Abstract
Background:
Craving induction in a controlled environment is helpful in the research of craving mechanism and its role in development of alcohol dependence (AD). We describe a novel tool Visual Image-induced Craving for Ethanol (VICE) and its effects on brain activation with pilot functional magnetic resonance imaging (fMRI).
Materials and Methods:
Alcohol-related visual cues (ARCs) in 5 scenarios were photographed, which included pictures of bars, alcoholic beverage bottles, pouring of alcohol into glasses, glasses filled with alcohol, and scenes of people sipping alcohol, counterbalanced with neutral pictures (involving water, milk etc.,). Craving scores were obtained from 15 hospitalized patients with AD to validate this tool. In the pilot fMRI (3-Tesla) study, 5 patients were examined using VICE in a symptom provocation model. Group level-fixed effect analysis of brain activation differences was done using SPM8.
Results:
VICE showed a high internal consistency with Cronbach's α coefficient of 0.86, which confirmed its reliability. Concurrent validity of VICE was demonstrated via its convergence with the Penn Alcohol Craving Scale. ARCs had significantly greater mean craving scores than neutral cues in all the 5 scenarios (intentional validity). In the pilot fMRI, patients were found to have greater activation while viewing ARCs compared to the neutral cues in right insular cortex and deficient activation in right orbitofrontal cortex.
Conclusions:
The VICE is a reliable and valid measure of alcohol craving with promising clinical and translational research implications. Preliminary fMRI findings indicate it can be used as a symptom provocation tool for fMRI experiments.
Keywords: Alcohol, craving, imaging, neurobiology, symptom provocation
INTRODUCTION
Drug craving is defined as a strong or persistent desire for a substance, and is known to play a central role in initiation, maintenance, and as well in triggering a relapse from sobriety. Craving for alcohol among most individuals can be explained based on 3 psychobiological models;[1] reward pathway (dopaminergic/opioidergic dysregulation) where craving is for the stimulating and/or enhancing effects of alcohol, relief pathway (GABAergic/glutamatergic dysregulation) where craving serves to reduce negative hedonic effects or arousal caused by the withdrawal state, and finally, obsessive pathway (serotonin deficiency) which relates to a lack of control over repetitive and intrusive thoughts about drinking.
All 3 pathways of craving may be triggered by exposure to an object, environment, or emotion that an individual has come to associate with alcohol consumption by the ways of classical conditioning. These stimuli are called alcohol-related cues (ARCs). These stimuli range from visual, olfactory, gustatory to auditory cues.
Craving induction in a controlled environment using these ARCs proves very helpful in the research of craving mechanism and its role in development of alcohol dependence;[2] it also helps predict treatment response and prevents relapse by clinical simulation of high-risk situation while in hospital. Various techniques have been used in research to induce craving for alcohol in laboratory settings. These include exposing patients to actual alcoholic beverages/visual representations of alcoholic beverages and, by manipulating mood states using individualized guided imagery exposure of their own recent stressful or relaxing scenarios.[3] The intensity of craving can be rated either by subjective self-reports or objectively by clinicians through behavioral observations or the measurement of autonomic physiological responses. However, success in inducing craving in the laboratory has been inconsistent due difficulty in replicating the induction techniques. Hence, there is a need to develop a novel tool based on subjective self-report scores and then to objectively test it with more proximal physiologic measures (e.g. functional neuroimaging techniques) to get a better insight into the neurobiology of alcohol cue reactivity and craving.
The present study has 2 phases: The first phase concerns with the development and validation of a novel tool - the Visual Image-induced Craving for Ethanol (VICE),[4] which would measure self-reported craving for alcohol among patients with alcohol dependence syndrome (ADS) in Indian settings. In the second phase of the study, this tool was used in a symptom provocation model while patients underwent functional magnetic resonance imaging (fMRI) to compare the brain activation patterns to ARCs and neutral images. Based on the earlier studies, we hypothesized that the neural activation in patients will be significantly different for ARCs compared to neutral cues in orbitofrontal cortex (OFC),[5,6,7,8,9] dorsolateral prefrontal cortex (DLPFC),[10,11] anterior cingulate cortex (ACC),[9,12] insula,[5,12,13] and amygdala.[3,9,14] The study was approved by the Ethics Committee of the National Institute of Mental Health and Neuro Sciences (NIMHANS), Bangalore, India.
MATERIALS AND METHODS
Part 1
Preparation of the tool
A total of 40 alcohol-related visual cues in 5 scenarios were photographed and were counterbalanced with neutral pictures for each image with inputs from 5 qualified psychiatrists and literature review.[2,15] We restricted ARCs to visual stimuli in order to ensure its simplicity and easy replicability for future use. These cues consisted of two types of stimuli — contextual and discrete. The contextual cues are those, which are commonly associated with drug-seeking/procuring rather than drug-taking experiences. These contextual cues could include common locations of bars/liquor storefronts etc. Discrete cues represent images of alcohol itself. The discrete cues were created in 4 stages, which included, unopened alcoholic beverage containers/bottles, pouring of alcohol into glasses, glasses filled with alcohol, and finally images of people sipping alcoholic beverages. We also used stronger ‘appetitive’ neutral cues involving popular non-alcoholic beverages, milk, coffee, tea, and water control for activation related to non-addictive motivated or acquisitive behaviors.
Participants
Fifteen consenting male patients admitted for treatment at the Center for Addiction Medicine, NIMHANS were recruited. They had a primary diagnosis of ADS according to ICD 10. Exclusion criteria were dependence on a substance other than alcohol or nicotine, a lifetime diagnosis of a psychotic disorder and those on disulfiram, anti-depressant, neuroleptic, or antianxiety medications, which may possibly confound the study.
Procedures
Participants were explained that the study was concerned with assessing their craving to various images of alcoholic beverages. Informed consent was obtained. The Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA-II) was used to assess the physical, psychological, and social manifestations of alcohol abuse or dependence and other psychiatric disorders. Craving scores were obtained during an initial assessment phase before active treatment commenced. Penn Alcohol Craving Scale (PACS)[16] was applied before and after VICE was administered. VICE cues were displayed on a 15 inch LCD monitor kept about 60 cm away by means of a laptop. The order of image delivery was fixed and did not change between participants. Each picture was scored on a scale 0 to 5 with a descriptive response. [0-I don't feel like drinking; 1 — If someone offered me my favorite alcohol, I will drink; 2 — If someone offered me alcohol, I will drink; 3 — If I go out of the hospital now, I will drink; 4 — I want to leave now and drink; 5 — Even if you tried to stop me, I would like to leave now and drink]. Patients were asked to vocally respond to the images with scores [Figure 1].
Figure 1.
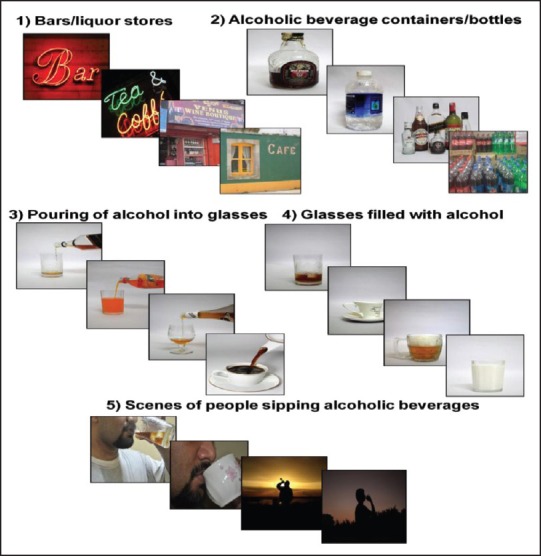
Schematic depiction of alcohol-related visual cues and neutral cues in 5 scenarios
Part 2
Participants
Five admitted and treatment-seeking male patients were recruited after obtaining written informed consent. These were different from the 15 subjects recruited for the first phase. They had a primary diagnosis of ADS with the same inclusion and exclusion criteria as earlier. Those with history of head injury or independent neurological illness or history of hereditary neurological illness, MMSE score <24, any contraindication for undergoing MRI procedure were also excluded. They underwent treatment-as-usual inpatient detoxification with benzodiazepine (for 7-10 days) followed by a drug wash out period of 5 days. Alcohol withdrawal symptoms were rated using the Clinical Institute Withdrawal Assessment for Alcohol-revised (CIWA-Ar).[17] Patients were required to have a score of zero on CIWA-Ar at the end of the detoxification to be included in the fMRI protocol.
VICE fMRI paradigm
A set of 40 images (20 alcohol-related cues and 20 neutral images) were selected from the pool of validated 80 images. The selection was based on higher subjective craving scores for each image reported during the first phase. All patients participated in a 6 minute VICE task, which was run twice. They viewed images presented through a display monitor on to a reflecting mirror mounted on the head coil while undergoing MRI. Patients viewed twelve 30 seconds alternating blocks of alcohol-related cues (discrete and contextual, 4 blocks of 5 images each); neutral images (non-alcoholic beverages, 4 blocks of 5 images each) and control block (blank black screen displayed for 30 seconds). Each image stayed on the screen for 6 seconds. Within this time, patients had to indicate by means of button presses whether they experienced craving for alcohol after seeing the picture or not. The order in which these blocks were presented was fully counterbalanced, all scans started with the control block. The beginning of each block of images was preceded by relevant instruction slide.
Patients underwent a brief training procedure prior to the scan where they were familiarized with the instructions and the task. The pictures used for training were different from what were shown in the scanner.
Image acquisition: Functional MRI (fMRI) scans were obtained with a Siemens 3T Skyra MRI using a 32 channel head coil using a gradient echo EPI sequence. First three scans of each functional time series before the task began were discarded. The scan parameters were as follows: TR = 2000 ms; TE = 30 ms; flip angle = 78; slice thickness = 3 mm; slice order: Descending; number of slice = 37; gap = 25%; matrix = 64 * 64 * 64 mm 3, FOV = 192 * 192, voxel = 3.0 mm isotropic). A total of 360 scans were obtained during the two runs of the paradigm. The blood-oxygen level- dependent (BOLD) response was measured as outcome criterion. The scan data were assessed using Statistical Parametric Mapping (SPM) 8 (http://www.fil.ion.ucl.ac.uk/spm). SPM combines the General Linear Model and Gaussian field theory to draw statistical inferences from BOLD response data regarding deviations from the null hypothesis in three-dimensional brain space. All scans were re-aligned to correct for between-scan movements, slice time corrected, spatially normalized, and smoothed using a 8 mm (FWHM) isotropic Gaussian Kernel followed by first-level design specification and estimation where brain activation differences (ARCS- Neutral Cue/Neutral Cue - ARC) were generated for each subject. Group level-fixed effect analysis was performed to examine for differences in BOLD responses between ARCs and neutral cues. Inclusion masks comprising OFC, DLPFC, ACC, insula, and amygdala were used for analysis based on the a priori regions of interest. These masks were created using automated software.[18] The coordinates of significant areas of activation were transformed from MNI space into the stereotactic space of Talairach and Tournoux using non-linear transformation. With the help of automated software, brain regions were localized from the Talairach and Tournoux coordinates.[19] Activations larger than 10 voxel in size, surviving uncorrected P < 0.001, with 10 mm sphere small volume correction (α = 0.05) were reported.
RESULTS
Part 1
Socio-demographic characteristics
The patients were all male, right-handed, with an average age of 33.1 years. The mean years of education were 11.2 years. The average age at which the patients started drinking was 16.6 years; the average age at onset of alcohol-related problems was 19.8 years. The average number of drinking days per month within the past year was 285 days, and the average amount of alcohol consumed per drinking day in the past year was 18 standard drinks. A family history of alcohol dependence was noted in 48% of the patients.
Reliability
VICE showed a high internal consistency with a Cronbach's Alpha of 0.86, which confirmed its reliability.
Concurrent validity
Concurrent validity of the VICE scale was demonstrated via its convergence with a commonly used measure for assessing craving -Penn Alcohol Craving Scale (PACS) (r = 0.61, P = 0.008).
Intentional validity
Alcohol-related cues (ARC) (1.37 ± 0.32) had significantly greater mean craving scores compared to non-alcohol neutral cues (0.03 ± 0.04) in all the 5 scenarios, which proves the intentional validity of the tool and that the constructs we chose adequately represented what we intend to study (t = 15.89, P < 0.001). The images of bars/liquor stores, alcoholic beverage containers/bottles, and pouring of alcohol into glasses were scored the highest and had significant positive correlation with PACS. Mean craving scores reported were less than half of the scale maximum [Figure 2].
Figure 2.
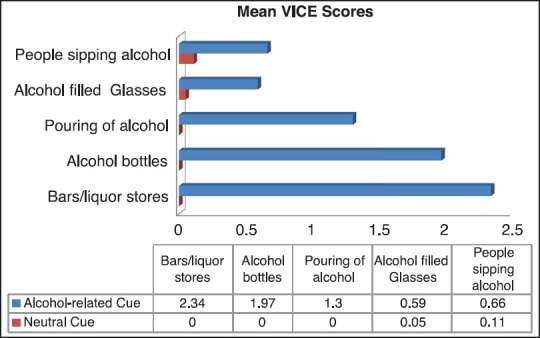
Mean VICE scores in 5 scenarios for alcohol-related cues and neutral cues
Part 2
Socio-demographic characteristics
The patients were all male, with an average age of 34.2 years. The mean years of education were 11.4 years. The average age at which the patients started drinking was 19.2 years; the average age at onset of alcohol-related problems was 21.4 years. The average number of drinking days per month within the past year was 255.5 days, and the average amount of alcohol consumed per drinking day in the past year was 15 standard drinks. A family history of alcohol dependence was noted in all patients.
Image analysis
Patients were found to have significantly greater activation on viewing ARCs compared to the neutral cues in right insular cortex (Brodmann Area 13, Talairach and Tournoux co-ordinates: X = 40, Y = −30, Z = 18; T value = 3.77) and significantly deficient activation in right orbitofrontal cortex (Brodmann Area 47, Talairach and Tournoux co-ordinates: X = 40, Y = 25, Z = −6; T value = 3.90) [Figures 3 and 4].
Figure 3.
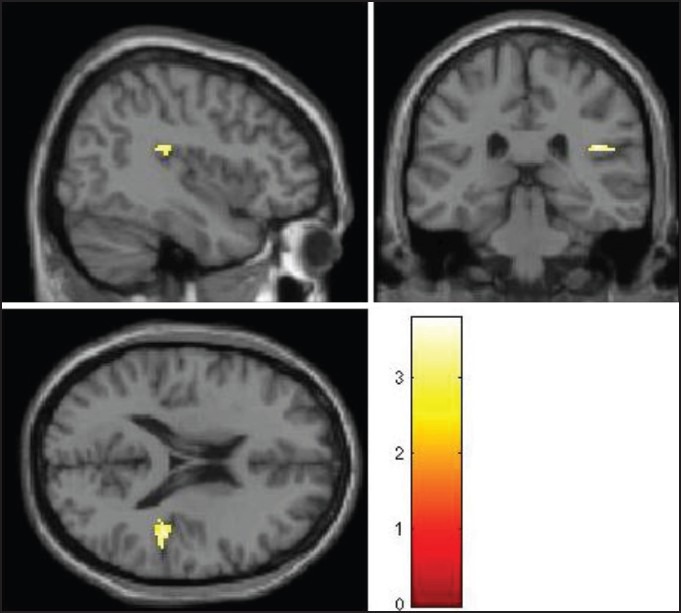
Significantly greater activation with alcohol-related cues compared to the neutral beverage cues in right insular cortex
Shown at uncorrected P < 0.005. The colored bars are representative of t scores mentioned in the text.
Figure 4.
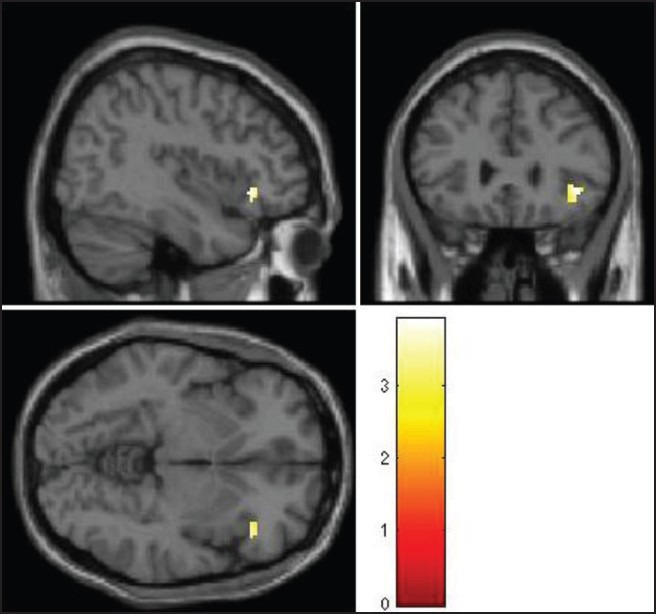
Right orbitofrontal cortex (OFC) with significantly deficient activation with alcohol-related cues compared to the neutral beverage cues
Shown at uncorrected P < 0.005. The colored bars are representative of t scores mentioned in the text.
DISCUSSION
Part 1
We examined the reliability and validity of a novel visual ARCs-based tool in a small sample of patients having ADS. The VICE was found to have good psychometric properties. Its internal consistency, as assessed by Cronbach's α coefficient, is excellent. The convergent validity was also demonstrated via correlation analyses with a known alcohol craving scale — PACS. The tool also demonstrates a high intentional validity of the construct chosen. Traditionally, cue reactivity paradigms have mostly used discrete stimuli (drug/alcohol cues itself). But, our study revealed that contextual cues (common locations of bars/liquor storefronts) scored significantly higher on craving than discrete stimuli. One possible reason is that drug-seeking/procuring experiences may lead to more intense anticipatory rush causing more craving. In addition, these visual cues may more closely resemble ‘real life’ cues than just pictures of alcohol in a plain background. Mean craving scores reported were less than half of the scale maximum, possible reasons for this includes reduction in perceived opportunity to drink being in a controlled setting, and fear that a high craving score might cause clinicians or family members to worry about relapse leading to longer hospital stay.
Part 2
The evaluation of a craving inducing paradigm developed in and for use in Indian settings by means of functional MRI is a first, to the best of our knowledge. Since we have restricted the stimuli to visual ARCs, it ensures easy replicability. In our pilot fMRI study, we found that in the patient population studied, the presentation of ARCs versus neutral cues elicited a significantly differential activation of right insula and a relative deficient activation in the right orbitofrontal cortex as evidenced by its increased activation during neutral cues in comparison with ARCs.
The brain activation patterns found in our preliminary study are consistent with other published alcohol cue reactivity neuroimaging studies. Consistent activation have been found in several brain region including mid-brain/limbic system[20,21,22] (ventral striatum, ventral tegmental area, amygdala, insula, and ACC) and prefrontal cortex (PFC)[10] structures (OFC, DLPFC, and ventromedial PFC).
Most studies on neurobiology of alcohol addiction have focused on subcortical reward pathways; however, there is recent surge of interest in the role of insula in the conscious urges to take drugs.[23,24] The insular cortex is believed to store a representation of the pleasurable interoceptive effects of drug use (e.g. alleviation of negative hedonic effects or arousal caused by the withdrawal state such as a tachycardia, sweating, tremor) and that this representation is reactivated by exposure to contextual and discrete cues that have previously been associated with drug use rituals. This processing of the interoceptive effects of drug use by the insula contributes to the explicit motivation to take drugs (e.g. conscious craving impulses) and also influences the decision-making processes. The relative deficient activation of OFC in our subject group could further lead to impaired top-down regulation of these craving impulses, which may finally precipitate a relapse.
The tool will be clinically useful if one can establish predictive validity, i.e. the ability to predict a relapse in the future. One study examined the treatment effects of Naltrexone and Ondansetron on brain activation patterns using ARCS induced craving paradigms found that patients with ADS treated with naltrexone, either alone or in combination with ondansetron, did not experience the ventral striatum activation seen in the placebo-treated patients.[25] It will be interesting to examine whether brain activation during VICE performance (measured using fMRI) can be used predict relapse by doing a follow-up study with a larger sample size.
Strengths of the methodology include the following: Patients were homogenous and free from other co-morbid psychiatric disorders, use of contextual stimuli to account for drug-seeking/procuring experiences, use of stronger ‘appetitive’ neutral cues to control for non-addictive motivated or acquisitive behaviors, 5-day drug wash out period following completion of detoxification to control for effects of alcohol withdrawal and benzodiazepines used. However, the study is limited by its small sample size and lack of healthy control group in both phases. A longitudinal study design would have been ideal to examine the predictive validity. Also, the study has not examined female ADS patients.
To conclude, the VICE is a reliable and valid measure of alcohol craving with promising clinical and translational research implications. Preliminary fMRI findings indicate that the VICE can also be used as a symptom provocation tool for functional brain imaging experiments to facilitate better understanding of the neurobiological underpinnings of craving in ADS.
ACKNOWLEDGMENTS
This study was funded by Center for Addiction Medicine, Department of Psychiatry, NIMHANS. SMA and SVK are supported by the Wellcome Trust / DBT India Alliance.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol. 1999;34:197–222. doi: 10.1093/alcalc/34.2.197. [DOI] [PubMed] [Google Scholar]
- 2.Litt MD, Cooney NL. Inducing craving for alcohol in the laboratory. Alcohol Res Health. 1999;23:174–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 4.Holla B, Viswanath B, Venkatasubramanian G, Maroky AS, Jayarajan D, Benegal V, editors. San Francisco, CA, USA: 35th Annual Research Society on Alcoholism Scientific Meeting; 2012. Visual Image induced Craving for Ethanol (VICE) Poster Presentation. [Google Scholar]
- 5.Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–84. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cerebral cortex. 2000;10:318–25. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med. 2012;4:116ra6. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- 8.Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex: Systematic review of behavioural decision-making and neuroimaging studies. Br J Psychiatry. 2005;187:209–20. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- 9.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 10.George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–52. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- 11.Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: A double-blind, sham-controlled study. Drug Alcohol Depend. 2008;92:55–60. doi: 10.1016/j.drugalcdep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Padula CB, Simmons AN, Matthews SC, Robinson SK, Tapert SF, Schuckit MA, et al. Alcohol attenuates activation in the bilateral anterior insula during an emotional processing task: A pilot study. Alcohol Alcohol. 2011;46:547–52. doi: 10.1093/alcalc/agr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta-analysis and systematic review. Addict Biol. 2013;18:121–33. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(Suppl 2):S189–210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23:1289–95. [PubMed] [Google Scholar]
- 17.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 18.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 19.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 21.Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–83. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- 22.Braus DF, Wrase J, Grüsser S, Hermann D, Ruf M, Flor H, et al. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm. 2001;108:887–94. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- 23.Naqvi NH, Bechara A. The hidden island of addiction: The insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naqvi NH, Bechara A. The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–50. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–75. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]


