Background: Flagellins with little sequence variability in multiple-flagellin systems nonetheless differ substantially in function.
Results: Motility differences resulting from S. oneidensis flagellin paralogs FlaA and FlaB are due to amino acid composition rather than regulation.
Conclusion: Two residue positions predominantly dictate differences in motility mediated by the two flagellins.
Significance: This work provides great insight into our understanding of functional difference between duplicated proteins.
Keywords: Cell Motility, Gene Regulation, Post-translational Modification, Protein Structure, Protein Synthesis, Flagellin, Shewanella
Abstract
Nearly half of flagellated microorganisms possess a multiple-flagellin system. Although a functional filament can be formed from one of multiple flagellins alone in many bacteria, it is more common that one flagellin is the major constituent and others contribute. Underlying mechanisms proposed for such scenarios cover flagellin regulation of various levels, including transcription, translation, post-translational modification, secretion, and filament assembly. In Shewanella oneidensis, the flagellar filament is composed of FlaA and FlaB flagellins; the latter is the major one in terms of motility. In this study, we showed that regulation of all levels except for filament assembly is indistinguishable between these two flagellins. Further analyses revealed that two amino acid residues predominantly dictated functional difference with respect to motility. Given that Shewanella prefer a solid surface-associated life style, of which filaments consisting of either FlaA or FlaB are equally supportive, we envision that roles of flagella in surface adhesion and formation of bacterial communities are particularly important for their survival and proliferation in these specific niches.
Introduction
Among flagellated microorganisms, ∼45% possess multiple flagellin genes, ranging from 2 to 15, which are originated from a single gene by duplication (1–3). Consequently, in multiple-flagellin systems, the functional redundancy appears to be inevitable. Indeed, a functional filament can be formed from one of the flagellins alone, as in Vibrio parahemeolyticus, Aeromonas hydrophila, Borrelia burgdorferi, Bdellovibrio bacteriovorus, and Caulobacter crescentus, to name a few (3–7). However, it is more common that one flagellin is the major constituent of the flagellar filament in such systems, especially those containing two flagellins (4, 8–13). In cases reported thus far, this scenario is attributable to flagellin regulation of various levels, including transcription, translation, post-translational modification, secretion, and filament assembly (4, 7, 12–17).
σ factors play a predominant role in directly controlling transcription of multiple flagellin genes among many regulators, given that the flagellar genes are arranged into transcription hierarchy (14). It has been reported that σ70, σ28 (FliA), and σ54 (RpoN) are involved in recognition of flagellin promoters. In multiple-flagellin systems that have been characterized well thus far, the major flagellins are mostly under the control of σ28, such as in Salmonella enterica serovar Typhimurium, Helicobacter pylori, Campylobacter jejuni, V. parahemeolyticus, Sinorhizobium meliloti, Brachyspira hyodysenteriae, and Shewanella oneidensis (4, 7, 13, 16, 18–20). However, exceptions exist as in V. cholerae and V. fischeri, where σ54 controls expression of the major flagellin (21, 22). Although transcriptional control of flagellin expression is of primary significance and omnipresence, other levels of regulation may be particularly important in certain species or strains. In B. burgdorferi, RNA-binding protein CsrA specifically mediates synthesis of the major flagellin by inhibiting translation initiation of the transcript (17). In C. jejuni, the flagellin secretion efficiency modulated by the N-terminal sequence plays a critical role in motility (12).
Shewanella, extensively studied for redox transformations, have become a research model for more generalized studies due to their widespread distribution, the availability of genome sequences and a related database, and their amenability to genetic manipulation (23). Most of Shewanella species are motile by a polar flagellum, as typified in the model species S. oneidensis, but some possess an additional lateral flagellar system whose assembly is condition-specific (13, 24–29). The polar system in all sequenced Shewanella contains two flagellins except for S. baltica, whose four flagellins most likely arise from transposition events (13). In terms of flagellin size, the polar flagellar systems within the genus are remarkably heterogeneous, with ∼270 amino acids (aa)2 for the majority, with FlaA (273 aa) and FlaB (272 aa) of S. oneidensis as examples. These flagellins are rather small, given that the minimum length for any flagellin is ∼250 aa (30). Interestingly, FlaA and FlaB differ substantially in their ability to propel cells, although they share a sequence identity of ∼89% (13, 29) (Fig. 1). FlaB is undoubtedly the major flagellin because a flaB mutant (flaA+B−) only retains a small share of its swimming capability, whereas the removal of FlaA (flaA−B+) has little or no effect on motility under the conditions tested.
FIGURE 1.
Sequence alignment of S. oneidensis FlaA and FlaB. Domains are named according to FliC of S. enterica serovar Typhimurium (StFliC) with modifications. The ND1 domain contains ND1a and ND1b only. Residues highlighted in green are glycosylation sites, and those highlighted in gray are ones that mainly underlie the functional difference between FlaA and FlaB.
Although these findings provide insights into understanding of the S. oneidensis flagellins, differences hidden in the nearly identical sequences that functionally distinguish these two flagellins remain elusive. In this study, we continue our investigation into the S. oneidensis flagellar system with an emphasis on flagellins. We found that the differences in functionality between FlaA and FlaB could not be readily explained by their distinct expression levels or secretion efficiencies. Instead, two amino acid residues are largely responsible for the difference in motility. These unexpected observations have important implications, not just for elucidation of structural and functional characteristics of small flagellins but for the insight that may be provided into the process of protein evolution.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Plasmids, PCR Primers, and Culture Conditions
A list of all bacterial strains and plasmids used in this study is given in Table 1. Primers used for generating mutant PCR products are given in supplemental Table 1. Escherichia coli and S. oneidensis strains were grown under aerobic conditions in Luria-Bertani (LB; Difco) medium at 37 and 30 °C for genetic manipulation, respectively. Where needed, antibiotics were added at the following concentrations: ampicillin at 50 μg/ml, kanamycin at 50 μg/ml, and gentamycin at 15 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| S. oneidensis strains | ||
| MR-1 | Wild type | Laboratory stock |
| HG3237 | flaB deletion mutant derived from MR-1; ΔflaB | Ref. 13 |
| HG3238 | flaA deletion mutant derived from MR-1; ΔflaB | Ref. 13 |
| HG3961 | rpoN deletion mutant derived from MR-1; ΔrpoN | Ref. 32 |
| FFM | flaA and flaB deletion mutant derived from MR-1; ΔflaAΔflaB | Ref. 13 |
| Other bacterial strains | ||
| WM3064 | E. coli donor strain for conjugation; ΔdapA | W. Metcalf (University of Illinois at Urbana—Champaign) |
| JB580v | Y. enterocolitica wild type | Ref. 36 |
| GY4757 | Y. enterocolitica JB580v ΔyplAB, pYV8081− | Ref. 36 |
| Plasmids | ||
| pHG101 | Promoterless broad-host Kmr vector | Ref. 13 |
| pHG102 | pHG101 containing the S. oneidensis arcA promoter | Ref. 13 |
| pBBR-Cre | Helper vector for removing antibiotic marker | Ref. 32 |
| pHGE-Ptac-PetAGFP | Donor vector for the gfp gene | Ref. 34 |
| pHGEI01 | pHGC01 containing the full-length E. coli lacZ gene | Ref. 33 |
| pHGEI02 | pHGC01 containing the full-length gfp gene | Ref. 33 |
| pHG101-flaB | pHG101 containing the S. oneidensis flaB gene and its native promoter | Ref. 13 |
| pHG101-flaA | pHG101 containing the S. oneidensis flaA gene and its native promoter | This study |
| pHG101-PflaB-flaA | pHG101 containing the S. oneidensis flaA gene and the flaB promoter | This study |
| pHG102-flaA | pHG102 containing the S. oneidensis flaA gene | This study |
| pHGEI01-flaA | pHGEI01 containing the S. oneidensis flaA gene and its native promoter | This study |
| pHGEI01-flaB | pHGEI01 containing the S. oneidensis flaB gene and its native promoter | This study |
| pCSP50 | the E. coli--Y. enterocolitica shuttle vector | Ref. 36 |
| pCSP50-flaA-yplA | pCSP50 containing the fused flaA-yplA | This study |
| pCSP50-flaB-yplA | pCSP50 containing the fused flaB-yplA | This study |
Mutagenesis
Plasmid pHG101-flaB was used as the template for site-directed mutagenesis with a QuikChange II XL site-directed mutagenesis kit (Stratagene) as described previously (28). For swapping large fragments between flaA and flaB, PCR products for hybrid flagellins were generated by fusion PCR (31), subsequently cloned into pHG101 by conventional digestion-ligation, and transformed into E. coli WM3064. After sequencing verification, the resulting vectors were transferred into the S. oneidensis strains by conjugation. Impacts of all mutant flagellins on growth were examined in LB broth, and none of these introduced a significant difference in growth rate compared with the wild type flagellin expressed from the same vector.
Motility Assay
Motility testing (swimming) was performed with semisolid LB agar plates (0.25% agar) as described previously (13, 28). Briefly, mid-log phase cultures were adjusted to an equivalent A600 of 0.4 for each strain with fresh LB broth, 5 μl of which was spotted onto a swimming plate by piercing it with a thin pipette tip. Plates were incubated at room temperature for 18 h before photography. To be consistent, the diameter of the area of motility for each strain was measured and used to calculate its relative motility by normalizing to the diameter for the wild type strain on the same plate. Determination of the swimming speed of the cells was carried out essentially as described elsewhere (26). Micrographs were captured with a Moticam 2306 charge-coupled device camera and Motic Images Advanced version 3.2 software.
Extraction and Analysis of Extracellular Proteins
Isolation of extracellular proteins, which were used for immunoblot analysis, was carried out using trichloroacetic acid (TCA) precipitation as described before (32). In brief, a 250-ml bacterial batch culture was vortexed gently for 1 min and then centrifuged at 18,500 × g for 15 min at 4 °C. TCA was added to the resulting supernatants to a final concentration of 8%, and the mixture was chilled on ice for 60 min and centrifuged at 15,000 × g for 10 min for protein precipitation. The pellets were suspended with 1 ml of cold acetone and centrifuged at 10,000 × g for 5 min. Acetone washing was repeated twice to remove TCA from the precipitates completely. The pellets were dried in a SpeedVac apparatus (Eppendorf) and kept at −20 °C. Prior to SDS-PAGE and immunoblot analyses, the protein samples were dissolved, and the protein concentration was determined using a Bradford assay with bovine serum albumin (BSA) as a standard (Bio-Rad). SDS-PAGE with Coomassie Brilliant Blue stain and immunoblot analyses with polyclonal antibodies that recognize both the FlaA and FlaB subunits and SO1072 with detection by chemiluminescence were done as described previously (13, 32).
Extraction, Purification, and Analysis of Flagellins
Extraction and purification of flagellins were carried out as described before (28). Briefly, a 250-ml bacterial batch culture was centrifuged at 5,000 × g for 10 min at 4 °C. The cell pellet was resuspended in 5 ml of phosphate-buffered saline (PBS), pH 7.0, and vortexed for 10 min to shear off flagella. The cells were removed by centrifugation at 10,000 × g for 30 min at 4 °C, and the supernatant containing flagella was filtered through a 0.45-μm pore filter. The filtrate was centrifuged at 100,000 × g for 2 h, and the pellet containing purified flagella was resuspended in double-distilled water. Sample purity was checked by SDS-PAGE, and the protein concentration was determined using a Bradford assay with bovine serum albumin (BSA) as a standard (Bio-Rad). LC/MS/MS analysis of flagellins was carried out essentially the same as described before (28).
Expression Analyses
To examine the activity of the S. oneidensis flaA and flaB promoters, fragments containing these promoters were generated from the genomic DNA by PCR, digested, and inserted into integrative reporter vectors pHGEI01 and pHGEI02 (33). The resulting vectors were transformed into E. coli WM3064, verified by sequencing, and transferred into S. oneidensis strains by conjugation. Correct integration of the promoter fusion constructs was confirmed by PCR. To eliminate the antibiotic marker, the helper plasmid pBBR-Cre was transferred into the strains carrying the correct integrated construct. Colonies without the integrated antibiotic marker were screened and verified by PCR, followed by the loss of pBBR-Cre as described previously (34).
Microscopy
GFP-expressing bacteria were visualized by a confocal laser scanning microscope as described previously (35). Briefly, 100 μl of the mid-log culture was dropped onto a layer of 3% agar on a slide for immobilization. After the droplet dried, a glass coverslip was placed on top. Expression and localization of GFP fusions were visualized using a Zeiss LSM-510 confocal laser scanning microscope equipped with a 636 oil immersion objective (numerical aperture 1.4). GFP was excited using 488-nm irradiation from an argon ion laser, and fluorescent emission was monitored by collection across windows of 505–530 nm. For counting flagellar filaments, cells were prepared, stained for filaments, and visualized under a Motic BA310 phase-contrast microscope as described previously (28).
Yersinia enterocolitica YplA Fusion Protein Secretion Assay
To determine whether FlaA and FlaB were exported at different efficiencies in a heterologous system, we utilized the YplA fusion protein secretion assay (12, 36, 37). In this assay, the first 108 bp of the 5′ regions of both flaA and flaB genes were PCR-amplified from S. oneidensis chromosomal DNA and fused to the truncated yplA (lacking 150 bp encoding the native T3S signal) within the E. coli-Y. enterocolitica shuttle vector pCSP50 (36). The pCSP50-derived vectors were transformed into E. coli WM3064 and confirmed by PCR fragment size and sequence analysis. The vectors were conjugated into a Y. enterocolitica yplAB mutant and confirmed by restriction enzyme mapping. Secretion of the YplA fusion proteins was assayed in essentially the same manner as those previously prescribed (12, 36).
Bioinformatics and Statistical Analyses
Sequences of flagellins for alignments were obtained from GenBankTM. Alignments were performed using Clustal Omega. Sequence logos were generated using WebLogo (38). Three-dimensional structures of S. oneidensis FlaA and FlaB were predicted using Phyre. The Phyre server predicts the three-dimensional structure of a protein sequence by “threading” the protein sequence through known structures and scoring the predicted structure for compatibility (39). The available three-dimensional structure of StFliC was chosen as the template for structure prediction on the basis of high primary sequence identity and similarity between the protein and FlaA and FlaB. The predicted structures were then visualized by PyMOL (Schroedinger, LLC, New York). Values are presented as means ± S.D. Student's t test was performed for pairwise comparisons of groups.
RESULTS
The flaA and flaB Genes Are Transcribed at Similar Levels
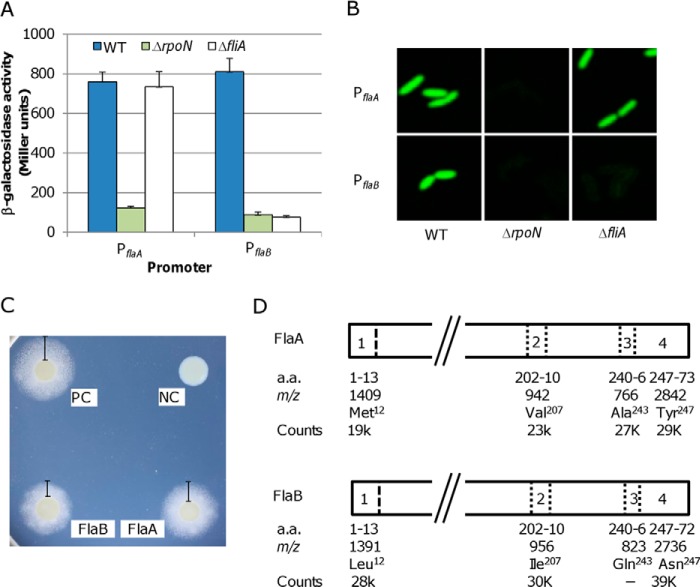
In S. oneidensis, a flagellin-free mutant (FFM; ΔflaAΔflaB) is completely non-motile, whereas the ΔflaA strain is as motile as the wild type, and the ΔflaB strain retains residual motility, ∼20% of the wild type level (13). Because transcriptional control is the major means of regulation in prokaryotes, we first intended to determine whether this level of regulation has a significant impact on the phenotypic difference between the ΔflaA and ΔflaB strains. Previously, we have characterized the expression of the flaA and flaB genes using a plasmid-based lacZ reporter system for Shewanella (13, 40). The flaB gene was expressed at levels that were ∼2.5-fold higher than those of the flaA gene. Unfortunately, due to the high level of sequence similarity between these two genes, independent quantitative RT-PCR analysis could not be applied to validate the observation. Moreover, because the vector used in the study exists as multiple copies, the observed difference in expression levels may not be a true reflection. To avoid interference of antibiotics on growth and copy numbers of plasmids on lacZ expression, in this study, a markerless integrative lacZ reporter system, pHGEI01, was used (33). To measure transcription levels of the flaA and flaB genes, their promoters were transcriptionally fused to the full-length E. coli lacZ gene in the pHGEI01. After verification by sequencing, the resulting vector was introduced to S. oneidensis for integration at the nrfBCD locus on the chromosome, and then the antibiotic marker was removed. Results revealed that the β-galactosidase activities driven by these two promoters were not significantly different from each other (Fig. 2A). To confirm this, we examined the activities of these two promoters in strains deficient in σ54 (ΔrpoN) or σ28 (ΔfliA). In most bacteria with a polar flagellum, the master regulator FlrA at the top tier activates σ54-dependent transcription of genes at tier II, including flrBC and fliA, whose products control transcription of genes at tiers III and IV, respectively (21, 22). As a result, in an RpoN-deficient strain, which is flagellum-less, genes at both tiers III and IV are not transcribed (21, 22, 32). Consistent with this, neither flaA nor flaB was expressed at the physiological relevant levels in the ΔrpoN strain. In contrast, expression of the flaB rather than the flaA gene was abolished in the absence of FliA (σ28), indicating that only the flaB gene is σ28-dependent. Similar results were obtained with the GFP gene in place of the E. coli lacZ gene (Fig. 2B). These results indicate that both flaA and flaB genes are actively transcribed at similar levels, ruling out that transcription is accountable for their functional difference.
FIGURE 2.
Expression, secretion, and assembly into filaments of FlaA and FlaB. A, promoter activities of the flaA and flaB genes were determined by measuring β-galactosidase levels using PflaA-lacZ and PflaB-lacZ reporter constructs in the wild type (WT), ΔrpoN, and ΔfliA strains. Whole-cell lysates were prepared from S. oneidensis cultures in mid-exponential growth phase and assayed. Quantification of the promoter activities was normalized to the total protein in each strain. The values are the mean ± S.D. (error bars) (n = 5). B, promoter activities were monitored using PflaA-gfp and PflaB-gfp reporter constructs in the wild type (WT), ΔrpoN, and ΔfliA strains. Experiments were repeated three times, and similar results were obtained. C, YplA secretion assay. Flagellin-YplA fusion proteins were composed of the N-terminal 36 residues of either flagellin and Y. enterocolitica YplA lacking its native T3S signal. Positive control (PC) was the Y. enterocolitica wild type strain JB580v, and negative control (NC) was the Y. enterocolitica ΔyplAB strain harboring the pCSP50 vector. The experiments were performed three times, and similar results were obtained. D, abundance of the signature peptides identified by LC-MS/MS analysis of tryptic digests of flagellins from the wild type filament. The approximate m/z values of these tryptic peptides were given according to our previous study (28). The third peptide of FlaB was missed in the analysis. Signal intensities by counts were used to calculate the abundance of FlaA and FlaB in the wild type filament.
Both Flagellins FlaA and FlaB Are Major Constituents of Filaments
Because flagellin secretion efficiency influences motility in C. jejuni (12), we determined the efficiency of FlaA and FlaB secretion employing a Y. enterocolitica phospholipase (YplA) reporter secretion assay. The system is like the flagellum in that it is a type III secretion system, allowing bacteria to secrete proteins from the cytosol to the extracellular environment (41). Because the information for secretion of a protein via the flagellar apparatus is stored within its N-terminal sequence (36), we created pCSP50-derived vectors pCSP50/flaA-yplA and pCSP50/flaB-yplA, which express hybrid proteins containing the first 36 residues of FlaA and FlaB with the N-terminal truncated YplA, respectively. A Y. enterocolitica yplA mutant was transformed with the resulting vectors and assayed (Fig. 2C). In comparison with the Y. enterocolitica strains carrying the wild type yplA (positive control) and the 5′-truncated yplA (negative control), we saw that the N-terminal sequence of FlaA or FlaB was able to drive secretion of YplA. Interestingly, efficiencies were not significantly different. The N-terminal sequences of FlaA and FlaB resulted in secretion efficiencies of ∼74 and ∼69% relative to the positive control.
Similar levels of expression and secretion suggest that the filament of the wild type strain should be composed of both flagellins of similar amounts. To determine the precise flagellin composition of the filaments, we employed an LC-MS/MS analysis of tryptic peptides from purified flagellar filaments (28). Among unique signature peptides observed during the analysis, four pairs of ion peaks at m/z 1,409/1,391, 942/956, 766/823, and 2,842/2,736 can be unambiguously assigned to FlaA and FlaB as fragments covering residues 1–13 (expected molecular mass, 1,410/1,392 Da; amino acid difference, Met12/Leu12), 202–210 (943/957 Da; Val207/Ile207), 240–246 (767/824 Da; Ala243/Gln243), and 247–273 (247–272 in the case of FlaB; 2,843/2,737 Da; Tyr247/Asn247 and Gly273/none) (Fig. 2D). Averaged intensities of fragments in pairs were 19,000/28,000, 23,000/30,000, 27,000/not detected, and 29,000/39,000, which conferred sufficient accuracy for quantification. The ratio of FlaA to FlaB is ∼0.73, indicating that FlaA and FlaB make up 42.2 and 57.8% of the S. oneidensis filament, respectively. Because this difference apparently exceeds those observed at transcription, translation, and secretion, it is possible that these two flagellins differ from each other in their abilities to assembly into flagellar filaments.
Flagellin FlaA in Excess Could Not Enhance Motility as FlaB Does
The observation that the ΔflaB strain still retains some motility indicates that the FlaA flagellin is not completely non-functional with respect to motility (13). Given that overproduction of FlaB flagellins results in augmented motility (13, 28), we therefore expected that FlaA in excess may enhance motility. To test this, we utilized three different promoters, including S. oneidensis PflaA, ParcA, and PflaB, to drive expression of the flaA gene in the flaA−flaB− background such that different amounts of FlaA flagellins can be produced. FFM carrying any of these promoters retained residual motility similar to that of the ΔflaB strain (Fig. 3A). To confirm FlaA production at the protein level, Western blotting was performed (Fig. 3B). In agreement with the previous findings, the flaB promoter (PflaB) on multiple-copy plasmid pHG101 substantially overproduced FlaA, whereas the arcA promoter, which is constitutively active, had a modest improvement (13, 28, 40). Results also revealed that, like PflaB, the flaA promoter on plasmid pHG101 was able to overproduce FlaA, ruling out the possibility that the quantity of FlaA is a determining factor for its function. These data, collectively, suggest that the severely impaired function of FlaA is intrinsic in its amino acid sequence. It is worth mentioning that these flagellin genes within pHG101 are expressed significantly less than other genes (nrfA and crp, encoding nitrite reductase and a global regulator, respectively) studied previously (34, 42).
FIGURE 3.
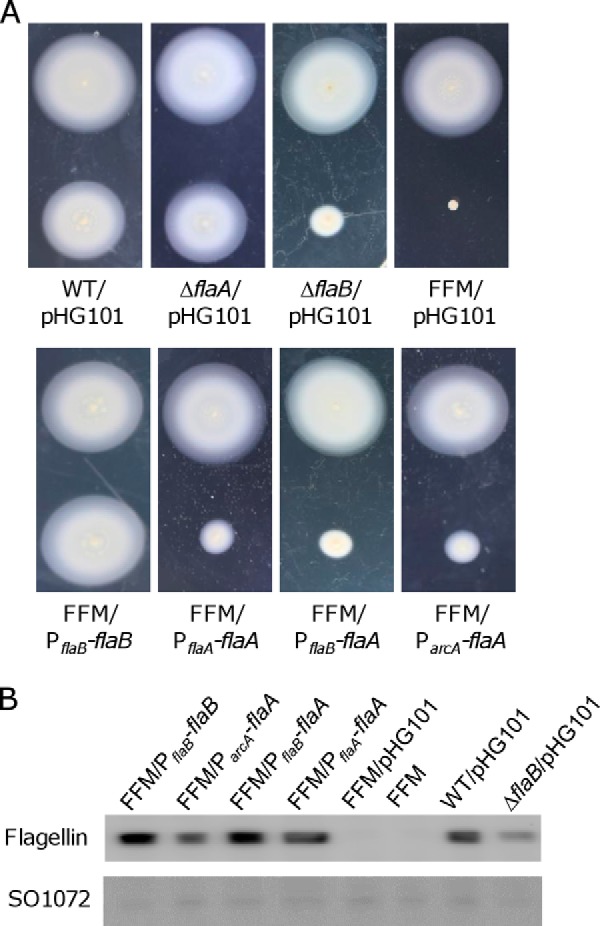
Overproduction of FlaA does not improve motility. A, motility of various S. oneidensis strains. The wild type strain carrying pHG101-flaB (PflaB-flaB) was included (top) on the same soft agar plates in all motility analyses. Compared with the wild type with empty vector, overexpression of flaB resulted in increased motility as reported before (13). FFM (ΔflaAΔflaB) was used to evaluate impacts of overexpressed flaA driven by three different promoters. B, to determine the extent of FlaA overproduction in the strains from A, extracellular protein extracts were prepared from mid-exponential cells. Immunoblot analysis was performed to determine the levels of flagellin, and extracellular protein SO1072 was used as internal control. The antibody recognizes both FlaA and FlaB, and FFM is the flagellin-free mutant (ΔflaAΔflaB) used as a negative control.
Structural Analysis of FlaA and FlaB
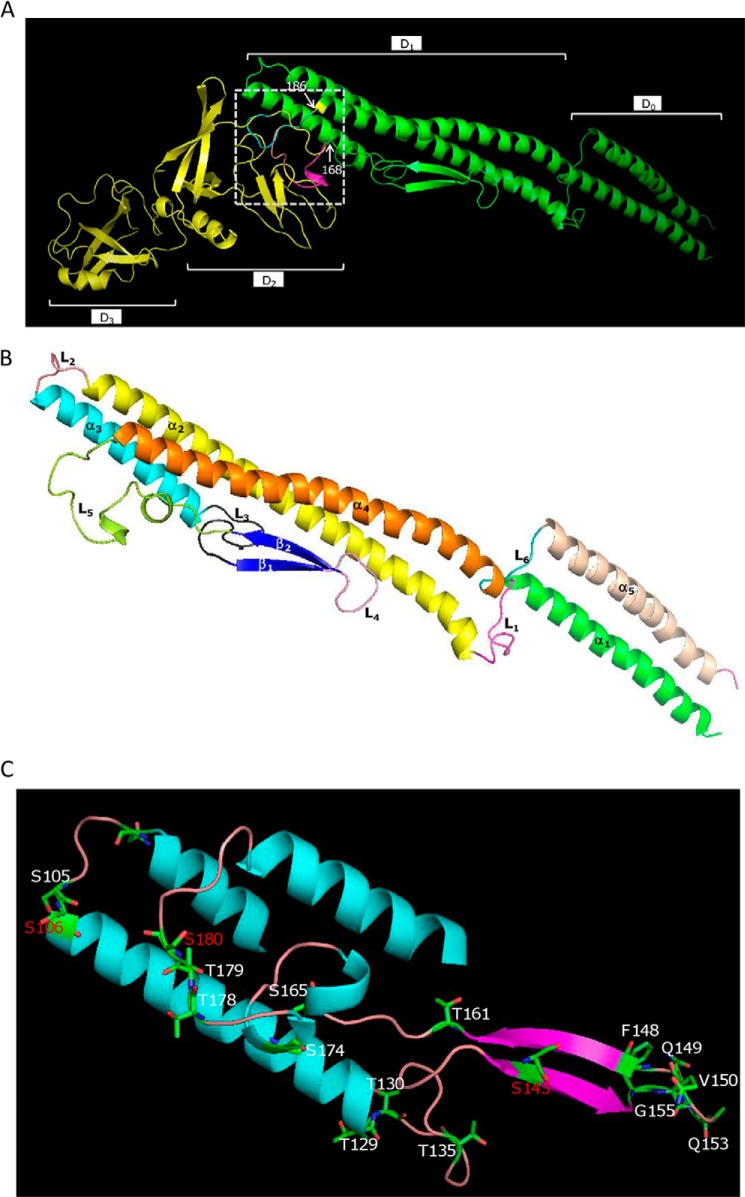
To determine residues that underlie the functional difference between FlaA and FlaB, a structural and functional understanding of S. oneidensis flagellins is a prerequisite. The best understood flagellin is FliC of S. enterica serovar Typhimurium (StFliC hereafter for simplicity), which is composed of 495 amino acid residues and organized into seven domains: D0-D1-D2-D3-D2-D1-D0 (43). In comparison with StFliC, S. oneidensis flagellins retain only four domains that are functionally essential, D0-D1-D1-D0, and a ∼20-residue linker in place of D2-D3-D2 domains of StFliC (13). The significance of individual residues within StFliC for functionality has been evaluated by deletion and alanine scanning mutational analyses (44, 45), and most of the sites that are functionally critical are located within D0 and the highly conserved segment of D1. In contrast, the importance of residues within the D2-D3-D2 domains in flagellar assembly remains undefined.
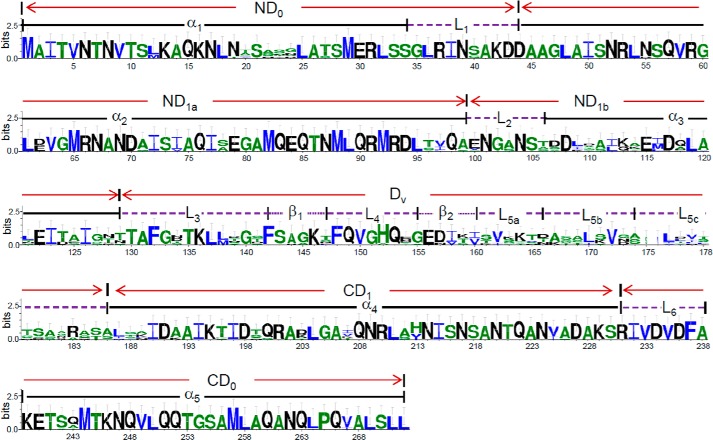
To provide necessary structural and functional understanding of flagellins of the minimum size, we performed two in silico analyses. First, the three-dimensional structure of FlaB was predicted using Phyre with StFliC as the template (39). As shown in Fig. 4A, the structural differences among StFliC, FlaA, and FlaB resided in the fragments between residues 168 and 186, which form the D2 and D3 domains in StFliC but flexible loops in both FlaA and FlaB. As functional flagellin, FlaB is composed of five α-helixes, two β-sheets, and six loops (Fig. 4, B and C). Based on the domain structure of StFliC (43), the N-terminal D0 (ND0) domain of FlaB consists of helix 1 (α1) and loop 1 (L1, also called the spoke region), the C-terminal D1 (CD1) domain is composed of helix 4 (α4) only, and the C-terminal D0 (CD0) domain covers loop 6 (L6) and helix 5 (α5). In the case of the N-terminal D1 (ND1) domain, some modification was made to better reflect sequence conservation and function. ND1 is constituted of ND1a and ND1b, which contain helix 2 (α2) and loop 2 (L2), and helix 3 (α3), respectively. The remaining segment was named the hypervariable domain (Dv; Fig. 5), consisting of loop 3 (L3), β-sheet 1 (β1), loop 4 (L4), β-sheet 2 (β2), and long loop 5 (L5). Both N- and C-terminal D0 and D1 domains of FlaB and StFliC overlapped nearly perfectly, suggesting strong conservation for functionality. Second, FlaB proteins from 14 sequenced Shewanella strains that host flagellins of ∼270 aa were aligned to identify conserved segments and residues (Fig. 5). Expectedly, D0 and CD1 domains were highly conserved, and loop L5 displayed remarkable variations. Interestingly, L3, β1, L4, and β2 showed a level of sequence conservation that was comparable with ND1b, but L5 was much less conserved.
FIGURE 4.
Structural superposition of the flagellin FlaA (blue) and FlaB (purple) models with StFliC (yellow). The structural alignment and figure were produced with PyMOL. A, the domains were named according to StFliC. The sequences between two labeled residues (in FlaA and FlaB) represent the distinct portions among these proteins. B, the S. oneidensis FlaB domain structure according to StFliC with modifications. The structural alignment and figure were produced with PyMOL. C, the location of residues replaced by an alanine that caused a significant impact on motility. Residues in red represent the glycosylation sites.
FIGURE 5.
Sequence conservation of Shewanella flagellins that are 270 amino acids in length. The domains are named according to StFliC with modifications. The ND1 domain contains ND1a and ND1b only. The Dv domain includes the rest of ND1 and the segment that is in place of D2-D3-D2 of StFliC. The sequence logo was produced with WebLogo.
Mutational Analysis of FlaB
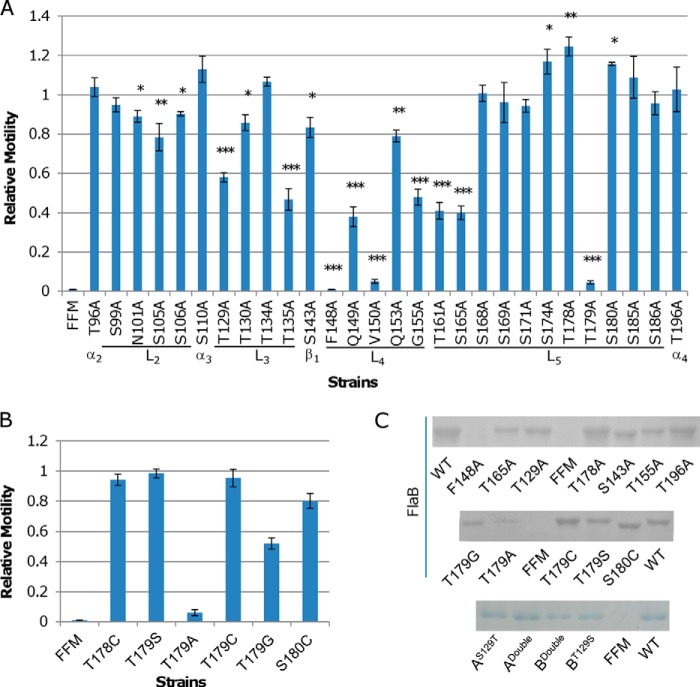
To assess the functional significance of individual residues within the less conserved segment of S. oneidensis FlaB (residues 95–196), we selected 28 residues for alanine scanning analysis. It is worth mentioning that the residues within L4 are not only highly conserved in Shewanella flagellins but also in StFliC, implicating that these residues are crucial for functionality. Consequently, five residues within L4 (Phe148, Gln149, Val150, Gln153, and Gly155) were included for the mutational analysis. The flaB gene on pHG101 was used as the template for the site-directed mutagenesis. The mutated flaB was introduced into FFM, and motility on soft agar LB plates was examined.
Alanine substitutions at 15 sites caused a significant decrease in motility, varying from 80% of the wild type level to completely non-motile. Intriguingly, three alanine point mutations resulted in an increase in motility (refer to Fig. 6 for details). The importance of residues to motility within the C-terminal α2 (Thr96) and L2 (Ser99, Asn101, Ser105, and Ser106) of S. oneidensis FlaB flagellin was modest, indicating that these mutations per se do not impede flagellin assembly substantially. In contrast, alanine substitutions at most of these sites within StFliC (Ser99, Asn101, Asn104, Ser105, and Ser106) resulted in much stronger reduction in motility, with S105A losing motility completely (44). This distinct difference in functionality suggests that L2 is more critical in flagellins of S. enterica serovar Typhimurium than of S. oneidensis for maintaining correct structural conformation. A possible explanation is that the large D2-D3-D2 domains of StFliC, which project out from the filament core, have a more rigid requirement for positioning and are thereby more susceptible to mutations in neighboring domains (43).
FIGURE 6.
Effect of alanine substitutions in FlaB. A, the importance of 28 sites beyond domains D0 and D1 in motility was evaluated by alanine scanning. The swimming motility of FFM hosting each of the mutant flagellins on 0.25% LB agar plates was assayed. Asterisks, statistically significant difference (*, p < 0.01; **, p < 0.001; ***, p < 0.0001; n ≥ 3). B, to determine whether the hydroxyl group of Thr179 is of significance in motility, the residue was replaced by serine, cysteine, or glycine, and the resulting flagellins were assayed for motility. Residues before and after Thr179 were also examined to reinforce the importance of Thr179. In both A and B, relative motility represents the ratio of mutant FlaB motility to wild type FlaB motility, which is the diameter of the area of motility obtained from the same plate as shown in Fig. 3A, and data are representative of at least three independent experiments with S.D. as the error bar. C, SDS-PAGE analysis of flagellins extracted from the indicated strain. Cultures at the same growing phase (mid-log) were adjusted to the same optical density to ensure that a similar number of cells was used for extraction. FlaBS143A and FlaBS180C mutant proteins migrate faster than others because of the loss of one glycosylation modification. AS129T, ADouble, BDouble, and BT129S refer to Fig. 7A.
Substitutions at three of four sites within L3 caused significantly impaired motility. FFM carrying T129A and T135A mutations only retained about half motile abilities compared with FFM with FlaBWT. Apparently, L3 is more crucial than L2 to the function of S. oneidensis flagellum. Unlike L2, which connects two structurally stable α helixes, L3 is located at the beginning of Dv, possibly having an important role in determining the orientation of the remaining portion of Dv. Moreover, L3, which is relatively long, may interact with other loops to stabilize the conformation of Dv.
Sheet β1, loop L4, and sheet β2 form a β-hairpin that interacts with helixes α2 and α4 to determine the two distinct states of flagellin with L- and R-type repeat (43, 46). Given high levels of sequence conservation in the hairpins from both StFliC and S. oneidensis flagellins, the structure has been proposed to be essential for correct assembly of a filament. As expected, substitutions at all of the six sites (S134A, F148A, Q149A, V150A, Q153A, and G155A) tested within the hairpin caused a striking decrease in motility. Among them, F148A and V150A mutants completely abolish motility. It is worth mentioning that the replacement of Ser143 by threonine caused a reduction of motility much more severe (28). These data suggest that the structural conformation of the hairpin can be significantly influenced by individual residues.
L5 can be further divided into L5a (residues 160–165), L5b (residues 166–174), and L5c (residues 175–186) because L5b is unlike the other two in that it is composed of two half-helixes, which are missing in the predicted three-dimensional structure of FlaA (Fig. 4, A–C). Physiological impacts of point mutations within L5 differed substantially. Two mutations (T161A and S165A) within L5a caused severe defects in flagellar filament function, with only 40% capacity remaining. The L5b loop may not be critical because none of the four mutations (S168A, S169A, S171A, and S174A) showed a negative impact on motility. Although the majority of substitutions by alanine within L5c did not negatively affect motility, T179A surprisingly displayed an extremely severe reduction in motility (∼5% capacity remaining). These data indicate that the L5a loop is more critical in general than L5b and L5c in terms of motility. Because mutations of T178A and S180A did not cause reduced motility and Thr179 is unlikely to be glycosylated (28, 29), we speculate that the non-motile phenotype of FFM carrying T179A may result from the loss of the polar hydroxyl group in threonine. To test this, Thr178, Thr178, and Ser180 were replaced by cysteine. Except that cysteine cannot be modified by o-linked glycosylation, the residue is not only structurally similar to serine and threonine but also contains a polar thiol group, which is also able to participate in hydrogen bond formation, similar to the polar hydroxyl group. As shown in Fig. 6B, FFM strains carrying T178C or T179C were as motile as FFM having FlaBWT, whereas FFM hosting S180C showed decreased motility, a result of the loss of glycosylation (28). To further assess the impact of the polar hydroxyl group on motility, Thr179 was replaced by either a serine or a glycine. The soft agar assay revealed that the motilities of FFM hosting T179C and T179S were identical and that FFM carrying T179G displayed a substantial decrease in motility (∼50% of the FlaBWT level) (Fig. 6B). Taken together, these data indicate that Thr179 is critical for FlaB function, with particular importance attached to its polar hydroxyl group.
Mutations That Impair Motility Affect Flagellin Assembly
Given that all of the FlaB mutants are expressed from the same vector in the same host strain and share the same N-terminal sequence, a similar level of expression and secretion efficacy is expected. Conceivably, mutations in filament subunits may impact flagellin assembly into the filament and the filament function, resulting in altered amount of flagellins and swimming speed of cells, respectively. To examine the assembly efficacy, flagellar filaments were extracted from cells of a similar number and analyzed as reported previously (28, 32). Using SDS-PAGE, we observed that amounts of flagellin varied significantly (Fig. 6C). A general trend was that amounts of FlaB mutants decreased with motility. In the extreme case of FlaBF148A or FlaBT179A, there were hardly any flagellin proteins. Moreover, cells hosting FlaB mutants were subjected to flagellar staining and swimming speed assessment (Table 2). Compared with the wild type, FlaB mutants with significantly reduced motility had varying decreases in the percentage of flagellated cells in the entire population. In addition, cell swimming speed of these FlaB mutants was lessened concurrently. These data suggest that mutations in FlaB cause a decrease in motility by mainly influencing the efficacy of flagellin assembly into the filament.
TABLE 2.
Characteristics of S. oneidensis cells expressing mutant flagellins
For swimming speed, a minimum of 10 flagellated motile cells were measured.
| Straina | Motility | Flagellated cells | Swimming speed |
|---|---|---|---|
| % | % | μm s−1 | |
| WT/FlaBWT | 100 | 55 ± 7 | 51 ± 9 |
| FFM (ΔflaAΔflaB) | 0 | 0 | 0 |
| FFM/FlaBWT | 100 | 56 ± 5 | 53 ± 6 |
| FFM/FlaBT129A | 57 ± 5 | 43 ± 8 | 36 ± 4 |
| FFM/FlaBS143A | 83 ± 4 | 48 ± 4 | 48 ± 8 |
| FFM/FlaBF148A | 0 | 0 | 0 |
| FFM/FlaBT155A | 48 ± 6 | 31 ± 6 | 35 ± 5 |
| FFM/FlaBT165A | 40 ± 5 | 27 ± 5 | 29 ± 3 |
| FFM/FlaBT178A | 123 ± 7 | 59 ± 7 | 51 ± 6 |
| FFM/FlaBT179A | 4 ± 0.5 | 6 ± 0.5 | 5 ± 0.6 |
| FFM/FlaBT179G | 52 ± 8 | 36 ± 5 | 37 ± 5 |
| FFM/BT129S | 62 ± 6 | 46 ± 7 | 37 ± 5 |
| FFM/AS129T | 61 ± 5 | 44 ± 5 | 36 ± 7 |
| FFM/BDouble | 22 ± 4 | 42 ± 5 | 21 ± 5 |
| FFM/ADouble | 88 ± 8 | 56 ± 5 | 49 ± 5 |
Two Residues Predominantly Dictate Functional Difference between Flagellins FlaA and FlaB
In flagellated bacteria, flagellins are often post-translationally modified via glycosylation and/or methylation (47). The S. oneidensis FlaB flagellins undergo both types of modification (28). With respect to motility, glycosylation has proven to be crucial, whereas the significance of methylation is negligible. It is therefore possible that glycosylation differentiates FlaA from FlaB functionally. Among five serine residues (Ser106, Ser143, Ser171, Ser180, and Ser185) within FlaB that are glycosylated (28, 29), residue 106, which is replaced by threonine in FlaA, is the only difference (Fig. 1). A FlaA mutant, T106S, was then constructed, and its ability in motility was assayed in FFM. The motility of FFM carrying FlaAWT and FlaAT106S was identical (Fig. 7A), suggesting that glycosylation is unlikely to be significant in functionally distinguishing FlaA and FlaB from each other.
FIGURE 7.
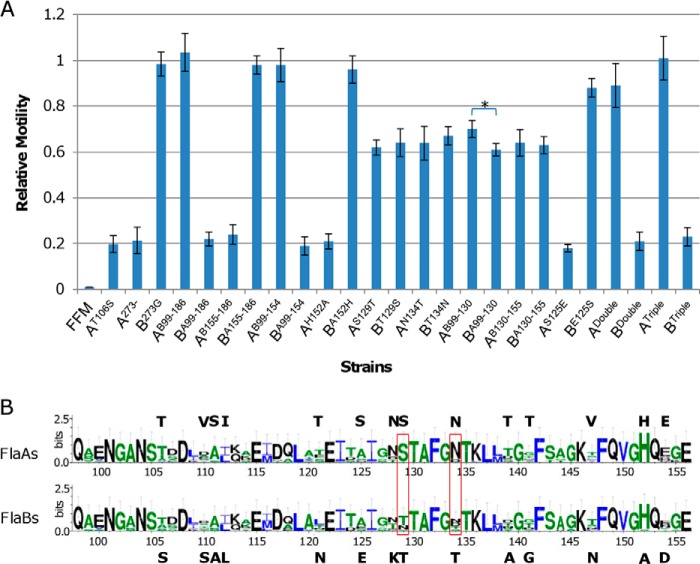
Effect of residue and fragment swappings between FlaA and FlaB. A, to determine the residues accountable for functional difference of FlaA and FlaB, the relative swimming motility was calculated as the ratio of mutant flagellin (FlaA or FlaB) motility to wild type FlaB motility. A and B in normal type represent FlaA and FlaB, respectively. Letters and numbers in superscript represent amino acid residues and location, respectively. For example, AT106S, A273−, and AB99–186 represent FlaA harboring mutation T106S, removal of residue 273, and the fragment of residues 99–186 of FlaB, respectively. Double (residues 129 and 134) and Triple (residues 125, 129, and 134) represent multiple exchanges between two flagellins. Data are representative of at least three independent experiments with S.D. as the error bar. *, statistically significant difference (p < 0.01, n ≥ 3). B, conservation of residues that differ within FlaA and FlaB. Sequence conservation of Shewanella flagellins that are ∼270 amino acids in length was shown (residues 98–156). The actual residues in S. oneidensis FlaA (top) and FlaB (bottom) are given. The boxed residues dictated the functional difference. The sequence logo was produced with WebLogo.
We then reasoned that some residues other than those with glycosylation potential underlie the drastic difference in functionality between FlaA and FlaB. In Shewanella, FlaA flagellins are characterized by an extra G at the C-terminal end (Fig. 1). This hydrophilic residue, projected into the central channel, is believed to facilitate the passage of unfolded flagellin monomers (43). However, neither its removal from FlaA nor its addition to FlaB elicited any difference in functionality (Fig. 7A). Because a sequence alignment of FlaA and FlaB and the predicted three-dimensional structures of these two flagellins converged to indicate that the difference between these two flagellins mainly resided in the fragment (residues 99–186) between helixes α2 and α4, we swapped this fragment between FlaA and FlaB (Figs. 1 and 4A). Soft agar assays on FFM/FlaAB99–186 and FFM/FlaBA99–186 demonstrated that the fragment swapping resulted in a functional swap, indicating that residues underlying the functional difference between FlaA and FlaB indeed are within residues 99–186 (Fig. 7A).
According to the predicted three-dimensional models presented above, the segments of loop L5 structurally differentiate FlaB from FlaA. Interestingly, although loop L5 is hypervariable within flagellins in general, FlaA is distinct from FlaB in this loop only with 5 residues (5 of 27), a level even lower than that of the entire fragment of residues 99–186 (19 of 87) (Fig. 1). Nevertheless, we reasoned that variations in sequence within loop L5 are most likely accountable for the functional difference. To test this, exchange of loop L5 between FlaA and FlaB was performed. Surprisingly, FFM/FlaBA155–186 showed similar motility relative to FFM/FlaB, and so did FFM/FlaAB155–186 relative to FFM/FlaA, ruling out loop L5 as the cause (Fig. 7A). Consequently, the residues underlying the functional difference of FlaA and FlaB are confined to the fragment of residues 99–154. Indeed, FFM/FlaAB99–154 showed motility similar to that of FFM/FlaB, whereas FFM/FlaBA99–154 had residual motility the same as FFM/FlaA (Fig. 7A).
In total, there are 14 residues in FlaA and FlaB fragments of residues 99–154 that are different. To facilitate the identification of residues underlying the functional difference, we aligned FlaA and FlaB from 14 sequenced Shewanella strains that possess ∼270-aa flagellins (Fig. 7B). Within the fragment of residues 99–154, 11 of these 14 sites are considerably variable, implying that the residues at these sites may not be critical for functionality. In contrast, residues 129 (SFlaA/TFlaB) and 134 (NFlaA/TFlaB) appear to be highly conserved in FlaA flagellins only, and His152 is perfectly conserved in all of these Shewanella flagellins except in FlaB of S. oneidensis, which has an alanine at the site confirmed by resequencing multiple times. We therefore swapped these three residues between FlaA and FlaB individually or in combination and examined the abilities of the resulting flagellins to propel FFM cells.
Neither substitution of His152 within FlaA into alanine nor substitution of Ala152 within FlaB into histidine had any effect on motility, indicating that the residue is not critical for function (Fig. 7A). This observation appears striking, given the extremely high conservation of the residue in S. oneidensis flagellins. It is widely accepted that mutations in conserved residues generally cause a large decrease in protein fitness, presumably by reducing stability, and reverting residues that deviate from the consensus amino acid can increase stability, thereby protein fitness (48). In contrast, residue exchanges at 129 and 134 caused a similar result; FFM carrying mutated FlaBT129S or FlaBT134N had a considerable decrease in motility, whereas FFM with mutated FlaAS129T or FlaAN134T displayed a drastic increase in motility. Intriguingly, in both cases, levels of motility were ∼65% relative to FlaBWT. To test the possibility that only residues 129 and 134 are relevant, we swapped fragments of residues 99–130 and 130–155 between two flagellins. Although similar results in motility, ∼65% relative to FlaBWT, were obtained from hybrid flagellins FlaAB130–155 and FlaBA130–155, FFM with FlaAB99–130 and FlaBA99–130 showed a slight but significant difference in motility; they had relative motility of ∼70 and ∼61%, respectively (Fig. 7A). These data suggest that impacts of single exchange at residue 134 on motility are equivalent to those of swapping fragments (residues 130–155) containing the corresponding residue, but the fragment of residues 99–130 may contain residues other than residue 129 that are relevant to the functional difference of flagellins, albeit only slightly. A further residue-exchanging assay on seven different residues (106, 110, 111, 112, 121, 125, and 128) within this fragment revealed that FlaBE125S was the only mutated flagellin with reduced motility, to ∼89% relative to FlaBWT. Interestingly, there was no difference in motility observed when FFM was equipped with FlaAS125E (Fig. 7A).
We then tested whether double (residues 129 and 134) or triple (residues 125, 129, and 134) exchanges at these sites can further the functional conversion. Indeed, FlaADouble enabled FFM to acquire motility ∼88% relative to FlaBWT, a level coincident with that of FFM/FlaBE125S, and FlaATriple was undistinguishable from FlaBWT in their motile capacities. This notion was supported by the finding that FFM/FlaBDouble and FFM/FlaBTriple cells had motility similar to that of the ΔflaB strain. To examine whether the flagellar assembly is affected by these replacements, we assessed the amount of flagellins, calculated the percentage of flagellated cells, and measured cell swimming speed (Fig. 6B and Table 2). Although FFM/FlaBDouble retains ∼20% of the wild type motility, the percentage of flagellated cells was reduced only modestly (Table 2). This coincided with the relatively abundant flagellins observed on SDS-PAGE, suggesting that mutations at these two sites do not cause severe damage on flagellin assembly. In contrast, these mutations introduced substantial reduction in cell swimming speed, implying that the filament per se is functionally impaired. Collectively, all of these data suggest that residues 129 and 134 between FlaA and FlaB predominantly account for the functional difference of two flagellins in S. oneidensis.
DISCUSSION
Thanks to the availability of a large number of bacterial genome sequences, we now know profound variations in flagellins from three aspects: (i) widespread (∼45% of bacteria host multiple copies of flagellins); (ii) gene copies (the maximum number of flagellin genes in one single genome is 15); and (iii) flagellin size (whereas the largest is 670 aa in length, 250 aa is the minimum required for functionality) (3, 30). Given the high level of sequence similarity among multiple flagellins in any bacterial species, these subunits are undoubtedly originated from gene duplications (1). During evolution, bacteria must be able to assemble filaments while allowing mutations to accumulate such that one of the flagellins emerges as predominant in the end. Multiple mechanisms are accountable for the difference in the roles that each flagellin plays in filament assembly and motility.
It appears to be a common scenario that the major flagellin gene is under the control of a regulator distinct from those for other flagellin genes (14–15). Such an arrangement ensures that multiple flagellin genes in a bacterium are differently transcribed, with the major flagellin expressed at a significantly elevated level. In most bacteria with a polar-flagellar filament, flagellar genes are transcribed in a four-tiered hierarchy (15, 21, 22). A σ54-associated transcription activator is the master regulator at the top level, controlling transcription of genes in the second tier, which encode components of the MS ring-switch complex as well as the two-component regulatory system FlrBC and σ28 (15). Flagellin genes belong to either the third tier whose transcription relies on FlrBC or the fourth tier controlled by σ28. We showed previously that the synthesis of the flagellum in S. oneidensis is similar to that in Vibrio, the paradigm for the polar flagellum, whose major flagellin gene is under the control of σ54 (13). Not surprisingly, the S. oneidensis flaA and flaB flagellin genes are dependent on distinct regulators for expression. However, the bacterium differs from others hosting a polar-flagellar filament in that the transcription of its major flagellin gene flaB does not exceed that of the flaA gene significantly.
The difference in transcription between the major and other flagellin genes, which has been widely accepted as the explanation for the different contribution of each flagellin in filament assembly and functionality, to some extent hampers investigations into alternative mechanisms. As a result, just recently it has been reported that secretion efficiency of flagellins makes a difference in certain bacteria (12). In this study, we demonstrated that both flaA and flaB genes are translated equally well, that secretion of their products is comparable, and that post-translational modification plays a dispensable role, eliminating the possibility that these processes are important for functionally differentiating the major flagellin FlaB from FlaA in S. oneidensis. Instead, the ability of each flagellin to assemble into the flagellar filament is apparently critical because the percentage of FlaB is significantly greater than that of FlaA within the filament. This idea is supported by findings that we reported previously and discovered in this study. In the case of glycosylation-defective FlaB mutants (in which one of the glycosylation sites is replaced by a cysteine residue), reduction in their motility is proportionally correlated to the amount of flagellins assembled into the filament (28). Similarly, most of the FlaB mutants with impaired motility by the alanine-scanning analysis produce the flagellin proteins at levels significantly lower than the wild type.
Although this mechanism is, to some extent, responsible for the functional difference of S. oneidensis flagellins in regard to motility, it is certainly not the decisive factor, given that FlaA in excess is unable to enhance the motile ability. Our study demonstrates that a couple of intrinsic variations in amino acid sequences, residues 129 and 134, are sufficient to distinguish FlaB from FlaA functionally. Such a feat would most likely require the segment in which these two residues are located to have sequence conservation of a proper level such that mutations not only are allowed to some degree but also cause a significant impact on motility. This idea is supported by two observations. First, residues within the flexible loop L5, where FlaA and FlaB differ from each other structurally, are generally not important for motility. This loop is apparently the most amenable to mutations on the basis of sequence conservation of relatively low levels, which may explain why it is replaced by D2-D3-D2 domains in StFliC (43–44, 46). Second, little variation is allowed in highly conserved segments, such as loop L4 (residues 148–155), the majority of whose residues are crucial for motility. It is worth mentioning that exchanges of residues 129 and 134 individually or together between FlaA and FlaB have a rather modest impact on flagellin assembly. Instead, the filament per se is functionally impaired, a characteristic of FlaA. This is in contrast to what we observe for most alanine substitution mutations in FlaB, which reduce motility mainly by lessening the amount of flagellins to be assembled into the filaments.
Alanine scanning has been routinely used to identify residues that are important for flagellar function (44, 49). By using this method, we found that 13 of 22 serine- and threonine-substituting mutants showed phenotypes of significantly reduced motility. However, a previous examination of these residues by cysteine substitution revealed that half of them had no obvious phenotype compared with the wild type with respect to motility, suggesting that the mutation to alanine generally introduced more profound physiological impacts than that to cysteine (28). This is best exemplified by T179A, which nearly abolishes motility completely. Alanine is structurally similar to both serine and threonine, but it lacks the polar hydroxyl group, which is not only subject to o-linked glycosylation but is also involved in hydrogen bond formation. As a result, the alanine replacement for serine and threonine residues may mix these two effects, veiling the result of either individual process. Interestingly, the substitution by alanine at Ser143 introduces an impact less severe than substitution by cysteine, indicating that the extra -SH at the site negatively affects the flagellar assembly. Nevertheless, our data suggest that the cysteine scanning is more practicable for identification of serine and threonine residues that are important for protein function, glycosylation in particular.
In evolutionary divergence, one of the main mechanisms is the accumulation of point mutations. This may be particularly true for flagellins because thousands of such units are required for motility at one time, and thus small changes in sequence would generate large functional divergence. Intriguingly, we observed that residues 129 and 134, as well as residue 152, are more conserved in FlaA than in FlaB in all sequenced Shewanella. Although accepted as a locomotive organelle, bacterial flagella are involved in many additional processes, including adhesive properties, biofilm formation, and modulation of the immune system of eukaryotic cells (50). Often, mutations causing a severe impact on one biological aspect may not affect others. For example, the structural requirements for the binding of Toll-like receptor 5 to flagellins, which recognizes a combinatorial surface on flagellins relying on the sum of a large group of residues, are much less rigid than for motility in S. enterica serovar Typhimurium (44, 51). In S. oneidensis, cells with flagellar filaments composed of either FlaA or FlaB are indistinguishable with respect to biofilm formation and extracellular protein production, whereas the loss of the filament has a profoundly different effect (32). Shewanella mostly reside in redox-stratified environments, where a living style of biofilm on a solid surface prevails (23, 52). With the present findings, we envision that flagellum of S. oneidensis, in addition to being an organelle for locomotion, is an important factor contributing to colonization in these specific niches, presumably with respect to their roles in surface adhesion and formation of bacterial communities.
Supplementary Material
Acknowledgment
We thank Dr. Michael E. Konkel (Washington State University) for providing the YplA secretion assay package.
This work was supported by the Major State Basic Research Development Program (973 Program Grant 2010CB833803), National Natural Science Foundation of China Grant 31270097, and Doctoral Fund of the Ministry of Education of China Grant 20130101110142 (to H. G.).

This article contains supplemental Table 1.
- aa
- amino acid(s)
- FFM
- flagellin-free mutant.
REFERENCES
- 1. Liu R., Ochman H. (2007) Stepwise formation of the bacterial flagellar system. Proc. Natl. Acad. Sci. U.S.A. 104, 7116–7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kanehisa M., Araki M., Goto S., Hattori M., Hirakawa M., Itoh M., Katayama T., Kawashima S., Okuda S., Tokimatsu T., Yamanishi Y. (2008) KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36, D480–D484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faulds-Pain A., Birchall C., Aldridge C., Smith W. D., Grimaldi G., Nakamura S., Miyata T., Gray J., Li G., Tang J. X., Namba K., Minamino T., Aldridge P. D. (2011) Flagellin redundancy in Caulobacter crescentus and its implications for flagellar filament assembly. J. Bacteriol. 193, 2695–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCarter L. L. (2001) Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 65, 445–462, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canals R., Vilches S., Wilhelms M., Shaw J. G., Merino S., Tomás J. M. (2007) Non-structural flagella genes affecting both polar and lateral flagella-mediated motility in Aeromonas hydrophila. Microbiology 153, 1165–1175 [DOI] [PubMed] [Google Scholar]
- 6. Iida Y., Hobley L., Lambert C., Fenton A. K., Sockett R. E., Aizawa S.-I. (2009) Roles of multiple flagellins in flagellar formation and flagellar growth post bdelloplast lysis in Bdellovibrio bacteriovorus. J. Mol. Biol. 394, 1011–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li C., Xu H., Zhang K., Liang F. T. (2010) Inactivation of a putative flagellar motor switch protein FliG1 prevents Borrelia burgdorferi from swimming in highly viscous media and blocks its infectivity. Mol. Microbiol. 75, 1563–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pleier E., Schmitt R. (1991) Expression of two Rhizobium meliloti flagellin genes and their contribution to the complex filament structure. J. Bacteriol. 173, 2077–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suerbaum S. (1995) 1995. The complex flagella of gastric Helicobacter species. Trends Microbiol. 3, 168–170; discussion 170–171 [DOI] [PubMed] [Google Scholar]
- 10. Klose K. E., Mekalanos J. J. (1998) Distinct roles of an alternative σ factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol. Microbiol. 28, 501–520 [DOI] [PubMed] [Google Scholar]
- 11. Deakin W. J., Parker V. E., Wright E. L., Ashcroft K. J., Loake G. J., Shaw C. H. (1999) Agrobacterium tumefaciens possesses a fourth flagellin gene located in a large gene cluster concerned with flagellar structure, assembly and motility. Microbiology 145, 1397–1407 [DOI] [PubMed] [Google Scholar]
- 12. Neal-McKinney J. M., Christensen J. E., Konkel M. E. (2010) Amino-terminal residues dictate the export efficiency of the Campylobacter jejuni filament proteins via the flagellum. Mol. Microbiol. 76, 918–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu L., Wang J., Tang P., Chen H., Gao H. (2011) Genetic and molecular characterization of flagellar assembly in Shewanella oneidensis. PLoS One 6, e21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chevance F. F., Hughes K. T. (2008) Coordinating assembly of a bacterial macromolecular machine. Nat. Rev. Microbiol. 6, 455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCarter L. L. (2006) Regulation of flagella. Curr. Opin. Microbiol. 9, 180–186 [DOI] [PubMed] [Google Scholar]
- 16. Kutsukake K., Ohya Y., Iino T. (1990) Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J. Bacteriol. 172, 741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sze C. W., Morado D. R., Liu J., Charon N. W., Xu H., Li C. (2011) Carbon storage regulator A (CsrABb) is a repressor of Borrelia burgdorferi flagellin protein FlaB. Mol. Microbiol. 82, 851–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jagannathan A., Constantinidou C., Penn C. W. (2001) Roles of rpoN, fliA,and flgR in expression of flagella in Campylobacter jejuni. J. Bacteriol. 183, 2937–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leying H., Suerbaum S., Geis G., Haas R. (1992) Cloning and genetic characterization of a Helicobacter pylori flagellin gene. Mol. Microbiol. 6, 2863–2874 [DOI] [PubMed] [Google Scholar]
- 20. Scharf B., Schuster-Wolff-Bühring H., Rachel R., Schmitt R. (2001) Mutational analysis of the Rhizobium lupini H13-3 and Sinorhizobium meliloti flagellin genes: importance of flagellin A for flagellar filament structure and transcriptional regulation. J. Bacteriol. 183, 5334–5342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prouty M. G., Correa N. E., Klose K. E. (2001) The novel σ54- and σ28-dependent flagellar gene transcription hierarchy of Vibrio cholerae. Mol. Microbiol. 39, 1595–1609 [DOI] [PubMed] [Google Scholar]
- 22. Syed K. A., Beyhan S., Correa N., Queen J., Liu J., Peng F., Satchell K. J., Yildiz F., Klose K. E. (2009) The Vibrio cholerae flagellar regulatory hierarchy controls expression of virulence factors. J. Bacteriol. 191, 6555–6570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fredrickson J. K., Romine M. F., Beliaev A. S., Auchtung J. M., Driscoll M. E., Gardner T. S., Nealson K. H., Osterman A. L., Pinchuk G., Reed J. L., Rodionov D. A., Rodrigues J. L., Saffarini D. A., Serres M. H., Spormann A. M., Zhulin I. B., Tiedje J. M. (2008) Towards environmental systems biology of Shewanella. Nat. Rev. Microbiol. 6, 592–603 [DOI] [PubMed] [Google Scholar]
- 24. Wang F., Wang J., Jian H., Zhang B., Li S., Wang F., Zeng X., Gao L., Bartlett D. H., Yu J., Hu S., Xiao X. (2008) Environmental adaptation: genomic analysis of the piezotolerant and psychrotolerant deep-sea iron reducing bacterium Shewanella piezotolerans WP3. PLoS One 3, e1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paulick A., Koerdt A., Lassak J., Huntley S., Wilms I., Narberhaus F., Thormann K. M. (2009) Two different stator systems drive a single polar flagellum in Shewanella oneidensis MR-1. Mol. Microbiol. 71, 836–850 [DOI] [PubMed] [Google Scholar]
- 26. Bubendorfer S., Held S., Windel N., Paulick A., Klingl A., Thormann K. M. (2012) Specificity of motor components in the dual flagellar system of Shewanella putrefaciens CN-32. Mol. Microbiol. 83, 335–350 [DOI] [PubMed] [Google Scholar]
- 27. Koerdt A., Paulick A., Mock M., Jost K., Thormann K. M. (2009) MotX and MotY are required for flagellar rotation in Shewanella oneidensis MR-1. J. Bacteriol. 191, 5085–5093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun L., Jin M., Ding W., Yuan J., Kelly J., Gao H. (2013) Post-translational modification of flagellin FlaB in Shewanella oneidensis. J. Bacteriol. 195, 2550–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bubendorfer S., Ishihara M., Dohlich K., Heiss C., Vogel J., Sastre F., Panico M., Hitchen P., Dell A., Azadi P., Thormann K. M. (2013) Analyzing the modification of the Shewanella oneidensis MR-1 flagellar filament. PLoS One 8, e73444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beatson S. A., Minamino T., Pallen M. J. (2006) Variation in bacterial flagellins: from sequence to structure. Trends Microbiol. 14, 151–155 [DOI] [PubMed] [Google Scholar]
- 31. Jin M., Jiang Y., Sun L., Yin J., Fu H., Wu G., Gao H. (2013) Unique organizational and functional features of the cytochrome c maturation system in Shewanella oneidensis. PLoS One 8, e75610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shi M., Wu L., Xia Y., Chen H., Luo Q., Sun L., Gao H. (2013) Exoprotein production correlates with morphotype changes of nonmotile Shewanella oneidensis mutants. J. Bacteriol. 195, 1463–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fu H., Jin M., Ju L., Mao Y., Gao H. (2014) Evidence for function overlapping of CymA and the cytochrome bc1 complex in the Shewanella oneidensis nitrate and nitrite respiration. Environ. Microbiol. 10.1111/1462-2920.12457 [DOI] [PubMed] [Google Scholar]
- 34. Fu H., Chen H., Wang J., Zhou G., Zhang H., Zhang L., Gao H. (2013) Crp-dependent cytochrome bd oxidase confers nitrite resistance to Shewanella oneidensis. Environ. Microbiol. 15, 2198–2212 [DOI] [PubMed] [Google Scholar]
- 35. Luo Q., Dong Y., Chen H., Gao H. (2013) Mislocalization of rieske protein PetA predominantly accounts for the aerobic growth defect of tat mutants in Shewanella oneidensis. PLoS One 8, e62064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Christensen J. E., Pacheco S. A., Konkel M. E. (2009) Identification of a Campylobacter jejuni-secreted protein required for maximal invasion of host cells. Mol. Microbiol. 73, 650–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Warren S. M., Young G. M. (2005) An amino-terminal secretion signal is required for YplA export by the Ysa, Ysc, and flagellar type III secretion systems of Yersinia enterocolitica biovar 1B. J. Bacteriol. 187, 6075–6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crooks G. E., Hon G., Chandonia J.-M., Brenner S. E. (2004) WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kelley L. A., Sternberg M. J. (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4, 363–371 [DOI] [PubMed] [Google Scholar]
- 40. Gao H., Wang X., Yang Z. K., Chen J., Liang Y., Chen H., Palzkill T., Zhou J. (2010) Physiological roles of ArcA, Crp, and EtrA and their interactive control on aerobic and anaerobic respiration in Shewanella oneidensis. PLoS One 5, e15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cornelis G. R. (2006) The type III secretion injectisome. Nat. Rev. Microbiol. 4, 811–825 [DOI] [PubMed] [Google Scholar]
- 42. Dong Y., Wang J., Fu H., Zhou G., Shi M., Gao H. (2012) A Crp-dependent two-component system regulates nitrate and nitrite respiration in Shewanella oneidensis. PLoS One 7, e51643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yonekura K., Maki-Yonekura S., Namba K. (2003) Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature 424, 643–650 [DOI] [PubMed] [Google Scholar]
- 44. Smith K. D., Andersen-Nissen E., Hayashi F., Strobe K., Bergman M. A., Barrett S. L., Cookson B. T., Aderem A. (2003) Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 4, 1247–1253 [DOI] [PubMed] [Google Scholar]
- 45. Malapaka R. R., Adebayo L. O., Tripp B. C. (2007) A deletion variant study of the functional role of the Salmonella flagellin hypervariable domain region in motility. J. Mol. Biol. 365, 1102–1116 [DOI] [PubMed] [Google Scholar]
- 46. Samatey F. A., Imada K., Nagashima S., Vonderviszt F., Kumasaka T., Yamamoto M., Namba K. (2001) Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature 410, 331–337 [DOI] [PubMed] [Google Scholar]
- 47. Nothaft H., Szymanski C. M. (2010) Protein glycosylation in bacteria: sweeter than ever. Nat. Rev. Microbiol. 8, 765–778 [DOI] [PubMed] [Google Scholar]
- 48. Tokuriki N., Tawfik D. S. (2009) Stability effects of mutations and protein evolvability. Curr. Opin. Struct. Biol. 19, 596–604 [DOI] [PubMed] [Google Scholar]
- 49. Hirai H., Takai R., Iwano M., Nakai M., Kondo M., Takayama S., Isogai A., Che F.-S. (2011) Glycosylation regulates specific induction of rice immune responses by Acidovorax avenae flagellin. J. Biol. Chem. 286, 25519–25530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Duan Q., Zhou M., Zhu L., Zhu G. (2013) Flagella and bacterial pathogenicity. J. Basic Microbiol. 53, 1–8 [DOI] [PubMed] [Google Scholar]
- 51. Yoon S.-I., Kurnasov O., Natarajan V., Hong M., Gudkov A. V., Osterman A. L., Wilson I. A. (2012) Structural basis of tlr5-flagellin recognition and signaling. Science 335, 859–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yuan J., Chen Y., Zhou G., Chen H., Gao H. (2013) Investigation of roles of divalent cations in Shewanella oneidensis pellicle formation reveals unique impacts of insoluble iron. Biochim. Biophys. Acta 1830, 5248–5257 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.