Background: The functional differences among astrocyte subtypes are uncharacterized.
Results: Only a subset of astrocytes expresses TRPV4 and regulates neurons.
Conclusion: TRPV4+ astrocytes release ATP and glutamate to regulate neurons.
Significance: Astrocytes can be classified by TRPV4 expression and by function.
Keywords: Astrocytes, ATP, Glutamate, Synapses, TRP Channels, TRPV4, Gliotransmitter
Abstract
Astrocytes play active roles in the regulation of synaptic transmission. Neuronal excitation can evoke Ca2+ transients in astrocytes, and these Ca2+ transients can modulate neuronal excitability. Although only a subset of astrocytes appears to communicate with neurons, the types of astrocytes that can regulate neuronal excitability are poorly characterized. We found that ∼30% of astrocytes in the brain express transient receptor potential vanilloid 4 (TRPV4), indicating that astrocytic subtypes can be classified on the basis of their expression patterns. When TRPV4+ astrocytes are activated by ligands such as arachidonic acid, the activation propagates to neighboring astrocytes through gap junctions and by ATP release from the TRPV4+ astrocytes. After activation, both TRPV4+ and TRPV4− astrocytes release glutamate, which acts as an excitatory gliotransmitter to increase synaptic transmission through type 1 metabotropic glutamate receptor (mGluR). Our results indicate that TRPV4+ astrocytes constitute a novel subtype of the population and are solely responsible for initiating excitatory gliotransmitter release to enhance synaptic transmission. We propose that TRPV4+ astrocytes form a core of excitatory glial assembly in the brain and function to efficiently increase neuronal excitation in response to endogenous TRPV4 ligands.
Introduction
Astrocytes provide metabolic support and eliminate waste products from extracellular spaces (1). The astrocyte-mediated clearance of extracellular glutamate and K+ and regulation of extracellular volume are known to be perfectly coupled with synaptic activities (2, 3). These cells also regulate blood flow within the brain in response to neuronal activity (4, 5). In addition to their basic physiological roles, astrocytes are essential for bidirectional communication with neurons. Astrocytes can detect, respond to, and modulate neuronal activity (6, 7). Specifically, they actively regulate neural processing by releasing gliotransmitters such as ATP and glutamate (8). Increases in intracellular Ca2+ levels induce glutamate release from astrocytes (9). Glutamate increases synaptic activity and modulates behaviors such as epilepsy and anxiety (2, 10). An increase in intracellular Ca2+ also induces astrocytes to release ATP, and this suppresses synaptic activity in the form of heterosynaptic depression (11). Dysfunction of astrocytes affects spatial working memory and pain perception (2). Although it is becoming clear that astrocytes contribute to neuronal function, the detailed functional characteristics of astrocytes remain poorly understood. In particular, the identity of the astrocytes that regulate neuronal activity is unknown. Furthermore, there is controversy as to whether astrocytes are functionally homogeneous or heterogeneous, although astrocytes can be clearly classified as either protoplasmic or fibrous on the basis of their morphology (12, 13).
TRPV42 is a non-selective cation channel that was first described as an osmosensor for detection of hypotonic stimuli (14–18). TRPV4 can also be activated by heat (>27–34 °C), the phorbol ester derivative 4α-phorbol-12,13-didecanoate (4αPDD), or metabolites of arachidonic acid (19–23). We have previously reported that physiological temperatures activate TRPV4 in some subtypes of hippocampal neurons and that this activation enhances neuronal activity (19). Moreover, we and others have reported that, in addition to neurons, astrocytes also express TRPV4 (19, 24–26). Calcium signaling is crucial for astrocyte function. An increase in the intracellular Ca2+ concentration mediated by the inositol 1,4,5-triphosphate receptor in the endoplasmic reticulum drives the release of gliotransmitters such as ATP and glutamate. Astrocytic TRPV4 is thought to be the upstream molecule that regulates intracellular Ca2+ levels because it is a highly permeable Ca2+ channel (20). The possibility exists that TRPV4 expression is restricted to a specific subtype of astrocytes. In this study we examined the expression pattern of TRPV4 in astrocytes and characterized the physiological function of TRPV4+ astrocytes.
EXPERIMENTAL PROCEDURES
Animals
C57BL/6J was utilized as the background strain for TRPV4-deficient (TRPV4KO) mice. All animal care procedures and experimental protocols were performed in accordance with the guidelines of the National Institute of Health, the National Institute for Physiological Sciences, and Gunma University.
Immunohistochemical Analysis, in Situ Hybridization, and Cell Counts
Immunohistochemistry and in situ hybridization were performed as previously described (19, 27). We used a TRPV4 mRNA probe that has been described in previous reports (19). The following antibodies were used: mouse monoclonal anti-glial fibrillary acidic protein (GFAP) antibody (1:500, Sigma) and mouse monoclonal anti-S100β antibody (1:500, Sigma). To determine the ratio of TRPV4-positive astrocytes, we used 12 slides of stained hippocampal tissue (each slide contained 6 brain slices). We used a microscope to count the number of TRPV4−/astrocyte-marker+ and TRPV4+/astrocyte-marker+ astrocytes in the CA1 region. To count the cells, we combined nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate-mediated detection of mRNA with diaminobenzidine-mediated detection of protein.
Culture of Dissociated Astrocytes and Neurons
Cortical astrocytes and hippocampal neurons were prepared from postnatal day 0 (P0) wild type (WT) or TRPV4KO mice as previously described (19). Hippocampal neurons from TRPV4KO mice were co-cultured with astrocytes (WT or TRPV4KO) to examine the changes in neuronal activity induced by TRPV4-positive astrocytes.
Fluorescent Measurements and Electrophysiology
Fura-2 fluorescence was measured by Fura-2-AM (Molecular Probes, Carlsbad, CA) in a standard bath solution containing 140 mm NaCl, 5 mm KCl, 2 mm MgCl2, 2 mm CaCl2, 10 mm HEPES, and 10 mm glucose, pH 7.4. The 340:380-nm ratio was recorded. Because spontaneous Ca2+ spikes were always below 0.1, we defined TRPV4-activated Ca2+ spikes as those at or above a value of 0.1. The standard bath solution for the patch clamp experiments was the same as that used for fluorescence measurements. The reversal potential was measured by using voltage ramps (−100 to +100 mV in 5-s intervals). Pipette solutions for whole-cell recordings contained 120 mm potassium gluconate, 20 mm KCl, 0.5 mm EGTA, 2 mm Mg-ATP, 2 mm K2-GTP, and 10 mm HEPES, pH 7.4. Whole-cell recordings were sampled at 10 kHz and filtered at 5 kHz for analysis (Axon 200B amplifier with pCLAMP software, Axon Instruments, Foster City, CA). Data were statistically analyzed by using the unpaired t test, analysis of variance, or Duncan's multiple range test.
Visualization of ATP Dynamics in Cultured Astrocytes
Cultured astrocytes were seeded on poly-l-lysine-coated glass slides at confluence. The glass slides were placed in 3 × 10-mm chambers made from silicon. The culture media in the chambers were replaced with a Ringer solution containing a luciferin-luciferase mixture (Checklite HS set, Kikkoman, Noda, Japan). Because changing the medium causes excessive stress to astrocytes, our experiments were performed 20 min after the medium was exchanged to allow ATP release from the astrocytes to stabilize. Bioluminescence emitted by the ATP/luciferin-luciferase reaction was detected by an upright microscope (BX51WI, Olympus, Tokyo, Japan) equipped with a cooled CCD camera (Evolve 512, Roper), an image intensifier (C8600, Hamamatsu Photonics, Hamamatsu, Japan), and a 34× (NA 0.28) dry objective. Images of ATP release (512 × 512 pixels) were acquired every 500 ms and averaged >10 frames by using Meta-Morph (Version 6.5; MDS Inc., Toronto, Canada). All experiments were performed at 30–34 °C in a perfectly dark room.
Analysis of Glutamate Release in Cultured Astrocytes
Standard bath solutions with or without 4αPDD were applied to cultured astrocytes for 10 min, and then the conditioned media were collected. After filtration, the amino acid composition of the media was determined with an automatic amino acid analyzer (Hitachi, Ibaraki, Japan).
RESULTS
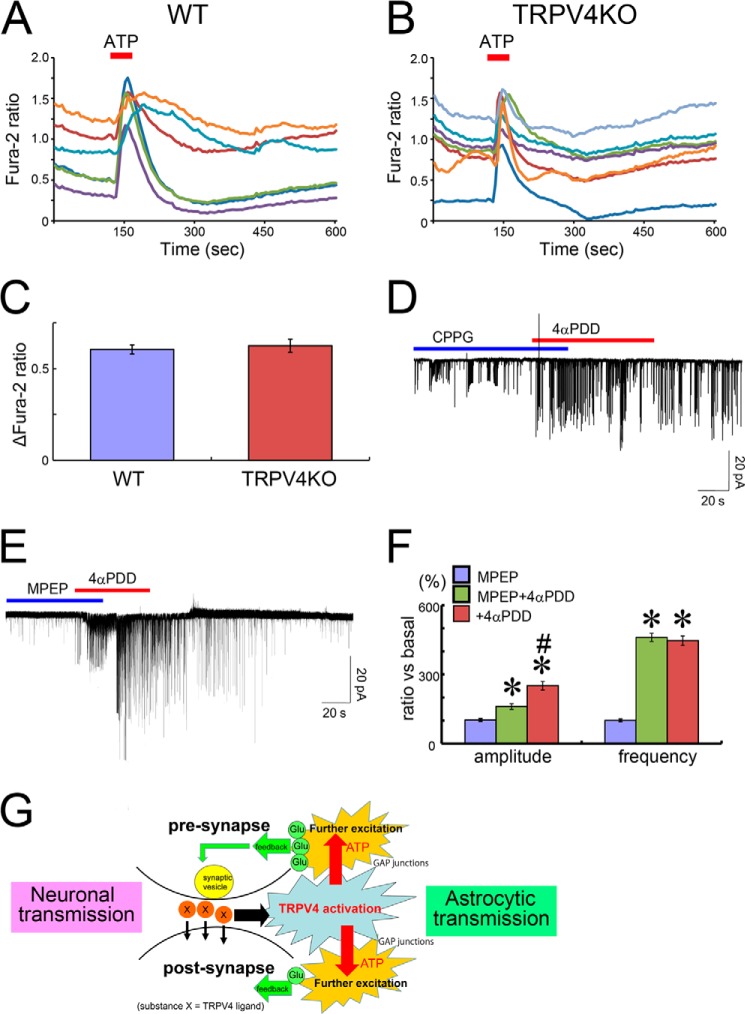
We and others have previously reported that TRPV4 is an important regulator of neuronal excitability in hippocampal neurons and that TRPV4 is expressed in both astrocytes and neurons (19, 24, 28, 29). Therefore, we sought to determine which types of astrocytes express TRPV4 by combining in situ hybridization and immunohistochemistry in adult mouse hippocampus. TRPV4 expression (arrowheads in Fig. 1, A and B) was restricted to a subset of astrocytes distinguished by GFAP expression (Fig. 1A, arrowheads) or S100β expression (Fig. 1B). Interestingly, only 30% of GFAP-positive astrocytes (323 of 1155 cells, n = 12 slides) expressed TRPV4. We also quantified the ratio of TRPV4-positive astrocytes to S100β-positive astrocytes. Consistent with the findings of TRPV4 expression in astrocytes expressing GFAP, only 20% of S100β-positive astrocytes (209 of 952 cells, n = 12 slides) expressed TRPV4 (Fig. 1B, arrowheads). To confirm that TRPV4 mRNA is expressed in a subset of astrocytes, we examined its expression in primary cultures of astrocytes (∼95% of the cells were astrocytes). Consistent with the aforementioned findings (Fig. 1, A and B), only a subset of cultured S100β+ astrocytes expressed TRPV4 (Fig. 1C, arrowheads). We propose that astrocytes can be classified into functionally heterogeneous groups on the basis of the expression of markers such as TRPV4.
FIGURE 1.
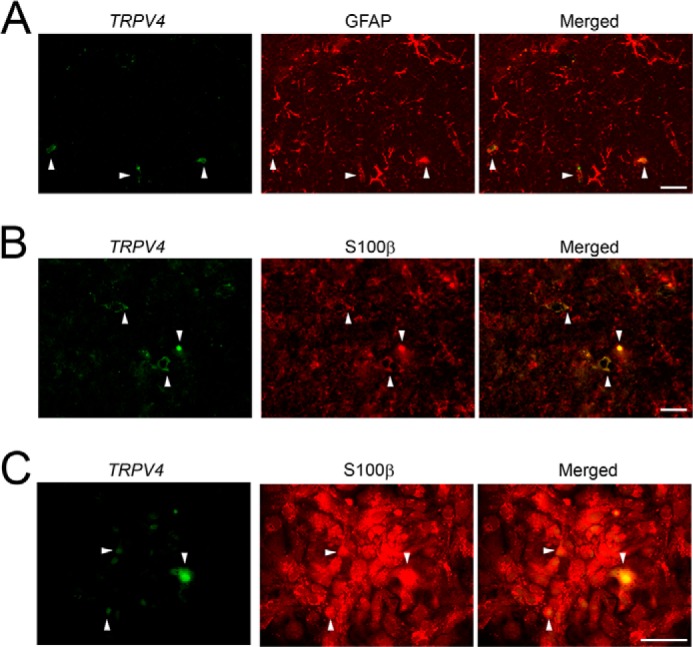
TRPV4 is expressed in a restricted subpopulation of astrocytes. A and B, TRPV4 mRNA (green) and GFAP (red) expression (A) or TRPV4 mRNA (green) and S100β (red) expression (B) in adult mouse hippocampus (CA1). White arrowheads indicate TRPV4+ astrocytes. Scale bars, 100 μm. C, TRPV4 mRNA expression (green) in cultured astrocytes. Astrocytes were identified by S100β expressions (red). White arrowheads indicate TRPV4 + astrocytes. Scale bar, 50 μm.
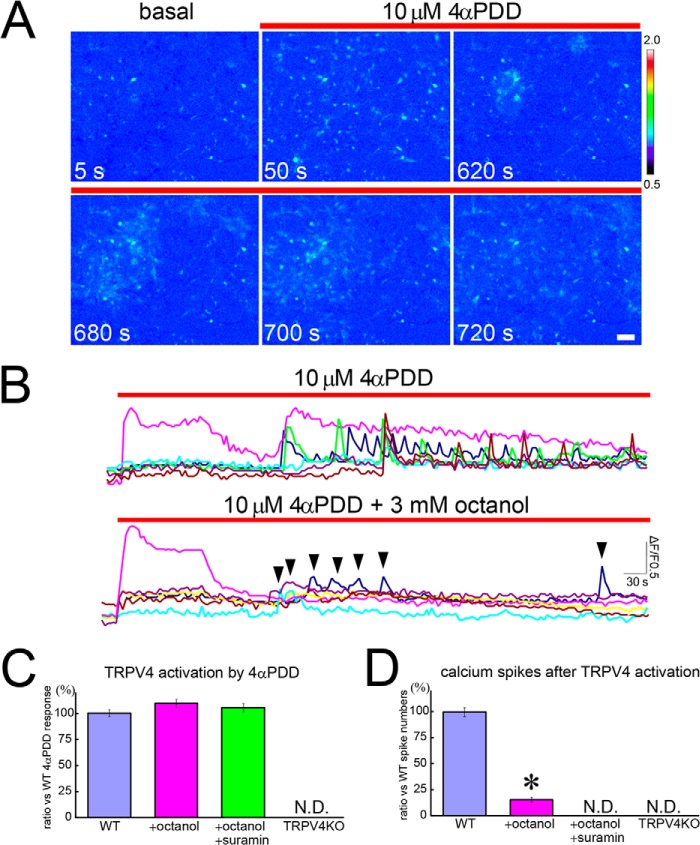
Next, we examined the response of astrocytes to chemical ligands of TRPV4, as the use of heat is not suitable for analysis of TRPV4 function in astrocytes (29) unlike hippocampal neurons (19). Application of a TRPV4-specific ligand, 4αPDD, caused an acute increase in intracellular Ca2+ ([Ca2+]i) in TRPV4+ astrocytes (<20% of the total number of astrocytes) within 1 min after 4αPDD was applied (Fig. 2, A and B, pink trace; supplemental Movie 1). These acute responses most likely resulted from activation of TRPV4 because they were not detected in astrocytes from TRPV4KO mice (Fig. 2C). After the initial increase in [Ca2+]i in TRPV4+ astrocytes, substantial Ca2+ oscillations with a spike-like appearance were observed in the majority of the astrocytes (Fig. 2, A and B, excluding the pink trace; supplemental Movie 1). This finding indicates that communication among astrocytes was initiated after TRPV4 was activated. Under normal conditions in our assay system, the cultured astrocytes exhibited very rare (1–2 times/10 min) and very small (<0.1ΔF/F0) spontaneous Ca2+ oscillations. Therefore, we defined the TRPV4-evoked Ca2+ spikes as those >0.1ΔF/F0. Moreover, TRPV4 activation and TRPV4-dependent Ca2+ oscillations were never observed in TRPV4KO astrocytes after exposure to 4αPDD (Fig. 2, C and D). The presence of 3 mm octanol, a gap junction blocker, significantly reduced the later Ca2+ oscillations (Fig. 2B, excluding the pink trace, and Fig. 2D), although the initial, presumably TRPV4-mediated responses (pink traces in Fig. 2, B and C) were not affected by exposure to octanol.
FIGURE 2.
A specific subpopulation of astrocytes responds to a chemical TRPV4 agonist and initiates Ca2+ oscillations. A, representative pictures of Ca2+ imaging experiments (see supplemental Movie 1). Cultured astrocytes were exposed to 4αPDD. Ca2+ influx was observed only in TRPV4+ populations (at 50 s). After the observed increase in [Ca2+]i exclusively in TRPV4+ astrocytes, significant Ca2+ oscillations were observed in most of the other astrocytes (at 620–720 s). B, representative traces from cells exposed to 4αPDD (10 μm) are shown in the upper graph. The pink trace is from a TRPV4+ cell. Representative traces from cells exposed to both 4αPDD (10 μm) and octanol (3 mm) are shown in the lower graph. The pink trace is from a TRPV4+ cell. Arrowheads indicate Ca2+ spikes in TRPV4− cells. C, quantification of 4αPDD-evoked activation of TRPV4 compared with the response in WT astrocytes. D, quantification of the number of Ca2+ spikes evoked by exposure to 4αPDD compared with the number evoked in WT astrocytes. *, p < 0.01, t test (versus WT).
To prove that the astrocytes producing the acute responses to the TRPV4 agonist were TRPV4-positive, we combined Ca2+ imaging and whole-cell patch clamp experiments (Fig. 3). First, we treated cells with another TRPV4-specific chemical ligand, GSK1016790A (GSK, 1 μm) then performed Ca2+ imaging. This approach allowed us to identify both the astrocytes producing acute responses to GSK and those producing non-acute responses. The results of these experiments (Fig. 3A and supplemental movie 2) were equivalent to those obtained with 4αPDD (Fig. 2, A and B, pink trace; supplemental movie 1). These data indicate that the increase in [Ca2+]i is a specific response to TRPV4 activation. After the astrocytes were identified by Ca2+ imaging, we applied 3 μm GSK then performed whole-cell patch clamp recordings from the identified cells. To avoid desensitization of TRPV4 by repeated exposure to agonist, we increased the dose of GSK in the patch clamp recordings. Consistent with our expectation, all of the astrocytes that produced an acute response to GSK evoked TRPV4 currents, but astrocytes that did not respond acutely did not evoke any such currents (Fig. 3B). These findings strongly indicate that the astrocytes that produce an acute response to TRPV4 agonists express TRPV4.
FIGURE 3.
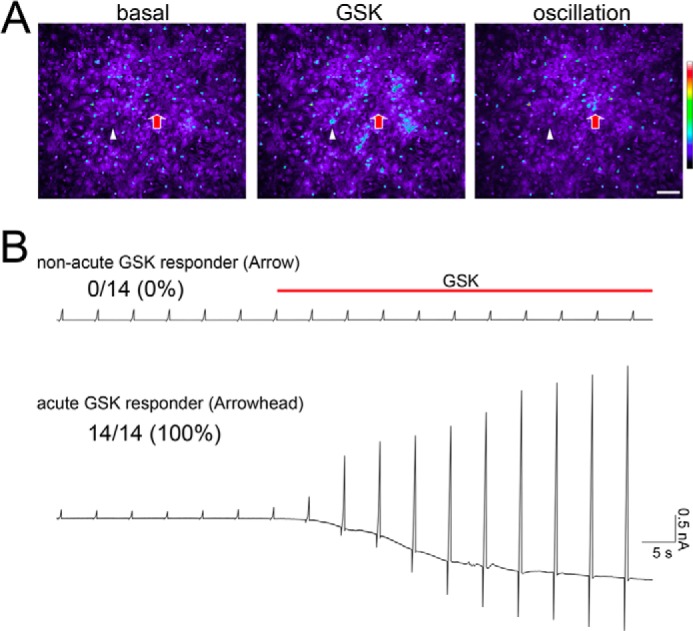
Only TRPV4+ astrocytes display an acute increase in [Ca2+]i in response to a chemical TRPV4 agonist. A, representative pictures from Ca2+ imaging experiments (see supplemental Movie 2). The initial time point (basal), the moment just after the application of 1 μm GSK (GSK), and the late Ca2+ oscillations (oscillation) are shown in representative pictures (see supplemental movie 2). White arrowhead indicates a cell that responds acutely to GSK, and the red arrow indicates a cell with a non-acute response to GSK. B, representative whole cell currents (at −60 mV holding potential) after application of 3 μm GSK in acute GSK responder cells (white arrowhead in panel A) or the non-acute GSK responder cells (red arrow in panel A). During whole-cell patch clamp recordings, we applied ramp pulses from −100 mV to +100 mV at 5-s intervals. The ratios indicate the number of astrocytes that displayed GSK-evoked currents per the total number of sampled cells, with the percentages in parentheses.
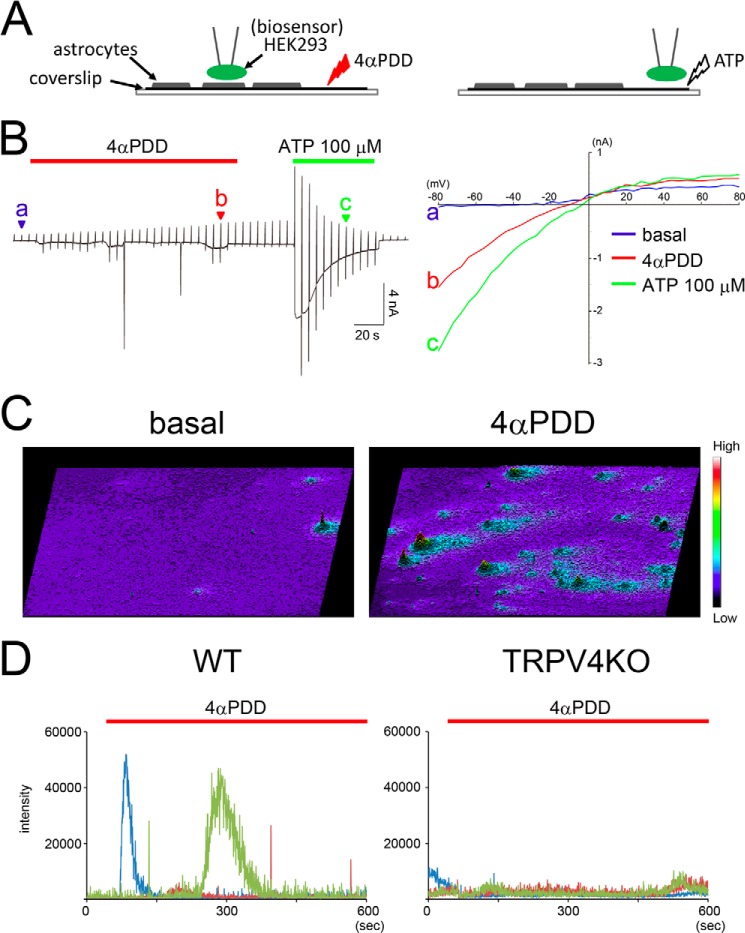
The failure of octanol to inhibit all Ca2+ oscillations caused by TRPV4+ astrocytes (Fig. 2B, arrowheads, and 2D) indicates that gliotransmitters are released from the astrocytes. The residual Ca2+ oscillations were completely abolished when octanol and 100 μm suramin (an ATP receptor blocker; Fig. 2D) were applied simultaneously, although TRPV4 remained activated (Fig. 2C). These results strongly suggest that excitation of TRPV4+ astrocytes is transmitted through gap junctions and ATP release. To directly determine whether activation of TRPV4 causes ATP release from astrocytes, we utilized an ATP biosensor system (30). We have previously utilized this biosensor technique to monitor ATP release from skin keratinocytes (30) as locally released ATP is very difficult to detect (exoATPases immediately degrade released ATP). In this system ionotropic receptors expressed in HEK293 cells detect molecules released from adjacent astrocytes that have been stimulated with ligand (Fig. 4A). During 4αPDD-mediated activation of TRPV4 in WT astrocytes at a holding potential of −60 mV, P2X purinoreceptor type2 (P2X2)-like currents were recorded only from whole-cell patch clamped HEK293 cells that were in close proximity to the astrocytes (located within 10 μm; Fig. 4B; n = 6 of 43 trials). Using a whole-cell patch clamp recording technique, we applied ramped pulses from −80 mV to +80 mV (Fig. 4B) at 5-s intervals. Exposure to 4αPDD evoked an inwardly rectified current-voltage relationship (Fig. 4B, red arrowhead and the red trace labeled b in the graph on the right), although the basal current-voltage relationship had a linear pattern (Fig. 4B, blue arrowhead and the blue trace labeled a in the graph on the right). The expression of functional P2X2 receptors in the HEK293 cells was confirmed by applying 100 μm ATP to HEK293 cells (to avoid the possible effects of ATP-induced ATP release from astrocytes, these cells were not in close proximity to any astrocytes). In addition, we determined that no P2X2-like inwardly rectifying currents were recorded from TRPV4KO astrocytes. Exposure to ATP evoked an inwardly rectified current-voltage relationship (Fig. 4B, green arrowhead and the green trace labeled c in the graph on the right). Taken together these results indicate that ATP is released from TRPV4+ astrocytes when the channel is activated.
FIGURE 4.
Activation of TRPV4 causes ATP release from cultured astrocytes. A, schematics representing the biosensor system. HEK293 cells expressing P2X2 (the biosensor) are placed in close proximity to the astrocytes, then 4αPDD (10 μm) is applied to the astrocytes. At the end of each experiment, the reliability of the biosensors is confirmed by directly applying ATP to a location that is distant to the astrocytes. B, representative traces showing that application of 4αPDD evokes whole-cell current responses (left panel) with inward rectification (red traces labeled b in the right panel) in HEK293 cells transfected with a P2X2 cDNA. Application of ATP (100 μm) was used to confirm P2X2-mediated responses (left panel) with inward rectification (green traces labeled c in the right panel). Blue traces labeled a in the right panel represent control currents. Ramp pulses (from −80 mV to +80 mV for 500 ms) were applied at 5-s intervals. The holding potential was −60 mV. The traces are representative of a typical experiment (6 of 43 trials). C, real-time ATP imaging was performed in cultured astrocytes (almost confluent). We stacked all of the images after the experiments were completed. The ratio ATP release is represented as pseudo color images. In this figure the height and color (as shown by the scale bars) represent the amount of ATP that is released. Representative images show ATP release after application of 4αPDD (right panel) and in basal conditions (left panel). D, quantification of ATP release in three representative cultures of WT (left panel) and TRPV4KO (right panel) astrocytes after application of 4αPDD.
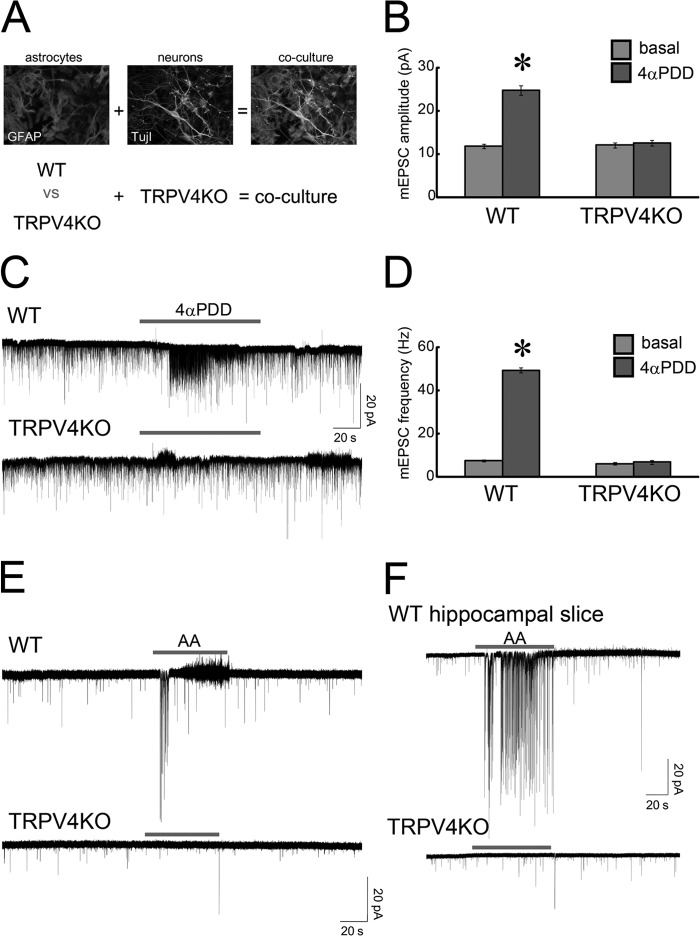
Unfortunately, the biosensor system does not indicate which astrocytes release ATP. To obtain this information, we used the luciferin-luciferase system to image ATP release in real time (31). In this imaging, we put cultured astrocytes (on glass coverslips) into a tiny chamber (3 × 10 mm) made from silicon. Then the culture medium was replaced with a Ringer solution containing a luciferin-luciferase mixture. Bioluminescence emitted by the ATP luciferin-luciferase reaction was detected by an upright microscope equipped with a cooled-CCD camera in dark room. Under basal conditions, very rare spontaneous ATP release was observed (Fig. 4C). In contrast, when TRPV4 was activated in astrocytes exposed to 4αPDD, this activation evoked strong ATP release in many astrocytes (Fig. 4C and supplemental Movie 3). The amount and duration of ATP release differed between astrocytes (Fig. 4, C and D). Consistent with the results of our experiments with the biosensor system (Fig. 4B), some astrocytes displayed both weak/sustained and high/transient ATP release (Fig. 4D, shown by the dark red trace). Astrocytes from TRPV4KO mice did not release any ATP (Fig. 4D). This finding indicates that ATP release by WT astrocytes upon application of 4αPDD was a TRPV4-dependent response and leads us to hypothesize that TRPV4+ astrocytes might regulate neuronal activity, as excited astrocytes have been shown to directly modulate neuronal functions (32). Because neurons also express TRPV4 (19), we could not use patch clamp recordings of slices from WT or TRPV4KO mice to examine this possibility. To eliminate the contributions from TRPV4 expressing neurons, we prepared a unique co-culture system. First, we cultured astrocytes from WT or TRPV4KO mice, and TRPV4KO hippocampal neurons were then added to the astrocytes (Fig. 5A). When 4αPDD was applied to the cultures, miniature excitatory post synaptic currents (mEPSCs) increased significantly in neurons cultured with WT astrocytes but not in those cultured with TRPV4KO astrocytes (Fig. 5, B–D). In addition, the amplitude of evoked mEPSCs was 2 times larger in cultures with WT astrocytes than in those with TRPV4KO astrocytes (Fig. 5, B and C; WT:basal, 12.1 ± 0.7 pA versus 4αPDD, 25.1 ± 1.8 pA; TRPV4KO:basal, 13.2 ± 0.8 pA versus 4αPDD, 13.7 ± 1.2 pA; n = 16 for each genotype). As with the amplitude, the frequency of mEPSCs evoked by treating the cultures with 4αPDD was 7 times larger in cultures with WT astrocytes than in those with TRPV4KO cells (Fig. 5, C and D; WT:basal, 7.5 ± 0.5 Hz versus 4αPDD, 49.3 ± 1.2 Hz; TRPV4KO:basal, 6.1 ± 0.6 Hz versus 4αPDD, 6.8 ± 0.7 Hz; n = 16 for each genotype). Arachidonic acid has been reported to be an endogenous ligand for TRPV4 (23). Therefore, we tested the effects of arachidonic acid in our co-culture system. Similar to the results obtained with 4αPDD, mEPSCs in cultures with WT astrocytes increased significantly after exposure to arachidonic acid (10 μm), but an increase was not observed in cultures containing TRPV4KO astrocytes (Fig. 5E). Because we opted to use a co-culture system to evaluate the function of TRPV4 in astrocytes, we cannot exclude the possibility that our results might be artificial. Therefore, we recorded mEPSCs in acute hippocampal slices prepared from WT and TRPV4KO mice. Puff application of arachidonic acid (10 μm) to neighboring patch-clamped cells significantly increased mEPSCs in slices from WT mice but not in those from TRPV4KO mice (Fig. 5F).
FIGURE 5.
Activation of TRPV4 in astrocytes enhances synaptic activity. A, co-culture of TRPV4KO neurons with WT or TRPV4KO astrocytes to analyze the physiological significance of TRPV4 signaling in astrocytes. B, quantification of the amplitudes of mEPSCs in cultures with WT or TRPV4KO astrocytes (basal conditions or after application of 10 μm 4αPDD). *, p < 0.01, t test (versus basal). C, representative traces of a typical experiment that measured mEPSCs in cultures with WT or TRPV4KO astrocytes at a holding potential of −60 mV (n = 16 for each genotype). D, quantification of the frequency of mEPSCs in cultures with WT or TRPV4KO astrocytes (basal conditions or after application of 10 μm 4αPDD). *, p < 0.01, t test (versus basal). E, representative traces of mEPSCs in cultures with WT or TRPV4KO astrocytes after application of 10 μm arachidonic acid (AA) at a holding potential of −60 mV. F, representative traces of mEPSCs in hippocampal slices from WT or TRPV4KO mice after localized application of 10 μm arachidonic acid at a holding potential of −60 mV.
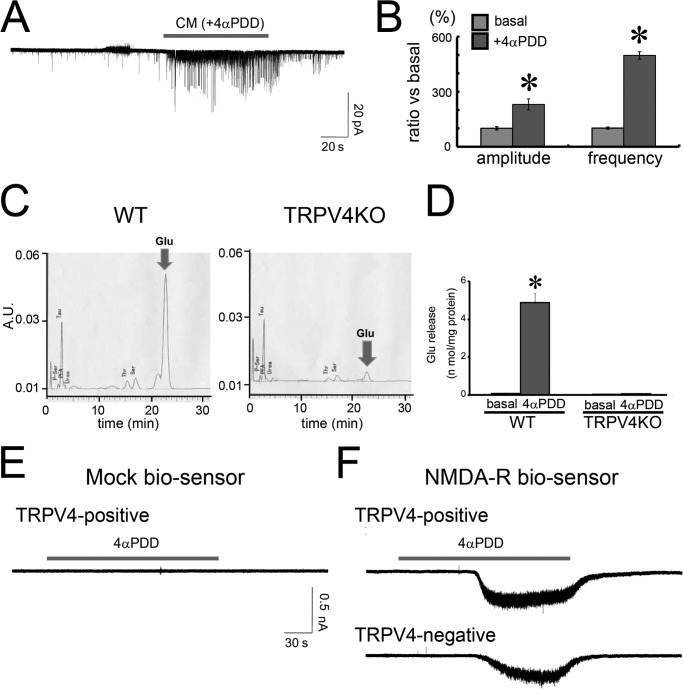
The results described above strongly suggest that activation of TRPV4 in astrocytes causes them to release factors that enhance synaptic function. To confirm this possibility, we prepared conditioned media from astrocytes exposed to 4αPDD. Unfortunately, TRPV4KO neurons exposed to conditioned medium did not exhibit any changes in the frequency or amplitude of evoked mEPSCs. We hypothesized that the gliotransmitters in the conditioned media were too dilute because they were secreted outside of the normally restricted synaptic spaces. Hence, we concentrated the conditioned media by lyophilization then reconstituted the media in a standard bath solution. Both the amplitude and frequency of mEPSCs were significantly increased in cultures exposed to this solution (Fig. 6, A and B). These results support the hypothesis that a gliotransmitter acting as a positive regulator is released from astrocytes when TRPV4 is activated. ATP is not a likely candidate, as it has been reported that ATP release from astrocytes actually inhibits synaptic function (33), and ATP is not retained after lyophilization. Therefore, we searched for another gliotransmitter with the ability to enhance synaptic function after activation of TRPV4. We focused on amino acids and utilized an amino acid analyzer to examine the contents of conditioned media from cultures of WT and TRPV4KO astrocytes. Surprisingly, glutamate release was significantly enhanced in cultures with WT astrocytes after exposure to 4αPDD, although the release of other amino acids was not changed (Fig. 6, C and D). Moreover, glutamate release was not enhanced in cultures of TRPV4KO cells treated similarly (Fig. 6, C and D). These results strongly indicate that activation of TRPV4 in astrocytes causes them to release glutamate. To identify the cells that release glutamate, we performed biosensor experiments of the GluN1/N2B NMDA receptor in cultures containing TRPV4+ or TRPV4− astrocytes, which we identified by the Ca2+ imaging after exposure to 4αPDD. Both TRPV4+ and TRPV4− astrocytes evoked inward, NMDA-derived currents in the biosensor cells (Fig. 6F), although the mock biosensor cells did not produce such currents (Fig. 6E). These results indicate that both TRPV4+ and TRPV4− astrocytes release glutamate, although TRPV4+ astrocytes are solely responsible for initiating glutamate release.
FIGURE 6.
Activation of TRPV4 in astrocytes enhances synaptic activity through release of excitatory glutamate. A, a representative trace of mEPSCs in TRPV4KO hippocampal neurons evoked after application of conditioned media from cultured astrocytes. The conditioned media (CM) were prepared after 4αPDD (10 μm) was applied. B, comparison of the amplitude and frequency of evoked mEPSCs to those detected in basal conditions. *, p < 0.01, t test (versus basal). C, a representative trace of the analysis of amino acids present in the conditioned media from WT or TRPV4KO astrocytes exposed to 10 μm 4αPDD. A.U., absorbance units. D, quantification of glutamate release (in basal media or conditioned media from cells exposed to 4αPDD) from WT or TRPV4KO astrocytes. *, p < 0.01, t test (versus WT basal). E and F, currents from NMDA receptor (NMDA-R) biosensor cells (GluN1- and GluN2B-expressing HEK293 cells). The biosensor cells sense glutamate release from TRPV4-positive or -negative astrocytes, which are identified by Ca2+ imaging after exposure to 10 μm 4αPDD (F). As a negative control, the mock biosensor cells (HEK293 cells transfected with the pCAG vector) did not display inward currents after 10 μm 4αPDD was applied to TRPV4+ astrocytes (E).
The lack of ATP or glutamate release from astrocytes from TRPV4KO mice (Figs. 4 and 6) might be responsible for the functional abnormalities of these cells. To confirm this idea, we applied ATP (1 μm) to WT and TRPV4KO astrocytes and then performed Ca2+ imaging. Both WT and TRPV4KO cells responded to ATP (Fig. 7, A and B), and the amplitudes of the responses were similar in both types of cells (Fig. 7C). These results indicate that the mutant cells are fully functional and that the observed lack of ATP and glutamate release from the mutant astrocytes is caused by a deficiency of TRPV4. If glutamate is a major gliotransmitter capable of positively regulating synaptic activity, then the effects should be inhibited by antagonists of type 1 mGluR, as previously described (34). To examine this issue, we pretreated co-cultures of TRPV4KO neurons and WT astrocytes with (RS)-α-cyclopropyl-4-phosphonophenylglycine (CPPG), a type 2/3 mGluR antagonist, or 2-methyl-6-(phenylethynyl)pyridine (MPEP), a type 1 mGluR antagonist, then applied 4αPDD. Treatment with CPPG had no inhibitory effects (Fig. 7D). The amplitudes of mEPSCs evoked in cultures exposed to both CPPG and 4αPDD were not significantly different from those of mEPSCs evoked in cultures exposed to 4αPDD alone (CPPG+4αPDD: 24.5 ± 1.5 pA; 4αPDD alone: 25.2 ± 1.9 pA; n = 6). In contrast, the potentiation of synaptic activity (both the frequencies and amplitudes of EPSCs) was significantly inhibited in cultures exposed to both MPEP and 4αPDD, and washout of MPEP significantly increased 4αPDD-evoked EPSCs (Fig. 7, E and F). These results indicate that the glutamate released from astrocytes in response to activation of TRPV4 signals through the type 1 mGluR. Taken together, we conclude that astrocytic TRPV4 is an important regulator of synaptic transmission (Fig. 7G).
FIGURE 7.
Glutamate released after activation of astrocytic TRPV4 enhances synaptic activity by signaling through type 1 mGluR. A and B, representative traces evoked by exposing cells to ATP (1 μm) are shown for WT and TRPV4KO astrocytes. C, comparison of ATP-evoked activation in WT and TRPV4KO astrocytes. D, a representative trace of 4αPDD-evoked mEPSCs in TRPV4KO hippocampal neurons with or without CPPG (50 μm). E, a representative trace of 4αPDD-evoked mEPSCs in TRPV4KO hippocampal neurons with or without MPEP (1 μm). F, comparison of mEPSC amplitude and frequency in cells exposed to MPEP, 4αPDD, or MPEP and 4αPDD. *. p < 0.01, Duncan's multiple range test (versus MPEP). #, p < 0.01, Duncan's multiple range test (versus MPEP+4αPDD). G, schematic representation of our findings. TRPV4+ astrocytes, shown by the blue color, are specifically localized in the brain; activation of TRPV4 in these astrocytes causes excitation in neighboring astrocytes through gap junctions and ATP release, shown as red arrows. These cells form a unit of excitatory astrocytes that release glutamate, shown as green arrows. The glutamate that is released signals through type 1 mGluRs at presynaptic sites to enhance neurotransmitter release.
DISCUSSION
The present study revealed that a unique subtype of astrocytes regulates neuronal activity. We examined TRPV4 expression in both brain slices and cultured astrocytes and found that expression of this ion channel was restricted to 20–30% of the astrocyte population (Fig. 1). Using Ca2+ imaging and whole-cell patch clamp recordings, we confirmed the restricted functional expression of TRPV4 to 20% of astrocytes (Figs. 1–3). Astrocytes have been reported to be electrophysiologically homogeneous, although their morphologies and the expression of biochemical markers are different (12). We agree with these findings because the in vitro electrophysiological properties of protoplasmic and fibrous astrocytes are similar.3 Hence, we hypothesize that all astrocytes have the same electrophysiological properties regardless of their morphological differences; however, subsets of astrocytes might synthesize different collections of gliotransmitters. Thus, we propose that the expression of markers such as TRPV4 can be used to classify astrocytes into functionally heterogeneous groups. This idea is at odds with data from previous studies. Benfenati et al. (24) reported that most astrocytes express TRPV4; however, these results were obtained with a commercial antibody, and the specificity of the antibody was never assessed in samples from TRPV4KO mice. Additionally, Benfenati et al. (24) did not use Ca2+ imaging to determine how TRPV4 ligands affect astrocytes. In contrast to their experiments, ours used an in situ hybridization method that has previously been confirmed by our group and others (35, 36) and that has been demonstrated to produce no background in neural samples from TRPV4KO mice. Given the functional evidence (Figs. 1–4), it is clear that only a restricted subset of astrocytes express TRPV4. Moreover, sonic hedgehog has recently been shown to regulate the development of a subtype of astrocytes (37), and this finding demonstrates that astrocyte subtypes are specified early in development.
In this study we determined that TRPV4+ astrocytes communicate with surrounding astrocytes through gap junctions and/or ATP release (Figs. 2–4). Although it has been reported that astrocyte-derived ATP is inhibitory and is rarely a positive regulator of synaptic function (31, 33), we did not observe such an effect in our experiments. In addition to ATP, we identified glutamate as a TRPV4-induced excitatory gliotransmitter in astrocytes (Fig. 6, C and D). Opposing actions for astrocytic ATP and glutamate have been reported (7, 33). Our results indicate that the effects of glutamate on synaptic function are stronger than those of ATP (Fig. 6). If this is the case, then ATP-mediated regulation might only be required for astrocyte-astrocyte communication (Figs. 2–4). We also found that glutamate release enhanced presynaptic activity by signaling through type 1 mGluRs (Fig. 7, D–F). This type of positive regulation of synaptic activity by astrocyte-derived glutamate and the presynaptic expression of type 1 mGluRs, both, is supported by other reports (6, 34). In addition to these reports, glutamate released from astrocytes located near axons has recently been reported to modulate the duration of action potentials and enhance presynaptic neurotransmitter release (13, 38). Furthermore, an important finding of our study is that activation of TRPV4 in a subset of astrocytes elicits an increase in synaptic events through gliotransmitter release (Figs. 2–7) and that TRPV4+ astrocytes may be present throughout the brain. The unique function and localization of TRPV4+ astrocytes might form a localized core that triggers neural excitation in response to the release of one type of neurotransmitter (Fig. 7G). Thus, TRPV4+ astrocytes might synchronize neuronal excitability and help increase the diversity of synaptic information and brain function. Very recently, the pathophysiological function of TRPV4 in astrocytes has been reported (25, 26, 28). According to these reports, TRPV4 signaling in astrocytes prevents the progression of brain damage, but the physiological importance of astrocytic TRPV4 under normal conditions has remained unclear. We have demonstrated that an endogenous TRPV4 ligand, arachidonic acid, is sufficient to enhance synaptic activity (Fig. 5, E and F). We speculate that the homeostatic temperature of the brain weakly and constitutively activates TRPV4 in astrocytes (29) and that activation of TRPV4 by endogenous chemical ligands such as arachidonic acid can modulate synaptic activity through the release of gliotransmitters (Fig. 7G). In a separate study we have shown that another thermo TRP member, TRPV2 (39, 40), is expressed in all astrocytes (29) and that astrocytic TRPV2 responds to lysophosphatidylcholine. Arachidonic acid is generated at postsynaptic sites at the same time as lysophosphatidylcholine and might excite astrocytes by activating TRPV4. Therefore, astrocytic TRPV4 might be activated in response to increased lipid metabolism near synapses.
We visualized TRPV4-dependent ATP release from astrocytes by using both the biosensor and ATP imaging methods (Fig. 4). These results are very similar to those of our other reports, in which urinary bladder epithelial cells and esophageal keratinocytes released ATP in response to activation of TRPV4 (36, 41). However, this study provides information about the unique features of ATP release from astrocytes. Reduced ATP release from hippocampal astrocytes has recently been reported to trigger depression, but it remains unknown which types of astrocytes are involved (42). Our findings might help to identify the characteristics of ATP-releasing, purinergic astrocytes that are related to brain function. In astrocytes, TRPV4 is thought to enhance vasodilation and contribute to local Ca2+ oscillations in endfeet (43). Because TRPV4 can be activated by the metabolites of many lipids, the contribution of ATP release to vasodilation might depend on the status of these metabolites as reported (44). Furthermore, we recently reported that activation of TRPV4 promotes water efflux in the choroid plexus (45). Since astrocytes express various members of the aquaporin-4 family (1), TRPV4 might function similarly in all astrocytes that express this channel.
In our study, treating astrocytes with MPEP (a type 1 mGluR antagonist) partially blocked the enhanced synaptic transmission resulting from activation of TRPV4 (Fig. 7, E and F), although CPPG (a type 2/3 mGluR antagonist) did not have an inhibitory effect (Fig. 7D). Furthermore, we have identified another excitatory gliotransmitter candidate by using MALDI-TOF mass spectrometry analysis to compare conditioned media from cultures of WT and TRPV4KO astrocytes.3 These results strongly suggest that other gliotransmitters exist. Thus, we hypothesize that TRPV4+ astrocytes might release other excitatory gliotransmitters to positively regulate neuronal activity. Future studies will identify these new gliotransmitters and characterize their contribution to synaptic function.
Supplementary Material
Acknowledgments
We thank Drs. T. Mori (The National Institute for Basic Biology (NIBB), Okazaki, Japan), N. Inamura (National Institute for Physiological Sciences, Okazaki, Japan), S. Mizuno (Gunma University), and the technical division of NIBB for technical assistance and Drs. K. Yamada (Hirosaki University), B. MacVicar (British Columbia University), and our laboratory members for helpful discussions. The TRPV4KO mice were kindly provided by Dr. A. Mizuno (Jichi Medical University).
This work was supported by Grants-in-aid for Scientific Research (KAKENHI Projects 21200012, 20399554, 24111507, and 26111702 “Brain Environment” and 26117502 “glial assembly” (to K. S.), 23650159 (to Y. I.), and 18077012 (to M. T.)) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology, by grants from the Uehara Memorial Foundation, Sumitomo Foundation, Brain Science Foundation, Narishige Neuroscience Research Foundation, and Salt Science Research Foundation no. 13C2 (all to K. S.), and by a grant from the Takeda Science Foundation (to K. S. and Y. I.).

This article contains supplemental Movies 1–3.
S. Mizuno and K. Shibasaki, manuscript in preparation.
- TRPV4
- transient receptor potential (TRP) vanilloid 4
- 4αPDD
- 4α-phorbol-12,13-didecanoate
- GSK
- GSK1016790A
- GFAP
- glial fibrillary acidic protein
- P2X2
- P2X purinoreceptor type 2
- mEPSC
- miniature excitatory post synaptic current
- CPPG
- (RS)-α-cyclopropyl-4-phosphonophenylglycine
- MPEP
- 2-methyl-6-(phenylethynyl)pyridine.
REFERENCES
- 1. Kettenmann H, R. B., eds. (2005) Neuroglia, pp. 135–148, Oxford University Press, New York [Google Scholar]
- 2. Pannasch U., Rouach N. (2013) Emerging role for astroglial networks in information processing: from synapse to behavior. Trends Neurosci. 36, 405–417 [DOI] [PubMed] [Google Scholar]
- 3. Pannasch U., Vargová L., Reingruber J., Ezan P., Holcman D., Giaume C., Syková E., Rouach N. (2011) Astroglial networks scale synaptic activity and plasticity. Proc. Natl. Acad. Sci. U.S.A. 108, 8467–8472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takano T., Tian G. F., Peng W., Lou N., Libionka W., Han X., Nedergaard M. (2006) Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 9, 260–267 [DOI] [PubMed] [Google Scholar]
- 5. Mulligan S. J., MacVicar B. A. (2004) Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431, 195–199 [DOI] [PubMed] [Google Scholar]
- 6. Piet R., Jahr C. E. (2007) Glutamatergic and purinergic receptor-mediated calcium transients in Bergmann glial cells. J. Neurosci. 27, 4027–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sakatani S., Seto-Ohshima A., Shinohara Y., Yamamoto Y., Yamamoto H., Itohara S., Hirase H. (2008) Neural-activity-dependent release of S100B from astrocytes enhances kainate-induced γ oscillations in vivo. J. Neurosci. 28, 10928–10936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newman E. A. (2003) New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 26, 536–542 [DOI] [PubMed] [Google Scholar]
- 9. Kang N., Xu J., Xu Q., Nedergaard M., Kang J. (2005) Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 94, 4121–4130 [DOI] [PubMed] [Google Scholar]
- 10. Sasaki T., Beppu K., Tanaka K. F., Fukazawa Y., Shigemoto R., Matsui K. (2012) Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation. Proc. Natl. Acad. Sci. U.S.A. 109, 20720–20725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serrano A., Haddjeri N., Lacaille J. C., Robitaille R. (2006) GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J. Neurosci. 26, 5370–5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mishima T., Hirase H. (2010) In vivo intracellular recording suggests that gray matter astrocytes in mature cerebral cortex and hippocampus are electrophysiologically homogeneous. J. Neurosci. 30, 3093–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sasaki T., Kuga N., Namiki S., Matsuki N., Ikegaya Y. (2011) Locally Synchronized Astrocytes. Cereb. Cortex 21, 1889–1900 [DOI] [PubMed] [Google Scholar]
- 14. Liedtke W., Choe Y., Martí-Renom M. A., Bell A. M., Denis C. S., Sali A., Hudspeth A. J., Friedman J. M., Heller S. (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103, 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strotmann R., Harteneck C., Nunnenmacher K., Schultz G., Plant T. D. (2000) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2, 695–702 [DOI] [PubMed] [Google Scholar]
- 16. Wissenbach U., Bödding M., Freichel M., Flockerzi V. (2000) Trp12, a novel Trp related protein from kidney. FEBS Lett. 485, 127–134 [DOI] [PubMed] [Google Scholar]
- 17. Nilius B., Prenen J., Wissenbach U., Bödding M., Droogmans G. (2001) Differential activation of the volume-sensitive cation channel TRP12 (OTRPC4) and volume-regulated anion currents in HEK-293 cells. Pflugers Arch. 443, 227–233 [DOI] [PubMed] [Google Scholar]
- 18. Suzuki M., Mizuno A., Kodaira K., Imai M. (2003) Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 278, 22664–22668 [DOI] [PubMed] [Google Scholar]
- 19. Shibasaki K., Suzuki M., Mizuno A., Tominaga M. (2007) Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J. Neurosci. 27, 1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Güler A. D., Lee H., Iida T., Shimizu I., Tominaga M., Caterina M. (2002) Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 22, 6408–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watanabe H., Davis J. B., Smart D., Jerman J. C., Smith G. D., Hayes P., Vriens J., Cairns W., Wissenbach U., Prenen J., Flockerzi V., Droogmans G., Benham C. D., Nilius B. (2002) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem. 277, 13569–13577 [DOI] [PubMed] [Google Scholar]
- 22. Watanabe H., Vriens J., Suh S. H., Benham C. D., Droogmans G., Nilius B. (2002) Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J. Biol. Chem. 277, 47044–47051 [DOI] [PubMed] [Google Scholar]
- 23. Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424, 434–438 [DOI] [PubMed] [Google Scholar]
- 24. Benfenati V., Amiry-Moghaddam M., Caprini M., Mylonakou M. N., Rapisarda C., Ottersen O. P., Ferroni S. (2007) Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience 148, 876–892 [DOI] [PubMed] [Google Scholar]
- 25. Benfenati V., Caprini M., Dovizio M., Mylonakou M. N., Ferroni S., Ottersen O. P., Amiry-Moghaddam M. (2011) An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc. Natl. Acad. Sci. U.S.A. 108, 2563–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Butenko O., Dzamba D., Benesova J., Honsa P., Benfenati V., Rusnakova V., Ferroni S., Anderova M. (2012) The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS ONE 7, e39959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shibasaki K., Takebayashi H., Ikenaka K., Feng L., Gan L. (2007) Expression of the basic helix-loop-factor Olig2 in the developing retina: Olig2 as a new marker for retinal progenitors and late-born cells. Gene Expr. Patterns 7, 57–65 [DOI] [PubMed] [Google Scholar]
- 28. Shi M., Du F., Liu Y., Li L., Cai J., Zhang G. F., Xu X. F., Lin T., Cheng H. R., Liu X. D., Xiong L. Z., Zhao G. (2013) Glial cell-expressed mechanosensitive channel TRPV4 mediates infrasound-induced neuronal impairment. Acta Neuropathol. 126, 725–739 [DOI] [PubMed] [Google Scholar]
- 29. Shibasaki K., Ishizaki Y., Mandadi S. (2013) Astrocytes express functional TRPV2 ion channels. Biochem. Biophys. Res. Commun. 441, 327–332 [DOI] [PubMed] [Google Scholar]
- 30. Mandadi S., Sokabe T., Shibasaki K., Katanosaka K., Mizuno A., Moqrich A., Patapoutian A., Fukumi-Tominaga T., Mizumura K., Tominaga M. (2009) TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 458, 1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee H. U., Yamazaki Y., Tanaka K. F., Furuya K., Sokabe M., Hida H., Takao K., Miyakawa T., Fujii S., Ikenaka K. (2013) Increased astrocytic ATP release results in enhanced excitability of the hippocampus. Glia 61, 210–224 [DOI] [PubMed] [Google Scholar]
- 32. Theodosis D. T., Poulain D. A., Oliet S. H. (2008) Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol. Rev. 88, 983–1008 [DOI] [PubMed] [Google Scholar]
- 33. Koizumi S., Fujishita K., Tsuda M., Shigemoto-Mogami Y., Inoue K. (2003) Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc. Natl. Acad. Sci. U.S.A. 100, 11023–11028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Navarrete M., Araque A. (2010) Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126 [DOI] [PubMed] [Google Scholar]
- 35. Gevaert T., Vriens J., Segal A., Everaerts W., Roskams T., Talavera K., Owsianik G., Liedtke W., Daelemans D., Dewachter I., Van Leuven F., Voets T., De Ridder D., Nilius B. (2007) Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J. Clin. Invest. 117, 3453–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mochizuki T., Sokabe T., Araki I., Fujishita K., Shibasaki K., Uchida K., Naruse K., Koizumi S., Takeda M., Tominaga M. (2009) The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J. Biol. Chem. 284, 21257–21264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garcia A. D., Petrova R., Eng L., Joyner A. L. (2010) Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J. Neurosci. 30, 13597–13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sasaki T., Matsuki N., Ikegaya Y. (2011) Action-potential modulation during axonal conduction. Science 331, 599–601 [DOI] [PubMed] [Google Scholar]
- 39. Shibasaki K., Murayama N., Ono K., Ishizaki Y., Tominaga M. (2010) TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J. Neurosci. 30, 4601–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mihara H., Boudaka A., Shibasaki K., Yamanaka A., Sugiyama T., Tominaga M. (2010) Involvement of TRPV2 activation in intestinal movement through nitric oxide production in mice. J. Neurosci. 30, 16536–16544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mihara H., Boudaka A., Sugiyama T., Moriyama Y., Tominaga M. (2011) Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J. Physiol. 589, 3471–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cao X., Li L. P., Wang Q., Wu Q., Hu H. H., Zhang M., Fang Y. Y., Zhang J., Li S. J., Xiong W. C., Yan H. C., Gao Y. B., Liu J. H., Li X. W., Sun L. R., Zeng Y. N., Zhu X. H., Gao T. M. (2013) Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 19, 773–777 [DOI] [PubMed] [Google Scholar]
- 43. Dunn K. M., Hill-Eubanks D. C., Liedtke W. B., Nelson M. T. (2013) TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc. Natl. Acad. Sci. U.S.A. 110, 6157–6162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gordon G. R., Choi H. B., Rungta R. L., Ellis-Davies G. C., MacVicar B. A. (2008) Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456, 745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takayama Y., Shibasaki K., Suzuki Y., Yamanaka A., Tominaga M. (2014) Modulation of water efflux through functional interaction between TRPV4 and TMEM16A/anoctamin 1. FASEB J., in press [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.