Abstract
Leukemias and other cancers have been proposed to contain a subpopulation of cells that display characteristics of stem cells, and which maintain tumor growth. That most anti-cancer therapy is directed against the bulk of the tumor, and possibly spares the cancer stem cells, may lie at the heart of treatment failures with conventional modalities. Leukemia stem cells are fairly well described for acute myeloid leukemia (AML), but their existence and relevance for acute lymphoblastic leukemia (ALL) is less clear. Several reports describe subpopulations with primitive phenotypes in clinical ALL samples. However, it has also been suggested that the majority of leukemic subfractions can propagate leukemia in the appropriate experimental setting, and that their hierarchical organization is less strict than in AML. In addition, it is uncertain whether cancer stem cells arise from malignant transformation of a tissue-specific stem cell, or from committed progenitors or differentiated cells that re-acquire a stem cell-like program. In common childhood ALL, current evidence points towards the cell of origin being a committed lymphoid progenitor. In this review, we highlight recent findings relating to the question of leukemia stem cells in ALL.
History of the Cancer Stem Cell Hypothesis
It has been known for decades that the malignant cells within a tumor can show differences in morphology, resemble different stages in maturation of the tissue of origin, and have different proliferative potential. Over the past few years, it has been recognized that many tumors contain a particular subpopulation of cells with biological features that are reminiscent of stem cells. The modern concept of the “cancer stem cell” or “cancer initiating cell” was promoted by the work of John Dick and colleagues 1, 2, who showed that cells with the ability to transfer human AML to NOD/SCID mice are frequently found exclusively in the CD34+, CD38− compartment, and they can be subdivided into more or less primitive subpopulations that are organized in a hierarchy that resembles that of the normal hematopoietic system. Since then, an ever expanding list of solid tumors has been reported to contain a cancer stem cell subpopulation: breast cancer 3, ovarian cancer, pancreatic cancer 4, colon cancer 5, 6, 7, 8, prostate cancer9, hepatocellular carcinoma10, 11, 12, melanoma13, 14, lung cancer15, glioblastoma16, other brain tumors17, and neuroblastoma18. Proof of the existence of a rare stem cell-like population maintaining acute lymphoblastic leukemia (ALL) has been more elusive. This review will discuss current evidence for and against an ALL stem cell.
Biological and Experimental Definition of Cancer Stem Cells
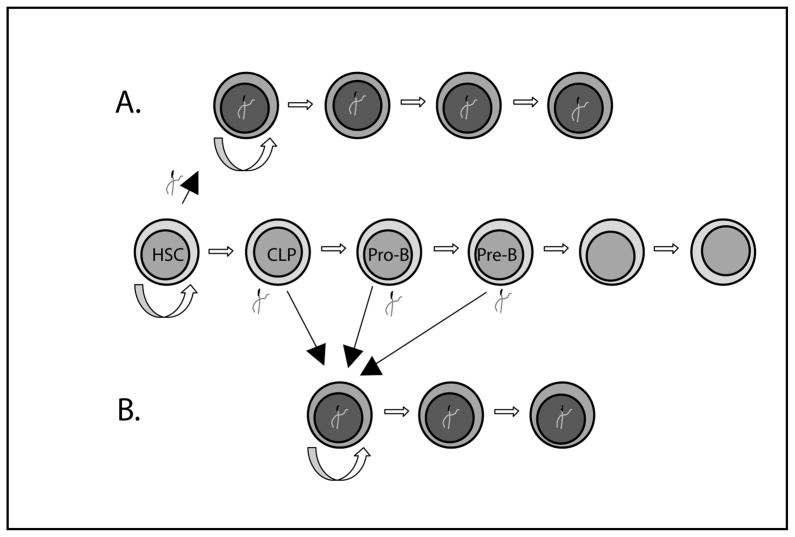
Cancer stem cells have been biologically defined as a rare subpopulation within a tumor that exhibits biological characteristics similar to those of non-malignant stem cells. In this model, the cancer stem cell is able to self-renew and to give rise to more differentiated progeny that includes every (malignant) cell type within the tumor. While these features are reminiscent of the characteristics of stem cells, they do not necessarily imply that all cancers initially arose from a stem cell (Figure 1A). Rather, it is possible that progenitors, or even terminally differentiated cells, acquire earlier, stem-cell-like programs that reactivate their ability to self-renew and sustain the growth of the tumor (Figure 1B). There has been considerable semantic confusion with regards to the definition of the cancer stem cell, and the cell in which the initial molecular event leading to malignant transformation occurred. For this review, we will call the cell from which the tumor arose the “cell of origin”.
Fig. 1. Possible cells of origin in different leukemia stem cell models.
A. A genetic event occurring in a stem cell leads to malignant transformation. The stem cell possesses (and contributes) the ability to self renew. The leukemic cells continue to develop along the lymphoid lineage and arrest at the pro or pre-B stage. B. A genetic event occurs in a committed progenitor (CLP, pro-B or pre-B cell), leading to a stem cell like genetic program. The leukemia cells develop further and arrest at a pro or pre-B cell stage.
There also has been debate on how to define a cancer stem cell experimentally. A 2006 AACR work-shop addressed this issue and defined guidelines for the minimal experimental evidence that should be obtained before calling a specific subpopulation a “cancer stem cell”19. An important part of the definition is the ability to define a unique population(s) of cells that can initiate tumors in immunocompromised mice. Still, the validity of this assay is not undisputed20,21. Central to concerns regarding this assay is that the frequency of tumor-initiating cells also heavily depends on the mouse strain, the degree of immune compromise20, and whether recipient mice were sublethally irradiated. In addition, expansion in semi-solid media prior to injection22, co-injection with irradiated supporting tumor cells23, injection into special sites (intrafemoral in the case of leukemias24), as well as cell loss and other aspects related to the flow cytometric isolation of different subfractions25 all appear to significantly influence the frequency of tumor initiating cells.
The Search for the Leukemia Stem Cell and the Cell of Origin in ALL
ALL defines a group of leukemias that express predominantly lymphoid cell surface markers and most often have rearrangements of immunoglobulin or T-cell receptor genes confirming commitment to the lymphoid lineage. Cytogenetic abnormalities and biological behavior differ widely, and the prognosis ranges from excellent (hyperdiploid, TEL/AML rearrangement) to dismal (BCR/ABL translocation, MLL rearrangement). Published reports assessing whether there is a cancer stem cell like subpopulation in ALL, and what the cell of origin is, have been contradictory. Some of the discrepancies may be related to the heterogeneity of ALL itself. It is possible that the marked differences in response to therapy are related to the different biologic characteristics of the cell of origin, and on the existence and relative importance of a primitive stem cell like population for a given ALL subtype. Therefore we will discuss the current state of evidence for and against the existence and biological relevance of a putative ALL stem cell by subtype.
TEL/AML [ETV6/RUNX1, t(12;21)]
TEL/AML rearranged ALL is among the best studied subtypes of ALL. Multiple investigational teams have performed flow cytometric evaluation of different B-cell precursor and more primitive hematopoietic compartments in patient samples, and analyzed the frequency of TEL/AML1+ cells as well as the frequency of cells capable of establishing leukemia in SCID mouse models. Figure 2 shows the normal differentiation of B-lineage lymphocytes. Very early, TEL/AML1 positive CD34+ cells that carried no surface markers of lymphocytic differentiation (CD19 or CD10) cells were reported by several groups26,27, and Cox et al.26 described reliable (and exclusive) leukemic engraftment of CD34+CD19− or CD34+CD10− population. Hotfilder et al.27 found the frequency of FISH positive cells in the CD34+CD19− population to be low, potentially attributable to contaminating cells.
Fig. 2.
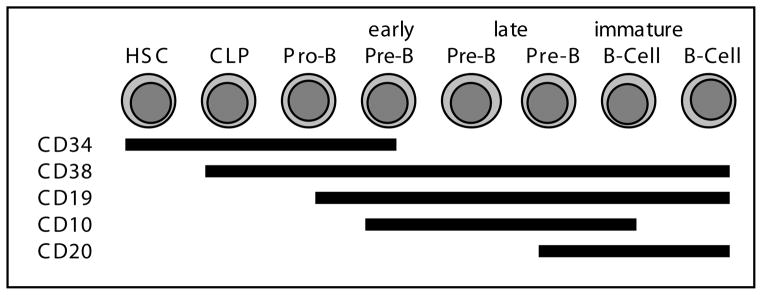
Schematic demonstrating expression of cell surface antigens that can be used to characterize stages of B-lymphoid differentiation.
The earliest population that consistently, across experiments confered leukemia to immunocompromised mice has been defined as CD34+, CD38−, and CD19+24, 28, 27. Le Viseur et al.24 also reported engraftment of a more differentiated, CD34−CD19+ subpopulation at robust frequencies in one patient sample. Both fractions gave rise to CD34+CD19+ and CD34− CD19+ cells, questioning the existence of a strict hierarchy in this type of ALL (Figure 3D).
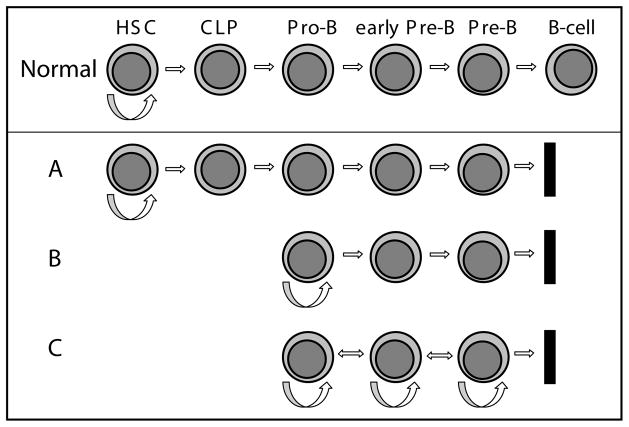
Fig. 3. Hierarchy and differentiation in different leukemia stem cell models.
Normal Differentiation toward the B-cell lineage is shown. A. Leukemia sustained by a very early leukemia stem cell (similar to a hematopoietic stem cell or very early progenitor). The leukemic cells continue to develop along the lymphoid lineage and arrest at the pre-B stage.
B. Leukemia sustained by a leukemia stem cell at the developmental stage of a committed progenitor (here a pro-B cell), with further differentiation of its progeny along the lymphoid lineage, and arrest at the pre-B stage. C. Leukemia maintained by “leukemia stem cells” corresponding to different stages in early B-cell development. Multiple subpopulations possess the ability to self-renew and propagate the leukemia.
TEL/AML is alone not sufficient to cause leukemic transformation in human hematopoietic stem or progenitor cells. The frequency of TEL/AML in healthy infants far exceeds the incidence of ALL, suggesting that normal individuals may have B-cells harboring a TEL/AML fusion that never undergo full leukemic transformation29. These findings have led to the development of a “multi-hit” model of leukemogenesis, in which TEL/AML + precursor cells constitute a preleukemic pool, and leukemia develops if a cell of the pool acquires other genetic events. Examples of the second event could be small point mutations such as flt-3 activating mutations30–32, or deletions or duplications of larger genomic segments 33,34. Based on this model, the concept of a pre-leukemic stem cell has recently been proposed. Hong et al. 35 investigated a pair of twins who both had evidence of a small, pre-leukemic pool of TEL/AML positive CD34+CD38−CD19+ cells detectable in their peripheral blood. One twin developed ALL, the other twin remained healthy while maintaining an abnormal, CD19+, TEL/AML +, pre-leukemic population. This preleukemic population showed evidence of self-renewal and hierarchical differentiation: only the preleukemic CD34+CD38−CD19+, not an also detectable CD34+CD38+CD19+, population, engrafted in primary and secondary recipients and gave rise to both populations. The preleukemic clone carried a DJ immunoglobulin rearrangement only, while the leukemic clone carried the identical DJ rearrangement (except for one point mutation), but had also rearranged the V-segment. The authors concluded that there is a “pre-leukemic stem cell” showing characteristics of self renewal and differentiation, and that the cell that established ALL had continued to develop further along the B-cell lineage when the presumed “second hit” occurred. Thus, in TEL/AML1 ALLs there is evidence for stem cell like properties in cells possessing both immature and more mature B-cell immunophenotypes.
Hyperdiploid ALL
Quijano et al.36 reported leukemic cells in the CD34+, CD 38−, CD19− fraction in 5 out of 19 hyperdiploid ALL samples by florescence in situ hybridization (FISH). Le Viseur et al.24 included two hyperdiploid patient samples in their study; one patient was later upgraded to high risk based on high minimal residual disease (MRD) which demonstrated that this was an unusual hyperdiploid ALL case. Blasts were sorted into an earlier, CD19+ CD20−/low, and a later, CD19+CD20 high fraction, both of which were engrafted in NOD/scid/Il2Rnull mice. While the data are not extensive for this subtype of ALL, it appears some cases of hyperdiploid ALL have leukemia initiating activity in populations, consistent with later mid-stage B-cell development.
Normal Karyotype
ALL samples with normal karyotype were included in the studies of Cox et al.26. Transplantation of sorted cedll fractions into sublethally irradiated NOD/SCID mice showed results comparable to their results for TEL/AML1 ALL. Only CD34+CD10− or CD34+CD19− cells resulted in reliable leukemic engraftment. Considering the range of results for TEL/AML1+ ALL reported by different investigators, it is unclear if this finding reflects a fundamentally different biology for this subtype of ALL, or rather technical differences among laboratories.
BCR/ABL [t(9;22), Ph+ ALL]
In contrast to hyperdiploid or TEL/AML+ categories of ALL, involvement of the myeloid compartment has been reported for BCR/ABL t(9;22) ALL 37. The Philadelphia chromosome could be detected in both mature myeloid cells and myeloid colony forming units, suggesting that at least in some patients the leukemia-initiating event occurs in a primitive cell that has not yet undergone lineage commitment 38. An alternative explanation would be that BCR/ABL ALL blasts re-acquire specific lineage promiscuity.
Castor et al.28 also analyzed the CD34+CD38−CD19 positive and negative compartment in t(9;22) ALL. They report no involvement (defined by FISH) of the CD34+CD38−CD19− compartment in 4 patients with P190 BCR/ABL ALL, but extensive involvement in 5 patients with P210 BCR/ABL ALL. The P210 BCR/ABL transcript could also be identified in CD34+CD33+ and CD34−CD33+ myeloid precursors, which was not the case for the P190 transcript. It is not clear from these data whether cells re-acquired their more primitive immune phenotype, or the more likely alternative explanation of the leukemia-initiating event occurring in an earlier cell of origin. Although CD34+CD38−CD19− P210 positive cells could be identified, they did not induce leukemia in NOD-SCID mice. This negative finding contrasts with the results of Cobaleda et al.39, who found NOD-SCID engrafting-leukemic cells only in the CD34+CD38−, not the CD34+ CD38+ subfraction (all P190).
George et al.40 reported leukemic cells (as assayed by T-cell receptor rearrangement) in the CD34+, CD38−, CD19− fraction in remission marrows of1 out of 3 children with t(9;22) ALL40.
Hotfilder et al.41 found a high frequency of FISH-positive in the CD34+CD19− compartment of 8 patients with BCR/ABL positive ALL, ranging from 14 to 95 percent, and detectable in all samples analyzed. [Au: not clear what “FISH-positivity” means in preceding sentence] Analysis of the P190 versus 210 transcript was not done, but as the P190 is the more common transcript in Ph+ ALL, likely several of the samples would contain this breakpoint. Despite the large number of FISH-positive cells in the CD34+CD19− fraction, only very few BCR/ABL+ myeloid colonies could be isolated. Furthermore, the fraction of FISH-positive cells within these colonies was very low, suggesting that they did not arise from a single BCR/ABL+ multipotent or myeloid committed cell, but rather from contaminating leukemic cells within these colonies. These results suggest that Ph+ALL is maintained by a cell that, while able to assume a very primitive (CD34+CD19−) immunophenotype, has likely committed to the lymphoid lineage.
MLL rearrangement: MLL-AF4 t(4;11), MLL-ENL t(11;19) and others
Coexpression of myeloid markers is a very common finding in this type of ALL, and several translocations involving MLL can be found in both AML and ALL, suggesting the possibility that an earlier cell may be involved. Similar to the findings in Ph+ ALL, Hotfilder et al.41 reported MLL-AF4-positive cells in high frequencies in the CD34+CD19− compartment (28 to 93% by FISH in 12/12 patients). However, as in Ph+ALL, these cells carrying an immature phenotype, which suggests a developmental stage prior to commitment to the lymphoid lineage and inability to establish true myeloid colonies in methylcellulose assays.
le Viseur et al.24 analyzed leukemic subpopulations in 4 patients with t(4;11) and one patient with t(11;19). Robust engraftment of the CD34+ CD19−, the CD34+Cd19+ and the CD34−CD19+ subfractions could be obtained in three of the assayed samples. Limiting dilution transplantation allowed the calculation of a frequency of about 1 in 2000 leukemi- initiating cells, which was relatively constant over the three fractions analyzed. These experimental findings suggest that the CD34+CD19− subpopulation, which does not show immunophenotypic characteristics of lymphoid commitment, contains a significant number of cells that are able to initiate lymphoblastic leukemia. Further studies to evaluate whether this immature immune phenotype correlates with developmental potential towards the myeloid lineage were not done, but the phenotype observed in secondary and tertiary recipients continued to be exclusively pre-B-ALL. In contrast to many prior publications, but consistent with these author’s results for other types of ALL, all three subfractions were observed in the recipient mice in proportions that mirrored the original leukemia regardless of which fraction was originally transplanted. Thus preliminarily it appears that MLL-rearranged ALL samples can exhibit stem cell-like characteristics over a range of developmental phenotypes (Figure 3C).
Two recent papers suggest that the microenviroment may play a role in determining lineage fate in MLL rearranged leukemias: Wei et al. reported that retroviral transduction of CD34+ cord blood cells with MLL-AF9 induced leukemic transformation, yielding both ALL and AML in immunocompromised mice. The authors were able to nudge lineage fate decisions by influencing ex vivo culture conditions and the in vivo milieu 42. The development of ALL and AML after the transplantation of MLL-AF9 transduced lineage-negative cord blood into immunocompromised mice has also been reported by Barabe et al., who, in addition, observed several clones capable of lineage switch in response to different culture conditions43. While these results are intriguing, remains unclear whether the pluripotent HSC and early progenitors transduced by a retroviral approach overlap meaningfully with the cell population in which the initial MLL rearrangement occurs in patients, and further study is needed to determine the extent to which environmental, genetic, and epigenetic changes influence the clinical phenotypes of MLL-rearranged leukemias.
Clinical Implications
The possible existence of a rare stem-cell-like population of cells within a much larger pool of malignant cells has presented new questions as to the biology of leukemia relapse and resistance. Most somatic stem cells are assumed to be quiescent at steady state, and to express a number of membrane transporters with broad specificity linked to drug resistance, such as MDR-1 (multi-drug resistance protein -1). Assuming that cancer stem cells recapitulate these two aspects of stem cells, quiescence and inherent drug resistance are likely to make the cancer stem cell population the most difficult to eradicate fraction within a tumor. The possible or questionable existence of an ALL stem cell also has implications for drug development. The more specific the target or pathway that is inhibited, the more important it will be that the right cell population is targeted. A current example would be the development of a calicheamycin conjugated anti-CD22 antibody 44.
Conclusions
The existence and biological relevance of a rare primitive ALL stem cell population remains controversial. Reports in the literature vary as much by subtype of ALL as by technique. Models range from proposing the existence of a very immature leukemia stem cell that does not possess many immunophenotypic characteristics of the lymphoid lineage (Figure 3A) 26, to a model in which B-ALL cells retain leukemogenic and self-renewal potential over a considerable range of developmental stages along the B-lymphocytic lineage (Figure 3C) 24. Similarly, the “cell or origin” in different ALL subtypes, and how it relates to the putative leukemia stem cell, is still elusive in most cancers including ALL.
A few principles, however, are emerging: Consistently over may subtypes and studies, and with few exceptions, ALL seems to be propagated and maintained by a lymphocyte-committed progenitor. Despite commonly reported coexpression of myeloid markers for certain subtypes, and despite the possible existence of leukemia cells that do not express any measurable characteristics of the lymphoid lineage, no true myeloid development has been observed in any of the reported studies. With good consistency, BCR/ABL- and MLL-rearranged leukemia cells have more robust involvement of earlier (in most cases lymphoid) subpopulations, and they engraft better in immunocompromised mice, than does ALL with a better prognosis. Further research is needed to determine whether this property is a function of the developmental stage at which the initial transforming event occurred, or a reflection of the type of genetic program activated by a specific cytogenetic/genetic abnormality. Defining and understanding different ALL subpopulations may be particularly important for the understanding of resistant and relapsed disease, and for targeted drug development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 2.Lapidot T, Sirard C, Vormoor J, Murdoch B, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Heidt DG, Dalerba P, Burant CF, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 5.Dalerba P, Dylla SJ, Park IK, Liu R, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ieta K, Tanaka F, Haraguchi N, Kita Y, et al. Biological and genetic characteristics of tumor-initiating cells in colon cancer. Ann Surg Oncol. 2008;15:638–648. doi: 10.1245/s10434-007-9605-3. [DOI] [PubMed] [Google Scholar]
- 7.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 9.Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98:756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suetsugu A, Nagaki M, Aoki H, Motohashi T, et al. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- 11.Ma S, Chan KW, Hu L, Lee TK, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Chiba T, Kita K, Zheng YW, Yokosuka O, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 13.Dou J, Pan M, Wen P, Li Y, et al. Isolation and identification of cancer stem-like cells from murine melanoma cell lines. Cell Mol Immunol. 2007;4:467–472. [PubMed] [Google Scholar]
- 14.Schatton T, Frank MH. Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res. 2008;21:39–55. doi: 10.1111/j.1755-148X.2007.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Beier D, Hau P, Proescholdt M, Lohmeier A, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 17.Singh SK, Hawkins C, Clarke ID, Squire JA, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 18.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke MF, Dick JE, Dirks PB, Eaves CJ, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 20.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy JA, Barabe F, Poeppl AG, Wang JC, Dick JE. Comment on “Tumor growth need not be driven by rare cancer stem cells”. Science. 2007;318:1722. doi: 10.1126/science.1149590. author reply 1722. [DOI] [PubMed] [Google Scholar]
- 22.Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Hewitt HB, Blake E, Proter EH. The effect of lethally irradiated cells on the transplantability of murine tumours. Br J Cancer. 1973;28:123–135. doi: 10.1038/bjc.1973.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.le Viseur C, Hotfilder M, Bomken S, Wilson K, et al. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell. 2008;14:47–58. doi: 10.1016/j.ccr.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taussig DC, Miraki-Moud F, Anjos-Afonso F, Pearce DJ, et al. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood. 2008;112:568–575. doi: 10.1182/blood-2007-10-118331. [DOI] [PubMed] [Google Scholar]
- 26.Cox CV, Evely RS, Oakhill A, Pamphilon DH, et al. Characterization of acute lymphoblastic leukemia progenitor cells. Blood. 2004;104:2919–2925. doi: 10.1182/blood-2004-03-0901. [DOI] [PubMed] [Google Scholar]
- 27.Hotfilder M, Rottgers S, Rosemann A, Jurgens H, et al. Immature CD34+CD19− progenitor/stem cells in TEL/AML1-positive acute lymphoblastic leukemia are genetically and functionally normal. Blood. 2002;100:640–646. doi: 10.1182/blood.v100.2.640. [DOI] [PubMed] [Google Scholar]
- 28.Castor A, Nilsson L, Astrand-Grundstrom I, Buitenhuis M, et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat Med. 2005;11:630–637. doi: 10.1038/nm1253. [DOI] [PubMed] [Google Scholar]
- 29.Mori H, Colman SM, Xiao Z, Ford AM, et al. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci U S A. 2002;99:8242–8247. doi: 10.1073/pnas.112218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong SA, Mabon ME, Silverman LB, Li A, et al. FLT3 mutations in childhood acute lymphoblastic leukemia. Blood. 2004;103:3544–3546. doi: 10.1182/blood-2003-07-2441. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong SA, Kung AL, Mabon ME, Silverman LB, et al. Inhibition of FLT3 in MLL. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell. 2003;3:173–183. doi: 10.1016/s1535-6108(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 32.Stubbs MC, Armstrong SA. FLT3 as a therapeutic target in childhood acute leukemia. Curr Drug Targets. 2007;8:703–714. doi: 10.2174/138945007780830782. [DOI] [PubMed] [Google Scholar]
- 33.Mullighan CG, Goorha S, Radtke I, Miller CB, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 34.Steinemann D, Cario G, Stanulla M, Karawajew L, et al. Copy number alterations in childhood acute lymphoblastic leukemia and their association with minimal residual disease. Genes Chromosomes Cancer. 2008;47:471–480. doi: 10.1002/gcc.20557. [DOI] [PubMed] [Google Scholar]
- 35.Hong D, Gupta R, Ancliff P, Atzberger A, et al. Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science. 2008;319:336–339. doi: 10.1126/science.1150648. [DOI] [PubMed] [Google Scholar]
- 36.Quijano CA, Moore D, 2nd, Arthur D, Feusner J, et al. Cytogenetically aberrant cells are present in the CD34+CD33-38-19- marrow compartment in children with acute lymphoblastic leukemia. Leukemia. 1997;11:1508–1515. doi: 10.1038/sj.leu.2400754. [DOI] [PubMed] [Google Scholar]
- 37.Schenk TM, Keyhani A, Bottcher S, Kliche KO, et al. Multilineage involvement of Philadelphia chromosome positive acute lymphoblastic leukemia. Leukemia. 1998;12:666–674. doi: 10.1038/sj.leu.2400986. [DOI] [PubMed] [Google Scholar]
- 38.Anastasi J, Feng J, Dickstein JI, Le Beau MM, et al. Lineage involvement by BCR/ABL in Ph+ lymphoblastic leukemias: chronic myelogenous leukemia presenting in lymphoid blast vs Ph+ acute lymphoblastic leukemia. Leukemia. 1996;10:795–802. [PubMed] [Google Scholar]
- 39.Cobaleda C, Gutierrez-Cianca N, Perez-Losada J, Flores T, et al. A primitive hematopoietic cell is the target for the leukemic transformation in human philadelphia-positive acute lymphoblastic leukemia. Blood. 2000;95:1007–1013. [PubMed] [Google Scholar]
- 40.George AA, Franklin J, Kerkof K, Shah AJ, et al. Detection of leukemic cells in the CD34(+)CD38(−) bone marrow progenitor population in children with acute lymphoblastic leukemia. Blood. 2001;97:3925–3930. doi: 10.1182/blood.v97.12.3925. [DOI] [PubMed] [Google Scholar]
- 41.Hotfilder M, Rottgers S, Rosemann A, Schrauder A, et al. Leukemic stem cells in childhood high-risk ALL/t(9;22) and t(4;11) are present in primitive lymphoid-restricted CD34+CD19− cells. Cancer Res. 2005;65:1442–1449. doi: 10.1158/0008-5472.CAN-04-1356. [DOI] [PubMed] [Google Scholar]
- 42.Wei J, Wunderlich M, Fox C, Alvarez S, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13:483–495. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barabe F, Kennedy JA, Hope KJ, Dick JE. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316:600–604. doi: 10.1126/science.1139851. [DOI] [PubMed] [Google Scholar]
- 44.Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia. 2007;21:2240–2245. doi: 10.1038/sj.leu.2404866. [DOI] [PubMed] [Google Scholar]