Background: FSP27 is a lipid droplet-associated protein.
Results: Expression of FSP27 in human adipocytes reversely correlates with ATGL levels. Mechanistically, FSP27 increases the inhibitory effect of Egr1 on the ATGL promoter.
Conclusion: FSP27 controls lipolysis by regulating ATGL transcription.
Significance: Our study provides a new model of regulation of lipolysis in adipocytes.
Keywords: Adipocyte, Adipose Triglyceride Lipase (ATGL), Early Growth Response Protein 1 (EGR1), Lipid Droplets, Lipolysis
Abstract
Lipolysis in fat tissue represents a major source of circulating fatty acids. Previously, we have found that lipolysis in adipocytes is controlled by early growth response transcription factor Egr1 that directly inhibits transcription of adipose triglyceride lipase, ATGL (Chakrabarti, P., Kim, J. Y., Singh, M., Shin, Y. K., Kim, J., Kumbrink, J., Wu, Y., Lee, M. J., Kirsch, K. H., Fried, S. K., and Kandror, K. V. (2013) Mol. Cell. Biol. 33, 3659–3666). Here we demonstrate that knockdown of the lipid droplet protein FSP27 (a.k.a. CIDEC) in human adipocytes increases expression of ATGL at the level of transcription, whereas overexpression of FSP27 has the opposite effect. FSP27 suppresses the activity of the ATGL promoter in vitro, and the proximal Egr1 binding site is responsible for this effect. FSP27 co-immunoprecipitates with Egr1 and increases its association with and inhibition of the ATGL promoter. Knockdown of Egr1 attenuates the inhibitory effect of FSP27. These results provide a new model of transcriptional regulation of ATGL.
Introduction
Current epidemics of metabolic diseases, such as type 2 diabetes, cardiac dysfunction, hypertension, hepatic steatosis, etc., are largely caused by widespread obesity. Although obesity can affect human health via several different mechanisms (1), the best established connection between obesity and metabolic disease is abnormal levels of circulating fatty acids (FA).3 FA play important physiological roles in energy production and the synthesis of most lipids; nonetheless, their oversupply is highly detrimental as it leads to insulin resistance, oxidative stress, and other pathophysiological effects via mechanisms that are currently under intense investigation (1–5).
Normally, dietary FA are partitioned into adipose tissue, converted into triglycerides, and stored in lipid droplets (LDs) that represent dynamic intracellular organelles consisting of a core of triglycerides and cholesterol esters, surrounded by a monolayer of phospholipids. Several proteins are associated with this monolayer, notably the PAT family proteins, PLIN 1–5 (6, 7), and fat-specific protein 27 (FSP27, also known as CIDEC) (8–10). The latter protein plays an essential role in the regulation of LD morphology. Depletion of FSP27 in adipocytes leads to fragmentation of LDs (11, 12), whereas overexpression of FSP27 increases the size of LDs while decreasing their number (8, 9, 12) by promoting LD fusion (13) and exchanging lipids from one droplet to another (14).
It has also been demonstrated by us and others that FSP27 has anti-lipolytic activity (8, 9, 12, 15–18). Lipolysis in adipose tissue is the major source of circulating FA (19–24). Correspondingly, unrestricted lipolysis in adipose tissue represents a serious metabolic defect and a causative factor of insulin resistance, diabetes mellitus, and other metabolic diseases (3, 25–27). As the mechanism of the anti-lipolytic activity of FSP27 is not completely clear, we decided to focus on this problem.
Our recent study showed that FSP27 directly interacts with ATGL and regulates its lipase activity (18). Another study predicted that FSP27-mediated fusion of LDs might limit the access of intracellular lipases to the LD surface due to decreased surface area of larger LDs (17). As ATGL represents a major lipolytic enzyme (28), reducing its contact with LDs may suppress lipolysis.
Here, we report another mechanism of the anti-lipolytic action of FSP27 in human adipocytes. We have found that FSP27 inhibits expression of ATGL at the level of transcription by stimulating the effect of its transcriptional repressor Egr1.
EXPERIMENTAL PROCEDURES
Cell Culture
Human preadipocytes were procured from the Boston Nutrition Obesity Research Center adipocyte core. Cells were seeded at a density of 5,000 cells/ml/well in a 12-well plate, in growth medium, which was made using 13.5 g of α-MEM powder (Life Technologies) with 10% FBS (Life Technologies), 100 units/ml of penicillin and streptomycin (Life Technologies), and 25 mm sodium bicarbonate (Fisher Scientific) reconstituted in double-distilled water (ddH2O) to 1 liter, pH 7.2–7.3. This growth medium was changed every 2 days, until cells reached 90% confluence. To allow the cells to differentiate, the GM was then replaced by complete differentiation medium, which consisted of: 13.5 g of DMEM powder/F12 (Life Technologies), 100 units/ml of penicillin and streptomycin (Life Technologies), 15 mm Hepes (Sigma), 25 mm sodium bicarbonate (Fisher Scientific), 33 μm biotin (Sigma), 17 μm pantothenate (Sigma), 0.5 mm isobutylmethylxanthine (Sigma), 10 mg/liter transferrin (Sigma), 1 μm Rosiglitazone (BIOMOL), 100 nm dexamethasone (Sigma), 100 nm human insulin (recombinant), 2 nm triiodo-l-thyronine (Sigma), reconstituted in ddH2O to 1 liter, pH 7.4. The day cells were first given complete differentiation medium is designated as day 0. The medium was changed every 2 days until the preadipocytes were fully differentiated into mature human white adipocytes that were filled with lipid droplets. Following differentiation, the cells were maintained in maintenance medium until fully differentiated with the medium being changed every 2 days. Maintenance medium was made of the similar constituents as complete differentiation medium, except without Rosiglitazone, isobutylmethylxanthine, triiodo-l-thyronine, and transferrin. Additionally, the concentrations of human insulin and dexamethasone used were 10 nm each.
3T3-L1 preadipocytes were cultured, differentiated, and maintained as described previously (29). HEK 293T cells were grown in DMEM supplemented with 10% fetal bovine serum in 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin.
siRNA Treatment and Adenoviral Transduction of Human Adipocytes
The following siRNA constructs were used for experiments: siScr (from Qiagen) All Stars Negative Control; human siFSP27 (from Dharmacon): AACUGUACAGACAGAAGAGUAUU. On day 9 following differentiation, cultured adipocytes were transfected with 40 nm siRNA, 5 μl/well PLUS reagent and 3 μl/well Lipofectamine reagent, all from Invitrogen, in a total volume of 350 μl/well (100 μl of siRNA mixture, 250 μl of maintenance medium). Twenty-four hours after transfection, 500 μl of maintenance medium was added to cells. The medium was then changed 24 h later with 1 ml of maintenance medium. Cells were further incubated for 3 days (day 14) before being harvested for analysis. FSP27-HA tagged adenoviruses were generated at the Adenoviral Vector Core Facility at Tufts Medical Center. FSP-CFP adenovirus was a kind gift from Drs. Carole Sztalryd and Da-Wei Gong from the University of Maryland. The adenovirus was added to adipocytes on either day 9 (if the experiment was solely studying effects of overexpression) or day 14 (for re-expression analysis) of differentiation at multiplicity of infection of 100. Cells were then harvested 48 h after infection for analysis.
RNA Isolation and Quantitative PCR
Total RNA was isolated from human adipocytes from each well (12-well plate) with 1 ml of TRIzol (Invitrogen). Total RNA isolation was performed as described in the manufacturer's protocol, and RNA concentrations were measured on a NanoDropTM ND-1000 spectrophotometer (Thermo Scientific). For quantitative PCR (mRNA analysis), 1 μg of RNA was reverse-transcribed with Transcriptor first strand cDNA synthesis kit (Roche Applied Science). The cDNA was diluted 1:10, and 1 μl was used for each reaction (per well, 384-well plate) in triplicates. Primers (TaqMan) were used with Roche LightCycler 480 Probes Master kit (Roche Applied Science) at 0.25 μl, with 2.5 μl of master stock and 1.25 μl of ddH2O per reaction. Thermal cycling conditions were: 10 min at 95 °C for activation, 45 cycles of 10 s at 95 °C, 30 s at 60 °C, and 1 s at 72 °C for reactions, and 5 min at 37 °C for cooling down. The comparative ΔCt method was used to calculate relative changes in mRNA abundance.
Transient Transfections and Reporter Gene Assays
The cDNA for FSP27 was purchased from Open Biosystems; ATGL promoter luciferase constructs (30) and pcDNA3 expression plasmids for Egr1 (31) have been described earlier. Transient transfections with cDNA were performed using Lipofectamine 2000 (Life Technologies) according to the manufacturer's instructions. Briefly, ∼80% confluent HEK293T cells were transfected with 1 μg of luciferase reporter constructs, FSP27 cDNA, Egr1 cDNA, and 100 ng of eGFP cDNA in a 6-well plate format. All experiments were performed in triplicate. After 48 h of transfection, cells were harvested in reporter lysis buffer (Promega). Luciferase activity was determined in whole cell lysates using the Promega luciferase assay kit and expressed as relative light units. Expression of eGFP was measured fluorometrically. Firefly luciferase was normalized by eGFP fluorescence to correct for transfection efficiency.
siRNA Treatment of HEK293T Cells
cells in 60-mm dishes were transfected with siScr SMARTpool ON-TARGETplus and human siEgr1 SMARTpool ON-TARGETplus (Dharmacon Inc., Lafayette, CO) using Lipofectamine 2000 according to the manufacturer's protocol.
Site-directed Mutagenesis
Nucleotides −44, −42, −41, −40, and −36 in the ATGL promoter were replaced for T's using QuikChange II-XL site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). The primers m1 and m2 for site-directed mutagenesis were synthesized by Eurofins MWG Operon, Huntsville, AL: m1, 5′-CAAAATTCTGAGCCAGGCGCCCtGtttTCAtCCGCACTAAAACACCTCCTC-3′; m2, 5′-CTTACGCGTGCTAGCGCCCtGtttTCAtCCGCACTAAAACACCTCCTC-3′ (mutations are shown in small case and underlined). The ATGL promoter constructs 2979/+21 and −48/+21 were used as template for the PCR reaction to produce mutations. The nucleotide sequence of all mutant constructs was confirmed by sequencing.
Chromatin immunoprecipitation (ChIP) Assay
ChIP was carried out using EZ-Magna ChIP HiSens kit (Millipore, MA) as described in the manufacturer's protocol. Briefly, HEK293T cells (75% confluent) were transfected with wild type (WT) and mutated ATGL promoter constructs (−2979/+21), Egr1 cDNA, and Myc-Fsp27 cDNA. Forty-eight hours after transfection, cells were treated with formaldehyde (final concentration 1%) for 10 min and then with glycine to quench excess formaldehyde. Cells were collected and suspended in hypotonic nuclei isolation buffer. Nuclei were sonicated using Bioruptor (Diagenode, NJ) with 8 pulses of 15 s each at high setting and centrifuged for 10 min. Supernatant was immunoprecipitated with 2 μg of the polyclonal antibody against Egr1 (Cell Signaling) or Myc (Millipore) using Magna CHIP protein A/G magnetic beads (10 μl) at 4 °C overnight. Rabbit IgG antibody (Millipore) was used as control. Antibody-bound chromatin fragments were washed three times with low stringency immunoprecipitation wash buffer and eluted from magnetic beads with 50 μl of ChIP elution buffer. Protein-DNA cross-links were treated with proteinase K at 65 °C for 2 h and then at 95 °C for 15 min. DNA was subjected to PCR to amplify the region between −102 and +21 of the ATGL promoter using primers described in Ref. 32.
Immunoprecipitation
HEK293T cells were co-transfected with Egr1 and HA-tagged FSP27 expression vectors in a 60-mm tissue culture dish. Twenty-four hours after transfection, cell lysates were harvested in the lysis buffer (1% Triton X-100, 50 mm Tris, 10 mm EDTA, 150 mm NaCl, protease inhibitor cocktail from Roche Applied Science). Cell lysates were immunoprecipitated with mouse anti-HA antibody (Roche Applied Science) or nonspecific mouse antibody and anti-mouse Dynabeads overnight at 4 °C on an orbital shaker. Proteins were eluted with Laemmli sample buffer and analyzed by Western blotting.
Lipolysis Assay
For analysis of total cellular glycerol and fatty acid release, cells were incubated in Krebs-Ringer buffer (24.6 mm NaHCO3, 1.11 mm KH2PO4, 130 mm NaCl, 4.7 mm KCl, 1.24 mm MgSO4, 3.3 mm CaCl2, 4% w/v BSA, 5 mm glucose, pH 7.4) for 2.5 h. Then, buffer was collected and analyzed for glycerol and fatty acid release under basal conditions. Glycerol and fatty acids were measured by using the serum triglyceride determination kit from Sigma-Aldrich and BioVision kit, respectively, according to the manufacturer's instructions.
Protein Quantification and Analysis
Cells were lysed in cell lysis buffer (Cell Signaling) with 5% SDS, protease inhibitor cocktail (Roche Applied Science) and phosphatase inhibitor cocktail (Roche Applied Science). Protein lysates were then briefly sonicated, incubated in a 37 °C water bath for 30 min, and then centrifuged at 10,000 rpm for 10 min. The clear middle phase of the solution was then collected as the protein sample. Protein quantification was performed using the Pierce BSA protein assay kit (Thermo Scientific). Immunoblots (Western blot) were performed using 10 and 15% Tris-HCl gels (Bio-Rad), run at 100 volts for 90 min, and transferred to PVDF membranes (Bio-Rad), run at 60 volts, for 120 min. Primary antibodies used were all diluted in 2% BSA in TBS-T. Antibodies used were: mouse anti-β-tubulin (Invitrogen, 1:5000 dilution), rabbit anti-FSP27 (1:4000 dilution), and rabbit IgG anti-ATGL (1:5000 dilution). Secondary antibodies were purchased from Santa Cruz Biotechnology (1:10000 dilution).
Immunostaining and Confocal Microscopy
Detection of endogenous FSP27 for immunostaining was performed using rabbit FSP27 polyclonal antibody as shown in our recent study (16). Microscopy was performed using a Zeiss LSM 710-Live Duo scan (Carl Zeiss, Oberkochen, Germany) with a 100× oil immersion objective. Cells cultured on glass coverslips were washed twice with PBS and fixed with 4% paraformaldehyde. Images were processed using MetaMorph imaging software, version 6.1 (Universal Imaging, Downingtown, PA).
Statistics
Quantitative data are presented as mean ± S.D. Statistical significance was calculated using paired Student's t test. A p value of less than 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
It has been well established that overexpression of FSP27 suppresses, whereas ablation of FSP27 increases lipolysis (8, 9, 12, 15–18). Intriguingly, depletion of FSP27 leads to changes in expression of multiple genes, although the mechanism of this effect remains unknown (12, 33, 34). Given that the rates of lipolysis tightly correlate with the levels of ATGL (35–45), we decided to examine whether FSP27 has any role in the regulation of ATGL expression.
Cultured human adipocytes were treated either with siRNA directed against FSP27 or the scrambled siRNA as control. Cells were harvested 5 days after transfection, and expression of FSP27 and ATGL was measured by quantitative PCR and Western blotting.
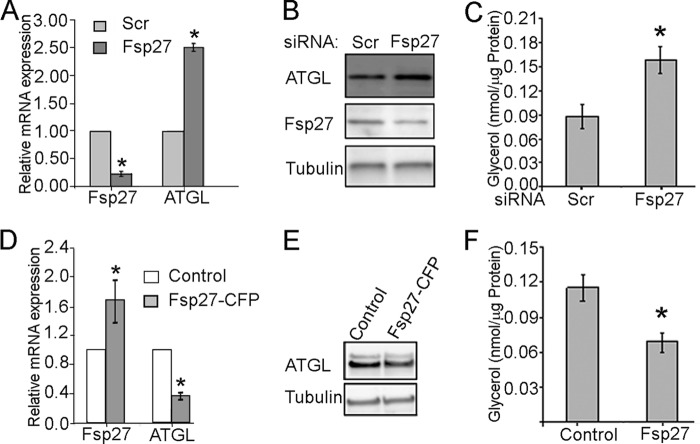
As shown in Fig. 1, A and B, FSP27 siRNA treatment resulted in 70% knockdown of FSP27 mRNA and 80% knockdown of the FSP27 protein. This decrease in FSP27 expression was accompanied by a 2.8-fold increase in ATGL mRNA, a 1.8-fold increase in ATGL protein, and an increase in basal lipolysis measured by glycerol release (Fig. 1C). Basal FA release was 5–7-fold smaller than glycerol release (supplemental Fig. S1A), suggesting high rates of re-esterification in human adipocytes under our experimental conditions (see also Ref. 40).
FIGURE 1.
FSP27 negatively regulates ATGL expression and lipolysis in human adipocytes. Human adipocytes were cultured and differentiated as described under “Experimental Procedures.” A, RNA was extracted from control and siRNA-treated adipocytes, and mRNA levels were measured by quantitative PCR and normalized by GAPDH mRNA. The data show an average of three independent experiments. B, protein lysates from control and siRNA-treated adipocytes were loaded at 15 μg/lane and probed with antibodies against FSP27, ATGL, or β-tubulin. Image is representative of at least three independent experiments. C, FSP27 knockdown increases basal lipolytic rate measured during 2.5-h incubation in Krebs-Ringer buffer with 4% BSA in human adipocytes. D, RNA was extracted from adipocytes treated with adenovirus expressing FSP27-CFP or control adenovirus, and mRNA levels were measured by quantitative PCR and normalized by GAPDH mRNA. The data show an average of three independent experiments. E, protein lysates from adipocytes treated with adenovirus expressing FSP27-CFP or control adenovirus were loaded at 15 μg/lane and probed with antibodies against ATGL and β-tubulin. Image is representative of experiment performed in triplicates. F, FSP27-CFP expression decreases basal lipolytic rate measured during 2.5-h incubation in Krebs-Ringer buffer with 4% BSA in human adipocytes. All values are mean ± S.D.; *, p < 0.05 as estimated by unpaired t test.
Next, we treated cultured human adipocytes with adenoviral preparation of CFP-tagged FSP27 or control adenovirus. As shown in Fig. 1, D and E, even a modest 50% increase in total FSP27 mRNA (endogenous and FSP27-CFP) and protein (not shown) resulted in a decrease in the ATGL protein and a corresponding decrease in basal glycerol release (Fig. 1F; see also supplemental Fig. S1B for FA release).
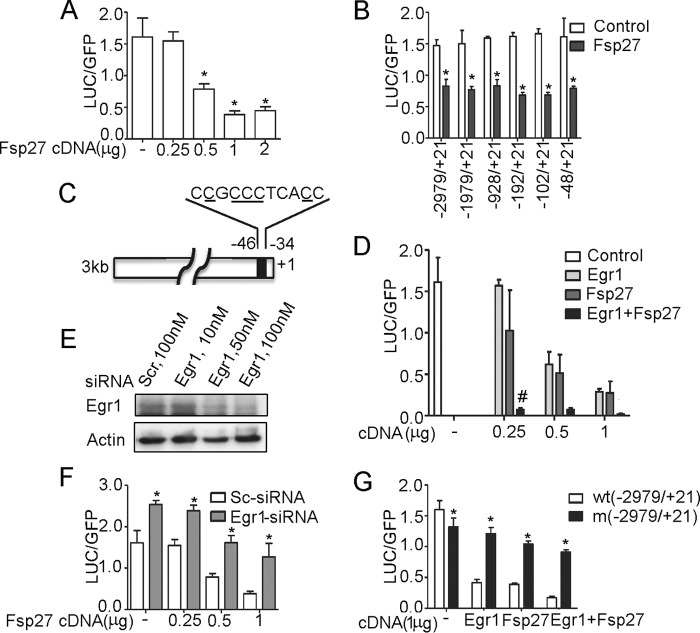
Overall, results shown in Fig. 1 suggest that FSP27 may regulate ATGL expression in human adipocytes at the level of transcription. To dissect the mechanism of this regulation, we examined the direct effect of FSP27 on the ATGL promoter activity in HEK293T cells that do not express this protein endogenously. We found that FSP27 inhibited activity of the ATGL promoter in vitro in a dose-dependent fashion (Fig. 2A). Truncation analysis showed that the negative effect of FSP27 on transcription of the reporter gene was maintained even in the case of the shortest ATGL promoter that included only the proximal 48 bp (Fig. 2B). This region of the ATGL promoter contains the binding site for the transcription factor Egr1 (Fig. 2C) that directly binds to and inhibits activity of the ATGL promoter (32). Indeed, upon expression in HEK293T cells, FSP27 and Egr1 demonstrated a synergistic effect in the inhibition of the ATGL promoter activity (Fig. 2D). Knockdown of endogenous Egr1 in HEK293T cells with siRNA (Fig. 2E) attenuated the inhibitory effect of exogenously expressed FSP27 (Fig. 2F). Replacement of 5 nucleotides (Fig. 2C, underlined) in the consensus Egr1 binding site for T's strongly decreased the inhibitory effect of exogenously expressed FSP27 and Egr1 (Fig. 2G). These results suggest that FSP27 inhibits ATGL transcription by potentiating the inhibitory effect of Egr1 on the ATGL promoter.
FIGURE 2.
FSP27 inhibits ATGL promoter activity via Egr1. A, B, D, F, and G, HEK293T cells cultured in 12-well dishes were transfected with the full-length (−2979/+21), C→T mutated, or truncated ATGL luciferase (LUC) promoter constructs, cDNA for eGFP; cDNAs for Fsp27 and Egr1; as well as scrambled siRNA and Egr1 siRNA as indicated. After 48 h, cells were washed three times in cold PBS and harvested in the reporter lysis buffer. Luciferase activity in cell lysates was assayed and normalized by eGFP fluorescence. Data are presented for triplicate samples as mean ± S.D.; *, p < 0.05 as estimated by unpaired t test. In D, # indicates the synergistic effect between Egr1 and FSP27 with p < 0.05. Experiments were repeated at least three times (A, B, D, and G) and two times (F). C, schematic representation of the proximal region of ATGL promoter with the consensus Egr1 binding site. Nucleotides that have been chosen for the site-directed mutagenesis are underlined. E, HEK293T cells growing in 35-mm dishes were transfected with scrambled or Egr1 siRNA. Cell lysates were collected 48 h after transfection, separated by 12.5% PAGE, and immunoblotted with Egr1 and actin antibodies. The experiment was repeated at least two times.
In agreement with this model, we were able to co-immunoprecipitate Egr1 with HA-tagged FSP27 upon expression of both proteins in HEK293T cells (Fig. 3A), indicating that FSP27 may bind to Egr1. Replacement of HA tag for Myc tag in FSP27 did not affect the results of co-immunoprecipitation (supplemental Fig. S2).
FIGURE 3.
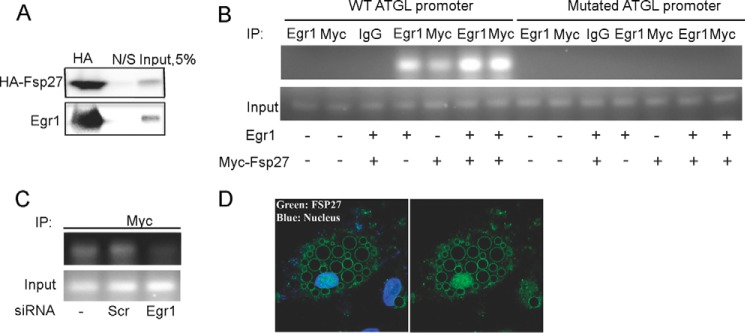
FSP27 interacts with Egr1 and facilitates its recruitment to the ATGL promoter. A, HEK293T cells were co-transfected with Egr1 (2 μg) and HA-tagged FSP27 (4 μg) expression vectors in a 60-mm tissue culture dish. Twenty-four hours after transfection, cell lysates (500 μg) were immunoprecipitated with mouse anti-HA antibody (1 μg) or nonspecific (N/S) mouse antibody and anti-mouse Dynabeads overnight at 4 °C on an orbital shaker. Proteins were eluted with Laemmli sample buffer and analyzed by Western blotting. The experiment was repeated at least three times. B, ChIP assays were performed in HEK293T cells transfected with either wild type (WT) or mutated ATGL promoter (0.5 μg) and Egr1 and Myc-Fsp27 cDNAs (2 μg each). Isolated chromatin was immunoprecipitated (IP) using polyclonal antibodies against Egr1, Myc, or nonspecific rabbit IgG; PCR-amplified cDNA fragments were separated in 2% agarose gel and visualized using ethidium bromide. The experiment was repeated three times. C, ChIP assay was performed in HEK293T cells transfected with the wild type ATGL promoter (0.5 μg), scrambled siRNA, and Egr1 siRNA. The experiment was repeated two times. D, endogenous FSP27 (green) was visualized in cultured human adipocytes by immunofluorescence as described under “Experimental Procedures” with (left) or without (right) DAPI nuclear staining. The experiment was repeated at least three times.
To get insight into the mechanism of FSP27 action, we carried out a chromatin immunoprecipitation assay using HEK293T cells transfected with Egr1 and Myc-tagged FSP27 in different combinations. In these experiments, we used full-length (−2979/+21) wild type and mutated ATGL promoters, and we amplified a 200-bp-long region that included the proximal Egr1 binding site shown in Fig. 2C. As expected, Egr1 interacted with its own binding site, and mutation of this site blocked binding (Fig. 3B), which is consistent with results shown in Fig. 2G. Interestingly, co-transfection of Egr1 together with Myc-FSP27 significantly increased binding of Egr1 to the wild type ATGL promoter (Fig. 3B). Note that exogenously expressed Myc-FSP27 was also associated with the wild type but not mutated Egr1 binding site of the ATGL promoter (Fig. 3B). To determine whether this interaction is mediated by the endogenously expressed Egr1, we carried out ChIP experiments using Egr1 knockdown cells (Fig. 2E). We found that in the absence of Egr1, the interaction of FSP27 with the ATGL promoter is abrogated (Fig. 3C), suggesting that FSP27 interacts with DNA not directly, but via Egr1.
Previous studies have established that most of FSP27 in adipocytes is associated with LDs (8–10), although some of this protein has also been detected in other intracellular compartments (16) and in particular, in the cell nucleus (46). Indeed, our subsequent analysis showed that a significant fraction of FSP27 is localized in the nucleus of human adipocytes (Fig. 3D). Thus, we conclude that FSP27 inhibits lipolysis, at least in part, by potentiating the effect of Egr1 on ATGL transcription. As expression of both Egr1 (32) and FSP27 (47) is strongly up-regulated by insulin, this regulatory axis may contribute to the anti-lipolytic effect of insulin, which is essential for normal lipid partitioning and overall metabolic health (26).
The functional connection between FSP27, Egr1, and lipolysis described in this study may represent a regulatory loop that couples the size of lipid stores in the adipocyte to transcription in the nucleus. Indeed, in obesity, fat tissue mass can expand by an order of magnitude and more. A proportional increase in lipolysis and circulating FA would be life-threatening. Fortunately, fasting plasma FA levels demonstrate a much more modest increase and are largely unrelated to body fat mass (48), although the mechanisms of this compensation are not known. One such mechanism may be the suppression of ATGL expression (32, 49) by Egr1 and FSP27 that are both up-regulated by high fat feeding (32, 50). This may represent a compensatory response in adipocytes that keeps circulating FA within physiological range and protects the body from detrimental effects of obesity.
Our observations may mechanistically explain many of the previously described physiological functions of FSP27. Thus, FSP27-deficient mice are protected from diet-induced obesity and insulin resistance (11, 12, 51), the phenotype that is mimicked by adipose-specific overexpression of ATGL (52). Furthermore, the link between FSP27 knock-out, fat catabolism, and mitochondrial oxidation (11) may also be mediated by ATGL that up-regulates mitochondrial activity by producing a yet unidentified ligand for peroxisome proliferator-activated receptor α (PPARα) (53).
Finally, we would like to point out that while our experiments were in progress, Wang et al. (54) reported that FSP27 interacts with and co-activates CCAAT/enhancer-binding protein β (C/EBPβ). Thus, FSP27 may emerge as a novel regulator of adipocyte transcription.
Supplementary Material
Acknowledgments
We thank Drs. Susan Fried and Valentina Perissi (Boston University Medical School) for helpful and insightful discussions.
This work was supported by Grants DK52057 and AG039612 from the National Institutes of Health, Grant 7-11-BS-76 from the American Diabetes Association, and a research award from the Allen Foundation (to K. V. K.); National Institutes of Health Grants R56DK094815 and 8KL2TR000158 (from the parent grant UL1-TR000157 to the Clinical and Translational Science Institute, Boston University); and Boston Nutrition and Obesity Research Center Pilot Grant P30DK046200 (to V. P.).

This article contains supplemental Figs. S1 and S2.
- FA
- fatty acids
- LD
- lipid droplet
- FSP27
- fat-specific protein 27
- ATGL
- adipose triglyceride lipase
- ddH2O
- double-distilled water
- eGFP
- enhanced green fluorescent protein.
REFERENCES
- 1. Qatanani M., Lazar M. A. (2007) Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 21, 1443–1455 [DOI] [PubMed] [Google Scholar]
- 2. Guilherme A., Virbasius J. V., Puri V., Czech M. P. (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Savage D. B., Petersen K. F., Shulman G. I. (2007) Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 87, 507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stumvoll M., Goldstein B. J., van Haeften T. W. (2008) Type 2 diabetes: pathogenesis and treatment. Lancet 371, 2153–2156 [DOI] [PubMed] [Google Scholar]
- 5. Coen P. M., Goodpaster B. H. (2012) Role of intramyocelluar lipids in human health. Trends Endocrinol. Metab. 23, 391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brasaemle D. L. (2007) Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48, 2547–2559 [DOI] [PubMed] [Google Scholar]
- 7. Ducharme N. A., Bickel P. E. (2008) Lipid droplets in lipogenesis and lipolysis. Endocrinology 149, 942–949 [DOI] [PubMed] [Google Scholar]
- 8. Keller P., Petrie J. T., De Rose P., Gerin I., Wright W. S., Chiang S. H., Nielsen A. R., Fischer C. P., Pedersen B. K., MacDougald O. A. (2008) Fat-specific protein 27 regulates storage of triacylglycerol. J. Biol. Chem. 283, 14355–14365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Puri V., Konda S., Ranjit S., Aouadi M., Chawla A., Chouinard M., Chakladar A., Czech M. P. (2007) Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J. Biol. Chem. 282, 34213–34218 [DOI] [PubMed] [Google Scholar]
- 10. Brasaemle D. L., Dolios G., Shapiro L., Wang R. (2004) Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279, 46835–46842 [DOI] [PubMed] [Google Scholar]
- 11. Toh S. Y., Gong J., Du G., Li J. Z., Yang S., Ye J., Yao H., Zhang Y., Xue B., Li Q., Yang H., Wen Z., Li P. (2008) Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS One 3, e2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishino N., Tamori Y., Tateya S., Kawaguchi T., Shibakusa T., Mizunoya W., Inoue K., Kitazawa R., Kitazawa S., Matsuki Y., Hiramatsu R., Masubuchi S., Omachi A., Kimura K., Saito M., Amo T., Ohta S., Yamaguchi T., Osumi T., Cheng J., Fujimoto T., Nakao H., Nakao K., Aiba A., Okamura H., Fushiki T., Kasuga M. (2008) FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest. 118, 2808–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jambunathan S., Yin J., Khan W., Tamori Y., Puri V. (2011) FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation. PLoS One 6, e28614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gong J., Sun Z., Wu L., Xu W., Schieber N., Xu D., Shui G., Yang H., Parton R. G., Li P. (2011) Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol. 195, 953–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ranjit S., Boutet E., Gandhi P., Prot M., Tamori Y., Chawla A., Greenberg A. S., Puri V., Czech M. P. (2011) Regulation of fat specific protein 27 by isoproterenol and TNF-α to control lipolysis in murine adipocytes. J. Lipid Res. 52, 221–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grahn T. H., Zhang Y., Lee M. J., Sommer A. G., Mostoslavsky G., Fried S. K., Greenberg A. S., Puri V. (2013) FSP27 and PLIN1 interaction promotes the formation of large lipid droplets in human adipocytes. Biochem. Biophys. Res. Commun. 432, 296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang X., Heckmann B. L., Zhang X., Smas C. M., Liu J. (2013) Distinct mechanisms regulate ATGL-mediated adipocyte lipolysis by lipid droplet coat proteins. Mol. Endocrinol. 27, 116–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grahn T. H., Kaur R., Yin J., Schweiger M., Sharma V. M., Lee M. J., Ido Y., Smas C. M., Zechner R., Lass A., Puri V. (2014) FSP27 interacts with ATGL to regulate lipolysis and insulin sensitivity in human adipocytes. J. Biol. Chem. 289, 12029–12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H. S. (2007) Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 27, 79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lafontan M., Langin D. (2009) Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 48, 275–297 [DOI] [PubMed] [Google Scholar]
- 21. Lass A., Zimmermann R., Oberer M., Zechner R. (2011) Lipolysis: a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 50, 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zechner R., Zimmermann R., Eichmann T. O., Kohlwein S. D., Haemmerle G., Lass A., Madeo F. (2012) Fat signals: lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15, 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zimmermann R., Lass A., Haemmerle G., Zechner R. (2009) Fate of fat: The role of adipose triglyceride lipase in lipolysis. Biochim. Biophys. Acta 1791, 494–500 [DOI] [PubMed] [Google Scholar]
- 24. Coleman R. A., Mashek D. G. (2011) Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chem. Rev. 111, 6359–6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Delarue J., Magnan C. (2007) Free fatty acids and insulin resistance. Curr. Opin Clin. Nutr. Metab. Care 10, 142–148 [DOI] [PubMed] [Google Scholar]
- 26. McGarry J. D. (1992) What if Minkowski had been ageusic? An alternative angle on diabetes. Science 258, 766–770 [DOI] [PubMed] [Google Scholar]
- 27. McGarry J. D. (2002) Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51, 7–18 [DOI] [PubMed] [Google Scholar]
- 28. Zechner R., Kienesberger P. C., Haemmerle G., Zimmermann R., Lass A. (2009) Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 50, 3–21 [DOI] [PubMed] [Google Scholar]
- 29. Xu Z., Kandror K. V. (2002) Translocation of small preformed vesicles is responsible for the insulin activation of glucose transport in adipose cells. Evidence from the in vitro reconstitution assay. J. Biol. Chem. 277, 47972–47975 [DOI] [PubMed] [Google Scholar]
- 30. Kim J. Y., Tillison K., Lee J. H., Rearick D. A., Smas C. M. (2006) The adipose tissue triglyceride lipase ATGL/PNPLA2 is downregulated by insulin and TNF-α in 3T3-L1 adipocytes and is a target for transactivation by PPARγ. Am. J. Physiol. Endocrinol. Metab. 291, E115–E127 [DOI] [PubMed] [Google Scholar]
- 31. Kumbrink J., Kirsch K. H., Johnson J. P. (2010) EGR1, EGR2, and EGR3 activate the expression of their coregulator NAB2 establishing a negative feedback loop in cells of neuroectodermal and epithelial origin. J. Cell. Biochem. 111, 207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chakrabarti P., Kim J. Y., Singh M., Shin Y. K., Kim J., Kumbrink J., Wu Y., Lee M. J., Kirsch K. H., Fried S. K., Kandror K. V. (2013) Insulin inhibits lipolysis in adipocytes via the evolutionary conserved mTORC1-Egr1-ATGL-mediated pathway. Mol. Cell. Biol. 33, 3659–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li D., Zhang Y., Xu L., Zhou L., Wang Y., Xue B., Wen Z., Li P., Sang J. (2010) Regulation of gene expression by FSP27 in white and brown adipose tissue. BMC Genomics 11, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Puri V., Virbasius J. V., Guilherme A., Czech M. P. (2008) RNAi screens reveal novel metabolic regulators: RIP140, MAP4k4 and the lipid droplet associated fat specific protein (FSP) 27. Acta Physiol. (Oxf.) 192, 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smirnova E., Goldberg E. B., Makarova K. S., Lin L., Brown W. J., Jackson C. L. (2006) ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 7, 106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306, 1383–1386 [DOI] [PubMed] [Google Scholar]
- 37. Villena J. A., Roy S., Sarkadi-Nagy E., Kim K. H., Sul H. S. (2004) Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 279, 47066–47075 [DOI] [PubMed] [Google Scholar]
- 38. Jenkins C. M., Mancuso D. J., Yan W., Sims H. F., Gibson B., Gross R. W. (2004) Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279, 48968–48975 [DOI] [PubMed] [Google Scholar]
- 39. Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., Kratky D., Wagner E. F., Klingenspor M., Hoefler G., Zechner R. (2006) Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312, 734–737 [DOI] [PubMed] [Google Scholar]
- 40. Bezaire V., Mairal A., Ribet C., Lefort C., Girousse A., Jocken J., Laurencikiene J., Anesia R., Rodriguez A. M., Ryden M., Stenson B. M., Dani C., Ailhaud G., Arner P., Langin D. (2009) Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J. Biol. Chem. 284, 18282–18291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miyoshi H., Perfield J. W., 2nd, Obin M. S., Greenberg A. S. (2008) Adipose triglyceride lipase regulates basal lipolysis and lipid droplet size in adipocytes. J. Cell. Biochem. 105, 1430–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kershaw E. E., Hamm J. K., Verhagen L. A., Peroni O., Katic M., Flier J. S. (2006) Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes 55, 148–157 [PMC free article] [PubMed] [Google Scholar]
- 43. Grönke S., Mildner A., Fellert S., Tennagels N., Petry S., Müller G., Jäckle H., Kühnlein R. P. (2005) Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 1, 323–330 [DOI] [PubMed] [Google Scholar]
- 44. Kurat C. F., Natter K., Petschnigg J., Wolinski H., Scheuringer K., Scholz H., Zimmermann R., Leber R., Zechner R., Kohlwein S. D. (2006) Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J. Biol. Chem. 281, 491–500 [DOI] [PubMed] [Google Scholar]
- 45. Chakrabarti P., English T., Shi J., Smas C. M., Kandror K. V. (2010) The mTOR complex 1 suppresses lipolysis, stimulates lipogenesis and promotes fat storage. Diabetes 59, 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yonezawa T., Kurata R., Kimura M., Inoko H. (2011) Which CIDE are you on? Apoptosis and energy metabolism. Mol. Biosyst. 7, 91–100 [DOI] [PubMed] [Google Scholar]
- 47. Kim J. Y., Liu K., Zhou S., Tillison K., Wu Y., Smas C. M. (2008) Assessment of fat-specific protein 27 in the adipocyte lineage suggests a dual role for FSP27 in adipocyte metabolism and cell death. Am. J. Physiol. Endocrinol. Metab. 294, E654–E667 [DOI] [PubMed] [Google Scholar]
- 48. Karpe F., Dickmann J. R., Frayn K. N. (2011) Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 60, 2441–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oliver P., Caimari A., Díaz-Rúa R., Palou A. (2012) Diet-induced obesity affects expression of adiponutrin/PNPLA3 and adipose triglyceride lipase, two members of the same family. Int. J. Obes. (Lond.) 36, 225–232 [DOI] [PubMed] [Google Scholar]
- 50. Puri V., Ranjit S., Konda S., Nicoloro S. M., Straubhaar J., Chawla A., Chouinard M., Lin C., Burkart A., Corvera S., Perugini R. A., Czech M. P. (2008) Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc. Natl. Acad. Sci. U.S.A. 105, 7833–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rubio-Cabezas O., Puri V., Murano I., Saudek V., Semple R. K., Dash S., Hyden C. S., Bottomley W., Vigouroux C., Magré J., Raymond-Barker P., Murgatroyd P. R., Chawla A., Skepper J. N., Chatterjee V. K., Suliman S., Patch A. M., Agarwal A. K., Garg A., Barroso I., Cinti S., Czech M. P., Argente J., O'Rahilly S., Savage D. B., and LD Screening Consortium (2009) Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol. Med. 1, 280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ahmadian M., Duncan R. E., Varady K. A., Frasson D., Hellerstein M. K., Birkenfeld A. L., Samuel V. T., Shulman G. I., Wang Y., Kang C., Sul H. S. (2009) Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes 58, 855–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haemmerle G., Moustafa T., Woelkart G., Büttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P. C., Zierler K., Schreiber R., Eichmann T., Kolb D., Kotzbeck P., Schweiger M., Kumari M., Eder S., Schoiswohl G., Wongsiriroj N., Pollak N. M., Radner F. P., Preiss-Landl K., Kolbe T., Rülicke T., Pieske B., Trauner M., Lass A., Zimmermann R., Hoefler G., Cinti S., Kershaw E. E., Schrauwen P., Madeo F., Mayer B., Zechner R. (2011) ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 17, 1076–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang W., Lv N., Zhang S., Shui G., Qian H., Zhang J., Chen Y., Ye J., Xie Y., Shen Y., Wenk M. R., Li P. (2012) Cidea is an essential transcriptional coactivator regulating mammary gland secretion of milk lipids. Nat. Med. 18, 235–243 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.