Abstract
Study Objective:
Sleep-disordered breathing (SDB) and speech difficulties are common problems in children with craniofacial malformations (CFM). The present study was designed to investigate whether resonance issues identified during speech assessment are associated with parental report of SDB symptoms in children with CFM.
Methods:
Children aged 2-18 years with congenital CFM attending at the Craniofacial Anomalies Program from March 2007 to April 2011 were screened for SDB symptoms using the Sleep-Related Breathing Disturbance Scale of the Pediatric Sleep Questionnaire. Speech evaluation, based on the Pittsburgh Weighted Speech Scale score, was the tool used to investigate velopharyngeal dysfunction (VPD) based on speech perceptual assessment.
Results:
A total of 488 children with congenital CFM were included. Overall 81% were Caucasian and 24% were overweight/obese. Twenty-four percent of children screened positive for SDB and 35% had VPD. Children with VPD were no more likely to screen positive for SDB than children without VPD (26% vs. 23%, p = 0.38). However, children with previous sphincter pharyngoplasty (SP) were more likely to have hyponasality (51% vs. 12%, p = 0.0001) and reduced or absent nasal emission (33% vs. 16%, p = 0.008). In a logistic regression, the adjusted odds ratio for SDB for those with hyponasality was 2.10 (95%CI 1.21-3.61, p = 0.008) and for those with reduced or absent nasal emission was 1.75 (95%CI 1.06-2.88, p = 0.028).
Conclusion:
Symptoms of sleep disordered breathing are common in children with craniofacial malformations especially if they have undergone sphincter pharyngoplasty; many of these children can be identified by measures of resonance on routine speech evaluation.
Citation:
Moraleda-Cibrián M, Berger M, Edwards SP, Kasten SJ, Buchman SR, O'Brien LM. Association between symptoms of sleep-disordered breathing and speech in children with craniofacial malformations. J Clin Sleep Med 2014;10(6):671-676.
Keywords: craniofacial malformations, sleep-disordered breathing, speech, velopharyngeal dysfunction
Craniofacial development is one of the most complex processes during the fetal period. Appropriate gene-environment interactions during early pregnancy are crucial to the normal development of the head and face.1–3 Independently of the type of craniofacial malformation (CFM) the distortion of normal development of the head and face, particularly those including orofacial anomalies, is frequently associated with upper airway obstruction, feeding difficulties, growth failure, and speech difficulties.4
Unfortunately, some of the comorbidities related to upper airway dysfunction persist or even increase following surgeries performed to repair the congenital defect. For example, the prevalence of velopharyngeal dysfunction (VPD), one of the main contributors involved in speech intelligibility in children with CFM, is estimated to be around 25%.5–7 The velopharyngeal port, including lateral and posterior oropharyngeal walls and soft palate, plays an essential role in speech, deglutition and nasal breathing since it represents a functional port between the oropharynx and the nasopharynx. Velopharyngeal insufficiency (VPI) occurs when there is an inability to completely close the velopharyngeal valve during the production of oral sounds resulting in leakage of air into the nasal cavity during speech or hypernasality. The most common surgical procedures performed to correct the residual VPI are pharyngeal flap (PF) and sphincter pharyngoplasty (SP). The goals of surgery are to restore oral speech productions and improve communication acceptability. Nevertheless, some studies have suggested that any surgical procedure performed to improve speech might potentially reduce the cross-sectional dimensions of upper airway.8,9 Moreover, it has raised the concern that some of these operations may lead to sleep-disordered breathing (SDB).
BRIEF SUMMARY
Current Knowledge/Study Rationale: Speech difficulties and snoring are common problems in children with craniofacial malformations. These two functions share anatomical structures and likely risk factors. Nevertheless, no study has investigated the association between resonance issues and SDB symptoms in this pediatric population.
Study Impact: Findings from this study demonstrate that children with CFM and hyponasality or reduced/absent nasal emission during speech assessment are at increased risk for SDB symptoms. Given that children with CFM are routinely assessed for speech problems, speech-language pathologists may play a role in identifying children who may benefit from evaluation for SDB.
Sleep-disordered breathing describes a spectrum of nocturnal breathing difficulties ranging from snoring at one end of the spectrum to obstructive sleep apnea at the other. The latter, most severe form of SDB, is characterized by intermittent hypoxemia, disruption of ventilation, and sleep fragmentation. In a typically developing pediatric population, SDB is a frequent medical condition with an estimated prevalence up to 11%.10 However, the frequency of SDB in children with CFM is believed to be significantly higher possibly due to upper airway dimensions and/or the impact of airway operations. Indeed, small studies suggest that approximately 20% to 40% of children with cleft and 40% to 50% of children with other cranio-facial anomalies have risk for SDB.11–16 Until recently habitual snoring, the main symptom of SDB, was considered benign,17 but studies now demonstrate that habitual snoring even in the absence of intermittent hypoxemia is associated with behavioral and cognitive difficulties.18,19
Speech and breathing are two functions with anatomy and embryonic development in common. The close pathophysiology between these two functions prompted the hypothesis that common risk factors can impact speech and breathing difficulties. Therefore, the aim of this study was to assess whether resonance issues identified during speech assessment are associated with SDB symptoms in children with CFM.
METHODS
Participants
All children aged 2-18 years with congenital CFM who attended the Craniofacial Anomalies Program at C.S. Mott Children's Hospital, University of Michigan between March 2007 and April 2011 were eligible for this cross-sectional study. During the clinical appointment, and as part of routine clinical care, children were screened for SDB and underwent clinical speech evaluation. Children with acquired craniofacial anomalies and those < 2 or > 18 years old were excluded. Approximately 30 children are evaluated each month through the Craniofacial Anomalies Program. Given that children are generally seen on an annual basis, only the first visit during the above time period was included in the current analysis. This study was approved by the University of Michigan Institutional Review Board.
Measures
Sleep-disordered breathing
Parents or guardians completed during a clinic visit the Sleep-Related Breathing Disturbance (SRBD) scale of the Pediatric Sleep questionnaire,20 a validated instrument to screen for SDB in the pediatric population. In typically developing children, this instrument has a sensitivity of 0.85 and a specificity of 0.87. The SRBD scale contains 22 items that ask about nocturnal and diurnal symptoms of SDB in the last month. Choices for each response are “yes,” “no,” or “don't know.” The ratio of positive responses to total responses was calculated, and a threshold score ≥ 0.33 identified children with high risk for objective evidence of SDB. Based on the SRBD scale, subjects were categorized into positive and negative SDB risk.
Speech evaluation
Speech assessment was performed by a single certified speech-language pathologist (MB) as part of the routine clinical evaluation. The Pittsburgh Weighted Speech Scale21 was the standardized instrument used to identify VPI in the study population. This scale evaluates 5 universal speech domains: nasal emission, nasal grimace, nasality, phonation, and articulation.22,23 Every item in each category has a weighted score that varies from 0 to 4. The global scoring was obtained by the sum of the maximum score in each speech domain. First, the study population was divided into 4 groups according to the VPI status (0 = competent velopharyngeal mechanism, 1-2 = borderline competent, 3-6 = borderline incompetent, 7 and up = incompetent velopharyngeal mechanism). Next, these groups were dichotomized into competent/borderline competent and incompetent/borderline incompetent. In addition, specific characteristics related to abnormal resonance, such as nasality and nasal emission (NE), were also analyzed in order to investigate obstructive symptoms during speech assessment. The first domain assessed, nasality had the following options: normal, mild/moderate/severe hypernasality, hypo/hypernasality, cul de sac, and hyponasality. Hypernasality is considered the key speech feature in the diagnosis of velopharyngeal insufficiency. The second domain assessed, NE, had 7 options: not present, inconsistent, consistent, nasal escape on nasal appropriate reduced or absent, audible, and nasal turbulence. As the Pittsburgh Weighted Speech Scale was designed to assess VPI, nasality patterns or NE characteristics that suggest VPI (hyper-nasality, NE consistent, audible or nasal turbulence) have higher scores, while those that might suggest upper airway obstruction (hyponasality, NE reduced or absent) have the lower scores. Objective measurements of the speech assessment were not included in the current analysis since they were not part of the routine evaluation.
Medical and Anthropometric Information
Data obtained from medical records also included gender, age, height, and weight. Body mass index (BMI = weight in kg/height in m2) and BMI percentiles, adjusted for age and sex, were calculated. The BMI percentile threshold recommended by the Centers for Disease Control (CDC) for pediatric populations24 was used to classify the sample into 3 weight groups: underweight (BMI < 5th percentile), normal weight (BMI ≥ 5th percentile and < 85th percentile), and overweight/ obese (BMI ≥ 85th percentile). In addition, type of craniofacial anomaly was noted in order to explore the frequency of positive screening for SDB in the two main groups: syndromic and nonsyndromic CFM and in children with and without cleft palate. Surgical procedures on the airway were extracted from medical records for all children. The most common surgical procedure performed to correct VPI at the University of Michigan is sphincter pharyngoplasty (SP).
Data Analysis
Statistical analyses were performed with SPSS software, version 19 (IBM, Armonk, NY). Means and standard deviations for continuous variables, and percentages for categorical variables were used to summarize the results. T-tests were conducted on continuous variables, and χ2 analyses were used to examine bivariate differences in frequency of positive screening for SDB and VPI for categorical variables such as gender (males vs. females), and type of craniofacial anomalies (syndromic vs. non-syndromic; cleft palate vs. no cleft palate). A logistic regression model was used to examine the risk for SBD in the study population after adjusting for potential confounders such as BMI percentile and previous SP. Statistical significance was set at p < 0.05.
RESULTS
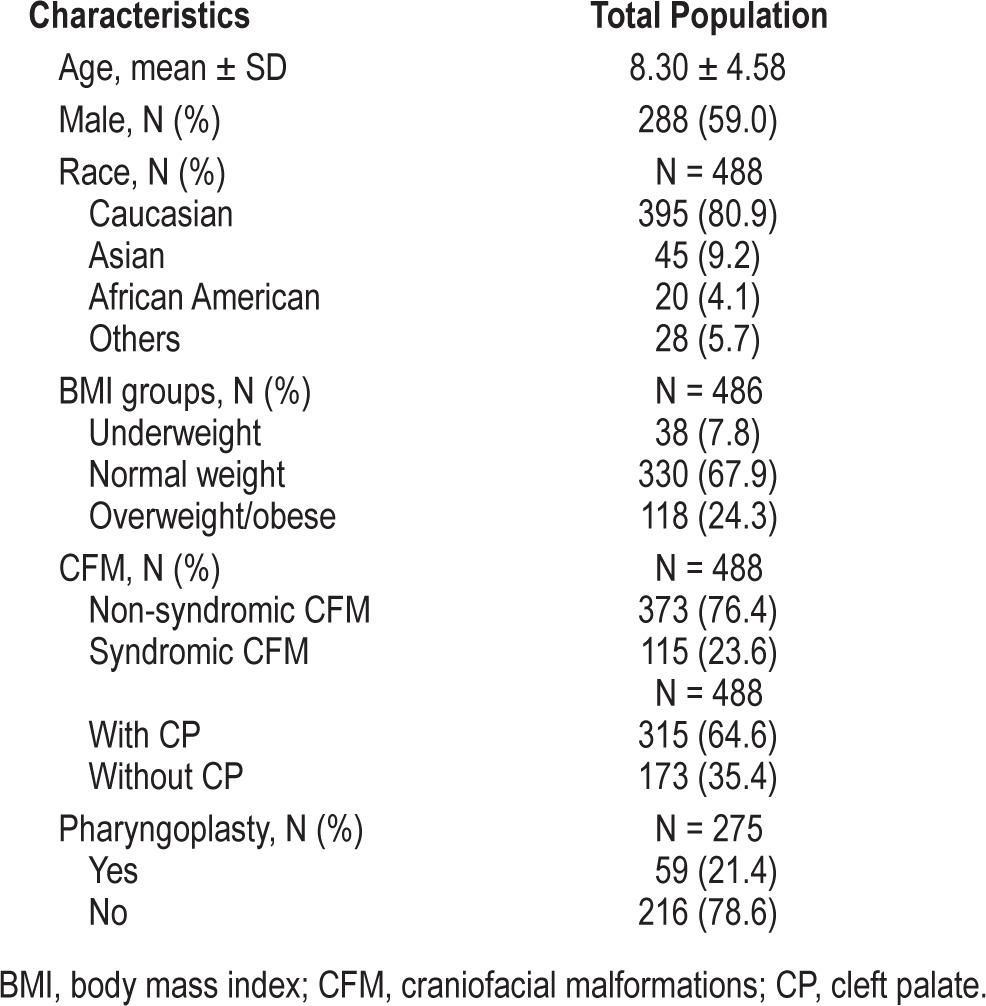
During the study period, a total of 575 unique children with congenital CFM were screened for SDB and of those, 488 (85%) had a speech assessment. The remaining 15% of children were excluded as they were referrals from other institutions such that their speech evaluations were not performed by the same speech pathologist (MB). Therefore, the final analytic sample included 488 children with congenital CFM. Table 1 summarizes the characteristics of the study population. Overall, 24% of children screened positive for SDB. Velopharyngeal insufficiency was present in 35% of children.
Table 1.
Demographic, anthropometric, and anatomical characteristics of the study population
Positive Screening for SDB
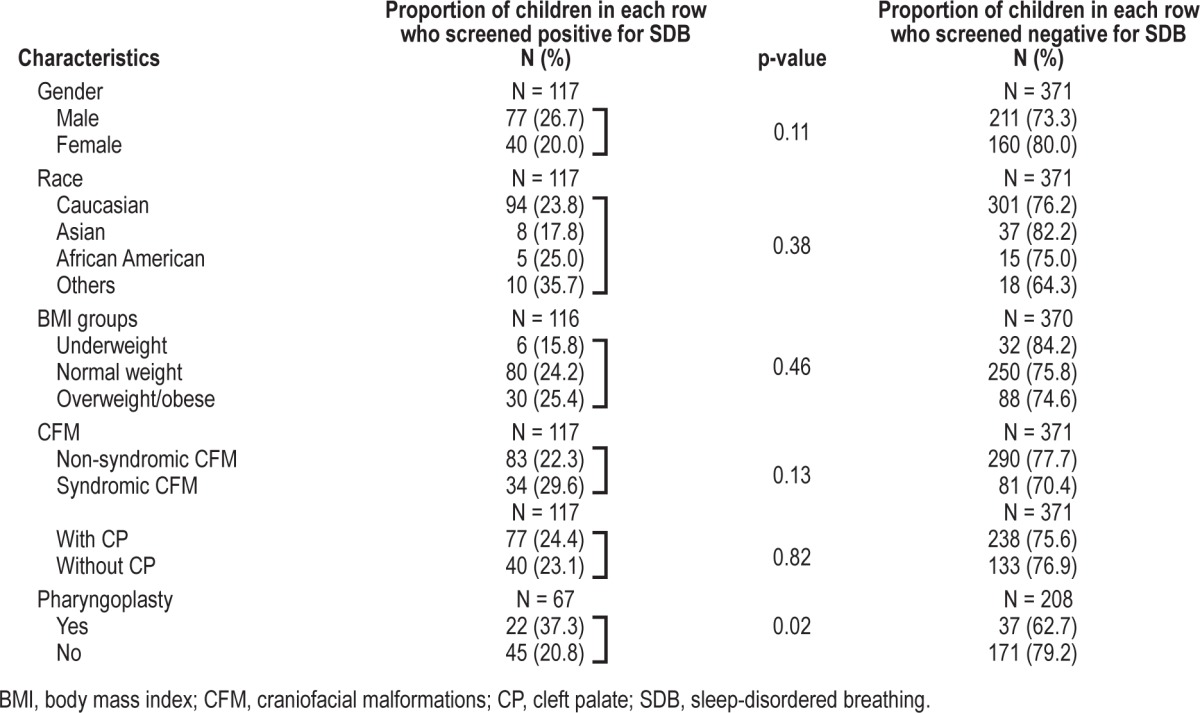
There were no differences in the frequency of positive screening for SDB between males and females (27% vs. 20%, p = 0.11), racial background (Caucasian 24%, Asian 18%, and African American 25%, p = 0.38), or BMI groups (normal weight 24%, overweight/obese 25%, p = 0.46). In the study population, neither the presence of syndromes nor the presence of cleft palate was associated with a significantly increased frequency of positive screening for SDB (30% vs. 22%, p = 0.13 and 24% vs. 23%, p = 0.82 respectively). See Table 2.
Table 2.
Differences in the study population according to the presence of positive and negative screening for SDB
VPI Status and Speech Characteristics
The main characteristics of the study population according to the presence of incompetent/borderline incompetent velopharyngeal mechanism (VPI) or competent/borderline competent velopharyngeal mechanism are shown in Table 3.
Table 3.
Differences in the study population according to the presence of VPI
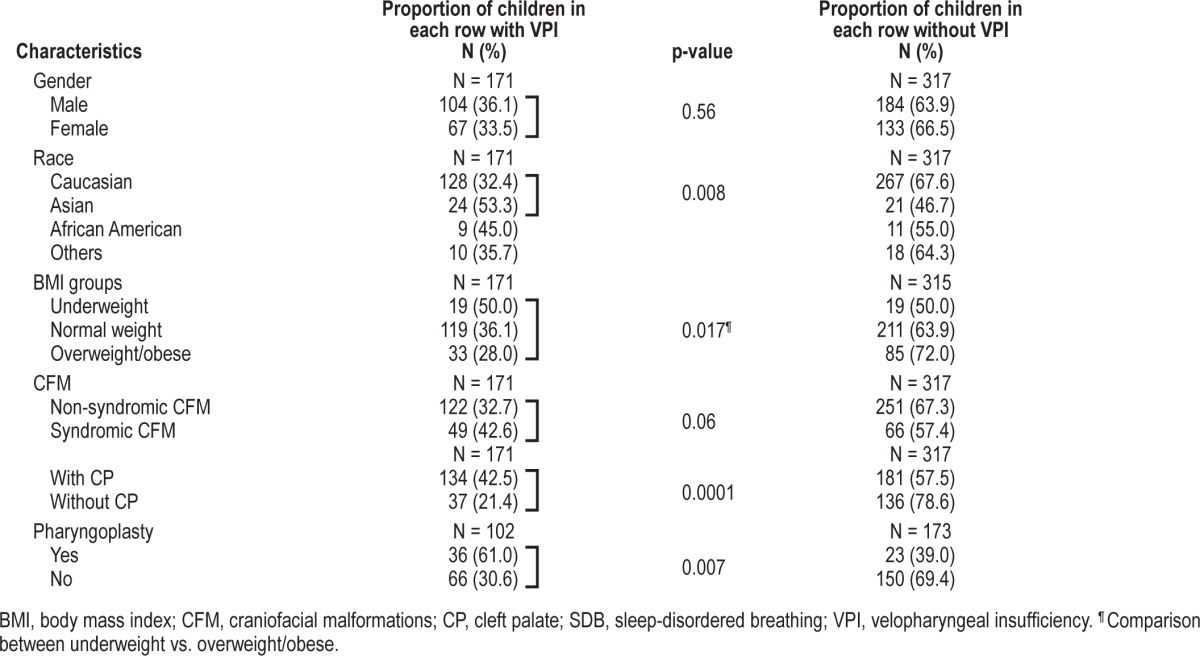
The distribution of the different nasality patterns in children with CFM (N = 487; 1 child with CFM had missing data for nasality patterns) was as follows: 218 children had normal nasality (45%), 172 hypernasality (35%), 39 hypo/ hypernasality (8%), 5 cul de sac (1%), and 53 hyponasality (11%). As expected, children with VPI were more likely to have hypernasality compared to those with competent or borderline competent velopharyngeal mechanism (73% vs. 27%, p = 0.0001). In a subgroup of N = 275 children whose sphincter pharyngoplasty (SP) status was documented, 59 children were undergone SP. The distribution of nasality patterns in this subgroup of children who had a SP (N = 59) was as follows: 7 had normal nasality (12%), 22 had hypernasality (37%), and 30 had hyponasality (51%). In children in whom it was not necessary to alter the velopharyngeal port (N = 216) the distribution of the nasality patterns was different: 107 had normal nasality (49.5%), 82 had hypernasality (38%), and 27 had hyponasality (12.5%).
In the study population nasal emission was reduced or absent in 91 children (19%), consistent visible in 93 (19%) and 10 children (2%) had audible NE or nasal turbulence. As expected, children with consistent NE or audible/nasal turbulence were more likely to have VPI than children with other NE patterns (97% or 100% vs. 18%, p = 0.0001). However, children with previous SP, compared to those without, were more likely to have reduced or absent NE (33% vs. 16%, p = 0.008). Finally, 75% of children with hyponasality had reduced or absent NE, compared to only 6% with hypernasality or 7% with normal nasality (p = 0.0001).
Association between VPI, Speech Patterns, and SDB Symptoms
There were no differences in the frequency of SDB symptoms between children with VPI and children with competent velopharyngeal mechanisms (26% vs. 23%, p = 0.38). Nevertheless, children who had a previous SP were more likely to screen positive for SDB compared to those children in whom it was not necessary to alter the velopharyngeal port (37% vs. 21%, p = 0.02).
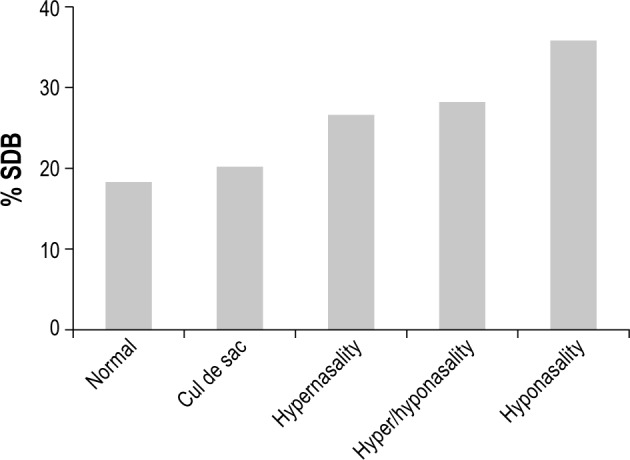
Interestingly, a higher frequency of positive screening for SDB was reported in children with hyponasality compared to children with normal nasality (36% vs. 18%; p = 0.01). Figure 1 shows the frequency of positive screening for SDB among the 5 nasality patterns: 18% of children with normal nasality screened positive for SDB, 20% of children with cul de sac nasality screened positive for SDB, 27% of children with hypernasality screened positive for SDB, 28% of children with hypo/hypernasality screened positive for SDB, and 36% of children with hyponasality screened positive for SDB. The latter group was the only group to reach statistical significance. In parallel, children with reduced or absent NE were more likely to screen positive for SDB compared to children with other NE patterns (33% vs. 22%, p = 0.03).
Figure 1. Frequency of positive screening for SDB according to nasality patterns during speech assessment.
In a logistic regression of the total population, using hyponasality as the independent variable and SDB as the dependent variable, children with hyponasality had an odds ratio of 2.10 (95%CI 1.21-3.61, p = 0.008) for SDB. After adjusting for BMI percentile and the presence of CP the odds ratio for SDB in children with hyponasality did not change substantially, 2.16 (95%CI 1.24-3.78, p = 0.007). In similar regression model of the subgroup of children in whom SP status was known (n = 275), the unadjusted odds ratio for SDB, using hyponasality as the independent variable, was 2.95 (95%CI 1.44-6.07, p = 0.003). After adjusting for BMI percentile, and the presence of CP and SP, hyponasality remained the only variable independently associated with SDB, with an odds ratio of 2.32 (95%CI 1.01-4.99, p < 0.05).
The above logistic regression models were repeated using reduced/absent NE as the independent variable. Children with reduced/absent NE had an odds ratio of 1.75 for SDB (95%CI 1.06-2.88, p = 0.028). After adjusting for the BMI percentile and the presence of CP, the odds ratio for SDB did not change substantially, 1.79 (95%CI 1.08-2.95, p = 0.023). In similar regression model of the subgroup of children in whom SP status was known (n = 275), the unadjusted odds ratio for SDB, using reduced/absent NE as the independent variable, was 2.73 for SDB (95%CI 1.44-5.16, p = 0.002). After adjusting for BMI percentile, the presence of CP, and SP, reduced/absent NE, and previous SP were the only variables independently associated with SDB with odds ratios of 2.53 (95%CI 1.32-4.88, p < 0.0005) and 1.98 (95%CI 1.04-3.78, p = 0.38), respectively.
DISCUSSION
These data suggest that measures of resonance obtained on routine speech evaluation may have clinical utility in the identification of children with CFM who may require further workup for undiagnosed SDB. The presence of a previous SP has been reported to increase the risk for SDB and while our data add further support to these findings, we also suggest that the resultant resonance outcomes following SP may provide additional information for SDB risk. As such, speech pathologists may have a role in early identification and referral of children at risk for SDB.
Despite the high frequency of SDB symptoms in pediatric populations with craniofacial anomalies the limited data available up to now suggest that these children are infrequently referred to the sleep clinic. In the present study, the frequency of positive screening for SDB in unselected children with CFM from the craniofacial clinic was 24%, which is approximately five times higher than the 5% reported at well-child pediatric visits using the same screening tool.25 These results are consistent with previous studies performed in children with cleft palate and other CFM.9,11,26–28 Rose et al. found that significant morphometric differences of the upper airway in children with cleft were often associated with increased upper airway resistance during sleep.29 Moreover, it is suspected that any surgical procedure to improve VPI might be a potential risk factor for SDB.8,30 Our data suggest that resonance patterns may have utility beyond the determination of need for a further speech related surgery in that it may also be an important indicator of the risk for SDB in pediatric populations with craniofacial anomalies.
In our study population VPI was present in one third of children. Despite the lack of consensus, SP is considered by some as the indicated surgical management in severe VPI.8 Consequently, in cases of an insufficient velopharyngeal mechanism with severe hypernasality and unintelligible speech, the resolution of VPI could be associated with an excessive velopharyngeal closure and reduction of cross-sectional dimensions of the airway. When that happens, modification of the morphology of upper airway may result not only in changes of resonance and nasal emission during production of sounds, but also in nocturnal symptoms of upper airway obstruction. Our results are consistent with this hypothesis. In fact we found that while the presence of VPI or hypernasality was not associated with positive screening for SDB, children with previous SP, which often resulted in hyponasality and reduced/absent NE, were more likely to have symptoms of upper airway obstruction during sleep. These findings suggest that post-surgery speech characteristics in children with CFM could provide us important information about the VPI status, but also about nocturnal upper airway obstruction.
Speech and breathing are two functions of the upper airway that share not only anatomical structures, but also a common embryonic development. Thus, a better understanding of the association between these two functions is important particularly in children with increased risk for speech problems and SDB. Davidson, in a study from an evolutionary perspective, speculated that anatomical changes of the larynx in relation to the development of speech might contribute to upper airway obstruction at night due to changes of soft tissue anatomy and cranial base angulation.31 In 1992, Warren—who described speech as a modified breathing behavior that uses the respiratory system to provide an energy source to produce meaningful sounds—reported that upper airway structures affected in children with cleft during breathing might also play a role in speech difficulties in this population.32 Interestingly, De Serres reported for the first time that hyponasality and snoring were frequently present in pediatric population with cleft.8 Nevertheless, to our knowledge the association between these two common problems in children with CFM has never been studied. Indeed, the vast majority of studies in pediatric populations with cleft or other craniofacial anomalies have focused on investigating the percentage of VPI resolution after surgery and the most common morbidities related to the surgical procedures.8,30
There are two major strengths to our study. First, to our knowledge this study is the first specifically designed to investigate the association between SDB symptoms and speech problems in a large clinical sample of children with CFM. The presence of SDB symptoms was based on the Pediatric Sleep Questionnaire,20 a validated pediatric SDB screening tool, and speech assessment, performed by an experienced speech pathologist, was also based on a standardized instrument. Importantly, children were non-selected for sleep problems and were representative of those attending craniofacial anomalies clinics. Secondly, classification of the study population according to speech problems was based not only on VPI status, but also in other speech domains. Nonetheless, the study is not without limitation. First, symptoms of SDB were reported by parents. Although good agreement has been found between SDB symptoms and objective data from polysomnography,20 the SRBD scale used has not been validated in the craniofacial population. However, in cases where symptoms of obstructive sleep apnea are well documented polysomnography is not recommended.33 Secondly, although the speech evaluation instrument is a routinely used clinical tool, it does not involve acoustic measurements or direct assessment utilizing imaging techniques. Therefore objective measures of resonance were not used. Nonetheless, our findings from standard measurements obtained by a trained speech-language pathologist are relevant to clinical practice where objective measures are not always available or able to be used in a routine manner.
In conclusion, children with CFM and hyponasality or reduced/absent nasal emission, which are often consequences of SP, are at increased risk for SDB. Routine speech evaluation may play a role in identifying children who may benefit from evaluation for SDB.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the staff of the Craniofacial Anomalies Clinic at the University of Michigan, particularly Marlene Chesney, Clinic Manager, and Jason Saims, Program Coordinator. The authors also thank the children and families whose participation made this study possible.
REFERENCES
- 1.Stanier P, Moore GE. Genetics of cleft lip and palate: syndromic genes contribute to the incidence of non-syndromic clefts. Hum Mol Genet. 2004;13:73–81. doi: 10.1093/hmg/ddh052. [DOI] [PubMed] [Google Scholar]
- 2.Wantia N, Rettinger G. The current understanding of cleft lip malformations. Facial Plast Surg. 2002;18:147–53. doi: 10.1055/s-2002-33061. [DOI] [PubMed] [Google Scholar]
- 3.Schutte BC, Murray JC. The many faces and factors of orofacial clefts. Hum Mol Genet. 1999;8:1853–9. doi: 10.1093/hmg/8.10.1853. [DOI] [PubMed] [Google Scholar]
- 4.Beals SP, Joganic EF. Form and function in craniofacial deformities. Semin Pediatr Neurol. 2004;11:238–42. doi: 10.1016/j.spen.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Peterson-Falzone SJ, Hardin-Jones MA, Karnell MP. 2nd ed. Philadelphia: BC Decker; 1990. Cleft palate speech. [Google Scholar]
- 6.Smith B, Guyette TW. Evaluation of cleft palate speech. Clin Plast Surg. 2004;31:251–60. doi: 10.1016/S0094-1298(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 7.Kuehn DP MK. Speech and language issues in the cleft palate population: the state of the art. Cleft Palate Craniofac J. 2000;37:448–33. [Google Scholar]
- 8.de Serres LM, Deleyiannis FW, Eblen LE, Gruss JS, Richardson MA, Sie KC. Results with sphincter pharyngoplasty and pharyngeal flap. Int J Pediatr Otorhinolaryngol. 1999;48:17–25. doi: 10.1016/s0165-5876(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 9.Muntz H, Wilson M, Park A, Smith M, Grimmer JF. Sleep disordered breathing and obstructive sleep apnea in the cleft population. Laryngoscope. 2008;118:348–53. doi: 10.1097/MLG.0b013e318158195e. [DOI] [PubMed] [Google Scholar]
- 10.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoeve LJ, Pijpers M, Joosten KF. OSAS in craniofacial syndromes: an unsolved problem. Int J Pediatr Otorhinolaryngol. 2003;67:S111–3. doi: 10.1016/j.ijporl.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Robison JG, Otteson TD. Increased prevalence of obstructive sleep apnea in patients with cleft palate. Arch Otolaryngol Head Neck Surg. 2011;137:269–74. doi: 10.1001/archoto.2011.8. [DOI] [PubMed] [Google Scholar]
- 13.MacLean JE, Hayward P, Fitzgerald DA, Waters K. Cleft lip and/or palate and breathing during sleep. Sleep Med Rev. 2009;13:345–54. doi: 10.1016/j.smrv.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Luna-Paredes C, Anton-Pacheco JL, Garcia Hernandez G, Martinez Gimeno A, Romance Garcia AI, Garcia R., II Screening for symptoms of obstructive sleep apnea in children with severe craniofacial anomalies: assessment in a multidisciplinary unit. Int J Pediatr Otorhinolaryngol. 2012;76:1767–70. doi: 10.1016/j.ijporl.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Cloonan YK, Kifle Y, Davis S, Speltz ML, Werler MM, Starr JR. Sleep outcomes in children with hemifacial microsomia and controls: a follow-up study. Pediatrics. 2009;124:313–21. doi: 10.1542/peds.2008-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rustemeyer J, Thieme V, Bremerich A. Snoring in cleft patients with velopharyngoplasty. Int J Oral Maxillofac Surg. 2008;37:17–20. doi: 10.1016/j.ijom.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Lofaso F, Coste A, d'Ortho MP, et al. Nasal obstruction as a risk factor for sleep apnoea syndrome. Eur Respir J. 2000;16:639–43. doi: 10.1034/j.1399-3003.2000.16d12.x. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114:44–9. doi: 10.1542/peds.114.1.44. [DOI] [PubMed] [Google Scholar]
- 19.Blunden S, Lushington K, Kennedy D, Martin J, Dawson D. Behavior and neurocognitive performance in children aged 5-10 years who snore compared to controls. J Clin Exp Neuropsychol. 2000;22:554–68. doi: 10.1076/1380-3395(200010)22:5;1-9;FT554. [DOI] [PubMed] [Google Scholar]
- 20.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 21.McWilliams BJ, Philips BJ. Philadelphia: WB Saunders; 1979. Velopharyngeal incompentence: Audio seminars in speech pathology. [Google Scholar]
- 22.Lohmander A, Willadsen E, Persson C, Henningsson G, Bowden M, Hutters B. Methodology for speech assessment in the Scandcleft project--an international randomized clinical trial on palatal surgery: experiences from a pilot study. Cleft Palate Craniofac J. 2009;46:347–62. doi: 10.1597/08-039.1. [DOI] [PubMed] [Google Scholar]
- 23.Henningsson G, Kuehn DP, Sell D, Sweeney T, Trost-Cardamone JE, Whitehill TL. Universal parameters for reporting speech outcomes in individuals with cleft palate. Cleft Palate Craniofac J. 2008;45:1–17. doi: 10.1597/06-086.1. [DOI] [PubMed] [Google Scholar]
- 24.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 S:164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 25.Archbold KH, Pituch KJ, Panahi P, Chervin RD. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97–102. doi: 10.1067/mpd.2002.119990. [DOI] [PubMed] [Google Scholar]
- 26.Pijpers M, Poels PJ, Vaandrager JM, et al. Undiagnosed obstructive sleep apnea syndrome in children with syndromal craniofacial synostosis. J Craniofac Surg. 2004;15:670–4. doi: 10.1097/00001665-200407000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics. 1991;88:132–9. [PubMed] [Google Scholar]
- 28.Kakitsuba N, Sadaoka T, Motoyama S, et al. Sleep apnea and sleep-related breathing disorders in patients with craniofacial synostosis. Acta Otolaryngol Suppl. 1994;517:6–10. doi: 10.3109/00016489409124330. [DOI] [PubMed] [Google Scholar]
- 29.Rose E, Thissen U, Otten JE, Jonas I. Cephalometric assessment of the posterior airway space in patients with cleft palate after palatoplasty. Cleft Palate Craniofac J. 2003;40:498–503. doi: 10.1597/1545-1569_2003_040_0498_caotpa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 30.Liao YF, Chuang ML, Chen PK, Chen NH, Yun C, Huang CS. Incidence and severity of obstructive sleep apnea following pharyngeal flap surgery in patients with cleft palate. Cleft Palate Craniofac J. 2002;39:312–6. doi: 10.1597/1545-1569_2002_039_0312_iasoos_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 31.Davidson TM. The Great Leap Forward: the anatomic basis for the acquisition of speech and obstructive sleep apnea. Sleep Med. 2003;4:185–94. doi: 10.1016/s1389-9457(02)00237-x. [DOI] [PubMed] [Google Scholar]
- 32.Warren DW, Drake AF, Davis JU. Nasal airway in breathing and speech. Cleft Palate Craniofac J. 1992;29:511–9. doi: 10.1597/1545-1569_1992_029_0511_naibas_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 33.Section on Pedaitric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 109(4):704–12. doi: 10.1542/peds.109.4.704. [DOI] [PubMed] [Google Scholar]


