Abstract
Introduction:
Mobile phones hold considerable promise for delivering evidence-based smoking cessation interventions that require frequent and objective assessment of smoking status via breath carbon monoxide (Breath CO) measurement. However, there are currently no commercially available mobile-phone-based Breath CO meters. We developed a mobile-phone-based Breath CO meter prototype that attaches to and communicates with a smartphone through an audio port. We then evaluated the reliability and the validity of Breath CO measures collected with the mobile meter prototype and assessed the usability and acceptability of the meter.
Methods:
Participants included 20 regular smokers (≥10 cigarettes/day), 20 light smokers (<10 cigarettes/day), and 20 nonsmokers. Expired air samples were collected 4 times from each participant: twice with the mobile meter and twice with a commercially available Breath CO meter.
Results:
Measures calculated by the mobile meter correlated strongly with measures calculated by the commercial meter (r = .96, p < .001). Additionally, the mobile meter accurately distinguished between smokers and nonsmokers. The area under the receiver-operating characteristic curve for the mobile meter was 94.7%, and the meter had a combined sensitivity and specificity of 1.86 at an abstinence threshold of ≤6 ppm. Responses on an acceptability survey indicated that smokers liked the meter and would be interested in using it during a quit attempt.
Conclusions:
The results of our study suggest that a mobile-phone-based Breath CO meter is a reliable, valid, and acceptable device for distinguishing between smokers and nonsmokers.
INTRODUCTION
Mobile phones have been adopted more rapidly than any other consumer technology in human history (Rainie & Wellman, 2012). Worldwide, the number of mobile phone subscriptions is approaching 7 billion (International Telecommunication Union, 2013). In the United States, more than 90% of all adults own cell phones (Rainie, 2013), and among adults with diverse demographic profiles—including racial and ethnic minorities, individuals living in rural communities, individuals from lower income households, and individuals with no college education—cell phone ownership either approaches or exceeds 90%.
One factor contributing to the rapid dissemination of mobile phone technology is the rise in popularity of smartphones. The majority of U.S. adults, 56%, now own smartphones, and this percentage is substantially higher among young adults (18–29 years of age). Even among young adults with annual household incomes less than $30,000, 77% own smartphones (Smith, 2013). Because smartphones are widely used and well integrated into the daily routines of millions of cigarette smokers, they are promising tools for delivering evidence-based smoking cessation interventions via text messaging (see Whittaker et al., 2012 for a review) and smartphone applications (i.e., “apps”; Backinger & Augustson, 2011).
Although many of the smoking cessation interventions that are currently available on smartphones are not evidence based (Abroms, Padmanabhan, Thaweethai, & Phillips, 2011), a growing number of researchers are capitalizing on advances in information and communication technology by delivering evidence-based smoking cessation interventions (e.g., contingency management) via mobile phones (Hertzberg et al., 2013) and personal computers (e.g., Meredith, Grabinski, & Dallery, 2011; see Dallery & Raiff, 2011 for a review). An important feature of these interventions is objective assessment of smoking status through frequent monitoring of breath carbon monoxide (Breath CO; Stitzer & Bigelow, 1982), a biochemical marker of cigarette smoking (Benowitz et al., 2002). Objective assessment of smoking is needed because smokers often misclassify themselves as nonsmokers during quit attempts (Noonan, Jiang, & Duffy, 2013; Sillett, Wilson, Malcolm, & Ball, 1978). In addition, some evidence suggests that Breath CO monitoring alone can help promote smoking reduction (Beard & West, 2012). However, the therapeutic benefits of this practice are likely enhanced when combined with other behavioral treatment strategies (e.g., awarding financial incentives contingent on abstinence; Meredith & Dallery, 2013).
Because mobile phones give researchers and practitioners unprecedented access to smokers’ behavior, this communication technology has the potential to significantly enhance behavioral interventions that require frequent and sustained Breath CO monitoring. Yet, to our knowledge, there are no commercially available mobile-phone-based Breath CO meters. Thus, the purpose of the current study was to evaluate the reliability, validity, and acceptability of a mobile-phone-based Breath CO meter prototype to assess smoking status.
We developed a compact and portable Breath CO detector that attaches to and communicates with a smartphone through an existing audio port using HiJack technology (Kuo, Verma, Schmid, & Dutta, 2010). In addition, we developed an app that can be used to calibrate the CO sensor, display Breath CO measures, and send CO data to a remote server. The current manuscript describes an investigation of the device’s usability and acceptability, as well as an evaluation of the reliability and validity of the device’s measurements.
METHODS
Participants
Participants (n = 60) were recruited from Gainesville, FL, and surrounding communities through print media and word of mouth. They were divided into three groups based on self-reported frequency of cigarette smoking during an initial phone screening: regular smokers (≥10 cigarettes/day; n = 20), light smokers (1–9 cigarettes/day; n = 20), and nonsmokers (0 cigarettes/day; n = 20). Qualified smokers reported smoking their last cigarette within the previous 24hr of phone screening. The University of Florida Institutional Review Board approved all study procedures.
Materials
Expired air samples were collected using a custom mobile CO meter prototype and a piCO+ Smokerlyzer® (Bedfont Scientific Ltd.). The mobile meter prototype consisted of two components: a smartphone and an attachment containing a CO sensor. The smartphone was an Apple iPhone 4 running iOS. (Notably, any iOS device [e.g., iPad or iPod] will work with the attachment.) The smartphone was loaded with a custom application that displayed instructions for use. It also displayed the current and most recent maximum CO concentrations (in ppm to the hundredths decimal place). The application facilitated 2-point calibration (i.e., 0 and 20 ppm CO), it allowed users to reset the maximum CO concentration to 0 prior to each use, and it allowed users to transfer data over a cellular or WiFi connection for remote data collection. The attachment connected to the smartphone through an existing audio headset port. The port had two outputs (right and left audio channels) and one input (a microphone channel). These channels were used to power and configure the CO sensor and receive data from the sensor. The attachment included an electrochemical CO sensor cell (2CF CiTiceL®, City Technology, Ltd.), signal conditioning electronics, embedded processing, and support circuitry, all housed in a custom 3D-printed enclosure (1.25″ × 2″ × 2″). Cell output was conditioned using analog front end (AFE) LMP91000 (Texas Instruments, Inc.). This integrated circuit converted a small current that the sensor cell generated into a digital value. A microcontroller (MSP430F1611, Texas Instruments, Inc.) configured and read the AFE and communicated with the smartphone application. Data were digitally encoded and processed by the microcontroller and smart phone application. Power and data circuits and communication were based on the HiJack system (Kuo et al., 2010). An image of the mobile-phone-based Breath CO meter prototype can be found in the Supplementary Material.
The Smokerlyzer® and mobile meter were calibrated at least every 6 months using gas with a 20 ppm CO concentration (per manufacturers’ recommendations). In addition, the 20 ppm gas was used in quality control (QC) checks of the mobile meter and Smokerlyzer® on the morning of every session day. Meters were recalibrated when results of QC checks were outside a margin of error of ±2 ppm.
Procedure
At intake, participants provided informed consent and completed a psychosocial questionnaire that included questions about demographics and smoking history, including the Fagerström Test for Nicotine Dependence (FTND)—a six-item questionnaire assessing nicotine dependence with a scale ranging from 0 to 10 (higher scores representing greater dependence; Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
Each participant provided four breath samples: two samples were collected and analyzed with the mobile CO meter prototype and two with the piCO+ Smokerlyzer®. The sequence of measurements was standardized across all participants, such that the first sample was collected with the mobile meter and, thereafter, samples were analyzed by alternating between the Smokerlyzer® and mobile meter (i.e., ABAB design).
Before providing a breath sample, participants were instructed to take a deep breath, hold it for 15 s, and exhale slowly into the meter. Research staff recorded the exhale duration and Breath CO level (in ppm) of each sample. A minimum 5 m inter-sample interval was required to elapse between each breath sample. Smokers completed a usability and acceptability survey about the mobile CO meter prototype immediately following the first Breath CO measurement. Participants rated the usability and acceptability of the mobile meter on a Visual Analog Scale (VAS; range 0–100, wherein 0 = strongly disagree and 100 = strongly agree) across several dimensions (e.g., ease of use, portability, and likelihood of using the device during a quit attempt), and they also answered several open-ended questions (e.g., “What did you like least about this device?”). At the end of each experimental session, participants received a $40 retail gift card.
Data Analysis
Mixed factorial analyses of variance (ANOVAs) were conducted to assess differences in Breath CO levels and duration of exhalation between the three smoking groups (i.e., regular smokers, light smokers, and nonsmokers) and between the four breath samples (i.e., two from the mobile meter and two from the Smokerlyzer®). Post-hoc comparisons were conducted when main effects were observed. Independent-samples t tests were conducted to assess differences in FTND scores, the number of cigarettes smoked per day, and time since last cigarette. Results of the main effects were deemed statistically significant at p < .05. Results of post-hoc analyses were deemed statistically significant according to Bonferroni error corrections.
Pearson product-moment correlation coefficients were calculated to assess the relationship between Breath CO measures obtained within and across the mobile meter and the Smokerlyzer®. In addition, because previous research suggests that duration of exhalation may influence Breath CO in expired air (Raiff, Faix, Turturici, & Dallery, 2010), correlations were calculated for the within-subject differences between Breath CO measures and differences between corresponding durations of exhalation to determine if changes in exhale duration were correlated with changes in Breath CO.
The sensitivity and specificity of the mobile meter and Smokerlyzer® were calculated at different Breath CO abstinence thresholds. In this context, sensitivity and specificity refer to the ability of the instrument to accurately detect recent smoking or abstinence, respectively. Sensitivity was calculated by determining the proportion of Breath CO samples provided by smokers that were positive (i.e., above the CO abstinence threshold; true positives) across a range of CO values. Specificity was calculated by determining the proportion of Breath CO samples provided by nonsmokers that were negative (i.e., at or below the CO abstinence threshold; true negatives) across a range of CO values. Self-reported smoking status was used as the standard to classify Breath CO measures as true positives, false positives, true negatives, or false negatives.
Receiver-operating characteristics (ROC) curves were generated for both the mobile meter and the Smokerlyzer® by plotting the percentage of true positives (i.e., sensitivity) against the percentage of false positives (i.e., 100 − specificity). Area-under-the-curve (AUC) and SE were calculated for each plot and compared using a nonparametric method to assess differences in two or more dependent ROC curves (DeLong, DeLong, & Clarke-Pearson, 1988). The ROC analysis was conducted with MedCalc Statistical Software V12.7.2. All other statistical analyses were conducted with IBM® SPSS® Statistics V21.0.
RESULTS
During phone screenings, all participants who were classified as regular smokers or light smokers reported smoking their last cigarette within the previous 24hr. However, self-report data collected from participants during experimental sessions, which typically occurred several days following phone screenings, showed that three light smokers had not smoked a cigarette within the previous 24hr. Due to the short half-life of Breath CO (3–6hr; Benowitz et al., 2002), breath analysis would not be expected to detect CO from cigarette smoking among these subjects (Javors, Hatch, & Lamb, 2005); thus, their data were excluded from analyses.
Smoking Characteristics
Regular smokers and light smokers differed in FTND scores [t(34) = −4.83, p < .001], number of cigarettes smoked per day [t(34) = −6.21, p < .001], and time since last cigarette [t(35) = 2.25, p < .05]. Regular smokers scored higher than light smokers on the FTND (M = 6.3±1.28 vs. M = 3.2±2.43), smoked more cigarettes than light smokers (M = 21±9.12 vs. M = 6.7±2.73), and reported significantly shorter average time since last cigarette than light smokers (M = 22.5±15.79 vs. M = 121.6±196.88).
Breath CO
A mixed factorial ANOVA revealed significant differences in Breath CO across the four within-subject breath samples [i.e., two measures from the mobile meter and two from the Smokerlyzer®; F(3, 162) = 46.43, p < .001], and across the three smoking groups [i.e., regular smokers, light smokers, and nonsmokers; F(2, 54) = 46.43, p < .001], as well as a significant interaction between Breath CO and smoking group [F(6, 165) = 32.09, p < .001]. With the mobile meter, regular smokers provided significantly higher Breath CO measures (M = 29.9 ppm ± 12.28) than light smokers (M = 13.0 ppm ± 12.30, p < .001), and both regular smokers and light smokers provided significantly higher Breath CO measures than nonsmokers (M = 3.2 ppm ± .82, p < .001). Similarly, with the Smokerlyzer®, regular smokers provided significantly higher Breath CO measures (M = 43.5 ppm ± 18.15) than light smokers (M = 13.8 ppm ± 12.06, p < .001), and both regular smokers and light smokers provided significantly higher Breath CO measures than nonsmokers (M = 4.2 ppm ± 1.81, p < .001). No significant differences were observed in duration of exhalation between the four breath samples or between the three smoking groups.
Post-hoc analyses revealed that, across all smoking groups, the first and second Breath CO measures from the mobile meter prototype were similar and not significantly different from each other (M = 15.4 and 15.6 ppm, respectively, p = .616). However, the second measure from the Smokerlyzer® was significantly higher than the first measure (M = 19.9 and 21.7 ppm, p < .001). In addition, Breath CO measures from the Smokerlyzer® were significantly higher than those from the mobile meter [t(113) = −7.13, p < .001].
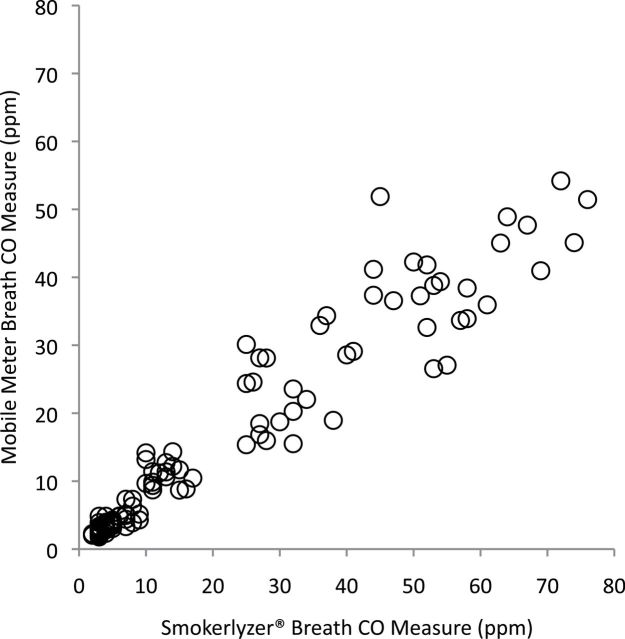
The first and second Breath CO measures taken with the mobile meter were strongly correlated with each other (r = .98, p < .001), as were the first and second measures taken with the Smokerlyzer® (r = .99, p < .001). As shown in Figure 1, the Breath CO measures taken with the mobile meter were also strongly correlated with the measures taken with the Smokerlyzer® (r = .96, p < .001).
Figure 1.
Correlation between breath carbon monoxide (CO) measures taken with the mobile meter prototype and the Bedfont piCO+ Smokerlyzer®.
Differences in Breath CO measures and differences in durations of exhalation observed across the two measures taken with the mobile meter were moderately correlated with one another (r = .51, p < .001). Differences in Breath CO measures and differences in durations of exhalation across the two measures taken with the Smokerlyzer® were also moderately correlated (r = .36, p < .01). Thus, a modest relationship between Breath CO and duration of exhalation was observed, such that longer durations of exhalation corresponded with higher Breath CO measures among samples taken with the same meter. However, this relationship was considerably weaker across the two meters. The differences in Breath CO and the differences in durations of exhalation across the first measures from each meter were not significantly correlated (r = .21, p = .123), and these differences across the second measures taken with each meter were only weakly correlated (r = .27, p < .05).
Sensitivity and Specificity
Sensitivity and specificity among several potential Breath CO abstinence thresholds for both the mobile meter and the Smokerlyzer® are shown in Table 1. For the mobile meter, the abstinence threshold with the highest combined sensitivity and specificity (1.86) was ≤6 ppm. Thus, an abstinence threshold of ≤6 ppm was the optimal cutoff for distinguishing between smokers and nonsmokers. For the Smokerlyzer®, the abstinence threshold with the highest combined sensitivity and specificity (1.83) was ≤9 ppm. Although only the most clinically relevant thresholds (≤1–10 ppm) are displayed in Table 1, thresholds up to ≤15 ppm were tested. Conclusions were not altered when these additional thresholds were included in the analysis.
Table 1.
Sensitivity and Specificity of Mobile-Phone-Based Breath CO Meter Prototype and Bedfont piCO+ Smokerlyzer®
| Abstinence thresholds for mobile meter prototype (ppm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Sensitivity | 1.00 | 1.00 | 0.98 | 0.93 | 0.86 | 0.86 | 0.85 | 0.83 | 0.79 | 0.74 |
| Specificity | 0.00 | 0.03 | 0.43 | 0.85 | 0.95 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Sensitivity + specificity | 1.00 | 1.03 | 1.41 | 1.78 | 1.81 | 1.86 | 1.85 | 1.83 | 1.79 | 1.74 |
| Abstinence thresholds for Smokerlyzer® (ppm) | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Sensitivity | 1.00 | 1.00 | 0.98 | 0.91 | 0.90 | 0.88 | 0.85 | 0.83 | 0.83 | 0.79 |
| Specificity | 0.00 | 0.10 | 0.45 | 0.70 | 0.85 | 0.85 | 0.93 | 0.95 | 1.00 | 1.00 |
| Sensitivity + specificity | 1.00 | 1.10 | 1.43 | 1.61 | 1.75 | 1.73 | 1.78 | 1.78 | 1.83 | 1.79 |
Note. CO = carbon monoxide. Abstinence thresholds with highest combined sensitivity and specificity are in bold.
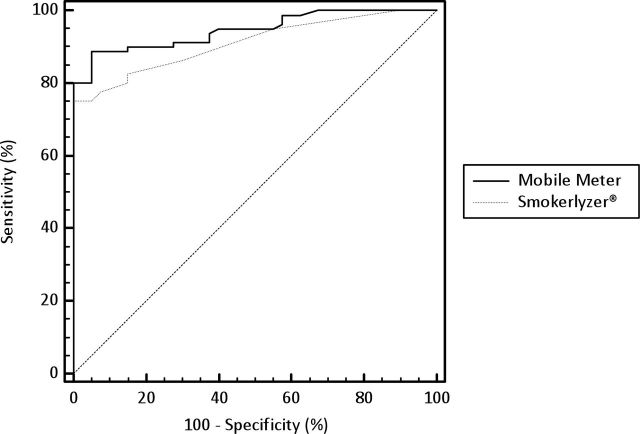
Figure 2 shows the ROC curves for the mobile meter and Smokerlyzer®. The AUCs for the mobile meter (94.7%, SE = 1.9%) and the Smokerlyzer® (91%, SE = 2.5%) were significantly different (p < .05).
Figure 2.
Receiver-operating characteristics curves for the mobile meter prototype and the Bedfont piCO+ Smokerlyzer®.
Usability and Acceptability
Data from smokers (n = 37) who completed a usability and acceptability survey are shown in Table 2. Notably, these participants had a mean age of 42 (SD = 13), 43% of them were female, 14% were Hispanic, and 30% were White. In addition, 62% had no college education and 68% earned $200 or less per month. These participants rated the mobile meter favorably on a VAS across all usability and acceptability dimensions that were evaluated, including ease of use (M = 86±19) and portability (M = 84±22). In addition, many smokers indicated that they would be interested in using the device for self-monitoring during a quit attempt (M = 74±28) or to allow clinicians to monitor their smoking status within the context of a contingency management intervention (M = 78±25). In response to open-ended questions, participants indicated that what they liked best about the device was that it was easy to use (e.g., “It was easy”; n = 15), it was compact or portable (e.g., “Portability”; n = 9), it was informative (e.g., “It let me know what’s in my lungs”; n = 8), it was quick (e.g., “Quick readings and results”; n = 7), it was accurate (e.g., “Looks accurate”; n = 4), and it was quiet (“It was quiet”; n = 1). Participants indicated that what they liked least about the device was holding their breath or exhaling for several seconds (e.g., “Having to hold breath”; n = 4), that the numbers on the display were too small (e.g., “Numbers are small”; n = 2), that the mouthpiece was attached directly to the phone (e.g., “Scared to blow it off the phone”; n = 2), that it was difficult to use or understand (“Technical terms to lay person”; n = 1), that it was too small (“Should be larger”; n = 1), and that it was only compatible with one type of mobile phone (“So far, it can be used with only one brand of cell phone”; n = 1). Notably, 62% of smokers indicated that they disliked nothing about the device (e.g., “Nothing,” “No dislikes at all”; n = 23). Lastly, changes to the prototype that were suggested by participants included making the numbers on the display easier to read (e.g., “Bigger numbers”; n = 7), making the device more compact (e.g., “The device should be made sleek and small for portable pocket travel”; n = 5), making it compatible with other mobile phones (e.g., “Make it work with all carriers/types of cell phones”; n = 2), making the results private (“A privacy screen”; n = 1), making the app talk (“Have it talk”; n = 1), and modifying the app to store and track CO measures (“Record the daily smoking results to show progress”; n = 1).
Table 2.
Usability and Acceptability of Mobile-Phone-Based Breath CO Meter Prototype
| Survey item | M | SD |
|---|---|---|
| This device works well with few or no errors | 78 | 20 |
| This device is easy to use | 86 | 19 |
| This device is compact and portable | 84 | 22 |
| This device works quickly | 87 | 18 |
| The display on this device is easy to read | 85 | 21 |
| The display on this device is easy to understand | 85 | 21 |
| If I wanted to quit smoking, I would use this device on a daily basis to track my smoking | 74 | 28 |
| If I wanted to quit smoking, I would use this device on a daily basis so clinicians could track my smoking and provide incentives when my readings indicate that I am smoke free | 78 | 25 |
Note. CO = carbon monoxide. Rating scale ranged from 0 (strongly disagree) to 100 (strongly agree).
DISCUSSION
The results of the current study show that Breath CO measures collected with a mobile-phone-based Breath CO meter prototype are reliable. The two measures that were collected with the mobile meter were strongly correlated with each other (r = .98). Further, the measures were strongly correlated with a commercially available Breath CO meter, the Bedfont piCO+ Smokerlyzer® (r = .96; see Figure 1). Notably, the largest differences observed between Breath CO measures taken with the two meters occurred among regular smokers. On average, regular smokers provided higher Breath CO measures with the Smokerlyzer® (M = 43.5 ppm) than the mobile meter (M = 29.9 ppm). Importantly, however, this difference was less pronounced among nonsmokers and light smokers (i.e., smokers with Breath CO levels within the clinically important range that includes abstinence thresholds). Nevertheless, future studies should examine whether there is a restriction of range problem with the mobile meter. That is, whether the meter is less accurate at higher CO levels.
Overall, the results of the study suggest that measures taken with the mobile meter were valid. Although Breath CO measures collected with the mobile meter were consistently lower than measures collected with the Smokerlyzer® (Figure 1), in the context of a smoking cessation intervention, the most important function of a Breath CO meter is to detect recent smoking or abstinence. The mobile meter performed this function well. In fact, the area under the ROC curve was even higher for the mobile meter than it was for the Smokerlyzer® (see Figure 2). Using an abstinence threshold of ≤6 ppm, the mobile meter had a combined sensitivity and specificity of 1.86—a sum that is comparable with commercially available Breath CO meters. For example, the highest combined sensitivity and specificity for the Vitalograph (Vitalograph Inc.) was 1.94 at ≤2 ppm (Cropsey, Eldridge, Weaver, Villalobos, & Stitzer, 2006), 1.7 at ≤4 ppm (Perkins, Karelitz, & Jao, 2013), and 1.56 at ≤2 ppm (Javors et al., 2005); the highest combined sensitivity and specificity for the Bedfont EC-50 Smokerlyzer® was 1.73 at ≤6 ppm (Deveci, Deveci, Acik, & Ozan, 2004) and 1.77 at ≤5 ppm (Middleton & Morice, 2000); and the highest combined sensitivity and specificity for the piCO+ Smokerlyzer® was 1.92 at ≤4 ppm (MacLaren et al., 2010), 1.89 at ≤4 ppm (Raiff et al., 2010), 1.88 at ≤7 ppm (Erb, Raiff, Meredith, and Dallery, in press), and 1.83 at ≤9 ppm (in the current study).
Notably, the variability in the abstinence threshold recommendations that have emerged from research and industry suggests that a determination of which threshold to use in a smoking cessation intervention should be based, in part, on the make and model of the instrument being used to measure Breath CO. However, as indicated above, even studies that used the same model instrument have found optimal abstinence thresholds that are substantially different from one another. In such cases, these discrepancies could be due to differences in the versions of the instrument used in each study (i.e., even products with the same brand name are periodically updated by manufacturers with new firmware, software, or hardware). For example, the piCO+ Smokerlyzer® that was used in MacLaren et al. (2010) and Raiff et al. (2010) was discontinued in 2011 (i.e., prior to the release of the model used in the current study; J. Aversano, coVita™, personal communication, August 28, 2013). Advances in technology adopted by manufacturers of the Smokerlyzer® (e.g., reducing cross-sensitivity to hydrogen; automating calibration) may have influenced the sensitivity and/or specificity of this instrument.
Variability among empirically derived Breath CO abstinence thresholds could also be due to other variables (e.g., ambient CO in the local environment [Crowley, Andrews, Cheney, Zerbe, & Petty, 1989]; duration of breath-holding [West, 1984]). Some environments, populations, and instruments may require the use of higher or lower abstinence thresholds than others. Thus, researchers and practitioners may benefit from conducting their own investigations into the optimal abstinence threshold for a smoking cessation intervention given their unique environment, target population, breath sampling procedure, and Breath CO meter. A determination of which abstinence threshold to use may also depend on treatment goals. Researchers or practitioners may find it more important to capture all true negatives and less important to capture all true positives (or vice versa). Notably, Table 1 shows that, for both meters, a range of CO values (e.g., ≤4–10 ppm) could function as relatively accurate abstinence thresholds (i.e., combined sensitivity and specificity > 1.6). Nevertheless, more systematic investigations of optimal Breath CO abstinence thresholds are still needed; specifically, more prospective empirical studies designed to collect repeated measures of Breath CO among larger samples of light smokers and nonsmokers. To date, many investigations of optimal abstinence thresholds have relied on secondary analyses of preexisting datasets that included only a limited number of within-subject measurements collected from light smokers.
Results from the current study show that the correlation between Breath CO and exhale duration difference scores was slightly stronger among measures taken with the mobile meter (r = .51) relative to the Smokerlyzer® (r = .36). Although this difference may reflect greater sensitivity of the mobile meter to exhale duration, it may also reflect a difference in how Breath CO measures were calculated with the mobile meter relative to calculations made by the Smokerlyzer®. That is, measurements taken with the mobile meter were calculated to two decimal places and, thus, had greater precision than the whole number values that were calculated by the Smokerlyzer®.
In addition to demonstrating the reliability and validity of Breath CO measurements taken with the mobile meter prototype, results of the current study also demonstrate that smokers liked the mobile meter and that they would be interested in using the device during a quit attempt (see Table 2). Participants’ comments and responses to open-ended questions indicated that many of them were enthusiastic about the device. For example, in response to the question, “What did you like least about this device?” one participant wrote, “That I don’t have one!” Another participant remarked that the mobile meter is “amazing,” and one participant suggested that the meter should be sold “in stores and at a low price because a lot of low-income people are smokers and would want to use it.” The favorable opinions of the mobile meter that were reported by smokers as well as their self-reported willingness to use the device during a quit attempt are important findings given that reinforcing participant engagement in technology-based smoking cessation interventions is critical to treatment success (Richardson et al., 2013).
The results of the current study should be interpreted within the context of several limitations. First, the research staff relied on self-report as the standard for distinguishing between smokers and nonsmokers. Future studies evaluating new Breath CO meters should examine participants’ salivary or urinary cotinine levels to verify smoking status. Second, many factors that have been shown to contribute to elevated Breath CO were not measured or analyzed among participants in the current study. These factors include: recent smoking of cannabis (Wu, Tashkin, Djahed, & Rose, 1988), recent exposure to passive tobacco smoke (Jarvis, Russell, & Feyerabend, 1983) or other ambient sources of CO (e.g., air pollution; Crowley et al., 1989), lung capacity (Terheggen-Lagro, Bink, Vreman, & van der Ent, 2003), chronic lung disease (e.g., chronic obstructive pulmonary disease; Sato et al., 2003), and other health conditions (e.g., lactose intolerance; McNeill, Owen, Belcher, Sutherland, & Fleming, 1990). Future studies designed to evaluate optimal Breath CO abstinence thresholds should control for these variables. Third, breath samples were collected in a controlled laboratory setting. Future studies should evaluate the reliability and validity of the mobile meter in smokers’ natural environments. Future studies should also evaluate how frequently the mobile meter needs to be recalibrated both within the laboratory and in the natural environment.
Another potential limitation of the current study is the sequence in which breath samples were collected and analyzed. Samples were collected from each participant with the mobile meter first. This procedure allowed researchers to administer the usability and acceptability questionnaire before introducing the commercially available meter to participants. Although some previous research suggests that there are no significant sequence effects on Breath CO measures when repeated measurements are taken within close temporal proximity (Raiff et al., 2010), results from the current study showed that the second measure taken with the Smokerlyzer® (M = 21.7 ppm) was significantly higher than the first measure (19.9 ppm). Importantly, Jarvis, Belcher, Vesey, and Hutchison (1986) also found that subsequent Breath CO measures were higher than preceding measures. Thus, future studies designed to compare the accuracy of multiple Breath CO meters should control for potential sequence effects.
The results of the current study suggest that a mobile-phone-based Breath CO meter is a reliable, valid, and acceptable device for distinguishing between smokers and nonsmokers. Moreover, because the majority of the population owns smartphones, this device holds exceptional promise as a remote-monitoring tool to help researchers and practitioners deliver evidence-based smoking cessation interventions that require objective assessment of smoking status.
SUPPLEMENTARY MATERIAL
Supplementary Material can be found online at http://www.ntr.oxfordjournals.org
FUNDING
This research was supported by the National Institutes of Drug Abuse grants P30DA029926 (Center for Technology and Behavioral Health) and T32DA007209, the National Science Foundation grants 0964120 (“CNS-Ne TS”) and 1059372 (“CI-ADDO-NEW”), the Development Impact Lab (USAID Cooperative Agreements AID-OAA-A-13-00002 and AID-OAA-A-12-00011), the U.S. Agency for International Development, and a gift from Texas Instruments. The funding sources had no other role in the research, in manuscript preparation, or in the decision to publish. Any findings, conclusions, or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the funding agencies.
DECLARATION OF INTERESTS
AR has consulted with CO2Meter, Inc. PD has consulted with Seeed Technology Inc. and IntelliQuit, LLC.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge B. Jarvis and R. Patel for their help with participant recruitment and data collection.
REFERENCES
- Abroms L. C., Padmanabhan N., Thaweethai L., Phillips T. (2011). iPhone apps for smoking cessation: A content analysis. American Journal of Preventive Medicine, 40, 279–285. 10.1016/j.amepre.2010.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backinger C. L., Augustson E. M. (2011). Where there’s an app, there’s a way? American Journal of Preventive Medicine, 40, 390–391. 10.1016/j.amepre.2010.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard E., West R. (2012). Pilot study of the use of personal carbon monoxide monitoring to achieve radical smoking reduction. Journal of Smoking Cessation, 7, 12. 10.1017/jsc.2012.1 [Google Scholar]
- Benowitz N. L., Jacob P., Ahijevych K., Jarvis M. F., Hall S., LeHouezec J, … Velicer W. (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4, 149–159. 10.1080/14622200210123581 [DOI] [PubMed] [Google Scholar]
- Cropsey K. L., Eldridge G. D., Weaver M. F., Villalobos G. C., Stitzer M. L. (2006). Expired carbon monoxide levels in self-reported smokers and nonsmokers in prison. Nicotine & Tobacco Research, 8, 653–659. 10.1080/14622200600789684 [DOI] [PubMed] [Google Scholar]
- Crowley T. J., Andrews A. E., Cheney J., Zerbe G., Petty T. L. (1989). Carbon monoxide assessment of smoking in chronic obstructive pulmonary disease. Addictive Behaviors, 14, 493–502. :10.1016/0306-4603(89)90069-5 [DOI] [PubMed] [Google Scholar]
- Dallery J., Raiff B. R. (2011). Contingency management in the 21st century: Technological innovations to promote smoking cessation. Substance use & Misuse, 46, 10–22. 10.3109/10826084.2011.521067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong E. R., DeLong D. M., Clarke-Pearson D. L. (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics, 44, 837–845 [PubMed] [Google Scholar]
- Deveci S. E., Deveci F., Acik Y., Ozan A. T. (2004). The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respiratory Medicine, 98, 551–556. :10.1016/j.rmed.2003.11.018 [DOI] [PubMed] [Google Scholar]
- Erb P., Raiff B. R., Meredith S. E., Dallery J.(in press). The accuracy of a lower-cost breath carbon monoxide meter in distinguishing smokers from nonsmokers. Journal of Smoking Cessation. [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K.-O. (1991). The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. British Journal of Addiction, 86, 1119–1127 [DOI] [PubMed] [Google Scholar]
- Hertzberg J. S., Carpenter V. L., Kirby A. C., Calhoun P. S., Moore S. D., Dennis M. F, … Beckham J. C. (2013). Mobile contingency management as an adjunctive smoking cessation treatment for smokers with posttraumatic stress disorder. Nicotine & Tobacco Research, 15, 1934–1938. 10.1093/ntr/ntt060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Telecommunication Union. (2013). The world in 2013 Retrieved from www.itu.int/en/ITU-D/Statistics/Documents/facts/ICTFactsFigures2013.pdf
- Jarvis M. J., Belcher M., Vesey C., Hutchison D. C. (1986). Low cost carbon monoxide monitors in smoking assessment. Thorax, 41, 886–887. :10.1136/thx.41.11.886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis M. J., Russell M. A., Feyerabend C. (1983). Absorption of nicotine and carbon monoxide from passive smoking under natural conditions of exposure. Thorax, 38, 829–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javors M. A., Hatch J. P., Lamb R. J. (2005). Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction (Abingdon, England), 100, 159–167. 10.1111/j.1360-0443.2004.00957.x [DOI] [PubMed] [Google Scholar]
- Kuo Y., Verma S., Schmid T., Dutta P. (2010). Hijacking power and bandwidth from the mobile phone’s audio interface Retrieved from http://web.eecs.umich.edu/~prabal/pubs/papers/kuo10hijack.pdf
- MacLaren D. J., Conigrave K. M., Robertson J. A., Ivers R. G., Eades S., Clough A. R. (2010). Using breath carbon monoxide to validate self-reported tobacco smoking in remote Australian indigenous communities. Population Health Metrics, 8, 1–24. 10.1186/1478-7954-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A. D., Owen L. A., Belcher M., Sutherland G., Fleming S. (1990). Abstinence from smoking and expired-air carbon monoxide levels: Lactose intolerance as a possible source of error. American Journal of Public Health, 80, 1114–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith S. E., Dallery J. (2013). Investigating group contingencies to promote brief abstinence from cigarette smoking. Experimental and Clinical Psychopharmacology, 21, 144–154. 10.1037/a0031707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith S. E., Grabinski M. J., Dallery J. (2011). Internet-based group contingency management to promote abstinence from cigarette smoking: A feasibility study. Drug and Alcohol Dependence, 118, 23–30. 10.1016/j.drugalcdep.2011.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton E. T., Morice A. H. (2000). Breath carbon monoxide as an indication of smoking habit. Chest, 117, 758–763. :10.1378/chest.117.3.758 [DOI] [PubMed] [Google Scholar]
- Noonan D., Jiang Y., Duffy S. A. (2013). Utility of biochemical verification of tobacco cessation in the Department of Veterans Affairs. Addictive Behaviors, 38, 1792–1795. 10.1016/j.addbeh.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins K. A., Karelitz J. L., Jao N. C. (2013). Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine & Tobacco Research, 15, 978–982. 10.1093/ntr/nts205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff B. R., Faix C., Turturici M., Dallery J. (2010). Breath carbon monoxide output is affected by speed of emptying the lungs: Implications for laboratory and smoking cessation research. Nicotine & Tobacco Research, 12, 834–838. 10.1093/ntr/ntq090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainie L. (2013). Cell phone ownership hits 91% of adults Retrieved from www.pewresearch.org/fact-tank/2013/06/06/cell-phone-ownership-hits-91-of-adults/
- Rainie L., Wellman B. (2012). Networked: The new social operating system. Cambridge, MA: MIT Press [Google Scholar]
- Richardson A., Graham A. L., Cobb N., Xiao H., Mushro A., Abrams D., Vallone D. (2013). Engagement promotes abstinence in a web-based cessation intervention: Cohort study. Journal of Medical Internet Research, 15, e14. 10.2196/jmir.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Nishimura K., Koyama H., Tsukino M., Oga T., Hajiro T., Mishima M. (2003). Optimal cutoff level of breath carbon monoxide for assessing smoking status in patients with asthma and COPD. Chest, 124, 1749–1754. :10.1378/chest.124.5.1749 [DOI] [PubMed] [Google Scholar]
- Sillett R. W., Wilson M. B., Malcolm R. E., Ball K. P. (1978). Deception among smokers. British Medical Journal, 2, 1185–1186. :10.1136/bmj.2.6146.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. (2013). Smartphone ownership—2013 update Retrieved from http://pewinternet.org/Reports/2013/Smartphone-Ownership-2013/Findings.aspx
- Stitzer M. L., Bigelow G. E. (1982). Contingent reinforcement for reduced carbon monoxide levels in cigarette smokers. Addictive Behaviors, 7, 403–412. :10.1901/jaba.1984.17-477 [DOI] [PubMed] [Google Scholar]
- Terheggen-Lagro S. W., Bink M. W., Vreman H. J., van der Ent C. K. (2003). End-tidal carbon monoxide corrected for lung volume is elevated in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine, 168, 1227–1231. 10.1164/rccm.200302-248OC [DOI] [PubMed] [Google Scholar]
- West R. J. (1984). The effect of duration of breath-holding on expired air carbon monoxide concentration in cigarette smokers. Addictive Behaviors, 9, 307–309. :10.1016/0306-4603(84)90026-1 [DOI] [PubMed] [Google Scholar]
- Whittaker R., McRobbie H., Bullen C., Borland R., Rodgers A., Gu Y. (2012). Mobile phone-based interventions for smoking cessation. Cochrane Database of Systematic Reviews (Online), 11, CD006611. 10.1002/14651858.CD006611.pub3 [DOI] [PubMed] [Google Scholar]
- Wu T. C., Tashkin D. P., Djahed B., Rose J. E. (1988). Pulmonary hazards of smoking marijuana as compared with tobacco. The New England Journal of Medicine, 318, 347–351. 10.1056/NEJM198802113180603 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.