Summary
Eating behaviors are highly cue-dependent. Changes in mood states and exposure to palatable food both increase craving and consumption of food. Vagal activity supports adaptive modulation of physiological arousal and has an important role in cue-induced appetitive behaviors. Using high-frequency heart rate variability (HF HRV), this preliminary study compared vagal activity during positive and negative mood induction, and presentation of preferred high-calorie food items between obese (n = 12; BMI ≥ 30) and non-obese individuals (n = 14; 18.5 < BMI < 30). Participants completed two laboratory sessions (negative vs. positive mood conditions). Following 3-hours of food deprivation, all participants completed a mood induction, and then were exposed to their preferred high-calorie food items. HF HRV was assessed throughout. Obese and non-obese individuals were not significantly different in HF HRV during positive or negative mood induction. Obese individuals showed significantly greater levels of HF HRV during presentation of their preferred high-calorie food items than non-obese individuals, particularly in the positive mood condition. This is the first study to demonstrate increased vagal activity in response to food cues in obese individuals compared with non-obese individuals. Our findings warrant further investigation on the potential role of vagally-mediated cue reactivity in overeating and obesity.
Keywords: Obesity, Cue Reactivity, Mood, Heart Rate Variability, Vagal Activity
Introduction
The current global obesity epidemic has been attributed partly to overconsumption of palatable, high-calorie foods [1]. Eating behaviors are known to be highly cue-dependent [2]. Human laboratory studies have demonstrated enhanced consumption of high-calorie foods in response to internal cues, such as psychological stress [3–7] or changes in negative and positive mood states [8–11]. Additional studies have examined the effect of stress on the wanting and liking values of foods [12–15]. Only a few experimental studies have compared the effects of stress or mood induction on eating behaviors by obesity status, and these studies have reported mixed findings [11, 16]. Exposure to palatable food cues may also increase food craving [17, 18], motivation to eat [19], and subsequent food intake [20], with some of these effects being stronger in obese individuals than lean individuals. Thus, cue-induced overeating of palatable foods may importantly contribute to obesity.
Neuroimaging studies have suggested altered central regulation of cue reactivity in obesity. In response to food cues and stress, obese individuals showed greater activation of brain regions involved in reward and motivation, compared with lean individuals [20–22]. In addition to central regulation of arousal response, autonomic regulation of physiological arousal responses also plays an important role in cue-induced appetitive behaviors [23, 24]. In coordination with the sympathetic nervous system, vagal activity (i.e., parasympathetic nervous activity) flexibly responds to internal and external stimuli partly through changes in heart rate [24], which is important for adaptive regulation of affective and cognitive states [25–28].
Animal models of diet-induced obesity reported blunted decreases in vagal activity in response to stress [29, 30]. Only a few human studies compared vagal activity to a stressor in obesity. One study reported that a greater body mass index (BMI) was associated with reduced high frequency heart rate variability (HF HRV) reactivity to a mental stressor [31], while another study did not find significant differences in HF HRV reactivity to a physical stressor by obesity status [32]. Thus, evidence for vagal regulation of physiological arousal in obesity is equivocal. It is also unknown whether vagal response to changes in mood states differs by obesity status. Modifying a well-established laboratory model of smoking lapse behaviors [33], our previous study was the first study to demonstrate that obese individuals showed less ability to resist eating (i.e., shorter latency to start eating after preferred food presentation) and increased consumption of high-calorie foods in response to positive mood induction compared with negative mood induction; non-obese individuals, on the other hand, consumed more calories after negative mood induction compared with positive mood induction [11]. When considered along withclinical and empirical obeservations that both positive and negative moods were associated with dieting relapse crisis [34], this supports the importance of positive mood states in overeating and obesity, in addition to negatively-valenced cues. The current study further compared vagal activity during mood induction between obese and non-obese individuals to examine whether vagal modulation of mood state might be altered in obese individuals.
In the current study, we also examined vagal response to highly palatable food cue presentation. Vagal activity in response to food cues has not been studied, but elevated vagal response to cue exposure has been observed in other appetitive behaviors, such as alcohol use disorders. For example, greater vagal activity following alcohol cue exposure has been reported in chronic heavy drinkers, compared with healthy samples [35, 36]. It is possible that obese individuals would also show heightened vagal activity in response to highly palatable food cues, compared with non-obese individuals.
HRV, changes in the time between beat-to-beat intervals, has been used to measure the influence of autonomic activity on the heart [37, 38]. The high frequency range (HF HRV; 0.15–0.4 Hz) of the power spectrum reflects beat-to-beat activity controlled by the vagus nerve, as well as phase variation in vagal effects on the heart associated with respiration [37, 38]. This preliminary study aimed to examine: 1) whether HF HRV during negative and positive mood inductions differed between obese and non-obese individuals, and 2) whether HF HRV activity during mood-primed food presentation differed between obese and non-obese individuals. It was hypothesized that obese individuals would show attenuated decreases in HF HRV (i.e., less decrease in vagal activity) after negative mood induction and greater HF HRV in response to food cue presentation, compared with non-obese individuals. We did not have specific hypotheses regarding the effect of positive mood induction on HRV and the effects of mood induction on HF HRV during food cue presentation, due to lack of previous research in relation to obesity.
Methods
Participants
Our sample consisted of 26 participants (mean age = 35.2 ± 13.4 years old; 41 % women, 55% Caucasian) who took part in a study that compared the effects of mood induction on the ability to resist eating high-calorie foods, total calorie consumption, and food cravings by weight status (N = 30) [11] and completed assessment of HRV. Eligible participants had to be between 18 to 65 years of age and have a BMI between 30 and 45 (obese group) or between 18.5 and 29.9 (non-obese group). Exclusion criteria included: current diagnosis of Axis I psychiatric disorders (except nicotine dependence), including anorexia nervosa and/or bulimia nervosa, significant medical conditions including metabolic disorders, and current use of psychotropic or illicit drugs. The majority of participants reported holding a high school degree (95 %) and an annual income of less than $60,000 (71 %).
Procedures
The experimental protocol was approved by the Yale Human Investigation Committee, and the procedures were in compliance with the Declaration of Helsinki for human subjects. Written informed consent was obtained from all the participants (see Udo et al., [11] for the complete description of the study procedures).
Intake assessment and script development sessions
During the intake session, the Structured Clinical Interview for DSM-IV Axis I Psychiatric Disorders (SCID) [39] was used to assess current psychiatric disorders. Participants were screened for metabolic disorders with basic blood chemistry tests.
Eligible participants completed a script development session for a personalized guided imagery procedure for the negative and positive mood inductions [40]. In brief, participants were asked to provide a detailed description about a recent negative mood-inducing experience occurring in the past six months that they perceived as “most stressful’ (negative mood induction), or a personal positive mood-inducing situation, such as sitting at the beach or reading in the park (positive mood induction). Scripts were developed by a PhD-level clinician, audio-taped for presentation, and were approximately five minutes in length [see also 11, 41]. At the end of this session, the participants were asked to provide a list of their preferred high-calorie sweet and salty foods as presentation of personalized cues was a critical component of this laboratory model of eating behaviors [33].
Laboratory sessions
Each participant individually completed two identical 9-hour laboratory sessions (positive mood vs. negative mood session, order counterbalanced). Participants were compensated up to $390 for completing the entire study.
Prior to each laboratory session, participants were instructed not to eat past 10:00 PM the night before the laboratory session. After completing a urine drug screen and baseline assessments at the start of the laboratory session, at 8:00 AM, participants received an equi-caloric breakfast that consisted of a juice box and a granola to control caloric intake and the time since last food consumption. This was followed by three hours of food deprivation. During the food deprivation period, participants were allowed to watch TV and read. At 11:00 AM, participants completed the guided imagery procedure. In brief, the participant listened to a 5-minute script (negative or positive) that was based on the script development session described above and pre-recorded by a research assistant over headphones. Participants were told to imagine the described situation as if it were happening “right now” (see [11, 41] for detailed procedures).
At 11:30 AM, the ad-lib eating period began by presenting the individual’s preferred three choices of high-calorie sweet foods (e.g., cookies, snack cakes, chocolate candy) and three choices of high-calorie salty foods (e.g., potato chips, pretzels, nuts). Snacks were portioned to five servings of each item. One main goal of the original study was to examine differences between obese and non-obese individuals in their ability to resist eating (i.e., latency to start eating) after the mood inductions [11]; therefore, participants were told that they could start eating at any time they wished and eat as much as they wished over the next three hours. Alternatively, for each minute the participants delayed or “resisted” eating, they received monetary rewards ($0.20/min for the first hour, $0.10/min for the second hour, and $0.05/min for the third hour). Parallel to the smoking lapse model [29], monetary reinforcement was a critical component of this model to provide incentive for not eating, and to provide a sensitive test of the relative reinforcing value of highly-palatable foods.
Measures
Heart rate variability
An ambulatory electrocardiography (ECG) monitor (Holter; GE Marquette SEER digital system) was used to record ECG during the session. Recordings were digitally sampled and analyzed using a GE-Marquette system. Tapes were manually reviewed to identify R-R intervals to edit artifacts and irregular beats, and then processed and analyzed with customized software as in prior work [42–44]. Recordings with greater than 20% interpolated segments were excluded from further analysis. The R-R interval time series were sampled using a boxcar window [45] to obtain 1,024 samples per 5 minutes (3.41333 Hz). RRI spectra were calculated through Fourier analysis [46, 47] with a Parzen window, on 4-minute segments with a 1-minute sliding window, corrected for attenuation due to windowing and sampling [48], and integrated over five standard frequency bands [39]. To assess vagal activity, HF HRV was calculated separately for pre-mood induction (averaged over 15 min), mood induction (averaged over 5 min), post-mood induction (averaged over 15 min), before food presentation (averaged over 5 min), and food presentation (averaged over 15 min). To correct skewness and kurtosis, the natural logarithm transformation was used on HF HRV.
Hunger levels
During the experiment, subjective hunger levels were assessed by one item, “how hungry do you feel right now?”, and participants were asked to rate on a 100-mm visual analog scale (VAS).
Statistical analysis
A repeated measures analysis of covariance (ANCOVA) was used to compare levels of HF HRV during mood induction and food presentation between obese and non-obese individuals (between-subject factor) in the negative and positive mood conditions (within-subject factor). In all analyses, levels of HF HRV at pre-mood induction (for mood induction) or at pre-food cue presentation (for food cue presentation) were included as covariates to adjust for individual differences at pre-mood and cue exposure. Age was also controlled in the analyses as it is known to affect HRV [49], and substantially reduced residuals. The results of eating behaviors (i.e., the ability to resist eating and total calorie consumptions) for a larger sample are reported elsewhere [11], thus they are not included in this study. Due to the pilot nature of the study, no corrections for multiple comparisons were made.
Results
Participant characteristics
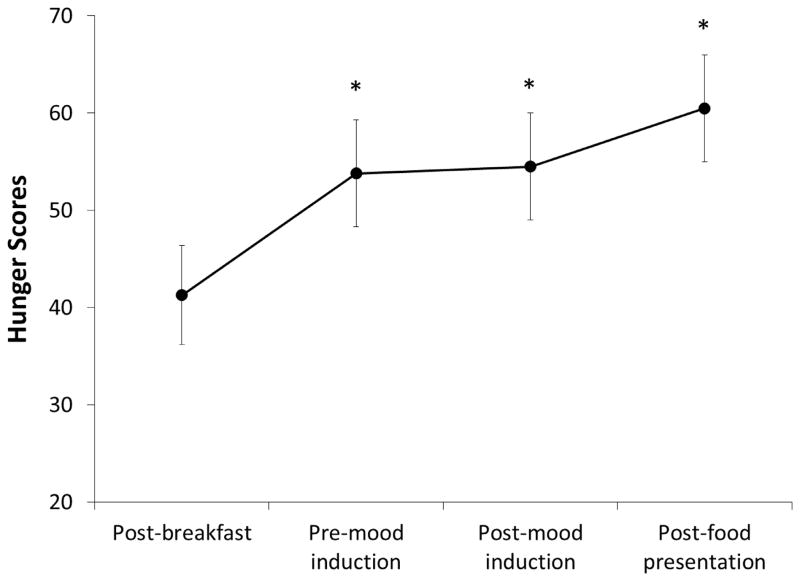
Table 1 summarizes the baseline sample characteristics. Except for BMI, there were no significant differences in sociodemographic characteristics between obese and non-obese individuals. There were no significant differences in pre-mood induction HF HRV by obesity status or session order for either mood induction session. Regardless of the mood conditions and obesity status, hunger scores significantly increased at pre-mood induction, post-mood induction, and after food presentation, compared with after an equi-caloric breakfast, F(3, 21) = 5.52, p < .05, partial η2 = .44.
Table 1.
Participants Characteristics.
| Obese (n = 12) | Non-obese (n = 14) | |
|---|---|---|
| Age | 40.0 (12.1) | 32.2 (12.2) |
| % female | 45 | 36 |
| Race | ||
| % Caucasian | 56 | 54 |
| % African-American | 44 | 46 |
| BMI | 35.8 (3.8) * | 22.9 (2.0) |
| HF HRV at pre-mood induction 1 | ||
| Negative mood condition | 5.90 (1.41) | 6.35 (0.99) |
| Positive mood condition | 5.90 (1.32) | 5.90 (1.06) |
| HF HRV at post-mood induction 1 | ||
| Negative mood condition | 5.97 (0.17) | 5.93 (0.13) |
| Positive mood condition | 5.93 (0.16) | 5.84 (0.13) |
Notes. Numbers in parentheses indicate standard errors. BMI = body mass index; HF HRV = high frequency heart rate variability (HRV).
natural log-transformation was used for HF HRV.
significantly different by obesity status at p < .05.
Heart rate variability during mood induction
Adjusting for HF HRV at pre-mood induction, there were no significant main or interactive effects of obesity status or mood condition on levels of HF HRV (p > .05; Table 1).
Heart rate variability during food presentation
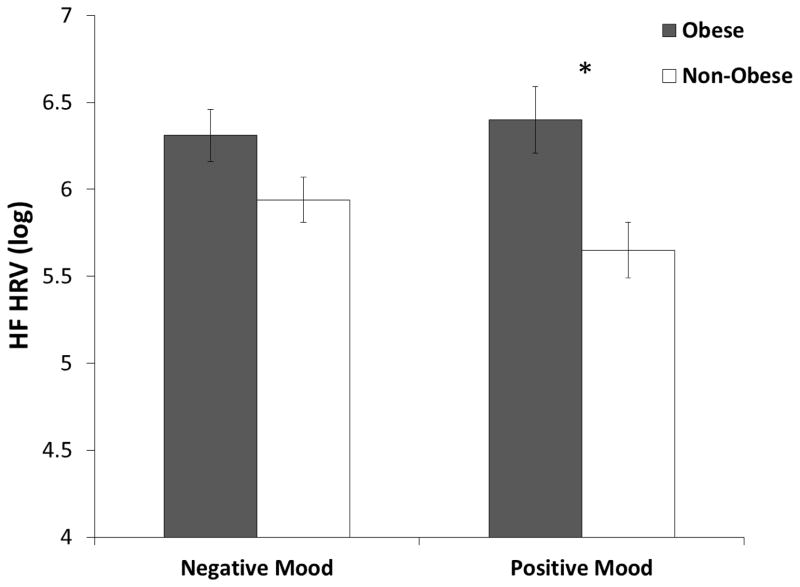
Across the mood conditions, adjusting for vagal activity at pre-food presentation, obese individuals showed significantly greater levels of HF HRV during presentation of their preferred food compared with non-obese individuals, F(1, 16) = 4.67, p < .05, partial η2 = .23. The main effect of mood conditions and interaction of mood conditions with obesity status on levels of HF HRV during food cue presentation was not significant (p > .05).
Given our previous findings that positive and negative mood distinctively changed eating behaviors by obesity status, we conducted a-priori comparisons of HF HRV levels to food cues between obese and non-obese individuals, separately for positive and negative mood condition. In the positive mood condition, obese individuals showed significantly greater levels of HF HRV during their preferred food presentation, compared with non-obese individuals, F(1, 22) = 5.07, p < .05, partial η2 = .21 (Figure 1). In the negative mood condition, obese individuals showed non-significant but greater levels of HF HRV during their preferred food presentation, compared with non-obese individuals, F(1, 22) = 3.29, p = .08, partial η2 = .15.
Figure 1.
Means and standard errors of hunger scores by mood conditions after breakfast, pre-mood induction, post-mood induction, and after food cue presentation. * = Significantly different from post-breakfast assessment at p = .05.
Discussion
This pilot study was the first to compare vagal activity during positive and negative mood inductions and presentation of preferred high-calorie foods between obese and non-obese individuals. Obese individuals showed greater vagal activity to presentation of their favorite high-calorie foods, compared with non-obese individuals, particularly in the positive mood condition. While greater attention has been paid to the link between stress/negative mood, changing eating behaviors, and obesity [e.g., 50], both clinical and experimental studies have also demonstrated the role of positive mood as a cue to induce overeating palatable food [9–11, 34]. One may argue that an equi-caloric meal before a laboratory experiment could have different impacts on energy balance between obese and non-obese individuals, leading to different vagal response to food cues. However, hunger scores, which indicate subjective feeling of energy state, did not differ between obese and non-obese individuals across mood conditions. Future research incorporating changes in ghrelin, an appetite-stimulating gut hormone [51], may help clarify the relationship. The current findings on enhanced vagal activity to palatable food cues under positive mood states add further support for the importance of positive mood in understanding vulnerability to obesity.
Increased vagal activity to food cues in obese individuals corresponds with the cue reactivity studies demonstrating greater cue-elicited vagal activity in alcoholics compared with healthy individuals [36, 52]. Furthermore, in treatment-seeking heavy drinkers, greater cue-elicited HF HRV has been linked with attentional bias towards alcohol cues [53], and was predictive of post-treatment relapse [54]. Thus, heightened cue-elicited vagal activity may be part of shared underlying mechanisms that increase vulnerability to unhealthy appetitive behaviors, including overeating of high-calorie foods.
The present study did not find significant differences in HF HRV between obese and non-obese individuals following either positive or negative mood induction. Laederach-Hofmann et al. [31] reported altered vagal activity to a mental stressor in obese individuals, compared with lean individuals. However, this study did not exclude those with other psychological and medical conditions that could influence HRV (e.g., depression, diabetes). A study that similarly focused on obese individuals without serious medical conditions also did not find altered vagal activity to a cold stressor [32]. Personalized positive and negative mood-inducing cues, rather than generic mental or physical stressors, might have also contributed to inconsistent findings. Further research is needed to clarify the relationships between vagal activity, and stress and mood dysregulation in obese individuals.
As this investigation was a pilot study, the sample size was relatively small, but was similar to previous studies [31, 32], and robust effects were demonstrated. Replication with a larger sample will be important, with the aims extended to examine the relationship between vagal activity and eating behaviors. In particular, whether observed differential changes in vagal response to food presentation after mood induction in obese individuals may be associated with the differences in eating behaviors seen in our prior study is an important avenue of future research in a larger sample. The present study did not assess respiration, and thus the influence of respiration on vagal traffic was not controlled. However, respiration rate does not have a strong influence on HF HRV response to experimental manipulations irrespective of whether the breathing was controlled or spontaneous [e.g., 55, 56, 57]. Therefore, the significance of our findings should not be affected by the lack of control for respiration. In addition, the study participants consisted of a wide range of age, which could affect HF HRV [49]. Since the analyses were adjusted for baseline HF HRV, absolute baseline differences based on age would not be expected to influence the results. Finally, the current study did not have a neutral mood condition or a control condition for food cue presentation. Inclusion of such reference conditions may refine our understanding of the specificity of the relationship between mood states and vagal reactivity to cue presentation.
Despite the limitations, this was the first study to demonstrate heightened vagal activity during presentation of preferred high-calorie food in obese individuals. Given the role of the vagus nerve in satiety signaling [58, 59], a comprehensive evaluation of concurrent changes between cardiac vagal activity and appetite-regulating peptides, along with subjective report on mood and appetite, may help us better understand the mechanistic pathways by which reactivity to food cue presentation affects eating behaviors in obese individuals. In conclusion, our findings highlight that food cue-elicited vagal activity, in addition to changes in internal mood states, may be important for consumption of high-calorie, palatable foods in obese individuals.
Figure 2.
Level of HF HRV (log) during preferred food presentation by obesity status in negative mood and positive mood conditions. HF HRV at pre-food presentation and age were controlled as covariates. * = Significantly different between obese and non-obese individuals at p < .05.
Acknowledgments
This study was supported by the NIDA grants RL1DA024857 and K12DA031050, an Interdisciplinary Research Education Grant (RL5DA024858, PI: Mazure); and CTSA grant UL1RR024139. The content is solely the responsibility of the authors, and the National Institute on Drug Abuse or the National Institutes of Health had no role other than financial support.
Footnotes
Authors have no conflict of interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pandit R, de Jong JW, Vanderschuren LJ, Adan RA. Neurobiology of overeating and obesity: the role of melanocortins and beyond. European Journal of Pharmacology. 2011;660(1):28–42. doi: 10.1016/j.ejphar.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 2.Wardle J. Conditioning processes and cue exposure in the modification of excessive eating. Addictive Behaviors. 1990;15(4):387–93. doi: 10.1016/0306-4603(90)90047-2. [DOI] [PubMed] [Google Scholar]
- 3.Habhab S, Sheldon JP, Loeb RC. The relationship between stress, dietary restraint, and food preferences in women. Appetite. 2009;52(2):437–44. doi: 10.1016/j.appet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Lattimore P, Caswell N. Differential effects of active and passive stress on food intake in restrained and unrestrained eaters. Appetite. 2004;42(2):167–73. doi: 10.1016/j.appet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosomatic Medicine. 2000;62(6):853–65. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Wallis DJ, Hetherington MM. Emotions and eating. Self-reported and experimentally induced changes in food intake under stress. Appetite. 2009;52(2):355–62. doi: 10.1016/j.appet.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Wallis DJ, Hetherington MM. Stress and eating: the effects of ego-threat and cognitive demand on food intake in restrained and emotional eaters. Appetite. 2004;43(1):39–46. doi: 10.1016/j.appet.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Schotte DE, Cools J, McNally RJ. Film-induced negative affect triggers overeating in restrained eaters. Journal of Abnormal Psychology. 1990;99(3):317–20. doi: 10.1037//0021-843x.99.3.317. [DOI] [PubMed] [Google Scholar]
- 9.Cools J, Schotte DE, McNally RJ. Emotional arousal and overeating in restrained eaters. Journal of Abnormal Psychology. 1992;101(2):348–51. doi: 10.1037//0021-843x.101.2.348. [DOI] [PubMed] [Google Scholar]
- 10.Yeomans MR, Coughlan E. Mood-induced eating. Interactive effects of restraint and tendency to overeat. Appetite. 2009;52(2):290–8. doi: 10.1016/j.appet.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Udo T, Grilo CM, Brownell KD, Weinberger AH, DiLeone RJ, McKee SA. Modeling the effects of positive and negative mood on the ability to resist eating in obese and non-obese individuals. Eating Behaviors. 2013;14(1):40–6. doi: 10.1016/j.eatbeh.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Acute stress-related changes in eating in the absence of hunger. Obesity. 2009;17(1):72–7. doi: 10.1038/oby.2008.493. [DOI] [PubMed] [Google Scholar]
- 13.Lemmens SG, Rutters F, Born JM, Westerterp-Plantenga MS. Stress augments food ‘wanting’ and energy intake in visceral overweight subjects in the absence of hunger. Physiology & Behavior. 2011;103(2):157–63. doi: 10.1016/j.physbeh.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Finlayson G, King N, Blundell J. The role of implicit wanting in relation to explicit liking and wanting for food: implications for appetite control. Appetite. 2008;50(1):120–7. doi: 10.1016/j.appet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Finlayson G, King N, Blundell JE. Is it possible to dissociate ‘liking’ and ‘wanting’ for foods in humans? A novel experimental procedure. Physiology & Behavior. 2007;90(1):36–42. doi: 10.1016/j.physbeh.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Appelhans BM, Pagoto SL, Peters EN, Spring BJ. HPA axis response to stress predicts short-term snack intake in obese women. Appetite. 2010;54(1):217–220. doi: 10.1016/j.appet.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: A fresh approach to the study of binge eating. Appetite. 2005;44(3):253–61. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Ferriday D, Brunstrom JM. How does food-cue exposure lead to larger meal sizes? British Journal of Nutrition. 2008;100(6):1325–32. doi: 10.1017/S0007114508978296. [DOI] [PubMed] [Google Scholar]
- 19.Ferriday D, Brunstrom JM. ‘I just can’t help myself’: effects of food-cue exposure in overweight and lean individuals. International Journal of Obesity. 2011;35(1):142–9. doi: 10.1038/ijo.2010.117. [DOI] [PubMed] [Google Scholar]
- 20.Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN. Neural correlates of stress- and food cue-induced food craving in obesity: Association with insulin levels. Diabetes Care. 2013;36(2):394–402. doi: 10.2337/dc12-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, 3rd, Horwitz B. Effective connectivity of a reward network in obese women. Brain Research Bulletin. 2009;79(6):388–95. doi: 10.1016/j.brainresbull.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, et al. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One. 2012;7(2):e31089. doi: 10.1371/journal.pone.0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–40. [PubMed] [Google Scholar]
- 24.Tiffany ST. Potential functions of classical conditioning in drug addiction. In: Drummond DC, Tiffany ST, Glautier S, Remington B, editors. Addictive Behaviour: Cue Exposure Theory and Practice. Oxford, England: John Wiley & Sons; 1995. pp. 47–71. [Google Scholar]
- 25.Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Review of General Psychology. 2006;10(3):229–40. [Google Scholar]
- 26.Elliot AJ, Payen V, Brisswalter J, Cury F, Thayer JF. A subtle threat cue, heart rate variability, and cognitive performance. Psychophysiology. 2011;48:1340–5. doi: 10.1111/j.1469-8986.2011.01216.x. [DOI] [PubMed] [Google Scholar]
- 27.Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37:141–53. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 28.Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Wazir YM, Li SG, Smith R, Silcox DL, Brown DR, Randall DC. Parasympathetic response to acute stress is attenuated in young Zucker obese rats. Autonomic Neuroscience. 2008;143(1–2):33–9. doi: 10.1016/j.autneu.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levin BE, Richard D, Michel C, Servatius R. Differential stress responsivity in diet-induced obese and resistant rats. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2000;279(4):R1357–64. doi: 10.1152/ajpregu.2000.279.4.R1357. [DOI] [PubMed] [Google Scholar]
- 31.Laederach-Hofmann K, Mussgay L, Ruddel H. Autonomic cardiovascular regulation in obesity. Journal of Endocrinology. 2000;164(1):59–66. doi: 10.1677/joe.0.1640059. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto T, Miyawaki T, Ue H, Kanda T, Zenji C, Moritani T. Autonomic responsiveness to acute cold exposure in obese and non-obese young women. International Journal of Obesity and Related Metabolic Disorders. 1999;23(8):793–800. doi: 10.1038/sj.ijo.0800928. [DOI] [PubMed] [Google Scholar]
- 33.McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology. 2009;14(1):99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grilo CM, Shiffman S, Wing RR. Relapse crises and coping among dieters. Journal of Consulting and Clinical Psychology. 1989;57:488–495. doi: 10.1037//0022-006x.57.4.488. [DOI] [PubMed] [Google Scholar]
- 35.Ingjaldsson JT, Thayer JF, Laberg JC. Craving for alcohol and pre-attentive processing of alcohol stimuli. International Journal of Psychophysiology. 2003;49:29–39. doi: 10.1016/s0167-8760(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 36.Rajan I, Murthy PJNV, Ramakrishnan AG, Gangadhar BN, Janakiramaiah N. Heart rate variability as an index of cue reactivity in alcoholics. Biological Psychiatry. 1998;43(7):544–6. doi: 10.1016/s0006-3223(97)00399-5. [DOI] [PubMed] [Google Scholar]
- 37.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 38.Task Force. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. European Heart Journal. 1996;17:354–81. [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV, Patient Edition. Washington, D. C: American Psychiatric Press; 1995. [Google Scholar]
- 40.Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction Biology. 2009;14(1):84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of Psychopharmacology. 2011;25(4):490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashare RL, Sinha R, Lampert R, Weinberger AH, Anderson GM, Lavery ME, et al. Blunted vagal reactivity predicts stress-precipitated tobacco smoking. Psychopharmacology. 2011;220(2) doi: 10.1007/s00213-011-2473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85(1):164–71. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 44.Lampert R, Ickovics J, Horwitz R, Lee F. Depressed autonomic nervous system function in African Americans and individuals of lower social class: a potential mechanism of race- and class-related disparities in health outcomes. American Heart Journal. 2005;150(1):153–60. doi: 10.1016/j.ahj.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Berger RD, Akselrod S, Gordon D, Cohen RJ. An efficient algorithm for spectral analysis of heart rate variability. IEEE Transactions on Bio-Medical Engineering. 1986;33(9):900–4. doi: 10.1109/TBME.1986.325789. [DOI] [PubMed] [Google Scholar]
- 46.Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. Journal of Physiology. 1999;517 (Pt 2):617–28. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98(6):547–55. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- 48.Hamming R. Numerical Methods for Scientists and Engineers. 2. New York: Dover Publications, Inc; 1973. [Google Scholar]
- 49.Ryan SM, Goldberger AL, Pincus SM, Mietus J, Lipsitz LA. Gender- and age-related differences in heart rate dynamics: are women more complex than men? Journal of the American College of Cardiology. 1994;24(7):1700–7. doi: 10.1016/0735-1097(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 50.Greeno CG, Wing RR. Stress-induced eating. Psychological Bulletin. 1994;115(3):444–464. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- 51.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 52.Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: Relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54(12):1427–36. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- 53.Garland EL, Franken IH, Sheetz JJ, Howard MO. Alcohol attentional bias is associated With autonomic indices of stress-primed alcohol cue-reactivity in alcohol-dependent patients. Experimental and Clinical Psychopharmacology. 2012;20(3):225–35. doi: 10.1037/a0027199. [DOI] [PubMed] [Google Scholar]
- 54.Garland EL, Franken IH, Howard MO. Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernardi L, Wdowczyk-Szulc J, Valenti C, Castoldi S, Passino C, Spadacini G, et al. Effects of controlled breathing, mental activity and mental stress with or without verbalization on heart rate variability. Journal of the American College of Cardiology. 2000;35(6):1462–9. doi: 10.1016/s0735-1097(00)00595-7. [DOI] [PubMed] [Google Scholar]
- 56.Madden K, Savard GK. Effects of mental state on heart rate and blood pressure variability in men and women. Clinical Physiology. 1995;15(6):557–69. doi: 10.1111/j.1475-097x.1995.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 57.Pagani M, Mazzuero G, Ferrari A, Liberati D, Cerutti S, Vaitl D, et al. Sympathovagal interaction during mental stress. A study using spectral analysis of heart rate variability in healthy control subjects and patients with a prior myocardial infarction. Circulation. 1991;83(4 Suppl):II43–51. [PubMed] [Google Scholar]
- 58.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterology and Motility. 2008;20:64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owyang C, Heldsinger A. Vagal control of satiety and hormonal regulation of appetite. Journal of Neurogastroenterology and Motility. 2011;17:338–348. doi: 10.5056/jnm.2011.17.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]