Abstract
Background and Objectives
Uridine-diphosphate glucuronosyltransferase 1A (UGT1A) is a key enzyme involved in irinotecan metabolism, and polymorphisms in the UGT1A gene are associated with irinotecan-induced toxicity. The aim of this study was to elucidate the allele frequencies of UGT1A polymorphisms in healthy Uzbek volunteers, and to compare them with those of the Japanese population.
Method
A total of 97 healthy volunteers from Uzbekistan were enrolled and blood samples were collected from each participant. Genotyping analysis was performed by fragment size analysis for UGT1A1*28, direct sequencing for UGT1A7*3 and UGT1A9*22, and TaqMan assays for UGT1A1*93, UGT1A1*6, UGT1A1*27, UGT1A1*60, and UGT1A7*12. The frequencies of polymorphisms were compared with the Japanese population by using the data previously reported from our study group.
Results
When the Uzbek and Japanese populations were compared, heterozygotes or homozygotes for UGT1A1*28, UGT1A1*60, and UGT1A1*93 were significantly more frequent in the Uzbek population (P < 0.01). The rate of UGT1A7*12 was not significantly different between the two populations, whereas UGT1A1*6 and UGT1A9*22 were significantly less frequent in the Uzbek population (P < 0.05). UGT1A7*1 were less prevalent in the Uzbek population than in the Japanese population (P < 0.01).
Conclusion
The Uzbek population has different frequencies of polymorphisms in UGT1A genes compared with the Japanese population. A comprehensive study of the influence of UGT1A1 polymorphisms on the risk of irinotecan-induced toxicity is necessary for optimal use of irinotecan treatment.
Introduction
Irinotecan in combination with fluorouracil and leucovorin prolongs the progression-free survival and overall survival of patients with metastatic colorectal cancer compared with fluorouracil and leucovorin alone. However, grade 3/4 diarrhea and neutropenia can occur during the course of the treatment, jeopardizing approximately 20–25 and 25–55 % of patients, respectively [1, 2]. The toxicity of irinotecan is influenced by several non-genetic factors [3, 4]. A number of recent studies have found a strong relationship between the adverse effects of irinotecan-based treatment and polymorphisms of the uridine-diphosphate glucuronosyltransferase 1A (UGT1A) gene, which encodes a crucial enzyme in irinotecan metabolism [5–13].
Administered irinotecan is activated and transformed through hydrolysis into 7-ethyl-10-hydroxycamptothecin (SN-38), which is a potent inhibitor of topoisomerase I and is the metabolite responsible for adverse reactions to irinotecan-based treatment [14]. SN-38 is then converted into an inactive form, 10-O-glucuronyl-SN-38 (SN-38G), by various UGT1A isoforms including UGT1A1, 1A6, 1A7, 1A8, 1A9, and 1A10 [15–17]. Therefore, irinotecan-induced toxicities could be due to a reduced glucuronidation rate of SN-38 caused by genetic variants of UGT1A. One of the representative polymorphisms that strongly influences irinotecan clearance is UGT1A1*28, which has (TA)7 in the promoter region instead of (TA)6 as in UGT1A1*1 [18]. Individuals with UGT1A1*28 demonstrate reduced irinotecan clearance [10, 12] due to reduced transcriptional activity of UGT1A1 [18]. The UGT1A1*28 polymorphism may increase the clinical risk of severe irinotecan-induced toxicity in colorectal cancer patients [5, 6]. In contrast to the effect of UGT1A1*28 on transcriptional efficacy, UGT1A1*6 has G replaced by A at position +211 relative to the UGT1A1 transcription start site, which results in decreased irinotecan metabolism [19].
Several polymorphisms, especially UGT1A1, 1A7, and 1A9, are associated with an alteration in irinotecan metabolism and are receiving increasing attention in clinical settings [8, 20], but only UGT1A1*28 and UGT1A1*6 have been extensively studied. We recently examined irinotecan metabolism-related UGT1A polymorphisms and reported their frequency in three geographically different regions in Japan [21]. We consider that studying the inter-ethnic diversity of these polymorphisms will lead to an understanding of the inconsistent drug responses observed between different populations, and will ultimately help establish the best strategies for application of new drugs in these populations. In this context, this study was designed to determine the allele frequency of UGT1A1*27, UGT1A1*60, UGT1A1*93, UGT1A7*2 (N129K), UGT1A7*3 (co-occurrence of N129K and W208R), UGT1A7*12, and UGT1A9* 22 as well as UGT1A1*28 and UGT1A1*6 in healthy volunteers of the Republic of Uzbekistan, a country located between East Asia and Europe (Table 1; Fig. 1).
Table 1.
Primers and probes for genotyping
| Polymorphism | Position | WT>variant | Primer or Probe | |
|---|---|---|---|---|
| UGT1A1*6 | 211 | G>A | C 559715 20 | |
| UGT1A1*27 | 686 | C>A | C 2307598 20 | |
| UGT1A1*28 | TATA box | TA6>TA7 |
F-FAM R |
5′-gtgacacagtcaaacattaacttgt-3′ 5′-gcctttgctcctgccagaggtt-3′ |
| UGT1A1*60 | −3279 | T>G | C 1432134 10 | |
| UGT1A1*93 | −3156 | G>A |
F R FAM VIC |
5′-acttaacattgcagcacagg-3′ 5′-atgggcaaaagccttgaact-3′ 5′-cctgtccaagctca-3′ 5’-cacctgtctaagctca-3’ |
| UGT1A7*2 (N129K) UGT1A7*3 (W208R) |
387 622 |
T>G T>C |
F R |
5′-tacactctggaggatcagga-3′ 5′-tattgggcatcacgggtttg-3′ |
| UGT1A7*12 | −57 | T>G | C 287265 10 | |
| UGT1A9* 22 | −188 | T9>T10 | F | 5′-acttaacattgcagcacagg-3′ |
| R | 5′-atgggcaaaagccttgaact-3′ |
Nucleotide positions are relative to the transcription start site
Transposition of T to G at position 387 (or N129K) is termed UGT1A7*2. Transposition of T to C at position 622 is termed W208R, and co-occurrence of N129K and W208R is termed UGT1A7*3
TA6 6 TA repeats, TA7 7 TA repeats, F-FAM forward primer-labeled reporter 1 probe, F forward primer, R reverse primer, FAM reporter 1 probe, VIC reporter 2 probe, WT wild type
Fig. 1.
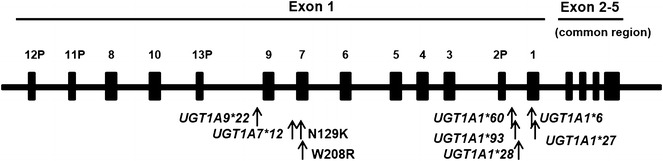
Schematic illustration of the UGT1A gene showing the locations of polymorphisms investigated in the present study. 13P, 12P, 11P, and 2P represent pseudogenes present in exon 1. Exons 2–4 are common exons. The enzyme, UGT1A, is produced through splicing of exon 1
Methods
Subjects
This study was approved by the ethics committee of Yamaguchi University, and written informed consent was obtained from all participants. We recruited 97 volunteers from Uzbekistan. A 7 ml sample of peripheral blood was collected from each individual and stored in ethylenediaminetetraacetic acid (EDTA). The 150 healthy Japanese volunteers were from three districts in Japan, and we have investigated and reported on their polymorphisms previously [21].
Genotyping
Genomic DNA from peripheral blood anti-coagulated with EDTA was extracted by using the conventional NaI method. UGT1A1*28 was identified using fragment size analysis, and UGT1A7*3 and UGT1A9*22 were detected using direct sequencing. A TaqMan assay was performed for the identification of UGT1A1*93, UGT1A1*6, UGT1A1*27, UGT1A1*60, and UGT1A7*12. Primers and probes used in the study are listed in Table 1.
For fragment size analysis, polymerase chain reaction (PCR) reactions using 800 ng of template DNA were performed according to the manufacturer’s instructions (Ex Taq; Takara, Tokyo, Japan). The PCR products of TA6 (6 TA repeats; 94 bp) and TA7 (7 TA repeats; 96 bp) were mixed with Hi-Di formamide and the internal size standard (GeneScan LIZ-500, Applied Biosystems, CA, USA) at a ratio of 1:10 (v/v). After electrophoresis in the ABI Prism 3100 Genetic Analyzer (Applied Biosystems), fragment sizes were compared with the internal size standard and determined by the local Southern algorithm and by GeneMapper software version 3.5 (Applied Biosystems).
For direct sequencing, PCR was conducted using the Gene Amp PCR System PC808 (ASTEC, Tokyo, Japan) with Ex Taq polymerase. Purification of PCR products was performed with ExoSAP-IT (Amersham Bioscience, Tokyo, Japan) for 20 min at 37°C followed by 20 min at 80°C. Sequencing reactions were conducted with a BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Tokyo, Japan), and, after the reaction products were purified with ethanol, analysis was performed using an ABI 3100-Avant Genetic Analyzer (Applied Biosystems).
As described in Table 1, the transposition of T to G at position 387 (or N129K), C to A at position 391, and G to A at position 392 are termed UGT1A7*2. These three polymorphisms are genetically linked, and only N129K is described in this manuscript. The transposition of T to C at position 622 is termed W208R, and co-occurrence of N129K and W208R is termed UGT1A7*3, whereas the occurrence of W208 alone is termed UGT1A7*4. Thus, in the case of heterozygous N129K and heterozygous W208R in the same individual, the possible genotypes are UGT1A7*1/*3 and *2/*4. However, because UGT1A7*4 is known to be quite rare in non-Uzbek populations [9, 17], the statistical analysis was performed on the assumption that all the genotypes were UGT1A7*1/*3.
For TaqMan assays for UGT1A1*93, forward and reverse PCR primers and TaqMan probes were custom-synthesized by Applied Biosystems. Primers and probes for UGT1A1*6, UGT1A1*27, UGT1A1*60, and UGT1A7*12 were obtained from Applied Biosystems (TaqMan SNP Genotyping Assays). Reaction mixtures were loaded into 384-well plates and analyzed in an ABI Prism 7900HT Sequence Detection System (Applied Biosystems).
Statistical Analysis
Each nucleotide polymorphism was evaluated for the Hardy–Weinberg equilibrium, and by linkage disequilibrium (LD) analysis using Haploview 4.2 software (Massachusetts Institute of Technology, Cambridge, MA, USA; http://www.broadinstitute.org/scientific-community/science/programs/medical-and-population-genetics/haploview/haploview). The Lewontin’s coefficient D′ and correlation coefficient, r 2, were calculated as measures of LD. The proportions of subjects with homozygous wild-type alleles or heterozygous or homozygous variant alleles were calculated with 95 % Agresti–Coull confidence intervals (95 % CI). Fisher’s exact test with a two-sided significance level of 0.05 was used for comparing the areas. For a two-sided 95 % CI for a binomial proportion whose true value is varied from 0.5 to 0.1, a sample size of 50 yields a half-width of, at most, 14 % in any situations of the true value. These analyses were performed using SAS v9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline Characteristics, Hardy–Weinberg Equilibrium, and Linkage Disequilibrium
The average age of the participants was 41.1 (range 19–78) years in Uzbekistan, and 39.9 (range 18–67) years in Japan. The 97 volunteers from Uzbekistan comprised 37 female and 60 males, while 125 of the 150 Japanese volunteers were female. As described previously [21], the Japanese volunteers were mainly recruited from nurses, which is an occupation traditionally chosen by women in Japan.
All of the UGT1A polymorphisms were in Hardy–Weinberg equilibrium (P > 0.05). Perfect linkages were observed between W208 and UGT1A7*12 in both the Uzbek and the Japanese populations (data not shown). UGT1A1*28 and UGT1A1*93 showed very strong linkage in the Uzbek population (r 2 = 0.95, D′ = 1.00) and they matched completely in the Japanese population (Fig. 2). Close linkage was also observed between N129K and UGT1A9*22, both in the Uzbek (r 2 = 0.94, D′ = 1.00), and in the Japanese (r 2 = 0.92, D′ = 1.00) populations.
Fig. 2.
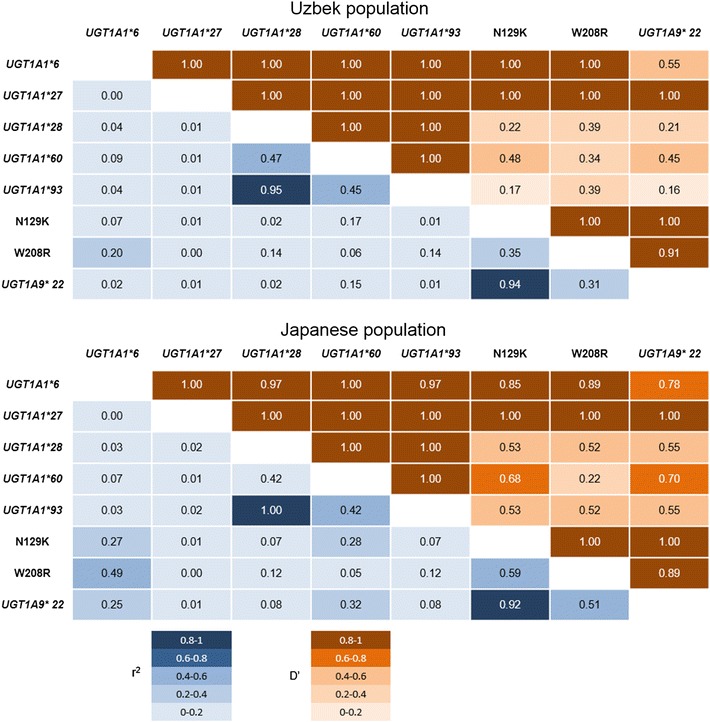
Linkage disequilibrium analysis for UGT1A1, 1A7, and 1A9 polymorphisms. Strong linkages are shown between UGT1A1*28 and UGT1A1*93, and between N129K and UGT1A1*93, respectively, in both Uzbek and Japanese populations. Each square is colored according to the value of D′ (upper brown) and r 2 (lower blue)
Frequencies of UGT1A1 Polymorphisms
Table 2 lists the frequencies of UGT1A1 polymorphisms, UGT1A1*6, *28, *60, *27, and *93. The two polymorphisms that have been extensively studied for their role in irinotecan metabolism, UGT1A1*28 and UGT1A1*6, showed different incidences in the Uzbek and Japanese populations. UGT1A1*28 showed a significantly higher incidence and UGT1A1*6 showed a significantly lower incidence in the Uzbek and the Japanese populations (P < 0.01, P = 0.012, respectively). Neither UGT1A1*36 (5TA repeat in the promoter region) nor UGT1A1*37 (8TA repeat in the promoter region) were identified among the Uzbek volunteers. Among the other UGT1A1 polymorphisms examined, UGT1A*27 was very rare in both Uzbek and Japanese populations, occurring at a rate of 0.005 and 0.003, respectively, and UGT1A1*60 and UGT1A1*93 were significantly more prevalent in the Uzbek population than in the Japanese population (both P < 0.01).
Table 2.
UGT1A1 polymorphisms
| UGT1A1*6 (P = 0.012) | Allele frequency of UGT1A1*6 | |||
|---|---|---|---|---|
| G/G | G/A | A/A | ||
| Uzbekistan | 82 (85, 79–93) | 13 (13, 8–22) | 2 (2, 0–8) | 0.09 |
| Japan | 103 (69, 61–76) | 43 (29, 22–36) | 4 (3, 0–7) | 0.17 |
| P value | 0.007 | 0.005 | 1.000 | |
| UGT1A1*27 (P = 0.394) | Allele frequency of UGT1A1*27 | |||
|---|---|---|---|---|
| C/C | C/A | A/A | ||
| Uzbekistan | 96 (99, 94–100) | 1 (1, 0–6) | 0 (0, 0–3) | 0.005 |
| Japan | 149 (99, 96–100) | 1 (1, 0–4) | 0 (0, 0–2) | 0.003 |
| P value | 0.394 | 0.394 | – | |
| UGT1A1*28 (P < 0.01) | Allele frequency of UGT1A1*28 | |||
|---|---|---|---|---|
| (TA)6/(TA)6 | (TA)6/(TA)7 | (TA)7/(TA)7 | ||
| Uzbekistan | 45 (46, 37–56) | 43 (44, 35–54) | 9 (9, 5–17) | 0.31 |
| Japan | 115 (77, 69–83) | 33 (22, 16–29) | 2 (1, 0–5) | 0.12 |
| P value | <0.01 | <0.01 | 0.008 | |
| UGT1A1*60 (P < 0.01) | Allele frequency of UGT1A1*60 | |||
|---|---|---|---|---|
| T/T | T/G | G/G | ||
| Uzbekistan | 25 (26, 18–35) | 48 (49, 40–59) | 24 (25, 17–34) | 0.50 |
| Japan | 79 (53, 45–60) | 66 (44, 36–52) | 5 (3, 1–8) | 0.25 |
| P value | <0.01 | 0.434 | <0.01 | |
| UGT1A1*93 (P < 0.01) | Allele frequency of UGT1A1*93 | |||
|---|---|---|---|---|
| G/G | G/A | A/A | ||
| Uzbekistan | 46 (47, 38–57) | 43 (44, 35–54) | 8 (8, 4–16) | 0.30 |
| Japan | 115 (77, 69–83) | 33 (22, 16–29) | 2 (1, 0–5) | 0.12 |
| P value | <0.01 | <0.01 | 0.016 | |
Polymorphisms of UGT1A1 are shown. For each genotype, the values represent the number of subjects (percentage of subjects, 95 % CI). Heterozygous and homozygous UGT1A1*28, *60, and *93 were observed significantly more frequently in the Uzbek population than in the Japanese population. In contrast, heterozygous and homozygous UGT1A1*6 were significantly less prevalent in the Uzbek population than in the Japanese population. The occurrence of UTG1A1*27 was rare in both populations
Frequencies of UGT1A7 and UGT1A9 Polymorphisms
Table 3 lists the frequencies of UGT1A7*3 (N129K and W208R), UGT1A7*12, and UGT1A9*22. The Uzbek population had a significantly higher incidence of homozygous N129K than did the Japanese population (P < 0.01). However, the incidences of W208R and UGT1A7*12 were not significantly different between the two populations; furthermore, W208R and UGT1A7*12 showed evidence of genetic linkage, which is reflected in their identical values in Table 3. No Uzbek individual carrying heterozygous UGT1A7*1/*4 or *3/*4, or homozygous UGT1A7*4 was detected. UGT1A7*1 is less prevalent in Uzbekistan than in Japan (P < 0.01). UGT1A9*22 was significantly less prevalent in the Uzbek population than in the Japanese population (P < 0.01).
Table 3.
UGT1A7 and UGT1A9 polymorphisms
| N129K (P < 0.01) | Allele frequency of UGT1A7 N129K | |||
|---|---|---|---|---|
| T/T | T/G | G/G | ||
| Uzbekistan | 18 (19, 12–28) | 46 (47, 38–57) | 33 (34, 25–44) | 0.58 |
| Japan | 63 (42, 34–50) | 67 (45, 37–53) | 20 (13, 9–20) | 0.36 |
| P value | <0.01 | 0.696 | <0.01 | |
| W208R (P = 0.149) | Allele frequency of UGT1A7 W208R | |||
|---|---|---|---|---|
| T/T | T/C | C/C | ||
| Uzbekistan | 47 (48, 39–58) | 37 (38, 29–48) | 13 (13, 8–22) | 0.32 |
| Japan | 86 (57, 49–65) | 54 (36, 29–44) | 10 (7, 4–12) | 0.25 |
| P value | 0.192 | 0.788 | 0.115 | |
| UGT1A7*2 or *3 (P < 0.01) | ||||||
|---|---|---|---|---|---|---|
| *1/*1 | *1/*2 | *1/*3(*2/*4) | *2/*2 | *2/*3 | *3/*3 | |
| Uzbekistan | 18 (19, 12–28) | 24 (25, 17–34) | 22 (23, 15–32) | 5 (5, 2–12) | 15 (15, 9–24) | 13 (13, 8–22) |
| Japan | 63 (42, 34–50) | 22 (15, 10–21) | 45 (30, 23–38) | 1 (1, 0–4) | 9 (6, 3–11) | 10 (7, 4–12) |
| P value | <0.01 | 0.065 | 0.242 | 0.036 | 0.026 | 0.115 |
| UGT1A7*12 (P = 0.149) | Allele frequency of UGT1A7*12 | |||
|---|---|---|---|---|
| T/T | T/G | G/G | ||
| Uzbekistan | 47 (48, 39–58) | 37 (38, 29–48) | 13 (13, 8–22) | 0.32 |
| Japan | 86 (57, 49–65) | 54 (36, 29–44) | 10 (7, 4–12) | 0.25 |
| P value | 0.192 | 0.788 | 0.115 | |
| UGT1A9*22 (P < 0.01) | Allele frequency of UGT1A9*22 | |||
|---|---|---|---|---|
| T9/T9 | T9/T10 | T10/T10 | ||
| Uzbekistan | 31 (32, 23–42) | 47 (49, 39–58) | 19 (20, 13–29) | 0.44 |
| Japan | 16 (11, 7–17) | 69 (46, 38–54) | 65 (43, 36–51) | 0.66 |
| P value | <0.01 | 0794 | <0.01 | |
For each genotype, the values represent the number of subjects (percentage of subjects, 95 % CI). The occurrence of N129 alone is termed UGT1A7*2, and the occurrence of W208 alone is termed UGT1A7*4, whereas co-occurrence of N129K and W208R is termed UGT1A7*3. In the case of heterozygous N129K and heterozygous W208R, the possible genotypes are UGT1A7*1/*3 and *2/*4; however, because UGT1A7*4 is known to be quite rare in non-Uzbek populations, the statistical analysis was performed on the assumption that all the genotypes were UGT1A7*1/*3
Discussion
UGT1A is an essential enzyme for the elimination of numerous endogenous and exogenous compounds through glucuronidation [22]. Here, we analyzed the allele frequencies of several UGT1A polymorphisms in 97 healthy Uzbek volunteers and compared them with those of the Japanese population.
The polymorphism UGT1A1*28 ([TA]7 in the UGT1A1 promoter region) has been extensively investigated [23, 24], particularly in terms of inter-individual variability of irinotecan-induced gastrointestinal and hematological toxicity [5–12]. Because inactivation of the active form of irinotecan (SN-38) protects against severe irinotecan-related toxicity, decreased transcriptional activity of UGT1A1 due to UGT1A1*28 is considered to be a risk factor for adverse reactions to irinotecan treatment. Here, we showed that the incidence of UGT1A1*28 homozygotes in the Uzbek population was 9.3 %, which was significantly higher than that in the Japanese population that we studied previously [21]. In published reports, the incidence of UGT1A1*28 homozygotes is high in Europe (5–14.8 %), Africa (5.9–17.9 %), and India (12.8–19.3 %); and less frequent in East Asia and Japan (0–5.9 %) [5–8, 25–31]; the allele frequency of UGT1A1*28 shows a similar trend to the incidence of homozygotes (Table 4). Horsfall et al. [23] and Premawardhena et al. [24] reported further diversity in the numbers of TA repeats (i.e. [TA]5 termed UGT1A1*36, and [TA]8 termed UGT1A1*37) among individuals from Africa and those with varying degrees of African ancestry in North and Central America. UGT1A1*36 and UGT1A1*37 are also present in Caucasian and Indian populations at a very low rate [24, 30]; however, to our knowledge, they have not been detected in Japan. Neither UGT1A1*36 nor UGT1A1*37 were identified among the 97 Uzbek volunteers.
Table 4.
Allele frequency of UGT1A polymorphisms in different ethnic groups
| Origin | ||||||
|---|---|---|---|---|---|---|
| Africa | Europe | Uzbekistan | India | East Asia | Japan | |
| Reference | 23–25, 32, 41 | 7, 8, 17, 23–25, 28, 32, 35, 36, 41 | Present study | 24, 30, 31, 40 | 27, 29, 31, 32, 38, 39 | 5, 6, 9, 12, 13, 21, 25, 26, 33, 34, 37 |
| UGT1A1*6 | 0 | 0.007–0.01 | 0.09 | 0.066–0.16 | 0.13–0.24 | 0.14–0.23 |
| UGT1A1*27 | 0 | 0 | 0.005 | 0 | 0.015–0.02 | 0.003 |
| UGT1A1*28 | 0.26–0.4 | 0.23–0.38 | 0.31 | 0.28–0.41 | 0.07–0.17 | 0.04–0.14 |
| UGT1A1*60 | 0.85 | 0.44–0.62 | 0.5 | 0.43 | 0.235–0.34 | 0.23–0.26 |
| UGT1A1*93 | 0.29 | 0.23–0.35 | 0.3 | 0.35 | 0.12 | 0.12 |
| UGT1A7*1 | 0.38 | 0.34–0.43 | 0.422 | 0.26 | 0.58–0.63 | 0.59–0.65 |
| UGT1A7*2 | 0.39 | 0.24–0.28 | 0.252 | 0.36 | 0.22–0.27 | 0.1–0.14 |
| UGT1A7*3 | 0.23 | 0.31–0.35 | 0.325 | 0.36 | 0.15 | 0.21–0.29 |
| UGT1A7*4 | 0.01 | 0–0.019 | 0 | 0.032 | 0 | 0–0.03 |
| UGT1A9*22 | 0.44 | 0.39–0.41 | 0.44 | – | 0.42 | 0.6–0.66 |
Different ethnic groups have different allele frequencies of UGT1A polymorphisms. Note that individual cohorts contributing to the ethnic group data comprised healthy volunteers or cancer patients or both. Europe includes individuals from several different European countries and Caucasians from non-European countries. East Asia includes China and Korea. All allele frequencies obtained from our previous study of the Japanese population are within the range of reported studies (Tables 2, 3)
The polymorphism UGT1A1*6 has also been extensively studied and has been shown to be associated with decreased irinotecan metabolism [19]. The current study showed the rates of heterozygotes and homozygotes for UGT1A1*6 in Uzbekistan were 13.4 and 2.1 %, respectively, which was significantly lower than the equivalent rates in the Japanese population we studied previously [21]. In a previous study, only two heterozygotes among 150 Caucasians and no individuals among 150 African-Americans were found to carry UGT1A1*6 [25]. The incidence of UGT1A1*6 polymorphisms in the Uzbek population was more frequent that that previously reported in Caucasian and African populations (Table 4).
The variant 686C>A (UGT1A1*27) is a very rare polymorphism that is associated with a significant reduction in UGT1A activity in vitro [19]. No UGT1A1*27 allele was detected in 150 Caucasian or 150 African-American healthy volunteers [25], or in more than 300 Indian healthy volunteers [30, 31] (Table 4). In contrast, studies from Japan identified heterozygous UGT1A1*27 in 2.6 % of Japanese colorectal cancer patients [5], in 0.7 % of Japanese or African-American healthy volunteers [21, 25], and in 2.3 % of a mixed population of Japanese arrhythmic patients and cancer patients [26]. The current study demonstrated that the incidence of UGT1A1*27 heterozygotes was also low in the Uzbek population (1 of 97 individuals).
UGT1A1*60 and UGT1A*93 are the polymorphisms that result in reduced UGT1A1 enzyme efficacy and may be related to the hematological toxicity observed after irinotecan treatment [8, 32]. The allele frequency of UGT1A1*60 in the Uzbek population (0.5) was higher than that in various reports of the Japanese population (0.23–0.26), including our previous study. The frequency was similar to that reported for Caucasian (0.44–0.62) and Indian (0.43) populations, and lower than that reported for the African population (0.85) (Table 4). Heterozygous or homozygous UGT1A1*93 was observed significantly more frequently in the Uzbek population than in the Japanese population that we studied previously [21]. In a small study of the Chinese population [27], UGT1A1*93 was detected at a frequency of 0.12, which is similar to that reported in various studies of the Japanese population, including our previous study. In contrast, UGT1A1*93 is relatively common in Europe, Africa, India, and Uzbekistan.
UGT1A9*22 is characterized by a one-base thymidine insertion in the promoter region of UGT1A9, which increases the UGT1A9 levels and causes a higher clearance rate of SN-38G than that observed for UGT1A9*1 [33]. Our previous study [21] revealed that the allele frequency of UGT1A9*22 in healthy Japanese volunteers was 0.66, which is comparable to that in Japanese colorectal cancer patients (0.54–0.66) [9, 33, 34]. A study of colorectal cancer patients who were mainly of Caucasian origin reported a UGT1A9*22 allele frequency of 0.4 [8, 35], which is similar to that observed here for the healthy Uzbek volunteers.
In the present study, no Uzbek individual carrying UGT1A7*1/*4, *3/*4, or *4/*4 was detected. The experimental methods used in the current study cannot directly determine UGT1A7*1, *2, *3, or *4. Therefore, when both heterozygous N129K and heterozygous W208R were detected in the same individual, the possible genotypes were UGT1A7*1/*3 or *2/*4. However, because the frequency of UGT1A7*4 is known to be quite rare among Caucasian [17, 36], Japanese [9, 26, 37], and Chinese [38] populations, we assumed that the only possible genotype was UGT1A7*1/*3. Accordingly, the allele frequency of UGT1A7*1 in the Uzbek population was lower than that reported for East Asia and Japan [9, 26, 38], slightly higher than that reported for India [40], and similar to that reported for both Africa and Europe [12, 32, 41]. UGT1A7*3 is associated with reduced enzymatic activity compared with the wild-type [42], and might be related to the irinotecan toxicity in colorectal cancer patients [20, 34].
The relationship between UGT1A polymorphisms and irinotecan-induced toxicities remains controversial. For instance, several early studies reported a strong association between UGT1A1*28 with severe (grade 3 or 4) toxicity in Japanese [5, 6, 11] and Caucasian colorectal patients [10]; however, another study found that UGT1A1*28 had little or no effect on the rate of adverse effects in such patients [7, 8, 27]. Inconsistent patient responses to irinotecan imply that factors such as age, organ function, concomitant therapy [3, 4], and dose of irinotecan [7] might modulate the therapeutic and adverse effects of this drug. However, the variable responses might also be explained by the synergic effect of UGT1A1*28 and other UGT1A polymorphisms, such as UGT1A1*6 and UGT1A7*3 [12, 13, 20, 28]. Our finding that there is a significantly different distribution of UGT1A polymorphisms in Uzbekistan and Japan strengthens this hypothesis, and provides further evidence that extensive studies of the relationships between UGT1A haplotypes and the risk of irinotecan toxicity would be worthwhile.
One limitation of this study was that the number of the Uzbek healthy volunteers examined was small. Thus, the rate of detection of rare polymorphisms, such as UGT1A1*36, UGT1A1*37, and UGT1A1*27, might not be representative of the whole Uzbek population. However, for other polymorphisms, the numbers of volunteers was sufficient to show very strong statistical differences between the Uzbek population and the Japanese population. Another limitation of the study was that a statistical comparison of the frequency of UGT1A polymorphisms in the Uzbek population and other populations that were studied using different genotyping methods was not possible. Our ongoing study of various ethnic groups from Asia to Europe will allow such direct comparisons and may clarify the movement of people throughout history [21].
LD analysis in the present study revealed strong linkage between some UGT1A genotypes. Recently, strong linkage between N129K and UGT1A9*22, as we have observed in Uzbek and Japanese populations, was also reported in a Caucasian population [8]. In contrast, the linkage between UGT1A1*28 and UGT1A1*93 was stronger in both Uzbek and Japanese populations (r 2 = 0.94 and 0.92, respectively) than in a Caucasian population (r 2 = 0.6782) [8]. This suggests that the difference in haplotype frequencies between different populations reflects both genotype (polymorphism) frequencies and the strength of LD. It is also plausible that the different strength of linkage is one of the reasons for the inconsistency observed between the adverse effects of irinotecan and the presence of UGT1A polymorphisms in treated patients. Thus, further investigation is required of UGT1A LD and haplotypes.
Our results revealed that UGT1A1*28 is more frequent in the Uzbek population than in the Japanese population, and is present at a similar rate to that reported for Caucasian and African populations. In contrast, UGT1A1*6 is less prevalent in the Uzbek population than in the Japanese population, but has a higher incidence than previous reports in Caucasians. Although UGT1A1*27 has not been reported in African and Caucasian populations, it was identified in a single Uzbek volunteer. UGT1A1*60 and *93 were detected more frequently in the Uzbek population than in the Japanese population, and the rate was similar to that reported for Caucasians. Finally, UGT1A9*22 was identified in the Uzbek population at a lower rate than that in the Japanese population. These findings confirm the inter-ethnic diversity of polymorphisms in UGT1A and lead us to speculate that the Uzbekistan population has genetic characteristic between those of East Asia and European countries, consistent with its geographical location (Table 4). We anticipate that further comprehensive studies revealing the relationship between UGT1A1 polymorphisms and the risk of irinotecan-induced toxicity will facilitate individual treatment option decisions.
Acknowledgments and Disclosures
This study was supported in part by a non-profit organization, Epidemiological and Clinical Research Information Network (ECRIN), as well as a Grant-in-Aid for Scientific Research of the Ministry of Education, Science, Sports and Culture of Japan (project No. 21591725 and 19591545). The authors have no conflicts of interest that are directly relevant to the content of this article.
Author contributions
All authors equally contributed to this study.
References
- 1.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/S0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 2.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 3.Dranitsaris G, Shah A, Spirovski B, Vincent M. Severe diarrhea in patients with advanced-stage colorectal cancer receiving FOLFOX or FOLFIRI chemotherapy: the development of a risk prediction tool. Clin Colorectal Cancer. 2007;6:367–373. doi: 10.3816/CCC.2007.n.006. [DOI] [PubMed] [Google Scholar]
- 4.Shiozawa T, Tadokoro J, Fujiki T, Fujino K, Kakihata K, Masatani S, Morita S, Gemma A, Boku N. Risk factors for severe adverse effects and treatment-related deaths in Japanese patients treated with irinotecan-based chemotherapy: a postmarketing survey. Jpn J Clin Oncol. 2013;43:483–491. doi: 10.1093/jjco/hyt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ando Y, Saka H, Ando M, Sawa T, Muro K, Ueoka H, Yokoyama A, Saitoh S, Shimokata K, Hasegawa Y. Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res. 2000;60:6921–6926. [PubMed] [Google Scholar]
- 6.Okuyama Y, Hazama S, Nozawa H, Kobayashi M, Takahashi K, Fujikawa K, Kato T, Nagata N, Kimura H, Oba K, Sakamoto J, Mishima H. Prospective phase II study of FOLFIRI for mCRC in Japan, including the analysis of UGT1A1 28/6 polymorphisms. Jpn J Clin Oncol. 2011;41:477–482. doi: 10.1093/jjco/hyr001. [DOI] [PubMed] [Google Scholar]
- 7.Toffoli G, Cecchin E, Corona G, Russo A, Buonadonna A, D’Andrea M, Pasetto LM, Pessa S, Errante D, De Pangher V, Giusto M, Medici M, Gaion F, Sandri P, Galligioni E, Bonura S, Boccalon M, Biason P, Frustaci S. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J Clin Oncol. 2006;24:3061–3068. doi: 10.1200/JCO.2005.05.5400. [DOI] [PubMed] [Google Scholar]
- 8.Cecchin E, Innocenti F, D’Andrea M, Corona G, De Mattia E, Biason P, Buonadonna A, Toffoli G. Predictive role of the UGT1A1, UGT1A7, and UGT1A9 genetic variants and their haplotypes on the outcome of metastatic colorectal cancer patients treated with fluorouracil, leucovorin, and irinotecan. J Clin Oncol. 2009;27:2457–2465. doi: 10.1200/JCO.2008.19.0314. [DOI] [PubMed] [Google Scholar]
- 9.Inoue K, Sonobe M, Kawamura Y, Etoh T, Takagi M, Matsumura T, Kikuyama M, Kimura M, Minami S, Utsuki H, Yamazaki T, Suzuki T, Tsuji D, Hayashi H, Itoh K. Polymorphisms of the UDP-glucuronosyl transferase 1A Genes are associated with adverse events in cancer patients receiving irinotecan-based chemotherapy. Tohoku J Exp Med. 2013;229:107–114. doi: 10.1620/tjem.229.107. [DOI] [PubMed] [Google Scholar]
- 10.Iyer L, Das S, Janisch L, Wen M, Ramírez J, Karrison T, Fleming GF, Vokes EE, Schilsky RL, Ratain MJ. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J. 2002;2:43–47. doi: 10.1038/sj.tpj.6500072. [DOI] [PubMed] [Google Scholar]
- 11.Hazama S, Nagashima A, Kondo H, Yoshida S, Shimizu R, Araki A, Yoshino S, Okayama N, Hinoda Y, OkaPhase M. Phase I, study of irinotecan and doxifluridine for metastatic colorectal cancer focusing on the UGT1A1*28 polymorphism. Cancer Sci. 2010;101:722–727. doi: 10.1111/j.1349-7006.2009.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araki K, Fujita K, Ando Y, Nagashima F, Yamamoto W, Endo H, Miya T, Kodama K, Narabayashi M, Sasaki Y. Pharmacogenetic impact of polymorphisms in the coding region of the UGT1A1 gene on SN-38 glucuronidation in Japanese patients with cancer. Cancer Sci. 2006;97:1255–1259. doi: 10.1111/j.1349-7006.2006.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onoue M, Terada T, Kobayashi M, Katsura T, Matsumoto S, Yanagihara K, Nishimura T, Kanai M, Teramukai S, Shimizu A, Fukushima M, Inui K. UGT1A1*6 polymorphism is most predictive of severe neutropenia induced by irinotecan in Japanese cancer patients. Int J Clin Oncol. 2009;14:136–142. doi: 10.1007/s10147-008-0821-z. [DOI] [PubMed] [Google Scholar]
- 14.Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187–4191. [PubMed] [Google Scholar]
- 15.Gupta E, Lestingi TM, Mick R, Ramirez J, Vokes EE, Ratain MJ. Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Res. 1994;54:3723–3725. [PubMed] [Google Scholar]
- 16.Iyer L, King CD, Whitington PF, Green MD, Roy SK, Tephly TR, Coffman BL, Ratain MJ. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest. 1998;101:847–854. doi: 10.1172/JCI915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacheco PR, Brilhante MJ, Ballart C, Sigalat F, Polena H, Cabral R, Branco CC, Mota-Vieira L. UGT1A1, UGT1A6 and UGT1A7 genetic analysis: repercussion for irinotecan pharmacogenetics in the São Miguel Island Population (Azores, Portugal) Mol Diagn Ther. 2009;13:261–268. doi: 10.1007/BF03256331. [DOI] [PubMed] [Google Scholar]
- 18.Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP, Chowdhury NR. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 19.Gagné JF, Montminy V, Belanger P, Journault K, Gaucher G, Guillemette C. Common human UGT1A polymorphisms and the altered metabolism of irinotecan active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38) Mol Pharmacol. 2002;62:608–617. doi: 10.1124/mol.62.3.608. [DOI] [PubMed] [Google Scholar]
- 20.Hazama S, Mishima H, Tsunedomi R, Okuyama Y, Kato T, Takahashi KI, Nozawa H, Ando H, Kobayashi M, Takemoto H, Nagata N, Kanekiyo S, Inoue Y, Hamamoto Y, Fujita Y, Hinoda Y, Okayama N, Oba K, Sakamoto JI, Oka M. UGT1A1*6, 1A7*3, and 1A9*22 genotypes predict severe neutropenia in FOLFIRI-treated metastatic colorectal cancer in two prospective studies in Japan. Cancer Sci. 2013 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 21.Kobayashi M, Hazama S, Takahashi K, Oba K, Okayama N, Nishioka M, Hinoda Y, Oka M, Okamoto K, Maeda H, Nakamura D, Sakamoto J, Mishima H. Is there diversity among UGT1A1 polymorphism in Japan? World J Gastrointest Oncol. 2012;4:170–175. doi: 10.4251/wjgo.v4.i7.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke DJ, Burchell B. The Uridine diphosphate glucronosyltransferase multigene families: function and regulation. In: Kaufmann FC, editor. Handbook of experimental pharmacology. Berlin: Springer; 1994. pp. 3–43. [Google Scholar]
- 23.Horsfall LJ, Zeitlyn D, Tarekegn A, Bekele E, Thomas MG, Bradman N, Swallow DM. Prevalence of clinically relevant UGT1A alleles and haplotypes in African populations. Ann Hum Genet. 2011;75:236–246. doi: 10.1111/j.1469-1809.2010.00638.x. [DOI] [PubMed] [Google Scholar]
- 24.Premawardhena A, Fisher CA, Liu YT, Verma IC, de Silva S, Arambepola M, Clegg JB, Weatherall DJ. The global distribution of length polymorphisms of the promoters of the glucuronosyltransferase 1 gene (UGT1A1): hematologic and evolutionary implications. Blood Cells Mol Dis. 2003;31:98–101. doi: 10.1016/S1079-9796(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 25.Kaniwa N, Kurose K, Jinno H, Tanaka-Kagawa T, Saito Y, Saeki M, Sawada J, Tohkin M, Hasegawa R. Racial variability in haplotype frequencies of UGT1A1 and glucuronidation activity of a novel single nucleotide polymorphism 686C> T (P229L) found in an African-American. Drug Metab Dispos. 2005;33:458–465. doi: 10.1124/dmd.104.001800. [DOI] [PubMed] [Google Scholar]
- 26.Saeki M, Saito Y, Jinno H, Sai K, Ozawa S, Kurose K, Kaniwa N, Komamura K, Kotake T, Morishita H, Kamakura S, Kitakaze M, Tomoike H, Shirao K, Tamura T, Yamamoto N, Kunitoh H, Hamaguchi T, Yoshida T, Kubota K, Ohtsu A, Muto M, Minami H, Saijo N, Kamatani N, Sawada JI. Haplotype structures of the UGT1A gene complex in a Japanese population. Pharmacogenomics J. 2006;6:63–75. doi: 10.1038/sj.tpj.6500335. [DOI] [PubMed] [Google Scholar]
- 27.Zhang A, Xing Q, Qin S, Du J, Wang L, Yu L, Li X, Xu L, Xu M, Feng G, He L. Intra-ethnic differences in genetic variants of the UGT-glucuronosyltransferase 1A1 gene in Chinese populations. Pharmacogenomics J. 2007;7:333–338. doi: 10.1038/sj.tpj.6500424. [DOI] [PubMed] [Google Scholar]
- 28.Lamas MJ, Duran G, Balboa E, Bernardes B, Candamio S, Vidal Y, Mosquera A, Giraldez JM, Lopez R, Carracedo A, Barros F. The value of genetic polymorphisms to predict toxicity in metastatic colorectal patients with irinotecan-based regimens. Cancer Chemother Pharmacol. 2012;69:1591–1599. doi: 10.1007/s00280-012-1866-2. [DOI] [PubMed] [Google Scholar]
- 29.Han JY, Lim HS, Shin ES, Yoo YK, Park YH, Lee JE, Jang IJ, Lee DH, Lee JS. Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol. 2006;24:2237–2244. doi: 10.1200/JCO.2005.03.0239. [DOI] [PubMed] [Google Scholar]
- 30.D’Silva S, Colah RB, Ghosh K, Mukherjee MB. UDP-glucuronosyltransferase 1A1 (UGT1A1) gene haplotypes and their effect on serum bilirubin concentration in healthy Indian adults. Gene. 2013;513:36–39. doi: 10.1016/j.gene.2012.10.081. [DOI] [PubMed] [Google Scholar]
- 31.Teh LK, Hashim H, Zakaria ZA, Salleh MZ. Polymorphisms of UGT1A1*6, UGT1A1*27 & UGT1A1*28 in three major ethnic groups from Malaysia. Indian J Med Res. 2012;136:249–259. [PMC free article] [PubMed] [Google Scholar]
- 32.Innocenti F, Kroetz DL, Schuetz E, Dolan ME, Ramírez J, Relling M, Chen P, Das S, Rosner GL, Ratain MJ. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol. 2009;1(27):2604–2614. doi: 10.1200/JCO.2008.20.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamanaka H, Nakajima M, Katoh M, Hara Y, Tachibana O, Yamashita J, McLeod HL, Yokoi T. A novel polymorphism in the promoter region of human UGT1A9 gene (UGT1A9*22) and its effects on the transcriptional activity. Pharmacogenetics. 2004;14:329–332. doi: 10.1097/00008571-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Fujita K, Ando Y, Nagashima F, Yamamoto W, Eodo H, Araki K, Kodama K, Miya T, Narabayashi M, Sasaki Y. Genetic linkage of UGT1A7 and UGT1A9 polymorphisms to UGT1A1*6 is associated with reduced activity for SN-38 in Japanese patients with cancer. Cancer Chemother Pharmacol. 2007;60:515–522. doi: 10.1007/s00280-006-0396-1. [DOI] [PubMed] [Google Scholar]
- 35.Carlini LE, Meropol NJ, Bever J, Andria ML, Hill T, Gold P, Rogatko A, Wang H, Blanchard RL. UGT1A7 and UGT1A9 polymorphisms predict response and toxicity in colorectal cancer patients treated with capecitabine/irinotecan. Clin Cancer Res. 2005;11:1226–1236. [PubMed] [Google Scholar]
- 36.Köhle C, Möhrle B, Münzel PA, Schwab M, Wernet D, Badary OA, Bock KW. Frequent co-occurrence of the TATA box mutation associated with Gilbert’s syndrome (UGT1A1*28) with other polymorphisms of the UDP-glucuronosyltransferase-1 locus (UGT1A6*2 and UGT1A7*3) in Caucasians and Egyptians. Biochem Pharmacol. 2003;65:1521–1527. doi: 10.1016/S0006-2952(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 37.Araki J, Kobayashi Y, Iwasa M, Urawa N, Gabazza EC, Taguchi O, Kaito M, Adachi Y. Polymorphism of UDP-glucuronosyltransferase 1A7 gene: a possible new risk factor for lung cancer. Eur J Cancer. 2005;41:2360–2365. doi: 10.1016/j.ejca.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 38.Huang MJ, Yang SS, Lin MS, Huang CS. Polymorphisms of uridine-diphosphoglucuronosyltransferase 1A7 gene in Taiwan Chinese. World J Gastroenterol. 2005;11:797–802. doi: 10.3748/wjg.v11.i6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang KS, Chiu HF, Chen HH, Eng HL, Tsai CJ, Teng HC, Huang CS. Link between colorectal cancer and polymorphisms in the uridine-diphosphoglucuronosyltransferase 1A7 and 1A1 genes. World J Gastroenterol. 2005;11:3250–3254. doi: 10.3748/wjg.v11.i21.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma R, Ahuja M, Panda NK, Khullar M. Interactions among genetic variants in tobacco metabolizing genes and smoking are associated with head and neck cancer susceptibility in North Indians. DNA Cell Biol. 2011;30:611–616. doi: 10.1089/dna.2010.1184. [DOI] [PubMed] [Google Scholar]
- 41.Zheng Z, Park JY, Guillemette C, Schantz SP, Lazarus P. Tobacco carcinogen-detoxifying enzyme UGT1A7 and its association with orolaryngeal cancer risk. J Natl Cancer Inst. 2001;93:1411–1418. doi: 10.1093/jnci/93.18.1411. [DOI] [PubMed] [Google Scholar]
- 42.Strassburg CP, Strassburg A, Nguyen N, Li Q, Manns MP, Tukey RH. Regulation and function of family 1 and family 2 UDP-glucuronosyltransferase genes (UGT1A, UGT2B) in human oesophagus. Biochem J. 1999;338:489–498. doi: 10.1042/0264-6021:3380489. [DOI] [PMC free article] [PubMed] [Google Scholar]


