Abstract
Study Objectives:
Pediatric obstructive sleep apnea (OSA) is associated with cardiovascular consequences, including accelerated atherosclerosis and endothelial dysfunction. Increased lipid peroxidation, a marker of oxidative stress, has been identified in adults with OSA in a severity-dependent manner, with attenuation following treatment with continuous positive airway pressure therapy. Studies on oxidative stress in children with OSA are sparse and results are inconclusive. The objective of this study was to compare lipid peroxidation in children with OSA to non-OSA children.
Methods:
A prospective cross-sectional study of 26 children with polysomnography-confirmed OSA (oAHI ≥ 5/h TST) was conducted. Thirty age- and body mass index z-score-matched children with primary snoring (PS) served as a comparison group (oAHI ≤ 1/h TST). Fasting blood samples were obtained on the morning following the sleep study. Plasma oxidized low-density lipoprotein (oxLDL) concentrations were measured by enzyme-linked immunosorbent assay.
Results:
There were no group differences in patient characteristics and their lipid profiles. The mean oxLDL levels of the OSA group were significantly higher than those of the comparison group (53.1 ± 13.0 vs. 45.7 ± 10.0 U/L, respectively, p = 0.02). There was a significant positive correlation between plasma oxLDL and the apnea hypopnea index (r = 0.29, p = 0.03) and between oxLDL and the oxygen desaturation index (r = 0.51, p = 0.003), and a significant negative correlation between SpO2 nadir and oxLDL (r = −0.29, p = 0.03).
Conclusions:
OSA in children is associated with increased lipid peroxidation in a severity-dependent manner. Lipid peroxidation levels correlate with the degree of intermittent hypoxia.
Citation:
Tauman R, Lavie L, Greenfeld M, Sivan Y. Oxidative stress in children with obstructive sleep apnea syndrome. J Clin Sleep Med 2014;10(6):677-681.
Keywords: obstructive sleep apnea, sleep disordered breathing, oxLDL, oxidative stress, children
Obstructive sleep apnea (OSA) is a prevalent condition that affects up to 3% of all children.1–3 Similar to adults, pediatric OSA is associated with an increased risk of cardiovascular morbidities. Altered autonomic function, systemic hypertension, decreased left ventricular function, accelerated atherogenesis, and endothelial dysfunction have all been documented in children with OSA.4–12 A large body of evidence has demonstrated that OSA in adults is strongly associated with oxidative stress, which is a major contributor to cardiovascular morbidity.13 The drastic changes in oxygen tension that accompany repeated breathing cessations in OSA promote oxidative stress similar to hypoxia/reoxygenation injury,13,14 resulting in increased production of reactive oxygen species (ROS) which damages cellular components and various biomolecules, such as lipids, proteins, DNA, and carbohydrates, and alters their biological functions. Of these, lipids are the most prone to oxidation and are also a surrogate marker of atherosclerosis and cardiovascular morbidity in adults and in children.15–21 Increased lipid peroxidation has been repeatedly shown in adults with OSA in a severity-dependent manner, with attenuation following treatment with positive airway pressure (CPAP).22–29
BRIEF SUMMARY
Current Knowledge/Study Rationale: OSA in adults is strongly associated with oxidative stress, a major contributor to cardiovascular morbidity. Studies on oxidative stress in children with OSA are sparse and results are inconclusive.
Study Impact: The results of the present study show that childhood OSA is associated with increased lipid peroxidation, a marker of oxidative stress, and cardiovascular morbidity in a severity-dependent manner. The degree of lipid peroxidation correlates with the degree of intermittent hypoxia.
Oxidative stress is also associated with a variety of other conditions, such as obesity, smoking, hypertension, hyperlipidemia, and diabetes, that frequently appear concomitantly with OSA in the adult population,13 but which are much less likely to affect children. As such, in the context of cardiovascular morbidity and oxidative stress, investigating oxidative stress in children with OSA may have a particular added value. Studies on oxidative stress in children with OSA are relatively sparse and their results are inconclusive, most likely due to different methodologies.30–36 Moreover, since there is some evidence to suggest that snoring without OSA may also be associated with long-term consequences,37,38 it is important to investigate the differences between snoring associated with OSA syndrome and gas exchange abnormalities and habitual snoring without OSA, i.e., primary snoring (PS). Oxidized low-density lipoprotein (oxLDL) is a marker of lipid peroxidation and oxidative stress and, more importantly, a key mediator of atherosclerosis and cardiovascular morbidity with prognostic value/quality.17–21
The aim of the current study was to investigate lipid peroxidation, a marker of oxidative stress, by measuring plasma oxLDL in children with polysomnography (PSG)-confirmed OSA. We hypothesized that children who snore and have OSA will exhibit increased lipid peroxidation in comparison to children who snore and do not have OSA.
METHODS
Study Participants
Healthy snoring children who were referred for PSG evaluation due to suspected sleep disordered breathing were recruited prospectively. Children with any chronic medical condition such as diabetes, hypertension, dyslipidemia, chronic inflammatory diseases, or any genetic, neuromuscular, or craniofacial syndrome were excluded. In addition, children with asthma on medications and on asthma exacerbation (by history and physical examination) were also excluded.
Data on height and weight were obtained from each participant and calculated for the body mass index (BMI) and the BMI-z score. Obesity was defined as a BMI z-score > 1.65. Data on family history of cardiovascular diseases, passive smoking, asthma, and tonsil size on physical examination were also collected. The study was approved by the institutional review board of the Tel Aviv Medical Center. Informed consent was obtained from the parents of all participants.
Overnight PSG
A standard overnight multichannel PSG evaluation was performed on each patient in the sleep laboratory. Chest and abdominal wall movement were monitored by respiratory impedance or inductance plethysmography, and heart rate was monitored by electrocardiography. Air flow was measured by end-tidal capnography, which also provided breath-by-breath assessment of end-tidal carbon dioxide levels ([PETCO2]; BCI SC-300, Menomonee Falls, WI), as well as by a nasal pressure transducer and an oronasal thermistor. Arterial oxygen saturation (SpO2) was assessed by pulse oximetry (Nellcor N 100; Nellcor Inc., Hayward, CA), with simultaneous recording of the pulse waveform. A bilateral electrooculogram, 8 channels of electroencephalogram, and chin and anterior tibial electromyograms were also monitored. All measures were digitized using a commercially available PSG system (EMBLA, MedCare diagnostics, Amsterdam, The Netherlands). Digital time-synchronized video recording was performed, and sleep architecture was assessed by standard techniques.39
The proportion of time spent in each sleep stage was expressed as percentage of total sleep time (TST). Central, obstructive, and mixed apneic events were counted. Obstructive apnea was defined as the absence of airflow with continued chest wall and abdominal movement for ≥ 2 breaths. Hypopneas were defined as a decrease in oronasal flow ≥ 50% on either the thermistor or nasal pressure transducer signal, with a corresponding decrease in SpO2 ≥ 3% and/or arousal.40,41 The obstructive apnea-hypopnea index (oAHI) was defined as the number of apneas and hypopneas per hour of TST. Moderate-severe OSA was defined as an oAHI > 5/h of TST, while normal PSG was defined as an oAHI < 1/h of TST. Children with moderate-severe OSA were included in the OSA group, and children with normal PSG results served as a comparison group.
Lipid Peroxidation
Fasting blood samples were drawn on the morning following the sleep study. Blood samples were immediately centrifuged, and plasma was frozen at −80°C until assay. Plasma oxLDL levels were measured by commercially available enzyme-linked immunosorbent assay (ELISA) methods (Mercodia, Sweden) according to manufacturer's instructions and by using 3 external standard samples with known values. The lipid profile (cholesterol, triglycerides, LDL, and high-density lipo-protein [HDL]) was also assessed.
Analysis
The statistical analyses were performed with SPSS (version 19.0; SPSS Inc., Chicago, IL). Children with moderate-severe OSA were compared to age- and BMI z-score-matched children who had normal PSG findings (primary snoring [PS]). Comparisons of variables according to group assignment (i.e., OSA or PS) were performed with independent t-tests and the Mann-Whitney nonparametric test for continuous variables, and χ2 analyses for categorical variables. Spearman correlations were calculated between variables since most variables were not normally or log normally distributed. Multiple regression analysis was performed with oxLDL as a dependent variable. All reported p-values are 2-tailed, with statistical significance set at p < 0.05.
RESULTS
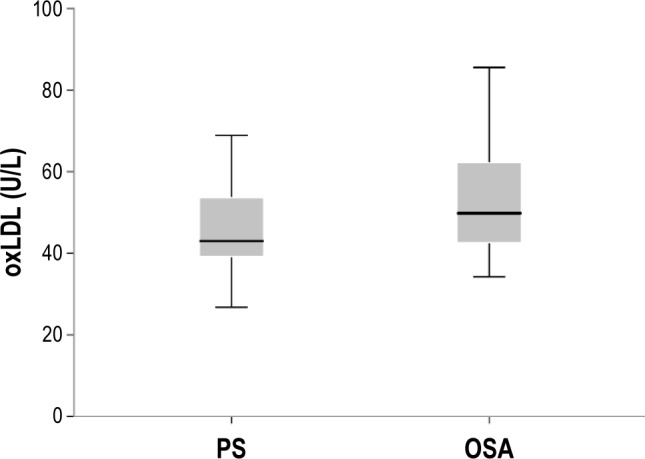
Twenty-six children with moderate-severe OSA were compared to 30 children with PS. There were no differences in the characteristics, PSG results, and lipid profiles between groups (Table 1). The mean oxLDL levels were significantly higher in the OSA group compared to the PS group (53.1 ± 13.0 vs. 45.7 ± 10.0 U/L, respectively, p = 0.02; Figure 1). There was a significant positive correlation between the oAHI and oxLDL levels (r = 0.29, p = 0.03), and a significant negative correlation between SpO2 nadir and oxLDL levels (r = −0.29, p = 0.03). Due to technical problems, we were able to retrieve data on the oxygen desaturation index (ODI) and the time spent in SpO2 < 90% for only 32 subjects (16 OSA and 16 PS), but SpO2 nadir was available for all participants.
Table 1.
Subject characteristics, polysomnographic measures, and lipid profile of 26 children with obstructive sleep apnea (OSA) and 30 children with primary snoring (PS).

Figure 1. Mean plasma oxidized low-density lipoprotein (oxLDL) concentrations in 26 children with obstructive sleep apnea (OSA) and 30 children with primary snoring (PS) (53.1 ± 13.0 vs. 45.7 ± 10.0, p = 0.02).
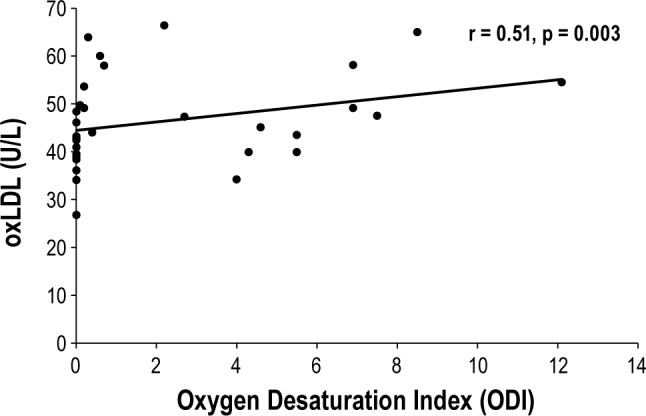
As expected, the mean values of both variables were significantly higher in the OSA group compared to the PS group (4.5 ± 3.4 vs. 0.06 ± 0.1, p < 0.0001 for ODI, and 0.12 ± 0.18 vs. 0.01 ± 0.0%, p < 0.03 for time spent with SpO2 < 90%, respectively). A significant positive correlation was found between oxLDL levels and ODI (r = 0.51, p = 0.003; Figure 2). In addition, there were significant positive correlations between oxLDL and LDL levels (r = 0.70, p < 0.0001), oxLDL and cholesterol levels (r = 0.66, p < 0.0001), and a significant negative correlation between oxLDL and HDL levels (r = −0.28, p = 0.04). No correlations were found between oxLDL levels and maximum EtCO2 levels or arousal index.
Figure 2. Correlation between plasma oxidized low-density lipoprotein (oxLDL) concentrations and oxygen desaturation index (ODI) for 32 children (r = 0.51, p = 0.03).
Multiple regression analysis with oxLDL as a dependent variable and PSG measures, family history of CVS morbidity, age, BMI-z score, passive smoking, tonsil size, and asthma as independent variables revealed that mean SpO2 and family history of CVS morbidity were the only predictive variables for oxLDL levels (p = 0.007 and p = 0.03, respectively), accounting for 26% of the variable.
DISCUSSION
The results of the present study show that childhood OSA is associated with increased lipid peroxidation, a marker of oxidative stress and cardiovascular morbidity in a severity-dependent manner. Notably, our results show that the degree of lipid peroxidation correlates with the degree of intermittent hypoxia, as reflected by the ODI, which is most likely the mechanism underlying this association.
Studies in adults have shown that OSA is associated with oxidative stress in a severity-dependent manner, with attenuation following treatment with CPAP.22–29 Since obesity, smoking, diabetes, and hypertension are relatively common in adults with OSA and concurrently and independently contribute to oxidative stress, our findings in children provide information on the relationship of OSA and oxidative stress that is unaffected by the coexistence of these conditions.
Several studies have thus far investigated oxidative stress in children. The first study, by Dogruer et al., showed higher serum levels of malondialdehyde and increased activity of antioxidant defense mechanisms (superoxide dismutase and glutathione peroxidase) in the pre-tonsillectomy compared with the post-tonsillectomy period.30 Their study, however, did not include an objective assessment of OSA and OSA severity. Verhulst et al. measured serum uric acid as a biomarker of tissue hypoxia and oxidative stress in overweight and obese children with OSA and found significant correlations between OSA severity and uric acid concentrations independently of adiposity.31 Similar to our finding, a more recent publication from the same group has shown positive correlation between uric acid concentration and ODI and reduction in these levels following weight loss in obese children.35 Biltagi et al. reported increased levels of 8-isoprostane (another biomarker of lipid peroxidation) in breath condensates of children with OSA, a positive correlation between clinical scores of disease severity and 8-isoprostane levels, and a positive correlation between 8-isoprostane levels and the degree of cardiac dysfunction.32 In a recent publication by Malakosioti et al., increased morning levels of hydrogen peroxide in exhaled breath condensate were found in children with moderate-severe OSA.34 In contrast, Montgomery-Downs et al. used urinary F2-isoprostance metabolite levels, a specific product of lipid peroxidation, and did not find any correlation between oxidative stress and the severity of OSA-related PSG measures.33 One explanation for the negative results in their study could be the relatively small number of children with moderate-severe OSA investigated (7 children), as reflected by the mean oAHI of the study group (1.8 ± 4.6).33
In the present study, we measured plasma oxLDL, a product of lipid peroxidation that had not been previously determined in children with PSG-confirmed OSA. In addition to being a marker of lipid peroxidation, oxLDL is also involved in the progression of atherosclerosis. Given that accelerated atherosclerosis is one of the known long-term consequences of OSA, our evidence of a link between OSA and oxLDL may have clinical implications. Our results showed that oxLDL levels are elevated in children with OSA compared to children with PS and that oxLDL levels correlate with the oAHI, which reflects the severity of the disorder. Importantly, similar to previous findings in obese children, we showed that oxLDL levels correlate with ODI, a PSG measure of the degree of intermittent hypoxia.
Although it has been suggested that PS may be associated with long-term consequences,37,38 our results indicate that snoring in the absence of OSA is not associated with the same level of lipid peroxidation as in OSA, suggesting that sleep fragmentation is most likely the mechanism underlying the long-term effects of PS. The chain of events that promotes oxidative stress in OSA is most likely initiated by repeated breathing cessations accompanied by drastic changes in oxygen tension, similar to a hypoxia/reoxygenation injury, resulting in increased ROS production. The excess ROS damages cellular components and various biomolecules, particularly lipids, and alters their biological functions.13
Several studies have indicated that childhood OSA is associated with accelerated atherogenesis and endothelial dysfunction.8–12 Lipid peroxidation is also a marker of cardiovascular morbidity; therefore, the findings of the current study support this notion. Several studies have shown that oxLDL is taken up by macrophages, leading to macrophage foaming and the progression of atherosclerosis.17–21,42,43 Others have shown that intermittent hypoxia increases the generation of ROS in the vascular wall and induces lipid peroxidation and formation of oxLDL, creating a substrate for atherosclerotic plaques.17–21,44,45
Our cohort consists of a relatively small number of children, and technical restraints precluded our providing the ODI and the time spent with SpO2 less than 90% for more than 32 of the 56 children. Nevertheless, our demonstration of such a significant positive correlation between oxLDL and ODI warrants further studies on larger patient populations and investigation into the effect of treatment (e.g., adenotonsillectomy).
In conclusion, OSA in children is associated with increased lipid peroxidation, a marker of oxidative stress and cardiovascular morbidity in a severity-dependent manner. The degree of lipid peroxidation correlates with the degree of intermittent hypoxia.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was supported by the Israel Science Foundation (grant No. 707/12). The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Rosen CL, Larkin EK, Kirchner EL, et al. Prevalence and risk factors of sleep-disordered breathing in 8- to 10- year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 2.Ali NJ, Pitson DJ, Stardling JR. Snoring, sleep disturbance, and behavior in 4-5 year olds. Arch Dis Child. 1993;68:360–6. doi: 10.1136/adc.68.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gislason T, Benediktsdóttir B. Snoring, apneic episodes, and nocturnal hypoxemia among children 6 months to 6 years old. An epidemiologic study of lower limit of prevalence. Chest. 1995;107:963–6. doi: 10.1378/chest.107.4.963. [DOI] [PubMed] [Google Scholar]
- 4.Amin RS, Carroll JL, Jeffries JL, et al. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Resp Crit Care Med. 2004;169:950–6. doi: 10.1164/rccm.200309-1305OC. [DOI] [PubMed] [Google Scholar]
- 5.Tal A, Leiberman A, Margulis G, Sofer S. Ventricular dysfunction in children with obstructive sleep apnea: radionuclide assessment. Pediatr Pulmonol. 1988;4:139–43. doi: 10.1002/ppul.1950040304. [DOI] [PubMed] [Google Scholar]
- 6.Goldbart AD, Levitas A, Greenberg-Dotan S, et al. B-type natriuretic peptide and cardiovascular function in young children with obstructive sleep apnea. Chest. 2010;138:528–35. doi: 10.1378/chest.10-0150. [DOI] [PubMed] [Google Scholar]
- 7.Amin R, Somers VK, McConnell K, et al. Activity-adjusted 24-hour ambulatory pressure and cardiac remodelling in children with sleep disordered breathing. Hypertension. 2008;51:84–91. doi: 10.1161/HYPERTENSIONAHA.107.099762. [DOI] [PubMed] [Google Scholar]
- 8.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics. 2004;113:e564–9. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- 9.Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–84. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien LM, Serpero LD, Tauman R, Gozal D. Plasma adhesion molecules in children with sleep-disordered breathing. Chest. 2006;129:947–53. doi: 10.1378/chest.129.4.947. [DOI] [PubMed] [Google Scholar]
- 11.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116:2307–14. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 12.Kheirandish-Gozal L, Bhattachariee R, Kim J, Clair HB, Gozal D. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182:92–7. doi: 10.1164/rccm.200912-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–84. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 14.Lavie L. Obstructive sleep apnea syndrome - an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 15.Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea and emerging risk factor for atherosclerosis. Chest. 2011;140:534–42. doi: 10.1378/chest.10-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stocker R, Keaney JF. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 17.Jarvisalo MJ, Lehtimaki T, Raitakari OT. Determinants of arterial nitrate-mediated dilatation in children: role of oxidized low density lipoprotein, endothelial function and carotid intima-media thickness. Circulation. 2004;109:2885–9. doi: 10.1161/01.CIR.0000129304.98566.D8. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Wei JJ, Wang F, Chen MT, Zhang MZ. Elevated levels of oxidized low-density lipoprotein correlate positively with C-reactive protein in patients with acute coronary syndrome. Cell Biochem Biophys. 2012;62:365–72. doi: 10.1007/s12013-011-9295-0. [DOI] [PubMed] [Google Scholar]
- 19.Tsimikas S. Oxidative biomarkers in the diagnosis and prognosis of cardiovascular disease. Am J Cardiol. 2006;98:9P–17P. doi: 10.1016/j.amjcard.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Wallenfeldt K, Fagerberg B, Wlkstrand J, Hulth J. Oxidized LDL in plasma is a prognostic marker of subclinical atherosclerosis development in clinically healthy men. J Intern Med. 2004;256:413–20. doi: 10.1111/j.1365-2796.2004.01402.x. [DOI] [PubMed] [Google Scholar]
- 21.Hulthe J, Fagerberg B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR study) Arterioscler Thromb Vasc Biol. 2002;22:1162–7. doi: 10.1161/01.atv.0000021150.63480.cd. [DOI] [PubMed] [Google Scholar]
- 22.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27:123–8. [PubMed] [Google Scholar]
- 23.Hackenhaar FS, Martinez D, Medeiros TM, et al. Oxidized LDL and paraoxonase-1 as biomarkers of coronary artery disease in patients with sleep disordered breathing. Curr Med Chem. 2012;19:4359–66. doi: 10.2174/092986712802884312. [DOI] [PubMed] [Google Scholar]
- 24.Kizawa T, Nakamura Y, Takahashi S, Sakurai S, Yamaucho K, Inoue H. Pathogenic role of angiotensin II and oxidized LDL in obstructive sleep apnea. Eur Respir J. 2009;34:1390–8. doi: 10.1183/09031936.00009709. [DOI] [PubMed] [Google Scholar]
- 25.Barcelo A, Miralles C, Barbe F, Vila M, Pons S, Agustí AG. Abnormal lipid peroxidation in patients with sleep apnea. Eur Respir J. 2000;16:644–7. doi: 10.1034/j.1399-3003.2000.16d13.x. [DOI] [PubMed] [Google Scholar]
- 26.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Resp Crit Care Med. 2002;165:934–9. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 27.Schulz R, Mahmoudi S, Hattar K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Resp Crit Care Med. 2000;162:566–70. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 28.Minoguchi K, Yokoe T, Tanaka A, et al. Association between lipid peroxidation and inflammation in obstructive sleep apnea. Eur Respir J. 2006;28:378–85. doi: 10.1183/09031936.06.00084905. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi M, Nakano H, Maekawa J, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127:1674–9. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]
- 30.Dogruer ZN, Unal M, Eskandari G, et al. Malondialdehyde and antioxidant enzymes in children with obstructive adenotonsillar hypertrophy. Clin Biochem. 2004;37:718–21. doi: 10.1016/j.clinbiochem.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Verhulst SL, Hoeck KV, Schrauwen N, et al. Sleep-disordered breathing and uric acid in overweight and obese children and adolescents. Chest. 2007;132:76–80. doi: 10.1378/chest.06-2930. [DOI] [PubMed] [Google Scholar]
- 32.Biltagi MA, Maguid MA, Ghafar MA, Farid E. Correlation of 8-isoprostane, interleukin-6 and cardiac functions with clinical score in childhood obstructive sleep apnea. Acta Pediatr. 2008;97:1397–405. doi: 10.1111/j.1651-2227.2008.00927.x. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery-Downs HE, Krishna J, Roberts LJ, Gozal D. Urinary F2-isoprostane metabolite levels in children with sleep-disordered breathing. Sleep Breath. 2006;10:211–5. doi: 10.1007/s11325-006-0079-5. [DOI] [PubMed] [Google Scholar]
- 34.Malakosioti G, Alexopoulos E, Befani C, et al. Oxidative stress and inflammatory markers in the exhaled breath condensate of children with OSA. Sleep Breath. 2012;16:703–8. doi: 10.1007/s11325-011-0560-7. [DOI] [PubMed] [Google Scholar]
- 35.Van Hoorenbeeck K, Franckx H, Debode P, et al. Weight loss and sleep-disordered breathing in childhood obesity: effects on inflammation and uric acid. Obesity. 2012;20:172–7. doi: 10.1038/oby.2011.282. [DOI] [PubMed] [Google Scholar]
- 36.Vlasic V, Trifunovic J, Cepelak I, et al. Urates in exhaled condensate of children with obstructive sleep apnea. Biochem Med. 2011;21:139–44. doi: 10.11613/bm.2011.022. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114:44–9. doi: 10.1542/peds.114.1.44. [DOI] [PubMed] [Google Scholar]
- 38.Gottlieb DJ, Chase C, Vezina RM, et al. Sleep disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145:458–64. doi: 10.1016/j.jpeds.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 39.Rechtschaffen A, Kales A. Washington, DC: US Government Printing Office; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 40.American Thoracic Society. Standard indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 41.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 42.Podrez EA, Poliakov E, Shen Z, et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–23. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 43.Podrez EA, Poliakov E, Shen Z, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277:38503–16. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 44.Jun J, Savransky V, Nanayakkara A, et al. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1274–81. doi: 10.1152/ajpregu.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savransky V, Jun J, Li J, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res. 2008;103:1173–80. doi: 10.1161/CIRCRESAHA.108.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]


