Background: The transcriptional regulation of human dendritic cells (DCs) differentiation and maturation remains incompletely understood.
Results: VentX knockdown impaired DCs differentiation and maturation whereas VentX overexpression counteracted the inhibitory effects of corticosteroid on DC activation.
Conclusion: VentX is critical for DCs differentiation and maturation.
Significance: VentX may be a novel target to modulate DCs functions and manage inflammatory diseases.
Keywords: Dendritic Cells, Development, Differentiation, Gene Regulation, Monocytes
Abstract
Dendritic cells (DCs) are specialized antigen presentation cells that play critical roles in the initiation and regulation of immune responses. The molecular determinants of DC differentiation and maturation are target of extensive investigation. VentX is a human homeobox transcriptional factor that regulates proliferation and differentiation of hematopoietic cells. In the current study, we report that ablation of VentX expression in monocytes significantly impaired their differentiation into DCs. Conversely, overexpression of VentX in monocytic THP1 cells accelerated their differentiation toward DCs. We showed that VentX regulates DC differentiation, in part, through modulating IL6 expression. Clinically, we found that VentX expression was elevated in intestinal lamina propria DCs (LPDCs) of inflamed mucosa from inflammatory bowel disease patients. Knockdown experiments suggested that VentX is essential for the maturation of LPDCs. In addition, corticosteroid treatment markedly decreased VentX expression in LPDCs and enforced expression of VentX counteracted the effects of corticosteroid on DCs maturation. Our data suggest that VentX is a critical transcriptional regulator of DC differentiation and maturation, and a potential target of immune regulation and therapy.
Introduction
Dendritic cells (DCs)2 are the most potent professional antigen-presenting cells that play critical roles in initiation of immune responses and maintenance of immune homeostasis. Derived from bone marrow hematopoietic stem/multipotent progenitor cells, DCs reside in an immature state in peripheral nonlymphoid tissues (1). These immature DCs are able of efficient taking up and processing various antigens. Upon stimulation with inflammatory cytokines or microbial components, such as lipopolysaccharide (LPS) of bacteria, immature DCs undergo a maturation process and begin to migrate to local lymph nodes, where they interact with CD40 ligand on antigen specific T cells via CD40 and mature into potent immuno-stimulatory or immuno-tolerogenic cells (2). Maturation of DCs correlates with up-regulated expression of MHC class II and co-stimulatory molecules, production of immuno-stimulatory cytokines, and acquisition of capability to stimulate naive and antigen-specific T cells (3).
Intestinal mucosa is enriched with DCs, which reside in the lamina propria and regulate immune response to pathogen invasion. Maturation and function of intestinal DCs are regulated by both host factors and microbial components (4). Intestinal DCs detect the presence of pathogens through pattern recognition receptors, such as members of the toll-like receptor (TLR) family (5). Aberrant maturation and activation of intestinal DCs has been postulated to play a pathogenic role in auto-immune/inflammatory conditions, such as inflammatory bowel disease (IBD). It has been shown that expression of TLR2, TLR4, and CD40 is enhanced in DCs isolated from inflamed mucosa of IBD patients (6). Moreover, aberrant TLR signaling has been found to predispose patients to Crohn disease (7). Evidence has been accumulating to suggest a potential role of targeting DCs in management of autoimmune/inflammatory conditions. However, little is known about the intrinsic DC factors that can be manipulated to modulate DC maturation and functions.
Circulating monocytes derived DCs have been found in human inflammatory fluids (8) and in vitro monocytes derived dendritic cells have been widely used as a model system to explore the molecular mechanisms of human DCs differentiation. Previous studies showed that granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL4 drive peripheral blood CD14+ monocytes differentiation to CD14−CD1a+ DCs (2, 9). Several cytokines such as IL6, IL10, and γ-IFN have been shown to negatively regulate the monocytes differentiation into DCs (2, 10–12), whereas other cytokines are reported to promote DCs differentiation (2, 13). Recent gene profiling analysis revealed a vast number of differentially expressed genes during induced human CD14+ monocytes differentiation into DCs (14). Nevertheless, the key transcriptional regulatory mechanisms underlying human monocytes to DCs differentiation remain poorly understood.
VentX is a human homologue of the Xenopus homeobox gene Xom of the BMP4 signaling pathway, and has been defined as a novel hematopoietic transcriptional factor controlling proliferation and differentiation of hematopoietic and immune cells (15–17). Initially identified as a novel LEF/TCF associated antagonist of the canonical Wnt signaling through reverse genetic modeling of Xenopus embryogenesis, VentX was found to be a transcriptional activator of the p53/p21 and p16ink4a/Rb tumor suppression pathways (18, 19). The critical role of VentX in hematopoietic cells development was indicated by its function in controlling the proliferation and differentiation of CD34+ cells and monocyte to macrophage terminal differentiation (16, 17).
In our current study, we provided evidence showing that VentX regulated DC differentiation and maturation through an IL6 mediated mechanism. The clinical importance of the findings was revealed by the elevated expression of VentX in DCs isolated from inflamed mucosa of IBD patients, and that VentX is a downstream target of corticosteroid treatment. Our data suggest that VentX is a key regulator of DC differentiation and maturation and may serve as a target of intervention for inflammatory disorders and immune therapy.
EXPERIMENTAL PROCEDURES
Human Primary Cells Isolation and Treatment
Peripheral blood mononuclear cells (PBMC) from healthy adult donors at Dana-Farber Cancer Institute were isolated by Ficoll density gradient centrifugation. CD14+ monocytes were purified from PBMCs using anti-CD14 antibody-coated magnetic microbeads (Miltenyi Biotec, Auburn, CA). Monocytes were cultured in 12-well plates at 1 × 106 cells/ml with RPMI 1640 medium containing 10% fetal bovine serum (FBS), GM-CSF (100 ng/ml), and IL4 (20 ng/ml) (PeproTech, Rocky Hill, NJ). Cytokines were added to cultures every 2 or 3 days for a total of 5 days to induce dendritic cell differentiation. Neutralizing antibody against IL6 was purchased from R&D Systems (Minneapolis, MN) and used at a daily dose of 2.5 μg/ml. Intestinal mucosa was obtained from surgically resected specimens from patients diagnosed with inflammatory bowel diseases including Crohn disease and ulcerative colitis. Specimens were taken from both inflamed and non-inflamed mucosa and were confirmed macroscopically and microscopically. Lamina propria mononuclear cells were isolated using previously described techniques (20, 21). LPDCs were purified as the fraction of CD19−CD1c+ cells with magnetic microbeads (Miltenyi Biotec). To promote maturation of DCs, 100 ng/ml of Escherichia coli LPS (Sigma-Aldrich) was added to the medium and further cultured for 24 h. Experiments with human materials were performed in accordance with guidelines approved by the institutional review committee of Brigham and Women's Hospital.
VentX and IκB Knockdown
Human primary monocytes were transfected with Morpholino (MO) antisense oligonucleotides using the Human Monocyte Nucleofector Kit (Lonza, Walkersville, MD) according to the manufacturer's instructions. Briefly, 10 × 106 monocytes were resuspended into 100 μl of nucleofector solution with 2.5 nmol of either VentX MO oligonucleotides (VentX MO: 5′-TACTCAACCCTGACATAGAGGGTAA-3′ or VentX MO-2: 5′-GAGCCCGGTTTGCATACACGGCTAA-3′) or a standard control MO oligonucleotides and electroporated with the Nucleofector II Device (Lonza). Cells were then immediately removed from the device and incubated overnight with 1 ml of pre-warmed Human Monocyte Nucleofector Medium containing 2 mm glutamine and 10% FBS. Cells were then resuspended into complete RPMI medium and treated with appropriate cytokines to induce differentiation into DCs. All the MO oligonucleotides were ordered from Gene Tools (Philomath, OR). LPDCs were transfected with siRNA targeting VentX as described in our previous study (17). Knockdown of IκBα expression in THP1 cells was achieved through electroporation transfection of SignalSilence® IκBα siRNA I from Cell Signaling (Danvers, MA).
Generation of THP1 Cell Line Conditionally Expressing VentX
Human monocytic leukemia cell line THP1 was obtained from American Type Culture Collection (ATCC; Manassas, VA). The doxycycline inducible retroviruses expressing GFP.VentX or GFP have been described in our previous study (17). The THP1 cell line conditionally expressing GFP.VentX was generated through co-transduction of pRetroX-GFP.VentX and pRetroX-Tet-On Advanced retroviruses and GFP.VentX-positive cells were sorted by FACSAria high-speed sorter (BD Bioscience, San Jose, CA) after incubation with 1.0 μg/ml doxycycline for 24 h (Dana-Farber Cancer Institute Flow Cytometry Core Facility). Sorted cells were then maintained in RPMI 1640 medium in the absence of doxycycline. The THP1 cell line conditionally expressing GFP was generated similarly as a control. To induce differentiation of THP1 cells toward DCs, cells were treated with the cytokines mixture as described previously (22) with some modifications. Briefly, cell were grown in 12-well plate in RPMI1640 medium supplemented with 10% FBS, 100 ng/ml GM-CSF, 50 ng/ml IL4, and TNF-α, 100 ng/ml ionomycin for 2 days. Under this sub-optimal condition, only mild DCs differentiation was observed in GFP-expressing THP1 cells, which allowed us to determine the effect of VentX expression on the DCs differentiation in this model cell line.
FACS Analysis
Phenotypic analyses of DCs and THP1 cells were performed with flow cytometry after immunostaining of cells with fluorescence dye conjugated antibodies (eBioscience, San Diego, CA). The following FITC or PE conjugated antibodies were used: anti-CD1a, CD1b, CD1c, CD11c, CD14, CD16, CD36, CD40, CD64, CD80, CD83, CD86, CD116, CCR7, HLA-DR, TLR2, and TLR4. Intracellular staining of CCL3, CCL5, IL6 and IL12, p70, and TNFα were performed with PE-conjugated antibodies following the protocol provided by manufacture. Isotope control staining was performed in parallel for all experiments and used for gating. Cell cycle analysis was carried out by propidium iodide (PI) staining. Stained cells were analyzed with FACScan flow cytometer (BD Bioscience) using FlowJo software. Results are expressed as the percentage of positive cells or mean fluorescence intensity (MFI) values after subtraction of the MFI obtained from the isotype control antibody.
Luciferase Reporter Assay
The −592 bp fragment of human IL6 promoter region was amplified with forward primer: 5′- GTAACGCGTTTCTACAACAGCCGCTCACAG-3′ and reverse primer: 5′-GATAGAGCTTCTCTTTCGTTC-3′. The −225 bp and −80 bp promoters were amplified with the same reverse primer and the following forward primers respectively: 5′-GTAACGCGTCAATGACGACCTAAGCTGCAC-3′ and 5′-GTAACGCGTGTGGGATTTTCCCATGAGTC-3′. The amplified products were digested with restriction enzymes Mlu I and XhoI, and digested fragments were subsequently cloned into pGL3 luciferase reporter. Transfection of reporter plasmid with pcDNA-VentX plasmid or control pcDNA-GFP plasmid into primary monocytes was carried out through electroporation. Reporter plasmid was also transfected into U2OS cells, which stably express tetracycline-inducible VentX (19), and reporter activity was evaluated in the absence or presence of tetracycline. 10 ng of Renilla luciferase plasmid was included for each transfection to normalize reporter activity. Cells were harvested 48 h after transfection or addition of tetracycline and analyzed with Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Mutation of NFκB binding site of luciferase reporters was achieved through QuikChange® Site-directed Mutagenesis Kit from Stratagene (Santa Clara, CA).The wild type NFκB binding sequence 5′-GGGATTTTCC-3′ was mutated to 5′-GGGATTTTAG-3′ as reported previously (23).
ChIP Assay
THP1 cell lines conditionally expressing GFP or GFP.VentX were employed to detect if VentX expression impairs the NFκB binding to IL6 promoter region. Cells were treated with 1.0 μg/ml doxycycline for 2 days and harvested for chromatin immunoprecipitation (ChIP) assay. The ChIP procedure was performed with SimpleChIP® Enzymatic Chromatin IP Kit from Cell Signaling following the manufacturer's instructions. The NFκB/p65 antibody (Cell Signaling) was used for the immunoprecipitation. Human IL6 promoter region containing the NFκB binding site was amplified by quantitative PCR with forward primer: 5′-GGACGTCACATTGCACAATC-3′ and reverse primer: 5′-GCCTCAGACATCTCCAGTCC-3′.
Cell Migration Assay
The dendritic cells migration assay was performed with CytoSelect™ 24-Well Cell Migration Assay kit from Cell Biolabs, Inc. (San Diego, CA).
PCR Array, Real Time-PCR, Western Blot, and Mixed Lymphocyte Reaction
Dendritic & Antigen Presenting Cell PCR Array was purchased from SABiosciences (Valencia, CA). Real time PCR was performed on a LightCycler® (480 Real-Time PCR System; Roche). The primers used for the detection of mRNA levels of VentX, IL6, IL12, p21, c-myc, CCL2, CCL3, CCL5, CCL19, CXCL1, CXCL12, CD1a, TNFα, TLR2, and TLR4 have been shown previously (17). Western blotting analysis was conducted as described in our prior study (17). Primary antibodies against p21 and c-Myc were from Cell Signaling, and anti-VentX antibody was purchased from Abcam (Boston, MA). Mixed lymphocyte reaction was performed as described previously (17) except that BrdU was added to culture to determine T cells proliferation. Incorporated BrdU was detected with PE-conjugated anti-BrdU antibody and analyzed with flow cytometry.
Statistical Analysis
The Student's t test was used to calculate statistical significance, and p < 0.05 was considered statistically significant.
RESULTS
VentX Is Required for Human Primary Monocytes to Dendritic Cell Differentiation
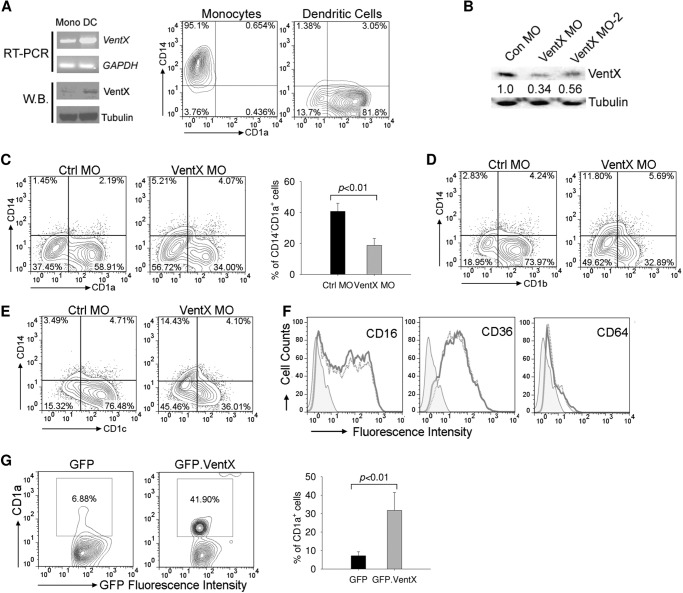
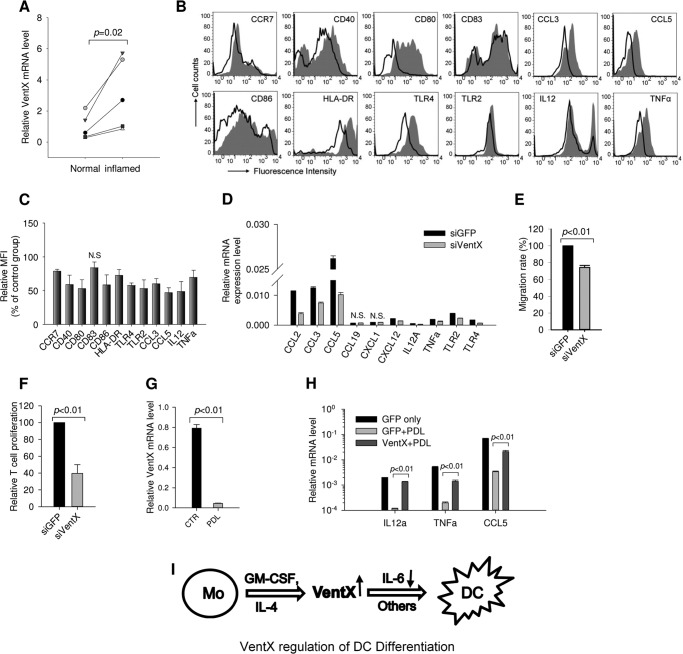
Following our recent finding that VentX is expressed in human primary monocytes (17), we examined VentX expression during primary monocytes to dendritic cell differentiation induced by GM-CSF and IL-4 treatment. We found that VentX expression underwent a marked increase at both mRNA and protein levels during the induced DC differentiation (Fig. 1A). To explore potential role of VentX in DC differentiation, the effects of VentX knockdown on in vitro DCs differentiation were examined. VentX siRNA produced marginal VentX knockdown efficiency in monocytes after 5 days of transfection, whereas differentiation of DCs from monocytes in vitro requires 5∼7 days of incubation with GM-CSF and IL4 (2, 9). To obtain a long-lasting knockdown effect of VentX in monocytes, we developed an MO-mediated VentX knockdown strategy. Two different MO antisense oligonucleotides were designed (VentX MO and VentX MO-2) and tested for the knockdown efficiency of VentX. As shown in Fig. 1B, both MO oligonucleotides inhibited VentX expression in DCs compared with control MO 5 days after transfection. Freshly isolated CD14+CD1a− monocytes differentiate in vitro into immature CD14−CD1a+ DCs when cultured with GM-CSF and IL-4 (2, 9). We therefore examined surface expression of CD1a antigen in monocytes transfected with control MO or VentX MO, respectively. As shown in Fig. 1 C, transfection of VentX MO led to a substantial reduction in the percentage of CD14−CD1a+ cells (Fig. 1C) in comparison with the control MO. The surface expression of CD1b and CD1c, which are considered as additional differentiation markers of monocytes-derived DCs (2, 9), were similarly downregulated in VentX MO transfected cells (Fig. 1, D–E). Transfection of VentX MO did not cause excess cell death in monocytes (17), which ruled out the possibility that the decreased differentiation was due to compromised cell viability. The expression of other surface antigens such as CD16, CD36, and CD64 remained unchanged in VentX MO transfected DCs (Fig. 1F), further supporting that VentX MO transfection exerted specific effects on DC differentiation. To corroborate the role of VentX in regulating DCs differentiation, we ectopically expressed VentX in primary monocytes and determined the CD14−CD1a+ cells after 3 days of cytokines treatment. Overexpression of VentX in monocytes greatly accelerated the DCs differentiation (Fig. 1G). Taken together, these data demonstrated that VentX is a critical regulator of primary monocytes to DCs differentiation.
FIGURE 1.
VentX is required for primary human monocyte to dendritic cell differentiation. A, human primary monocytes isolated from peripheral blood were cultured in the presence of GM-CSF plus IL4 for 5 days. Total RNA and whole cell lysates were isolated from fresh monocytes and differentiated dendritic cells for analysis of VentX mRNA level by RT-PCR and protein level by Western blot (left panel). The flow cytometry results of fresh monocytes and differentiated dendritic cells are shown in the right panel. B, primary human monocytes (10 m each) were transfected with MO oligonucleotides targeting VentX (VentX MO and VentX MO-2) or control MO oligonucleotides through electroporation as described under “Experimental Procedures.” Cytokines GM-CSF and IL4 were added 24 h later, and cells were collected 5 days after cytokines addition for Western blot analysis of VentX protein level. Tubulin was used as loading control. The quantification of VentX knockdown efficiency was determined by densitometry. C, human primary monocytes were transfected with MO oligonucleotides targeting VentX or the control MO as described under “Experimental Procedures.” Twenty-four hours after transfection, GM-CSF and IL4 were added to culture medium. Cells were harvested 5 days after cytokine addition, and the surface expression of CD1a and CD14 was analyzed by flow cytometry (left panel). Bar graph in right panel shows the percentage of CD14−CD1a+ cells from VentX MO or control MO-transfected monocytes. The results are mean ± S.D. of six independent experiments. D—F, monocytes were transfected with VentX MO or control MO oligonucleotides as described above. The surface expression of CD1b (D), CD1c (E), and CD16, CD36, CD64 (F) was analyzed by flow cytometry 5 days after addition of cytokines. Results shown were representative of at least five independent experiments. G, monocytes were transfected with pcDNA-GFP or pcDNA-GFP.VentX plasmids through electroporation. Cytokines were added to culture medium 2 h after transfection, and cells were harvested 3 days after cytokine addition for flow cytometry analysis. GFP-positive population was gated for analysis of CD1a expression (left panel). The mean ± S.D. of four independent experiments is shown in right panel.
VentX Promotes Dendritic Cell Differentiation
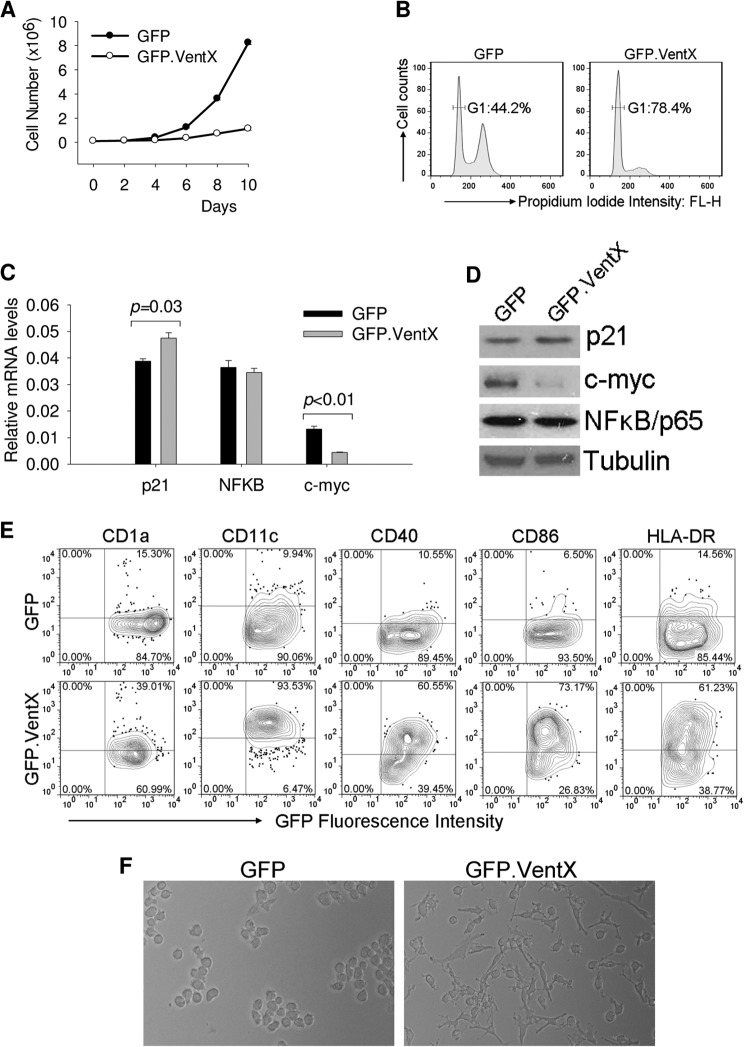
Human monocytic cell line THP1, under appropriate differentiation conditions, can be induced to differentiate into immature and mature DCs with phenotypic and functional properties similar to those of primary DCs (22, 24). To further explore the effects of VentX on dendritic cell differentiation, we generated stable THP1 cell lines expressing GFP or GFP.VentX under the control of doxycycline-inducible promoter. VentX has been reported to function as an anti-proliferation and pro-differentiation transcriptional factor (16–19). Thus, we first examined whether overexpression of VentX could exert an inhibitory role on the proliferation of THP1 cells. Consistent with our prior study (17, 19), expression of VentX in THP1 cells induced apparent growth inhibition (Fig. 2A) and G1 cell cycle arrest (Fig. 2B), which was associated with the down-regulation of c-myc and up-regulation of p21 expression by VentX (Fig. 2, C and D). To determine the effects of VentX expression on DC differentiation of THP1 cells, we developed a suboptimal induction condition under which a mild differentiation of GFP transduced THP1 cells could be observed (Fig. 2E, upper panel). Under this condition, overexpression of VentX significantly accelerated the DCs differentiation of THP1 cells. As shown in Fig. 2E, VentX transduced THP1 cells displayed a markedly increase of surface expression of CD1a, CD11c, CD40, CD86, and HLA-DR, indicating an enhanced DCs differentiation in these cells. Strikingly, VentX transduced THP1 cells became adherent and flattened with extensive dendrite formation (Fig. 2F, right panel), resembling the morphology of DCs derived from primary monocytes. In contrast, no such morphological changes were observed in GFP transduced cells (Fig. 2F, left panel).
FIGURE 2.
VentX overexpression promotes THP1 cell differentiation toward dendritic cells. A, THP1 cell lines expressing GFP or GFP.VentX under the control of tetracycline-inducible promoter were treated with 1.0 μg/ml doxycycline (DOX) for 10 days. Cell numbers at indicated days were counted and plotted. B, cell cycle profiles of THP1 cells expressing GFP or GFP.VentX after 10 days exposure to DOX. Cells were stained with propidium iodide and analyzed by flow cytometry. C and D, THP1 cells were treated with DOX for 3 days and harvested. The mRNA level (C) and protein level (D) of p21, NFκB, and c-Myc were analyzed by real-time PCR and Western blot, respectively. E and F, THP1 cells were treated with DOX and cultured under conditions described under “Experimental Procedures” to induce dendritic cell differentiation. E, 2 days after treatment, cells were harvested to analyze the surface expression of indicated antigens by flow cytometry. F, cells were photographed using phase contrast microscopy to show the morphological changes of VentX-expressing cells. Results shown are representative of at least three independent experiments.
IL-6 Mediates VentX Regulation of DCs Differentiation
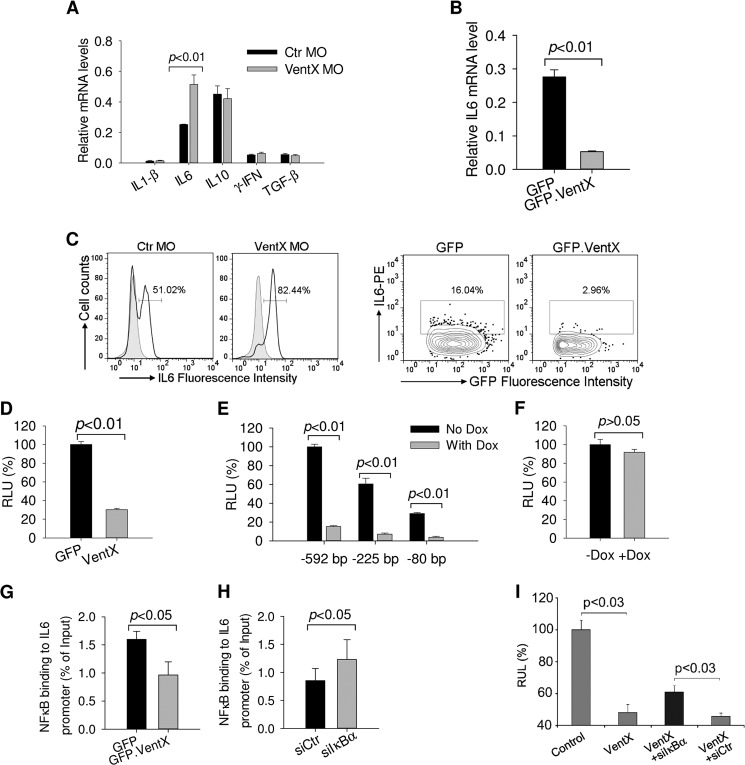
To elucidate the mechanisms underlying VentX regulated DCs differentiation, expression level of cytokines known to be important for DCs development was analyzed by reverse transcription PCR. Among the cytokines examined, IL6 expression was consistently increased in cells transfected with VentX MO (Fig. 3A). It has been shown that IL6 inhibits DC differentiation and maturation from both CD14+ monocytes and CD34+ progenitor cells (2, 25). To corroborate the results of knockdown experiments, we examined the effect of ectopic expression of VentX on expression level of IL6. Consistent with loss-of-function approach, overexpression of VentX reduced IL6 mRNA level (Fig. 3B). Intracellular staining experiments demonstrated that VentX inhibited IL6 production in primary monocytes (Fig. 3C). To determine whether VentX inhibits IL6 expression at the transcriptional level, we performed luciferase reporter assays with IL6 promoter. The effect of VentX on the activity of 592 bp IL6 promoter luciferase reporter and several deletion mutant constructs was assessed (26). As shown in Fig. 3D, VentX significantly inhibited the −592 bp IL6 promoter activity in primary monocytes. Previous studies have delineated several functional cis-regulatory elements in the human IL-6 promoter, including binding sites of AP1 (−283 to −277 bp), C/EBP (−158 to −145 bp and −87 to −76 bp) and NFκB (−75 to −63 bp) (26). To explore potential VentX responsible elements on the IL6 promote, the −592 bp and the truncated luciferase reporters were transfected into U2OS cells that express VentX under the control of doxycycline-inducible promoter (21). Upon induction of VentX expression with doxycycline, we found that the activity of −80 bp IL6 promoter reporter, which contains only NFκB binding site (26), was suppressed by VentX to a degree similar to that of −592 bp IL6 promoter (Fig. 3E). This finding indicated that VentX may target NFκB binding site to regulate IL6 expression. To test this hypothesis, we performed mutation analysis of the IL6 promoter and found that mutation of NFκB binding site on the −80 bp IL6 promoter abrogated the ability of VentX to inhibit the promoter activity (Fig. 3F). To determine whether NFκB is involved in VentX regulated IL6 expression, we examined the effects of VentX on NFκB expression and its interaction with IL6 promoter, using THP1 cells that express VentX under the control of doxycyclin-inducible promoter. We found that VentX did not affect the expression of NFκB at either mRNA or protein levels (Fig. 2, C and D). However, using ChIP and real-time PCR assays, we found that ectopic expression of VentX led to a reduced binding of NFκB to the IL6 promoter (Fig. 3G). To further explore potential involvement of NFκB in VentX regulated IL6 expression, IκBα siRNA was transfected into the VentX expressing THP1 cells to enhance NFκB activity. We found that transfection of IκBα siRNA increased NFκB binding to IL6 promoter (Fig. 3H). Consistently, we found that expression of IκBα siRNA attenuated VentX inhibition of IL6 promoter activation (Fig. 3I), These data suggest that VentX may compete with NFκB to suppress IL6 expression. To further investigate whether impaired DCs differentiation in VentX MO transfected monocytes could be ascribed to the increased IL6 secretion in these cells, neutralizing antibody against IL6 was added to the culture medium of VentX MO transfected monocytes and DCs differentiation was assessed through flow cytometry analysis of CD1a surface expression. As shown in Fig. 4, neutralizing IL6 activity with specific antibody significantly improved DCs differentiation defects in VentX MO transfected monocytes, indicating that VentX regulates DCs differentiation, at least in part, through modulating IL6 autocrine production.
FIGURE 3.
VentX suppresses IL6 expression. A, monocytes were transfected with VentX MO or control MO and GM-CSF and IL4 were added into culture medium for 5 days. Cells were harvested for quantitative analysis of mRNA level of indicated genes. B, monocyte was transfected with pcDNA-GFP or pcDNA-GFP.VentX through electroporation. Cells were then cultured in the presence of GM-CSF and IL4 for 3 days, and GFP-positive cells were sorted out to analyze IL6 mRNA level. C, monocytes were transfected and treated as above, and cells were harvested for intracellular staining followed by flow cytometry analysis of IL6 levels. Results shown are representative of at least three independent experiments. D, −592 bp human IL6 promoter reporter was transfected with pcDNA-GFP or pcDNA-VentX plasmid into primary monocytes through electroporation. Two days after transfection, cells were harvested to analyze the luciferase activity. E, various IL6 promoter reporters were transfected into U2OS cells that express VentX upon induction with doxycycline. Cells were harvested 2 days after DOX addition, and luciferase activity in the presence or absence of DOX was analyzed. F, −80 bp IL6 promoter reporter with mutant NFκB binding site was transfected into U2OS cells. Then DOX was added into medium or omitted, and luciferase activity was determined 2 days after transfection. G, THP1 cells expressing GFP or GFP.VentX under the control of tetracycline-inducible promoter were treated with DOX for 2 days and harvested for ChIP assay with anti-NFκB/p65 antibody. The human IL6 promoter region containing an NFκB binding site was amplified by real-time PCR as described under “Experimental Procedures.” H, THP1 cells expressing GFP.VentX were transfected with control siRNA or IκBα siRNA, and cells were harvested 2 days after transfection for ChIP assay with anti-NFκB/p65 antibody as described above. Results shown are mean ± S.D. of triplicates from one representative of three independent experiments. I, −592 bp human IL6 promoter reporter was transfected with pcDNA-VentX plasmid into U2OS cells that have been transfected with control siRNA or IκBα siRNA 12 h prior. Two days after transfection, cells were harvested, and luciferase activity was determined. Error bars indicate standard deviation of representative experiments carried out in triplicate.
FIGURE 4.
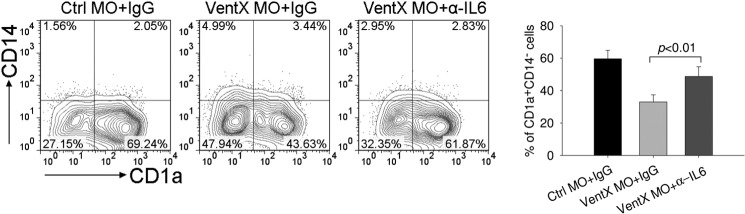
Enhanced auotcrine IL6 contributes to differentiation defects of dendritic cells with VentX knockdown. Human primary monocytes were transfected with VentX MO or control MO as indicated. Twenty-four hours post-transfection, cytokines GM-CSF plus IL4 were added into culture medium together with neutralizing IL6 antibody or control immunoglobulin (IgG). Cells were harvested 5 days after cytokine addition and analyzed for the expression of CD14 and CD1a by flow cytometry (left panel). The mean ± S.D. of four independent experiments is shown in the right panel.
VentX Promotes DC Maturation Response to LPS Stimulation
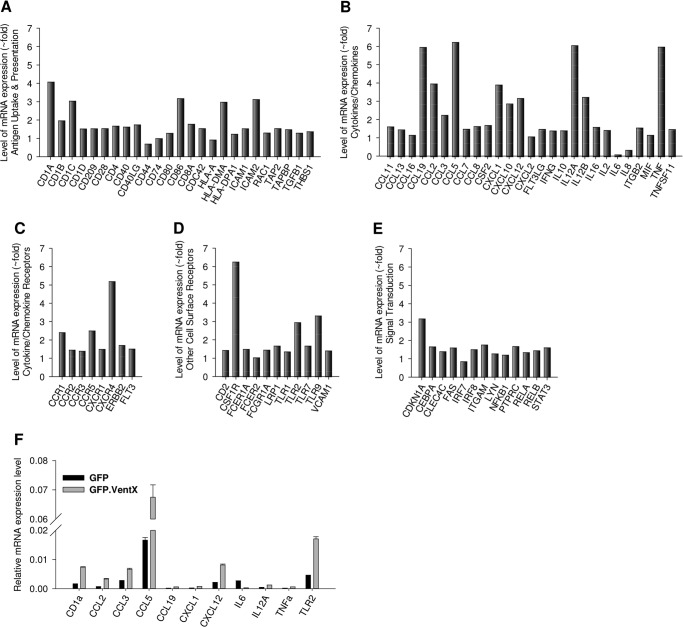
To explore whether VentX plays a role in DC maturation, we examined the effects of VentX on the changes of DCs gene expression profile during LPS induced maturation process, using a PCR array approach. The PCR array includes genes involved in antigen uptake and presentation, cytokines/chemokines and their receptors important for DC maturation. Cell surface receptors and signal transduction factors implicated in inflammation are also included in this array. As shown in Fig. 5, A–E, overexpression of VentX induced up-regulation of a wide variety of genes, such as CD1 antigens, co-stimulatory factors CD80 and CD86, HLA molecules, proinflammatory cytokines IL12, TNFα, chemokines CCL2(MCP-1), CCL3(MIP-1α), and CCL5 (RANTES), chemokine receptors CCR5 and CXCR4, and TLRs. In addition, the expressions of CSF1R (M-SCF receptor) and CDKN1A (p21) also increased as previously demonstrated (17, 19). The results of PCR array were further verified by independent PCR reaction, using different sets of primers. As shown in Fig. 5F, independent quantitative PCR experiments produced consistent results as PCR array; therefore suggesting a potential role of VentX during DCs maturation process.
FIGURE 5.
PCR array analysis of indicated genes in dendritic cells that overexpress VentX. Monocytes were transfected with pcDNA-GFP or pcDNA-GFP.VentX plasmids through electroporation. GM-CSF and IL4 were added to culture medium 2 h later to induce DCs differentiation. 3 days after transfection, LPS was added for overnight treatment, and cells were harvested and sorted with GFP through flow cytometry. A–E, total RNA was isolated, and the mRNA levels of indicated genes were analyzed by PCR array. Results shown are one representative of two independent experiments. F, mRNA levels of selected genes were determined by real-time PCR. Results shown are mean ± S.D. of triplicates, and the differences are significantly different between two groups (p < 0.05) for all genes.
VentX Regulates DC Maturation during Intestinal Mucosal Inflammation and Is a Treatment Target of Corticosteroid
Our findings that VentX regulates DCs differentiation and maturation led to our exploration of the relevance of our findings in pathogenesis of inflammation. To address the question, intestinal lamina propria DCs (LPDCs) were isolated from inflamed as well as control normal mucosa of patients with ulcerative colitis and Crohn disease. VentX expression levels in these DCs were determined by RT-PCR method. As shown in Fig. 6A, VentX expression levels were significantly elevated in LPDCs from mucosa of inflamed area in comparison with LPDCs from non-inflamed area (Fig. 6A). To determine whether the elevated expression of VentX is relevant to the aberrant maturation response of DCs, we tested the effects of VentX knockdown on maturation of LPDCs. As shown in Fig. 6, B and C, among the cell surface markers examined, knockdown of VentX in LPDCs significantly down-regulated expression of co-stimulatory factors CD40, CD80, and CD86, chemokine receptor CCR7, and pattern recognition receptors TLR2 and TLR4, which have previously been shown to be up-regulated in DCs from inflamed intestinal mucosa of IBD patients (6). Expression of CD83 was unaffected by VentX knockdown. Intracellular staining demonstrated that LPDCs with VentX knockdown produced less inflammatory cytokines IL12, TNFα, and chemokines CCL3, CCL5 (Fig. 6C). Consistently, RT-PCR revealed a significant decrease of mRNA level for CCL2, CCL3, CCL5, CXCL12, IL12, TNFα, and TLRs in LPDCs cells with VentX knockdown in comparison with control LPDCs cells (Fig. 6D). In addition to the phenotype characterization, functional analysis showed that DCs with VentX knockdown displayed significantly reduced migration capability (Fig. 6E) and weaker stimulation to primary T cells proliferation (Fig. 6F). Immune suppressants, such as corticosteroid, remain as the main treatment modality in managing mucosal inflammation in moderate to severe IBD patients. It has been reported that corticosteroid modulated DC maturation and function (11, 27). To determine whether VentX might be a downstream target of corticosteroid, we examined VentX expression levels in LPDCs treated with prednisolone (PDL) in vitro. As shown in Fig. 6G, corticosteroid treatment drastically decreased VentX expression in LPDCs, as well as the expression of pro-inflammatory cytokines, such as the IL12, TNFα, CCL5 (Fig. 6H). When VentX was ectopically expressed in DCs, the inhibitory effect of corticosteroid on the expression of pro-inflammatory cytokines was significantly diminished (Fig. 6H), suggesting that corticosteroid exert its anti-inflammatory in part through down-regulating VentX expression in DCs.
FIGURE 6.
VentX regulates maturation of lamina propria DCs. A, LPDCs were isolated from inflamed and control non-inflamed intestinal mucosa of five IBD patients. VentX expression level was determined by real-time PCR. B, LPDCs were transfected with siRNA targeting VentX or control GFP through electroporation. Two days post-transfection, cells were stimulated with LPS for overnight to induce maturation and harvested for flow cytometry analysis of surface markers and intracellular cytokines/chemokines. Filled histograms indicate siGFP transfection. C, bar graph showed the mean fluorescence intensity (MFI) of four independent experiments in B. Results are expressed as the percentage of MFI normalized to control. There was a significant difference between these two groups (p < 0.05) except for CD83, which was not significant (N.S.). D, LPDCs transfected with siGFP or siVentX were analyzed for the mRNA expression levels of indicated genes. There was a significant difference between the two groups (p < 0.05) except for CCL19 and CXCL1. E, knockdown of VentX impaired the migration capability of DCs. F, knockdown of VentX impaired DCs capability to stimulate allogenic T cells proliferation in a mixed lymphocyte reaction. G, DCs were treated with prednisolone (10 μg/ml) for 48 h or mock treated; then VentX level was determined with real time PCR. H, DCs were transfected with GFP or VentX and then treated with PDL as indicated. LPS was added 24 h later, and cells were harvested 48 h after transfection to analyze the mRNA levels of CCL5, IL12A, and TNFα. Results shown are mean ± S.D. of triplicates from one of two independent experiments. I, schematic of VentX regulation of monocyte (Mo)-derived DC differentiation.
DISCUSSION
The mechanism of DCs differentiation and maturation is the focus of great interests for its potential application in immune regulation and therapy. In the present study, we showed that the human homeobox protein VentX is a key regulator of DC differentiation and maturation. Recent studies demonstrated that VentX is a critical hematopoietic transcriptional factor during ontogenesis of different lineages of hematopoietic cells (16, 17, 28). Knockdown of VentX impairs early differentiation of hematopoietic CD34+ cells as well as monocytes terminal differentiation (16, 17). These data suggested that VentX is a general permissive factor for the differentiation of hematopoietic cells, upon exposure to different inducing factors. Our data showed that ectopic expression of VentX in U937 cell promoted macrophage development (17), whereas expression of VentX in THP1 cell promoted DCs development (Fig. 2), suggesting that the effects of VentX on differentiation of hematopoietic cells are cell type specific, and perhaps through different mechanisms.
The exploration of the mechanisms underlying VentX regulated DCs differentiation led our attention to IL6, a critical factor involved in DC differentiation (2, 10). Prior studies showed that modulation of DCs differentiation by γ-IFN, Wnt5a, HLA-G, and transcription factor ESE-3 related to the altered IL6 secretion or signaling (12, 29–31). Our data showed that IL6 expression was increased in DCs with VentX knockdown (Fig. 3, A and C), whereas overexpression of VentX inhibited IL6 expression (Fig. 3, B and C). Importantly, we found that impaired DC differentiation upon VentX knockdown could be partially rescued by diminished IL6 signaling through addition of IL6 antibody to culture media (Fig. 4). The mechanism underlying VentX regulated IL6 expression remained to be further defined. VentX does not bind to the IL6 promoter,3 thus, it is likely that VentX regulates IL6 expression through an indirect mechanism. Notably, previous studies have established the NFκB as a direct transcriptional activator of IL6 (32, 33). VentX is a direct transcriptional activator of the p53 and the p16, which are known antagonists of NFκB activation of IL6 (19, 34, 35). Thus, VentX may regulate IL6 expression in part through its inhibitory effects on NFκB binding and transactivation of IL6 promoter. In comparison with its effects in dendritic cells, our previous study showed that VentX promoted IL6 expression in macrophages (17). The mechanism underlying context dependent regulation of NFκB downstream genes by VentX during monocyte differentiation remains to be defined. It is noteworthy that our findings paralleled to the findings of Lee et al., who showed the context-specific regulation of NFκB target genes by EZH2 in breast cancers (36).
In addition to modulation of IL6 expression (Fig. 3), knockdown VentX also down-regulates GM-CSF receptor (CD116) expression.3 Therefore, multiple pathways have been implicated in VentX regulated dendritic cell differentiation. Our previous studies have revealed that VentX is a critical regulator of p53/p21 and the canonical Wnt signaling (18, 19), which have also been implicated in DC differentiation (37, 38). As such, we propose that VentX targets multiple pathways to regulate monocyte to DC differentiation (Fig. 6I).
Our findings that VentX expression was aberrantly elevated in DCs isolated from inflamed mucosa of IBD patients suggested the clinical relevance of the identification of VentX as a novel regulator of DC maturation during pathogenesis of IBD. Importantly, VentX was found to be a downstream target of corticosteroid and VentX mediated inhibitory effects of steroid on DCs function. Refractory to corticosteroid and other immune suppressants is a major issue of managing autoimmune and inflammatory diseases. Results of our studies suggest that targeting VentX may allow development of alternative strategies to manage corticosteroid resistance. Further studies are needed to define how VentX-regulated DCs differentiation and maturation is modulated by host and microbial factors. Such information would be critical for understanding localized damage of intestinal mucosa in IBD. Interestingly, similar change of VentX was observed in macrophages isolated from inflamed mucosa of IBD patients,3 further suggesting a potential role of VentX in managing mucosal inflammation.
The difference of the molecular mechanisms underlying human and murine blood and immune cell development and pathogenesis of inflammation has been increasingly appreciated (39) (40). VentX is a human hematopoietic homeobox transcriptional factor that lacks a murine homologue (41). Further investigation on the role of VentX in blood and immune cell development may help delineate the unique mechanisms of human hematopoiesis and immune regulation and open novel avenues for managing autoimmune inflammatory-disorders.
Acknowledgments
We thank Drs. D. Cohen and R. Blumberg for critical reagents and use of their facilities for this study and Shuanhu Zhou and Hongwei Gao for technical support.
This work was supported, in whole or in part, by grants from the National Institutes of Health, American Cancer Society, and a research fund from Brigham and Women's Hospital (to Z. Z.).
Z. Zhu, unpublished data.
- DC
- dendritic cell
- LPS
- lipopolysaccharide
- TLR
- toll-like receptor
- IBD
- inflammatory bowel disease
- MO
- morpholino
- LPDC
- lamina propria DC
- PDL
- prednisolone.
REFERENCES
- 1. Liu K., Nussenzweig M. C. (2010) Origin and development of dendritic cells. Immunol. Rev. 234, 45–54 [DOI] [PubMed] [Google Scholar]
- 2. Chomarat P., Banchereau J., Davoust J., Palucka A. K. (2000) IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 1, 510–514 [DOI] [PubMed] [Google Scholar]
- 3. Mellman I. (2005) Antigen processing and presentation by dendritic cells: cell biological mechanisms. Adv. Exp. Med. Biol. 560, 63–67 [DOI] [PubMed] [Google Scholar]
- 4. Bell S. J., Rigby R., English N., Mann S. D., Knight S. C., Kamm M. A., Stagg A. J. (2001) Migration and maturation of human colonic dendritic cells. J. Immunol. 166, 4958–4967 [DOI] [PubMed] [Google Scholar]
- 5. Kadowaki N., Ho S., Antonenko S., Malefyt R. W., Kastelein R. A., Bazan F., Liu Y. J. (2001) Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hart A. L., Al-Hassi H. O., Rigby R. J., Bell S. J., Emmanuel A. V., Knight S. C., Kamm M. A., Stagg A. J. (2005) Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology 129, 50–65 [DOI] [PubMed] [Google Scholar]
- 7. Hugot J. P., Chamaillard M., Zouali H., Lesage S., Cézard J. P., Belaiche J., Almer S., Tysk C., O'Morain C. A., Gassull M., Binder V., Finkel Y., Cortot A., Modigliani R., Laurent-Puig P., Gower-Rousseau C., Macry J., Colombel J. F., Sahbatou M., Thomas G. (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603 [DOI] [PubMed] [Google Scholar]
- 8. Segura E., Touzot M., Bohineust A., Cappuccio A., Chiocchia G., Hosmalin A., Dalod M., Soumelis V., Amigorena S. (2013) Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 38, 336–348 [DOI] [PubMed] [Google Scholar]
- 9. Sallusto F., Lanzavecchia A. (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179, 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mitani H., Katayama N., Araki H., Ohishi K., Kobayashi K., Suzuki H., Nishii K., Masuya M., Yasukawa K., Minami N., Shiku H. (2000) Activity of interleukin 6 in the differentiation of monocytes to macrophages and dendritic cells. Br. J. Haematol 109, 288–295 [DOI] [PubMed] [Google Scholar]
- 11. Piemonti L., Monti P., Allavena P., Sironi M., Soldini L., Leone B. E., Socci C., Di Carlo V. (1999) Glucocorticoids affect human dendritic cell differentiation and maturation. J. Immunol. 162, 6473–6481 [PubMed] [Google Scholar]
- 12. Delneste Y., Charbonnier P., Herbault N., Magistrelli G., Caron G., Bonnefoy J. Y., Jeannin P. (2003) Interferon-γ switches monocyte differentiation from dendritic cells to macrophages. Blood 101, 143–150 [DOI] [PubMed] [Google Scholar]
- 13. Gabriele L., Borghi P., Rozera C., Sestili P., Andreotti M., Guarini A., Montefusco E., Foà R., Belardelli F. (2004) IFN-α promotes the rapid differentiation of monocytes from patients with chronic myeloid leukemia into activated dendritic cells tuned to undergo full maturation after LPS treatment. Blood 103, 980–987 [DOI] [PubMed] [Google Scholar]
- 14. Le Naour F., Hohenkirk L., Grolleau A., Misek D. E., Lescure P., Geiger J. D., Hanash S., Beretta L. (2001) Profiling changes in gene expression during differentiation and maturation of monocyte-derived dendritic cells using both oligonucleotide microarrays and proteomics. J. Biol. Chem. 276, 17920–17931 [DOI] [PubMed] [Google Scholar]
- 15. Gao H., Wu B., Giese R., Zhu Z. (2007) Xom interacts with and stimulates transcriptional activity of LEF1/TCFs: implications for ventral cell fate determination during vertebrate embryogenesis. Cell Res. 17, 345–356 [DOI] [PubMed] [Google Scholar]
- 16. Gao H., Wu X., Sun Y., Zhou S., Silberstein L. E., Zhu Z. (2012) Suppression of Homeobox Transcription Factor VentX Promotes Expansion of Human Hematopoietic Stem/Multipotent Progenitor Cells. J. Biol. Chem. 287, 29979–29987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu X., Gao H., Ke W., Giese R. W., Zhu Z. (2011) The homeobox transcription factor VentX controls human macrophage terminal differentiation and proinflammatory activation. J. Clin. Invest. 121, 2599–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao H., Le Y., Wu X., Silberstein L. E., Giese R. W., Zhu Z. (2010) VentX, a novel lymphoid-enhancing factor/T-cell factor-associated transcription repressor, is a putative tumor suppressor. Cancer Res. 70, 202–211 [DOI] [PubMed] [Google Scholar]
- 19. Wu X., Gao H., Ke W., Hager M., Xiao S., Freeman M. R., Zhu Z. (2011) VentX trans-activates p53 and p16ink4a to regulate cellular senescence. J. Biol. Chem. 286, 12693–12701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamada N., Hisamatsu T., Okamoto S., Chinen H., Kobayashi T., Sato T., Sakuraba A., Kitazume M. T., Sugita A., Koganei K., Akagawa K. S., Hibi T. (2008) Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J. Clin. Invest. 118, 2269–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pignata C., Budillon G., Monaco G., Nani E., Cuomo R., Parrilli G., Ciccimarra F. (1990) Jejunal bacterial overgrowth and intestinal permeability in children with immunodeficiency syndromes. Gut 31, 879–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berges C., Naujokat C., Tinapp S., Wieczorek H., Höh A., Sadeghi M., Opelz G., Daniel V. (2005) A cell line model for the differentiation of human dendritic cells. Biochem. Biophys. Res. Commun. 333, 896–907 [DOI] [PubMed] [Google Scholar]
- 23. Grassl C., Luckow B., Schlöndorff D., Dendorfer U. (1999) Transcriptional regulation of the interleukin-6 gene in mesangial cells. J. Am. Soc. Nephrol 10, 1466–1477 [DOI] [PubMed] [Google Scholar]
- 24. Takayanagi H., Ogasawara K., Hida S., Chiba T., Murata S., Sato K., Takaoka A., Yokochi T., Oda H., Tanaka K., Nakamura K., Taniguchi T. (2000) T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 408, 600–605 [DOI] [PubMed] [Google Scholar]
- 25. Menetrier-Caux C., Montmain G., Dieu M. C., Bain C., Favrot M. C., Caux C., Blay J. Y. (1998) Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood 92, 4778–4791 [PubMed] [Google Scholar]
- 26. Faggioli L., Costanzo C., Donadelli M., Palmieri M. (2004) Activation of the Interleukin-6 promoter by a dominant negative mutant of c-Jun. Biochim. Biophys. Acta 1692, 17–24 [DOI] [PubMed] [Google Scholar]
- 27. de Jong E. C., Vieira P. L., Kalinski P., Kapsenberg M. L. (1999) Corticosteroids inhibit the production of inflammatory mediators in immature monocyte-derived DC and induce the development of tolerogenic DC3. J. Leukoc Biol. 66, 201–204 [DOI] [PubMed] [Google Scholar]
- 28. Rawat V. P., Arseni N., Ahmed F., Mulaw M. A., Thoene S., Heilmeier B., Sadlon T., D'Andrea R. J., Hiddemann W., Bohlander S. K., Buske C., Feuring-Buske M. (2010) The vent-like homeobox gene VENTX promotes human myeloid differentiation and is highly expressed in acute myeloid leukemia. Proc. Natl. Acad. Sci. U.S.A. 107, 16946–16951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chim C., Pang R., Liang R. (2008). Epigenetic Dysregulation of the Wnt Signaling Pathway in Chronic Lymphocytic Leukemia (CLL). J. Clin. Pathol. 61, 1214–1219 [DOI] [PubMed] [Google Scholar]
- 30. Appel S., Bringmann A., Grünebach F., Weck M. M., Bauer J., Brossart P. (2006) Epithelial-specific transcription factor ESE-3 is involved in the development of monocyte-derived DCs. Blood 107, 3265–3270 [DOI] [PubMed] [Google Scholar]
- 31. Valencia J., Hernández-López C., Martinez V. G., Hidalgo L., Zapata A. G., Vicente Á., Varas A., Sacedón R. (2011) Wnt5a skews dendritic cell differentiation to an unconventional phenotype with tolerogenic features. J. Immunol. 187, 4129–4139 [DOI] [PubMed] [Google Scholar]
- 32. Libermann T. A., Baltimore D. (1990) Activation of interleukin-6 gene expression through the NF-κB transcription factor. Mol. Cell Biol. 10, 2327–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanters E., Pasparakis M., Gijbels M. J., Vergouwe M. N., Partouns-Hendriks I., Fijneman R. J., Clausen B. E., Förster I., Kockx M. M., Rajewsky K., Kraal G., Hofker M. H., de Winther M. P. (2003) Inhibition of NF-κB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 112, 1176–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santhanam U., Ray A., Sehgal P. B. (1991) Repression of the interleukin 6 gene promoter by p53 and the retinoblastoma susceptibility gene product. Proc. Natl. Acad. Sci. U.S.A. 88, 7605–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang J., Kantrow S., Sai J., Hawkins O. E., Boothby M., Ayers G. D., Young E. D., Demicco E. G., Lazar A. J., Lev D., Richmond A. (2012) INK4a/ARF [corrected] inactivation with activation of the NF-κB/IL-6 pathway is sufficient to drive the development and growth of angiosarcoma. Cancer Res. 72, 4682–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee S. T., Li Z., Wu Z., Aau M., Guan P., Karuturi R. K., Liou Y. C., Yu Q. (2011) Context-specific regulation of NF-κB target gene expression by EZH2 in breast cancers. Mol. Cell 43, 798–810 [DOI] [PubMed] [Google Scholar]
- 37. Kramer J. L., Baltathakis I., Alcantara O. S., Boldt D. H. (2002) Differentiation of functional dendritic cells and macrophages from human peripheral blood monocyte precursors is dependent on expression of p21 (WAF1/CIP1) and requires iron. Br. J. Haematol 117, 727–734 [DOI] [PubMed] [Google Scholar]
- 38. Manicassamy S., Reizis B., Ravindran R., Nakaya H., Salazar-Gonzalez R. M., Wang Y. C., Pulendran B. (2010) Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 329, 849–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Doulatov S., Notta F., Laurenti E., Dick J. E. (2012) Hematopoiesis: a human perspective. Cell Stem Cell 10, 120–136 [DOI] [PubMed] [Google Scholar]
- 40. Seok J., Warren H. S., Cuenca A. G., Mindrinos M. N., Baker H. V., Xu W., Richards D. R., McDonald-Smith G. P., Gao H., Hennessy L., Finnerty C. C., López C. M., Honari S., Moore E. E., Minei J. P., Cuschieri J., Bankey P. E., Johnson J. L., Sperry J., Nathens A. B., Billiar T. R., West M. A., Jeschke M. G., Klein M. B., Gamelli R. L., Gibran N. S., Brownstein B. H., Miller-Graziano C., Calvano S. E., Mason P. H., Cobb J. P., Rahme L. G., Lowry S. F., Maier R. V., Moldawer L. L., Herndon D. N., Davis R. W., Xiao W., Tompkins R. G., and Inflammation and Host Response to Injury, Large Scale Collaborative Research Program (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U.S.A. 110, 3507–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhong Y. F., Holland P. W. (2011) The dynamics of vertebrate homeobox gene evolution: gain and loss of genes in mouse and human lineages. BMC Evol. Biol. 11, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]