up. Therefore data were reported on 7 subjects. Subjects' long-term NEPAP use based on the used devices collected, excepting the dropout, was 25 ± 6 (83%) of 30 nights. Two subjects spontaneously commented that they preferred NEPAP devices to their usual home CPAP.
There was significant improvement in sleepiness as shown on the Epworth Sleepiness Scale and OSAS symptoms/quality of life on the Obstructive Sleep Apnea-18 for the NEPAP responders after using the devices for 30 days (Table 3). There was a trend (p = 0.05) for improvement in externalizing behaviors as shown on the Child Behavior Checklist. Otherwise, there were no significant changes in behavioral and quality of life outcomes in this small sample.
Table 3.
Behavioral and quality of life measurements by visit (responders only)
Subjects tolerated the devices well without serious adverse events. One subject reported mild skin irritation due to the adhesive bandage. Discomfort was reported by 2 subjects during 2 of the nights devices were worn, which resolved with habituation.
In Vitro Testing
In vitro testing was performed on representative samples of the intact and placebo devices. Figure 3 illustrates the difference between an intact NEPAP device and the placebo. Generated pressure was low during inspiration, indicating low inspiratory resistance in both the intact and placebo device. During expiration, however, the intact NEPAP device had a high resistance that created the expiratory positive airway pressure effect for the user. For example, in Figure 3, the expiratory pressure created at −0.100 L/sec was approximately 4 cm H2O for the intact device. That was equivalent to the manufacturer's stated resistance value for the devices.
Figure 3. The bi-directional pressure versus flow characteristics of the nasal expiratory positive airway pressure (NEPAP) and placebo devices measured in vitro are shown.
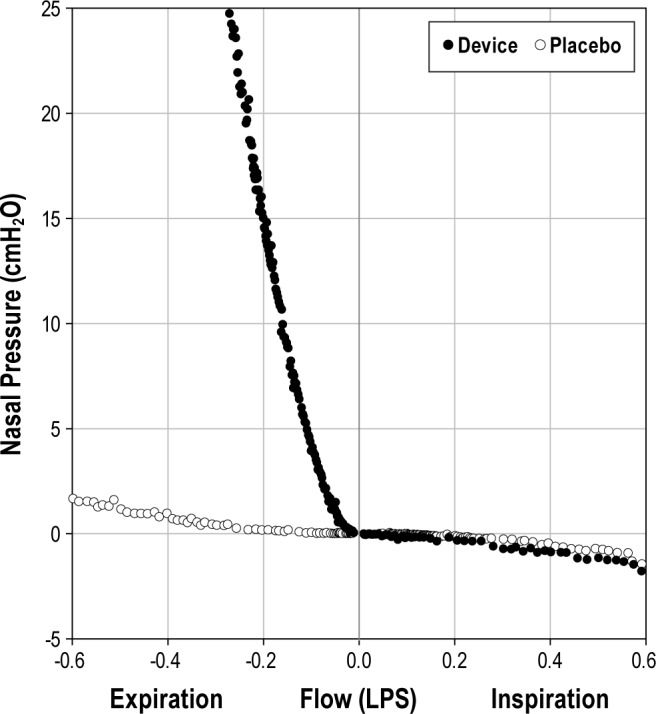
NEPAP device points are shown as solid circles, and placebo points as hollow circles.
DISCUSSION
This pilot study is the first pediatric study to our knowledge that utilized NEPAP devices as therapy for the treatment of OSAS. Our goals were to determine efficacy and tolerability of the devices. Previous adult studies have shown improvement with NEPAP in the AHI and oxygen desaturation index.7–9 The current study demonstrated a variable response to NEPAP in our pediatric cohort, with overall improvement in obstructive apneas and trends for improvements in some other polysomnographic parameters, but with persistent abnormalities remaining.
Approximately two-thirds of individuals studied had improvement in baseline OSAS with NEPAP, but only four subjects had normalization of their PSG, and two subjects worsened. Therefore, only a small proportion of individuals demonstrated a clinically important benefit from using the devices. Several predictors for response to NEPAP were analyzed; however, the only significant finding was that responders were older and had less percentage of total sleep time with ETCO2 > 50 mm Hg during the placebo PSG night than non-responders. There were four subjects who had an AHI < 5/h during both the placebo and NEPAP PSGs, despite having OSAS on their prior clinical studies. The reason for this is unclear. These subjects were included in the study as we initially suspected the placebo devices may have provided a small amount of therapeutic NEPAP. However, based on our in vitro data (Figure 3), the placebo devices only exerted a negligible pressure. However, it is possible a small amount of therapeutic pressure may have been provided in vivo.
The majority of the subjects tolerated the devices well, with a high adherence rate. However, subjects were requested to keep a daily log, and report daily use and return the used devices. It is possible that NEPAP adherence would be lower in the clinical setting. The number of nights with NEPAP use was similar to that of CPAP use in a similar population (24 ± 6 of 30 nights).5 However, those subjects were contacted every 2 weeks over a 3-month period. There was also no objective way to determine how many hours the NEPAP was worn per night. By parent and patient report, devices were worn most of the night.
Patients using NEPAP at home showed a significant improvement in sleepiness, symptoms of OSAS and OSAS-related quality of life after 30 days of home NEPAP use. A decrease in sleepiness has also been noted in studies of NEPAP in adults.8–10
There were certain limitations to the study. Since it was a pilot study, there were a limited number of subjects enrolled. Polysomnography results may have been affected by night to night variability. However, pediatric studies have not shown clinically relevant night-to-night variability.22,23 Children younger than 8 years old could not be evaluated due to the large size of the NEPAP devices. Results could not be compared to baseline PSGs as some of the subjects had been on CPAP for several years so had not had a true baseline PSG recently. Another limitation to the study was potential bias by the respondents during their follow-up questionnaires as subjects were not blinded during the home phase of the study.
CONCLUSION
This study has shown that some children respond to NEPAP, but there is individual variability in the response. Some children had a clinically meaningful response, with both PSG and symptomatic changes, and devices were well tolerated. NEPAP devices may be a potential alternative therapy for OSAS for a small subset of children with persistent OSAS following surgery or those who are not able to tolerate CPAP. Due to variability in individual responses, the efficacy of NEPAP should be evaluated with PSG. Since the study had a limited number of subjects, these findings should be explored further in a large scale study to characterize which children will respond to NEPAP devices. The efficacy and tolerability compared to CPAP should also be addressed in future studies.
DISCLOSURE STATEMENT
The study was supported in part by: NIH RO1 HL58585 and UL1RR024134. The authors utilized REDCap's database. Ventus Medical Incorporated. (Belmont, CA) supplied the NEPAP devices, placebo devices and cannulas but no other support or intellectual input. Dr. Marcus has received research support from Philips Respironics. The other authors have indicated no financial conflicts of interest. Abstract presented at the Associated Professional Sleep Societies, June 3, 2013, Baltimore, MD.
ACKNOWLEDGMENTS
We would like to thank the children and their families who participated in the study. We are also grateful to Caitlin Haas, Mary Cate Menello, and Ruth Bradford for their help in recruitment and study execution.
ABBREVIATIONS
- AHI
apnea hypopnea index
- CPAP
continuous positive airway pressure
- NEPAP
nasal expiratory positive airway pressure
- OSAS
obstructive sleep apnea syndrome
- PSG
polysomnography
- ETCO2
end-tidal CO2
REFERENCES
- 1.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117:e442–51. doi: 10.1542/peds.2005-1634. [DOI] [PubMed] [Google Scholar]
- 5.Marcus CL, Beck SE, Traylor J, et al. Randomized, double-blind clinical trial of two different modes of positive airway pressure therapy on adherence and efficacy in children. J Clin Sleep Med. 2012;8:37–42. doi: 10.5664/jcsm.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Donnell AR, Bjornson CL, Bohn SG, Kirk VG. Compliance rates in children using noninvasive continuous positive airway pressure. Sleep. 2006;29:651–8. [PubMed] [Google Scholar]
- 7.Colrain IM, Brooks S, Black J. A pilot evaluation of a nasal expiratory resistance device for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2008;4:426–33. [PMC free article] [PubMed] [Google Scholar]
- 8.Kryger MH, Berry RB, Massie CA. Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment for obstructive sleep apnea (OSA) J Clin Sleep Med. 2011;7:449–453B. doi: 10.5664/JCSM.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep. 2011;34:479–85. doi: 10.1093/sleep/34.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal L, Massie CA, Dolan DC, Loomas B, Kram J, Hart RW. A multicenter, prospective study of a novel nasal EPAP device in the treatment of obstructive sleep apnea: efficacy and 30-day adherence. J Clin Sleep Med. 2009;5:532–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Ventus Medical Incorporated. Provent therapy mechanism of action. http://www.proventtherapy.com/hcp/microvalve-mechanism-of-action.php.
- 12.Iber C, Ancoli-Israel S, Chesson A, Quan S. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for scoring of sleep and associated events: rules, terminology, and technical specifications. [Google Scholar]
- 13.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–75. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 14.Casat CD, Norton HJ, Boyle-Whitesel M. Identification of elementary school children at risk for disruptive behavioral disturbance: validation of a combined screening method. J Am Acad Child Adolesc Psychiatry. 1999;38:1246–53. doi: 10.1097/00004583-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Achenbach TM, Rescorla LA. Burlington: University of Vermont, Research Center for Children, Youth & Families; 2001. Manual for the ASEBA School-age Forms & Profiles. [Google Scholar]
- 16.Achenbach T, Rescorla L. Burlington: University of Vermont, Research Center for Children, Youth & Families; 2000. Manual for the ASEBA preschool forms and profiles. [Google Scholar]
- 17.Franco RA, Jr, Rosenfeld RM, Rao M. First place--resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;123:9–16. doi: 10.1067/mhn.2000.105254. [DOI] [PubMed] [Google Scholar]
- 18.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130:157–63. doi: 10.1016/j.otohns.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones B, Kenward M. London: Chapman & Hall; 2003. Design and Analysis of Cross-Over Trials. [Google Scholar]
- 22.Li AM, Wing YK, Cheung A, et al. Is a 2-night polysomnographic study necessary in childhood sleep-related disordered breathing? Chest. 2004;126:1467–72. doi: 10.1378/chest.126.5.1467. [DOI] [PubMed] [Google Scholar]
- 23.Katz ES, Greene MG, Carson KA, et al. Night-to-night variability of polysomnography in children with suspected obstructive sleep apnea. J Pediatr. 2002;140:589–94. doi: 10.1067/mpd.2002.123290. [DOI] [PubMed] [Google Scholar]